Abstract
Angiopoietin1 (Ang1) is a novel angiogenic factor with important actions on endothelial cell (EC) differentiation and vascular maturation. Ang1 has been shown to prevent EC apoptosis through activation of PI3-kinase/Akt, a pathway that is also known to activate endothelium nitric oxide synthase (eNOS). Therefore, we hypothesized that the angiogenic effects of Ang1 would also be dependent on the PI3-kinase/Akt pathway, possibly mediated by increased eNOS activity and NO release. Treatment of human umbilical vein endothelial cells with recombinant Ang1* (300 ng/ml) for 15 minutes resulted in PI3-kinase-dependent Akt phosphorylation, comparable to that observed with vascular endothelial growth factor (VEGF) (50 ng/ml), and increased NO production in a PI3-kinase/Akt-dependent manner. Capillary-like tube formation induced by Ang1* in fibrin matrix at 24 hours (differentiation index, DI: 13.74 ± 0.76 versus control 1.71 ± 0.31) was abolished in the presence of the selective PI3-kinase inhibitor, LY294002 (50 μmol/L) (DI: 0.31 ± 0.31, P < 0.01) or the NOS inhibitor, L-NAME (3 mmol/L) (DI: 4.10 ± 0.59, P < 0.01). In subcutaneous Matrigel implants in vivo, addition of recombinant Ang1* or wild-type Ang1 from conditioned media of COS-1 cells transfected with a pFLAG Ang1 expression vector, induced significant neovascularization to a degree similar to VEGF. Finally, angiogenesis in vivo in response to both Ang1 and VEGF was significantly reduced in eNOS-deficient compared with wild-type mice. In summary, our results demonstrate for the first time that endothelial-derived NO is required for Ang1-induced angiogenesis, and that the PI3-kinase signaling mediates the activation of eNOS and NO release in response to Ang1.
Angiopoietin-1 (Ang1) has recently been identified as a ligand of the endothelial selective receptor tyrosine kinase (RTK), Tie2. 1 Tie2 signaling has been shown to be required for later stages of embryonic blood vessel development, 2-4 including vascular remodeling, vessel integrity, and maturation. 1,5 In vitro experiments have shown that Ang1 induces endothelial cell (EC) sprouting in vitro, 6,7 and stimulates EC migration 8 and the formation and stabilization of capillary-like networks in culture systems. 9-11
One of the signaling cascades initiated by Tie2 phosphorylation is the recruitment of Dok-R, 12 a novel docking molecule that on phosphorylation binds to proteins containing Src homology 2 (SH2) domains. 13 The recruitment of a Nck-p21 activated kinase (Pak) complex to Dok-R, 14 mediates EC migration. 15 In addition, the p85 subunit of phosphatidylinositol (PI) 3-kinase can interact specifically with the phosphorylated Tie2 through its SH2 domain. Although an initial report suggested that activated Tie2 does not associate with PI3-kinase, 16 subsequent studies have confirmed that activation of Tie2 leads to PI3-kinase activation, 17 the lipid products of which participate in the regulation of cell survival by activation of the serine/threonine kinase, Akt. 18 Indeed, the PI3-kinase/Akt pathway has been shown to transduce the antiapoptotic effect of both vascular endothelial growth factor (VEGF) 19 and Ang1. 17,20
Recently, Akt has also been demonstrated to directly phosphorylate endothelial nitric oxide synthase (eNOS), leading to calcium/calmodulin-independent enzyme activation. 21,22 Endothelium-derived nitric oxide (NO) is a crucial mediator in the regulation of EC growth, survival, and angiogenesis. 23,24 Importantly, eNOS knockout (KO) mice have also been shown to exhibit marked impairment in postnatal angiogenesis in response to growth factors delivery and ischemia. 25,26
The goal of this study was to define the role of endothelium-derived NO in Ang1-mediated angiogenesis both in vitro and in vivo. We now report that Ang1 induced the activation of Akt and increased NO release in cultured ECs by a PI3-kinase-dependent mechanism. Moreover, Ang1-induced capillary-like tube formation in three-dimensional fibrin matrices in vitro and neovascularization of Matrigel implants in response to Ang1 in vivo were dependent on endothelium-derived NO.
Materials and Methods
Materials
Human Ang1* was kindly provided by Regeneron Pharmaceuticals, Inc. (Tarrytown, NY). Ang1* is a genetically engineered variant of naturally occurring Ang1 that retains similar properties in all assays. In Ang1*, the nonconserved cysteine at residue 245 has been mutated to the corresponding serine residue of Ang2, and the first 77 amino acids of human Ang1 have been replaced with the first 73 residues of Ang2. 1 The recombinant Ang1* protein was prepared in buffer containing 0.05 mol/L Tris-HCl pH 7.5, 150 mmol/L NaCl and 0.05% CHAPS. Native human Ang1 and VEGF165 were obtained from R&D Systems (Minneapolis, MN). Other sources of materials are indicated as mentioned.
Cell Lines and Culture
Human umbilical vein endothelial cells (HUVEC) and monkey kidney (COS-1) cells were obtained from the American Type Culture Collection (ATCC; Manassas, VA). HUVECs were maintained in culture in Ham’s 12 medium (Invitrogen/Gibco, Burlington, ON) supplemented with 15% fetal bovine serum (FBS), penicillin (500 U/ml), streptomycin (50 μg/ml) and heparin (100 μg/ml), (all from Invitrogen/Gibco), and EC growth factor (ECGF 20 μg/ml; Roche Diagnostics, Mannheim, Germany) and equilibrated with 95% air and 5% CO2 at 37°C. Cells between passages 13 and 18 were used in these experiments. COS-1 cells were grown in Dulbrecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS and antibiotics as indicated above.
Plasmid Transfection
Plasmid (pFLAG-Ang1 kindly provided by Dr. Injune Kim, University of South Korea), encoding the human Ang1, fused with a c-Myc tag at the C terminus, was expressed in COS-1 cell line. Transient transfection was performed using Superfect reagent (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions. Twenty hours after transfection, cells were incubated in serum-free DMEM for another 24 hours. The conditioned medium (CM) was harvested and concentrated 100× using Amicon Centricon 10-kd cutoff columns (Millipore Corp., Bedford, MA).
Animals
Male C57 (WT) and eNOS KO mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were housed in filter-topped cages, maintained with a day/night cycle of 12 hours under pathogen-free conditions, fed a standard diet of rodent chow, and given water ad libitum until they reached 6 to 8 weeks of age. All animal use was approved by and adhered closely to the guidelines set out by the Animal Care and Use Committee, St. Michael’s Hospital.
Preparation of Fibrin Gels
Endotoxin- and plasminogen-free human fibrinogen (10 mg/ml, Calbiochem-Novabiochem Corp., La Jolla, CA) was prepared as previously described. 27 After polymerization, gels were soaked in cultured medium containing 15% FBS for 2 hours at 37°C to inactivate the thrombin. EC were plated on the surface of the three-dimensional matrix and culture for 24 hours in the presence or absence of study agents as described above.
