Abstract
Organ-specific stem cells can be identified by the side population (SP) phenotype, which is defined by the property to effectively exclude the Hoechst 33342 dye. The ATP-binding cassette transporter ABCG2/BCRP1 mediates the SP phenotype. Because hepatic oval cells possess several characteristics of stem cells, we examined whether they have the SP phenotype using the 2-acetylaminofluorene/partial hepatectomy (PH) model. Fluorescence-activated cell sorting analysis showed that a population of non-parenchymal cells containing oval cells, prepared on day 7 after PH, carried a significant number of SP cells, whereas that of non-parenchymal cells without oval cells, prepared on day 0 after PH, did not. Northern blot analysis using total liver RNA obtained on various days after PH showed that the expression of ABCG2/BCRP1 mRNA increased after PH, reaching the highest level on day 7, and then gradually decreased. This pattern of changes in the ABCG2/BCRP1 mRNA level was well correlated to that in the number of oval cells. Furthermore, in situ hybridization revealed that oval cells were the sites of expression of ABCG2/BCRP1 mRNA. These results indicate that oval cells have the SP phenotype defined by expression of ABCG2/BCRP1, suggesting that oval cells may represent stem cells in the liver.
Hepatocytes proliferate after liver damage such as partial hepatectomy (PH), resulting in regeneration of the liver. 1,2 When hepatocytes are prevented from proliferating in response to liver damage, hepatic oval cells emerge from periportal areas, proliferate, and infiltrate into the surrounding parenchyma. 3-5 For example, many oval cells develop in the periportal areas and the surrounding parenchyma in the rat 2-acetylaminofluorene (AAF)/PH model, in which the AAF administration inhibits hepatocyte proliferation. 6-9 Since oval cells are capable of differentiating into both hepatocytes and cholangiocytes, 5,10 they are believed to participate in the liver regeneration under conditions in which the replication of hepatocytes is impaired. In addition to markers of fetal hepatocytes and cholangiocytes, such as α-fetoprotein and cytokeratin (CK)-19, oval cells have recently been shown to express hematopoietic stem cell markers, such as c-kit receptor tyrosine kinase, flt-3 receptor tyrosine kinase, CD34, and Thy-1. 9,11-13
In 1996, Goodell and colleagues 14 reported a new method of obtaining a cell population enriched with hematopoietic stem cells from adult mouse bone marrow in a single step. This method exploits the ability of hematopoietic stem cells to efflux Hoechst 33342, which generates, in fluorescence-activated cell sorting (FACS) analysis, a side population (SP) consisting of low fluorescent cells called SP cells. SP cells represent 0.05% of the whole bone marrow of adult mice and humans, and differentiate into all hematopoietic lineages. 14 These SP cells also give rise to endothelial cells and skeletal and cardiac muscles. 15,16 Moreover, the skeletal muscle contains SP cells that might be responsible for reconstituting both the skeletal muscle and the hematopoietic system of lethally irradiated recipients. 15 SP cells have recently been identified in the nervous system of mice 17 ; thus, it is likely that the SP phenotype represents a common molecular feature for stem cells possessing multiorgan plasticity in various organs.
Recently, Zhou and colleagues have shown that the ATP-binding cassette transporter ABCG2/BCRP1 is expressed in SP cells isolated from bone marrow cells, and that transduction of bone marrow cells with a vector expressing ABCG2/BCRP1 results in an increase in the SP population from 0.05% to 62.5%. 18 Moreover, they have demonstrated that the loss of ABCG2/BCRP1 expression lead to a significant reduction in the number of SP cells in the bone marrow and skeletal muscle. 19 These results indicate that ABCG2/BCRP1 mediates the phenotype of SP cells. Since oval cells have several features of stem cells, such as the capability of differentiating into both hepatocytes and cholangiocytes 5,10 and the expression of hematopoietic stem cell markers, 9,11-13 we here examined whether oval cells show the SP phenotype defined by expression of ABCG2/BCRP1.
Materials and Methods
Rats and Treatments
Male Fischer 344 rats 6 weeks of age were purchased from Japan SLC (Hamamatsu, Shizuoka, Japan). Oval cells were induced by the method of the AAF/PH model. 20,21 Briefly, AAF (Tokyo Chemical Industry, Tokyo, Japan) was daily administered to rats by gavage at 15 mg/kg body weight for 4 days. On day 5, a standard two-thirds PH was carried out, and then the daily administration of AAF at the same dosage continued for 5 days. Rats were sacrificed for liver analysis on days 0, 4, 7, 9, 11, 13, and 20 after PH. All surgical procedures were done under sevoflurane anesthesia. All experiments with rats were approved by the Animal Care Committee of the Hyogo College of Medicine, and were performed in accordance with the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Science.
