Abstract
Malignant melanomas of the skin are distinguished by their propensity for early metastatic spread via lymphatic vessels to regional lymph nodes, and lymph node metastasis is a major determinant for the staging and clinical management of melanoma. However, the importance of tumor-induced lymphangiogenesis for lymphatic melanoma spread has remained unclear. We investigated whether tumor lymphangiogenesis occurs in human malignant melanomas of the skin and whether the extent of tumor lymphangiogenesis may be related to the risk for lymph node metastasis and to patient survival, using double immunostains for the novel lymphatic endothelial marker LYVE-1 and for the panvascular marker CD31. Tumor samples were obtained from clinically and histologically closely matched cases of primary melanomas with early lymph node metastasis (n = 18) and from nonmetastatic melanomas (n = 19). Hot spots of proliferating intratumoral and peritumoral lymphatic vessels were detected in a large number of melanomas. The incidence of intratumoral lymphatics was significantly higher in metastatic melanomas and correlated with poor disease-free survival. Metastatic melanomas had significantly more and larger tumor-associated lymphatic vessels, and a relative lymphatic vessel area of >1.5% was significantly associated with poor disease-free and overall survival. In contrast, no differences in the density of tumor-associated blood vessels were found. Vascular endothelial growth factor and vascular endothelial growth factor-C expression was equally detected in a minority of cases in both groups. Our results reveal tumor lymphangiogenesis as a novel prognostic indicator for the risk of lymph node metastasis in cutaneous melanoma.
Malignant melanoma of the skin is a common and frequently lethal neoplasm with increasing worldwide incidence. 1 In 1998, 42,000 new cases of cutaneous malignant melanoma and 7300 related deaths were reported in the United States. 1 Complete surgical excision with wide margins represents the therapy of choice for primary melanomas. The vertical thickness 2 and the anatomical level of invasion (Clark level) 3 of the primary tumor are the most valuable prognostic indicators for the metastatic risk of cutaneous melanoma. 4-7 However, it is still difficult to predict the outcome after excision of the primary tumor, in particular in thin melanomas. 8,9 Hence novel indicators for the prognostic risk of metastatic melanoma spread are urgently needed.
Malignant melanomas metastasize via blood and lymphatic vessels, and the induction of tumor angiogenesis provides a possible explanation for how tumor cells escape their original site by invading the newly formed vascular bed. 10 Tumor vascularization has been observed in human melanoma both experimentally 11,12 and clinically. 13 The importance of tumor angiogenesis for the prognosis of primary malignant melanomas of the skin, however, has remained controversial. 14 Whereas several studies found an inverse correlation of tumor microvessel density with disease-free and overall survival, 15,16 other reports did not detect any significant differences of tumor microvessel density between metastasizing and nonmetastasizing melanomas. 17 Therefore, the potential prognostic value of tumor vascularization in malignant melanoma remains at present unclear.
Malignant melanomas of the skin are distinguished by their propensity for early metastatic spread via lymphatic vessels to regional lymph nodes, even at the early stages of tumor invasion. Hence lymph node metastasis, as determined by the analysis of sentinel lymph nodes, is a major determinant for the staging and clinical management of melanoma. 5,18 In contrast to the extensive studies on melanoma-associated angiogenesis, little is known about the mechanisms by which melanoma cells gain entry into the lymphatic system. Dilated tumor-associated lymphatic vessels, sometimes containing tumor cells, have been observed by routine histology and by electron microscopy in cutaneous melanoma. 19,20 However, the importance of tumor-induced lymphangiogenesis for lymphatic melanoma spread has remained unclear, and the very existence of melanoma-associated lymphangiogenesis has even been questioned. 21 Recent experimental evidence strongly suggests that tumors can actively induce lymphangiogenesis via production of lymphangiogenic factors such as vascular endothelial growth factor (VEGF)-C and VEGF-D, and that the extent of tumor lymphangiogenesis is directly correlated with the extent of experimental metastatic tumor spread to regional lymph nodes. 22,23 We have previously reported that VEGF-C is expressed in several human melanoma cell lines in vitro; 24 however, because of the lack of specific markers for the lymphatic endothelium in human cancers, the importance of tumor-associated lymphangiogenesis for melanoma progression has remained unclear. The recent discovery of the lymphatic endothelial hyaluronan receptor-1 (LYVE-1) as a specific marker for normal 25 and tumor-associated lymphatic vessels 26 has now provided the tool for a detailed analysis of tumor lymphangiogenesis in melanoma.
In the present study, we investigated whether tumor lymphangiogenesis occurs in human malignant melanomas of the skin and whether the extent of tumor lymphangiogenesis may be related to the risk for lymph node metastasis and to patient survival, using tumor samples obtained from clinically and histologically matched primary cutaneous malignant melanomas from patients who developed early lymph node metastasis and from patients who remained metastasis-free. Our results reveal, for the first time, a higher incidence of intra- and peritumoral lymphangiogenesis in metastatic melanoma, as compared with nonmetastatic melanomas. Moreover, multivariate proportional hazards analysis identified peritumoral lymphatic vascular density as a novel prognostic indicator for the risk of lymph node metastasis in cutaneous melanoma.
Materials and Methods
Patient Population and Histological Analyses
Patients were identified retrospectively through review of survival data from the German Melanoma Registry. Of a total of 1050 patients with primary cutaneous malignant melanoma, 19 patients with nonmetastatic primary melanoma (mean disease-free follow-up of 6.5 years; range, 34 to 119 months) were closely matched with a group of 18 patients who had documented early (<1 year) lymph node metastasis (Table 1) ▶ . The two groups were matched for gender, age at diagnosis, tumor thickness, Clark’s level of invasion, histological type, and presence of ulceration. Exclusion criteria were acrolentiginous melanoma and adjuvant therapy after the surgical excision of the primary tumor. The diagnosis of melanoma, the tumor thickness, and the level of tumor invasion were reconfirmed by two pathologists (SSD and MCM). Additional parameters such as the frequency of mitoses, tumor regression, vascular invasion, and microsatellites were also evaluated. Peritumoral inflammation was evaluated as absent, present (nonbrisk), and brisk. 27 The degree of inflammation was further graded as low (≤50% of tumor area) or high (>50% of tumor area).
Table 1.