In Vitro Angiogenesis
HUVECs were cultured on fibrin-matrix, pretreated with NG-nitro-l-arginine methyl ester (L-NAME, 3 mmol/L; 1 hour) or with LY294002 (50 μmol/L; 2 hours) before exposure to recombinant Ang1* (300 ng/ml). After 24 hours, total length of capillary-like structures >30 μm was derived using an Olympus BX50 inverted microscope (100×) for each of six randomly preselected fields. At the same time, the total area of residual EC monolayer was determined for the same fields, and differentiation index (DI) was calculated as the ratio of total tube length over cell area for each field. Images were taken using a digitized Sony CCD-IRIS/RGB camera (Cohu Inc., Japan) and analyzed by a computer-assisted morphometric analysis system (C-Imaging, Compix Inc., Cranberry Township, PA) by observers blinded to the experimental conditions.
Western Blot Analysis and Tie2 Phosphorylation
CM was collected from pFLAG (mock)-transfected or pFLAG-Ang1 transfected COS-1 cells. Ten microliters of 100× concentrated CM was subjected to SDS-PAGE, and proteins were transferred onto nitrocellulose membrane and probed with an anti c-Myc monoclonal antibody (1:5000 dilution according to the supplier’s instruction; Invitrogen, Carlsbad, CA).
For Tie2 phosphorylation studies, HUVECs were maintained overnight in F12 medium with 1% FBS, and stimulated for 10 minutes in serum-free medium with Ang1* protein (300 ng/ml), mock-CM or concentrated Ang1-CM (200 μl CM/ml). Cells were solubilized with radio immuno-precipitation assay (RIPA) lysis buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 20 mmol/L Tris, pH 7.6, 50 mmol/L sodium fluoride, 150 mmol/L sodium chloride, 1 mmol/L EDTA, 5 mmol/L benzamidine, 1 mmol/L sodium orthovanadate, 10 μg/ml aprotinin, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, and 1 μg/ml pepstatin). The lysates were immunoprecipitated with anti-Tie2 antibodies (C-20, Santa Cruz Biotechnology, Santa Cruz, CA). Immunocomplexes were recovered on Protein G-Sepharose and separated by SDS-PAGE. Proteins were transferred onto nitrocellulose membrane, blocked with 5% bovine serum albumen, 1X TBS, 0.1% Tween-20 for 2 hours, and probed with anti-phosphotyrosine antibodies 4G10 (1:2500, Upstate Biotechnology, Inc., Lake Placid, NY), stripped and re-probed with anti-Tie2 (1:1000). Specific bands were visualized using the enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotech). Densitometry was performed by scanning the immunoblots (imaging densitometer; BioRad, Hercules, CA) and the intensity of each band was analyzed using the Molecular Analyst software (BioRad).
Akt and eNOS Phosphorylation
HUVEC were grown until confluence, serum-starved for 12 hours in F12 medium containing 1% FBS, and then the medium was changed to serum-free F12 containing the inhibitors of PI3-kinase, wortmannin (100 nmol/L) or LY294002 (50 μmol/L) (both from Sigma, St. Louis, MO), or the selective Akt inhibitor, 1L-6-hydroxymethyl-chiro-inositol 2-(R)-2-O-methyl-3-O-octadecylcarbonate (20 to 75 μmol/L; Calbiochem-Novabiochem). After a 2-hour pretreatment with inhibitors, the cells were stimulated with Ang1* (100 to 600 ng/ml, and in some cases native Ang1 for comparison) for 15 to 30 minutes before lysis with 200 μl of SDS sample buffer (10 mmol/L Tris-HCl, pH 6.8, 4% SDS, 20% glycerol, 0.4% dithiothreitol, 1 mmol/L orthovanadate). Proteins (50 μg per lane) were separated on SDS-PAGE and transferred to nitrocellulose membrane. Membranes were blotted using antibodies specific to phospho-Akt (Ser473, 1:1000; New England Biolabs, Beverly, MA), or phospho-eNOS (Ser1177, 1:1000; Cell Signaling Technology, New England Biolabs) and developed by ECL as described above. The blots were then stripped and reprobed with Akt (1:1000, New England Biolabs), eNOS (1:2500, Transduction Laboratories, Lexington, KY), or Tie2 antibodies (1:1000, Santa Cruz) and the bands were scanned and analyzed by densitometry as described above.
Measurement of NOS Activity by Quantification of NO Release
The measurement of NO production in ECs were performed using the amino-700 NO sensor (Innovative Instruments, Inc., Tampa, FL) which is 100 times more sensitive than the Griess reagent (detection limit below 1 nmol/L). Cells were treated as described above and 50 μl of cell culture media was injected into an acid/iodide solution to reduce NO2 stoichiometrically to NO, detected by sensor. The exchange of electrons between NO and the electrode surface resulted in an electrical current, which was monitored and recorded. The analyzer was calibrated on the day of the experiment with nitrite (NO2) standards, and the results were normalized to the cell number in the plate. NO production was confirmed using the Griess reaction as previously described. 27
The Matrigel Plug Assay to Assess in Vivo Angiogenesis
Matrigel (0.5 ml, Collaborative Biomedical Products) containing Ang1* (300 ng/ml), VEGF (100 or 300 ng/ml, as indicated), or PBS was injected subcutaneously at either side of the abdominal midline of WT or eNOS KO mice (n = 12/group). For the CM experiments, 100 μl of concentrated CM (100×) were mixed with 400 μl of Matrigel and injected subcutaneously into the animals. On day 10, Matrigel plugs were harvested and subjected to histological analysis or determination of blood content (see below).
For histology, the Matrigel plugs were fixed in 10% buffered formalin for 24 hours and paraffin blocks were cut to 5-μm sections and stained with hematoxylin and eosin (H&E). Adjacent sections from each group of animals were also collected for immunohistochemistry using vWF polyclonal rabbit anti-human antibody (1:250 dilution, Dako Corp., Santa Barbara, CA) for 1 hour at 37°C and subsequently treated with goat anti-rabbit antibody (1:300 dilution; Vector Laboratories, Burlingame, CA) for 1 hour at room temperature and then treated with streptavidin-biotin-peroxidase complex (Vectastain ABC kit; Vector Laboratories) for 30 minutes at room temperature. Diaminobenzadine was used as the peroxidase substrate and hematoxylin as the nuclear counterstain. Negative controls were prepared by substituting preimmune rabbit serum for the primary antibody. The sections were stained for vWF and the images were captured under 20× objective with the use of a Cool SNAP camera (Photometrics, Tucson, AZ) connected to an inverted microscope. On average, 6 to 8 fields were taken from each section. Results were expressed as mean number of vWF-positive vessels per mm2.