Preparation of Parenchymal and Nonparenchymal Cells
Parenchymal and non-parenchymal cells of the liver were isolated by the two-step collagenase digestion method with some modifications. 22 Briefly, livers were perfused with 200 ml of Ca++-free Hanks’ balanced salt solution (HBSS), and then with 200 ml of Ca++-HBSS-containing 0.05% collagenase type IA (Sigma Chemical Co., St. Louis, MO) at 30 ml/minute at 37°C. The livers were promptly removed and minced with a razor. For isolation of parenchymal cells, the minced livers were incubated in 50 ml of Ca++-HBSS containing 0.1% collagenase type IA for 60 minutes at 37°C, filtered through a 60-μm mesh, and centrifuged at 50 × g for 1 minute. Pellets formed were used as a population of parenchymal cells containing hepatocytes. For isolation of non-parenchymal cells, the minced livers were incubated in 50 ml of Ca++-HBSS containing 0.1% collagenase type IA, 0.1% actinase E (Kaken Pharmaceutical Company, Tokyo, Japan), 0.01% deoxyribonuclease I (Sigma), 0.05% hyaluronidase (Sigma), 10−7 mol/L insulin (Sigma), and 10−8 mol/L dexamethasone (Sigma) for 60 minutes at 37°C, and filtered through a 60-μm mesh. The filtrate was centrifuged at 50 × g for 1 minute, and the supernatant was centrifuged again at 50 × g for 1 minute. The centrifugation was repeated one more time. The final supernatant was centrifuged at 190 × g for 8 minutes, and pellets formed were used as a population of non-parenchymal cells.
Hoechst 33342 Exclusion Assay
Non-parenchymal cells were resuspended at 1.0 × 106 cells/ml in pre-warmed Ca++-HBSS with 2% fetal calf serum and 1 mmol/L HEPES and divided into two portions. One portion was treated with 100 μmol/L verapamil for 20 minutes at 37°C, and the other was not. Both portions were incubated in Ca++-HBSS with 5 μg/ml Hoechst 33342 (Sigma), 2% fetal calf serum, and 1 mmol/L HEPES for 90 minutes at 37°C. The cells were kept on ice for 5 minutes, and analyzed for Hoechst 33342 efflux by FACS Vantage (Becton Dickinson, San Jose, CA). The Hoechst 33342 dye was excited at 350 nm ultraviolet and resultant fluorescence was measured at two wavelengths using a 424/44 BP and 675 LP filters for detection of Hoechst blue and red, respectively.
Isolation of Rat ABCG2/BCRP1 cDNA
Total RNA was extracted by using TRIZOL reagent (Life Technologies, Inc., Grand Island, NY), and reverse-transcribed by Superscript II reverse transcriptase (RT) (Life Technologies) in the presence of an anchor primer (5′-GCAATTAACCCTCACTAAAGAATTCAGTCAGTCA(T)17-3′). The resultant cDNA was amplified by polymerase chain reaction (PCR) using Taq Ex (Takara Shuzou, Kyoto, Japan) and four sets of primers (FW1 and RV1, FW2 and RV2, FW3 and RV3, and FW4 and RV4) (Table 1) ▶ with 25 cycles of 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 1 minute. The resultant four overlapping products were subcloned into the Eco RV site of Bluescript I KS (−) (Stratagene, La Jolla, CA). To obtain the 3′-end of ABCG2/BCRP1 cDNA, nested PCR was carried out using FW5 and slica1 as the first set of primers and FW6 and slica2 as the second set of primers (Table 1) ▶ . The product was subcloned into the Eco RV site of Bluescript I KS (−). Six clones were selected at random from each PCR product and analyzed for nucleotide sequences.
Table 1.
