Abstract
Angiogenesis is a key aspect of the dynamic changes occurring during the normal ovarian cycle. Hyperplasia and hypervascularity of the ovarian theca interna and stroma are also prominent features of the polycystic ovary syndrome (PCOS), a leading cause of infertility. Compelling evidence indicated that vascular endothelial growth factor (VEGF) is a key mediator of the cyclical corpus luteum angiogenesis. However, the nature of the factor(s) that mediate angiogenesis in PCOS is less clearly understood. Endocrine gland-derived (EG)-VEGF has been recently identified as an endothelial cell mitogen with selectivity for the endothelium of steroidogenic glands and is expressed in normal human ovaries. In the present study, we compared the expression of EG-VEGF and VEGF mRNA in a series of 13 human PCOS and 13 normal ovary specimens by in situ hybridization. EG-VEGF expression in normal ovaries is dynamic and generally complementary to VEGF expression in both follicles and corpora lutea. A particularly high expression of EG-VEGF was detected in the Leydig-like hilus cells found in the highly vascularized ovarian hilus. In PCOS ovaries, we found strong expression of EG-VEGF mRNA in theca interna and stroma in most of the specimens examined, thus spatially related to the new blood vessels. In contrast, VEGF mRNA expression was most consistently associated with the granulosa cell layer and sometimes the theca, but rarely with the stroma. These findings indicate that both EG-VEGF and VEGF are expressed in PCOS ovaries, but in different cell types at different stages of differentiation, thus suggesting complementary functions for the two factors in angiogenesis and possibly cyst formation.
Angiogenesis is a key aspect of normal cyclical ovarian function. Follicular growth and the development of the corpus luteum (CL) are dependent on the proliferation of new capillary vessels. 1 The process of selection of a dominant follicle in monovular species has been also associated with angiogenesis, as there is evidence that selected follicles possess a more elaborate microvascular network than other follicles. 2 The angiogenesis that accompanies CL development also plays a key role in the delivery of cholesterol to luteal cells for progesterone biosynthesis. 3 Subsequently, the blood vessels regress, suggesting the coordinated action of inducers as well as inhibitors of angiogenesis in the course of the ovarian cycle. 4,5
Angiogenesis is also a prominent feature of the polycystic ovary syndrome (PCOS), a leading cause of infertility affecting as many as 5 to 10% of women of reproductive age. PCOS was originally described as a disorder characterized by the association of hirsutism, obesity, reduced fertility, and enlarged polycystic ovaries. 6 Hyperplasia of the theca interna and stroma, with excessive production of androgens, are hallmarks of PCOS. 7 . Indeed, the ultrasonographic assessment of stromal area 8 and blood flow 9 is currently used as diagnostic test. Although PCOS was described more than 50 years ago, its etiology has remained primarily unclear. However, increased luteinizing hormone/follicle-stimulating hormone ratio, defective selection of a dominant follicle, and anovulation are considered to be key aspects of the pathogenesis. Recent evidence also indicates that PCOS is a part of a complex endocrine/metabolic disorder in which insulin resistance plays a major role. 10
Previous studies have shown that the vascular endothelial growth factor (VEGF) mRNA expression is temporally and spatially related to the proliferation of blood vessels in the normal rat, mouse, and primate ovary, suggesting that VEGF may be a mediator of the cyclical growth of blood vessels that occurs in the female reproductive tract. 11,12 Administration of VEGF inhibitors suppresses luteal angiogenesis 13-15 and delays follicular development 16 in rodents and primates. Furthermore, a few studies have implicated VEGF also in the angiogenesis associated with PCOS. 17
More recently, an endothelial cell mitogen with an even greater level of specificity than VEGF has been identified. This molecule, termed endocrine gland-derived (EG)-VEGF, is expressed in the human and primate ovary. 18 Intriguingly, adenovirus-mediated delivery of EG-VEGF induced a strong angiogenic response, accompanied by extensive cyst formation in the ovary, whereas it fails to have significant effects when delivered in other organs such as the skeletal muscle. 18 Similar to VEGF, the expression of EG-VEGF mRNA is up-regulated by hypoxia by a HIF-1α-dependent mechanism. 19 EG-VEGF represents one of a structurally related class of peptides ascribed multiple regulatory functions, including regulation of gastrointestinal motility and circadian rhythms. 19 The first of these molecules, venom protein A, (VPRA), 20 was purified from the venom of the black mamba snake as a nontoxic component. The other members of this family include the digestive enzyme, colipase, 21 the Xenopus head-organizer, dickkopf, 22 and the secreted protein from the toad Bombina variegata, designated Bv8. 23 EG-VEGF, 80% homologous to VPRA, is most likely the human orthologue of this molecule. EG-VEGF and VPRA are closely related, 71% and 76% homologous, respectively, to the Bv8 peptide. Mouse and human orthologues of Bv8 (also known as prokineticin-2) 24 have been recently described.
In the present study we have examined the expression of VEGF and EG-VEGF mRNA by in situ hybridization in a series of normal ovaries and PCOS specimens. The expression of KDR (VEGFR-2) mRNA and CD34 and CD31 proteins were used as markers of the endothelium of blood vessels. Results of these studies show that EG-VEGF may play a critical role, along with VEGF, in both normal and pathological ovarian function.
Materials and Methods
Specimens from 13 patients with PCOS and 13 patients with normal ovaries were used in this study. Tissues were obtained from the University of Michigan and Genomics Collaborative Inc. (Cambridge, MA). Normal and pathological specimens were removed from patients for therapeutic procedures unrelated to this study; they were provided after the appropriate institutional review board review. The PCOS tissues were selected because the ovaries displayed the histological features of PCOS, ie, fibrosis of the ovarian cortex and numerous maturing and atretic follicles. The ages of the PCOS patients ranged from 24 to 38 years, with a mean age of 33 years. Normal ovaries were chosen from women in their third and fourth decade of life. Cycle status was determined by histological criteria. All tissues were fixed in 4% buffered formalin and paraffin-embedded. Sections 5 μm thick were deparaffinized, deproteinated in 4 μg/ml proteinase K for 30 minutes at 37°C, and further processed for in situ hybridization as previously described. 25,26 [33P]UTP-labeled sense and anti-sense riboprobes were hybridized at 55°C overnight, followed by a high stringency wash at 55°C in 0.1× standard saline citrate for 2 hours. Before dipping the slides in photographic emulsion, the dry glass slides were exposed for 3 days at room temperature to Kodak BioMax MR autoradiographic film. Films from parallel sections of ovaries hybridized with VEGF and EG-VEGF probes were digitized on a flat-bed scanner (Canon FB1200S), selected images overlaid using PhotoShop, and false colored in red or green, and adjusted to enhance the signal-to-noise ratio. The slides were dipped in NTB2 nuclear track emulsion (Eastman Kodak, Rochester, NY), exposed in sealed plastic slide boxes containing desiccant for 2 to 4 weeks at 4°C, developed, and counterstained with hematoxylin and eosin (H&E). The VEGF probe was prepared as previously described. 27 Polymerase chain reaction primers were designed to amplify fragments of the genes of interest. A sequence spanning nucleotides 668 to 1617 of human VEGF (GenBank accession no. AF022375) was cloned in reverse orientations into two transcription vectors. Linearization of each with HindIII and transcription with SP6 generated sense and anti-sense transcripts. For EG-VEGF, KDR (VEGFR-2), and the human homologue of the Bv8 protein 23 (known also as prokineticin 2 24 ), the following probe templates were polymerase chain reaction amplified using the primers described below. Upper primers and lower primers had 27 nucleotide extensions appended to the 5′ ends encoding T7 RNA polymerase and T3 RNA polymerase promoters, respectively, for generation of sense and anti-sense transcripts. EG-VEGF probe: length 759 nucleotides; upper primer 5′-GCTTCGAGGGCTGCGGATGT-3′; lower primer 5′-TGCCTTGGGGTGACTGTCTGC-3′. KDR probe: length 814 nucleotides; upper primer 5′-AAATGTGAAGCGGTCAACAA-3′; lower primer 5′-CTGTCTTCAG TTCCCCTCCA T-3′. Bv8 probe: length 599 nucleotides; upper primer 5′-CTGCCGCACAAATACACCTAC-3′; lower primer 5′-TACAGGTACAAACGGCAATCC-3′.
