Abstract
Successful lung transplantation has been limited by the high incidence of acute graft rejection. There is mounting evidence that the stress response gene heme oxygenase-1 (HO-1) and/or its catalytic by-product carbon monoxide (CO) confers cytoprotection against tissue and cellular injury. This led us to hypothesize that CO may protect against lung transplant rejection via its anti-inflammatory and antiapoptotic effects. Orthotopic left lung transplantation was performed in Lewis rat recipients from Brown-Norway rat donors. HO-1 mRNA and protein expression were markedly induced in transplanted rat lungs compared to sham-operated control lungs. Transplanted lungs developed severe intraalveolar hemorrhage, marked infiltration of inflammatory cells, and intravascular coagulation. However, in the presence of CO exposure (500 ppm), the gross anatomy and histology of transplanted lungs showed marked preservation. Furthermore, transplanted lungs displayed increased apoptotic cell death compared with the transplanted lungs of CO-exposed recipients, as assessed by TUNEL and caspase-3 immunostaining. CO exposure inhibited the induction of IL-6 mRNA and protein expression in lung and serum, respectively. Gene array analysis revealed that CO also down-regulated other proinflammatory genes, including MIP-1α and MIF, and growth factors such as platelet-derived growth factor, which were up-regulated by transplantation. These data suggest that the anti-inflammatory and antiapoptotic properties of CO confer potent cytoprotection in a rat model of lung transplantation.
Lung transplantation has become an accepted treatment modality for end-stage lung disease. Compared to other solid organ transplants, the success of lung transplantation has been severely limited by the high incidence of acute and chronic graft rejection. 1 The frequency and severity of episodes of acute rejection are the predominant risk factors for chronic airway rejection, manifested as obliterative bronchiolitis. 2 Data from rodent allograft studies as well as from clinical lung transplantation show that the lung, in comparison to other solid organs, is highly immunogenic. 3 Despite advances in immunosuppression, the incidence of acute rejection in lung graft patients can be as high as 60% in the first postoperative month. 4,5
Heme oxygenase (HO) catalyzes the rate-limiting step in heme degradation, generating iron, carbon monoxide (CO), and biliverdin-IXa. 6 Recent attention has focused on the biological effects of the by-products of this enzymatic reaction, which have important cytoprotective functions. 7-9 Three isoforms of heme oxygenase (HO) have been described: an inducible isoform, HO-1, and two constitutively expressed isoforms, HO-2 and HO-3. 6 The induction of HO-1 occurs as an adaptive and beneficial response to many varied tissue and cellular injury models including sepsis, hyperoxia, hypoxia, and other oxidant-induced tissue injury. 10,11 Furthermore, expression of HO-1 in rodent liver, heart, and kidney allografts 12-15 and heart xenografts 7 correlates with long-term graft survival.
The precise mechanism by which HO-1 confers protection against organ transplantation rejection is not clear. Depending on the model of tissue and cellular injury, evidence has accumulated in support of functional roles for all three catalytic by-products of HO-1 (CO, ferritin from released iron, biliverdin) in mediating its cytoprotective effects. 16-22 In the current study we focus on the functional role of CO in a rat model of orthotopic lung transplantation. Given the anti-apoptotic and anti-inflammatory properties of both HO-1 and CO, we hypothesize that CO limits graft injury by maintaining cell viability and modulating tissue inflammation. We evaluated the role of CO in an acute model of rat lung transplantation, demonstrating that CO confers potent cytoprotection in the setting of lung transplantation.
Materials and Methods
Human Pulmonary Fibroblast Cultures
Primary cell cultures of lung fibroblasts were isolated from lung tissue obtained from endobronchial lung biopsies of lung transplant recipients under a protocol approved by the Institutional Review Board of the University of Pittsburgh. Fibroblasts were grown to confluence using Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), both from Invitrogen (Carlsbad, CA) prior to isolation of lysates for Western blot analysis for HO-1 expression, as described below.
Histological Grading of Human Lung Biopsy Specimen of Lung Transplant Recipients
Allograft rejection was graded according to the working formulation of the International Society of Heart and Lung Transplantation (ISHLT). 23 Acute rejection was graded in A0 to A4 according to the presence and the extent of perivascular and interstitial mononuclear cell infiltrates. All of the histological scoring was performed by a single investigator in a blinded fashion.
Carbon Monoxide Exposure
For animal exposure, CO at a concentration of 1% (10,000 parts per million-ppm) in compressed air was mixed with balanced air (21% oxygen) in a stainless steel mixing cylinder before delivery into the exposure chamber. A CO analyzer (Interscan Corporation, Chatsworth, CA) was used to measure CO levels in the chamber and there were no fluctuations in the CO concentrations after the chamber had equilibrated. Recipient rats were exposed to 500 ppm CO after transplantation procedures.
Rat Lung Transplantation
Male Lewis (LEW) and Brown-Norway (BN) viral antibody-free rats (250 to 300 g) were purchased from Harlan (Madison, WI). All procedures involving animals were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Orthotopic left lung transplantation was performed in LEW recipient from BN donor by means of the cuff technique as previously described. 24 Donor rats were anesthetized by intraperitoneal injection of sodium pentobarbital and intubated through a tracheotomy. Animals were mechanically ventilated with 100% oxygen, tidal volume of 10 ml/kg and respiratory rate of 70 breaths/minute. After heparinization (1000 units/kg intravenously, a median laparosternotomy was performed and the lungs were flushed through the main pulmonary artery with 20 ml of cold saline delivered from a height of 25 cm. The heart-lung block was removed with the lungs inflated at the tidal volume. The left lungs were then divided and cuffs prepared. 14-gauge catheters were placed into the pulmonary artery and pulmonary vein and secured with 8–0 polypropylene ligatures.
Recipient animals were premedicated by intramuscular injection of ketamine chloride (40 mg/kg) and atropine sulfate (0.25 mg/kg), and intubated orotracheally. The animals were maintained under general anesthesia with a mixture of isoflurane and oxygen. A left thoracotomy was performed through the fourth intercostal space. The hilum of the left lung was dissected, and the pulmonary artery, pulmonary vein, and the left main bronchus were clamped with microvascular clamps. The pulmonary artery and pulmonary vein were incised, and the cuffs of the donor lung were inserted and fixed with 6–0 silk suture. The left main bronchus was anastomosed by a running suture of 9–0 nylon. After graft reperfusion, the recipient’s native lung was removed, a temporary chest tube was placed, which was removed after recovery from anesthesia, and the thoracotomy was closed. After recovery from the anesthesia, animals were placed either in CO or air chamber.
Hematoxylin-Eosin Staining and Grading
Formalin-fixed, paraffin-embedded sections were used for conventional histology hematoxylin and eosin (H&E) staining. Allograft rejection was graded according to the working formulation of the ISHLT. 23 Acute rejection was graded in A0 to A4 according to the presence and the extent of perivascular and interstitial mononuclear cell infiltrates. All of the histological scoring was performed by a single investigator in a blinded fashion.
Myeloperoxidase Activity
Myeloperoxidase (MPO) is a constituent enzyme found principally in neutrophils that results in the formation of hypochlorous acid. Its presence only in neutrophils allows it to be extracted from tissues and represents a direct correlation to neutrophil content.
Rat lungs from both transplant and transplant/CO group were measured for MPO activity. 25 50 mg of frozen lung samples were homogenized in phosphate buffer containing 0.5% hexadecyltrimethylammonium bromide, sonicated for 15 seconds, and further disrupted by a freeze-thaw cycle in liquid nitrogen. The extract was centrifuged at 20,000 × g for 20 minutes at 4°C. The supernatants were assayed for MPO activity by measuring the absorbance at 460 nm, using a 0.167 mg/dl solution of o-dianisidine dihydrochloride in 0.0005% hydrogen peroxide as the substrate. A similar amount from the same lung was weighed and desiccated in a 60°C oven to determine the wet-to-dry lung weight. MPO activity is expressed per gram of dry weight.
HO-1 and Caspase-3 Immunohistochemical Staining
Formalin-fixed, paraffin-embedded lung tissue sections were deparaffinized with xylene, rehydrated gradually with graded alcohol solutions (100%, 95%, and 80%), and then washed with deionized water. For antigen unmasking, sections were treated in trypsin solution for 10 minutes at 37°C. Sections were then washed with deionized water and incubated with 3% H2O2 for 5 minutes. Sections were then incubated with a 1:300 dilution of the rabbit polyclonal anti-active caspase-3 (Promega, Madison, WI) or rabbit polyclonal anti-HO-1 (Stressgen, Victoria, BC, Canada) primary antibody overnight at 4°C. After three PBS washes, sections were incubated with the secondary antibody, a biotinylated goat anti-rabbit IgG, at 37°C for 30 minutes, and with peroxidase-conjugated strepavidin-biotin complex (Santa Cruz Biotechnology, Santa Cruz, CA) at 37°C for 30 minutes. Diaminobenzidine (DAB) substrate (Zymed, South San Francisco, CA) was applied as the chromogen, giving a brown reaction product, and the sections were counterstained with Mayer’s hematoxylin.