Oligonucleotide Primers Used in This Study
| Oligonucleotide | Sequence | Position |
|---|---|---|
| FW1 | 5′-AACTGCACCATTGAGAGAAAAC-3′ | 114–135* |
| FW2 | 5′-GAAAACTTACAGTTCTCAGCAGCTC-3′ | 428–452† |
| FW3 | 5′-ACAAAAGCTGAATTAGATCAACTT-3′ | 1052–1075† |
| FW4 | 5′-GCCAGTTCCATGGCACTGGCCATAG-3′ | 1577–1601† |
| FW5 | 5′-TACTTGATAAATCAGGGCATCG-3′ | 1862–1883† |
| FW6 | 5′-GAATCATGTGGCCCTGGCTTG-3′ | 1909–1929† |
| RV1 | 5′-CCCTGTTTAGACATCCTTTTCAGGA-3′ | 696–720† |
| RV2 | 5′-CATCGGAGCTGGTGACAGAAAGAGG-3′ | 1131–1155† |
| RV3 | 5′-GAGATTCACCAAGAGGCCAG-3′ | 1674–1693† |
| RV4 | 5′-AACAATTTCAGGTAGGCAATTGTGAG-3′ | 1946–1971† |
| slica1 | 5′-CAATTAACCCTCACTAAAGA-3′ | |
| slica2 | 5′-CTAAAGAATTCAGTCAGTCA-3′ |
*Number of uj11b07.y1 Sugano mouse kidney mkia Mus musculus cDNA (GenBank accession number AI226912).
†Number of mouse ABCG2/BCRP1 cDNA (GenBank accession number AF140218).
Northern Blot Analysis
The total RNA (20 μg) was fractionated by agarose-formaldehyde gel electrophoresis, transferred to a Hybond-N+ nylon membrane (Amersham International plc., Buckinghamshire, UK), and hybridized with the 32P-labeled rat ABCG2/BCRP1 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) 23 cDNA in Church’s buffer (1 mmol/L ethylenediamine-N,N,N′,N′-tetraacetic acid, 7% sodium dodecylsulfate, and 0.5 mol/L Na2HPO4, pH 7.2) at 65°C overnight. The blot was washed in 2X SSC (300 mmol/L NaCl and 30 mmol/L trisodium citrate, pH 7.4) at 65°C, and subjected to autoradiography. Analysis of ABCG2/BCRP1 mRNA in various tissues was conducted using a membrane of rat polyA+ RNA (2 μg) Northern blot-12 major tissues (OriGene Technologies, Inc., Rockville, MD).
Number of Oval Cells
Liver tissues were fixed with methacarn (methanol/chloroform/glacial acetic acid, 6:3:1), embedded in paraffin, and cut into 5 μm thick sections. The sections were treated with 0.3% H2O2 in methanol for 30 minutes to inactivate endogenous peroxidase, washed with Tris-buffered saline/Tween solution (50 mmol/L Tris-HCl, pH 7.6, 300 mmol/L NaCl, and 0.1% Tween 20), and incubated with normal goat serum to block nonspecific binding of antibodies. The sections were then incubated with a mouse monoclonal antibody against human CK-19 (Novocastra Laboratories, Ltd., Newcastle, UK), which was an oval cell marker, 4,24 at 4°C overnight. The sections were then incubated with Histofine Simple Stain MAX-PO(M) (Nichirei Corporation, Tokyo, Japan) for 60 minutes, and immunoreacted cells were visualized with Simple Stain DAB Solution (Nichirei Corporation). The sections were lightly counterstained with hematoxylin. A periportal field examined in this study was a 0.25 mm × 0.25 mm area containing one portal region and the surrounding parenchyma. The number of CK-19 positive oval cells was counted in ten periportal fields selected randomly in each CK-19 immunostained specimen, and the value was expressed as the number per periportal field.
In Situ Hybridization
Liver tissues were fixed with 4% paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.2) overnight, embedded in paraffin, and cut into 5-μm-thick sections. One section was used for in situ hybridization and the adjacent section was stained with hematoxylin and eosin. Complementary RNA probes of ABCG2/BCRP1 were prepared by using a DIG RNA Labeling Kit (Boehringer Mannheim, Mannheim, Germany). Hybridization was performed at 50°C for 16 hours, and signals were visualized with a DAKO Gen Point kit using the horseradish peroxidase-conjugated anti-digoxigenin antibody.
