Abstract
Vascular endothelial growth factor-3 (VEGFR-3) plays a critical role in embryonic cardiovascular development and is thought to be expressed exclusively on the lymphatic endothelium, high endothelial venules, and rarely on adult vascular endothelium. Recent evidence also suggests expression of VEGFR-3 on some tumor-associated macrophages. We have studied the expression of VEGFR-3, its ligand VEGF-C and the co-receptor neuropilin-2, in normal and inflamed corneas and characterized the phenotype and distribution of VEGFR-3+ cells. Our data demonstrate, for the first time, the expression of VEGFR-3 on corneal dendritic cells (DC) and its up-regulation in inflammation. VEGFR-3+ DC are CD11c+CD45+CD11b+, and are mostly major histocompatibility (MHC) class II−CD80−CD86−, indicating immature DC of a monocytic lineage. During inflammation, there is rapid increase in the number of VEGFR-3+ DC in the cornea associated with heightened membranous expression as compared to a mostly intracellular expression in uninflamed tissue. VEGFR-3+ DC in normal corneas are VEGF-C−neuropilin-2−, but express VEGF-C in inflammation. Interestingly, similar cells are absent both in the normal and inflamed skin. These data demonstrate, for the first time, the expression of VEGFR-3 and VEGF-C on tissue DC, which implicate a novel potential relationship between lymphangiogenesis and leukocyte trafficking in the eye.
The development of blood vessels (angiogenesis) has been studied extensively, whereas the development of lymphatic vessels (lymphangiogenesis), despite its critical relevance, has gained relatively little attention until recently. Lymphangiogenesis is a key process in conditions such as lymphedema, lymphangiectasia, lymphangioma, lymphangiosarcoma, and cancer metastasis. 1-3 Over the last few years, molecules expressed specifically by lymphatic endothelial cells have been characterized, 4-8 and knowledge about the lymphatic system has started to accumulate considerably. 9-11
Vascular endothelial growth factor (VEGF) is a secreted polypeptide that was initially identified by its ability to increase permeability of the vasculature. 12 It also stimulates endothelial cell and monocyte migration, and promotes survival of the newly formed vessels. 13 VEGF belongs to the platelet-derived growth factor (PDGF)/VEGF family, which is a potent inducer of angiogenesis. 13,14 The VEGF receptor (VEGFR) family consists of three members: VEGFR-1 (Flt-1), VEGFR-2 (KDR/Flk-1), and VEGFR-3 (Flt-4). At least five ligands (VEGF-A, VEGF-B, VEGF-C, VEGF-D, and VEGF-E) and placenta growth factor (PIGF) bind to one or two of these receptors. VEGF-A stimulates vascular endothelial cells through both VEGFR-1 and VEGFR-2, 15 and is indispensable for development of blood vessels. 13 VEGF-B binds to only VEGFR-1 and may be involved in regulation of extracellular matrix degradation, cell adhesion, and migration. 16 VEGF-C and VEGF-D do not bind to VEGFR-1, although both are ligands for VEGFR-2 and VEGFR-3. 17-19 VEGF-E, which is a ligand for VEGFR-2, is expressed as a native protein in mammalian cells and possesses similar bioactivities as VEGF-A. 20 VEGF-C and VEGF-D, the only ligands for VEGFR-3, 17,21 are distinguished by their capacity to stimulate the growth of lymphatic vessels. VEGF-C stimulates lymphangiogenesis in the mature avian chorioallantoic membrane and in the skin of transgenic mice. 5,22 In embryos, VEGFR-3 is initially expressed in all vasculature, but, during development, its expression in blood vessels decreases and becomes restricted to the developing lymphatic vessels; therefore it is thought to be expressed almost exclusively by the lymphatic endothelium (LE) and is thus considered a major regulator in lymphangiogenesis. 4 Recently, neuropilins, which mediate axonal guidance during neuronal development by binding to semaphorins, 23,24 have been found to bind to and mediate signaling for some VEGF species. 25,26 Neuropilin-2 is expressed on a subset of lymphatic vessels and acts as a co-receptor for VEGF-C. 27