Clinical and Pathological Characteristics of Patients with Melanoma
| Category | Nonmetastatic | Metastatic |
|---|---|---|
| Number of patients | 19 | 18 |
| Male | 11 | 10 |
| Female | 8 | 8 |
| Time of lymph node metastasis (months) | ||
| Mean | — | 24.4 |
| Range | — | 5–69 |
| Time of visceral organ metastasis (months) | ||
| Mean | — | 38.0 |
| Range | — | 14–76 |
| Age at diagnosis (years) | ||
| Mean | 53.8 | 54.9 |
| Range | 21–79 | 23–76 |
| Histologic type | ||
| Superficial spreading | 14 | 14 |
| Nodular | 4 | 4 |
| Lentigo maligna | 1 | 0 |
| Breslow thickness (mm) | ||
| <1.5 | 5 | 2 |
| ≥1.5 | 14 | 16 |
| Mean | 2.5 | 2.6 |
| Clark level of invasion | ||
| II | 2 | 1 |
| III | 4 | 6 |
| IV | 12 | 10 |
| V | 1 | 2 |
| Mean | 3.63 | 3.47 |
| Ulceration | ||
| Present | 4 | 4 |
| Absent | 15 | 14 |
| Site | ||
| Upper Extremity | 5 | 2 |
| Lower Extremity | 7 | 4 |
| Head and neck | 3 | 3 |
| Trunk | 4 | 9 |
| Mitoses (mm2) | ||
| >6.0 | 4 | 4 |
| 1–6 | 11 | 13 |
| 0 | 3 | 0 |
| Regression | ||
| Present | 4 | 2 |
| Absent | 15 | 15 |
| Peritumoral inflammation | ||
| Absent | 6 | 7 |
| Present | 9 | 9 |
| Brisk | 4 | 2 |
Immunostains and in Situ Hybridization
Paraffin sections (6 μm thickness) were dewaxed, hydrated, and treated with 0.01% protease XXIV (Sigma, St. Louis, MO) in phosphate-buffered saline for 20 minutes at 37°C. Sections were double-stained using a rabbit polyclonal antibody against human LYVE-1 (1:600) 25 and a mouse monoclonal anti-human CD31 antibody (1:40; DAKO, Carpinteria, CA), followed by incubation with the respective secondary antibodies that were labeled with either Texas Red (1:50) or with fluorescein isothiocyanate (1:50) (Jackson ImmunoResearch, West Grove, PA) as previously described. 24 Cell nuclei were counterstained with Hoechst bisbenzimide (Sigma) at 20 μg/ml. Additional immunohistochemical stains were performed using affinity-purified rabbit polyclonal antibodies against human VEGF-C (C-terminus; Zymed, San Francisco, CA) or against human LYVE-1, followed by incubation with conjugated rabbit anti-human immunoglobulin (1:200), using the 3-amino-9-ethylcabazole peroxidase substrate kit (Vector Laboratories, Burlingame, CA). To detect proliferating cells, the Zymed proliferating cell nuclear antigen (PCNA) 3,3′-diaminobenzidine staining kit was used as previously described. 28 For specificity controls, either the secondary antibody was omitted or the primary anti-VEGF-C antibody was preincubated with a 40-fold molar excess of recombinant human VEGF-C (a generous gift from Dr. K. Alitalo, University of Helsinki, Finland). In situ hybridization was performed as previously described, 29 using a riboprobe for human VEGF that detects all known VEGF splice variants 29 or a 808-bp human VEGF-C riboprobe described previously. 30 Transcription reactions were performed using the Riboprobe Gemini II kit (Promega, Madison, WI) in the presence of [α-35S]UTP. For autoradiography, slides were coated with NTB2 film emulsion and exposed for 4 weeks. Sections were examined using a Nikon E-600 microscope (Nikon, Melville, NY) and digital images were captured using a SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI).
Computer-Assisted Morphometric Vessel Analysis
To analyze the lymphatic and blood vessel density and size within and surrounding the 37 primary melanomas, we performed double-immunofluorescence stains for CD31 and LYVE-1. Sections were examined using a Nikon E-600 microscope and digital images were captured using a SPOT digital camera. For each tumor section, three fields with the highest lymphatic vascular density (hot spots) were evaluated at ×100 magnification. Digital images of tumor-associated lymphatic vessels and blood vessels were captured in the same field. Peritumoral lymphatic vessels were defined as LYVE-1-positive vessels within an area of 100 μm from the tumor border. Intratumoral lymphatic vessels were defined as LYVE-1-positive vessels located within the tumor mass and not confined by invagination of normal tissue. Tumor borders were determined on serial sections using Hoechst nuclear stains and hematoxylin and eosin stains. Morphometric analyses of lymphatic vessels and of blood vessels were performed using the IP-Lab software (Scanalytics, Fairfax, VA) to determine the vessel number per mm2, the average vessel size, and the relative tumor area occupied by vessels as described. 31 The relative lymphatic vascular area was determined in the peritumoral area and was calculated as the area covered by lymphatic vessels divided by the total area examined times 100, expressed in percent.
Statistical Analyses
The unpaired Student’s t-test was used to determine the statistical significance (P value) of the mean for all vascular parameters. The chi-square square test was used to evaluate differences in the frequency of intratumoral lymphatic vessels. Disease-free survival and overall survival intervals were determined as the time period from initial diagnosis to the time of first metastasis or the time of death. Patients with no events (ie, no lymph node metastasis) were censored and the disease-free interval for these patients was the same as their overall follow-up time. Disease-free survival and overall survival analyses were performed using the Kaplan-Meier method. The comparison between survival functions for different strata was assessed with the log-rank statistic. Univariate and multivariate analyses of prognostic factors were based on the Cox proportional hazards model with model selection based on the backwards elimination process.
Results
Detection of Intratumoral and Peritumoral Lymphatic Vessels in Malignant Melanomas
We closely matched two cohorts of patients with metastatic (n = 18) or with nonmetastatic (n = 19) primary cutaneous malignant melanoma for age, gender, tumor type, thickness, invasion level, and presence of ulceration (Table 1) ▶ . Histological analysis of hematoxylin and eosin-stained paraffin sections revealed that additional prognostic parameters such as mitotic activity, peritumoral inflammation, or regression also showed comparable distribution in both groups (Table 1) ▶ . Next, melanoma-associated lymphatic and blood vessels were simultaneously visualized in all primary melanomas, using immunofluorescence double stains for the lymphatic vessel marker LYVE-1 and for the panvascular marker CD31. Whereas CD31-positive/LYVE-1-negative blood vessels were homogeneously distributed throughout the tumors, CD31-positive/LYVE-1-positive lymphatic vessels were found in prominent hotspots both within and around primary cutaneous melanomas (Figure 1 ▶ ; A to F). Such hotspots were seen in thick melanomas (Figure 1 ▶ ; A to C) as well as in thin melanomas (Figure 1 ▶ ; D to F). Foci of intratumoral lymphatic vessels were preferentially localized near the tumor border in metastatic melanomas (Figures 1E and 2A) ▶ . In all cases, peritumoral lymphatic vessels with frequently open lumina were found within a distance of 100 μm from the tumor border (Figures 1E and 2A) ▶ . Intratumoral lymphatic vessels frequently exhibited a thin-walled, basket-like morphology (Figure 2B) ▶ resembling that seen in blood vessel networks during VEGF-induced angiogenesis. 32 Importantly, intratumoral lymphatics were found more frequently in metastatic melanomas (77.8% of all cases) than in nonmetastatic melanomas (36.8%, P = 0.01, chi-square test; Figure 2D ▶ ). Pigmented tumor cells within LYVE-1-positive intratumoral lymphatics were found in 2 of 18 (11%) metastatic melanomas (Figure 2, E and F) ▶ , whereas no intralymphatic tumor cells were detected in nonmetastatic melanomas. Differential immunostains for LYVE-1 and for PCNA revealed PCNA-positive nuclei in the majority of melanoma cells and in several LYVE-1-positive lymphatic endothelial cells (Figure 2C) ▶ , confirming the occurrence of active intratumoral lymphangiogenesis in primary human melanomas.