Results
A Population of Non-Parenchymal Cells Containing Oval Cells Includes a Significant Number of SP Cells
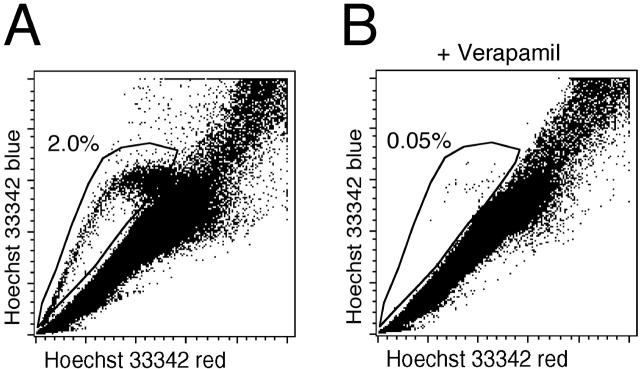
We showed previously that a population of non-parenchymal cells isolated from livers of rats sacrificed on day 0 after PH in the AAF/PH model did not contain oval cells, whereas cells from rats sacrificed on day 7 did. 9 To investigate whether oval cells show the SP phenotype, we performed Hoechst 33342 dye exclusion assays on non-parenchymal cell preparations from day 0 and day 7. SP was minimally detected with non-parenchymal cell preparation from day 0 (data not shown), whereas it was clearly observed with that from day 7 (Figure 1A ▶ ; 2.0% gated cells). This SP phenotype was essentially absent in the presence of verapamil, 14 which was the specific inhibitor of Hoechst 33342 transport (Figure 1B ▶ ; 0.05% gated cells). These observations suggest that oval cells exhibit the SP phenotype.
Figure 1.
SP profile of non-parenchymal cells containing oval cells. Non-parenchymal cells were isolated on day 7 after PH in the AAF/PH model, and analyzed for Hoechst 33342 efflux by FACS vantage. A: A clear SP is visible after Hoechst 33342 staining. B: Dye efflux from SP cells is inhibited in the presence of 100 μmol/L verapamil. SP-gated cells are 2.0% of the total number of cells analyzed in A, and 0.05% in B.
Isolation of Rat ABCG2/BCRP1 cDNA
It has recently been reported that the SP phenotype is characterized by the expression of ABCG2/BCRP1. 25-27 To analyze ABCG2/BCRP1 expression in oval cells, we first isolated rat ABCG2/BCRP1 cDNA. Four overlapping fragments of ABCG2/BCRP1 cDNA (FW1-RV1, FW2-RV2, FW3-RV3, and FW4-RV4) were amplified by RT-PCR using total RNA isolated from the liver of a rat sacrificed on day 7 after PH in the AAF/PH model, and cloned into Bluescript I KS (−) to be sequenced (Figure 2A) ▶ . The 3′-portion of ABCG2/BCRP1 cDNA was obtained by nested PCR, and cloned into Bluescript I KS (−) to be sequenced (Figure 2A) ▶ . The sequence of the entire coding region of rat ABCG2/BCRP1 cDNA is shown in Figure 2B ▶ (GenBank accession no. AB094089). The coding region of rat ABCG2/BCRP1 gene showed 93% and 82% homologies to the mouse 28 and human 29 counterparts, respectively. The predicted amino acid sequence (Figure 2B) ▶ was 93% and 82% homologous to mouse and human counterparts, respectively.
Figure 2.
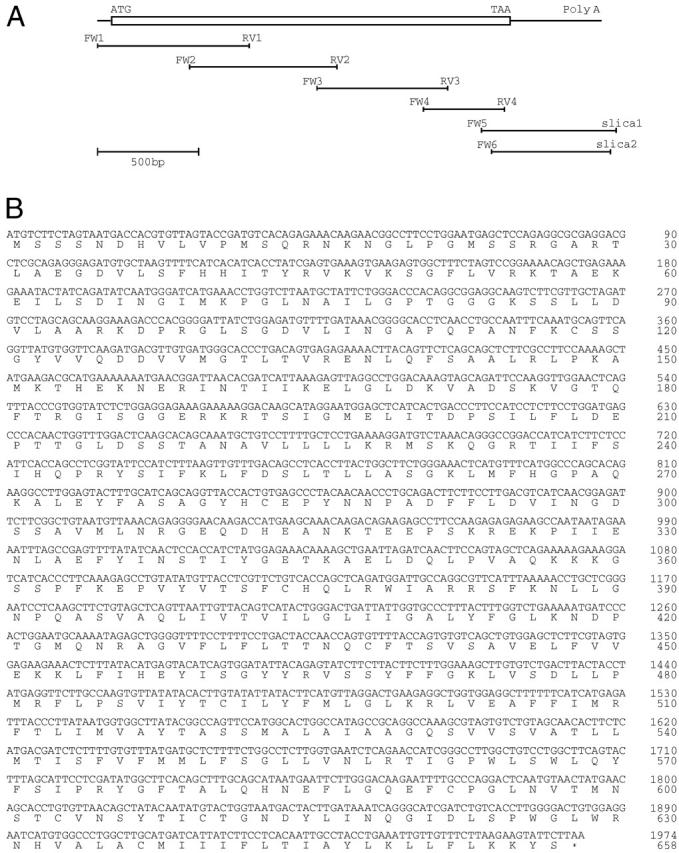
Rat ABCG2/BCRP1 cDNA. A: Schematic representation. An open reading frame of ABCG2/BCRP1 cDNA is indicated by box. Six overlapping partial clones are shown below with primers. B: Nucleotide sequence (GenBank accession no. AB094089). The DNA sequence is numbered from the initiation codon. The predicted amino acid sequence is displayed under the DNA sequence.