TUNEL Staining
We also confirmed apoptosis with terminal deoxynucleotidyl-transferase dUTP nick end-labeling (TUNEL) assay using the in situ cell death detection kit (Roche Molecular Biochemicals, Indianapolis, IN). Sections of formalin-fixed, paraffin-embedded lung tissue were deparaffinized and rehydrated, rinsed with PBS, and digested with proteinase K (Roche Molecular Biochemicals, Indianapolis, IN) at a concentration of 20 μg/ml for 20 minutes. After being washed, sections were incubated with TUNEL reaction mixture at 37°C for 1 hour and then incubated with converter-AP at 37°C for 30 minutes. Sections were washed, stained with NBT/BCIP substrate solution, and counterstained with nuclear fast red.
TUNEL/Macrophage Co-Staining
Formalin-fixed, paraffin-embedded lung tissue sections were deparaffinized with xylene, rehydrated gradually with graded alcohol solutions (100%, 95%, and 80%), and then washed with deionized water. Sections were then incubated with Apoptosis Detection System (Fluorescein; Promega) for 30 minutes followed by an incubation in normal goat serum (Sigma Chemie, Deisenhofen, Germany) for 40 minutes. For Macrophage co-staining, 1 hour incubation with mouse anti-rat ED1 (2 μg/ml) (Serotec, Raleigh, NC) was performed, followed by 1 hour incubation in goat anti-mouse cy3 (2 μg/ml) (Jackson Immunoresearch Laboratories, West Grove, PA). Confocal slices were taken using an Olympus Fluoview BX61 Scanning Confocal Microscope.
Protein Extraction
Tissue was homogenized on ice in cold lysis buffer [150 mmol/L NaCl, 50 mmol/L Tris, pH 7.6, 1% sodium dodecyl sulfate (SDS; Bio-Rad, Hercules, CA), 3% Nonidet P-40, 5 mmol/L ethylenediaminetetraacetic acid (EDTA), 1 mmol/L MgCl2, 2 mmol/L 1,3-dichloroisocoumarin, 2 mmol/L 1,10-phenanthroline, and 0.5 mmol/L E-64] and the homogenate was centrifuged at 10,000 × g for 10 minutes. The supernatant was decanted, and an aliquot was stored at −20°C for measurement of protein concentration. The remaining supernatant was mixed with an equal volume of double-strength Laemmli sample buffer [250 mmol/L Tris ·HCl (Bio-Rad), pH 6.8, 4% SDS, 10% glycerol, 0.006% bromphenol blue, 2% β-mercaptoethanol], divided into aliquots, and stored at −80°C.
Western Blot
Protein samples (50 μl) were boiled for 5 minutes and resolved by a 10% to 12.5% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), then electroblotted onto nitrocellulose membranes (Bio-Rad). The membranes were incubated with HO-1, (Stressgen), MIP-1α, MIF, and PDGF antibody (1:1000; Santa Cruz Biotech) for 2 hours, followed by incubation with horseradish peroxidase (HRP)-conjugated anti-rabbit antibody (1:5000) for 1.5 hours. The filters were treated with LumiGLO (New England Biolabs Inc., Beverly, MA) for signal development, and then exposed to X-ray film. All gels were repeated in triplicate. The level of HO-1, MIP-1α, MIF, and PDGF were normalized to the level of β-actin detected in the same membrane.
ELISA Measurement of IL-6
The concentration of IL-6 in the serum of transplanted rat at day 4 was measured by enzyme-linked immunosorbent assay (ELISA) as described by the manufacturer (R&D systems, Minneapolis, MN).
RNA Extraction and Northern Analysis of HO-1 and IL-6
Total RNA was isolated from transplanted lung tissue at day 4 using TRIzol reagent (Invitrogen, Carlsbad, California) according to the manufacturer’s instructions. Total RNA (10 μg per lane) was denatured, electrophoresed on 1.2% agarose formaldehyde gels, transferred to a positively-charged nylon membrane (Hybond N+; Amersham Pharmacia Biotech, Piscataway, NJ) and UV cross-linked (Stratalinker; Stratagene, La Jolla, CA). Membranes were prehybridized for 1 to 2 hours at 60°C and subsequently hybridized overnight at 60°C with random primer [32P]dCTP-labeled rat HO-1 cDNA and rat IL-6 cDNA, cDNA. The blots were washed three times with a solution containing 0.5% bovine serum albumin, 5% SDS, and 1 mmol/L EDTA in 0.2X standard saline citrate at 56°C to 60°C and then exposed to X-ray film at −80°C.
cDNA Expression Arrays Analysis
Total RNA (10 μg) from each group of 4 rat lungs was combined with Atlas Rat 1.2 specific RNA primers, cDNA synthesis (CDS) primer mix (Clontech, Palo Alto, CA). The solution was preheated to 94°C followed by annealing at 70°C for 10 minutes and extension at 49°C for 35 minutes]50 mmol/L of Tris–HCl, pH 8.3, 75 mmol/L KCl, 3 mmol/L MgCl2, 5.6 mmol/L DTT, 0.6 mmol/L dNTPs, 100 μCi [33P]dCTP (NEN, Boston, MA) and 200 units of Superscript II (Invitrogen)[. The reaction was stopped at 94°C for 5 minutes and then placed in an ice bath. Unincorporated [33P]dCTP was removed on a Microspin Sepharose G-50 gel filtration column (Amersham Pharmacia Biotech, Piscataway, NJ). [33P]-cDNA hybridization to Clontech Rat Atlas 1.2 membranes was done at 64°C in a microtube (Research Genetics, Huntsville, AL) containing 2 μg Cot-1 DNA. For hybridization of the labeled [33P]-cDNA product, 250,000 Cerenkov cpm at 0 to 260 mKeV were used. Phosphor screens were exposed for 36 hours; the arrays were wrapped and read in a Packard Cyclone phosphor imager (PerkinElmer Life Sciences, Boston, MA). The two array images were analyzed and compared using the Clontech AtlasImage 1.5 software. First, the phosphor image of each array was separately aligned and fine-tuned to the Atlas Image Grid Template. After normalizing the signal values of all of the genes on the arrays (global normalization), the two aligned arrays were compared with each other using the control group as the reference to generate a color schematic diagram of the changes in the treatment group and a tabular report of the data.
Statistical Analysis
Data are expressed as the mean ± SE. Differences in measured variables between experimental and control group were assessed using Student’s t-test. Statistical difference was accepted at P < 0.05.
Results
HO-1 Protein Levels are Increased in Lung Fibroblasts from Lung Transplant Patients
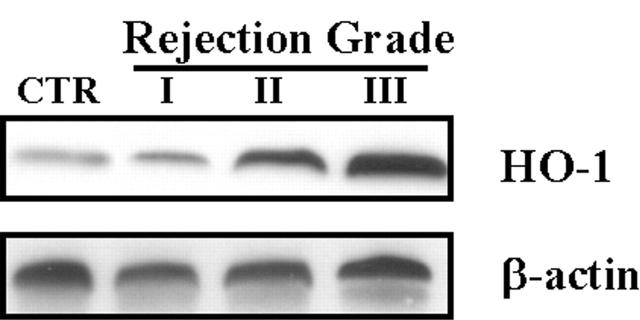
Lung fibroblasts were cultured from endobronchial lung biopsies of lung transplant recipients. Corresponding lung biopsy specimens were graded for rejection grade using the criteria for human lung transplantation rejection grade at the University of Pittsburgh as described in Methods. Cultured fibroblasts were grown to confluence before analysis of cell lysates by Western blot for HO-1 protein expression. As illustrated in Figure 1 ▶ , we observed increasing levels of HO-1 protein expression with increasing grades of acute rejection.
Figure 1.
HO-1 protein level in lung fibroblasts from human transplant recipients increased and correlated with acute rejection grade. Fibroblasts cultured from lung biopsy specimens of transplanted patients were harvested and cultured. Biopsy specimens were sent to pathology for acute rejection grading. Protein was extracted from cultured fibroblast and subjected to Western blot hybridization with HO-1 antibody as described in Methods. Each lane represents pooled samples from a group of five patients with acute rejection grade I to III; control was from normal patient lung. Cultured fibroblast was used at passage 2 to 3. The same membranes were probed with an antibody against β-actin to assure equal loading of the gel.
HO-1 mRNA and Protein Expression are Induced after Rat Lung Transplantation
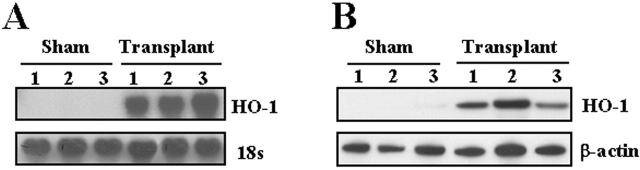
Having demonstrated increased HO-1 protein in fibroblasts cultured from human transplanted biopsy specimens, we examined HO-1 mRNA and protein levels in rat lung following transplantation. Total RNA was extracted from the transplanted lung at day 4 after rat orthotopic lung transplantation and subjected to Northern blot analyses for HO-1. Figure 2A ▶ demonstrates marked induction of HO-1 mRNA in the transplanted lung (at 4 days after transplantation; n = 3) compared to sham control lungs (n = 3) as assessed by Northern blot analyses. The increase in HO-1 mRNA expression correlated with increased HO-1 protein by Western blot analysis in the transplanted lungs as compared with sham-operated lungs (Figure 2B) ▶ . Immunohistochemical staining demonstrated markedly elevated HO-1 expression diffusely in alveolar and airway epithelial and vascular cells (brown staining in the transplanted lung section; Figure 3, C and D ▶ ) compared to the sham-operated control lung section (Figure 3, A and B) ▶ .