Figure 1.
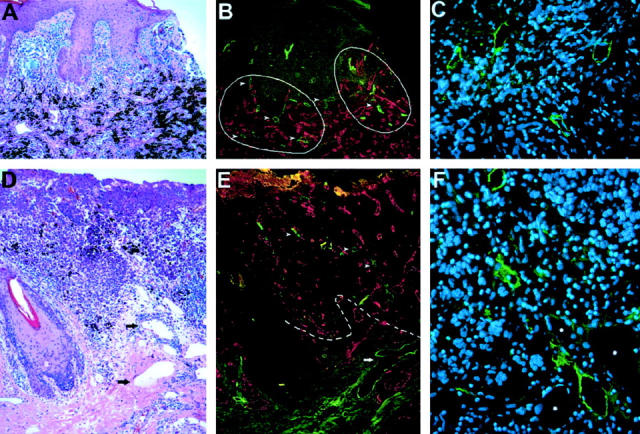
Detection of intratumoral and peritumoral lymphatic vessels in cutaneous malignant melanomas. A: Histology of a thick melanoma (4.5 mm) shows compact masses of frequently pigmented tumor cells. B: Immunofluorescent stain of a serial section of the same tumor for the lymphatic marker LYVE-1 (green) and the panvascular marker CD31 (red) reveals prominent hotspots of high lymphatic vessel density (hotspots are circled by solid line). In contrast, blood vessels are homogeneously distributed throughout the tumor. C: Thin-walled, LYVE-1-positive intratumoral lymphatic vessels with open lumina. D: Histology of a thin melanoma (≤1.5 mm) with dilated peritumoral lymphatics (arrows). E: Immunofluorescent stain of a serial section for LYVE-1 (green) and CD31 (red) reveals lymphatic vessels within (arrowheads) and surrounding (arrows) the tumor border (dotted line). F: LYVE-1-positive intratumoral lymphatic vessels with open lumina. The adjacent blood vessels (asterisks) are LYVE-1-negative. Cell nuclei are counterstained (blue) with Hoechst (C and F). Original magnifications: ×100 (A, B, D, and E); ×400 (C and F).
Figure 2.
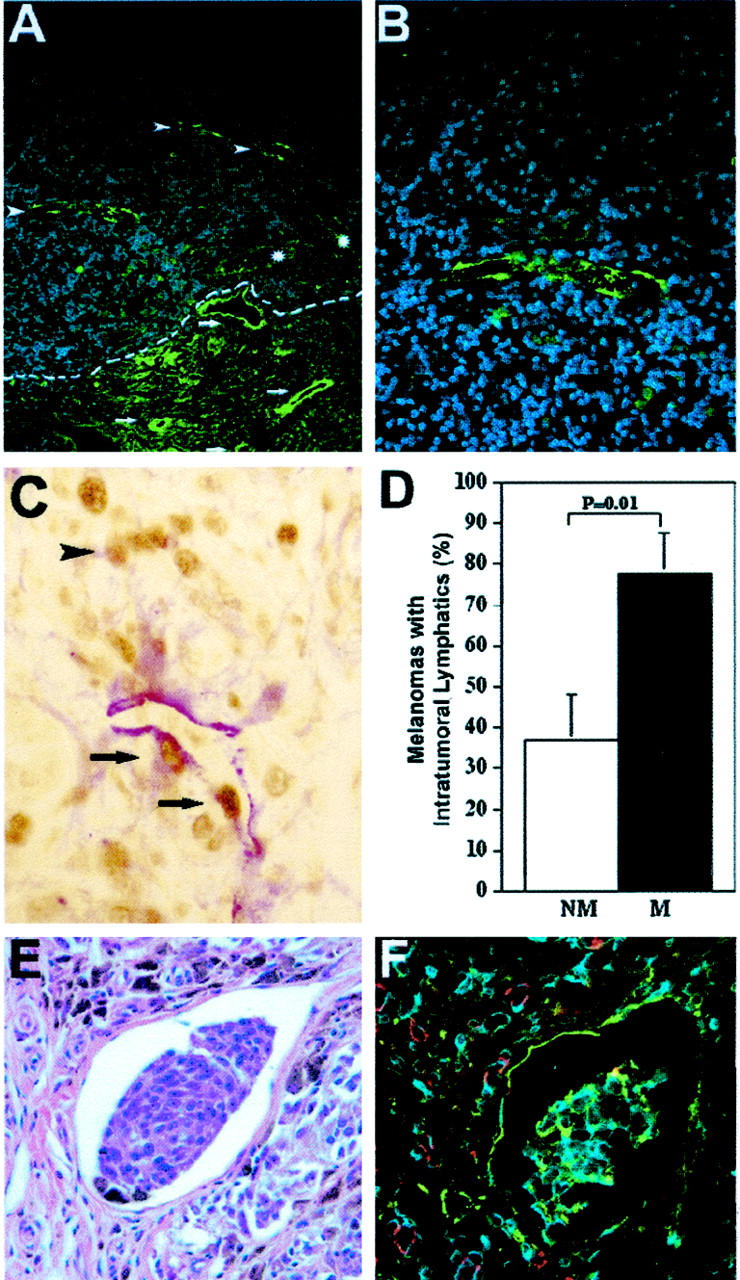
Higher frequency of intratumoral lymphangiogenesis in metastatic melanomas. A: Immunofluorescent stain for LYVE-1 (green) depicts thick-walled peritumoral (arrows) and thin-walled intratumoral lymphatics (arrowheads) in a thin melanoma (1.05 mm). The tumor border is indicated by a dotted line. Blood vessels (asterisks) are negative for LYVE-1. B: Higher magnification of intratumoral lymphatics reveals thin-walled, basket-like morphology. C: Double immunostain for LYVE-1 (red) and PCNA (brown) reveals an intratumoral lymphatic vessel with proliferating lymphatic endothelial cells (arrows) and adjacent melanoma cells (arrowhead). D: Significantly increased frequency of detectable intratumoral lymphatic vessels in metastatic melanomas (M; n = 18), as compared with nonmetastatic (NM; n = 19) tumors (mean ± SEM, chi-square test, P = 0.01). E: Detection of melanin-containing tumor cells within an intratumoral lymphatic vessel. H&E stain. F: Immunofluorescent stain of a serial section for LYVE-1 (green) and CD31 (red) confirms that tumor cells are located within a lymphatic vessel. Cell nuclei are counterstained blue with Hoechst (B and F). Original magnifications: ×200 (A); ×400 (B, C, E, F).