Expression of ABCG2/BCRP1
The expression of ABCG2/BCRP1 mRNA in various tissues was examined by using a membrane of rat polyA+ RNA Northern blot-12 major tissues. As shown in Figure 3A ▶ , strong signals of ABCG2/BCRP1 mRNA were observed in the liver, kidney, and testis, and weak signals were observed in the brain and small intestine.
Figure 3.
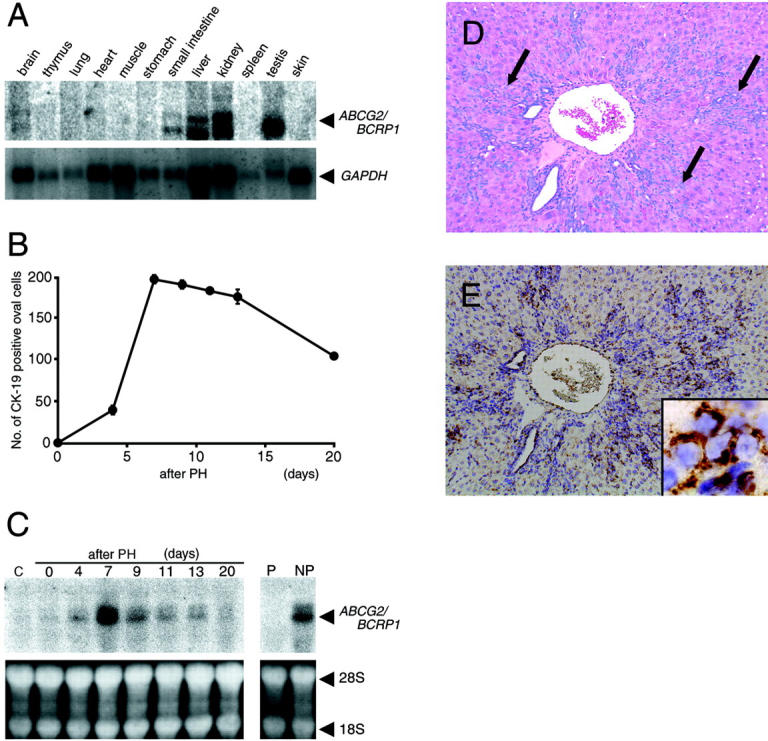
Expression of ABCG2/BCRP1 mRNA. A: Expression of ABCG2/BCRP1 mRNA in various tissues of adult rats. A membrane of rat polyA+ RNA (2 μg) Northern blot-12 major tissues was used for Northern blot analysis. GAPDH mRNA was analyzed as an internal standard. B: Time course analysis of CK-19-positive oval cells in livers following PH in the AAF/PH model. Rats were sacrificed on the indicated days after PH, and liver sections were immunostained with an anti-CK-19 antibody. The number of CK-19-positive oval cells was counted in ten periportal fields, and its value was expressed as the number per periportal field (0.25 mm × 0.25 mm). Each point represents the mean of three rats. Bars are standard errors (SE); the SE was sometimes too small to be shown by bars. C: Time course analysis of ABCG2/BCRP1 mRNA in livers following PH in the AAF/PH model. Total liver RNA (20 μg) from rats sacrificed on the indicated days after PH was analyzed by Northern blotting using ABCG2/BCRP1 cDNA as a probe. RNA from rats that did not undergo AAF/PH treatment was used as the control (C). Parenchymal cell fraction (P) and non-parenchymal cell fraction (NP) were isolated from livers on day 7 after PH, and total RNA (20 μg) was analyzed by Northern blotting. 18S and 28S were used as an internal standard. D: A section of the liver obtained on day 7 after PH stained with hematoxylin and eosin. The representative clusters of oval cells are shown by arrows. E: in situ hybridization analysis of ABCG2/BCRP1 mRNA using an adjacent section of D. Inset in E is high magnification, and shows oval cells expressing ABCG2/BCRP1 mRNA. Magnification, ×80 (D and E) and ×750 (inset of E).