Figure 2.
Rat lung transplantation increased HO-1 mRNA expression and protein level. A: Total RNA from the transplanted lung (day 4) was extracted and subjected to Northern blot hybridization with a 32P-labeled HO-1 cDNA probe as described in Methods. Each lane represents RNA extracted from one rat (n = 3). Normalization for RNA loading is shown by labeling 18S rRNA of the same membrane. B: Protein from the transplanted lung (day 4) was extracted and subjected to Western blot hybridization with HO-1 antibody as described in Methods. Each lane represents protein extracted from one rat (n = 3). The same membranes were probed with an antibody against β-actin to assure equal loading of the gel.
Figure 3.
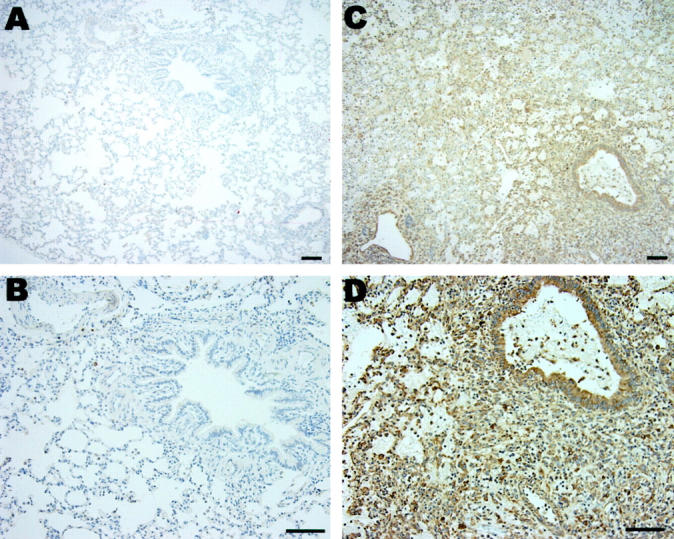
Expression of HO-1 in transplantation. Immunohistochemical staining for HO-1 demonstrated markedly elevated HO-1 expression (brown) in the transplanted lung section (C and D) compared to sham-operated lung section (A and B). Bar equals 100 μm.
Gross Anatomy of Transplanted Lungs
Given the known cytoprotective effects of CO, a byproduct of HO-1 activity, we examined whether exogenous CO could provide cytoprotection against lung injury in an acute model of orthotopic lung transplantation. Figure 4 ▶ shows a representative lung tissue 6 days after orthotopic lung transplantation in rats (n = 6), compared to lung tissue from rats receiving CO (500 ppm, n = 6) over this time period. In the absence of CO, the transplanted lung became markedly hyperemic and infarcted (Figure 4, A and B) ▶ , features which were notably absent in the transplanted lung exposed to CO (Figure 4, C and D) ▶ .
Figure 4.
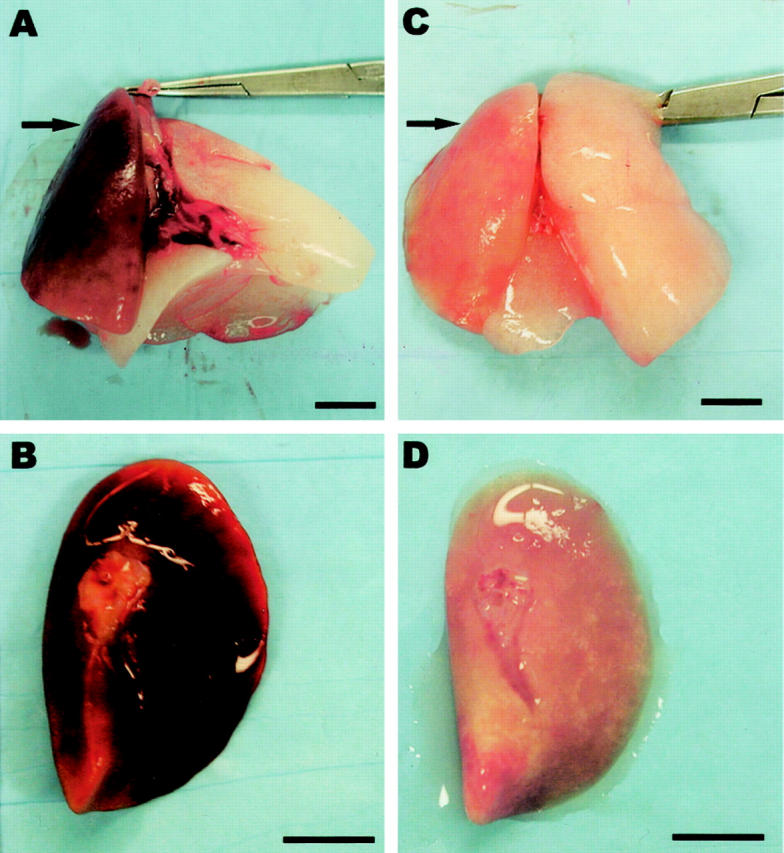
Gross anatomy of lungs from rats 6 days after transplantation (arrow in A, B), and lungs from rats 6 days after transplantation which received 500 ppm CO over this time period (arrow in C, D). In A and C, the arrow points to the left transplanted lung; the right lung is the remaining native lung. Note that in the absence of CO, transplanted lungs were noted to be markedly hyperemic (A and B), which was notably absent in the transplanted lung exposed to CO (C and D). Scale bar, 1 cm.
Histology and Grading of Transplanted Lungs
Sections of lung tissue from control rats 6 days after transplantation (Figure 5, A and B) ▶ , and lungs from rats that received 500 ppm CO over this same time period (Figure 5, C and D) ▶ were stained with H&E and graded for acute rejection using a current standard grading system in human specimens. 23 This was done by a pathologist in blinded approach (Table 1) ▶ . Sections were also examined for changes in architecture, hemorrhage, fibrosis, and thrombosis (Table 1) ▶ . We observed significant attenuation of lung hemorrhage, fibrosis, and thrombosis in tissues from CO-treated animals compared to control animals (Table 1) ▶ . Figure 5, A and B ▶ demonstrate pronounced perivascular and peribronchiole lymphocyte and mononuclear cell aggregates, severe intraalveolar hemorrhage and intravascular coagulation in transplanted lung in the absence of CO. Intraalveolar hemorrhage (Figure 5A) ▶ and intravascular coagulation (Figure 5B ▶ , arrow) were notably absent in the transplanted lung exposed to CO (500 ppm) (Figure 5, C and D) ▶ .
Figure 5.
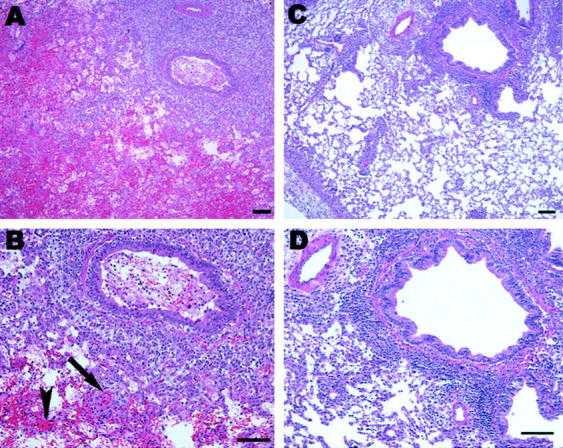
H&E-stained sections of lungs from rats 6 days after transplantation (A and B) and lungs from rats 6 days after transplantation that received 500 ppm CO over this time period (C and D). Note: rejection is present in both CO-treated and untreated transplanted lungs as made evident by the pronounced perivascular and peribronchiole lymphocyte aggregates. However, lung transplant in the absence of CO were noted to have severe intraalveolar hemorrhage (arrowhead in B) and intravascular coagulation (arrow in B) that were notably absent in the transplanted lung exposed to CO (500 ppm) (C and D). Scale bar, 100 μm.
Table 1.
Histology of Rat Lung Transplantation with H&E Staining
| Animal | Grading | Fibrosis/necrosis/thrombosis | Alveolar hemorrhage | |||
|---|---|---|---|---|---|---|
| RA | CO | RA | CO | RA | CO | |
| Day 6–1 | 4 | 2 | + | − | 2 | 0 |
| Day 6–2 | 4 | 3 | + | − | 2 | 0 |
| Day 6–3 | 4 | 4 | + | − | 1 | 2 |
| Day 6–4 | 4 | 3 | + | − | 1 | 0 |
| Day 6–5 | 4 | 2 | + | − | 2 | 0 |
| Day 6–6 | 4 | 3 | + | − | 2 | 0 |
| Average | 4 | 2.8* | + | − | 1.6 | 0.3* |
*P < 0.05.
RA, Room air control group; CO, Carbon monoxide (500 ppm) group; +, presence of; −, absence of.