Fluorescein isothiocyanate-dextran (FITC-dextran; molecular weight, 200-kd) was used as an index of functionality of the Matrigel neovessel. 28 FITC-dextran was prepared fresh (25 mg/ml in PBS) and 0.02 ml was injected into the lateral tail vein of each mouse 10 days after the Matrigel injection, and then the mice were sacrificed by cervical dislocation after 20 minutes, blood samples were collected by cardiac puncture into heparinized tubes, centrifuged immediately, and plasma was separated and protected from light at 4°C. The matrix plugs were then promptly removed and placed into tubes containing 1 ml of dispase (1:10 dilution in PBS), and incubated overnight, in the dark, in a 37°C shaker. The following day, the plugs were homogenized using a polytron and centrifuged at 2000 × g for 15 minutes, and the supernatant was saved and kept in the dark until fluorescence analysis. Fluorescence readings were obtained on FL600 fluorescence plate reader using a standard curve created by serial dilution of FITC-dextran used for injection. Angiogenic response was calculated as a ratio of Matrigel plug/plasma fluorescence and presented as fluorescent unit (FU).
Statistics
One-way analysis of variance was used to determine the significance of differences between groups, where appropriate, with post hoc Student’s t-test for unpaired observations and Bonferroni correction for multiple comparisons. Summary data are presented as means ± SEM or as percentage of control of the indicated numbers of observations. Statistical significance is defined as P < 0.05.
Results
Ang1-Induced Angiogenesis and NO
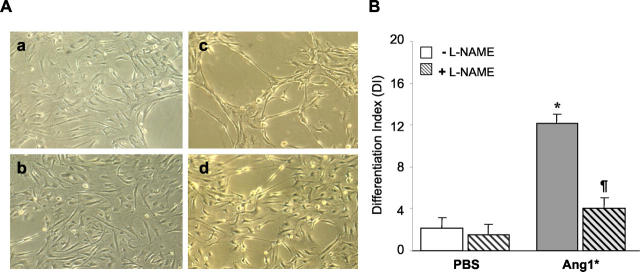
The addition of Ang1* to HUVEC cultured on 3-D fibrin matrices resulted in the formation of extensive capillary-like networks at 24 hours (Figure 1A ▶ , panel c), as has previously been reported. 9 Addition of the nonselective inhibitor of NOS, L-NAME, nearly completely inhibited this effect of Ang1* in cultured ECs (Figure 1A ▶ , panel d). Figure 1B ▶ shows summary data for six separate experiments. The addition of L-NAME markedly reduced differentiation index (DI) in Ang1*-treated wells to values not significantly different from control.
Figure 1.
Nitric oxide-synthase activity required for angiogenic effects of Ang1. A: Ang1*-induced capillary-like tube formation in the three-dimensional fibrin matrices is blocked by inhibition of endogenous eNOS activity. HUVECs were cultured on fibrin matrices in the presence (c and d) or absence (a and b) of recombinant Ang1*, with L-NAME (b and d) or without L-NAME (a and c) for 24 hours. A significant increase in tube length was observed in the presence of Ang1* which was inhibited by L-NAME (original magnification, ×100). B: Quantitative analysis of capillary-like network formation calculated as the ratio of total tube length over cell area for each field (DI, differentiation index). Scale bars represent a mean ± SEM of DI value obtained for each culture well from six independent experiments. The difference among groups were analyzed by analysis of variance followed by Student’s t-test. *P < 0.05 versus control, #P < 0.05 versus Ang1*. Ang1* (300 ng/ml), L-NAME (3 mmol/L).
Tie2 and Akt Phosphorylation Studies
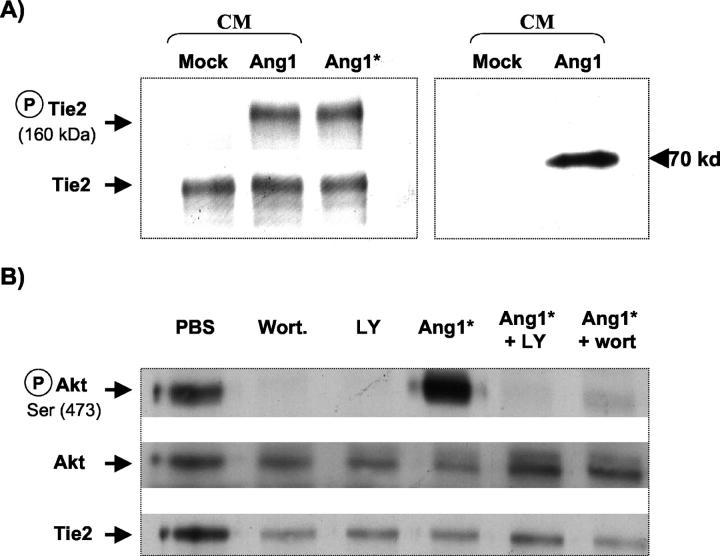
Stimulation of HUVECs with Ang1* (300 ng/ml) or Ang1-CM for 10 minutes induced a marked increase in Tie2 phosphorylation (Figure 2A ▶ , left). This was associated with Akt phosphorylation at Ser473 (Figure 2B) ▶ , which was nearly completely blocked by two specific inhibitors of PI3-kinase, wortmannin and LY294002. A 70-kd single band was observed in Ang1-CM but not in mock-CM subjected to Western blot analysis using anti-c-Myc antibody, consistent with the expression of the Ang1 c-Myc fusion protein (Figure 2A ▶ , right).
Figure 2.
Ang1 stimulates Tie2 and Akt phosphorylation. A: Tie2 receptor tyrosine phosphorylation by Ang1-CM or Ang1* in HUVECs visualized by immunoblot analysis with mouse monoclonal anti-phosphotyrosine antibodies (left panel, top), and anti-Tie2 antibody to normalize equal loading (bottom). Note that the similar level of phosphorylation of Tie2 could be detected following stimulation with Ang1-CM or Ang1* recombinant protein, whereas no phosphorylation was seen following stimulation of cells with mock-CM. Western blot analysis of the concentrated CM obtained from pFLAG (mock)-transfected COS-1 cells or cells expressing pFLAG-Ang1, shows a single 70-kd band, confirming the expression of Ang1 as a c-Myc fused protein (right). Data shown are representative of two independent experiments. B: Stimulation of HUVEC with Ang1* (300 ng/ml), increased Akt phosphorylation, which was blocked by the PI3-kinase inhibitors, LY294002 (50 μmol/L) or wortmannin (100 nmol/L). All figures are representative of three separate experiments. Akt phosphorylation (P-Akt, Ser 473, top); total Akt expression (center) and total Tie2 expression (bottom).
