To the Editor-in-Chief:
A severe reduction or absence of expression of major histocompatibility complex (MHC) molecules on tumor cells is frequently noted. 1–3 It is generally accepted that this down-regulation of MHC molecules by tumor cells impairs cellular immune recognition and contributes to inefficient cell-mediated tumor eradication. This low expression or lack of MHC expression is frequently observed in early developmental or embryonically derived tumor cells, and in certain types of hematopoietic cells. These include teratocarcinomas, choriocarcinomas, and neuroendocrine cancers such as neuroblastoma (NB), small cell lung cancer (SCLC), and erythroleukemic cells. 4–11 In addition to a strong reduction or absence of MHC class I expression, these types of tumors are recognized by a lack of interferon-γ (IFN-γ)-mediated induction of MHC class II molecules. This phenotype is generally not found in other tumor types that include melanomas, cervical carcinomas, renal cell carcinomas, head-neck squamous cell carcinomas, gliomas, lymphomas, and tumor virus-transformed normal skin fibroblasts. 6–12
The MHC is a large multigene family that encodes cell surface glycoproteins involved in binding and presentation of antigenic peptides to T lymphocytes. MHC class I molecules present (tumor) peptides to mainly CD8+ T lymphocytes, whereas the function of MHC class II molecules is to present peptides to CD4+ T lymphocytes. MHC class I molecules are expressed on almost all nucleated cells. In contrast, the constitutive expression of MHC class II molecules is restricted to specific immune cell types that include antigen-presenting cells such as dendritic cells, B-lymphocytes, macrophages, and thymic epithelial cells. Non-immune cells lack constitutive expression of MHC class II, however, in most of these cells MHC class II expression can be induced by IFN-γ. 13,14 Notably, expression of MHC class I molecules can also be enhanced by IFN-γ. Together, the up-regulated expression of MHC class I and class II molecules results in an increase in the immunogenic potential of cells. It is now well established that the class II transactivator (CIITA) plays a pivotal role in the expression of MHC class II and accessory genes (invariant chain and HLA-DM), whereas it has an ancillary function in the expression of MHC class I and β2-microglobulin genes. 15,16 Because both classes of MHC molecules are essential for the generation of antigen-specific immune responses by virtue of their ability to present antigenic peptides to T-lymphocytes, expression and subsequent functional activity of CIITA represents a critical step in the activation of the immune response.
The expression of CIITA in humans is controlled by at least three separate functional promoters, each coding for distinct first exons that drive expression of CIITA during differentiation and in overlapping subsets of different cell types. 17 CIITA promoter I (CIITA-PI) is the promoter used in dendritic cells. Promoter III of CIITA (CIITA-PIII) is constitutively expressed in B-lymphocytes and by activated T-lymphocytes, in monocytes and in dendritic cells. Furthermore, CIITA-PIII can drive CIITA expression after IFN-γ-stimulation in a number of different cell types, including endothelial cells and fibroblasts. 18 While the B-cell-specific expression of CIITA requires a small region directly upstream of the initiation codon, the IFN-γ induction requires an additional region located approximately 5 kb upstream of the transcriptional start site. 18 Promoter IV of CIITA (CIITA-PIV) is the principal IFN-γ inducible promoter. 17–22 Promoter II of CIITA is expressed at insignificant levels and is as yet functionally poorly understood. The cellular and temporal diversity in MHC class II expression is thus regulated via the differential usage of the CIITA promoters.
