Abstract
Recent studies have shown that caveolin-1 (Cav-1) plays an important role as a regulator of angiogenesis in vitro. Here, we use Cav-1 knockout (KO) mice as a model system to examine the in vivo relevance of these findings. A primary mediator of angiogenesis is basic fibroblast growth factor (bFGF). Thus, we studied bFGF-induced angiogenesis in Cav-1 KO mice using a reconstituted basement membrane system, ie, Matrigel plugs, supplemented with bFGF. In Cav-1 KO mice, implanted Matrigel plugs showed a dramatic reduction in both vessel infiltration and density, as compared with identical plugs implanted in wild-type control mice. We also examined the necessity of Cav-1 to support the angiogenic response of an exogenous tumor by subcutaneously injecting Cav-1 KO mice with the melanoma cell line, B16-F10. We show that tumor weight, volume, and vessel density are all reduced in Cav-1 KO mice, consistent with diminished angiogenesis. Ultrastructural analysis of newly formed capillaries within the exogenous tumors reveals a lack of endothelial caveolae and incomplete capillary formation in Cav-1 KO mice. These results provide novel evidence that Cav-1 and caveolae play an important positive role in the process of pathological angiogenesis in vivo.
One of the most conspicuous structures within the relatively thin endothelial cell layer of blood vessels are caveolae (also known as plasmalemmal vesicles). 1,2 These cholesterol/sphingolipid-enriched microdomains of the plasma membrane invaginate into the endothelial cell’s cytoplasm, forming submembranous vesicles. 3 These caveolar vesicles can also coalesce to generate trans-endothelial channels that act as conduits between the lumenal and basolateral membranes. Expression of the caveolin-1 (Cav-1) protein within endothelial cells is sufficient drive the formation of these caveolae membrane domains. In support of this notion, targeted disruption of the Cav-1 gene in mice, thereby producing a null mutation, results in the complete loss of caveolae within all Cav-1-expressing cells. 4,5
Recent studies using Cav-1 knockout (KO) mice have begun to address the in vivo functional role of endothelial cell caveolae. For example, ablation of Cav-1 gene expression results in alterations in tight-junction morphology and resultant microvascular hyperpermeability. 6 Furthermore, Cav-1 KO mouse aortas demonstrate enhanced vasorelaxation when subjected to acetylcholine treatment 7,8 because of constitutive activation of eNOS within Cav-1 KO endothelial cells. These data clearly demonstrate that Cav-1 and caveolae organelles play important roles in normal endothelial cell functions.
Angiogenesis is the formation of new blood vessels from pre-existing vessels. 9 The process of angiogenesis is characterized by the following progression of events: vasodilation, increased vascular permeability, extracellular matrix degradation, endothelial cell proliferation/migration/differentiation, and periendothelial maturation. Examination of the molecular underpinnings of these events has revealed that angiogenesis is tightly regulated by an array of stimulators and inhibitors, at multiple steps in the process.
Previous studies have shown that caveolae can fuse with each other to form larger vesicular structures as part of the angiogenic response. 10 Consistent with these studies, we have recently shown that recombinant overexpression of Cav-1 in cultured endothelial cells, thus driving enhanced caveolae formation, accelerates endothelial capillary tubule formation by nearly threefold. 11 Conversely, transfection of Cav-1 anti-sense oligonucleotides into endothelial cells results in the marked reduction of capillary tubule formation using a three-dimensional fibrin gel assay or the chorioallantoic membrane angiogenesis system. 12 Finally, using an in vitro Matrigel assay, we have demonstrated that down-regulation of Cav-1 via an anti-sense adenoviral approach reduces the number of capillary-like tubules more than 10-fold. 11 Consistent with these findings, we have observed that Cav-1 expression appears to be down-regulated during the proliferation phase of angiogenesis, and then markedly up-regulated during the differentiation phase, 11 as observed using endothelial cells in culture. Similarly, numerous previous reports show a lack of Cav-1 expression in transformed cells versus a high level of Cav-1 expression in fully differentiated cells. 13,14
In this report, we present the first in vivo evidence that Cav-1 and caveolae organelles play a positive regulatory role in the process of pathological angiogenesis, using Cav-1 KO mice as a model system.
Experimental Procedures
Animal Studies
All mice were housed in a barrier facility at the Institute for Animal Studies, Albert Einstein College of Medicine. The generation of Cav-1 KO and Cav-2 KO mice, in the C57B/6 background, was as previously described. 7,15-17 Twelve- to 16-week-old wild-type (WT), Cav-1 KO, or Cav-2 KO mice were used for all of the studies described below.
Matrigel Angiogenesis Assay
Mice were mildly anesthetized with isoflurane. Growth factor-reduced Matrigel (Becton Dickinson, Mountain View, CA) supplemented with basic fibroblast growth factor (bFGF) (500 ng/plug; Upstate Biotechnology, Inc., Lake Placid, NY) was injected subcutaneously into the mid-lower abdominal region of each mouse. Growth factor-reduced Matrigel without bFGF was used as a negative control. Plugs were excised after 1 or 2.5 weeks after injection. The abdominal skeletal muscle was removed intact with each plug for purposes of orientation. Matrigel plugs were then fixed in 10% neutrally buffered formalin. Vessel counts were determined from hematoxylin and eosin (H&E)-stained sections of paraffin-embedded plugs, at a magnification of ×20.
Tumor Angiogenesis Assay
We chose to use B16-F10 cells as they are derived from C57BL/6 mice and, thus, are immunologically compatible with the C57BL/6 mice (WT and Cav-1 KO) used in this study. B16-F10 cells (CRL-6475; ATCC, Rockville, MD) were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum. When B16-F10 cells reached ∼75% confluence, they were lightly trypsinized, washed twice, then resuspended in ice-cold phosphate-buffered saline. Mice were mildly anesthetized with isoflurane, then injected subcutaneously in both flanks with 0.75 × 106 cells per flank. Twelve days after injection, the tumors were excised from each mouse, and tumor weight and volume were determined. Tumor volume was assessed after excision using caliper measurements of three dimensions (Scienceware; Bel-Art Products). Tumors were then fixed in 10% neutrally buffered formalin. Vessel counts were determined from H&E-stained paraffin-embedded sections, at a magnification of ×40.