We next examined whether the level of ABCG2 mRNA correlated with the number of oval cells in livers in the AAF/PH model. As shown in Figure 3B ▶ , the number of CK-19 positive oval cells in the liver increased after PH, reaching a maximal level on day 7, and then gradually decreased. Northern blot analysis using total liver RNA showed that ABCG2/BCRP1 mRNA was hardly detectable on day 0, but was expressed at high levels on day 7, and then gradually decreased over time (Figure 3C) ▶ . These results show that the number of oval cells and the level of ABCG2/BCRP1 mRNA changed in a similar manner following PH, but the abundance of mRNA appeared to decline faster than the oval cell number after day 7. This may reflect decreases in transcription of ABCG2/BCRP1 mRNA as oval cells lose division potential.
It has been shown that oval cells are present in non-parenchymal cell fraction, but not parenchymal cell fraction. 22 Northern blot analysis of ABCG2/BCRP1 mRNA showed that there was no signal in parenchymal cells isolated from livers on day 7, but a strong signal in non-parenchymal cells isolated from the same livers (Figure 3C) ▶ .
Finally, we performed in situ hybridization analysis to identify the type of cells expressing ABCG2/BCRP1 mRNA in the liver on day 7. The signal of ABCG2/BCRP1 mRNA was associated with oval cells that were present at the periportal field and the surrounding parenchyma (Figure 3,D and E) ▶ .
Discussion
Oval cells are capable of differentiating into both hepatocytes and cholangiocytes. 5,10 In addition, they express hematopoietic stem cell markers, such as c-kit receptor tyrosine kinase, flt-3 receptor tyrosine kinase, CD34, and Thy-1. 9,11-13 Because of these characteristics, oval cells are considered candidates for stem cells in the liver. In this study, we examined whether oval cells show the SP phenotype, which has recently been shown to identify organ-specific stem cells. 18,19,25-27 Hoechst 33342 dye exclusion assays showed that a population of non-parenchymal cells isolated from livers on day 7 after PH in the AAF/PH model contained a significant number of SP cells, whereas no SP cells were found in the corresponding population isolated from livers on day 0. Since the former cell preparation contained numerous oval cells, but the latter did not, it seems that the oval cells have the SP phenotype.
This possibility was further examined by analyzing the expression of ABCG2/BCRP1, which has been shown to be the major component of the SP phenotype. 18,19,25-27 We first isolated the rat ABCG2/BCRP1 cDNA with an open reading frame of 1974 bases encoding a 657-amino acid protein, showing high homologies to mouse and human counterparts. We then conducted time course analysis of oval cells and ABCG2/BCRP1 mRNA in the liver following PH in the AAF/PH model. The results showed that the level of ABCG2/BCRP1 mRNA was well correlated to the number of oval cells. Furthermore, we confirmed that a non-parenchymal cell preparation with oval cells, but not a parenchymal cell preparation with hepatocytes, from livers on day 7 expressed ABCG2/BCRP1 mRNA. Finally, we showed by in situ hybridization that the signal of the ABCG2/BCRP1 mRNA localized in oval cells. These results taken together indicate that oval cells express ABCG2/BCRP1 mRNA and exhibit the SP phenotype. Whether these oval cells with the SP phenotype define stem cells requires further studies, such as testing the regenerative capacity of oval cells with SP phenotype by transplantation.
The biological function of ABCG2/BCRP1 in oval cells is not clear at present, but it is possibly as follows. ABCG2/BCRP1 belongs to the ATP-binding cassette family of transport proteins that are associated with resistance to various drugs, such as mitoxantrone, 28 topotecan, 28,30 and flavopiridol. 31 Based on these findings, it is conceivable that ABCG2/BCRP1 protects oval cells from cytotoxic agents. Alternatively, ABCG2/BCRP1 may regulate the development of oval cells by functioning as transporters of peptides involved in proliferation, differentiation, and apoptosis.