The first demonstration that lack of IFN-γ-mediated induction of MHC class II antigens was caused by the absence of expression of CIITA was made in fetal trophoblast-derived tumor cell lines. 4,5,9 It was shown that JEG-3 and JAR cells lacked induction of CIITA following exposure of these cells to IFN-γ explaining the absence of expression of all MHC class II isotypes in these tumor cells. Expression of CIITA following gene transfer resulted in the induction and subsequent cell surface expression of all isotypes of MHC class II molecules. 9 Furthermore, in a transfection assay it was shown that CIITA-PIV could be activated to levels similar to those observed in CIITA-inducible cell lines revealing that all transcription factors were present. 6,7,23
It was subsequently established that the mechanism leading to the lack of IFN-γ-mediated induction of endogenous CIITA was DNA hypermethylation of CIITA-PIII and CIITA-PIV. 6,9,23 This DNA hypermethylation impaired the binding of transcription factors critical to the activation of these promoters resulting in a bare promoter phenotype of CIITA-PIII and CIITA-PIV in trophoblast-derived tumor cells following IFN-γ-induction as established by in vivo genomic footprint analysis. 23 Subsequently, we and others also demonstrated that in other types of tumor cells the lack of IFN-γ-mediated induction of CIITA was caused by promoter hypermethylation both in humans 6 and in mice. 24 We noted that this phenomenon was found predominantly in human developmental tumor cell lines. 6 These developmental tumor cell types included neuroblastomas and teratocarcinomas. Treatment of these cell lines with t-azacytidine resulted in the restoration of both CIITA-PIII and CIITA-PIV promoter activity and resulting expression of CIITA congruent with induction of MHC class II genes. 6 We have argued that silencing of CIITA through promoter hypermethylation could represent a common mechanism of gene silencing that is frequently observed during early development. The lack of CIITA induction due to promoter hypermethylation in these types of tumor cell lines might therefore reflect their stage in early development and is not a result of oncogenic transformation.
In a recent paper in The American Journal of Pathology, Yazawa and co-workers 10 investigated the mechanism contributing to the absence of MHC class II expression in SCLC and NB following exposure to IFN-γ. Their findings show that in both of these neuroendocrine-derived tumor cells, the absence of MHC class II expression was caused by a deficient IFN-γ-induction of CIITA despite inducible IRF1 expression, immediate Stat1 phosphorylation, and USF-1 expression, which are critical events to CIITA-PIV activation. It reveals that the SCLC and NB cell lines studied are not deficient in the activation pathways that involve general transcription factors governing inducible CIITA expression. These observations are similar to the observation made by van der Stoep and co-workers 6 in the IMR-32 and NGP neuroblastoma tumor cell lines. Subsequently, Yazawa and co-workers 10 show that the bHLH factor HASH-1 is expressed to high levels in SCLC and NB and in addition, that the SCLC overexpressed L-myc and the NB cells N-myc. Furthermore, they show abundant expression of E12/E47 and Max proteins, which are the heterodimeric partners for HASH-1 and myc proteins. In a number of experiments it was suggested that these factors were involved in the repression of IFN-γ-induced CIITA-PIV activity and congruent MHC class II expression at the level of a transient CIITA-PIV-reporter assay in a large cell lung cancer line with well-defined IFN-γ-inducible CIITA expression. They also found that these factors bind to CIITA-PIV in vivo as explored by chromatin immunoprecipitation analysis. In addition, in using antisense technology little, if any, de-repression of the IFN-γ-inducibility CIITA-PIV is revealed. On the basis of these combined experiments they conclude that these factors play a role in the CIITA deficiency of SCMC and NB although their precise models of action remain an enigma.
Although these observations are of potential interest, several aspects of IFN-γ-mediated induction of CIITA were not fully addressed. First of all, IFN-γ-mediated induction of CIITA is not only exerted by the activation of CIITA-PIV but also by CIITA-PIII through the activity of an upstream interferon regulatory region. 18,21 In this respect it should be noted that for the detection of CIITA transcripts the authors refer to a previous article, which depicts primer pairs for the detection of CIITA transcripts driven by CIITA-PIII and a pan-CIITA primer pair, detecting CIITA transcripts driven from all promoters. 7 On the basis of the specificity of these primer pairs it can be concluded that the lack of CIITA after IFN-γ induction in the SCLC and NB cell lines investigated results from a deficiency in the IFN-γ-mediated induction of both CIITA-PIII and CIITA-PIV. Unfortunately, the authors do not discuss this issue because it infers that the proposed CIITA-PIV silencing mechanism should also affect the IFN-γ-mediated activation of CIITA-PIII. On the basis of sequence divergence and promoter architecture of the elements and the regions involved in the CIITA-PIV and CIITA-PIII activation, this seems unlikely.