In the eye, VEGFR-3 has been detected in the retina, 28 and very recently on the ocular surface. 29-31 The cornea, which is normally devoid of blood and lymphatic vessels, can be vascularized by a number of inflammatory stimuli such as surgical invasion, infection, contact lens wear, or burns, through penetration of new vessels from the neighboring conjunctiva which has a luxurious supply of both blood and lymphatic vessels. 32 From an immunological standpoint, the induction of lymphatic vessels into the cornea facilitates the delivery of antigen presenting cells (APC) to draining lymph nodes (LN) which can contribute to immunogenic inflammation such as rejection of corneal grafts. 33-35 However, despite the critical function of lymphatics in ocular immunity, little is known about the mechanisms by which leukocytes interface with afferent lymphatics. The presence of lymphatic vessels was shown in vascularized corneas over 30 years ago, 32,36-38 and has recently been confirmed through immunohistochemical studies with specific markers, including VEGFR-3, in vascularized human corneas and the conjunctiva. 29,30 Very recently, we and others have demonstrated that monocytic cells in the normal and inflamed conjunctiva, 29 and tumor-associated macrophages (TAM) associated with skin tumors express this receptor, 39,40 suggesting that VEGFR-3 expression is not limited exclusively to the vascular endothelium as previously thought. These VEGFR-3+ monocytes or macrophages are present, however, in vascularized tissues and are thought to play a role in lymphangiogenesis in tissues that unlike the cornea have a significant endowment of vascular endothelial cells. Very recently we have reported the novel presence of a heterogeneous population of APC in the normal cornea that has profoundly revised the paradigm of corneal DC recruitment and function. 41-44 In the epithelium, we have characterized a population of resident major histocompatibility (MHC) class II-negative Langerhans cells (LC) in the corneal center that become activated after inflammation. 41 Another lab has recently identified a population of resident macrophages in the normal stroma, 45 while we have demonstrated that bone marrow-derived cells in the stroma consist of several subsets: a largely immature population of resident myeloid (CD8α−) dendritic (CD45+CD11c+) cells (DC) in the anterior stroma, and a smaller population of monocytic (CD11b+CD11c−) macrophages in the posterior stroma, 42-44 and have reported their maturation during inflammation. 43,44 We have established that these DC are able to gain access to lymphatics and to migrate to draining LN, and stimulate T cells to corneal alloantigens. 46-48 We report herein, for the first time, that DC residing in the normal uninflamed cornea express VEGFR-3 and that the expression of this receptor, and its ligand VEGF-C, increase substantially in inflammation.
Materials and Methods
Animals
Seven to 12-week-old male BALB/c, C57BL/6, and C3H mice (Taconic Farms, Germantown, NY or from our own breeding facility) were used in these experiments. Most experiments were performed on BALB/c mice and experiments on other strains were performed only when noted. All protocols were approved by the Schepens Eye Research Institute Animal Care and Use Committee, and all animals were treated according to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. For each antibody staining study in the cornea, unless specified otherwise, three to five corneas were examined. For skin experiments, multiple sections derived from the skin of three mice were used per each double-staining study. All studies were repeated at least twice for confirmation.
Cauterization of Corneal Surface
Application of electric cautery to the ocular surface is a standard method of inducing corneal inflammation without associated neovascularization. 49-51 Mice were deeply anesthetized with an intraperitoneal injection of 3 to 4 mg ketamine and 0.1 mg xylazine and placed under the operating microscope. Using the tip of a hand-held thermal cautery (Aaron Medical Industries Inc., St. Petersburg, FL), five light burns were applied to the central 50% of the cornea as an experimental model for corneal inflammation as previously described, 51 followed by application of antibiotic ophthalmic ointment. Corneas were excised at 3, 7, and 14 days after cautery application and assessed in immunohistochemical studies as described below.
Induction of Inflammation in Skin
Application of 2,4-dinitro-1-fluorobenzene (DNFB) (0.2% dissolved in acetone:olive oil (4:1)) is a standard method of inducing inflammation in skin and was performed as described previously. 52-54 In brief, DNFB (Sigma Chemical Co., St. Louis, MO) was applied epicutaneously to shaved abdominal skin (50 μl). This induced cutaneous inflammation at the application site within 48 hours. The skin was excised after 48 hours and assessed in immunohistochemical studies as described below.