Multiphoton Confocal Microscopy
WT or Cav-1 KO mice were mildly anesthetized with isoflurane. Growth factor-reduced Matrigel supplemented with bFGF (500 ng/plug) was injected subcutaneously into the mid-lower abdominal region of each mouse. One week after Matrigel injection, each mouse was injected through the tail vein with 2 mg/ml of rhodamine-conjugated dextran (Sigma Chemical Co., St. Louis, MO). Plugs were then excised and immediately viewed using a Bio-Rad Radiance 2000 MP multiphoton confocal microscope (Albert Einstein College of Medicine, Analytical Imaging Facility).
Electron Microscopic Ultrastructural Analysis
Exogenous tumors were fixed with 2.5% glutaraldehyde in 0.1 mol/L of cacodylate buffer postfixed with OsO4, and stained with uranyl acetate and lead citrate. Samples were examined under a JEOL 1200EX transmission electron microscope (Albert Einstein College of Medicine, Analytical Imaging Facility).
Statistical Analysis
The Student’s t-test was applied to data from test groups. A P value of ≤0.05 was considered significant.
Results
Cav-1 KO Mice Show Reduced Vessel Infiltration into bFGF-Supplemented Matrigel Plugs
Basic fibroblast growth factor (bFGF) is a proven in vivo angiogenic factor. 18,19 Thus, we tested the necessity of Cav-1/caveolae for proper bFGF-induced vessel infiltration into Matrigel plugs. Matrigel supplemented with 500 ng/plug of bFGF was injected into the subcutaneous compartment of the lower abdominal region of WT or Cav-1 KO mice. The formed Matrigel plugs were then removed after 1 week.
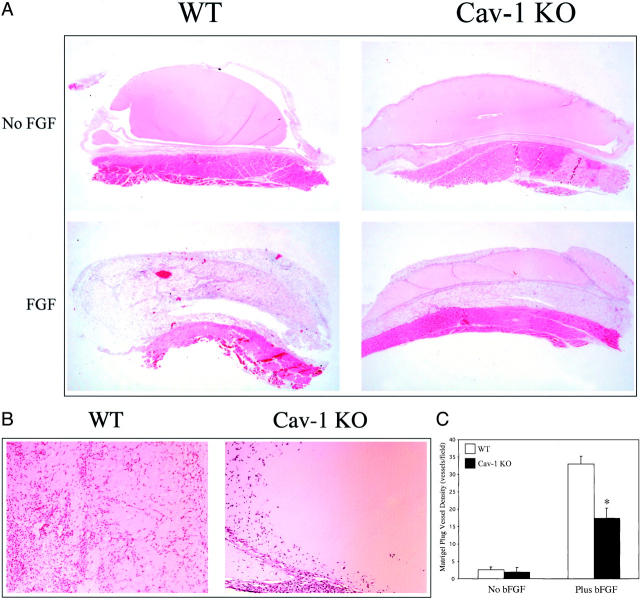
Many of the plugs removed from WT mice clearly showed gross signs of increased vascular and red blood cell content, whereas none of the Cav-1 KO plugs showed such macroscopic features (Figure 1A) ▶ . Histological analysis of Cav-1 KO plugs revealed marked cellular proliferation at the interface between the abdominal skeletal muscle and the outer boundary of the plug; interestingly, this localized cellular proliferation was not observed in WT plugs (Figure 1B ▶ , top). In addition, the number and depth of penetration of blood vessels was clearly reduced in Cav-1 KO mouse plugs, as compared with WT plugs (Figure 1B ▶ , bottom).
Figure 1.
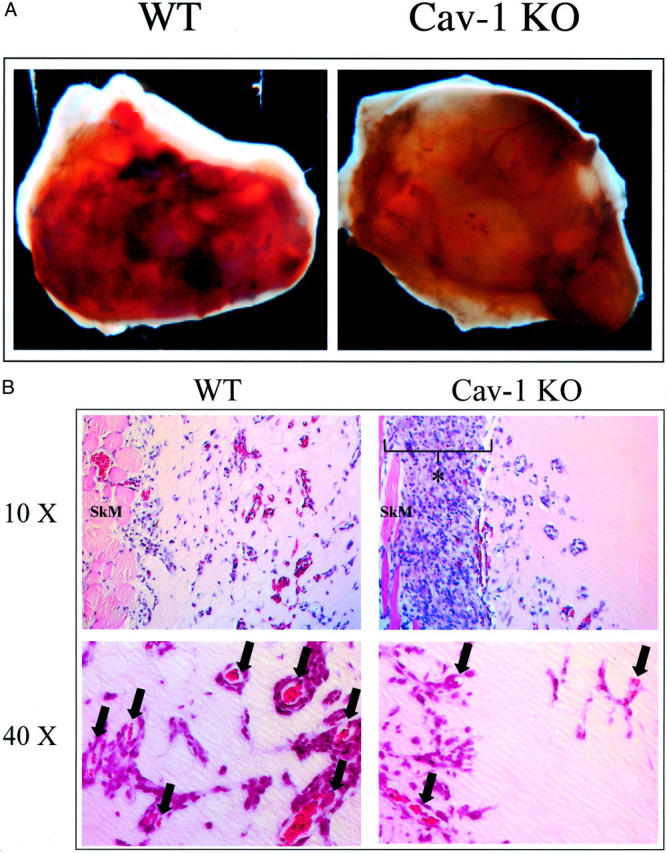
In Cav-1 KO mice, bFGF-supplemented Matrigel plugs show reduced vessel penetration and increased cellular proliferation adjacent to the plug. Matrigel plugs supplemented with 500 ng of bFGF/plug were injected into WT or Cav-1 KO mice and removed after 1 week. A: Gross appearance. Many WT plugs showed marked vascular content (vessels and heme coloration) and appeared hyperemic at the macroscopic level, whereas none of the plugs removed from Cav-1 KO mice showed this characteristic. B: Histological analysis (H&E staining). Note the marked cellular proliferation in the Cav-1 KO periplug region, at the interface between the abdominal skeletal muscle and the boundary of the Matrigel plug (top); an asterisk marks the region of increased cellular proliferation. In contrast, little or no cellular proliferation in this region was observed in plugs excised from WT animals. Also note that within the plug, the depth of penetration and vessel density was reduced in Cav-1 KO mice, as compared with WT mice (top and bottom); arrows mark newly formed blood vessels within the bFGF-supplemented Matrigel plugs. Original magnifications: ×10 (top); ×40 (bottom).