Increased Tumor Lymphangiogenesis in Metastatic Melanomas
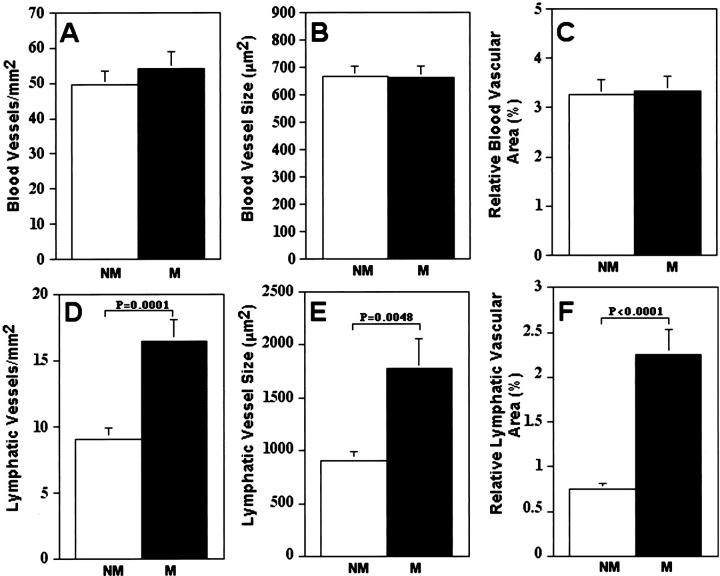
Metastatic melanomas exhibited more hotspots of intratumoral and peritumoral LYVE-1-positive lymphatic vessels than nonmetastatic tumors (Figure 1B) ▶ , whereas the extent of blood vascularization was uniform throughout both metastatic and nonmetastatic tumors. The density of both lymphatic and blood vessels was higher in melanomas than in neighboring normal human skin (data not shown). Computer-assisted morphometric analysis confirmed a comparable blood vascular density, average blood vessel size, and tumor area covered by blood vessels in metastatic and nonmetastatic melanomas (Figure 3 ▶ ; A to C). In contrast, the number of peritumoral lymphatic vessels was significantly increased in metastatic melanomas (16.5 ± 1.62 vessels/mm2) as compared with nonmetastatic melanomas (9.1 ± 0.76 vessels/mm2, P = 0.0002; Figure 3D ▶ ). Moreover, the average lymphatic vessel size was significantly larger in metastatic melanomas (1773.0 ± 280.9 μm2) than in nonmetastatic melanomas (908.6 ± 86.2 μm2, P = 0.0048; Figure 3E ▶ ). The relative peritumoral area covered by lymphatic vessels was threefold higher in metastatic melanomas (2.25 ± 0.27%) than in nonmetastatic melanomas (0.75 ± 0.07%, P < 0.0001; Figure 3F ▶ ).
Figure 3.
Computer-assisted image analysis of tumor-associated blood vessels and peritumoral lymphatic vessels in nonmetastatic versus metastatic melanomas. A–C: Comparable blood vascular density, average blood vessel size, and relative area occupied by blood vessels in metastatic (M; n = 18) and nonmetastatic (NM; n = 19) malignant melanomas. D–F: Significant increase of lymphatic vascular density, average lymphatic vessel size, and relative area occupied by lymphatics in metastatic malignant melanomas, as compared with nonmetastatic melanomas. Mean ± SEM.
VEGF-C Expression in Malignant Melanomas
Because VEGF-C expression has been recently linked to tumor lymphangiogenesis in experimental models, we next analyzed VEGF-C protein expression in all 37 primary melanomas by in situ hybridization and by immunohistochemistry. By in situ hybridization, we detected focal, low-level tumor cell expression of VEGF-C mRNA (Figure 4 ▶ ; A to C) in 44% of the metastatic melanomas and in 44% of the nonmetastatic melanomas. VEGF-C mRNA expression was frequently also detected in epidermal keratinocytes overlying the tumors and in peritumoral stromal cells (Figure 4D) ▶ . Moreover, mRNA expression of the angiogenesis factor VEGF was detected in 33.3% of the metastatic and in 30% of the nonmetastatic melanomas (data not shown). Focal cytoplasmic VEGF-C protein expression (Figure 5, A and B) ▶ was found more frequently in tumor cells of metastatic melanomas (50.0%) than in nonmetastatic melanomas (31.6%); however, these differences did not reach statistically significant levels (P > 0.05). VEGF-C expression was also detected in peritumoral dermal fibroblasts near the invasive edge of metastatic (55.6%) (Figure 5C) ▶ and of nonmetastatic melanomas (31.6%, P = 0.14).
Figure 4.
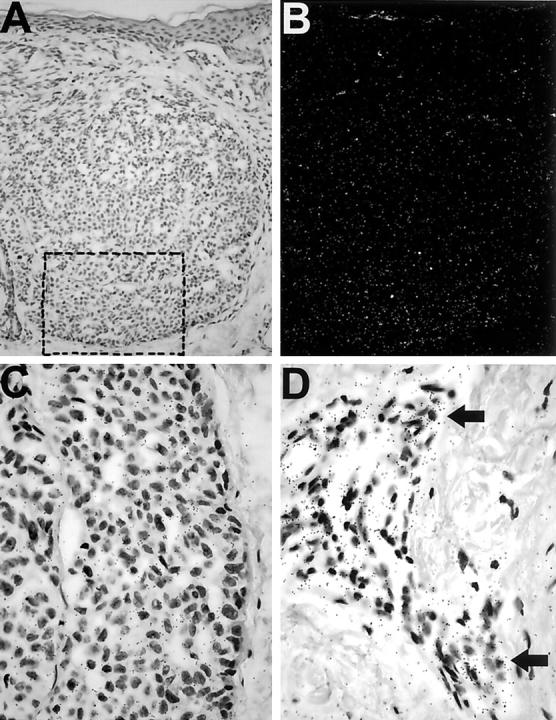
VEGF-C mRNA expression in cutaneous melanomas. A: Bright-field microscopy shows a dermal melanoma nodule. B: Dark-field microscopy reveals increased signal (white grains) in the tumor area but not the dermis. C: Bright-field microscopy shows increased black grains under a higher magnification from the rectangle in A. D: VEGF-C mRNA expression by peritumoral stromal cells. Arrows indicate areas of strong hybridization signals. Original magnifications: ×200 (A and B); ×600 (C and D).
Figure 5.
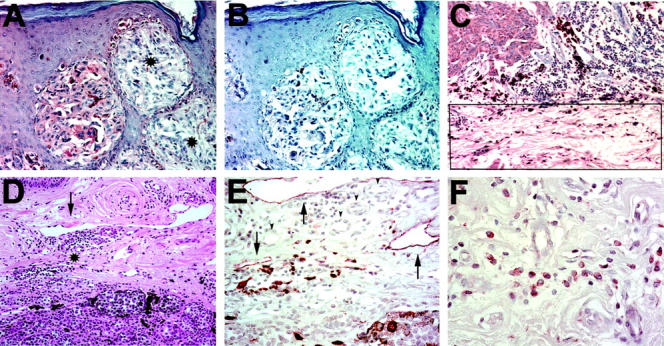
VEGF-C protein expression in cutaneous melanomas. A: Immunoperoxidase staining for VEGF-C (red) demonstrates focal expression in the cytoplasm of melanoma cells in one tumor nest but not in the adjacent nests (asterisks). B: Serial section negative control with omission of secondary antibody. C: Immunoperoxidase staining for VEGF-C (red) demonstrates VEGF-C expression by peritumoral fibroblasts (rectangle) at the invasive edge of a metastatic melanoma (top left) in the reticular dermis. D: H&E stain of the invasive tumor edge of a metastasizing cutaneous melanoma reveals mononuclear inflammation (asterisks) near dilated lymphatic vessel (arrow). E: Immunoperoxidase staining of a serial section for LYVE-1 (red) decorates dilated peritumoral lymphatics (arrow) and adjacent mononuclear infiltrate. Blood vessels are LYVE-1-negative (arrowheads). F: Immunoperoxidase staining for VEGF-C (red) stains the cytoplasm of peritumoral mononuclear cells in a granular pattern. Original magnifications: ×200 (A–D); ×400 (E); and ×600 (F).