Antibodies
The following antibodies (Abs) were used: purified rabbit anti-mouse FLT-4 (VEGFR-3), purified rabbit anti-human FLT-4, purified goat anti-mouse VEGF-C and purified rabbit anti-mouse neuropilin-2 (Santa Cruz Biotechnology, Santa Cruz, CA); purified rat anti-mouse VEGFR-3 (a kind gift from Dr. Hajime Kubo, University of Helsinki, Helsinki, Finland); FITC-conjugated rat anti-mouse DEC-205 (Cedarlane Laboratories Limited, Ontario, Canada); purified rabbit anti-mouse LYVE-1 (a kind gift from Dr. David G. Jackson, Institute of Molecular Medicine Oxford, Oxford, UK); FITC-conjugated hamster anti-mouse CD3 (T cell marker), FITC-conjugated rat anti-mouse CD14 (immature myeloid marker), FITC-conjugated rat anti-mouse CD11b (monocyte/macrophage marker); purified (immunohistochemistry) and PE-conjugated (flow cytometry) hamster anti-mouse CD11c (dendritic cell marker); purified rat anti-mouse CD45 (leukocyte common marker); FITC-conjugated hamster anti-mouse CD80 (costimulatory molecule; B7–1); PE-conjugated rat anti-mouse CD86 (costimulatory molecule; B7–2); FITC-conjugated rat anti-mouse GR-1 (neutrophil marker); FITC-conjugated rat anti-mouse CD8α (lymphoid DC marker); FITC-conjugated mouse anti-mouse IAd (major histocompatibility (MHC) class II) and FITC-conjugated mouse anti-human HLA-DR, DP, DQ. Secondary Abs were rhodamine-conjugated goat anti-rat IgG, rhodamine-conjugated donkey anti-rabbit IgG, rhodamine-conjugated goat anti-rabbit IgG, rhodamine-conjugated donkey anti-goat IgG, FITC-conjugated donkey anti-rabbit IgG, FITC-conjugated goat anti-rabbit IgG, FITC-conjugated donkey anti-goat IgG (Santa Cruz) and Cy5-conjugated goat anti-hamster IgG. Isotype-matched control Abs were FITC-conjugated mouse IgG3, FITC-conjugated rat IgG1, FITC-conjugated rat IgG2a, FITC-conjugated rat IgG2b, FITC-conjugated hamster IgG, FITC-conjugated mouse IgG2a, PE-conjugated rat IgG2a, purified hamster IgG, purified rat IgG2b, purified goat IgG, and purified rabbit IgG. All primary and secondary Abs (except where noted) and isotype matched controls were purchased from BD PharMingen (San Diego, CA). Anti-Flt-4 blocking peptide was obtained from Santa Cruz. A green-fluorescent dye for nuclear acids, YOYO-1 (DNA staining), was a kind gift from the Ksander Laboratory at the Schepens Eye Research Institute, Boston.
Immunohistochemical Studies
Corneal and limbal (intervening area between cornea and conjunctiva) tissue were excised, immersed in phosphate-buffered saline (PBS) and used as whole-mounts; skin was excised from the ear and abdomen of mice and 8-μm frozen sections were prepared. Twenty-μm horizontal sections of human eye bank corneas were also prepared. The tissues were fixed in acetone for 15 minutes at room temperature (RT) or used unfixed for staining where noted. Whole-mount corneas or tissue sections were then incubated in 2% bovine serum albumin (BSA) diluted in PBS (PBS-BSA) for 15 minutes. To block non-specific staining, whole-mounts or sections were blocked with anti-FcR mAb (CD16/CD32) for 30 minutes before they were immunostained with primary antibodies or isotype-matched control antibodies for 2 hours. Afterward, a second FITC- or PE-conjugated primary antibody, or secondary antibodies were added and incubated for 60 minutes (all diluted for optimal concentrations in PBS-BSA). All staining procedures were performed at room temperature. Whole-mounts or sections were covered with mounting medium (Vector, Burlingame, CA) and examined by a confocal microscope (Leica TCS 4D, Heidelberg, Germany). The YOYO-1 anti-nuclear dye, was added just before covering slides with mounting medium. To ensure specificity, negative controls were performed by omitting the primary antibody, or by using irrelevant primary antibodies of the same isotype or by incubating VEGFR-3 Ab with different concentrations of its blocking peptide for 2 hours at room temperature before staining. At least three to five different corneas were examined per each double staining experiment; representative data are presented below. All studies were repeated at least twice for confirmation.
Corneal Stroma Culture and Flow Cytometry
Corneal DC were procured using our standard protocol as previously described. 42,46 In brief, corneal buttons were excised and placed into a 6-well plate with 10 buttons per well after the epithelium was removed with a forceps. Buttons were cultured in 2.5 ml of RPMI-1640 medium with 10% FBS (Hyclone, Salt Lake City, UT), 10 mmol/L HEPES, 0.1 mmol/L nonessential amino acid, 100 units/ml of penicillin, 100 μg/ml of streptomycin (BioWhittaker, Walkersville, MD) and 1 × 10 −5M 2-mercaptoethonol (Sigma) and incubated at 37°C for 3 days. The non-adherent (dendritic) cells were isolated by centrifuging the culture supernatant, resuspending the cells in PBS and washing them once in PBS. The final suspension of non-adherent cells was filtered through a 70-μm nylon cell strainer to remove corneal fragments and then washed with cold PBS and counted. Non-adherent cells were blocked by anti-FcR mAb (CD16/CD32) before cells were labeled with PE-conjugated hamster anti-mouse CD11c (BD PharMingen), the above mentioned anti-VEGFR-3 antibody (Santa Cruz), and its secondary antibody (FITC-conjugated donkey anti-rabbit). For the isotype control, the cells were labeled with PE-conjugated hamster anti-mouse CD11c and rabbit IgG instead of VEGFR-3, using the same secondary antibody. Cells were washed and analyzed using an Epics XL flow cytometer (Coulter, Miami, FL). The analysis was done by gating on CD11c-positive cells using appropriate isotype and cell culture controls to adjust color compensation and gating parameters. Non-adherent cells of parallel spleen cell cultures were also used to evaluate relative VEGFR-3 expression. The splenic cultures were established with initially adherent cells from naive BALB/c mice incubated in culture for 90 minutes, washed, and then 2.5 ml of 10% FBS RPMI-1640 medium was added to the cultures. The non-adherent cells were collected as described above and the harvested cells were treated identically as cells derived from corneal explants. Cultures were incubated for 7 days at 37°C.