Alveolar hemorrhage: 0, none; 1, moderate; 2, severe.
Day 6-1 to Day 6-6 denotes separate animal sacrificed 6 days after transplantation.
CO Decreased Neutrophils in Transplanted Lung
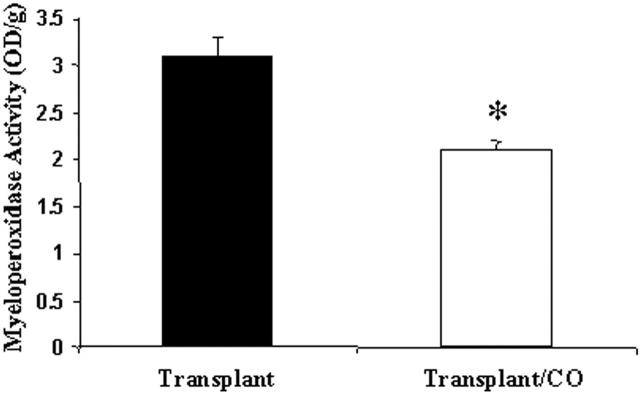
MPO is an enzyme responsible for the generation of hypochlorous acid; it is found principally in neutrophils. Because MPO is found mainly in neutrophils, determination of its enzyme activity yields an estimate of neutrophil content. Thus, we measured MPO activity in whole lung extracts from rats that had received a pulmonary transplantation. Lungs from rats treated with CO and transplantation had lower levels of MPO activity compared to control lungs (Figure 6) ▶ .
Figure 6.
Myeloperoxidase activity was decreased in the CO-treated lung from transplantation. Rat lungs (n = 3) from both transplant and transplant/CO group were measured for MPO activity as described in Methods. MPO activity is expressed per gram of dry weight. CO treatment decreased MPO level by 31%. (* P < 0.05 versus control).
TUNEL and Activated Caspase-3 Staining of Transplanted Lung
Given that CO decreased tissue injury in transplanted lung, the effect of CO on cell death was determined using TUNEL and caspase-3 staining. Staining for TUNEL demonstrated markedly elevated staining (blue; Figure 7, A and B ▶ ) in lungs from control rats after transplantation compared to lungs from transplantation that received CO (500 ppm; Figure 7, C and D ▶ ). Likewise, immunohistochemical staining revealed markedly-elevated activated caspase-3 expression (brown; Figure 8, A and B ▶ ) control transplanted lungs compared to transplanted lungs, which received 500 ppm CO (Figure 8, C and D) ▶ . These data suggest that the cytoprotective effect of CO in rat lung transplantation involves attenuation of cell death. Observation under high-power microscope indicated that the cell types that stained positive for TUNEL or caspase-3 included macrophages, endothelial, and epithelial cells. CO treatment attenuated the death of all these cell types.
Figure 7.
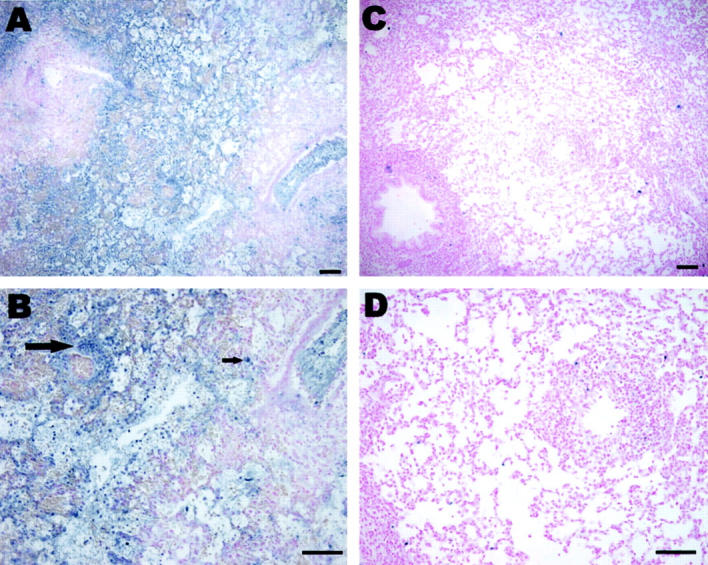
Effect of CO on transplantation-induced TUNEL staining. Markedly elevated TUNEL staining (A, arrow and blue staining in B) occurred in lungs from rats 6 days after transplantation compared to lungs (C and D) from transplantation which received 500 ppm CO over an equivalent time period. Scale bar, 100 μm.
Figure 8.
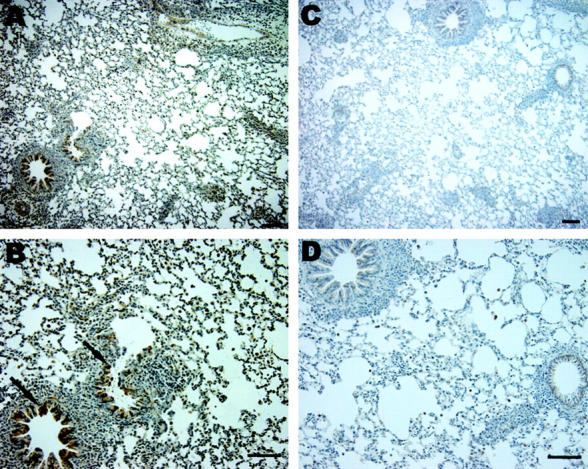
Effect of CO on transplantation-induced activated caspase-3 expression. Immunohistochemical staining demonstrated markedly elevated activated caspase-3 expression (A, arrow and brown staining in B) in lungs from rats 4 days after transplantation compared to lungs (C and D) from transplantation which received 500 ppm CO over an equivalent time period. Scale bar, 100 μm.
We also performed TUNEL/macrophage co-staining to determine the amount of macrophage apoptosis. There was markedly elevated TUNEL/macrophage staining (arrows point to double-positive) (Figure 9, C and D) ▶ in lungs from control rats after transplantation compared to lungs from transplantation that received CO (500 ppm; Figure 9, A and B ▶ ).
Figure 9.
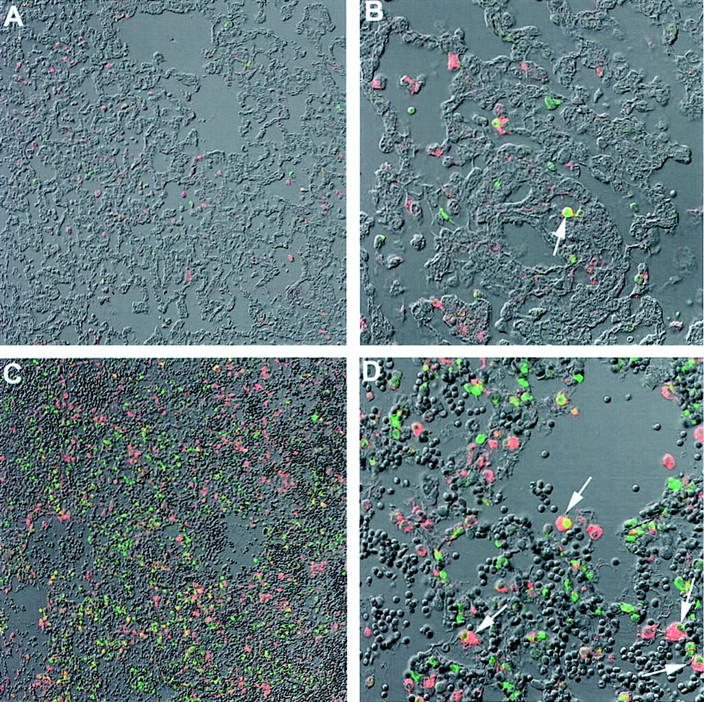
TUNEL/macrophage co-staining. Sections were co-stained for TUNEL (green) and macrophage (red). A and B: Lung from rats 6 days after transplantation in the presence of CO (500 ppm). C and D: Lung from transplantation in the absence of CO. Arrows in B and D: Double-positive staining. We demonstrated markedly elevated co-staining for TUNEL/macrophage staining in lungs from rats after transplantation (C and D) compared to lungs from transplantation that received CO (500 ppm; A and B). Magnifications: A and C, ×20; B and D, ×60.
CO Inhibited Transplantation-Induced IL-6 Expression
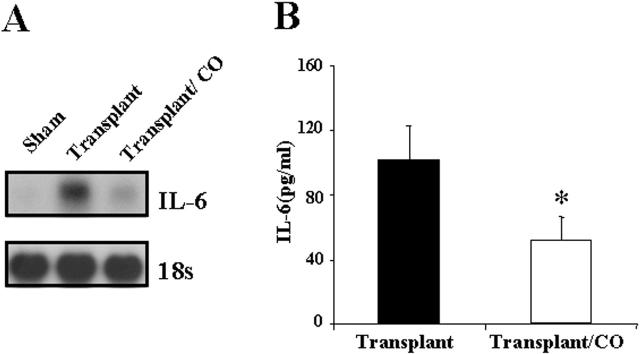
Pulmonary and systemic IL-6 levels have been previously shown to correlate with levels of lung injury after transplantation. 26-29 Total RNA from the transplanted lung was extracted at day 4 after transplantation and subjected to Northern blot hybridization. IL-6 mRNA expression was markedly induced in the transplanted lungs compared with sham-operated lungs. CO treatment (500 ppm) significantly attenuated the increase of IL-6 mRNA expression (Figure 10A) ▶ . Serum IL-6 levels at 4 days after transplantation analyzed by ELISA was markedly increased in the transplanted rat compared with sham-operated rat. Rats treated with CO (500 ppm) displayed a significant decrease in serum IL-6 levels compared with rats receiving lung transplants in the absence of CO (Figure 10B) ▶ .