Multiphoton Confocal Microscopic Analysis also Shows Reduced Vessel Density in Matrigel Plugs from Cav-1 KO Mice
Multiphoton fluorescence microscopy allows for the observation of fluorescent indicators deep within tissues. Thus, we used multiphoton fluorescence microscopy to assess the depth of vessel infiltration into Matrigel plugs. WT or Cav-1 KO mice containing bFGF-supplemented Matrigel plugs for 1 week were infused intravascularly with rhodamine-labeled dextran. Plugs were then removed and examined with the multiphoton confocal microscope at consecutively deeper planes.
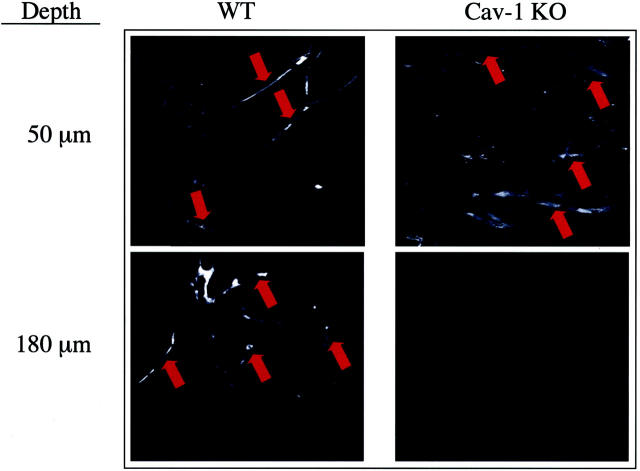
Figure 2 ▶ shows images captured from WT and Cav-1 KO mice at the surface and deeper levels of the plug. Optical sections were taken at 5-μm intervals until the fluorescence signal was undetectable. Note the similar levels of fluorescence outlining vessels at the surface (50-μm depth) in both WT and Cav-1 KO plugs. Although fluorescence levels became negligible at a depth of 180 μm in the Cav-1 KO plug, vessels were still apparent in the WT plug until the maximal depth obtainable with this technique, ie, 300 μm. Thus, vessel infiltration is clearly reduced in Cav-1 KO Matrigel plugs.
Figure 2.
Multiphoton confocal microscopic analysis of vessel depth within Matrigel plugs. Both WT and Cav-1 KO mice harboring bFGF-supplemented Matrigel plugs were intravenously injected with rhodamine-conjugated dextran. Plugs were then analyzed by multiphoton confocal microscopy. Images taken at a depth of 50 μm from the surface of the plug reveal multiple fluorescently labeled vessels in both the WT and Cav-1 KO plugs. Images taken at a depth of 180 μm also reveal vessels in the WT plug; however, no vessels were seen in the Cav-1 KO plug at this depth.
The Angiogenic Process Is Significantly Reduced in Cav-1 KO Mice
By 2.5 weeks, histological analysis reveals the entire WT bFGF Matrigel plug to have undergone angiogenesis, as well as a later-forming adipogenic response, whereas Cav-1 KO plugs clearly showed a diminished angiogenic response (Figure 3, A and B) ▶ . Importantly, Matrigel plugs not supplemented with bFGF failed to induce an angiogenic response in either WT or Cav-1 KO mice (Figure 3, A and B) ▶ . Quantitative analysis of the bFGF-supplemented Matrigel plugs from Cav-1 KO mice reveals an almost twofold reduction in the number of vessels per field, as compared with plugs from WT mice (WT, 32.9 ± 2.3 versus KO, 17.4 ± 2.9; vessels/field; Figure 3C ▶ , counted at a magnification of ×20). Thus, the angiogenic process is significantly reduced in Cav-1 KO mice.
Figure 3.
Reduced angiogenesis in Cav-1 KO mice harboring bFGF-supplemented Matrigel plugs. A: Microscopic appearance. Histological analysis (H&E staining) of WT and Cav-1 KO mouse Matrigel plugs removed after ∼2.5 weeks is shown. Note that no angiogenesis occurs in Matrigel plugs lacking bFGF supplementation, indicating that this process is clearly bFGF-dependent (top). In contrast, bFGF-supplemented Matrigel plugs excised from WT animals show a robust angiogenic response during this time period. However, this bFGF-dependent angiogenic response is clearly blunted in Cav-1 KO mice (bottom). B: Higher power views. Images of bFGF-supplemented Matrigel plugs excised from WT and Cav-1 KO mice are shown. C: Quantitative analysis. Vessel density within Matrigel plugs derived from WT and Cav-1 KO mice was quantitated. Matrigel plugs derived from both WT and Cav-1 KO mice were analyzed microscopically to quantitate the number of vessels/field. Note that Cav-1 KO mice showed a clear reduction in vessel density, as compared with WT controls (right). WT (n = 5) and Cav-1 KO (n = 6); an asterisk indicates a statistically significant difference between the two groups. Also, note that angiogenesis within the Matrigel plugs is strictly dependent on bFGF-supplementation (left). Original magnifications: ×4 (A); ×10 (B).
Exogenous Tumor Weight, Volume, and Vessel Density Is Reduced in Cav-1 KO Mice
bFGF has been shown to play an important role in angiogenesis during melanoma progression. 20,21 Therefore, we used the well-characterized technique of exogenous tumor formation to examine if tumor angiogenesis is also negatively affected in Cav-1 KO mice. 22 This approach has the benefit of excluding any effects that might result from the loss of Cav-1 in the tumor cells because Cav-1 has been identified as a tumor suppressor. 23 To this end, B16-F10 melanoma cells (0.75 × 106 cells) were injected subcutaneously into the flanks of WT or Cav-1 KO mice. We chose to use B16-F10 cells as they are derived from C57BL/6 mice and, thus, are immunologically compatible with the C57BL/6 mice (WT and Cav-1 KO) used in this study.