Correlation of Lymphangiogenesis with Peritumoral Inflammation
We next studied whether the degree of lymphangiogenesis in metastatic melanomas was correlated with the degree of peritumoral inflammation, as assessed by routine histology. We found that the lymphatic vascular area was significantly increased (P = 0.005) in melanomas with high-grade nonbrisk inflammation (3.15 ± 0.10%), as compared with melanomas with low-grade inflammation (1.18 ± 0.10%). LYVE-1-positive macrophages near dilated, LYVE-1-positive lymphatic vessels were frequently found in metastatic melanomas with high-grade nonbrisk inflammation (Figure 5E) ▶ . VEGF-C-expressing inflammatory cells were detected in 3 of 18 (16.7%) metastatic melanomas, but not in nonmetastatic tumors. The peritumoral mononuclear inflammatory cells, mostly located near the invasive tumor edge, expressed VEGF-C in a cytoplasmic granular pattern (Figure 5F) ▶ .
Lymphangiogenesis Is a Novel Prognostic Parameter for Melanoma Metastasis and Survival
Because metastatic melanomas were characterized by a significant increase of lymphatic vessel density and lymphatic vessel size, we next investigated whether the degree of tumor lymphangiogenesis might serve as a new prognostic parameter for the risk of melanoma metastasis. A univariate proportional hazard analysis revealed that the presence of intratumoral lymphatic vessels and a higher level of peritumoral lymphatic vascular area were significantly associated with a more rapid development of lymph node metastasis (Table 2) ▶ . A multivariate proportional hazard analysis, using backward elimination of all other variables, revealed that only the level of peritumoral lymphatic area was an independent predictor of the time to lymph node metastasis. An additional univariate proportional hazard analysis for the effect of different risk factors on overall survival identified the presence of intratumoral lymphatics and the extent of peritumoral lymphatic vascularization as the only two parameters that were significantly associated with reduced overall survival (Table 3) ▶ . Because both groups of metastatic and nonmetastatic melanomas were exactly matched for tumor thickness, no prognostic significance could be assigned to this parameter by the hazard analyses.
Table 2.
Univariate Analysis for the Effect of Different Risk Factors on the Development of Lymph Node Metastasis
| Covariate | Hazard ratio | P value |
|---|---|---|
| Intratumoral lymphatics* | 3.559 | 0.0263 |
| Lymphatic Vascular† Area (%) | 1.602 | 0.0002 |
| Vascular Invasion* | 1.971 | 0.372 |
| Ulceration* | 1.515 | 0.4344 |
| Microsatellites* | 1.165 | 0.882 |
| Peritumoral inflammation† | 0.835 | 0.5942 |
| Site† | 0.698 | 0.3115 |
| Regression* | 0.624 | 0.5292 |
| Mitoses (>6/mm2)* | 0.531 | 0.2305 |
* Dichotomous variable.
† Continuous variable.
Table 3.
Univariate Analysis for the Effect of Different Risk Factors on Overall Survival
| Covariate | Hazard ratio | P value |
|---|---|---|
| Intratumoral lymphatics* | 6.133 | 0.0180 |
| Lymphatic Vascular† Area (%) | 1.555 | 0.0028 |
| Vascular Invasion* | 4.511 | 0.0619 |
| Ulceration* | 1.986 | 0.2607 |
| Microsatellites* | NA | NS |
| Peritumoral inflammation† | 0.570 | 0.1763 |
| Site* | 0.935 | 0.8697 |
| Regression* | 0.994 | 0.9933 |
| Mitoses (>6/mm2)* | 0.696 | 0.5414 |
NA, not applicable; NS, not significant.
* Dichotomous variable.
† Continuous variable.
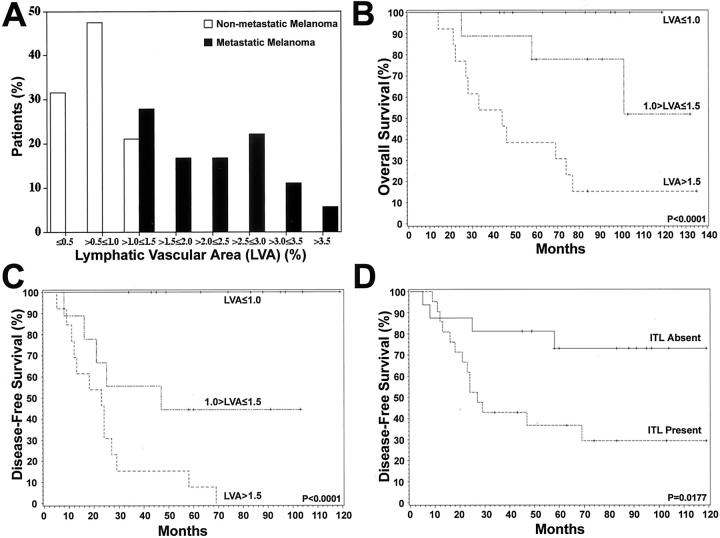
We next used Kaplan-Meier analyses to calculate the overall survival and the disease-free survival for patients with or without the detection of intratumoral lymphatics, and for patients with low (≤1.0%), medium (1.0% to ≤1.5%), or high (>1.5%) peritumoral lymphatic vascular area (Figure 6 ▶ ; B to D). This classification was based on the distribution of lymphatic vascular areas in our patient population (Figure 6A) ▶ . We found that a high level of tumor lymphangiogenesis was a significant prognostic factor for reduced overall survival (Figure 6B ▶ ; P < 0.0001) and for reduced disease-free survival (Figure 6C ▶ ; P < 0.0001). The estimates for 3, 5, and 10 years for each stratum were calculated with pair-wise comparisons, which were all significant (P < 0.05) for both overall and disease-free survival. Importantly, the detection of intratumoral lymphatic vessels was significantly associated with a poorer disease-free survival, as compared with melanomas without intratumoral lymphatics (Figure 6D ▶ ; P = 0.0177).
Figure 6.