Immunohistochemical staining for CD34 and CD31 was done using Target antigen retrieval (DAKO, Indianapolis, IN), 20 minutes at 99°C, anti-CD34 monoclonal QBEnd/10 (Novocastro, Newcastle, UK) at 0.125 μg/ml, or the anti-CD31 monoclonal JC/70A (DAKO) at 13 μg/ml, each detected sequentially with 2.5 μg/ml biotinylated horse anti-mouse secondary, Vectastain Elite (Vector Laboratories, Burlingame, CA), biotinyl-tyramide (DAKO) and Vectastain Elite. Reaction product was generated using metal-enhanced DAB (Pierce Chemical, Rockford, IL). Sections were lightly counterstained with hematoxylin, dehydrated, and coverslipped.
Results
Normal Ovaries
We have expanded our previous analysis of EG-VEGF expression in human and primate ovarian follicles 18 to include a wider range of human preovulatory and atretic follicular stages, and a range of CL stages. Expression of VEGF and EG-VEGF mRNA was detected by in situ hybridization in all of the specimens examined. Figure 1, A to I ▶ , illustrates representative examples of EG-VEGF and VEGF expression in preovulatory follicles from normal ovaries. Granulosa cells in primordial and primary follicles express EG-VEGF strongly (Figure 1B) ▶ , whereas VEGF expression is very weak or undetectable (Figure 1C) ▶ . VEGF expression is more uniformly detectable but still weak in secondary follicles with two to three layers of granulosa cells (Figure 1F) ▶ . As preovulatory follicles mature, VEGF expression appears to progressively increase, so that antral follicles show intense granulosa cell signal (Figure 1I) ▶ that is often associated with moderate or weak VEGF expression in the adjacent thecal layers (both the theca interna and externa can be VEGF-positive; Figure 1I ▶ ). As the secondary follicle matures, EG-VEGF expression in granulosa cells declines (Figure 1H) ▶ , although in a previous series we detected some EG-VEGF expression in granulosa cells in the cumulus oophorus surrounding the oocyte. 18 In antral follicles, variable EG-VEGF expression can be seen in the surrounding theca.
Figure 1.
VEGF and EG-VEGF expression in maturing follicles in normal ovaries. A–C: Primary and primordial follicles show strong expression of EG-VEGF (B) but little or no expression of VEGF (C). D–F: Maturing secondary follicles with multiple layers of granulosa cells maintain strong EG-VEGF expression, but show weak to moderate VEGF expression. G–I: Antral follicle (see arrowhead in Figure 5B ▶ ), with abundant mitotic figures (not shown) in both the granulosa and thecal layers, has minimum EG-VEGF expression surrounding the theca, but very intense VEGF expression in the granulosa cell layer and moderate VEGF expression (I) in the thecal cells. J–L: Antral follicle (see filled arrowhead in Figure 4B ▶ ) with heterogeneous EG-VEGF (K) and VEGF (L) expression; the right end of this follicle has a narrow rim of granulosa cells, some of which are degenerating and detached from the theca; these granulosa cells and the surrounding theca externa, lack the significant VEGF expression (L) seen elsewhere in the follicle; adjacent to the area of weak VEGF expression, EG-VEGF thecal expression is focally strong (K). M–O: Mature atretic follicle (see arrow in Figure 4B ▶ ) shows strong expression of EG-VEGF (N) in residual theca interna cells surrounding the glassy membrane (arrows) remnant of the follicular basal lamina. There is weak VEGF expression (O) in a subset of these cells. Scale bars: 100 μm (A–C); 50 μm (D–F); 200 μm (G–O).
Approximately 0.1% of the follicles present at birth mature to the point of ovulation, subsequently progressing to form CL. The remaining follicles mature to various preovulatory stages, then undergo degenerative changes, becoming atretic. 28 We examined expression of VEGF and EG-VEGF in atretic follicles at different stages of their evolution. Figure 1, M to O ▶ , illustrates a representative example of a mature (collapsed) atretic follicle, which typically strongly expresses EG-VEGF in the residual thecal cells surrounding the dense hyaline remnant of the follicular basal lamina. VEGF is only weakly expressed (Figure 1O) ▶ in a subset of these cells immediately adjacent to the follicular basal lamina. Atretic follicles in a less mature stage of evolution may retain a central lumen lacking intact granulosa cells, but surrounded by luteinized thecal cells. Follicles at this stage (not shown), typically expressed high levels of EG-VEGF in the thecal cells, but lacked VEGF expression. Follicles with a large central lumen lined by an intact granulosa cell layer are occasionally found to lack VEGF expression in the granulosa cell layer, but retain significant VEGF expression (and have EG-VEGF expression) in the theca interna (not shown). It is unclear whether follicles with this pattern represent a later stage of preovulatory follicle than illustrated in Figure 1, G to I ▶ , or whether they represent an early stage of follicular atresia.
CL derived from ovulatory follicles mature in a canonical 14-day pattern. 29 We examined EG-VEGF and VEGF expression in a series of CL representing time points ∼2 days to 14 days after ovulation. To convey a sense of the overall distribution of EG-VEGF and VEGF expression in individual ovaries, autoradiographic film results of parallel sections were digitized and the images corresponding to EG-VEGF and VEGF signals from representative ovary samples were false-colored green and red, respectively. At ∼2 to 3 days after ovulation (Figure 2 ▶ ; time points are inferred, according to the histological criteria of Corner 29 ), the EG-VEGF and VEGF expression resemble the pattern seen in the late preovulatory follicle: granulosa cells are intensely VEGF-positive, but lack significant EG-VEGF expression (Figure 2; C to F) ▶ . At ∼5 days after ovulation (Figure 3) ▶ , both VEGF (Figure 3, C and D) ▶ and EG-VEGF (Figure 3, E and F) ▶ are strongly expressed in a portion of granulosa lutein cells (theca lutein cells are not clearly distinct histologically at this stage; they may also express EG-VEGF and VEGF). At ∼8 days after ovulation (Figure 4) ▶ , EG-VEGF expression is intense in the theca lutein cells (Figure 4, E and F) ▶ , while VEGF expression has diminished to the point where only weak signal remains in the peripheral thecal cells (Figure 4, C and D) ▶ . Figure 5 ▶ illustrates a CL undergoing involutional changes (approximately day 14 after ovulation). Essentially no VEGF signal is present at this stage (Figure 5; A, C, and D) ▶ , and EG-VEGF expression is almost completely abolished in theca lutein cell layer (Figure 5; A, E, and F) ▶ .