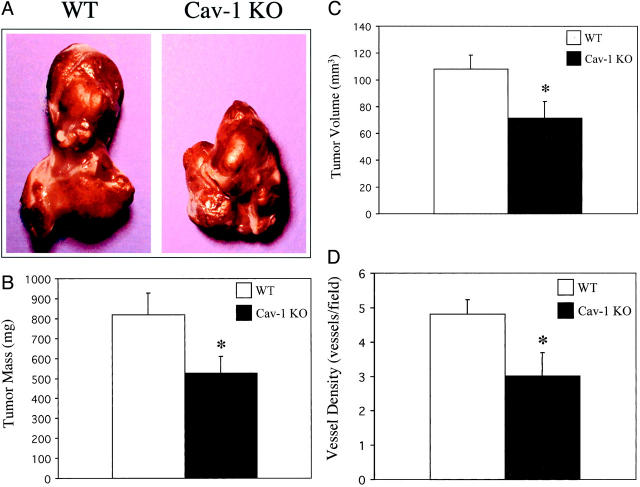
Figure 4A ▶ shows representative tumors removed from WT and Cav-1 KO mice 12 days after injection. Tumors grown in Cav-1 KO mice showed an overall reduction of ∼35% in tumor weight, as compared to WT controls (WT, 820 ± 110 versus KO, 528 ± 91, mg ± SEM; Figure 4B ▶ ). A corresponding decrease of ∼33% in tumor volume was also observed in Cav-1 KO mice versus WT controls (WT, 108.2 ± 10.7 versus KO, 71.3 ± 13.4, mm3 ± SEM; Figure 4C ▶ ).
Figure 4.
Exogenous tumors derived from Cav-1 KO mice are reduced in mass, volume, and vessel density. B16-F10 melanoma cells (0.75 × 106 cells) were injected subcutaneously into the flanks of WT or Cav-1 KO mice. We chose to use B16-F10 cells as they are derived from C57BL/6 mice and, thus, are immunologically compatible with the C57BL/6 mice (WT and Cav-1 KO) used in this study. Twelve days after injection, the tumors were excised from each mouse. A: Gross appearance. Overall, tumors derived from Cav-1 KO mice appeared grossly smaller than tumors derived from WT mice. Two examples are shown. B: Weight. Tumor weight was determined. Note that tumors grown in Cav-1 KO mice showed ∼35% reduction in their overall weight, as compared with WT controls. WT (n = 11 tumors) and Cav-1 KO (n = 9 tumors). C: Volume. Tumor volume was determined using multiple caliper measurements of three dimensions. Note that tumors grown in Cav-1 KO mice showed ∼33% reduction in their overall volume, as compared with WT controls. WT (n = 11 tumors) and Cav-1 KO (n = 9 tumors). D: Vessel density. Tumors were analyzed microscopically to determine the number of vessels/field. Vessel counts were determined from H&E-stained paraffin-embedded sections. Note that exogenous tumors from Cav-1 KO mice showed a reduction in vessel density, as compared with exogenous tumors from WT control mice. WT (n = 6 tumors) and Cav-1 KO (n = 6 tumors). In B–D, an asterisk indicates a statistically significant difference between the two groups.
Given the necessity of angiogenesis for optimal tumor growth, we next examined vessel density within these exogenous tumors. Blood-vessel density was quantified by counting the number of vessels per microscopic field of tumor sections. As predicted based on the reduction in tumor weight and volume, the vessel number was proportionally reduced in tumors from Cav-1 KO mice (WT, 4.81 ± 0.43 versus KO, 3.01 ± 0.67 vessels/field; Figure 4C ▶ , counted at a magnification of ×40).
Endothelial Cells Derived from Cav-1 KO Mice Lack Caveolae and Form Incomplete/Poorly-Organized Nascent Capillaries
To assess the phenotypic behavior of WT and Cav-1 KO endothelial cells, the vessels that infiltrated exogenous tumors grown in WT and Cav-1 KO mice were examined by transmission electron microscopy. Importantly, the capillary endothelium of the exogenous tumor has its origin from pre-existing vessels within the mouse into which tumor cells were injected.
High-magnification views of nascent capillary endothelial cells show abundant caveolae (50 to 100 nm vesicles and invaginations) in endothelial cells derived from WT mice, as expected. However, Cav-1 KO endothelial cells show a complete ablation of caveolae formation (Figure 5A) ▶ , in accordance with our previously published studies on lung endothelial cells. 7
Figure 5.
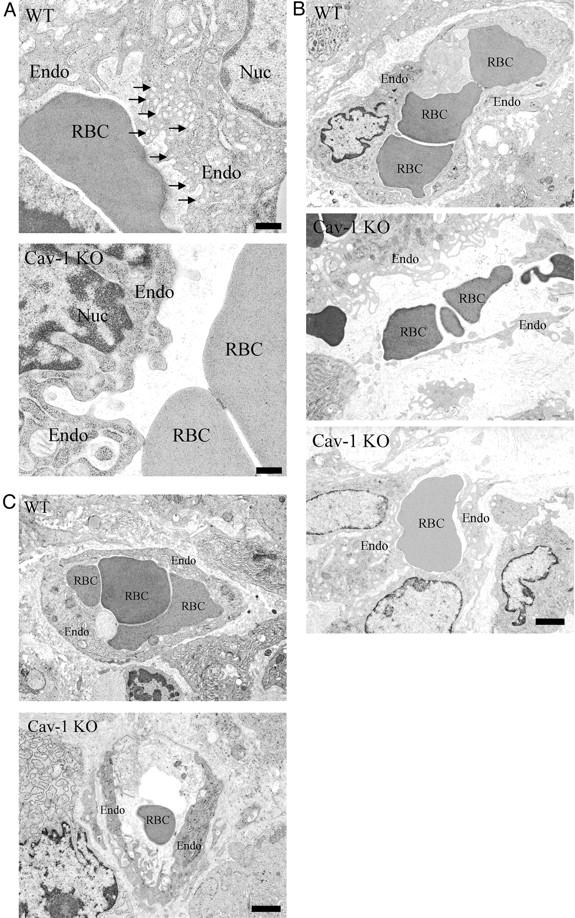
Ultrastructural analysis of endothelial cells infiltrating the exogenous tumors within WT and Cav-1 KO mice. Exogenous tumors were fixed with 2.5% glutaraldehyde in 0.1 mol/L of cacodylate buffer, postfixed with OsO4, and stained with uranyl acetate and lead citrate. Samples were examined under a JEOL 1200EX transmission electron microscope. A: Caveolar morphology. High-magnification electron micrographs of nascent capillaries formed within exogenous tumors are shown. Note that in WT endothelial cells, caveolae organelles (50- to 100-nm vesicles and invaginations) are particularly abundant (arrows); in contrast, in Cav-1 KO endothelial cells, caveolae formation is completely ablated. B and C: Nascent capillary formation. Low-magnification electron micrographs of nascent capillaries formed within exogenous tumors are shown. Note that nascent capillary formation is incomplete and disorganized in tumors grown in Cav-1 KO mice. B shows larger nascent capillaries, while C shows examples of smaller nascent capillaries. Endo, endothelial cell; RBC, red blood cell; Nuc, nucleus. Scale bars: 400 nm (A); 2 μm (B and C).