Kaplan-Meier analyses of overall and disease-free survival as a function of tumor lymphangiogenesis. A: The distribution of lymphatic vascular area (LVA) in the patient population indicates an overlap in the >1.0 to ≤1.5 range between the nonmetastatic and metastatic melanoma groups. B, C: Overall and disease-free survival curves were stratified by low (≤1.0%), medium (1.0 to ≤1.5%), or high (>1.5%) level of tumor lymphangiogenesis. Statistically significant correlation between the extent of tumor-associated lymphangiogenesis, expressed as percentage of area covered by lymphatic vessels, and reduced disease-free survival and overall survival are noted. D: The disease-free survival analysis revealed that the detection of intratumoral lymphatics was significantly associated with a poorer disease-free survival.
Discussion
The prognosis of cutaneous malignant melanoma is determined by its ability to metastasize, and the evaluation of the metastatic risk of a primary melanoma poses a challenge to clinicians and pathologists alike. Although a number of clinical, histological, 2,3,27,33 and molecular 34 prognostic indicators have been described, the current method of cutaneous melanoma prognostication is predominantly based on the tumor thickness. 5 However, the prognostic value of tumor thickness is limited because a considerable number of patients with thin melanomas die of metastatic disease whereas many of those with thick tumors experience long-term survival. This clinical observation was confirmed in our matched cohort study of 19 patients with nonmetastatic primary melanoma and of 18 patients with early (<1 year) lymph node metastasis that were selected from a pool of 1050 melanoma patients. Thus, novel prognostic indicators of metastasis for malignant melanoma are urgently needed. The discovery of the lymphatic endothelial hyaluronan receptor-1 (LYVE-1) as a specific marker for both normal 25 and tumor-associated lymphatics 26 has now paved the road to study tumor lymphangiogenesis not only in experimental tumor models, but also in spontaneously arising human tumors. The specificity of LYVE-1 as a lymphatic marker has been recently challenged because of its presence on hepatic sinusoidal endothelial cells that are involved in hyaluronan uptake. 35 However, recent studies, using differential immunostains for LYVE-1 and for the lymphatic-specific transcription factor Prox1 have confirmed that LYVE-1 is selectively expressed by lymphatic vessels, but not by blood vessels, in murine and in human tumors. 36-38
In our present study, using double immunostains for CD31 and LYVE-1, we detected both peri- and intratumoral lymphatic vessels in a large number of primary cutaneous melanomas. Although intratumoral lymphatics have been proposed to be nonfunctional in experimental mouse tumor models, 39,40 univariate proportional hazard analysis revealed that the presence of intratumoral lymphatics was a significant risk factor for the development of lymph node metastasis in patients with cutaneous melanoma. Not only did intratumoral lymphatics occur at a significantly higher incidence in metastatic melanoma, but their presence was also related to a significantly increased risk of lymph node metastasis. These results suggest that intratumoral lymphatics play a clinically significant role as conduits for the metastasis of cutaneous melanoma, proposing an active role of lymphangiogenesis as evidenced by hot spots of lymphatic vessels within and surrounding metastatic melanomas. In contrast, a traditional view asserts that lymphatic endothelium plays a passive role during tumor metastasis and that lymphatic invasion only occurs via infiltrating tumor cells invading pre-existing lymphatic vessels. 41 However, several studies in animal tumor models provide direct experimental evidence that increased tumor lymphangiogenesis promotes lymphatic tumor spread to regional lymph nodes. 22,23,42-44 Taken together, the results obtained in animal tumor models and in human tumors 26 support the recently proposed concept of an active lymphangiogenesis model for tumor metastasis. 45
We have previously reported that the lymphangiogenic factor VEGF-C is expressed by several melanoma cell lines in vitro and that overexpression of recombinant VEGF-C in human melanoma cell lines promoted tumor lymphangiogenesis after xenotransplantation in nude mice. 24 In the human melanomas studied in the present investigation, however, we only detected low-level and heterogeneous expression of VEGF-C by tumor cells that did not significantly correlate with the metastatic potential of the primary tumors. Similarly, only weak VEGF-C protein has been recently found in metastatic human squamous cell carcinomas of the head and neck, 26 whereas other studies found a correlation between the occurrence of tumor metastasis and tumor expression of VEGF-C mRNA in a number of epithelial cancers. 46 We also found detectable VEGF expression in only a minority of all melanomas examined, confirming previous studies reporting that VEGF was only expressed in some cases of primary melanomas. 47,48 Taken together, these results indicate that tumor-derived VEGF and VEGF-C likely do not represent the major source of angiogenic or lymphangiogenic activity in cutaneous melanomas. Therefore, it is tempting to speculate that the relative low levels of VEGF-C expression by melanoma cells are complimented by nontumoral, stromal sources such as dermal fibroblasts and peritumoral macrophages, as demonstrated in our study. Moreover, recent evidence indicates that peritumoral inflammation and VEGF-C production by inflammatory cells might also contribute to the induction of lymphangiogenesis. 24,49 Although we were unable to detect any expression of VEGF-D, 50 another known lymphangiogenic factor, in the 37 cases studied (data not shown), lymphatic vessel growth might be stimulated by other, yet unknown growth factors. The recent establishment of specific human lymphatic endothelial cell cultures 51-53 and the development of in vivo lymphangiogenesis assays 54 now provide the experimental tools for future in vitro and in vivo studies for the identification and characterization of novel lymphangiogenesis factors that might include members of the fibroblast growth factor family. 54
Surprisingly, we did not detect any significant differences in tumor angiogenesis between the metastatic and nonmetastatic matched cutaneous melanomas. The similarity in tumor angiogenesis may be because of the comparably weak levels of VEGF mRNA expression in both metastatic and nonmetastatic melanomas in our study that did not include cases with early organ metastasis. Moreover, both groups were exactly matched for tumor thickness, thereby excluding differences of local tumor progression that has been previously correlated with increased vascularity. 13,16,55,56 A recently published prospective study in 417 cutaneous melanoma patients found that tumor vascularity, as assessed on routine histological stains, was the most important determinant of overall survival. 20 In contrast, other investigators failed to detect any correlation between melanoma vascularization and prognosis. Using morphometric analysis of Ulex europaeus type I lectin-labeled sections obtained from 86 melanomas with no evidence of recurrence after a minimum follow-up period of 5 years and of 21 cases with locoregional recurrence and/or metastasis, one study found that tumor recurrence could not be predicted by any of the derived vascular parameters (vascular length, surface, and volume density) either independently or together with other histological and clinical features. 57 In another study of 60 cases of metastasizing and nonmetastasizing cutaneous melanomas that were matched for tumor thickness, age, sex, and anatomical site, there was no significant difference in the number of microvessels or in the pattern of vascular microarchitecture between metastasizing and nonmetastasizing tumors. 17 The reported increase of VEGF protein expression during the transition from horizontal growth to the vertical melanoma growth phase 55,56 suggests that angiogenesis may be important for promoting primary tumor growth whereas it seems to play a minor role if any in promoting lymphatic melanoma metastasis.