Secondly, because their SCLC and NB cell lines displayed inducible IRF1 expression, immediate Stat1 phosphorylation, and USF-expression, it would have been more appropriate if the CIITA-PIV reporter assays were performed in these SCLC and NB cell lines that lacked induction of endogenous CIITA after IFN-γ-treatment. This would have directly addressed the activity of CIITA-PIV in these SCLC and NB cells. Considering a previous report by van der Stoep and co-workers 6 in which CIITA promoter silencing was investigated in various tumor cell lines, including in NB, would have allowed Yazawa and co-workers to consider promoter hypermethylation as a more plausible explanation for the CIITA-deficiency in SCLC and NB. Unfortunately, despite several previous publications concerning CIITA promoter hypermethylation in human and mouse tumor cells, the authors have not addressed this issue at all. 6,9,23,24 Therefore, we feel that a more plausible explanation for the lack of IFN-γ-mediated induction of CIITA and congruent MHC class II expression in these SCLC and NB cell lines is promoter hypermethylation of CIITA-PIII and CITA-PIV. This mechanism secures a block on the transcriptional activation of CIITA and congruent MHC class II expression and does not interfere with other more general transcriptional activation pathways.
Author’s Reply:
We read with interest the comments and suggestions by Peter J. van den Elsen and Nienke van der Stoep regarding our recent report on the class II transactivator (CIITA) transcription deficiency mechanism in small cell lung cancer (SCLC) and neuroblastoma (NB). 1
As they described, it is generally accepted that the reduction of major histocompatibility complex (MHC) in tumor cells contributes to escape from anti-tumor immunity. Since Ostrand-Rosenberg et al 2 reported that class II MHC (MHC-II) as well as class I MHC (MHC-I) expressed on the tumor cells can participate in T-cell-dependent anti-tumor immunity, it seems to be paying more attention to the MHC-II expressed in the tumor cells. Previously, we investigated both histopathologically and immunohistochemically, the relations among the tumor infiltrating lymphocytes, MHC-II expression in cancer cells, and patient prognoses. 3,4 And as shown in the our previous report, the reduced level of MHC-II in lung cancer cells varied in each non-SCLC cell line, and the reduction was the most conspicuous in SCLC, which is clearly distinct from non-SCLC in terms of having neuroendocrine character. 5 NB is a highly aggressive infant neuroendocrine neoplasm and the CIITA and congruent MHC-II expression is severely repressed as well as is that of SCLC. Therefore, we investigated the CIITA transcription deficiency mechanism focused on the neuroendocrine cell-specific basic helix-loop-helix transcription factors, human achaete-scute homolog (HASH)-1, L-myc, and N-myc, which are specifically overexpressed in SCLC and NB. We obtained the results that these neuroendocrine cell-specific basic helix-loop-helix transcription factors competitively bind to the E-box in the CIITA promoter IV and repress transcription of CIITA, that interferon-γ (IFN-γ) up-regulates HASH-1, and that L-myc and N-myc up-regulate HASH-1 in SCLC and NB cells. From these results, we concluded that the complicated mechanisms of CIITA transcription deficiency act in SCLC and NB cells.