Interestingly, low-magnification electron microscopic analysis of the capillaries formed within B16-F10 tumors grown in Cav-1-null mice revealed that these nascent capillaries are poorly formed, very disorganized, and show increased matrix deposition, as compared with capillaries formed within B16-F10 tumors grown in WT mice (Figure 5, B and C) ▶ . These results are consistent with the idea that Cav-1-null mice have defects in angiogenesis, as the nascent capillaries that they form within tumors are clearly morphologically incomplete or are very poorly organized. Examples of larger nascent capillaries are shown in Figure 5B ▶ , whereas smaller nascent capillaries are shown in Figure 5C ▶ .
The Observed Angiogenesis Defect in Cav-1-Null Mice Is Specific for the Loss of Cav-1 Protein Expression and Is Not Related to a Loss of Cav-2 Protein Expression
Cav-1 deficiency in mice leads to an ∼95% reduction in Cav-2 protein levels, as Cav-1 protein expression is required to stabilize the Cav-2 protein product. 7 Therefore, Cav-1-null mice are essentially deficient in both Cav-1 and Cav-2. To determine whether the defective angiogenesis phenotype seen in Cav-1-null mice is because of the loss of Cav-1 or Cav-2, we also examined angiogenesis in Cav-2-null mice 17 using bFGF-supplemented Matrigel plugs.
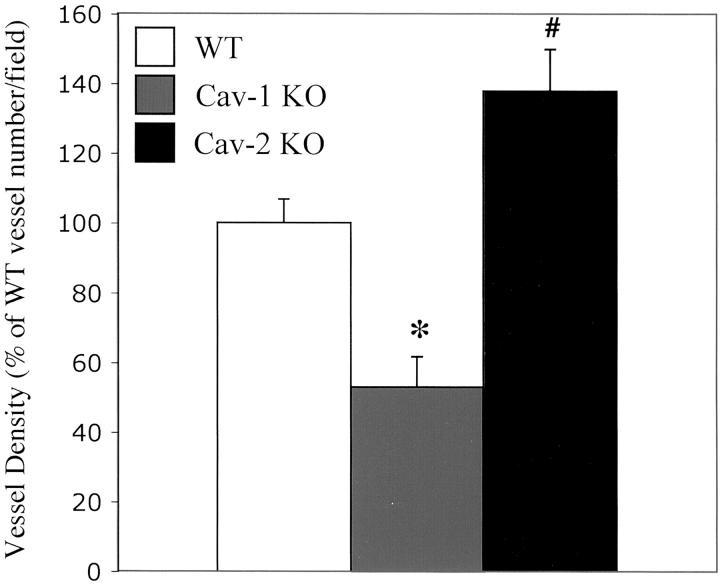
Importantly, Figure 6 ▶ shows that Cav-2-null mice do not show any decreases in vessel density; instead, surprisingly, they show an increase in vessel density compared to WT. These results clearly indicate that loss of Cav-1, and not Cav-2, is responsible for the angiogenesis defect observed in Cav-1-null mice.
Figure 6.
Quantitative analysis of vessel density within Matrigel plugs derived from WT, Cav-1 KO, and Cav-2 KO mice. bFGF-supplemented Matrigel plugs derived from WT, Cav-1 KO, and Cav-2 KO mice were analyzed microscopically to quantitate the number of vessels/field. Note that Cav-1 KO mice showed a clear reduction in vessel density, as compared with WT controls. WT, n = 5; Cav-1 KO, n = 6. As a control, Cav-2 KO (n = 6) mouse plug vessel density was also quantitated, and showed no decrease in vessel density; surprisingly, they show an increase in vessel density compared to WT. These results clearly indicate that loss of Cav-1, and not Cav-2, is responsible for the angiogenesis defect observed in Cav-1-null mice. Note that an asterisk indicates a statistically significant decrease as compared to WT, while a number sign indicates a statistically significant increase as compared to WT.
Discussion
The process of pathological angiogenesis requires the effect of proangiogenic factors to override the inhibitory influence of anti-angiogenic factors. 24 An early event in this process is increased permeability of the normal vasculature. 9 The resultant extravasation of plasma proteins is believed to create the scaffolding for endothelial migration. One mechanism for the extravasation of molecules through hyperpermeable vessels is Cav-1-containing caveolae and vesiculo-vacuolar organelles. 25,26 The microvasculature of angiogenic endothelium is characterized by an accumulation of these structures throughout the endothelial cell. 26-28
Although Cav-1 KO mice demonstrate vascular hyperpermeability, it is most likely because of altered tight-junction function, as demonstrated by ultrastructural analysis of tight-junctions in the lung microvasculature. 6 Therefore, it is possible that the intercellular hyperpermeability associated with Cav-1 loss is of a different nature and degree than that resulting from caveolae or vesiculo-vacuolar organelles intraendothelial passage, and is incommensurate with an angiogenic program. In addition, Cav-1 KO mice are hyperpermeable under baseline conditions, and it is not clear if basal hyperpermeability negatively affects the beginning stages of the angiogenic process. However, if caveolae and vesiculo-vacuolar organelles formation is a prerequisite for subsequent angiogenic phenomena, then loss of Cav-1 may in part explain the reduced angiogenesis observed in this study.