Figure 10.
CO inhibited transplantation-induced IL-6 mRNA expression and serum IL-6. A: Total RNA from the transplanted lung was extracted and subjected to Northern blot hybridization with a 32P-labeled IL-6 cDNA probe as described in Methods. Each lane represents pooled RNA extracted from 3 rats (n = 3). Normalization for RNA loading is shown by labeling 18S rRNA of the same membrane. B: Serum was collected 4 days after transplantation and analyzed for IL-6 levels by ELISA. CO treatment decreased IL-6 level by 51%. (* P < 0.05 versus control). Data represent the means ± SE of samples from three independent experiments.
Rat cDNA Array
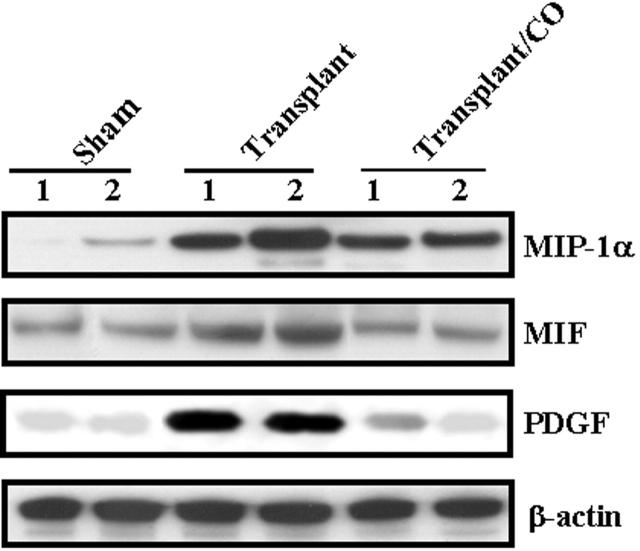
To identify other inflammatory cytokines CO may modulate, we performed cDNA array analysis in lung tissues 4 days after transplantation in the absence or presence of CO. Twenty-seven genes were up-regulated and 11 genes were down-regulated (Table 2) ▶ in transplanted lung versus sham-operated control. Furthermore, 4 genes were up-regulated and 16 genes were down-regulated (Table 3) ▶ in transplanted lung in the presence of CO (500 ppm) versus transplanted lung in the absence of CO. Summarizing the gene expression regulated by transplant and CO, there were 6 genes, including MIP-1α, MIF, and PDGF, that were up-regulated by transplant and down-regulated by CO and 1 gene down-regulated by transplant and up-regulated by CO (Table 4) ▶ . We used Western blot hybridization with MIP-1α, MIF, and PDGF antibodies to confirm the identified gene product of cDNA Array results (Figure 11) ▶ .
Table 2.
Rat cDNA Expression Array: Transplant vs. Control
| Up | Down | Gene | GenBank # |
|---|---|---|---|
| 2.5 | c-jun proto-oncogene; transcription factor AP1 | X17163 | |
| 1.7 | Inhibitor of DNA binding 1 (ID1) | D10862 | |
| 2.0 | Structure-specific recognition protein 1 (SSRP1); recombination signal sequence recognition protein; T160; CIIDBP | L08814 | |
| 5.1 | High mobility group protein 2 (HMG2) | D84418 | |
| 5.0 | Proliferating cell nuclear antigen (PCNA); cyclin | Y00047 | |
| 2.9 | Copper-zinc-containing superoxide dismutase1 (Cu-ZnSOD1) | Y00404 | |
| 1.8 | Heat shock 60-kDa protein (HSP60); 60-kDa chaperonin (CPN60); Gro EL homolog; mitochondrial matrix protein P1; p60 lymphocyte protein | X54793 | |
| 3.1 | Heat shock 70-kDa protein (HSP70) | Z27118 | |
| 2.2 | Signal transducer and activator of transcription 3 (STAT3) | X91810 | |
| 7.3 | NF-κ-B transcription factor p105 subunit (NFKBp105); NF-κ-B 1P84; NF-κ-B 1P98 (NFκB1); DNA-binding factor KBF1; EBP-1 | L26267 | |
| 10.1 | Protein kinase C β-I type (PKC-βI)+ protein kinase C β-II type (PKC-βII) | M19007 | |
| X04440 | |||
| 2.1 | Calcium/calmodulin-dependent protein kinase type IV (CAMKIV; catalyticchain); CAMkinase-GR | M63334 | |
| 1.9 | Transducin β-2 subunit; GTP-binding protein G(i)/G(s)/G(t) β subunit 2 (GNB2) | U34959 | |
| 2.0 | Calmodulin (CALM;CAM) | X13817 | |
| 2.2 | Leukocyte common antigen-related tyrosine phosphatase (LAR) | L11586 | |
| 2.9 | Tumor necrosis factor receptor 1 precursor (TNFR1) | M63122 | |
| 3.9 | Angiotensin converting enzyme (ACE); somatic; dipeptidyl carboxypeptidase I; kininase II | U03734 | |
| 7.6 | SR13 myelin protein; peripheral myelin protein 22 (PMP-22); CD25 protein | M69139 | |
| Up | Epidermal fatty acid-binding protein (E-FABP); cutaneous fatty acid-binding protein (C-FABP); DA11; FABP5 | U13253 | |
| 3.2 | Nonspecific lipid-transfer protein precursor (NSL-TP); sterol carrier protein 2 (SCP2); sterol carrier protein X (SCPX) | M34728 | |
| 4.0 | 60S ribosomal protein L44 (RPL44); L36A | M19635 | |
| 2.1 | Eukaryotic translation initiation factor 2 α subunit (EIF-2-α) | J02646 | |
| 2.5 | 60S ribosomal protein L21 | M27905 | |
| 2.0 | 60S ribosomal protein L19 (RPL19) | J02650 | |
| 1.8 | 40S ribosomal protein S11 | K03250 | |
| 2.7 | 40S ribosomal protein S17 (RPS17) | K02933 | |
| 8.5 | Leukocyte common antigen precursor (LCA); CD45 antigen T200; PTPRC | M10072 | |
| 4.4 | Fibronectin receptor β subunit precursor; integrin β 1 | U12309 | |
| 5.7 | Interleukin-4 receptor precursor (IL4R) | X69903 | |
| 2.0 | Transforming growth factor β II receptor precursor (TGF β II receptor; TGFBR2) | L09653 | |
| 3.3 | Na+/K+ATPase α 1 subunit | M28647 | |
| 3.7 | Small inducible cytokine A3 precursor (SCYA3); macrophage inflammatory protein 1 α precursor (MIP1-α; MIP1A) | U22414 | |
| 4.0 | PDGF-associated protein | U41744 | |
| 2.8 | Macrophage migration inhibitory factor (MIF); glutathione-binding 13-kDa protein | U62326 | |
| 2.3 | Tissue inhibitor of metalloproteinase 2 precursor (TIMP2) | L31884 | |
| 3.3 | Proteasome component C3 | J02897 | |
| 6.1 | Proteasome subunit RC6-1 | D30804 | |
| 2.0 | Metalloproteinase inhibitor 3 precursor; tissue inhibitor of metalloproteinase 3 (TIMP3) | U27201 | |
| 2.6 | Polyubiquitin | D16554 | |
| 2.6 | Phospholipase A2 precursor; phosphatidylcholine 2-acylhydrolase; PLA2G1B | D00036 | |
| 13.4 | Hypoxanthine-guanine phosphoribosyltransferase (HPRT) | M63983 | |
| 12.0 | Glycerol dehyde3-phosphate dehydrogenase (GAPDH) | M17701 | |
| 2.4 | Cytoplasmic β-actin (ACTB) | V01217 |
Table 3.