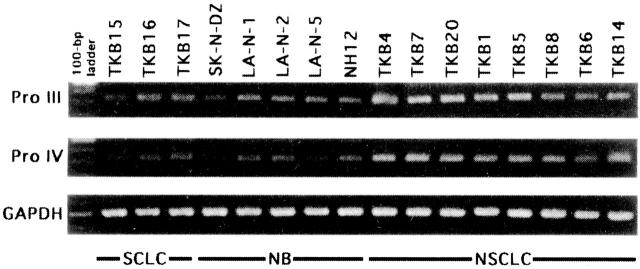
Piskurich et al and van der Stoep et al 6,7 reported that CIITA promoter III is activated by IFN-γ even in non-B cells, such as glioma cells and melanoma cells. Van der Stoep et al 7 also described that teratocarcinoma cells, neuroblastoma cells, choriocarcinoma cells, and erythroleukemia cells (they characterized these cell lines as developmental tumor cell lines) lack induction of CIITA despite 24 hours of 500 units/ml IFN-γ treatment. However, we described in our recent paper that weak CIITA transcripts were detectable both in SCLC and NB cell lines examined through 48 hours of 100 units/ml IFN-γ treatment. 1 Since the level of IFN-γ induction of MHC-II shows dose-dependent manner and since our previously used primer set amplified pan-CIITA cDNA as van den Elsen and van der Stoep pointed out, we re-examined the inducibility of CIITA in SCLC and NB cell lines using the CIITA promoter III- and promoter IV-specific primer sets and the same condition of IFN-γ treatment and RT-PCR as van der Stoep used (24-hour exposure of 500 units/ml IFN-γ, 38 cycles of PCR after reverse transcription reaction). 7 We consequently obtained the results that both promoter III- and promoter IV-derived CIITA transcripts are detectable in all SCLC and NB cell lines examined, that the signals were weaker in comparison with those of non-SCLC cell lines, and that the promoter III-derived CIITA transcripts are predominant in the CIITA induced by IFN-γ in SCLC and NB (Figure 1) ▶ .
Figure 1.
RT-PCR analysis on IFN-γ-inducible expression of promoter III-derived (Pro III) and promoter IV-derived (Pro IV) CIITA (38 cycles of PCR) and GAPDH (28 cycles of PCR) in small cell lung cancer (SCLC), neuroblastoma (NB), and non-SCLC cell lines. Cultured cancer cells were treated with 500 units/ml IFN-γ for 24 hours.
The methylation status of the CIITA promoter IV was also examined by the Southern blot analysis using methylation-sensitive restriction enzymes, and we obtained the results that severe methylation of CIITA promoter IV as shown in the report of van der Stoep et al 7 was not found in both SCLC and NB cell lines examined. Although some DNA methylation of CIITA promoter IV was found both in SCLC and NB as well as non-SCLC cell lines, the methylated level was not closely correlated to the inducibility of CIITA. Hence, we concluded that the IFN-γ induction of CIITA and methylation of CIITA promoter IV in our examined SCLC and NB cell lines is not severe in comparison with trophoblasts, teratocarcinoma cell lines, neuroblastoma cell lines, choriocarcinoma cell lines, and a pluripotent erythroleukemia cell line which van der Stoep et al used. 7,8 Van der Stoep and co-workers 7,8 described that CIITA gene silencing in developmental tumor cell lines, including NB, is caused by promoter hypermethylaton, since silencing of CIITA through promoter hypermethylation could represent a common mechanism of gene silencing that is frequently observed during early development. However, SCLC is an adult lung cancer with poor prognosis, and there are combined types of SCLC, such as the combination of SCLC and squamous cell carcinoma, adenocarcinoma, large cell carcinoma, or large cell neuroendocrine carcinoma. 9 As we reported previously, even in the combined type of SCLC, the SCLC element showed highly reduced MHC-II expression and no association with tumor infiltrating lymphocytes, while the non-SCLC (adenocarcinoma) element expressed a detectable level of MHC-II and was closely associated with tumor infiltrating lymphocytes. 5 These results suggest that the CIITA reduction is closely linked to the neuroendocrine differentiation.