Figure 2.
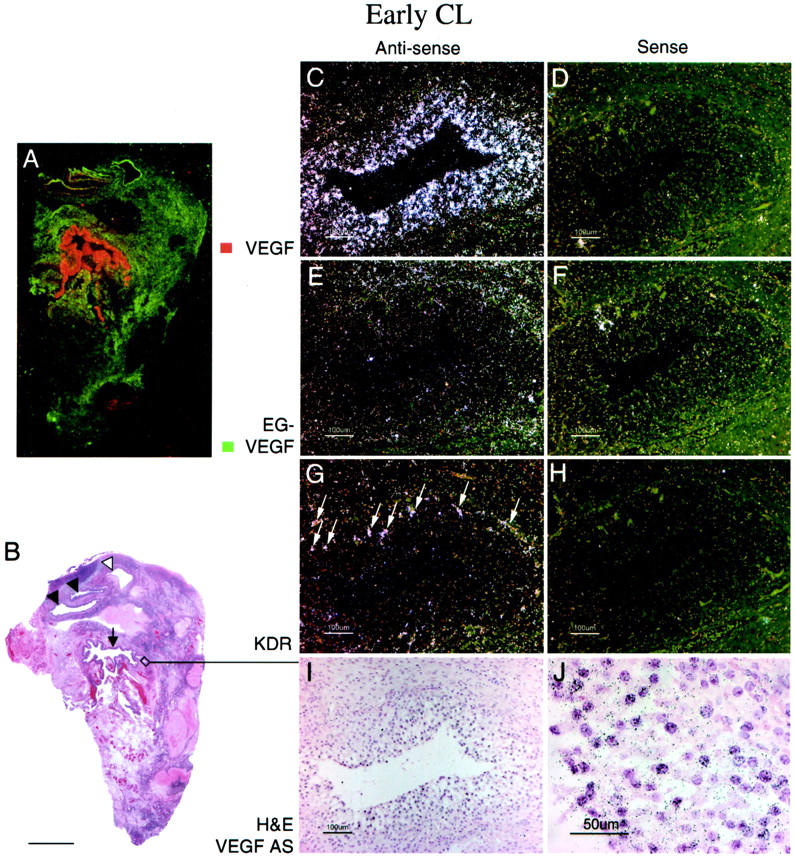
EG-VEGF and VEGF expression in normal ovary early-stage CL. An early-stage (approximately day 2 to 3 after ovulation) CL, characterized by incompletely developed vascularity in the granulosa lutein layer and by inapparent theca lutein cell differentiation (I, J), shows strong VEGF expression in the granulosa lutein cells. A: False-colored autoradiographic film results show intense VEGF expression (red) in the wall of the large cystic CL (B, arrow). Microscopic results show granulosa lutein cells are intensely VEGF-positive (C, dark field; J, bright field), but only weakly positive for EG-VEGF (E); the surrounding theca is only weakly positive for both VEGF and EG-VEGF. VEGFR-2 (KDR) expression (G) is present in small vessels at the boundary between the theca interna and granulosa cell layer, and in vessels invading the outermost granulosa cell layers (I, arrows). Other atretic follicles (A, B) with (closed arrowheads) and without (open arrowhead) intact granulosa cell linings (detail not shown) show prominent EG-VEGF expression in the theca interna. Scale bars: 5 mm (B); 100 μm (C–I); 50 μm (J).
Figure 3.
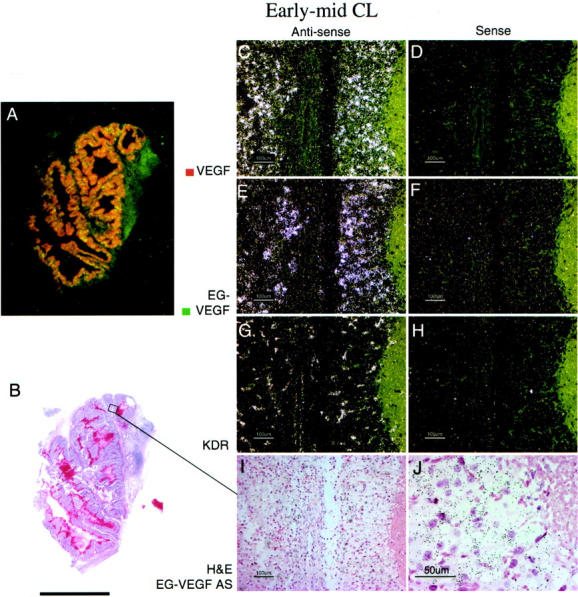
EG-VEGF and VEGF expression in normal ovary early- to mid-stage CL. A CL at approximately day 5 after ovulation, characterized microscopically by well-developed vascularity and early theca lutein cell differentiation (I), shows strong VEGF (C) and EG-VEGF (E) expression in spatially overlapping CL cell populations. A: False-colored autoradiographic film results show yellow-orange overlapping of VEGF (red) and EG-VEGF (green) expression in a large cystic CL. It was not possible to determine from this result whether VEGF and EG-VEGF were co-expressed in the same cells, or in separate cells in the CL. The distribution of EG-VEGF and VEGF signal across the entire CL is consistent with expression by theca granulosa cells, but theca lutein cells may also express EG-VEGF at this stage. Vascular VEGFR-2 (KDR) expression is intense in the CL (G). Scale bars: 5 mm (B); 100 μm (C–I); 50 μm (J).
Figure 4.
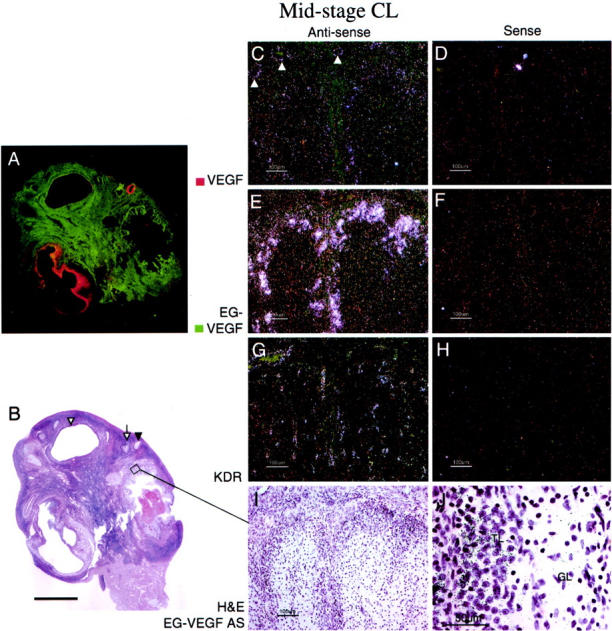
EG-VEGF and VEGF expression in normal ovary mid-stage CL. A: False-colored autoradiographic film results show intense EG-VEGF (green) expression in a narrow convoluted border surrounding a large CL. This mid-stage CL (approximately day 8 after ovulation), characterized by well-developed granulosa lutein vascularity (G) and distinct theca lutein cell differentiation (I), shows intense EG-VEGF expression in the theca lutein cell population at the CL perimeter, surrounding the vessels supplying the CL (E). EG-VEGF and VEGF expression (C) are weak or absent in the granulosa lutein cell layer. Note that VEGF is clearly expressed in this sample in the vascular smooth muscle of some small arterioles supplying the CL (arrowheads, C). VEGFR-2 (KDR) expression is still strong in vessels in all layers of the CL (G). Scale bars: 5 mm (B); 100 μm (C–I); 50 μm (J). GL, granulosa lutein; TL, theca lutein.