eNOS Phosphorylation Studies
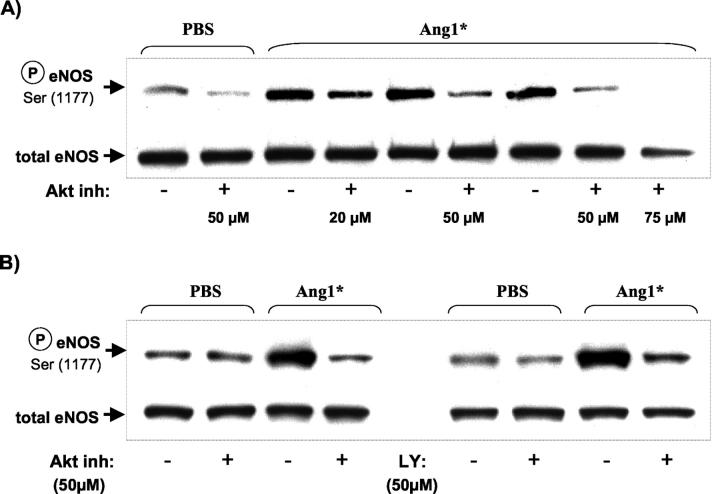
Densitometric analysis of the Western blot bands showed that Ang1*-stimulated eNOS phosphorylation in a dose-dependent manner (100 ng/ml: 2.96 ± 0.18, 300 ng/ml: 3.27 ± 0.24* and 600 ng/ml: 3.74 ± 0.37**; *P < 0.05, **P < 0.01 versus PBS: 1.63 ± 0.27) with a maximal response similar to that of native Ang1 (600 ng/ml: 4.39 ± 0.60, P < 0.01). Based on these experiments the 300 ng/ml concentration was used for the in vivo studies.
Next we tested the upstream pathways that were involved in eNOS phosphorylation by using selective inhibitors of Akt and PI3-kinase (Figure 3) ▶ . Addition of Akt inhibitor (20 to 75 μmol/L) dose-dependently inhibited eNOS phosphorylation in response to Ang1* (Figure 3A) ▶ ; however, at 75 μmol/L total eNOS expression was also reduced, possibly due to cytotoxicity. Therefore, in further experiments a concentration of 50 μmol/L was used. Inhibition of Akt (50 μmol/L) reduced eNOS phosphorylation in response to Ang1* to a similar degree as the inhibition of PI3-kinase (Figure 3B) ▶ .
Figure 3.
Ang1*-dependent phosphorylation of eNOS is blocked by PI3-kinase and Akt inhibitors. A: HUVEC were maintained overnight in F12 medium with 1% FBS, then pretreated for 2 hours in serum-free medium with an Akt inhibitor at various doses (20 to 75 μmol/L) to evaluate optimal conditions as indicated. B: HUVECs were cultured as above and pretreated either with an Akt inhibitor (50 μmol/L) or a PI3-kinase inhibitor LY294002 (50 μmol/L). In both panels the cells were either stimulated with Ang1* (300 ng/ml, 30 minutes) or buffer solution. After the stimulation, Western analysis was performed using antibodies specific to phosphorylated eNOS and total eNOS.
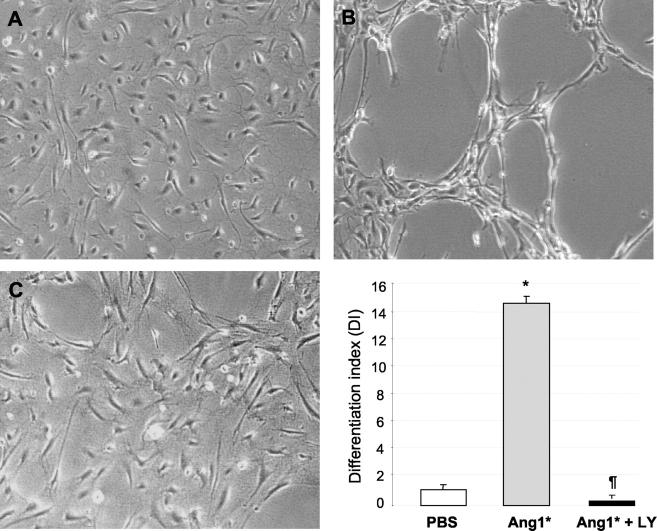
PI3-Kinase Inhibitors Significantly Reduce Ang1*-Dependent Tube Formation and NO Release
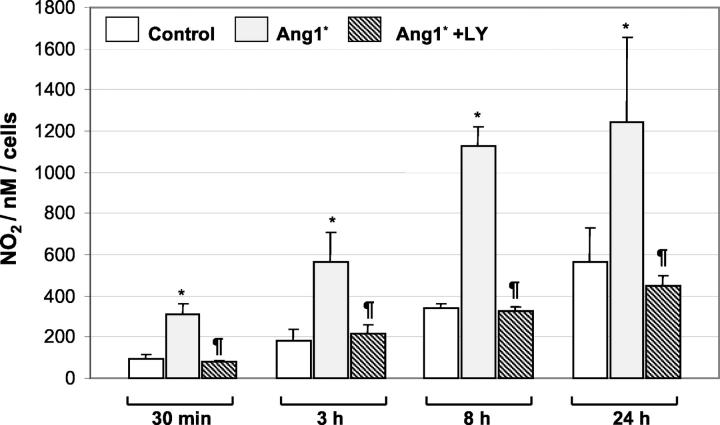
Capillary-like tube formation in the fibrin matrix model in response to Ang1* was completely inhibited by the PI3-kinase inhibitor, LY 294002 (50 μmol/L) (Figure 4C) ▶ . To determine whether the effect of Ang1* was associated with an increase in the release of NO, HUVECs were stimulated with Ang1* for up to 24 hours and the medium was collected for measurement of NO. As shown in Figure 5 ▶ , treatment of HUVECs with Ang1* resulted in an increase in NO production, which was apparent after only 30 minutes of exposure and was sustained for up to 24 hours. Increase in NO release after 24 hours of incubation with Ang1* was confirmed using the Griess reagent (data not shown). The ability of Ang1* to stimulate NO release was abrogated by pretreatment with the PI3-kinase inhibitor LY294002 (Figure 5) ▶ , which itself had no effect on basal NO levels (data not shown).
Figure 4.
Ang1*-induced activation of PI3-kinase/Akt pathway is required for endothelial cell tube formation. A: HUVEC plated on fibrin matrices were pretreated with 50 μmol/L of LY294002 for 2 hours before addition of Ang1* (300 ng/ml) or PBS, and then incubated for 24 hours. HUVEC grown on fibrin gel in the presence of PBS (control) showed no significant angiogenic response (A). In contrast, cells treated with Ang1* showed an extensive network of capillary-like tube formation (B), which was prevented by addition of LY294002 (C). Original magnification, ×100. Bars represent the mean ± SEM of four independent experiments. Statistical comparisons were performed using one-way analysis of variance followed by Student’s t-test. *P < 0.05 versus control, ¶P < 0.05 versus Ang1*.
Figure 5.
Ang1* activates NO production and release. HUVEC CM was collected after incubation with Ang1* (300 ng/ml) for the periods indicated, and assayed for NO production, as described in the Methods section. Results are expressed as the mean ± SEM of three independent experiments (five experiments for the 24-hour time point). *P < 0.05 versus PBS, ¶P < 0.05 versus Ang1*.