Recently, histone deacetylation has been highlighted as another gene silencing mechanism. It was reported that histone deacetylation of the promoter and exon was partially involved in the silencing of genes such as TGF-β receptor II in lung cancer including SCLC. 10,11 Since TGF-β is a factor to attenuate CIITA promoter III activity, 6,12 the TGF-β unresponsiveness in SCLC is considered to be unfavorable for interpreting repressed transcription of CIITA in SCLC. However, increase of heterochromatin in the nuclei is one of the morphological characteristics of SCLC and NB, 9,13 and we often experience that it is difficult to remove DNA-binding proteins in the process of DNA purification from SCLC and NB cells. Therefore, the histone deacetylation might be contribute to one of causes that the expression of both IFN-γ inducible promoter III- and promoter IV-derived CIITA transcripts of SCLC and NB are generally lower than those of non-SCLC. Further investigations are necessary for a complete understanding of the CIITA transcription deficiency mechanisms in SCLC and NB.
- 1.Yazawa T, Ito T, Kamma H, Suzuki T, Okudela K, Hayashi H, Horiguchi H, Ogata T, Mitsui H, Ikeda M, Kitamura H: Complicated mechanisms of class II transactivator transcription deficiency in small cell lung cancer and neuroblastoma. Am J Pathol 2002, 161:291-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrand-Rosenberg S, Thakur A, Clements V: Rejection of mouse sarcoma cells after transfection of MHC class II genes. J Immunol 1990, 144:4068-4071 [PubMed] [Google Scholar]
- 3.Kamma H, Fujii K, Ogata T: Lymphocytic infiltration in juvenile thyroid carcinoma. Cancer 1988, 62:1988-1993 [DOI] [PubMed] [Google Scholar]
- 4.Yazawa, Kamma H, Ogata T: Frequent expression of HLA-DR antigen in medullary carcinoma of the breast: a possible reason for its prominent lymphocytic infiltration and favorable prognosis. Appl Immunohistochem 1993, 1:289-296 [Google Scholar]
- 5.Yazawa T, Kamma H, Fujiwara M, Matsui M, Horiguchi H, Satoh H, Fujimoto M, Yokoyama K, Ogata T: Lack of class II transactivator causes severe deficiency of HLA-DR expression in small cell lung cancer. J Pathol 1999, 187:191-199 [DOI] [PubMed] [Google Scholar]
- 6.Piskurich JF, Linhoff MW, Wang Y, Ting JP-Y: Two distinct γ-interferon-inducible promoters of the major histocompatibility complex class II transactivator gene are differentially regulated by Stat1, interferon regulatory factor 1, and transforming growth factor β. Mol Cell Biol 1999, 19:431-440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Stoep N, Biesta P, Quinten E, Van den Elsen PJ: Lack of IFN-γ-mediated induction of the class II transactivator (CIITA) through promoter methylation is predominantly found in developmental tumor cell lines. Int J Cancer 2002, 97:501-507 [DOI] [PubMed] [Google Scholar]
- 8.Van den Elsen PJ, Van der Stoep N, Vietor HE, Wilson L, Van Zutphen M, Gobin SJ: Lack of CIITA expression is central to the absence of antigen presentation functions of trophoblast cells and is caused by methylation of the IFN-γ inducible promoter (PIV) of CIITA. Hum Immunol 2000, 61:850-862 [DOI] [PubMed] [Google Scholar]
- 9.Travis WD, Colby TV, Corrin B, Shimosato Y, Brambilla E: World Health Organization: Histological Typing of Lung and Pleural Tumours ed 3. 1999:pp 1-156 Springer-Verlag, Heidelberg
- 10.Hougaard S, Norgaard P, Abrahamsen N, Moses HL, Spang-Thomsen M, Skovgaard Poulsen H: Inactivation of the transforming growth factor β type II receptor in human small cell lung cancer cell lines. Br J Cancer 1999, 79:1005-1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osada H, Tatematsu Y, Masuda A, Saito T, Sugiyama M, Yanagisawa K, Takahashi T: Heterogeneous transforming factor (TGF)-β unresponsiveness and loss of TGF-β receptor type II expression caused by histone deacetylation in lung cancer cell lines. Cancer Res 2001, 61:8331-8339 [PubMed] [Google Scholar]
- 12.Piskurich JF, Wang Y, Linhoff MW, White LC, Ting JP: Identification of distance regions of 5′ flanking DNA that mediate constitutive, IFN-γ, STAT1, and TGF-β regulated expression of the class II transactivator gene. J Immunol 1998, 160:233-240 [PubMed] [Google Scholar]
- 13.Schwab M, Shimada H, Joshi V, Brodeur GM: Neuroblastic tumours of adrenal gland and sympathetic nervous system. Kleihues P Cabenee WK eds. Pathology and Genetics of Tumours of the Nervous System 2000:pp 153-16 International Agency for Research on Cancer, Lyon
Acknowledgments
We thank Dr. Ir. L.C. van Dinten for stimulating discussions. This study was supported by Dutch Cancer Society grant RUL 98–1732 to P.v.d.E. N.v.d.S. is a fellow of the Royal Netherlands Academy of Arts and Sciences.