Rat cDNA Expression Array: Transplant/+CO vs. Transplant
| Up | Down | Gene | GenBank # |
|---|---|---|---|
| 1.8 | Platelet-derived growth factor B-chain (PDGFb); c-sis | Z14117 | |
| 1.7 | Heat shock 70-kDa protein (HSP70) | Z27118 | |
| 1.7 | Interleukin-6 receptor β chain; membrane glycoprotein gp130 | M92340 | |
| 2.6 | Interferon regulatory factor 1 (IRF1) | M34253 | |
| 2.7 | Angiotensin converting enzyme (ACE); somatic; dipeptidyl carboxypeptidase I; kininase II | U03734 | |
| 2.8 | Neuropilin 2 | AF016297 | |
| 1.7 | Set β isoform + Set α isoform; neural plasticity-related protein | S68987 | |
| S68989 | |||
| 2.7 | Epidermal fatty acid-binding protein (E-FABP); cutaneous fatty acid-binding protein (C-FABP); DA11; FABP5 | U13253 | |
| 1.9 | Low-density lipoprotein receptor precursor (LDL receptor; LDLR) | X13722 | |
| 6.1 | Arachidonate 12-lipoxygenase (12-LOX; ALOX12) | L06040 | |
| 3.2 | β-arrestin 2 (ARRB2) | M91590 | |
| 2.0 | Leukocyte common antigen precursor (LCA); CD45 antigen; T200; PTPRC | M10072 | |
| 3.8 | Fibronectin receptor β sub unit precursor; integrin β 1 | U12309 | |
| 2.0 | Na,K-ATPase β 3 sub unit | D84450 | |
| 2.7 | Small inducible cytokine A3 precursor (SCYA3); macrophage inflammatory protein 1 α precursor (MIP1-α; MIP1A) | U22414 | |
| 2.4 | PDGF-associated protein | U41744 | |
| 2.0 | Macrophage migration inhibitory factor (MIF); glutathione-binding 13-kDa protein | U62326 | |
| 7.7 | Plasminogen activator inhibitor 1 precursor (PAI1; PLANH1) | M24067 | |
| 3.7 | Endothelin converting enzyme | D29683 | |
| 36.2 | Tissue-type plasminogen activator (t-PA) | M23697 | |
| 4.0 | Hypoxanthine-guanine phosphoribosyl transferase (HPRT) | M63983 | |
| 1.8 | Glyceraldehyde3-phosphate dehydrogenase (GAPDH) | M17701 |
Table 4.
Genes Regulated by Transplant and CO
| Regulated by transplant (fold) | Regulated by CO (fold) | Name of genes |
|---|---|---|
| ↑3.7 | ↓ 2.7 | Small inducible cytokine A3 precursor (SCYA3); macrophage inflammatory protein 1 α precursor (MIP1-α; MIP1A) |
| ↑4.0 | ↓ 2.4 | PDGF-associated protein |
| ↑3.8 | ↓ 2.0 | Macrophage migration inhibitory factor (MIF); glutathione-binding 13-kDa protein |
| up | ↓ 2.7 | Epidermal fatty acid-binding protein (E-FABP); cutaneous fatty acid-binding protein (C-FABP); DA11; FABP5 |
| ↑8.5 | ↓ 2.0 | Leukocyte common antigen precursor (LCA); CD45 antigen; T200; PTPRC |
| ↑4.4 | ↓ 3.8 | Fibronectin receptor β subunit precursor; integrin α 1 |
| ↓3.9 | ↑ 2.7 | Angiotensin converting enzyme (ACE); somatic; dipeptidyl-carboxypeptidaseI; kininase II |
↑, up-regulation;
↓, down-regulation.
Figure 11.
CO decreased protein levels of multiple chemokines/cytokines following lung transplantation. Western blots were performed to confirm changes in MIP-1α, MIF, and PDGF at the protein level, all of which demonstrated increase of mRNA expression in transplant group and decrease in transplant/CO group by cDNA array. Protein from the transplanted lung was extracted and subjected to Western blot hybridization with MIP-1α, MIF, and PDGF antibodies as described in Methods. The same membranes were probed with an antibody against β-actin to assure equal loading of the gel.
HO-1 mRNA and Protein Expression are Decreased in the CO-Treated Rat
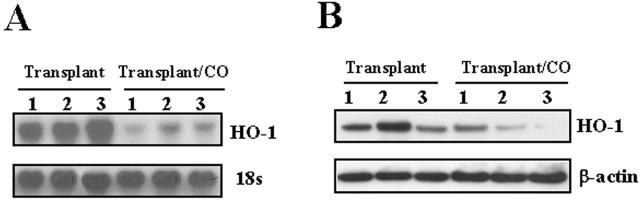
We have demonstrated an induction of HO-1 mRNA and protein expression after rat lung transplantation. We also examined HO-1 mRNA and protein levels in rat lung following transplantation in the presence of CO (500 ppm). Figure 12A ▶ demonstrates marked inhibition of HO-1 mRNA in CO-treated transplanted lung (at 4 days post transplantation, n = 3) compared to transplant control lungs (n = 3) as assessed by Northern blot analysis. The inhibition of HO-1 mRNA expression correlated with inhibition of HO-1 protein by Western blot analysis in the transplanted lungs as compared with transplant control lungs (Figure 12B) ▶ .
Figure 12.
CO decreased HO-1 mRNA expression and protein level. A: Total RNA from the transplanted lung (day 4) was extracted and subjected to Northern blot hybridization with a 32P-labeled HO-1 cDNA probe as described in Methods. Each lane represents RNA extracted from 1 rat (n = 3). Normalization for RNA loading is shown by labeling 18S rRNA of the same membrane. B: Protein from the transplanted lung (day 4) was extracted and subjected to Western blot hybridization with HO-1 antibody as described in Methods. Each lane represents protein extracted from 1 rat (n = 3). The same membranes were probed with an antibody against β-actin to assure equal loading of the gel.
Discussion
HO-1 performs the rate-limiting step in the oxidative catabolism of heme, and may represent a critical adaptive mechanism activated during cellular stress. The cytoprotection provided by HO-1 may result from the elimination of heme, a potential pro-oxidant, and/or the generation of three biologically active downstream mediators: biliverdin-IXα, CO, and ferritin from released iron. 10 HO-1 has been shown to confer cytoprotection in various in vitro and in vivo 6,30 models of cellular and tissue injury including graft rejection in animal models of transplantation. 15,30,31 For example, HO-1 overexpression exerts beneficial effects in a number of transplantation models, including antigen-independent ischemia/reperfusion injury, 32 acute and chronic allograft rejection, 15 and xenotransplantation. 7 Although the precise mechanism by which HO-1 confers cytoprotection in transplantation models is not clear, modulation by HO-1 of cell cycle control, 33 inflammation, 34,35 and apoptosis 9 may play critical roles. HO-1 exerts profound direct and indirect inhibitory effects on the cascade of host inflammatory responses mediated by neutrophils, macrophages, and lymphocytes. 10,35 These anti-inflammatory properties result in cytoprotection in many experimental models of graft injury, including ischemia/reperfusion, acute and chronic allograft, and xenograft rejection. 7,32 Furthermore, the HO-1 expression may simultaneously benefit both local graft function and host systemic immune responses. 36 Thus, the HO-1 system may potentially serve as a novel therapeutic in organ transplantation.
Our laboratory has further tested the hypothesis that the cytoprotective effects of HO-1 may be mediated by the by-products of HO catalysis of heme, namely biliverdin production, secondary of ferritin as a consequence of heme-iron release, and the gaseous molecule CO. We have focused on one such catalytic by-product, the endogenously derived CO from HO. In the present study, we tested the hypothesis that a low concentration of exogenous CO can confer cytoprotection against acute allograft lung rejection via its anti-inflammatory and anti-apoptotic effects. We first had to demonstrate that HO-1 is up-regulated in human lung transplantation and an animal model, to support the concept that HO-1 induction occurs as a stress response in lung transplantation. We demonstrated that the lung fibroblasts from transplanted patients exhibit increasing levels of HO-1 protein expression correlating with increasing grades of acute rejection (Figure 1) ▶ . In a rat orthotopic lung transplantation model, we also demonstrated an induction of HO-1 mRNA and protein expression after rat lung transplantation (Figure 2) ▶ . These data support the hypothesis that HO-1 is a stress-inducible gene and support previous observations that HO-1 expression increases during lung transplantation. 30
We then examined the cytoprotective effects of CO in a clinically relevant lung transplant model. Six days after rat lung orthotopic transplantation, the lungs were markedly hyperemic and infarcted (Figure 4, A and B) ▶ . However, the transplanted lungs of rats receiving inhalation CO (500 ppm) were dramatically well-preserved (Figure 4, C and D) ▶ . Similar preservation was confirmed by histological analysis. Examination for changes in architecture, hemorrhage, fibrosis, and thrombosis showed that lung transplants in the absence of CO displayed severe intraalveolar hemorrhage (Figure 5A) ▶ and intravascular coagulation (Figure 5B ▶ , arrow), features that were notably absent in the transplanted lung exposed to CO (500 ppm; Figure 5, C and D ▶ ). These data confirmed our hypothesis that CO plays a major role in the protective effect of HO-1.
Studies show that ischemia/reperfusion (I/R) or anoxia/reoxygenation (A/R), processes that mark the acute phase of organ transplantation, induce apoptosis in the lungs and vascular cells, respectively. 37,38 While apoptosis may initiate early in the onset of ischemia, there is evidence that the process is amplified during reperfusion. 39 It is well recognized that apoptosis occurs not only in I/R of the heart, kidney, intestine, liver, and brain injury but also in organ transplantation. 32 In addition, recent evidence with human lung transplantation implicates apoptosis as an important contributor to graft injury and severe organ dysfunction. 39 The modulation of apoptosis may provide an important avenue of therapeutic intervention in lung injury, including I/R lung injury and lung transplantation. Therefore, we studied whether CO conferred protection by modulating apoptosis. Immunohistochemical staining of the transplanted lung sections demonstrated marked elevations in TUNEL (Figure 7) ▶ and caspase-3 staining (Figure 8) ▶ in lungs after transplantation compared to lungs transplanted in the presence of CO (500 ppm) over an equivalent time period. Both methods suggested that the protective effects of CO were mediated, in part, by inhibiting apoptosis.