Effect of Ang1 on in Vivo Neovascularization
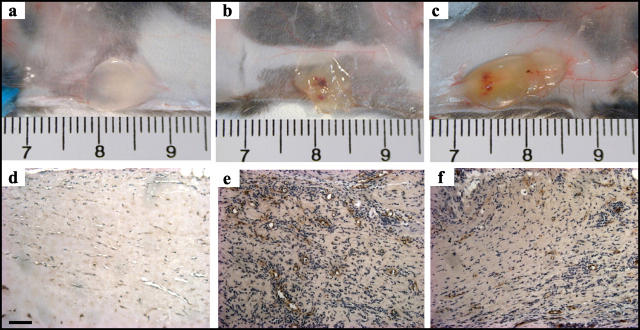
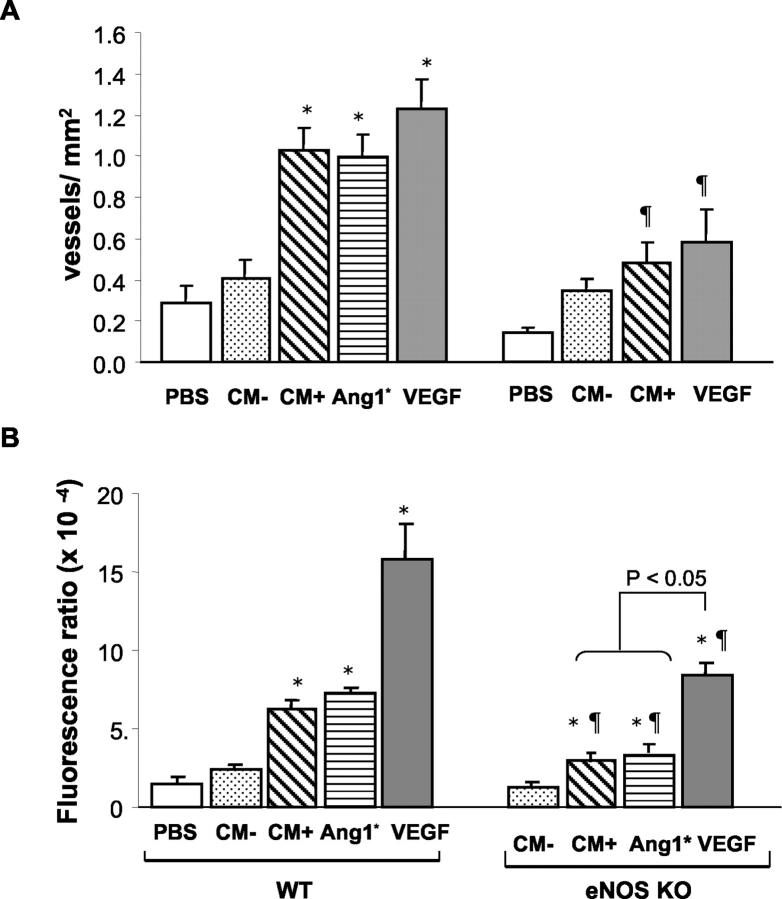
To assess the role of Ang1 on angiogenesis in vivo, we studied neovascularization of Matrigel implants supplemented with Ang1* (300 ng/ml) or VEGF (100 ng/ml). Both of the angiogenic factors induced neovascularization of the Matrigel plugs within 10 days (Figure 6,b and c) ▶ , while only minimal angiogenesis was observed in the control plugs (Figure 6a) ▶ . To determine the effect of wild-type Ang1, we added 100 μl of either concentrated CM from pFLAG-Ang1 transfected cells (Ang1-CM) or CM from mock-vector transfected cells (mock-CM) to the Matrigel (PBS served as a negative control). In WT mice, Ang1-CM resulted in greater neovascularization of the Matrigel implants compared to plugs containing mock-CM or PBS alone (Figure 7A) ▶ . Moreover, the response to Ang1-CM was not different from that observed with the purified recombinant Ang1* or VEGF protein (both 300 ng/ml). Similar results were seen when FITC-dextran perfusion was used as an index of Matrigel blood content (Figure 7B) ▶ ; however, in this case VEGF produced substantially greater increases in fluorescent signals than Ang1* or Ang1-CM, possibly due to differential effects on vascular permeability which may have resulted in greater extravasations of labeled dextran in the VEGF-treated inserts. To test whether the combination of these two factors would have additive effects, we measured the blood volume in the Matrigel plugs using FITC-dextran content as a marker. The results of this experiment suggested that the combination of these two agents was slightly less than additive (Ang1*: 17.27 ± 1.05, VEGF: 28.47 ± 1.47 and Ang1* plus VEGF: 33.82 ± 3.23 FU; all = P < 0.05 vs. PBS: 5.19 ± 0.61).
Figure 6.
Ang1-induced neovascularization of Matrigel plugs in vivo. Gross appearance of Matrigel plugs containing PBS (a), Ang1* (300 ng/ml, b) or VEGF (100 ng/ml, c) implanted for 10 days in WT mice. Histological sections of the Matrigel plugs retrieved from WT (d and e: mock-CM and Ang1-CM, respectively) or eNOS KO (f: Ang1-CM) mice and immunostained with vWF to identify neovessels.
Figure 7.
Quantative analysis of neovascularization of the Matrigel plugs at day 10. A: Number of vWF positive vessels per mm2 of Matrigel plugs implanted into WT or eNOS KO mice. B: Blood content in the Matrigel explants assessed by FITC-dextran perfusion in both WT and eNOS KO mice. Data are presented as mean ± SEM (6 animals per group). mock-CM (CM−), Ang1-CM (CM+), Ang1* and VEGF (both 300 ng/ml). *P < 0.05 versus PBS or CM−, P < 0.05 versus WT mice.
eNOS Plays a Critical Role in Ang1-Induced Angiogenesis in Vivo
The role of endothelium-derived NO in Ang1-induced neovascularization in vivo was tested in the Matrigel model. Ang1-CM resulted in significantly less neovascularization in eNOS KO (Figure 6f) ▶ compared to the WT (Figure 6e) ▶ mice as determined by the number of vWF positive noevessels. Summary data of the vessels numbers are shown in Figure 7A ▶ . These results were consistent with a significant decrease in FITC-dextran content of the Ang1* or Ang1-CM treated Matrigel plugs obtained from the eNOS KO mice compared to those collected from the WT mice (Figure 7B) ▶ .
Discussion
This is the first report to explore the importance of NO as a downstream mediator of the biological effects of Tie2 activation. We now show that the formation of a capillary-like network in vitro in response to Ang1 required a PI3-kinase/Akt-dependent increase in NO release and that Ang1-induced neovascularization in vivo was dependent on eNOS expression.