Results
VEGFR-3 Expression on Dendritiform Non-Endothelial Cells in the Normal Corneal Stroma
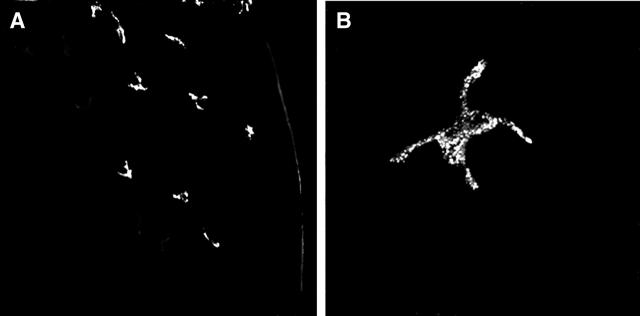
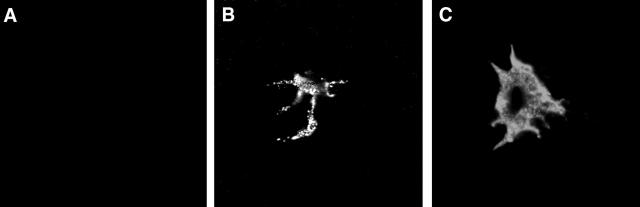
To screen for VEGFR-3 expression in the normal non-vascularized cornea, whole-mounts were initially single-stained with anti-VEGFR-3 Ab. Immunofluorescence studies showed expression of VEGFR-3 on cells in the peripheral cornea and limbus but not the center of the cornea (Figure 1A) ▶ . These cells were located in the stroma and had a dendritiform shape (Figure 1B) ▶ . Stainings with secondary antibody alone, or with isotype control instead of VEGFR-3 primary antibody, were negative. To further confirm that the results were not due to non-specific staining, a specific blocking peptide was used; VEGFR-3 staining was found to be specific since increasing concentrations of the blocking peptide abrogated all staining for VEGFR-3 (data not shown). To alleviate any lingering doubt that VEGFR-3 staining is associated with cellular structure, we double-stained corneas with VEGFR-3 and YOYO-1 (which stains all cell nuclei). Confocal microscopy of corneas showed VEGFR-3+ dendritiform nucleated cells in the stroma, interspersed between cells that were not stained with VEGFR-3 (Figure 2) ▶ , most probably representing stromal keratocytes. Our results with the VEGFR-3 antibody from Santa Cruz were compared with a monoclonal antibody to VEGFR-3 that was used in prior publications 55 and showed identical results (data not shown).
Figure 1.
VEGFR-3 expression on cells in the periphery of the corneal stroma. Confocal micrographs demonstrate VEGFR-3+ dendritic shaped cellular structures in the periphery (right) of the cornea, while the central areas (left) do not demonstrate such cells (A). Higher magnification, confirming the dendritic morphology of VEGFR-3+ cells in the cornea (B). Magnification, (A) ×200, (B) ×1000.
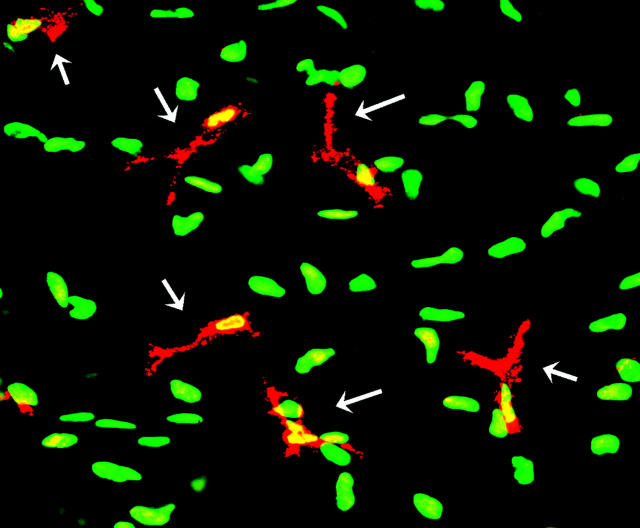
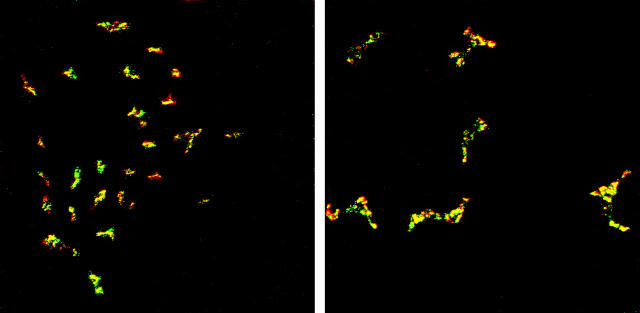
Figure 2.