Figure 5.
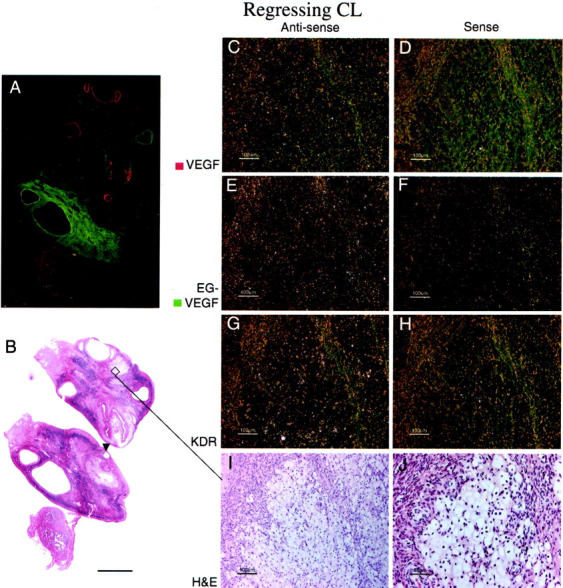
EG-VEGF and VEGF expression in normal ovary late-regressing CL. A regressing CL (approximately day 14 after ovulation), characterized by large, pale, vacuolated theca granulosa and theca lutein cells (I, J), shows absence of both VEGF (C) and EG-VEGF (E) expression. A: False-colored autoradiographic film results show absence of VEGF (red) and EG-VEGF (green) signal in an area that microscopically corresponds to the regressing CL. Only weak VEGFR-2 (KDR) expression (G) is noted in scattered vessels in the granulosa cell layer. A developing tertiary (antral) follicle (A and B, arrowhead) shows strong VEGF expression (see Figure 1 ▶ for details). Scale bars: 5 mm (B); 100 μm (C–I); 50 μm (J).
As noted in Figures 4 to 7 ▶ , EG-VEGF expression is consistently expressed in the ovarian stroma between follicles, generally at lower levels than in the theca immediately surrounding follicles, in agreement with our earlier report. 18 Near the ovarian hilum, particularly strong EG-VEGF expression is detected in clusters of cells consistent with Leydig-like hilus cells 30 (Figure 6) ▶ . As has been previously described, 31,32 these cells often occur in intimate association with blood vessels and unmyelinated nerves (Figure 6A ▶ , closed arrowhead) and large hilar vessels (Figure 6A ▶ , open arrowhead). VEGF mRNA was undetectable in hilus cells (data not shown).
Figure 6.
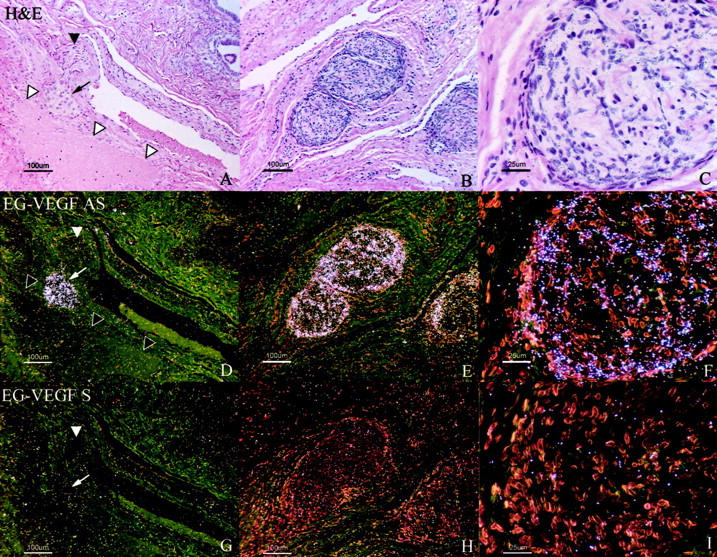
EG-VEGF expression in ovarian hilus cells. EG-VEGF expression (D–F) is very strong in cells, morphologically and biochemically similar to testicular Leydig cells, found in the ovarian hilus in association with blood vessels and unmyelinated nerves. A, D, G: H&E-stained section (A) shows a nest of hilus cells (small arrow) adjacent to a large vein (open arrowheads). In this example, an adjacent unmyelinated nerve fiber (filled arrowhead) lacks associated hilus cells. B, C, E, F: Hilus cells are intimately associated with a large unmyelinated nerve fiber in this section. Epithelium of the rete ovarii (glandular structures in A, top right) expresses neither VEGF nor EG-VEGF. Scale bars: 100 μm (A, B, D, E, G, H); 25 μm (C, F, I).
PCOS Ovaries
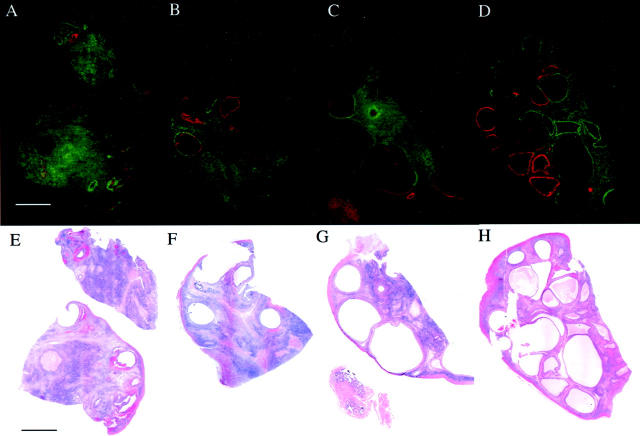
Both VEGF and EG-VEGF are expressed in all PCOS ovaries examined, but with an almost mutually exclusive expression pattern. Figure 7, A to D ▶ , presents these false-color images along with corresponding bright-field images (Figure 7; E to H) ▶ of H&E-stained, emulsion-dipped sections from each ovary. These images show that expression of VEGF mRNA is primarily limited to the cyst walls, with little or no expression in the stroma. Cysts appear either to express only VEGF, only EG-VEGF, or to express VEGF in an inner rim surrounded by an outer rim of EG-VEGF expression. Macroscopically identified corpora albicantia and the immediately surrounding stroma expressed neither VEGF nor EG-VEGF. The most consistent expression of EG-VEGF mRNA was localized to the ovarian stroma, where there were focal areas of greater intensity; EG-VEGF signal was consistently absent only in a narrow rim of cortical (subcapsular) stroma. A very similar pattern was observed in the remaining nine PCOS specimens.
Figure 7.
Differential expression of EG-VEGF (green) and VEGF (red) in four individual PCOS specimens. A–D: Parallel tissue sections were hybridized with [33P]-labeled probes, autoradiographic films were digitized, overlaid, and false-colored as described in Materials and Methods. H&E-stained sections (E–H) are also shown. Note the generally nonoverlapping expression pattern of each gene, with VEGF having a more restricted expression, most consistently associated with the lining of several individual cysts. EG-VEGF is expressed around a minority of VEGF-positive (A, B) and around all VEGF-negative cysts, as well as extensively in the stroma. Weak VEGF expression is noted in the fallopian tube mucosa (C, G), where EG-VEGF expression is absent. Scale bar, 5 mm.