Cav-1 KO mice have increased basal levels of endothelial nitric oxide synthase (eNOS) activity, and nitric oxide has been proposed to contribute to VEGF-mediated angiogenesis. 29,30 However, the angiogenic effects of bFGF has been shown to be independent of nitric oxide. 30 Similarly, VEGF and bFGF have also been shown to differ in the integrins they use to mediate angiogenesis. 31 It will be of great interest in the future to examine multiple angiogenic factors (including VEGF) within the Cav-1 KO background, to further define the role of Cav-1 in angiogenesis. Interestingly, a recent study shows the loss of caveolae through cholesterol depletion is associated with the inhibition of VEGF-mediated endothelial cell migration in vitro, 32 suggesting a role for caveolae during the endothelial invasive response of angiogenesis.
There is abundant evidence that integrin signaling plays an important role in angiogenesis. For example, αvβ3 expression is markedly up-regulated on vascular cells in tumors. 33 Disruption of αvβ3 ligation with antibodies or peptide antagonists inhibits blood vessel formation in angiogenesis assays, 34-36 and specifically abolishes bFGF-mediated angiogenesis. 31 Curiously, angiogenesis is enhanced in mice with a null mutation in the β3 or β3/β5 integrin genes. 37 Although an explanation that unifies these data awaits further investigation, they clearly show integrin involvement in the process of angiogenesis.
Cav-1 has been shown to co-immunoprecipitate with various integrin subunits. 38 It is thought that Cav-1 may serve as a membrane adaptor that couples the integrin α subunit to Fyn, which phosphorylates Shc, resulting in the downstream activation of the Ras-Erk pathway. 38,39 It is thus possible that loss of Cav-1 affects this signaling cascade, and thereby disrupts the migration and subsequent differentiation phase of the angiogenic process. 40
Cav-1 also co-immunoprecipitates with the glycolipid-anchored urokinase receptor (uPA-R). 41 The formation of complexes with the uPAR may help to mediate the proteolysis necessary for invasion and migration, as well as, the nonproteolytic cell adhesion events during angiogenesis. It has been shown that uPA-R forms a complex with Cav-1 and the β1 integrin, and loss of Cav-1 disrupts the uPA-R/integrin complex and focal adhesion formation. Interestingly, bFGF up-regulates uPA, the ligand for the uPA-R, mediating the migration of endothelial cells in the angiogenic process. 42,43 In turn, integrins can serve to reciprocally facilitate bFGF receptor signaling. 44 Blocking either bFGF or uPA activity is sufficient to inhibit angiogenesis. 21,45,46 Thus, Cav-1 may act at multiple steps in the bFGF-uPAR-integrin-mediated angiogenic process.
We have previously reported alterations in endothelial cell-cell and cell-matrix adhesion in the lung vasculature of the Cav-1 KO mouse. 6 Ultrastructural analysis in the present study shows a marked diminishment in caveolae and other caveolae-sized vesicular structures in exogenous tumors from Cav-1 KO mice. Tumors grown in Cav-1 KO mice have capillaries that are poorly formed and disorganized, as compared with capillaries formed within tumors grown in WT mice. This suggests that invading endothelial cells from the Cav-1 KO mouse have a delayed or diminished capacity to form a complete capillary structure. As previously reported, the endothelium of Cav-1 KO mice still contains some larger vesicular structures, but classically shaped and sized caveolae are absent in Cav-1 KO mice. 8 As relatively sizeable exogenous tumors are still able to form in both WT and Cav-1 KO mice, the endothelial disruption in Cav-1 KO mice is not sufficient to eliminate all capillary formation, but clearly affects the kinetics of tumor growth. The morphological alterations observed in Cav-1 KO capillaries is in addition to the already abnormal nature of tumor blood vessels, as compared with the organization of the normal vasculature. 47
Quantitation of vessels in the Matrigel plugs clearly shows that diminished angiogenesis is specific to the Cav-1 KO mouse. Surprisingly, Matrigel plugs implanted in Cav-2 KO mice showed an enhanced angiogenic response. These data argue that caveolar structures play an important role in angiogenesis, as Cav-2 KO mouse cells (including endothelia) still form normal caveolae through the homo-oligomerization of Cav-1. 17 Thus, there may be important differences in the capacity of Cav-1 homo-oligomeric caveolae versus Cav-1/Cav-2 hetero-oligomeric caveolae to facilitate the process of angiogenesis.
We have previously shown using an in vitro adenoviral approach and cultured endothelial cells that down-regulation of Cav-1 markedly reduces endothelial cell tube formation, whereas overexpression of Cav-1 enhances the generation of endothelial tubes. 11 These findings are consistent with the studies of Griffoni and colleagues 12 who showed that Cav-1 anti-sense oligonucleotides strongly suppress endothelial tube formation in a three-dimensional fibrin gel angiogenesis assay. In addition, it was shown that Cav-1 anti-sense oligonucleotides also dramatically reduce vessel formation using the chorioallantoic membrane assay. Thus, our current studies using Cav-1 KO mice and in vivo assays of angiogenesis directly support these earlier in vitro studies.
It is of interest that in Cav-1 KO mice, bFGF-supplemented Matrigel plugs showed marked cellular proliferation or hypercellularity directly adjacent to the plug. This is consistent with our previous work indicating a role for Cav-1 as an inhibitor of cellular proliferation. 14,23 In fact, one of the major phenotypes of the Cav-1 KO mouse is the hyperproliferation of the lung parenchymal and primordial endothelial cells. 7,8 Perhaps bFGF-supplemented Matrigel plugs illicit a similar hyperproliferative response in the Cav-1 KO cells in the periplug region. This hypothesis is consistent with our previous interpretations of in vitro experiments in which we speculated that down-regulation of Cav-1 is an important aspect of the proliferative component of angiogenesis, whereas up-regulation of Cav-1 facilitates differentiation later in the angiogenic process. These data also support a role for Cav-1 in the migratory and/or differentiation phases of angiogenesis. Consistent with this notion, we show here that vessel infiltration and density are significantly reduced by ∼35 to 50% in Cav-1 KO mice using two different in vivo angiogenesis assays (Matrigel plugs and exogenous tumor formation).