VEGFR-3 expression by dendritiform cells in the corneal stroma. Micrograph of double-stained cornea staining VEGFR-3 (red) and YOYO-1 (green) expression. Significant numbers of dendritic VEGFR-3+ cells (arrows) are present in the corneal stroma; these cells are nucleated (yellow) and are present amid VEGFR-3− cells (green). Magnification, ×400.
VEGFR-3+ Cells in the Corneal Stroma Represent Bone Marrow-Derived Dendritic Cells
The normal uninflamed corneal stroma is endowed with resident bone marrow-derived dendritic cells and macrophages, in addition to keratocytes, as recently shown. 42-46 We therefore used a number of antibodies to phenotype the VEGFR-3+ cells and to specify which of the stromal cells express this receptor. Double-staining for VEGFR-3 and CD45 (pan-leukocyte marker) demonstrated that VEGFR-3+ cells were uniformly CD45+ (Figure 3A) ▶ ; therefore bone marrow-derived, and not keratocytes. When staining was performed for VEGFR-3 and CD11c (dendritic cell maker), 56-60 we found VEGFR-3+ cells being mostly CD11c+ (Figure 3B) ▶ , and were located almost exclusively in the anterior stroma, indicating that the recently identified resident corneal DC express this receptor. VEGFR-3+ DC (CD45+CD11c+) were located exclusively in the peripheral cornea and the limbus, while resident DC in the paracentral and central areas of the stroma did not express VEGFR-3 (Figure 3B) ▶ . In addition, we double-stained for VEGFR-3 and CD11b (monocyte/macrophage marker). Results demonstrated that VEGFR-3+ cells were uniformly CD11b+, in accord with our previous findings that corneal DC are CD11b+ and of a monocytic lineage. 42-44 Moreover, to confirm the lineage of these DC, we performed double-staining experiments for VEGFR-3 and DEC-205 or CD14.VEGFR-3+ DC were uniformly negative for both DEC-205 and CD14 (data not shown). The negative expression of DEC-205 confirmed the myeloid monocytic lineage of stromal DC since DEC-205 is a reliable marker for lymphoid or plasmacytoid, but not myeloid DC. 61-67 However, a very small population of VEGFR-3+CD11b+CD11c− cells in the posterior stroma, representing monocytes/macrophages was also identified. VEGFR-3+ cells were further uniformly negative for GR-1 (neutrophil marker), CD3 (T cell marker) and CD8α (lymphoid DC marker). Studies in C57BL/6 and C3H mice demonstrated presence of similar VEGFR-3+ cells in the corneal stroma (data not shown).
Figure 3.
Myeloid dendritic cells in the corneal stroma express VEGFR-3. Whole-mounted corneas were double-stained with CD45 (green) and VEGFR-3 (red). Confocal micrograph of the anterior stroma shows VEGFR-3+ dendritic cells being CD45+ (yellow) (A). CD11c (red) expression of these VEGFR-3+ (green) cells provides evidence that they are dendritic cells, and that VEGFR-3 expression is limited to the periphery of the cornea (right), while corneal DC in central areas (red) are VEGFR-3− (lower left) (B). Additionally, CD11b expression (green) of VEGFR-3+ cells indicates the monocytic lineage of these cells (C). Magnification, (A and C) ×400. (B) ×160.
Our in vivo results showed that at least two populations of partially differentiated resident bone marrow-derived cells reside in the corneal stroma: dendritic cells and macrophages. Both cell types are known to migrate from tissue explants. 68 While dendritic cells or LC are known to be non-adherent in culture and to float into the supernatant, macrophages are known to adhere to plastic. 68 To further confirm the in situ results, normal corneas were excised, stripped of their epithelium to deplete LC, and placed in culture, using our standard protocol. 42,46 The non-adherent dendritic cells were harvested, and subjected to two-color staining (CD11c and VEGFR-3) for flow cytometric analysis. Similarly derived non-adherent spleen cells were used also. Eighty to 85% of CD11c+ corneal DC expressed VEGFR-3 after 3 days in culture (data not shown), similar to these cells’ acquisition of MHC class II expression in culture. 46 Non-adherent spleen cells, in contrast, did not express any VEGFR-3.