Microscopic examination of emulsion-dipped slides revealed that the most consistent and intense expression of VEGF mRNA was in granulosa cells bordering the cyst lumens (Figure 8, H and I) ▶ . Some VEGF expression was seen in theca interna (Figure 8, H and I) ▶ , although not as consistently as in granulosa cells. Diffuse, usually weak, expression was seen in ovarian cortex, with individual cells sometimes intensely positive. However, as was noted macroscopically, we rarely saw expression of VEGF mRNA in the stroma.
Figure 8.
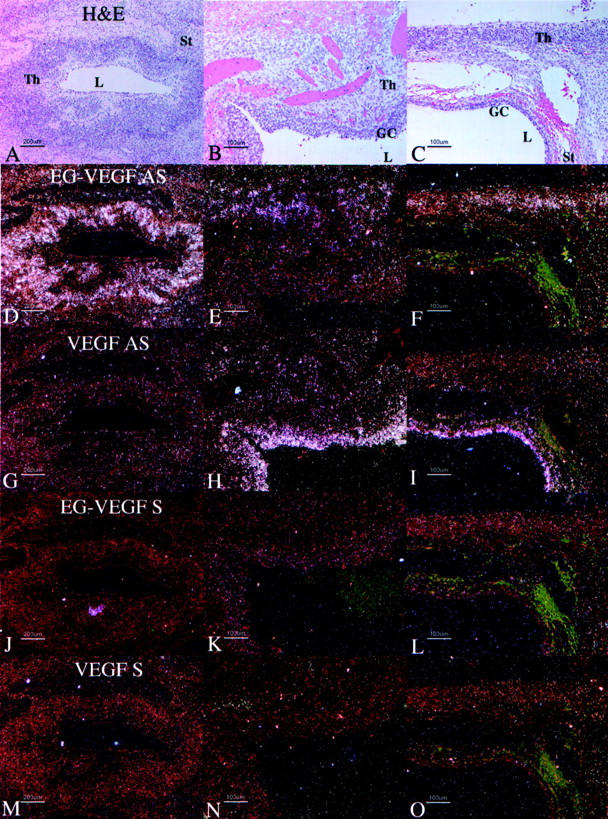
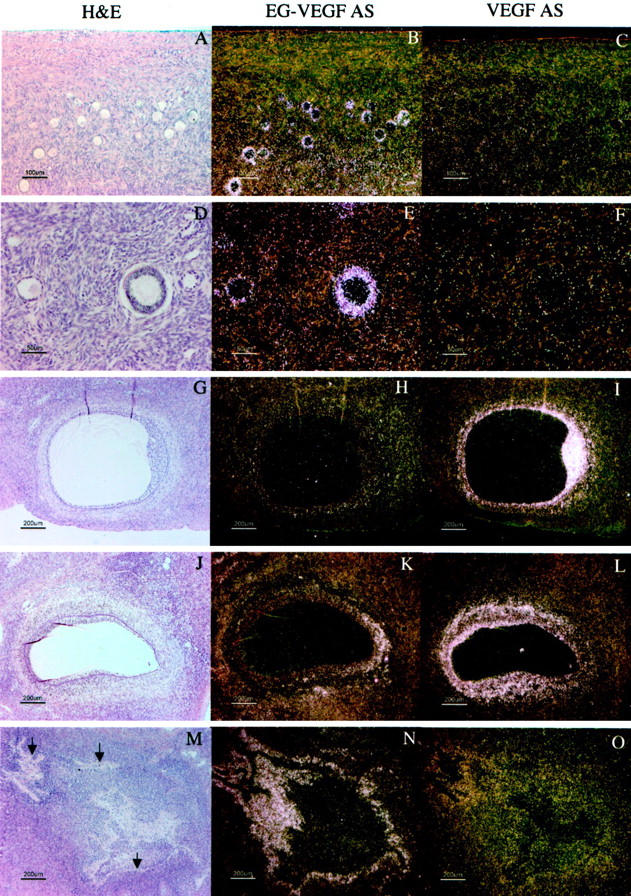
Distribution of VEGF and EG-VEGF mRNA in parallel sections of cysts in individual PCOS ovaries. Parallel section were hybridized with EG-VEGF anti-sense (D–F), VEGF anti-sense (G–I), EG-VEGF sense (J–L), and VEGF sense (M–O) riboprobes. H&E images (A–C) are shown for reference. A, D, G, J, M: Detail of late-stage atretic follicle from Figure 7A ▶ , boxed area 1; EG-VEGF (D) is strongly expressed in theca cells surrounding follicle lumen in which the granulosa cell layer has degenerated. B, E, H, K, N: Detail of early-stage atretic follicle from Figure 7A ▶ , boxed area 2; VEGF (H) is strongly expressed in granulosa cells surrounding the follicle lumen; some surrounding thecal cells are weakly VEGF-positive; EG-VEGF (C) is expressed in clusters of surrounding thecal cells. C, F, I, L, O: detail from Figure 7D ▶ , boxed area, of two atretic follicles at different stages of degeneration; VEGF (I) is strongly expressed in granulosa cells surrounding lower follicle lumen; the surrounding thin layer of thecal cells are weakly VEGF-positive, and EG-VEGF-negative; EG-VEGF (F) is expressed in the thecal cells of the upper follicle in which the granulosa cell layer has degenerated. Granulosa cells (GC), theca (Th), stroma (St), lumen (L). Scale bars: 200 μm (A, D, G, J, M); 100 μm (B, C, E, F, H, I, K, L, N, O).
In all of the samples of PCOS ovaries, EG-VEGF expression was strongest in theca interna of follicles in various stages of atresia (Figure 8; A to F) ▶ , with consistent but weaker expression in the stroma between follicles. As above noted, intense signal, albeit of lower magnitude than that detected in the theca, occurs in the stroma. EG-VEGF expression in PCO ovaries was noted in thecal cells surrounding atretic follicles in which the granulosa cell layer had degenerated (Figure 9, B and J) ▶ , and in residual thecal-like cells in late stage atretic follicles in which the follicular lumen was replaced with fibrovascular connective tissue (Figure 9, A and I) ▶ . Follicles at both these stages typically have weak or undetectable VEGF expression (Figure 9, M and N) ▶ . Importantly, thecal and stromal tissue expressing EG-VEGF maintain an abundant vascular supply, despite lacking significant VEGF expression. Endothelial immunostaining with anti-CD34 demonstrates persistent vascularity in these areas. Such a pattern is consistent with the establishment of a proangiogenic gradient directing new vessel growth toward the EG-VEGF-expressing cells (Figure 9; E to H) ▶ .
Figure 9.