Northern blot analysis using a membrane of rat polyA+ RNA (2 μg) Northern blot-12 major tissues showed that ABCG2/BCRP1 mRNA was expressed in the liver and several non-hepatic tissues. At the protein level, ABCG2/BCRP1 has not been shown to be present in non-hepatic tissues except for the placenta, gastrointestinal tract, breast, and venous and capillary endothelial cells of various organs, but in the case of the liver, ABCG2/BCRP1 has been shown to be localized specifically on the bile canalicular membrane, 32 suggesting that ABCG2/BCRP1 may be involved in excretion processes, similar to many other ATP-binding cassette transporters. However, the function of ABCG2/BCRP1 expressed in the liver and non-hepatic tissues remains subject to further investigations.
In summary, our results show that oval cells express ABCG2/BCRP1, indicating that the SP phenotype may serve as a useful marker in the isolation of oval cells, and suggesting that oval cells may represent stem cells in the liver.
Acknowledgments
We thank Michiko Kakihana, Naoko Hamaue, Kunihisa Hamada, and Susumu Tanaka of Hyogo College of Medicine (Nishinomiya, Hyogo, Japan) and Hiroshi Yamazaki of Osaka University Graduate School of Medicine (Suita, Osaka, Japan) for technical assistance.
Footnotes
Address reprint requests to Tohru Tsujimura, M.D., Ph.D., Department of Pathology, Hyogo College of Medicine, 1–1, Mukogawa-cho, Nishinomiya, Hyogo 663-8501, Japan. E-mail: tohru@hyo-med.ac.jp.
Supported in part by grants from the Ministry of Education, Science, Sports, and Culture of Japan.
References
- 1.Steer CJ: Liver regeneration. EMBO J 1995, :1396-1400 [DOI] [PubMed] [Google Scholar]
- 2.Fausto N: Hepatocyte differentiation and liver progenitor cells. Curr Opin Cell Biol 1990, 2:1036-1042 [DOI] [PubMed] [Google Scholar]
- 3.Sell S: Is there a stem cell? Cancer Res 1990, 50:3811-3815 [PubMed] [Google Scholar]
- 4.Thorgeirsson SS: Hepatic stem cells in liver regeneration. EMBO J 1996, 10:1249-1256 [PubMed] [Google Scholar]
- 5.Grisham JW, Thorgeirsson SS: Liver stem cells. Potten CS eds. Stem Cells. 1997:pp 233-282 Academic Press, London
- 6.Evarts RP, Nagy P, Nakatsukasa H, Marsden E, Thorgeirsson SS: In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res 1989, 49:1541-1547 [PubMed] [Google Scholar]
- 7.Thorgeirsson SS, Evarts RP, Bisgaard HC, Fujio K, Hu Z: Hepatic stem cell compartment: activation and lineage commitment. Proc Soc Exp Biol Med 1993, 204:253-260 [DOI] [PubMed] [Google Scholar]
- 8.Sarraf C, Lalani EN, Golding M, Anilkumar TV, Poulsom R, Alison M: Cell behavior in the acetylaminofluorene-treated regenerating rat liver: light and electron microscopic observations. Am J Pathol 1994, 145:1114-1126 [PMC free article] [PubMed] [Google Scholar]
- 9.Matsusaka S, Tsujimura T, Toyosaka A, Nakasho K, Sugihara A, Okamoto E, Uematu K, Terada N: Role of c-kit receptor tyrosine kinase in development of oval cells in the rat 2-acetylaminofluorene/partial hepatectomy model. Hepatology 1999, 29:670-676 [DOI] [PubMed] [Google Scholar]
- 10.Lazaro CA, Rhim JA, Yamada Y, Fausto N: Generation of hepatocytes from oval cell precursors in culture. Cancer Res 1998, 58:5514-5522 [PubMed] [Google Scholar]
- 11.Fujio K, Evarts RP, Hu Z, Marsden ER, Thorgeirsson SS: Expression of stem cell factor and its receptor, c-kit, during liver regeneration from putative stem cells in adult rat. Lab Invest 1994, 704:511-516 [PubMed] [Google Scholar]
- 12.Omori N, Omori M, Evarts RP, Teramoto T, Miller MJ, Hoang TN, Thorgeirsson SS: Partial cloning of rat CD34 cDNA and expression during stem cell-dependent liver regeneration in the adult rat. Hepatology 1997, 26:720-727 [DOI] [PubMed] [Google Scholar]
- 13.Petersen BE, Goff JP, Greenberger JS, Michalopoulos GK: Hepatic oval cells express the hematopoietic stem cell marker Thy-1 in the rat. Hepatology 1998, 27:433-445 [DOI] [PubMed] [Google Scholar]