References
- 1.Möller P, Hammerling GJ: The role of surface HLA-A, B, C molecules in tumour immunity. Cancer Surv 1992, 13:101-127 [PubMed] [Google Scholar]
- 2.Garrido F, Ruiz-Cabello F, Cabrera T, Perez-Villar JJ, Lopez-Botet M, Duggan-Keen M, Stern PL: Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol Today 1997, 18:89-95 [DOI] [PubMed] [Google Scholar]
- 3.Algarra I, Cabrera T, Garrido F: The HLA crossroad in tumor immunology. Hum Immunol 2000, 61:65-73 [DOI] [PubMed] [Google Scholar]
- 4.Morris AC, Riley JL, Fleming WH, Boss JM: MHC class II gene silencing in trophoblast cells is caused by inhibition of CIITA expression. Am J Reprod Immunol 1998, 40:385-394 [DOI] [PubMed] [Google Scholar]
- 5.Murphy SP, Tomasi TB: Absence of MHC class II antigen expression in trophoblast cells results from a lack of class II transactivator (CIITA) gene expression. Mol Reprod Dev 1998, 51:1-12 [DOI] [PubMed] [Google Scholar]
- 6.Van der Stoep N, Biesta P, Quinten E, Van den Elsen PJ: Lack of IFN-γ-mediated induction of the class II transactivator (CIITA) through promoter methylation is predominantly found in developmental tumor cell lines. Int J Cancer 2002, 97:501-507 [DOI] [PubMed] [Google Scholar]
- 7.Yazawa T, Kamma H, Fujiwara M, Matsui M, Horiguchi H, Satoh H, Fujimoto M, Yokoyama K, Ogata T: Lack of class II transactivator causes severe deficiency of HLA-DR expression in small cell lung cancer. J Pathol 1999, 187:191-199 [DOI] [PubMed] [Google Scholar]
- 8.Lu Y, Tschickardt ME, Schmidt BJ, Blanck G: IFN-γ inducibility of class II transactivator is specifically lacking in human tumour lines: relevance to retinoblastoma protein rescue of IFN-γ inducibility of the HLA class II genes. Immunol Cell Biol 1997, 75:325-332 [DOI] [PubMed] [Google Scholar]
- 9.Van den Elsen PJ, Van der Stoep N, Vietor HE, Wilson L, Van Zutphen M, Gobin SJ: Lack of CIITA expression is central to the absence of antigen presentation functions of trophoblast cells and is caused by methylation of the IFN-γ-inducible promoter (PIV) of CIITA. Hum Immunol 2000, 61:850-862 [DOI] [PubMed] [Google Scholar]
- 10.Yazawa T, Ito T, Kamma H, Suzuki T, Okudela K, Hayashi H, Horiguchi H, Ogata T, Mitsui H, Ikeda M, Kitamura H: Complicated mechanisms of class II transactivator transcription deficiency in small cell lung cancer and neuroblastoma. Am J Pathol 2002, 161:291-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu A, Takahashi M, Toba K, Zheng Z, Hashimoto S, Nikkuni K, Furukawa T, Koike T, Aizawa Y: Regulation of the expression of MHC class I and II by class II transactivator (CIITA) in hematopoietic cells. Hematol Oncol 1999, 17:149-160 [DOI] [PubMed] [Google Scholar]
- 12.Peijnenburg A, Gobin SJ, van Eggermond MC, Godthelp BC, van Graafeiland N, van den Elsen PJ: Introduction of exogenous class II trans-activator in MHC class II-deficient ABI fibroblasts results in incomplete rescue of MHC class II antigen expression. J Immunol 1997, 159:2720-2727 [PubMed] [Google Scholar]