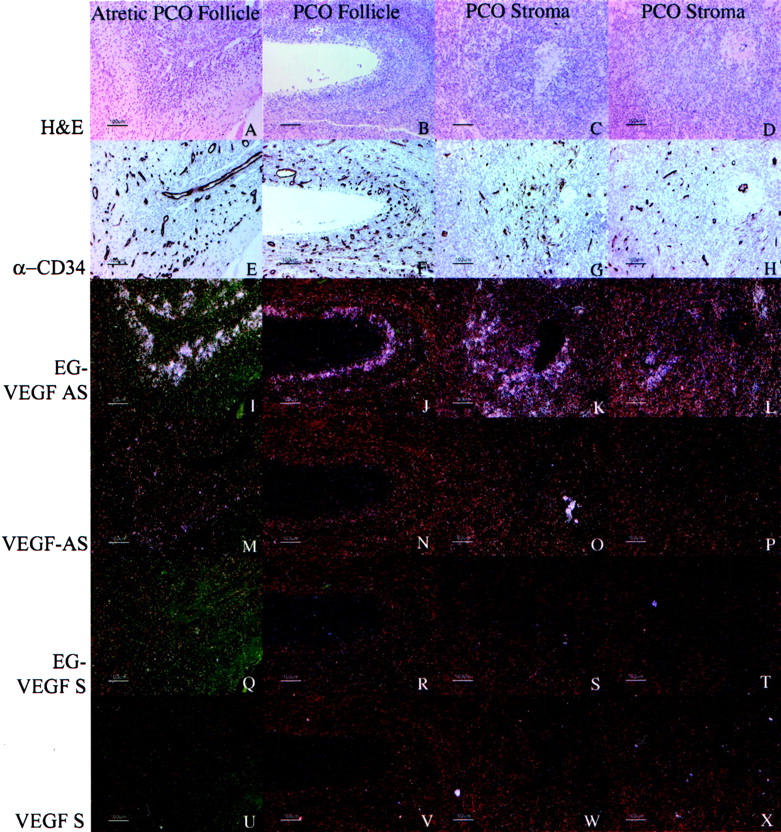
Correlation between expression of VEGF or EG-VEGF and vascularity, as assessed by expression of CD34, in representative PCOS specimens. Parallel sections were immunostained with anti-CD34 (QBEnd/10, E–H) or hybridized with EG-VEGF anti-sense (I–L), VEGF anti-sense (M–P), EG-VEGF sense (Q–T), and VEGF sense (U–X) riboprobes. H&E images (A–D) are shown for reference. In PCOS ovaries, EG-VEGF expression is high in the theca surrounding atretic follicle lumens (A, B, I, J) and diffusely in ovarian stroma (C, D, K, L), whereas VEGF expression in these areas (Q–T) is weak or absent. Vascularity in corresponding areas is illustrated by CD34 immunostaining (E–H). Similar, although weaker immunostaining was observed with anti-CD31 monoclonal antibody JC/70A (not shown). Scale bars, 100 μm.
We also examined whether Bv8 (known also as prokineticin 2), a gene prevalently expressed in the testis, encoding a protein with a high degree of homology to EG-VEGF, may be also expressed in normal or PCOS ovaries. Interestingly, Bv8 is, similar to EG-VEGF, a potent angiogenic factor. 33 However, no significant expression of the Bv8 gene was detected in any of the specimens examined in this study.
Discussion
The results presented in this study reveal a surprisingly dynamic pattern in the normal ovarian cycle in which EG-VEGF and VEGF are rarely expressed in the same population of cells, and then only transiently, suggesting distinct roles for these factors in the recruitment of a vascular supply, and perhaps other functions. This appears to be true during both follicular and luteal phases. EG-VEGF is expressed early on in primordial and primary follicles, when VEGF is undetectable or barely detectable. Therefore, EG-VEGF may be significant for the recruitment of follicles and may perhaps play some trophic function for oocytes. VEGF expression appears stronger in antral follicles but atretic follicles acquire an intense EG-VEGF message. The high expression of EG-VEGF in atretic follicles might relate to hypoxia (via HIF-1α) secondary to regressive/apoptotic changes occurring in these follicles and a signal for remodeling. A sequential activation of the two genes occurs also in the CL, but the order seems to be reversed. In agreement with previous studies, 34 we found that VEGF is strongly expressed early on, coincident with the initial recruitment and development of a capillary plexus, but becomes almost undetectable by mid-luteal phase. In contrast, EG-VEGF starts being expressed later than VEGF but persists at least through mid-luteal phase, when it is strongly expressed by theca lutein cells surrounding blood vessels. Therefore, EG-VEGF may be especially important for the formation of a more mature vascular bed that includes arterioles and thus for the persistence and adequacy of luteal function. In our initial report we did not detect significant expression of EG-VEGF in the CL. 18 The limited series examined and the stage-specific expression of EG-VEGF mRNA in the CL are likely explanations for such lack of detection.
Particularly high expression of EG-VEGF (but not VEGF) mRNA was demonstrated in hilus cells. 30 These cells are thought to be the functional equivalent of Leydig cells in the ovary, as hyperplastic or neoplastic changes affecting them are known to result in a masculinizing syndrome. 30-32 The intimate relationship of hilus cells with blood vessels and nerve terminals was noted even in the earliest studies. 31,32 Intriguingly, Bv8, a protein having a high degree of homology with EG-VEGF and able to interact with the same binding sites, 33 has been shown to have neurotrophic 35 and neuromodulator 36 functions. Although Bv8 mRNA is undetectable in the human ovary, it is tempting to speculate that EG-VEGF may play both an angiotrophic and neurotrophic role in this context.
However, these findings are correlative in nature and inhibition studies with monoclonal antibodies or other inhibitors, performed at different stages in the cycle, will be required to dissect the physiological roles of EG-VEGF in the ovary.
It is well established that increased ovarian mass, supported by new blood vessel proliferation in stroma and theca, is a key feature of PCOS. Indeed, there has been considerable interest in the identification of the mediators of such hypervascularity, but surprisingly little is known about the nature and distribution of such mediators. The present study may represent the most extensive series reported so far examining the in situ expression of candidate angiogenic factor genes in PCOS. Recent literature has focused on VEGF as one of the most likely candidates. Indeed, as previously mentioned, VEGF is a key regulator of normal and abnormal proliferation of blood vessels and has been shown to play a central role in ovarian angiogenesis. 37 Interestingly, VEGF levels have been reported to be elevated in the serum of PCOS patients compared to normal controls, although the degree of increase varied among different studies, being as little as 25% 38 or approximately twofold. 39 Furthermore, Kamat and colleagues 40 have reported in a series of three PCOS ovaries the expression of VEGF mRNA.
Given the hyperplasia and hypervascularity of the stroma in PCOS and the finding that EG-VEGF is expressed in the theca of atretic follicles and in the ovarian stroma, we were prompted to compare the expression of EG-VEGF and VEGF mRNAs in specimens of such disorder. A consistent finding of our study is that both VEGF and EG-VEGF are expressed in PCOS ovaries, but with a pattern that is almost mutually exclusive. The most intense and consistent expression of VEGF was in the granulosa cell layer of follicles, with a lower expression in the theca of some follicles. In contrast, EG-VEGF in PCOS follicles is never seen in the granulosa cells, but frequently in the theca surrounding follicles. This expression pattern is an exaggeration of the pattern seen in normal cycling ovaries, where our results show intense VEGF expression in the granulosa cells of antral follicles, with lower expression in the theca some atretic follicles; a complementary pattern of EG-VEGF expression shows strong granulosa cell signal in primordial and primary follicles, and strong thecal signal in atretic follicles. The arrested follicular development in PCOS reflects the lack of follicular maturation and CL development and acyclical gonadotropin stimulation. 41 Although there is debate whether most PCOS follicles are truly atretic, 42 they clearly have several features of atresia. 43 We detected a very low or undetectable VEGF hybridization signal in the stroma, a component that, like the theca, undergoes dramatic hyperplastic changes in PCOS. This is in contrast to the generally high expression of EG-VEGF mRNA in the stroma. Although we cannot rule out the possibility that matrix metalloproteinase-mediated proteolytic events may result in enhancement in the activity of low, constitutive, levels of VEGF, 44,45 our findings suggest that the hyperplastic/angiogenic changes occurring in PCOS are not likely solely because of VEGF and most likely EG-VEGF also participates in these events. In fact, our analysis indicates that, at least in terms of mRNA expression, EG-VEGF is the molecule that shows an even stronger correlation with hyperplasia and angiogenesis in this condition. We suggest that, although VEGF is an essential player in normal cycling ovaries, EG-VEGF might be of even greater pathophysiological importance in the acyclical angiogenesis occurring during chronic anovulation. Additional studies are clearly needed to verify this hypothesis. The availability of antibodies suitable for immunohistochemistry as well as sensitive assays to measure the EG-VEGF protein levels in the serum or other biological fluids will be helpful to extend these findings.