We have recently shown that Cav-1 KO mice are clearly more susceptible to tumor formation/cell transformation induced either by treatment with chemical carcinogens (DMBA) or by breeding with tumor-prone animals (MMTV-PyMT). 48,49 Despite the dramatic tumor suppressor effects we observed, it is important to note that these tumor suppressor effects may be underestimated due to the concomitant defects in angiogenesis in Cav-1 KO mice that we describe here.
Acknowledgments
We thank Dr. Radma Mahmood (Histotechnology and Comparative Pathology Facility at Albert Einstein College of Medicine) for her expertise with tissue processing and sectioning and Dr. Richard Kitsis for insightful discussions.
Footnotes
Address reprint requests to Dr. Michael P. Lisanti, M.D., Ph.D., Department of Molecular Pharmacology, Albert Einstein College of Medicine, 1300 Morris Park Ave., Golding Rm 202, Bronx, NY 10461. E-mail: lisanti@aecom.yu.edu.
Supported by grants from the National Institutes of Health, the Muscular Dystrophy Association, the American Heart Association, and the Breast Cancer Alliance, as well as a Hirschl/Weil-Caulier Career Scientist Award (all to M. P. L.); and by a National Institutes of Health Medical Scientist Training Grant (T32-GM07288; S. E. W. and T. M. W.).
References
- 1.Simionescu N, Simionsecu M: The cardiovascular system. Weiss L eds. Histology: Cell and Tissue Biology. 1983:pp 371-433 Elsevier Biomedical, New York
- 2.Simionescu N: Cellular aspects of transcapillary exchange. Physiol Rev 1983, 63:1536-1560 [DOI] [PubMed] [Google Scholar]
- 3.Razani B, Woodman SE, Lisanti MP: Caveolae: from cell biology to animal physiology. Pharmacol Rev 2002, 54:431-467 [DOI] [PubMed] [Google Scholar]
- 4.Razani B, Lisanti MP: Caveolin-deficient mice: insights into caveolar function and human disease. J Clin Invest 2001, 108:1553-1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galbiati F, Razani B, Lisanti MP: Emerging themes in lipid rafts and caveolae. Cell 2001, 106:403-411 [DOI] [PubMed] [Google Scholar]
- 6.Schubert W, Frank PG, Woodman SE, Hyogo H, Cohen DE, Chow CW, Lisanti MP: Microvascular hyper-permeability in caveolin-1 (−/−) knock-out mice: treatment with a specific NOS inhibitor, L-NAME, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem 2002, 277:40091-40098 [DOI] [PubMed] [Google Scholar]
- 7.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP: Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem 2001, 276:38121-38138 [DOI] [PubMed] [Google Scholar]
- 8.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV: Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 2001, 293:2449-2452 [DOI] [PubMed] [Google Scholar]
- 9.Conway EM, Collen D, Carmeliet P: Molecular mechanisms of blood vessel growth. Cardiovasc Res 2001, 49:507-521 [DOI] [PubMed] [Google Scholar]
- 10.Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W: Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol 1998, 140:947-959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Wang XB, Park DS, Lisanti MP: Caveolin-1 expression enhances endothelial capillary tubule formation. J Biol Chem 2002, 277:10661-10668 [DOI] [PubMed] [Google Scholar]
- 12.Griffoni C, Spisni E, Santi S, Riccio M, Guarnieri T, Tomasi V: Knockdown of caveolin-1 by antisense oligonucleotides impairs angiogenesis in vitro and in vivo. Biochem Biophys Res Commun 2000, 276:756-761 [DOI] [PubMed] [Google Scholar]
- 13.Koleske AJ, Baltimore D, Lisanti MP: Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci USA 1995, 92:1381-1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Razani B, Altschuler Y, Zhu L, Pestell RG, Mostov KE, Lisanti MP: Caveolin-1 expression is down-regulated in cells transformed by the human papilloma virus in a p53-dependent manner. Replacement of caveolin-1 expression suppresses HPV-mediated cell transformation. Biochemistry 2000, 39:13916-13924 [DOI] [PubMed] [Google Scholar]
- 15.Park DS, Lee H, Frank PG, Razani B, Nguyen AV, Parlow AF, Russell RG, Hulit J, Pestell RG, Lisanti MP: Caveolin-1-deficient mice show accelerated mammary gland development during pregnancy, premature lactation, and hyper-activation of the Jak-2/STAT5a signaling cascade. Mol Biol Cell 2002, 13:3416-3430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H, Park DS, Razani B, Russell RG, Pestell RG, Lisanti MP: Caveolin-1 mutations (P132L and null) and the pathogenesis of breast cancer: Cav-1 (P132L) behaves in a dominant-negative fashion and Cav-1 (−/−) null mice show mammary epithelial cell hyperplasia. Am J Pathol 2002, 161:1357-1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Razani B, Wang XB, Engelman JA, Battista M, Lagaud G, Zhang XL, Kneitz B, Hou H, Jr, Christ GJ, Edelmann W, Lisanti MP: Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol 2002, 22:2329-2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bikfalvi A, Klein S, Pintucci G, Rifkin DB: Biological roles of fibroblast growth factor-2. Endocr Rev 1997, 18:26-45 [DOI] [PubMed] [Google Scholar]
- 19.Kawaguchi N, Toriyama K, Nicodemou-Lena E, Inou K, Torii S, Kitagawa Y: De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proc Natl Acad Sci USA 1998, 95:1062-1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Straume O, Akslen LA: Importance of vascular phenotype by basic fibroblast growth factor, and influence of the angiogenic factors basic fibroblast growth factor/fibroblast growth factor receptor-1 and ephrin-A1/EphA2 on melanoma progression. Am J Pathol 2002, 160:1009-1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Compagni A, Wilgenbus P, Impagnatiello MA, Cotten M, Christofori G: Fibroblast growth factors are required for efficient tumor angiogenesis. Cancer Res 2000, 60:7163-7169 [PubMed] [Google Scholar]