- 14.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC: Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 1996, 183:1797-1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC: Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 1999, 401:390-394 [DOI] [PubMed] [Google Scholar]
- 16.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA: Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest 2001, 107:1395-1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hulspas R, Quesenberry PJ: Characterization of neurosphere cell phenotypes by flow cytometry. Cytometry 2000, 40:245-250 [PubMed] [Google Scholar]
- 18.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP: The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 2001, 7:1028-1034 [DOI] [PubMed] [Google Scholar]
- 19.Zhou S, Morris JJ, Barnes Y, Lan L, Schuetz JD, Sorrentino BP: Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci USA 2002, 99:12339-12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solt D, Farber E: New principle for the analysis of chemical carcinogenesis. Nature 1976, 263:701-703 [Google Scholar]
- 21.Evarts RP, Nagy P, Marsden E, Thorgeirsson SS: A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis 1987, 8:1737-1740 [DOI] [PubMed] [Google Scholar]
- 22.Yasui O, Miura N, Terada K, Kawarada Y, Koyama K, Sugiyama T: Isolation of oval cells from Long-Evans Cinnamon rats and their transformation into hepatocytes in vivo in the rat liver. Hepatology 1997, 25:329-334 [DOI] [PubMed] [Google Scholar]
- 23.Tso JY, Sun XH, Kao TH, Reece KS, Wu R: Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res 1985, 13:2485-2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiojiri N, Lemire JM, Fausto N: Cell lineages and oval cell progenitors in rat liver development. Cancer Res 1991, 51:2611-2620 [PubMed] [Google Scholar]
- 25.Scharenberg CW, Harkey MA, Torok-Storb B: The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood 2002, 99:507-512 [DOI] [PubMed] [Google Scholar]
- 26.Kim M, Turnquist H, Jackson J, Sgagias M, Yan Y, Gong M, Dean M, Sharp JG, Cowan K: The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res 2002, 8:22-28 [PubMed] [Google Scholar]
- 27.Lechner A, Leech CA, Abraham EJ, Nolan AL, Habener JF: Nestin-positive progenitor cells derived from adult human pancreatic islets of Langerhans contain side population (SP) cells defined by expression of the ABCG2(BCRP1) ATP-binding cassette transporter. Biochem Biophys Res Commun 2002, 293:670-674 [DOI] [PubMed] [Google Scholar]
- 28.Allen JD, Brinkhuis RF, Wijnholds J, Schinkel AH: The mouse Bcrp1/Mxr/Abcp gene: amplification and overexpression in cell lines selected for resistance to topotecan, mitoxantrone, or doxorubicin. Cancer Res 1999, 59:4237-4241 [PubMed] [Google Scholar]
- 29.Komatani H, Kotani H, Hara Y, Nakagawa R, Matsumoto M, Arakawa H, Nishimura S: Identification of breast cancer resistant protein/mitoxantrone resistance/placenta-specific, ATP-binding cassette transporter as a transporter of NB-506 and J-107088, topoisomerase I inhibitors with an indolocarbazole structure Cancer Res 2001, 61:2827-2832 [PubMed] [Google Scholar]
- 30.Jonker JW, Smit JW, Brinkhuis RF, Maliepaard M, Beijnen JH, Schellens JHM, Schinkel AH: Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst 2000, 92:1651-1656 [DOI] [PubMed] [Google Scholar]
- 31.Robey RW, Medina-Perez WY, Nishiyama K, Lahusen T, Miyake K, Litman T, Senderowicz AM, Ross DD, Bates SE: Overexpression of the ATP-binding cassette half-transporter, ABCG2 (MXR/BCRP/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin Cancer Res 2001, 7:145-152 [PubMed] [Google Scholar]
- 32.Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg ACLM, Schinkel AH, van de Vijver MJ, Scheper RJ, Schellens JHM: Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res 2001, 61:3458-3464 [PubMed] [Google Scholar]