Previous studies have shown that adenovirus-mediated delivery of EG-VEGF in the ovary elicits angiogenic effects as well as cyst formation of similar magnitude as that induced by VEGF. 18 Therefore, our findings suggest that EG-VEGF is potentially an important contributor to the angiogenesis and hyperplasia of PCOS. Thus, it is tempting to speculate that blockade of VEGF and EG-VEGF may be effective at reducing ovarian mass and androgen output before induction of ovulation with current protocols.
Finally, previous studies 46 have implicated VEGF also in the pathogenesis of ovarian hyperstimulation syndrome (OHSS), a potentially fatal condition characterized by ovarian enlargement, with multiple follicular cysts and increased vascular permeability. 47,48 PCOS is a well-established risk factor for OHSS. 49 However, other studies have cast doubt on the hypothesis that VEGF may be the causative factor in the vascular permeability associated with OHSS. 50 It is tempting to speculate that such discrepancies are because, at least in part, of the fact that although VEGF may be an important mediator in OHSS, it is by itself insufficient and the symptoms reflect the contribution of other factors, including EG-VEGF.
Footnotes
Address reprint requests to Napoleone Ferrara, M.D. (E-mail: nf@gene.com) or Franklin Peale, M.D. (E-mail: fpeale@gene.com),Genentech Inc., 1 DNA Way, South San Francisco, CA 94080.
References
- 1.Bassett DL: The changes in the vascular pattern of the ovary of the albino rat during the estrous cycle. Am J Anat 1943, 73:251-278 [Google Scholar]
- 2.Zeleznik AJ, Schuler HM, Reichert LE, Jr: Gonadotropin-binding sites in the rhesus monkey ovary: role of the vasculature in the selective distribution of human chorionic gonadotropin to the preovulatory follicle. Endocrinology 1981, 109:356-362 [DOI] [PubMed] [Google Scholar]
- 3.Carr BR, MacDonald PC, Simpson ER: The role of lipoproteins in the regulation of progesterone secretion by the corpus luteum. Fertil Steril 1981, 38:303-311 [DOI] [PubMed] [Google Scholar]
- 4.Goede V, Schmidt T, Kimmina S, Kozian D, Augustin HG: Analysis of blood vessel maturation processes during cyclic ovarian angiogenesis. Lab Invest 1998, 78:1385-1394 [PubMed] [Google Scholar]
- 5.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegend SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD: Angiopoietin-2, a natural antagonist for Tie-2 that disrupts in vivo angiogenesis. Science 1997, 277:55-60 [DOI] [PubMed] [Google Scholar]
- 6.Goldziher JW, Green JA: The polycistic ovary. I. Clinical and histologic features. J Clin Endocrinol Metab 1962, 22:325-332 [DOI] [PubMed] [Google Scholar]
- 7.Yen SSC: Adashi EY Rock JA Rosenwaks Z eds. A contemporary overview. Reproductive Endocrinology, Surgery, and Technology. 1996:pp 1117-1126 Lippincott-Raven, Philadelphia
- 8.Dewailly D, Robert Y, Helin I, Ardaens Y, Thomas-Desrousseaux P, Lemaitre L, Fossati P: Ovarian stromal hypertrophy in hyperandrogenic women. Clin Endocrinol (Oxf) 1964, 41:557-562 [DOI] [PubMed] [Google Scholar]
- 9.Fulghesu AM, Ciampelli M, Belosi C, Apa R, Pavone V, Lanzone A: A new ultrasound criterion for the diagnosis of polycystic ovary syndrome: the ovarian stroma/total area ratio. Fertil Steril 2001, 76:326-331 [DOI] [PubMed] [Google Scholar]
- 10.Dunaif A, Thomas A: Current concepts in the polycystic ovary syndrome. Annu Rev Med 2001, 52:401-419 [DOI] [PubMed] [Google Scholar]
- 11.Phillips HS, Hains J, Leung DW, Ferrara N: Vascular endothelial growth factor is expressed in rat corpus luteum. Endocrinology 1990, 127:965-967 [DOI] [PubMed] [Google Scholar]
- 12.Ravindranath N, Little-Ihrig L, Phillips HS, Ferrara N, Zeleznik AJ: Vascular endothelial growth factor messenger ribonucleic acid expression in the primate ovary. Endocrinology 1992, 131:254-260 [DOI] [PubMed] [Google Scholar]
- 13.Ferrara N, Chen H, Davis-Smyth T, Gerber H-P, Nguyen T-N, Peers D, Chisholm V, Hillan KJ, Schwall RH: Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat Med 1998, 4:336-340 [DOI] [PubMed] [Google Scholar]
- 14.Ryan AM, Eppler DB, Hagler KE, Bruner RH, Thomford PJ, Hall RL, Shopp GM, O’Neill CA: Preclinical safety evaluation of rhuMAbVEGF, an antiangiogenic humanized monoclonal antibody. Toxicol Pathol 1999, 27:78-86 [DOI] [PubMed] [Google Scholar]
- 15.Fraser HM, Dickson SE, Lunn SF, Wulff C, Morris KD, Carroll VA, Bicknell R: Suppression of luteal angiogenesis in the primate after neutralization of vascular endothelial growth factor. Endocrinology 2000, 141:995-1000 [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann RC, Xiao E, Husami N, Sauer MV, Lobo R, Kitajewski J, Ferin M: Short-term administration of antivascular endothelial growth factor antibody in the late follicular phase delays follicular development in the rhesus monkey. J Clin Endocrinol Metab 2001, 86:768-772 [DOI] [PubMed] [Google Scholar]
- 17.Ferrara N: Vascular endothelial growth factor: a key regulator of physiological angiogenesis. Augustin HG Iruela-Arispe L Rogers PAW Smith SK eds. Vascular Morphogenesis in the Female Reproductive System. 2001:pp 149-165 Birkhauser, Boston
- 18.LeCouter J, Kowalski J, Foster J, Hass P, Zhang Z, Dillard-Telm L, Frantz G, Rangell L, DeGuzman L, Keller G-A, Peale F, Gurney A, Hillan KJ, Ferrara N: Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature 2001, 412:877-884 [DOI] [PubMed] [Google Scholar]
- 19.LeCouter J, Lin R, Ferrara N: Endocrine gland-derived VEGF and the emerging hypothesis of organ-specific regulation of angiogenesis. Nat Med 2002, 8:913-917 [DOI] [PubMed] [Google Scholar]
- 20.Joubert FJ, Strydom DJ: Snake venom. The amino acid sequence of protein A from Dendroaspis polylepis polylepis (black mamba) venom. H-S Z Physiol Chem 1980, 361:1787-1794 [DOI] [PubMed] [Google Scholar]
- 21.Aravind L, Koonin EV: A colipase fold in the carboxy-terminal domain of the Wnt antagonists—the Dickkopfs. Curr Biol 1998, 8:477-478 [DOI] [PubMed] [Google Scholar]
- 22.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C: Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 1998, 391:357-362 [DOI] [PubMed] [Google Scholar]