Our lab has previously shown that CO confers anti-inflammatory 10,35 effects. Therefore, we also evaluated the anti-inflammatory effect of CO in an orthotopic lung transplantation model. Pulmonary and systemic IL-6 levels have been previously shown to correlate with levels of injury. 26-29 We demonstrated, using Northern blot and ELISA analysis, that IL-6 mRNA and protein expression were markedly induced in the transplanted lungs, but markedly inhibited by CO treatment (Figure 10) ▶ . We also evaluated the effect of transplantation and CO treatment on gene expression by cDNA gene array. Comparing RNA extracted from transplanted lung versus sham-operated control, 27 genes were up-regulated and 11 genes were down-regulated (Table 2) ▶ . Comparing RNA extracted from transplanted lung in the presence of 500 ppm CO versus transplanted lung in the absence of CO, 4 genes were up-regulated and 16 genes were down-regulated (Table 3) ▶ . There were 6 genes (MIP1-α, PDGF-associated protein, MIF, Leukocyte common antigen precursor (LCA), Fibronectin receptor-β subunit precursor; integrin β-1 epidermal fatty acid-binding protein (E- FABP); cutaneous fatty acid-binding protein (C-FABP, DA11, FABP5)) whose expressions were increased in the transplanted lung, but attenuated in the presence of CO. The expression of 1 gene (angiotensin-converting enzyme) decreased in the transplanted lung but increased in the presence of CO (Table 3) ▶ . We used Western blot hybridization with MIP-1α, MIF, and PDGF antibodies to confirm the cDNA array result (Figure 11) ▶ . It is interesting to note that MIP-1α, 40 MIF, 41,42 and PDGF 43,44 have all been implicated in the pathogenesis of human transplantation including lung transplantation and bronchiolitis obliterans. 40,43,44
It is well known that exposure to CO can be lethal at high concentrations in the context of industrial or accidental exposure. 45 Against this paradigm of CO toxicity, recent data have accumulated in the literature regarding the possibility that CO at low concentrations can behave as a regulatory molecule in cellular and biological processes. 10,33,35 The concentration used for our studies is less than one-tenth of the CO concentration used in humans during measurement of diffusion of lung for carbon monoxide (DLCO) in pulmonary function testing (eg, 3000 ppm CO used in DLCO tests). Furthermore, Stupfel 46,47 demonstrated that long-term (2 years) exposure of rodents to low levels of CO (500 ppm) found no significant alterations in physiological or biochemical parameter.
In summary, these data demonstrate that CO confers a potent protective effect in the setting of lung transplantation, which involves antiapoptotic and anti-inflammatory mechanisms. It is important to note that in our previous studies using other models of lung injury, 48-50 the cytoprotective effects of CO required pretreatment of CO. In this study, however, we observed cytoprotective effects of CO without CO pretreatment of either recipient or donor lungs, which makes it suitable for therapeutic use in the future. The data strongly suggest the formal possibility that exogenous administration of low concentrations of CO may represent a viable novel therapeutic option in lung transplantation. Studies are underway to test the efficacy of CO in larger animals (eg, pig) to support the application for human studies.
Acknowledgments
Supported by the National Institutes of Health (grants NIH HL55330, NIH HL60234, and NIH AI-42365 to A.M.K.C.).
Footnotes
Address reprint requests to Augustine M.K. Choi, M.D., Division of Pulmonary, Allergy and Critical Care Medicine, University of Pittsburgh School of Medicine, NW628 MUH, 3459 5th Avenue, Pittsburgh, PA 15213. E-mail: choiam@msx.upmc.edu.
References
- 1.Girgis RE, Tu I, Berry GJ, Reichenspurner H, Valentine VG, Conte JV, Ting A, Johnstone I, Miller J, Robbins RC, Reitz BA, Theodore J: Risk factors for the development of obliterative bronchiolitis after lung transplantation. J Heart Lung Transplant 1996, 15:1200-1208 [PubMed] [Google Scholar]
- 2.Bando K, Paradis IL, Similo S, Konishi H, Komatsu K, Zullo TG, Yousem SA, Close JM, Zeevi A, Duquesnoy RJ, Manzetti J, Keenan RJ, Armitage JM, Hardesty RL, Griffith BP: Obliterative bronchiolitis after lung and heart-lung transplantation: an analysis of risk factors and management. J Thorac Cardiovasc Surg 1995, 110:4-13 [DOI] [PubMed] [Google Scholar]
- 3.Alwayn IP, Xu R, Adler WH, Kittur DS: Does high MHC class II gene expression in normal lungs account for the strong immunogenicity of lung allografts? Transpl Int 1994, 7:43-46 [DOI] [PubMed] [Google Scholar]
- 4.Sibley RK, Berry GJ, Tazelaar HD, Kraemer MR, Theodore J, Marshall SE, Billingham ME, Starnes VA: The role of transbronchial biopsies in the management of lung transplant recipients. J Heart Lung Transplant 1993, 12:308-324 [PubMed] [Google Scholar]
- 5.Trulock EP: Management of lung transplant rejection. Chest 1993, 103:1566-1576 [DOI] [PubMed] [Google Scholar]
- 6.Otterbein LE, Choi AM: Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol 2000, 279:L1029-L1037 [DOI] [PubMed] [Google Scholar]
- 7.Sato K, Balla J, Otterbein L, Smith RN, Brouard S, Lin Y, Csizmadia E, Sevigny J, Robson SC, Vercellotti G, Choi AM, Bach FH, Soares MP: Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J Immunol 2001, 166:4185-4194 [DOI] [PubMed] [Google Scholar]
- 8.Otterbein LE, Mantell LL, Choi AM: Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol 1999, 276:L688-L694 [DOI] [PubMed] [Google Scholar]
- 9.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP: Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med 2000, 192:1015-1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morse D, Choi AM: Heme oxygenase-1: the “emerging molecule” has arrived. Am J Respir Cell Mol Biol 2002, 27:8-16 [DOI] [PubMed] [Google Scholar]
- 11.Choi AM, Otterbein LE: Emerging role of carbon monoxide in physiologic and pathophysiologic states. Antioxid Redox Signal 2002, 4:227-228 [DOI] [PubMed] [Google Scholar]
- 12.Coito AJ, Shaw GD, Li J, Ke B, Ma J, Busuttil RW, Kupiec-Weglinski JW: Selectin-mediated interactions regulate cytokine networks and macrophage heme oxygenase-1 induction in cardiac allograft recipients. Lab Invest 2002, 82:61-70 [DOI] [PubMed] [Google Scholar]
- 13.DeBruyne LA, Magee JC, Buelow R, Bromberg JS: Gene transfer of immunomodulatory peptides correlates with heme oxygenase-1 induction and enhanced allograft survival. Transplantation 2000, 69:120-128 [DOI] [PubMed] [Google Scholar]
- 14.Tullius SG, Nieminen-Kelha M, Bachmann U, Reutzel-Selke A, Jonas S, Pratschke J, Bechstein WO, Reinke P, Buelow R, Neuhaus P, Volk H: Induction of heme-oxygenase-1 prevents ischemia/reperfusion injury and improves long-term graft outcome in rat renal allografts. Transplant Proc 2001, 33:1286-1287 [DOI] [PubMed] [Google Scholar]
- 15.Avihingsanon Y, Ma N, Csizmadia E, Wang C, Pavlakis M, Giraldo M, Strom TB, Soares MP, Ferran C: Expression of protective genes in human renal allografts: a regulatory response to injury associated with graft rejection. Transplantation 2002, 73:1079-1085 [DOI] [PubMed] [Google Scholar]
- 16.Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, Zhao D, Zaky J, Melinek J, Lassman CR, Kolls JK, Alam J, Ritter T, Volk HD, Farmer DG, Ghobrial RM, Busuttil RW, Kupiec-Weglinski JW: Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest 1999, 104:1631-1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coito AJ, Buelow R, Shen XD, Amersi F, Moore C, Volk HD, Busuttil RW, Kupiec-Weglinski JW: Heme oxygenase-1 gene transfer inhibits inducible nitric oxide synthase expression and protects genetically fat Zucker rat livers from ischemia-reperfusion injury. Transplantation 2002, 74:96-102 [DOI] [PubMed] [Google Scholar]
- 18.Guo X, Shin VY, Cho CH: Modulation of heme oxygenase in tissue injury and its implication in protection against gastrointestinal diseases. Life Sci 2001, 69:3113-3119 [DOI] [PubMed] [Google Scholar]