- 22.Franco S, Segura I, Riese HH, Blasco MA: Decreased B16F10 melanoma growth and impaired vascularization in telomerase-deficient mice with critically short telomeres. Cancer Res 2002, 62:552-559 [PubMed] [Google Scholar]
- 23.Galbiati F, Volonté D., Engelman JA, Watanabe G, Burk R, Pestell R, Lisanti MP: Targeted down-regulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. EMBO J 1998, 17:6633-6648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carmeliet P: Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000, 6:389-395 [DOI] [PubMed] [Google Scholar]
- 25.Feng D, Nagy JA, Pyne K, Hammel I, Dvorak HF, Dvorak AM: Pathways of macromolecular extravasation across microvascular endothelium in response to VPF/VEGF and other vasoactive mediators. Microcirculation 1999, 6:23-44 [PubMed] [Google Scholar]
- 26.Dvorak AM, Feng D: The vesiculo-vacuolar organelle (VVO). A new endothelial cell permeability organelle. J Histochem Cytochem 2001, 49:419-432 [DOI] [PubMed] [Google Scholar]
- 27.Dvorak HF, Brown LF, Detmar M, Dvorak AM: Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 1995, 146:1029-1039 [PMC free article] [PubMed] [Google Scholar]
- 28.Vasile E, Qu H, Dvorak HF, Dvorak AM: Caveolae and vesiculo-vacuolar organelles in bovine capillary endothelial cells cultured with VPF/VEGF on floating Matrigel-collagen gels. J Histochem Cytochem 1999, 47:159-167 [DOI] [PubMed] [Google Scholar]
- 29.Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC: Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest 1997, 100:3131-3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, Bicknell R: Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest 1997, 99:2625-2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA: Definition of two angiogenic pathways by distinct alpha v integrins. Science 1995, 270:1500-1502 [DOI] [PubMed] [Google Scholar]
- 32.Labrecque L, Royal I, Surprenant DS, Patterson C, Gingras D, Beliveau R: Regulation of vascular endothelial growth factor receptor-2 activity by caveolin-1 and plasma membrane cholesterol. Mol Biol Cell 2003, 14:334-347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eliceiri BP: Integrin and growth factor receptor crosstalk. Circ Res 2001, 89:1104-1110 [DOI] [PubMed] [Google Scholar]
- 34.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA: Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994, 79:1157-1164 [DOI] [PubMed] [Google Scholar]
- 35.Brooks PC, Clark RA, Cheresh DA: Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 1994, 264:569-571 [DOI] [PubMed] [Google Scholar]
- 36.Brooks PC, Stromblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA: Antiintegrin alpha v beta 3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest 1995, 96:1815-1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM: Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med 2002, 8:27-34 [DOI] [PubMed] [Google Scholar]
- 38.Wary KK, Mariott IA, Zurzolo C, Giancotti FG: A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell 1998, 94:625-634 [DOI] [PubMed] [Google Scholar]
- 39.Mettouchi A, Klein S, Guo W, Lopez-Lago M, Lemichez E, Westwick JK, Giancotti FG: Integrin-specific activation of Rac controls progression through the G(1) phase of the cell cycle. Mol Cell 2001, 8:115-127 [DOI] [PubMed] [Google Scholar]
- 40.Preissner KT, Kanse SM, May AE: Urokinase receptor: a molecular organizer in cellular communication. Curr Opin Cell Biol 2000, 12:621-628 [DOI] [PubMed] [Google Scholar]
- 41.Wei Y, Yang X, Liu Q, Wilkins JA, Chapman HA: A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J Cell Biol 1999, 144:1285-1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Presta M, Moscatelli D, Joseph-Silverstein J, Rifkin DB: Purification from a human hepatoma cell line of a basic fibroblast growth factor-like molecule that stimulates capillary endothelial cell plasminogen activator production, DNA synthesis, and migration. Mol Cell Biol 1986, 6:4060-4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gualandris A, Presta M: Transcriptional and posttranscriptional regulation of urokinase-type plasminogen activator expression in endothelial cells by basic fibroblast growth factor. J Cell Physiol 1995, 162:400-409 [DOI] [PubMed] [Google Scholar]
- 44.Liu G, Eskin SG, Mikos AG: Integrin alpha(v)beta(3) is involved in stimulated migration of vascular adventitial fibroblasts by basic fibroblast growth factor but not platelet-derived growth factor. J Cell Biochem 2001, 83:129-135 [DOI] [PubMed] [Google Scholar]
- 45.Guo Y, Higazi AA, Arakelian A, Sachais BS, Cines D, Goldfarb RH, Jones TR, Kwaan H, Mazar AP, Rabbani SA: A peptide derived from the nonreceptor binding region of urokinase plasminogen activator (uPA) inhibits tumor progression and angiogenesis and induces tumor cell death in vivo. EMBO J 2000, 14:1400-1410 [DOI] [PubMed] [Google Scholar]
- 46.Bajou K, Noel A, Gerard RD, Masson V, Brunner N, Holst-Hansen C, Skobe M, Fusenig NE, Carmeliet P, Collen D, Foidart JM: Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat Med 1998, 4:923-928 [DOI] [PubMed] [Google Scholar]
- 47.Carmeliet P, Jain RK: Angiogenesis in cancer and other diseases. Nature 2000, 407:249-257 [DOI] [PubMed] [Google Scholar]
- 48.Capozza F, Williams TM, Schubert W, McClain S, Bouzahzah B, Sotgia F, Lisanti MP: Absence of caveolin-1 sensitizes mouse skin to carcinogen-induced epidermal hyperplasia and tumor formation. Am J Pathol 2003, 162:2029-2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams TM, Cheung MWC, Park DS, Razani B, Cohen AW, Muller WJ, Di Vizio D, Chopra NG, Pestell RG, Lisanti MP: Loss of caveolin-1 gene expression accelerates the development of dysplastic mammary lesions in tumor-prone transgenic mice. Mol Biol Cell 2003, 14:1027-1042 [DOI] [PMC free article] [PubMed] [Google Scholar]