- 23.Wechselberger C, Puglisi R, Engel E, Lepperdinger G, Boitani C, Kreil G: The mammalian homologues of frog Bv8 are mainly expressed in spermatocytes. FEBS Lett 1999, 462:177-181 [DOI] [PubMed] [Google Scholar]
- 24.Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY: Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol 2001, 59:692-698 [DOI] [PubMed] [Google Scholar]
- 25.Lu LH, Gillette N: An optimized protocol for in situ hybridization using PCR-generated 33P-labeled riboprobes. Cell Vis 1994, 7:53-64 [Google Scholar]
- 26.Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, Frantz GD, Tumas DB, Peale FV, Jr, Shelton DL, Hebert CC: FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J 2000, 19:4046-4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warren RS, Yuan H, Matli MR, Gillett NA, Ferrara N: Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest 1995, 95:1789-1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clement PB: Histology of the ovary. Am J Surg Pathol 1987, 11:277-303 [DOI] [PubMed] [Google Scholar]
- 29.Corner GWJ: The histological dating of the human corpus luteum of menstruation. Am J Anat 1956, 9:377-401 [DOI] [PubMed] [Google Scholar]
- 30.Clement PB: Histology of the ovary. Sternberg SS eds. Histology for Pathologists ed 2 1997:pp 929-959 Lippincott, Philadelphia
- 31.Sternberg WH: The morphology, androgenic function, hyperplasia and tumors of the ovarian hilus cells. Am J Pathol 1949, 25:493-521 [PMC free article] [PubMed] [Google Scholar]
- 32.Merrill JA: Ovarian hilus cells. Am J Obstet Gynecol 1959, 78:1258-1271 [Google Scholar]
- 33.LeCouter J, Lin R, Frantz G, Tejada M, Peale F, Hillan KJ, Ferrara N: The endocrine gland-derived VEGF homologue Bv8 promotes angiogenesis in the testis: localization of Bv8 receptors to endothelial cells. Proc Natl Acad Sci USA 2003, 100:2685-2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto S, Konishi I, Tsuruta Y, Nanbu K, Mandai M, Kuroda H, Matsushita K, Hamid AA, Yura Y, Mori T: Expression of vascular endothelial growth factor (VEGF) during folliculogenesis and corpus luteum formation in the human ovary. Gynecol Endocrinol 1997, 11:371-381 [DOI] [PubMed] [Google Scholar]
- 35.Melchiorri D, Bruno V, Besong G, Ngomba RT, Cuomo L, De Blasi A, Copani A, Moschella C, Storto M, Nicoletti F, Lepperdinger G, Passarelli F: The mammalian homologue of the novel peptide Bv8 is expressed in the central nervous system and supports neuronal survival by activating the MAP kinase/PI-3-kinase pathways. Eur J Neurosci 2001, 13:1694-1702 [DOI] [PubMed] [Google Scholar]
- 36.Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY: Prokineticin 2 transmits the behavioral circadian rhythm of the suprachiasmatic nucleus. Nature 2002, 417:405-410 [DOI] [PubMed] [Google Scholar]
- 37.Ferrara N: Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int 1999, 56:794-814 [DOI] [PubMed] [Google Scholar]
- 38.Tulandi T, Saleh A, Morris D, Jacobs HS, Payne NN, Tan SL: Effects of laparoscopic ovarian drilling on serum vascular endothelial growth factor and on insulin responses to the oral glucose tolerance test in women with polycystic ovary syndrome. Fertil Steril 2000, 74:585-588 [DOI] [PubMed] [Google Scholar]
- 39.Agrawal R, Sladkevicius P, Engmann L, Conway GS, Payne NN, Bekis J, Tan SL, Campbell S, Jacobs HS: Serum vascular endothelial growth factor concentrations and ovarian stromal blood flow are increased in women with polycystic ovaries. Hum Reprod 1998, 13:651-655 [DOI] [PubMed] [Google Scholar]
- 40.Kamat BR, Brown LF, Manseau EJ, Senger DR, Dvorak HF: Expression of vascular permeability factor/vascular endothelial growth factor by human granulosa and theca lutein cells. Role in corpus luteum development. Am J Pathol 1995, 146:157-165 [PMC free article] [PubMed] [Google Scholar]
- 41.Yen SSC: Polycystic ovary syndrome (hyperandrogenic chronic anovulation). Yen SSC Jaffe RB Barbieri RL eds. Reproductive Endocrinology. 1999:pp 436-478 W. B. Saunders, Philadelphia
- 42.Almahbobi G, Anderiesz C, Hutchinson P, McFarlane JR, Wood C, Trounson AO: Functional integrity of granulosa cells from polycystic ovaries. Clin Endocrinol 1996, 44:571-580 [DOI] [PubMed] [Google Scholar]
- 43.Cataldo NA, Dumesic DA, Goldsmith PC, Jaffe RB: Immunolocalization of Fas and Fas ligand in the ovaries of women with polycystic ovary syndrome: relationship to apoptosis. Hum Reprod 2000, 15:1889-1997 [DOI] [PubMed] [Google Scholar]
- 44.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D: Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2000, 2:737-744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoue M, Hager JH, Ferrara N, Gerber HP, Hanahan D: VEGF-A has a critical, nonredundant role in angiogenic switching and pancreatic beta cell carcinogenesis. Cancer Cell 2002, 1:193-202 [DOI] [PubMed] [Google Scholar]
- 46.McClure N, Healy DL, Rogers PA, Sullivan J, Beaton L, Haning RV, Connolly DT, Robertson DM: Vascular endothelial growth factor as a capillary permeability agent in ovarian hyperstimulation syndrome. Lancet 1994, 344:235-269 [DOI] [PubMed] [Google Scholar]
- 47.Engel T, Jewelewicz R, Dyrenfurth I, Speroff L, Vande Wiele RL: Ovarian hyperstimulation syndrome: report of one case with notes on pathogenesis and treatment. Am J Obstet Gynecol 1972, 112:1052-1057 [PubMed] [Google Scholar]
- 48.Haning RJ, Strawn E, Nolten W: Pathophysiology of the ovarian hyperstimulation syndrome. Obstet Gynecol 1985, 166:220-224 [PubMed] [Google Scholar]
- 49.Kemmann E, Tavakoli F, Shelden RM, Jones JR: Induction of ovulation with menotropins in women with polycystic ovary syndrome. Am J Obstet Gynecol 1981, 141:58-64 [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi H, Okada Y, Asahina T, Gotoh J, Terao T: The kallikrein-kinin system, but not vascular endothelial growth factor, plays a role in the increased vascular permeability associated with ovarian hyperstimulation syndrome. J Mol Endocrinol 1998, 20:363-374 [DOI] [PubMed] [Google Scholar]