In summary, our study demonstrates, for the first time, the presence of intra- and peritumoral lymphangiogenesis in primary human cutaneous melanomas. It also provides the first evidence that the extent of tumor lymphangiogenesis may be related to the risk of lymph node metastasis as well as patient survival, and that peritumoral lymphatic vascular density might serve as a novel prognostic indicator for the risk of lymph node metastasis of human cutaneous melanomas. The feasibility of CD31/LYVE-1 double immunostains on routine paraffin sections eliminates the need for fresh-frozen tumor samples and will greatly facilitate larger, prospective, multi-institutional clinical trials that are needed to further validate the prognostic value of tumor lymphangiogenesis for metastasis and patient survival of primary malignant melanomas of the skin and, potentially, of other human malignancies.
Acknowledgments
We thank L. Janes, M. Constant, D. Lipoff, and L. Nguyen for expert technical assistance; M. Streit for critical reading of the manuscript; R. Kunstfeldt, S. Hirakawa, and Y.-K. Hong for helpful discussions; and K. Alitalo for the gift of human VEGF-C.
Footnotes
Address reprint requests to Michael Detmar, M.D., Cutaneous Biology Research Center, Massachusetts General Hospital, Building 149, 13th St., Charlestown, MA 02129. E-mail: michael.detmar@cbrc2.mgh.harvard.edu.
Supported by the National Institutes of Health/National Cancer Institute (grants CA69184, CA86410, and CA92644 to M. D., and pathology training grant 5T32CA09216 to S. S. D.), the Susan G. Komen Breast Cancer Foundation (to M. D.), the American Cancer Society (program project grant 99-23901 to M. D.), the Deutsche Forschungsgemeinschaft (to T. P), the Association for International Cancer Research (grants 99-250 and 00-311to D. G. J.), and the Cutaneous Biology Research Center through the Massachusetts General Hospital/Shiseido Co. Ltd. Agreement (to M. D.).
References
- 1.Landis SH, Murray T, Bolden S, Wingo PA: Cancer statistics, 1998. CA Cancer J Clin 1998, 48:6-29 [DOI] [PubMed] [Google Scholar]
- 2.Breslow A: Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg 1970, 172:902-908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark WH, Jr, From L, Bernardino EA, Mihm MC: The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res 1969, 29:705-727 [PubMed] [Google Scholar]
- 4.Balch CM, Soong SJ, Milton GW, Shaw HM, McGovern VJ, Murad TM, McCarthy WH, Maddox WA: A comparison of prognostic factors and surgical results in 1,786 patients with localized (stage I) melanoma treated in Alabama, USA, and New South Wales, Australia. Ann Surg 1982, 196:677-684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A, Jr, Kirkwood JM, McMasters KM, Mihm MF, Morton DL, Reintgen DS, Ross MI, Sober A, Thompson JA, Thompson JF: Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001, 19:3635-3648 [DOI] [PubMed] [Google Scholar]
- 6.Barnhill RL, Fine JA, Roush GC, Berwick M: Predicting five-year outcome for patients with cutaneous melanoma in a population-based study. Cancer 1996, 78:427-432 [DOI] [PubMed] [Google Scholar]
- 7.Vollmer RT, Seigler HF: Using a continuous transformation of the Breslow thickness for prognosis in cutaneous melanoma. Am J Clin Pathol 2001, 115:205-212 [DOI] [PubMed] [Google Scholar]
- 8.Blessing K, McLaren KM, McLean A, Davidson P: Thin malignant melanomas (less than 1.5 mm) with metastasis: a histological study and survival analysis. Histopathology 1990, 17:389-395 [DOI] [PubMed] [Google Scholar]
- 9.Guitart J, Lowe L, Piepkorn M, Prieto VG, Rabkin MS, Ronan SG, Shea CR, Tron VA, White W, Barnhill RL: Histological characteristics of metastasizing thin melanomas: a case-control study of 43 cases. Arch Dermatol 2002, 138:603-608 [DOI] [PubMed] [Google Scholar]
- 10.Weidner N, Semple JP, Welch WR, Folkman J: Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 1991, 324:1-8 [DOI] [PubMed] [Google Scholar]
- 11.Warren BA, Shubik P: The growth of the blood supply to melanoma transplants in the hamster cheek pouch. Lab Invest 1966, 15:464-478 [PubMed] [Google Scholar]
- 12.Hubler WR, Jr, Wolf JE, Jr: Melanoma. Tumor angiogenesis and human neoplasia. Cancer 1976, 38:187-192 [DOI] [PubMed] [Google Scholar]
- 13.Barnhill RL, Fandrey K, Levy MA, Mihm MC, Jr, Hyman B: Angiogenesis and tumor progression of melanoma. Quantification of vascularity in melanocytic nevi and cutaneous malignant melanoma. Lab Invest 1992, 67:331-337 [PubMed] [Google Scholar]
- 14.Streit M, Detmar M: Melanoma angiogenesis, lymphangiogenesis and metastasis. Oncogene (in press) [DOI] [PubMed]
- 15.Srivastava A, Laidler P, Davies RP, Horgan K, Hughes LE: The prognostic significance of tumor vascularity in intermediate-thickness (0.76–4.0 mm thick) skin melanoma. A quantitative histologic study. Am J Pathol 1988, 133:419-423 [PMC free article] [PubMed] [Google Scholar]
- 16.Straume O, Salvesen HB, Akslen LA: Angiogenesis is prognostically important in vertical growth phase melanomas. Int J Oncol 1999, 15:595-599 [DOI] [PubMed] [Google Scholar]
- 17.Busam KJ, Berwick M, Blessing K, Fandrey K, Kang S, Karaoli T, Fine J, Cochran AJ, White WL, Rivers J: Tumor vascularity is not a prognostic factor for malignant melanoma of the skin. Am J Pathol 1995, 147:1049-1056 [PMC free article] [PubMed] [Google Scholar]
- 18.Gershenwald JE, Thompson W, Mansfield PF, Lee JE, Colome MI, Tseng CH, Lee JJ, Balch CM, Reintgen DS, Ross MI: Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol 1999, 17:976-983 [DOI] [PubMed] [Google Scholar]
- 19.Deutsch A, Lubach D, Nissen S, Neukam D: Ultrastructural studies on the invasion of melanomas in initial lymphatics of human skin. J Invest Dermatol 1992, 98:64-67 [DOI] [PubMed] [Google Scholar]
- 20.Kashani-Sabet M, Sagebiel RW, Ferreira CM, Nosrati M, Miller JR, III: Vascular involvement in the prognosis of primary cutaneous melanoma. Arch Dermatol 2001, 137:1169-1173 [DOI] [PubMed] [Google Scholar]
- 21.de Waal RM, van Altena MC, Erhard H, Weidle UH, Nooijen PT, Ruiter DJ: Lack of lymphangiogenesis in human primary cutaneous melanoma. Consequences for the mechanism of lymphatic dissemination. Am J Pathol 1997, 150:1951-1957 [PMC free article] [PubMed] [Google Scholar]
- 22.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M: Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 2001, 7:192-198 [DOI] [PubMed] [Google Scholar]
- 23.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG: VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 2001, 7:186-191 [DOI] [PubMed] [Google Scholar]
- 24.Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, Detmar M: Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol 2001, 159:893-903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG: LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol 1999, 144:789-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beasley NJ, Prevo R, Banerji S, Leek RD, Moore J, van Trappen P, Cox G, Harris AL, Jackson DG: Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res 2002, 62:1315-1320 [PubMed] [Google Scholar]