Ang1 and its receptor, Tie2, play a critical role in embryonic vascular development. Targeted disruption of these genes has been reported to produce embryonic lethality due to major abnormalities in vascular development. However, unlike VEGF, which is necessary for the differentiation of EC from the primitive mesoderm, 29 defects in Ang1/Tie2 KO mice involve a later stage of vascular development. 5 These embryos are characterized by large and poorly organized vessels, which lack mature basement membrane and exhibit partial failure of pericytes recruitment. 5 Ang1 has also been shown to induce postnatal angiogenesis in a variety of experimental models. In cultured cells, Ang1 alone induced EC chemotaxis, 8,14,15,30 capillary-like tube formation 9-11 and sprouting angiogenesis, 30 and enhanced EC survival. 11,20 In vivo reports have shown that Ang1 is unable to induce angiogenesis alone, but potentiated the angiogenic response to VEGF. 31-33 In contrast, in the present report, Ang1 resulted in robust neovascularization of Matrigel implants directly, and the combination with VEGF was at best additive. These results are consistent with previous reports showing Ang1-induced angiogenesis in the absence of exogenous VEGF. 34,35 The reason for the variable requirement for VEGF in these models is unclear, but may relate to differences in its endogenous expression of growth factors. Since Ang1 does not directly induce EC proliferation, 6,9,31 the action of an additional EC mitogen such as VEGF may be crucial in facilitating the angiogenic effects of Ang1. The cornea is normally avascular and thus unlikely to express angiogenic factors, whereas in the ischemic hindlimb model, tissue hypoxia results in up-regulation of angiogenic factors and their receptors. 36 Matrigel is derived from tumor basement membrane and contains low concentrations of multiple growth factors, possibly providing a suitable background to support the angiogenic actions of Ang1.
In a recent report, overexpression of Ang1 targeted to the skin was shown to result in a network of large venular-like dermal vessels 37 that were resistant to increases in permeability caused by inflammatory agents or concomitant VEGF overexpression. 38 This is consistent with the stabilizing effect of Ang1 on vascular endothelium and its underlying basement membrane. In the Matrigel model, the induction of angiogenesis in response to Ang1 also appeared to produce relatively non-leaky neovessels, because although VEGF and Ang1 resulted in similar increases in vessel number, VEGF-treated Matrigel implants contained significantly greater amounts of FITC-labeled dextran. This finding is consistent with differences in permeability characteristics between vessels produced by Ang1 or VEGF; the former resulted in more competent vascular structures, whereas VEGF-induced neovessels may have led to greater extravasation of dextran into the Matrigel. Thus, the apparent increase in blood content of the VEGF-containing Matrigel plugs may have been exaggerated by the hypermeability effects of this factor. This finding may also have relevance for therapeutic angiogenesis, since patients treated with VEGF for lower limb ischemia exhibited limb edema, presumably due to increased capillary leakiness caused by VEGF. 39 In our experiments, although both Ang1 and VEGF induced robust neovascularization in the Matrigel implant model, the combination of Ang1 and VEGF appeared to be less than additive. Of interest, Visconti et al 40 have recently reported that overexpression of Ang1 in a nonischemic transgenic model reduced rather than enhanced VEGF-induced angiogenesis in the heart, suggesting that the interactions between Ang1 and VEGF in vivo are complex and depend critically on the model and experimental conditions.
Tie2 Signal Transduction
Recently it has been recognized that the PI3-kinase/Akt pathway plays a pivotal role in signal transduction by a number of tyrosine kinase receptors including VEGF receptors (Flk/KDR) and Tie2. Enhanced EC survival in response to Ang1 has been shown to be dependent on activation of Akt by PI3-kinase (also known as PKB). 10,17,20,41 Akt promotes cell survival through its ability to phosphorylate Bad and procaspase-9. 42,43 In addition, a recent report has demonstrated that Ang1 increased EC survival by the induction of the apoptosis inhibitor, survivin, again through the PI3-kinase/Akt pathway. 41 Similarly, the angiogenic response to Ang1 has been shown to be dependent on PI3-kinase activity 15 and downstream activation of p125FAK. 30 In addition, it has been reported that phosphorylation of Y1106 on the Tie2 receptor recruits the Tie2-associated docking molecule Dok-R. 12 Recruitment of Dok-R to activated Tie2 leads to its phosphorylation and subsequent recruitment of Nck-p21 activated kinase (Pak) complex to the membrane. 14
Release of endothelium-derived NO requires the activation of eNOS, which until recently has been thought to be largely regulated by a Ca2+ and calmodulin. However, several reports have demonstrated that the activity of eNOS can be regulated by phosphorylation. The serine/threonine protein kinase Akt has been shown to increase human eNOS activity in ECs by Akt phosphorylation at Ser1179, 21 which leads to increase NO production even at low intracellular Ca2+ concentrations, whereas mutant eNOS (Ser1179A and Ser1177) is resistant to phosphorylation and Akt-dependent activation. 21,22
Role of NO in Angiogenesis
The mechanisms by which NO mediates angiogenesis have not been fully elucidated. Both clinical and experimental studies support an association between NO production and tumor progression, wherein the level of NOS protein and/or activity in the tumor has been positively correlated with the degree of malignancy for tumors. 44 The hemodynamic effects of this potent vasodilator may contribute to its angiogenic effects. Increased shear stress in the microcirculation may initiate angiogenesis by perturbing the luminal side of ECs, or by releasing growth factors or other vasoactive agents. 45 Increased NO production has also been shown to directly result in EC proliferation 23 and migration, 46 and to promote angiogenesis both in vitro and in vivo 27,47 by a cGMP-dependent mechanism. 48 Thus, NO has been implicated in nearly all levels of the angiogenic response. Moreover, a number of reports suggest that NO mediates the angiogenic activity of several angiogenic growth factors including TGF-β, bFGF, and VEGF. 24,27,49 EC proliferation, 24,50,51 migration, 50,52 and tube formation 24 in response to VEGF in vitro could be inhibited by the addition of NOS inhibitors to the cultured EC. Also, experiments using systemic inhibition of NOS 53 or eNOS KO animals 26 have provided evidence supporting an important role for NO in VEGF-induced angiogenesis in vivo. However, the possible role of NO in the angiogenic actions of the angiopoietins remains largely unexplored. An earlier study suggested that the NO pathway was not involved in the response to Ang1, 11 but this conclusion was based only on the measurement of cellular cGMP levels. In contrast, the results of the present study support an important role for NO release via the PI3-kinase/Akt pathway in the angiogenic response to Ang1. Pharmacological inhibitors of PI3-kinase and NOS blocked Ang1-induced NO release and prevented EC capillary-like network formation; therefore, signaling between Tie2 and PI3-kinase appears to be essential for Ang1-induced angiogenesis in vitro. This is in apparent conflict with the effects of Ang1 in a rodent model of diabetic retinopathy, 54 in which the diabetes-induced increases in retinal eNOS and NO levels were reduced by Ang1. However, this may have been a result of indirect anti-inflammatory effects of Ang1 in this model, outweighing the direct actions of Ang-1 on these pathways. 54
The role of endothelium-derived NO in Ang1-induced neovascularization was confirmed in vivo using eNOS KO mice. Although the in vitro angiogenesis models are useful in exploring the mechanisms underlying this complex process, they may not reproduce faithfully all of the events of the angiogenic cascade required for new blood vessel formation in vivo. The Matrigel implant model allows reliable estimation of the angiogenic response since these plugs are completely avascular on implantation and therefore any blood vessels penetrating into the Matrigel arise de novo. The present results from the eNOS KO model implicate the endothelial isoform directly in Ang1-induced postnatal angiogenesis, and provide the first data in vivo supporting a role for endothelium-derived NO in the angiogenic response to this factor. These data also raise a question concerning the possible role of NO in vasculogenesis during embryonic development since, by necessity, this must occur normally for viable offspring to be produced. However, the litter sizes of eNOS KO mice are much smaller that WT, 55 consistent with a high degree of embryonic lethality (unpublished data). Although the cause of embryonic wastage has not been fully elucidated, several reports point to abnormalities in vascular and cardiac development. 55,56
In summary, our results support an important role for NO in mediating the angiogenic response to Ang1, in a manner analogous to VEGF and other angiogenic growth factors. Specifically, Ang1 induced increased NO production and capillary-like network formation via activation of PI3-kinase/Akt, and the angiogenic response in vitro could be abrogated by inhibition of NOS or PI3-kinase activity. Also, we have confirmed that Ang1 alone induced robust neovascularization in vivo, which was largely absent in eNOS KO animals. These data represent the first demonstration of the NO-dependent nature of Ang1-induced angiogenesis and suggest that endothelial function and endothelium-derived NO release will be of critical importance for the full biological rein response to Ang1.