- 19.Hegazy KA, Dunn MW, Sharma SC: Functional human heme oxygenase has a neuroprotective effect on adult rat ganglion cells after pressure-induced ischemia. Neuroreport 2000, 11:1185-1189 [DOI] [PubMed] [Google Scholar]
- 20.Peng J, Lu R, Ye F, Deng HW, Li YJ: The heme oxygenase-1 pathway is involved in calcitonin gene-related peptide-mediated delayed cardioprotection induced by monophosphoryl lipid A in rats. Regul Pept 2002, 103:1-7 [DOI] [PubMed] [Google Scholar]
- 21.Kato H, Amersi F, Buelow R, Melinek J, Coito AJ, Ke B, Busuttil RW, Kupiec-Weglinski JW: Heme oxygenase-1 overexpression protects rat livers from ischemia/reperfusion injury with extended cold preservation. Am J Transplant 2001, 1:121-128 [PubMed] [Google Scholar]
- 22.Zhou J, Zhu X, Zhang G, Ling T: Protective effect of hemoglobin-induced heme oxygenase-1 on injured lungs caused by limb ischemia-reperfusion in rats. Chin J Traumatol 2002, 5:86-91 [PubMed] [Google Scholar]
- 23.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, Marchevsky A, Ohori NP, Ritter J, Stewart S, Tazelaar HD: Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: lung rejection study group. J Heart Lung Transplant 1996, 15:1-15 [PubMed] [Google Scholar]
- 24.Mizuta T, Kawaguchi A, Nakahara K, Kawashima Y: Simplified rat lung transplantation using a cuff technique. Transplant Proc 1989, 21:2601-2602 [PubMed] [Google Scholar]
- 25.Klebanoff SJ, Waltersdorph AM, Rosen H: Antimicrobial activity of myeloperoxidase. Methods Enzymol 1984, 105:399-403 [DOI] [PubMed] [Google Scholar]
- 26.Lu KC, Jaramillo A, Lecha RL, Schuessler RB, Aloush A, Trulock EP, Mendeloff EN, Huddleston CB, Alexander Patterson G, Mohanakumar T: Interleukin-6 and interferon-γ gene polymorphisms in the development of bronchiolitis obliterans syndrome after lung transplantation. Transplantation 2002, 74:1297-1302 [DOI] [PubMed] [Google Scholar]
- 27.Scholma J, Slebos DJ, Boezen HM, van den Berg JW, van der Bij W, de Boer WJ, Koeter GH, Timens W, Kauffman HF, Postma DS: Eosinophilic granulocytes and interleukin-6 level in bronchoalveolar lavage fluid are associated with the development of obliterative bronchiolitis after lung transplantation. Am J Respir Crit Care Med 2000, 162:2221-2225 [DOI] [PubMed] [Google Scholar]
- 28.Iacono A, Dauber J, Keenan R, Spichty K, Cai J, Grgurich W, Burckart G, Smaldone G, Pham S, Ohori NP, Yousem S, Williams P, Griffith B, Zeevi A: Interleukin-6 and interferon-γ gene expression in lung transplant recipients with refractory acute cellular rejection: implications for monitoring and inhibition by treatment with aerosolized cyclosporine. Transplantation 1997, 64:263-269 [DOI] [PubMed] [Google Scholar]
- 29.Yoshida Y, Iwaki YM, Pham S, Dauber JH, Yousem SA, Seevi A, Morita S, Griffith BP: Benefits of posttransplantation monitoring of interleukin 6 in lung transplantation. Ann Thorac Surg 1993, 55:89-93 [DOI] [PubMed] [Google Scholar]
- 30.Lu F, Zander DS, Visner GA: Increased expression of heme oxygenase-1 in human lung transplantation. J Heart Lung Transplant 2002, 21:1120-1126 [DOI] [PubMed] [Google Scholar]
- 31.Katori M, Busuttil RW, Kupiec-Weglinski JW: Heme oxygenase-1 system in organ transplantation. Transplantation 2002, 74:905-912 [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Bedard EL, Potter R, Zhong R, Alam J, Choi AM, Lee PJ: Mitogen-activated protein kinases regulate HO-1 gene transcription after ischemia-reperfusion lung injury. Am J Physiol Lung Cell Mol Physiol 2002, 283:L815-L829 [DOI] [PubMed] [Google Scholar]
- 33.Song R, Mahidhara RS, Liu F, Ning W, Otterbein LE, Choi AM: Carbon monoxide inhibits human airway smooth muscle cell proliferation via mitogen-activated protein kinase pathway. Am J Respir Cell Mol Biol 2002, 27:603-610 [DOI] [PubMed] [Google Scholar]
- 34.Song R, Ning W, Liu F, Ameredes BT, Calhoun WJ, Otterbein LE, Choi AMK: Regulation of IL-1β-induced GM-CSF production in human airway smooth muscle cells by carbon monoxide. Am J Physiol Lung Cell Mol Physiol 2003, 284:L50-56 [DOI] [PubMed] [Google Scholar]
- 35.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM: Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 2000, 6:422-428 [DOI] [PubMed] [Google Scholar]
- 36.Pileggi A, Molano RD, Berney T, Cattan P, Vizzardelli C, Oliver R, Fraker C, Ricordi C, Pastori RL, Bach FH, Inverardi L: Heme oxygenase-1 induction in islet cells results in protection from apoptosis and improved in vivo function after transplantation. Diabetes 2001, 50:1983-1991 [DOI] [PubMed] [Google Scholar]
- 37.Yue TL, Wang C, Gu JL, Ma XL, Kumar S, Lee JC, Feuerstein GZ, Thomas H, Maleeff B, Ohlstein EH: Inhibition of extracellular signal-regulated kinase enhances ischemia/reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ Res 2000, 86:692-699 [DOI] [PubMed] [Google Scholar]
- 38.Martinez-Mier G, Toledo-Pereyra LH, Bussell S, Gauvin J, Vercruysse G, Arab A, Harkema JR, Jordan JA, Ward PA: Nitric oxide diminishes apoptosis and p53 gene expression after renal ischemia and reperfusion injury. Transplantation 2000, 70:1431-1437 [DOI] [PubMed] [Google Scholar]
- 39.Ficher S, Mclean AA, Liu M, Cardella JA, Slutsky AS, Suga M, Moreira JM, Keshavjee S: Dynamic changes in apoptotic and necrotic cell death correlate with severity of ischemia-reperfusion injury in lung transplantation. Am J Respir Crit Care Med 2000, 162:1932-1939 [DOI] [PubMed] [Google Scholar]
- 40.Farver CF, Raychaudhuri B, Malur A, Drazba J, Maurer J, Tubbs R, Mehta AC, Schilz R, Thomassen MJ: Increased alveolar macrophage nuclear factor-κ B activation and macrophage inhibitory protein-1α levels in lung transplant patients. Transplantation 2000, 70:1599-1603 [DOI] [PubMed] [Google Scholar]
- 41.Brown FG, Nikolic-Paterson DJ, Chadban SJ, Dowling J, Jose M, Metz CN, Bucala R, Atkins RC: Urine macrophage migration inhibitory factor concentrations as a diagnostic tool in human renal allograft rejection. Transplantation 2001, 71:1777-1783 [DOI] [PubMed] [Google Scholar]
- 42.Hou G, Valujskikh A, Bayer J, Stavitsky AB, Metz C, Heeger PS: In vivo blockade of macrophage migration inhibitory factor prevents skin graft destruction after indirect allorecognition. Transplantation 2001, 72:1890-1897 [DOI] [PubMed] [Google Scholar]
- 43.Hertz MI, Henke CA, Nakhleh RE, Harmon KR, Marinelli WA, Fox JM, Kubo SH, Shumway SJ, Bolman RM, 3rd, Bitterman PB: Obliterative bronchiolitis after lung transplantation: a fibroproliferative disorder associated with platelet-derived growth factor. Proc Natl Acad Sci USA 1992, 89:10385-10389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aris RM, Walsh S, Chalermskulrat W, Hathwar V, Neuringer IP: Growth factor upregulation during obliterative bronchiolitis in the mouse model. Am J Respir Crit Care Med 2002, 166:417-422 [DOI] [PubMed] [Google Scholar]
- 45.Nicholls JC: Carbon monoxide: the elusive environmental toxicant. Med Hypotheses 2001, 57:591-592 [DOI] [PubMed] [Google Scholar]
- 46.Stupfel M, Bouley G: Physiological and biochemical effects on rats and mice exposed to small concentrations of carbon monoxide for long periods. Ann NY Acad Sci 1970, 174:342-368 [DOI] [PubMed] [Google Scholar]
- 47.Demaria Pesce VH, Stupfel M, Gourlet V, Lemercerre C: Age and survival of an acute carbon monoxide intoxication: an animal model. Sci Total Environ 1987, 65:41-51 [DOI] [PubMed] [Google Scholar]
- 48.Otterbein LE, Chin BY, Mantell LL, Stansberry L, Horowitz S, Choi AM: Pulmonary apoptosis in aged and oxygen-tolerant rats exposed to hyperoxia. Am J Physiol 1998, 275:L14-L20 [DOI] [PubMed] [Google Scholar]
- 49.Chapman JT, Otterbein LE, Elias JA, Choi AM: Carbon monoxide attenuates aeroallergen-induced inflammation in mice. Am J Physiol Lung Cell Mol Physiol 2001, 281:L209-L216 [DOI] [PubMed] [Google Scholar]
- 50.Otterbein LE, Haga M, Zuckerbaun BS, Liu F, Song R, Usheva A, Stachulak C, Bodyak N, Smith RN, Csizmadia E, Tyagi S, Akamatsu Y, Flavell RJ, Billiar TR, Tzeng E, Bach F, Choi AMK, Soares MP: Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med 2003, 9:183-190 [DOI] [PubMed] [Google Scholar]