Maturational State and Distribution of VEGFR-3+ DC in Normal Versus Inflamed Corneas
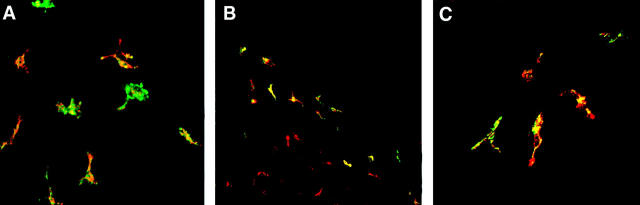
To characterize the maturational state and distribution of VEGFR-3+ stromal DC, in normal versus inflamed corneas, we performed double-staining of corneas for VEGFR-3 and MHC class II or B7 costimulatory markers CD80 (B7–1) and CD86 (B7–2). Double-staining for MHC class II and VEGFR-3 demonstrated that the majority of VEGFR-3+ cells were MHC class II-negative (Figure 4A) ▶ . As early as 72 hours after induction of inflammation, VEGFR-3+ DC were present in the central areas of the cornea and increased in numbers through day 14 when these cells could be located throughout the cornea (Figure 4B) ▶ . However, even during inflammation, the majority of VEGFR-3+ DC remained MHC class II-negative (Figure 4B) ▶ . When normal uninflamed corneas were double-stained for VEGFR-3 and B7 costimulatory markers, VEGFR-3+ DC were found to be mostly CD80− (Figure 4C) ▶ and CD86− (data not shown). During inflammation, similar to class II expression, a majority of VEGFR-3+ cells in the corneal center remained CD80− (Figure 4D) ▶ and CD86− (data not shown). Double staining for CD11c and VEGFR-3 in inflamed corneas confirmed that most VEGFR-3+ cells in inflamed corneas were CD11c+ DC, but that they were now present in both the periphery and center of the corneal stroma (Figure 4E) ▶ .
Figure 4.

Immature VEGFR-3+ DC are present throughout the inflamed cornea. Stacked optical sections of the anterior stroma show that the majority of VEGFR-3+ DC (red) do not express MHC class II (green) in normal corneas, and are exclusively located in the peripheral cornea (A). During inflammation, VEGFR-3+ DC (red) are also present in the corneal center as early as 72 hours after induction of inflammation, but are mostly negative for MHC class II (green) expression (B). Corneas double-stained with VEGFR-3 (red) and CD80 (green) show that VEGFR-3+ cells are mostly CD80− (C). After induction of inflammation, large numbers of VEGFR-3+ cells are also present in the corneal center (red), but generally do not co-express the maturation marker CD80 (green) (D). Double staining with CD11c (red) and VEGFR-3 (green) in inflamed corneas shows that the VEGFR-3 expression is present both in the periphery (upper right) and the center (lower left) of the cornea and that most DC now express VEGFR-3 (yellow) (E). Double-staining of human eye bank corneal sections with VEGFR-3 (red) and HLA-DR (green) confirms the presence of VEGFR-3 on stromal cells in the human cornea (F). Magnification, (A, B, E) ×160, (C, D, F) ×400.
VEGFR-3 Expression in Human Corneas
To investigate whether our findings in the murine cornea also applied to the human cornea and might be relevant in clinical settings, horizontal sections through human eye bank corneas were double-stained for HLA-DR and VEGFR-3 and analyzed as whole-mounts using confocal microscopy. The results showed that the human cornea, like the murine cornea, is endowed with VEGFR-3+ cells (Figure 4F) ▶ . These cells were present exclusively in the peripheral stroma, and nearly half were HLA-DR negative.
VEGFR-3 Expression in Normal and Inflamed Murine Skin
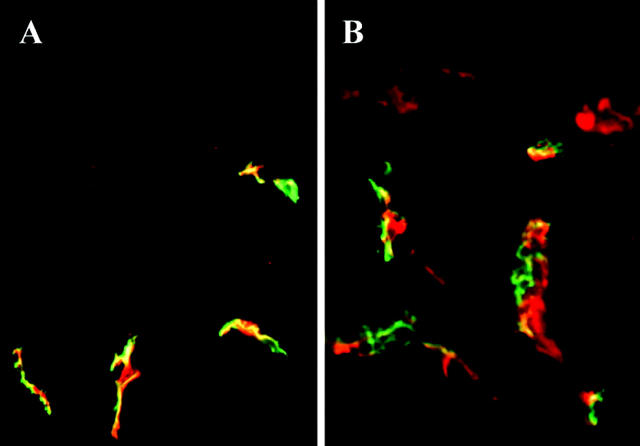
Finally, since the cornea shares embryological origins with the skin, in that it is partially ectodermally derived, and both tissues are richly endowed with DC, we evaluated the skin for VEGFR-3 expression. Initial immunofluorescence studies of skin double-stained with VEGFR-3 and CD11c showed no co-staining (data not shown). VEGFR-3 in the skin was limited to the lymphatic endothelium and skin leukocytes were uniformly VEGFR-3 negative, confirming previous data. 4 To confirm the findings that VEGFR-3 expression was limited to the lymphatic endothelium, we double-stained skin sections with VEGFR-3 and LYVE-1, a specific marker for lymphatic endothelium. 8 Results showed double-staining of lymphatic endothelium in the skin for both antibodies (Figure 5A) ▶ , while leukocytes, including DC, did not stain for either antibody. Since VEGFR-3+ DCs were found in increased numbers in inflamed corneas, we next evaluated inflamed skin to exclude the possibility that skin DC express VEGFR-3 in inflammation. However, in contrast to the cornea, where inflammation increases VEGFR-3 expression by DC, VEGFR-3 expression was still limited to lymphatic endothelium that co-stained with LYVE-1 (Figure 5B) ▶ . Our results with the VEGFR-3 antibody from Santa Cruz were compared with a monoclonal antibody to VEGFR-3 that was used in prior publications 55 and showed identical results (data not shown).