Acknowledgments
We thank Dr. Yancopoulus for providing Ang1* and Dr. Kim for providing the Ang1 construct.
Footnotes
Address reprint requests to D.J. Stewart, Director, Division of Cardiology, University of Toronto and Head, Department of Cardiology, St. Michael’s Hospital, 30 Bond Street, Toronto, Ontario M5B 1W8, Canada. E-mail: stewartd@smh.toronto.on.ca.
Supported by grant 10960 from the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Ontario (NA-4789). D.J.S. is the Dexter Man Chair of Cardiology at the University of Toronto.
S. Babaei and K. Teichert-Kuliszewska contributed equally to this work.
References
- 1.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD: Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 1996, 87:1161-1169 [DOI] [PubMed] [Google Scholar]
- 2.Puri MC, Rossant J, Alitalo K, Bernstein A, Partanen J: The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J 1995, 14:5884-5891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML: Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev 1994, 8:1897-1909 [DOI] [PubMed] [Google Scholar]
- 4.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y: Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 1995, 376:70-74 [DOI] [PubMed] [Google Scholar]
- 5.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD: Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 1996, 87:1171-1180 [DOI] [PubMed] [Google Scholar]
- 6.Kim I, Moon SO, Koh KN, Kim H, Uhm CS, Kwak HJ, Kim NG, Koh GY: Molecular cloning, expression, and characterization of angiopoietin-related protein: angiopoietin-related protein induces endothelial cell sprouting. J Biol Chem 1999, 274:26523-26528 [DOI] [PubMed] [Google Scholar]
- 7.Koblizek TI, Weiss C, Yancopoulos GD, Deutsch U, Risau W: Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr Biol 1998, 8:529-532 [DOI] [PubMed] [Google Scholar]
- 8.Witzenbichler B, Maisonpierre PC, Jones P, Yancopoulos GD, Isner JM: Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase Tie2. J Biol Chem 1998, 273:18514-18521 [DOI] [PubMed] [Google Scholar]
- 9.Teichert-Kuliszewska K, Maisonpierre PC, Jones N, Campbell AI, Master Z, Bendeck MP, Alitalo K, Dumont DJ, Yancopoulos GD, Stewart DJ: Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of Tie2. Cardiovasc Res 2001, 49:659-670 [DOI] [PubMed] [Google Scholar]
- 10.Hayes AJ, Huang WQ, Mallah J, Yang D, Lippman ME, Li LY: Angiopoietin-1 and its receptor Tie-2 participate in the regulation of capillary-like tubule formation and survival of endothelial cells. Microvasc Res 1999, 58:224-237 [DOI] [PubMed] [Google Scholar]
- 11.Papapetropoulos A, Garcia-Cardena G, Dengler TJ, Maisonpierre PC, Yancopoulos GD, Sessa WC: Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab Invest 1999, 79:213-223 [PubMed] [Google Scholar]
- 12.Jones N, Dumont DJ: The Tek/Tie2 receptor signals through a novel Dok-related docking protein, Dok-R. Oncogene 1998, 17:1097-1108 [DOI] [PubMed] [Google Scholar]
- 13.Pawson T, Scott JD: Signaling through scaffold, anchoring, and adaptor proteins. Science 1997, 278:2075-2080 [DOI] [PubMed] [Google Scholar]
- 14.Master Z, Jones N, Tran J, Jones J, Kerbel RS, Dumont DJ: Dok-R plays a pivotal role in angiopoietin-1-dependent cell migration through recruitment and activation of Pak. EMBO J 2001, 20:5919-5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones N, Master Z, Jones J, Bouchard D, Gunji Y, Sasaki H, Daly R, Alitalo K, Dumont DJ: Identification of Tek/Tie2 binding partners: binding to a multifunctional docking site mediates cell survival and migration. J Biol Chem 1999, 274:30896-30905 [DOI] [PubMed] [Google Scholar]
- 16.Huang L, Turck CW, Rao P, Peters KG: GRB2 and SH-PTP2: potentially important endothelial signaling molecules downstream of the TEK/TIE2 receptor tyrosine kinase. Oncogene 1995, 11:2097-2103 [PubMed] [Google Scholar]
- 17.Kontos CD, Stauffer TP, Yang WP, York JD, Huang L, Blanar MA, Meyer T, Peters KG: Tyrosine 1101 of Tie2 is the major site of association of p85 and is required for activation of phosphatidylinositol 3-kinase and Akt. Mol Cell Biol 1998, 18:4131-4140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toker A, Cantley LC: Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature 1997, 387:673-676 [DOI] [PubMed] [Google Scholar]
- 19.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N: Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway: requirement for Flk-1/KDR activation. J Biol Chem 1998, 273:30336-30343 [DOI] [PubMed] [Google Scholar]
- 20.Kim I, Kim HG, So JN, Kim JH, Kwak HJ, Koh GY: Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-Kinase/Akt signal transduction pathway. Circ Res 2000, 86:24-29 [DOI] [PubMed] [Google Scholar]
- 21.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC: Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 1999, 399:597-601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM: Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999, 399:601-605 [DOI] [PubMed] [Google Scholar]
- 23.Ziche M, Morbidelli L, Masini E, Granger H, Geppetti P, Ledda F: Nitric oxide promotes DNA synthesis and cyclic GMP formation in endothelial cells from postcapillary venules. Biochem Biophys Res Commun 1993, 192:1198-1203 [DOI] [PubMed] [Google Scholar]
- 24.Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC: Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest 1997, 100:3131-3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC: Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest 1998, 101:731-736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM: Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest 1998, 101:2567-2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babaei S, Teichert-Kuliszewska K, Monge JC, Mohamed F, Bendeck MP, Stewart DJ: Role of nitric oxide in the angiogenic response in vitro to basic fibroblast growth factor. Circ Res 1998, 82:1007-1015 [DOI] [PubMed] [Google Scholar]