- 27.Clemente CG, Mihm MC, Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N: Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 1996, 77:1303-1310 [DOI] [PubMed] [Google Scholar]
- 28.Streit M, Riccardi L, Velasco P, Brown LF, Hawighorst T, Bornstein P, Detmar M: Thrombospondin-2: a potent endogenous inhibitor of tumor growth and angiogenesis. Proc Natl Acad Sci USA 1999, 96:14888-14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Detmar M, Brown LF, Claffey KP, Yeo K-T, Kocher O, Jackman RW, Berse B, Dvorak HF: Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med 1994, 180:1141-1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skobe M, Brown LF, Tognazzi K, Ganju RK, Dezube BJ, Alitalo K, Detmar M: Vascular endothelial growth factor-C (VEGF-C) and its receptors KDR and flt-4 are expressed in AIDS-associated Kaposi’s sarcoma. J Invest Dermatol 1999, 113:1047-1053 [DOI] [PubMed] [Google Scholar]
- 31.Detmar M, Velasco P, Richard L, Claffey KP, Streit M, Riccardi L, Skobe M, Brown LF: Expression of vascular endothelial growth factor induces an invasive phenotype in human squamous cell carcinomas. Am J Pathol 2000, 156:159-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pettersson A, Nagy JA, Brown LF, Sundberg C, Morgan E, Jungles S, Carter R, Krieger JE, Manseau EJ, Harvey VS, Eckelhoefer IA, Feng D, Dvorak AM, Mulligan RC, Dvorak HF: Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability factor/vascular endothelial growth factor. Lab Invest 2000, 80:99-115 [DOI] [PubMed] [Google Scholar]
- 33.Elder D: Tumor progression, early diagnosis and prognosis of melanoma. Acta Oncol 1999, 38:535-547 [DOI] [PubMed] [Google Scholar]
- 34.Duncan LM, Deeds J, Cronin FE, Donovan M, Sober AJ, Kauffman M, McCarthy JJ: Melastatin expression and prognosis in cutaneous malignant melanoma. J Clin Oncol 2001, 19:568-576 [DOI] [PubMed] [Google Scholar]
- 35.Mouta Carreira C, Nasser SM, di Tomaso E, Padera TP, Boucher Y, Tomarev SI, Jain RK: LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res 2001, 61:8079-8084 [PubMed] [Google Scholar]
- 36.Hawighorst T, Oura H, Streit M, Janes L, Nguyen L, Brown LF, Oliver G, Jackson DG, Detmar M: Thrombospondin-1 selectively inhibits early-stage carcinogenesis and angiogenesis but not tumor lymphangiogenesis and lymphatic metastasis in transgenic mice. Oncogene 2002, 21:7945-7956 [DOI] [PubMed] [Google Scholar]
- 37.Oliver G, Detmar M: The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev 2002, 16:773-783 [DOI] [PubMed] [Google Scholar]
- 38.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G: An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J 2002, 21:1505-1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leu AJ, Berk DA, Lymboussaki A, Alitalo K, Jain RK: Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res 2000, 60:4324-4327 [PubMed] [Google Scholar]
- 40.Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ, Munn LL, Jain RK: Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 2002, 296:1883-1886 [DOI] [PubMed] [Google Scholar]
- 41.Carmeliet P, Jain RK: Angiogenesis in cancer and other diseases. Nature 2000, 407:249-257 [DOI] [PubMed] [Google Scholar]
- 42.Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, Orci L, Alitalo K, Christofori G, Pepper MS: Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J 2001, 20:672-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Yla-Herttuala S, Jaattela M, Alitalo K: Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res 2001, 61:1786-1790 [PubMed] [Google Scholar]
- 44.Mattila MM, Ruohola JK, Karpanen T, Jackson DG, Alitalo K, Harkonen PL: VEGF-C induced lymphangiogenesis is associated with lymph node metastasis in orthotopic MCF-7 tumors. Int J Cancer 2002, 98:946-951 [DOI] [PubMed] [Google Scholar]
- 45.Detmar M, Hirakawa S: The formation of lymphatic vessels and its importance in the setting of malignancy. J Exp Med 2002, 196:713-718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K: Lymphangiogenesis and cancer metastasis. Nat Rev Cancer 2002, 2:573-583 [DOI] [PubMed] [Google Scholar]
- 47.Salven P, Heikkila P, Joensuu H: Enhanced expression of vascular endothelial growth factor in metastatic melanoma. Br J Cancer 1997, 76:930-934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vlaykova T, Laurila P, Muhonen T, Hahka-Kemppinen M, Jekunen A, Alitalo K, Pyrhonen S: Prognostic value of tumour vascularity in metastatic melanoma and association of blood vessel density with vascular endothelial growth factor expression. Melanoma Res 1999, 9:59-68 [DOI] [PubMed] [Google Scholar]
- 49.Schoppmann SF, Schindl M, Breiteneder-Geleff S, Soleima A, Breitenecker G, Karner B, Birner P: Inflammatory stromal reaction correlates with lymphatic microvessel density in early-stage cervical cancer. Anticancer Res 2001, 21:3419-3423 [PubMed] [Google Scholar]
- 50.Achen MG, Williams RA, Minekus MP, Thornton GE, Stenvers K, Rogers PAW, Lederman F, Roufial S, Stacker SA: Localization of vascular endothelial growth factor-D in malignant melanoma suggests a role in tumor angiogenesis. J Pathol 2001, 193:147-154 [DOI] [PubMed] [Google Scholar]
- 51.Kriehuber E, Breiteneder-Geleff S, Groeger M, Soleiman A, Schoppmann SF, Stingl G, Kerjaschki D, Maurer D: Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J Exp Med 2001, 194:797-808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirakawa S, Hong YK, Harvey N, Schacht V, Matsuda K, Libermann T, Detmar M: Identification of lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol 2003, 162:575-586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K: Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J 2001, 20:4762-4773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kubo H, Cao R, Brakenhielm E, Makinen T, Cao Y, Alitalo K: Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci USA 2002, 99:8868-8873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erhard H, Rietveld FJ, van Altena MC, Brocker EB, Ruiter DJ, de Waal RM: Transition of horizontal to vertical growth phase melanoma is accompanied by induction of vascular endothelial growth factor expression and angiogenesis. Melanoma Res 1997, 7(Suppl 2):S19-S26 [PubMed] [Google Scholar]
- 56.Marcoval J, Moreno A, Graells J, Vidal A, Escriba JM, Garcia-Ramirez M, Fabra A: Angiogenesis and malignant melanoma. Angiogenesis is related to the development of vertical (tumorigenic) growth phase. J Cutan Pathol 1997, 24:212-218 [DOI] [PubMed] [Google Scholar]
- 57.Carnochan P, Briggs JC, Westbury G, Davies AJ: The vascularity of cutaneous melanoma: a quantitative histological study of lesions 0.85–1.25 mm in thickness. Br J Cancer 1991, 64:102-107 [DOI] [PMC free article] [PubMed] [Google Scholar]