Figure 5.
VEGFR-3 expression is limited to lymphatic endothelium in normal and inflamed skin and absent on skin DC. To determine whether VEGFR-3 is also expressed on DC in the skin, we examined normal skin tissue sections that were double-stained for VEGFR-3 (red) and LYVE-1 (green) (A). VEGFR-3 was present and co-stained with LYVE-1 on lymphatic vessels (yellow), but was not expressed by leukocytes, including DC. Similar stainings with VEGFR-3 (red) and LYVE-1 (green) in inflamed skin (B) did not induce novel expression of VEGFR-3 on DC. The expression of VEGFR-3 and LYVE-1 was limited to lymphatic endothelium (yellow) that co-stained for both antibodies. Magnification, (A and B) ×200 (Epidermis is on the top).
VEGFR-3 Staining in Corneal DC Is Intracellular in Normal Corneas and Is Expressed on the Surface in Inflamed Corneas
Because the data presented above were procured from acetone-fixed tissue, it was not clear to us whether the VEGFR-3 expression on corneal DC was intracellular or on the cell surface. Accordingly, we stained unfixed corneas with VEGFR-3 to prevent penetration of the antibody into the cytoplasm. Unfixed whole-mount corneas stained with VEGFR-3 failed to demonstrate its expression (Figure 6A) ▶ , suggesting that corneal DC did not express this receptor on their surface. To confirm this, we stained fixed whole-mount corneas with VEGFR-3 and performed serial confocal sections through single VEGFR-3+ cells. Our results with acetone fixation, that renders the cellular membrane permeable to antibodies, showed VEGFR-3 staining, but serial confocal sections showed granular staining pattern confined to the cytoplasm (Figure 6B) ▶ . Moreover, when we stained unfixed inflamed corneas, we detected membranous staining of VEGFR-3 (Figure 6C) ▶ , indicating that inflammation up-regulates the expression of this receptor on the cell surface.
Figure 6.
VEGFR-3 staining is intracellular in uninflamed corneas, but membranous in inflamed corneas. To localize the cellular expression of VEGFR-3, we stained unfixed whole-mount corneas with VEGFR-3. Unfixed tissue failed to express VEGFR-3 (A). Similar staining of acetone-fixed corneal whole-mounts, however, showed granular cytoplasmic staining as shown with serial confocal sections of a single cell (B). Staining of unfixed inflamed corneas demonstrated VEGFR-3 expression on the cell surface (C). Magnification, (A–C) ×1000.
VEGFR-3+ DC Co-Express VEGF-C during Inflammation
We double-stained normal and inflamed corneas for VEGFR-3 and VEGF-C expression. Immunofluorescense studies of whole-mount uninflamed corneas demonstrated no VEGF-C expression in the normal cornea (data not shown). This finding is in accord with previous published data showing the absence of VEGF-C expression in normal corneas. 30,31,69 However, VEGF-C expression was detectable as early as 72 hours after induction of inflammation, both in the periphery (Figure 7A) ▶ and in the center of the cornea on DC (Figure 7B) ▶ . VEGFR-3+ DC uniformly co-expressed VEGF-C and serial confocal sections through these cells showed that VEGF-C expression was not limited to the membrane alone but was also expressed in a similar pattern as VEGFR-3 in the cytoplasm.
Figure 7.
VEGFR-3+ DC uniformly co-express VEGF-C during inflammation. Staining of inflamed corneal tissue with VEGFR-3 (green) and VEGF-C (red) demonstrates that VEGFR-3+ DC uniformly co-express the ligand VEGF-C both in the periphery (A) and the center (B) of corneas. Magnification, (A) ×160, (B) ×400.
Neuropilin-2 Is Not Expressed by Corneal or Skin DC and Lymphatic Endothelium
We stained normal corneal whole-mounts for neuropilin-2, a co-receptor for VEGF-C, and VEGFR-3. Results demonstrated that corneas uniformly did not express neuropilin (data not shown), and DC in the periphery stained solely for VEGFR-3. Further, we investigated the expression of neuropilin-2 in inflamed corneas. Although the VEGFR-3 expression increased in corneal DC, neuropilin-2 was not expressed in the corneal tissue during inflammation. Lastly, we double-stained skin sections with VEGFR-3 and neuropilin-2. VEGFR-3+ vessels were negative for neuropilin-2, confirming previous data. 27 However, there was positive staining for neuropilin-2 in the proximity of hair shafts in the skin epidermis, which did not express VEGFR-3. Hence, neither skin nor corneal DC expressed neuropilin-2.