- 28.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS: Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest 2000, 105:R15-R24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW: Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996, 380:439-442 [DOI] [PubMed] [Google Scholar]
- 30.Kim I, Kim HG, Moon SO, Chae SW, So JN, Koh KN, Ahn BC, Koh GY: Angiopoietin-1 induces endothelial cell sprouting through the activation of focal adhesion kinase and plasmin secretion. Circ Res 2000, 86:952-959 [DOI] [PubMed] [Google Scholar]
- 31.Chae JK, Kim I, Lim ST, Chung MJ, Kim WH, Kim HG, Ko JK, Koh GY: Coadministration of angiopoietin-1 and vascular endothelial growth factor enhances collateral vascularization. Arterioscler Thromb Vasc Biol 2000, 20:2573-2578 [DOI] [PubMed] [Google Scholar]
- 32.Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, Yancopoulos GD, Isner JM: Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res 1998, 83:233-240 [DOI] [PubMed] [Google Scholar]
- 33.Zhu WH, MacIntyre A, Nicosia RF: Regulation of angiogenesis by vascular endothelial growth factor and angiopoietin-1 in the rat aorta model: distinct temporal patterns of intracellular signaling correlate with induction of angiogenic sprouting. Am J Pathol 2002, 161:823-830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shyu KG, Manor O, Magner M, Yancopoulos GD, Isner JM: Direct intramuscular injection of plasmid DNA encoding angiopoietin-1 but not angiopoietin-2 augments revascularization in the rabbit ischemic hindlimb. Circulation 1998, 98:2081-2087 [DOI] [PubMed] [Google Scholar]
- 35.McDonald DM: Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med 2001, 164:S39-S45 [DOI] [PubMed] [Google Scholar]
- 36.Rissanen TT, Vajanto I, Hiltunen MO, Rutanen J, Kettunen MI, Niemi M, Leppanen P, Turunen MP, Markkanen JE, Arve K, Alhava E, Kauppinen RA, Yla-Herttuala S: Expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 (KDR/Flk-1) in ischemic skeletal muscle and its regeneration. Am J Pathol 2002, 160:1393-1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD: Increased vascularization in mice overexpressing angiopoietin-1. Science 1998, 282:468-471 [DOI] [PubMed] [Google Scholar]
- 38.Detmar M, Brown LF, Schon MP, Elicker BM, Velasco P, Richard L, Fukumura D, Monsky W, Claffey KP, Jain RK: Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol 1998, 111:1-6 [DOI] [PubMed] [Google Scholar]
- 39.Baumgartner I, Pieczek A, Manor O, Blair R, Kearney M, Walsh K, Isner JM: Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation 1998, 97:1114-1123 [DOI] [PubMed] [Google Scholar]
- 40.Visconti RP, Richardson CD, Sato TN: Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF). Proc Natl Acad Sci USA 2002, 99:8219-8224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O’Connor DS, Li F, Altieri DC, Sessa WC: Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem 2000, 275:9102-9105 [DOI] [PubMed] [Google Scholar]
- 42.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME: Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997, 91:231-241 [DOI] [PubMed] [Google Scholar]
- 43.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC: Regulation of cell death protease caspase-9 by phosphorylation. Science 1998, 282:1318-1321 [DOI] [PubMed] [Google Scholar]
- 44.Jadeski LC, Hum KO, Chakraborty C, Lala PK: Nitric oxide promotes murine mammary tumour growth and metastasis by stimulating tumour cell migration, invasiveness and angiogenesis. Int J Cancer 2000, 86:30-39 [DOI] [PubMed] [Google Scholar]
- 45.Hudlicka O: Is physiological angiogenesis in skeletal muscle regulated by changes in microcirculation? Microcirculation 1998, 5:7-23 [PubMed] [Google Scholar]
- 46.Murohara T, Witzenbichler B, Spyridopoulos I, Asahara T, Ding B, Sullivan A, Losordo DW, Isner JM: Role of endothelial nitric oxide synthase in endothelial cell migration. Arterioscler Thromb Vasc Biol 1999, 19:1156-1161 [DOI] [PubMed] [Google Scholar]
- 47.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, Ledda F: Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest 1994, 94:2036-2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Babaei S, Stewart DJ: Overexpression of endothelial NO synthase induces angiogenesis in a co-culture model. Cardiovasc Res 2002, 55:190-200 [DOI] [PubMed] [Google Scholar]
- 49.Inoue N, Venema RC, Sayegh HS, Ohara Y, Murphy TJ, Harrison DG: Molecular regulation of the bovine endothelial cell nitric oxide synthase by transforming growth factor-β1. Arterioscler Thromb Vasc Biol 1995, 15:1255-1261 [DOI] [PubMed] [Google Scholar]
- 50.Shizukuda Y, Tang S, Yokota R, Ware JA: Vascular endothelial growth factor-induced endothelial cell migration and proliferation depend on a nitric oxide-mediated decrease in protein kinase Cδ activity. Circ Res 1999, 85:247-256 [DOI] [PubMed] [Google Scholar]
- 51.Morbidelli L, Chang CH, Douglas JG, Granger HJ, Ledda F, Ziche M: Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am J Physiol 1996, 270:H411-H415 [DOI] [PubMed] [Google Scholar]
- 52.Noiri E, Lee E, Testa J, Quigley J, Colflesh D, Keese CR, Giaever I, Goligorsky MS: Podokinesis in endothelial cell migration: role of nitric oxide. Am J Physiol 1998, 274:C236-C244 [DOI] [PubMed] [Google Scholar]
- 53.Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, Bicknell R: Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest 1997, 99:2625-2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joussen AM, Poulaki V, Tsujikawa A, Qin W, Qaum T, Xu Q, Moromizato Y, Bursell SE, Wiegand SJ, Rudge J, Ioffe E, Yancopoulos GD, Adamis AP: Suppression of diabetic retinopathy with angiopoietin-1. Am J Pathol 2002, 160:1683-1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee TC, Zhao YD, Courtman DW, Stewart DJ: Abnormal aortic valve development in mice lacking endothelial nitric oxide synthase. Circulation 2000, 101:2345-2348 [DOI] [PubMed] [Google Scholar]
- 56.Gregg AR, Schauer A, Shi O, Liu Z, Lee CG, O’Brien WE: Limb reduction defects in endothelial nitric oxide synthase-deficient mice. Am J Physiol 1998, 275:H2319-H2324 [DOI] [PubMed] [Google Scholar]