- 13.Benoist C, Mathis D: Regulation of major histocompatibility complex class-II genes: x, y, and other letters of the alphabet. Annu Rev Immunol 1990, 8:681-715 [DOI] [PubMed] [Google Scholar]
- 14.Glimcher LH, Kara CJ: Sequences and factors: a guide to MHC class-II transcription. Annu Rev Immunol 1992, 10:13-49 [DOI] [PubMed] [Google Scholar]
- 15.Gobin SJ, Peijnenburg A, Keijsers V, van den Elsen PJ: Site α is crucial for two routes of IFN-γ-induced MHC class I transactivation: the ISRE-mediated route and a novel pathway involving CIITA. Immunity 1997, 6:601-611 [DOI] [PubMed] [Google Scholar]
- 16.Martin BK, Chin KC, Olsen JC, Skinner CA, Dey A, Ozato K, Ting JP: Induction of MHC class I expression by the MHC class II transactivator CIITA. Immunity 1997, 6:591-600 [DOI] [PubMed] [Google Scholar]
- 17.Muhlethaler-Mottet A, Otten LA, Steimle V, Mach B: Expression of MHC class II molecules in different cellular and functional compartments is controlled by differential usage of multiple promoters of the transactivator CIITA. EMBO J 1997, 16:2851-2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piskurich JF, Wang Y, Linhoff MW, White LC, Ting JP: Identification of distince regions of 5′ flanking DNA that mediate constitutive, IFN-γ, STAT1, and TGF-β regulated expression of the class II transactivator gene. J Immunol 1998, 160:233-240 [PubMed] [Google Scholar]
- 19.Dong Y, Rohn WM, Benveniste EN: IFN-γ regulation of the type IV class II transactivator promoter in astrocytes. J Immunol 1999, 162:4731-4739 [PubMed] [Google Scholar]
- 20.Muhlethaler-Mottet A, Di Berardino W, Otten LA, Mach B: Activation of the MHC class II transactivator CIITA by interferon-γ requires cooperative interaction between Stat1 and USF-1. Immunity 1998, 8:157-166 [DOI] [PubMed] [Google Scholar]
- 21.Piskurich JF, Linhoff MW, Wang Y, Ting JP-Y: Two distinct γ-interferon-inducible promoters of the major histocompatibility complex class II transactivator gene are differentially regulated by Stat1, interferon regulatory factor 1, and transforming growth factor β. Mol Cell Biol 1999, 19:431-440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rohn W, Tang LP, Dong Y, Benveniste EN: IL-1 β inhibits IFN-γ-induced class II MHC expression by suppressing transcription of the class II transactivator gene. J Immunol 1999, 162:886-896 [PubMed] [Google Scholar]
- 23.Morris AC, Spangler WE, Boss JM: Methylation of class II trans-activator promoter IV: a novel mechanism of MHC class II gene control. J Immunol 2000, 164:4143-4149 [DOI] [PubMed] [Google Scholar]
- 24.Murphy SP, Holtz R, Lewandowski N, Tomasi TB, Fuji H: DNA alkylating agents alleviate silencing of class II transactivator gene expression in L1210 lymphoma cells. J Immunol 2002, 169:3085-3093 [DOI] [PubMed] [Google Scholar]