Discussion
The present study demonstrates, for the first time, that VEGFR-3 which has been described to be predominantly a lymphatic endothelial marker in adult tissues and tumors, is also expressed by corneal dendritic cells. Whereas the significance of VEGFR-3 and VEGF-C in lymphangiogenesis and tumors has been extensively investigated in recent years (see reviews 9-11,70-77 ), their expression on non-endothelial cells is just starting to evolve. VEGFR-3 was initially reported as the first specific LE marker in adult tissues. 4 In adults, VEGFR-3 was later also found to be expressed in a subset of capillary endothelia, although it is absent in all large blood vessels. 78 Wilting et al 79 were the first group to observe the expression of VEGFR-3 on non-endothelial cells in the cornea and podocytes of kidney glomeruli of 4-day-old quail embryos, but did not identify the cellular origin of these cells. More recently, Mimura et al, 31 Cursiefen et al, 30 and our group 29 have reported on VEGFR-3 expression by non-endothelial cells in the ocular surface. Mimura et al 31 initially detected VEGFR-3 and VEGF-C gene expression in neovascularized rat corneas with RT-PCR. They also reported VEGF-C expression on infiltrating (not resident) “inflammatory” cells. Later, Cursiefen et al 30 demonstrated the expression of VEGFR-3 and VEGF-C on LE in neovascularized human corneas with immunofluorescence microscopy and reported expression of VEGF-C and VEGFR-3 on unidentified “inflammatory” cells in neovascularized, but not normal, corneas; however these cells were not phenotyped. Very recently, we have characterized VEGFR-3+ non-endothelial cells in the normal and inflamed conjunctiva as monocytic bone marrow-derived cells. 29 Interestingly, VEGFR-3 expression has also been demonstrated on CD11b+ cells (presumably tumor-associated macrophages) in the skin adjacent to melanomas, but not in the normal skin. 40 To exclude the possibility that inflamed skin may harbor DC that express VEGFR-3, we evaluated both normal and inflamed skin tissue, but could not detect VEGFR-3 expression on any leukocytes, including DC. Moreover, the expression of VEGFR-3 on lymphatic endothelium was confirmed by co-staining with LYVE-1. In addition, expression of VEGF-C, VEGF-D, and VEGFR-3 on tumor-associated macrophages has also been reported in human cervical cancer. 39 Finally, VEGFR-3 expression has been demonstrated on blood monocytes and on immature DC derived from blood monocytes in vitro. 39,80 Given that the non-endothelial VEGFR-3+ cells in our study collectively exhibited identical expression (or lack of expression) of multiple cell surface markers (CD45+, CD11c+ CD11b+, GR-1−, CD3−, CD8α−, DEC-205−, and CD14−), we believe that they are monocytic bone marrow-derived myeloid DC that are identified as CD11c+CD11b+CD8a−DEC-205−. 61-67 To our knowledge, we are the first group to report the expression of VEGFR-3 and VEGF-C on resident DC in normal as well as inflamed tissues in vivo. Our findings are not strain-specific and preliminary experiments in human eyes confirm the presence of such VEGFR-3+ cells in the human eye. We caution, however, that we have not phenotyped VEGFR-3+ human ocular cells as thoroughly as we have in the mouse, since detailed phenotyping in the human cornea requires extensive experimentation with freshly procured tissues from healthy donors, a difficult task that has not been completed by us given that normally available eye bank eyes for research purposes have been retained in medium for some time before their release, and DC are known to migrate out of tissues into the medium. 68
Our novel data presented herein indicate that VEGFR-3 and VEGF-C are, in addition to their expression on vessels during lymphangiogenesis, cancer metastasis, and tumors and normal tissues as previously described, 9-11,70 also expressed on non-lymphoid tissue dendritic cells. Further, we show that after induction of inflammation the number of VEGFR-3+ corneal DC increases significantly and that their presence is not limited to the peripheral cornea, but rather extends throughout the corneal stroma, while similar findings could not be reproduced in inflamed skin. The absence of MHC class II and B7 costimulatory molecules on most of these cells indicates that they are predominantly immature, and that the up-regulation of VEGFR-3 and VEGF-C expression in inflammation by these cells may reflect a pathway independent from that which regulates acquisition of MHC class II expression, since corneal DC are known to rapidly up-regulate MHC class II expression in inflammation. 46 The absence of VEGF-C in normal corneas, using our antibody, corresponds with previous findings showing no expression of VEGF-C in normal corneas. 30,31,69 Further, our finding that resident corneal DC express VEGF-C in inflammation is novel, and represents a feature previously related to uncharacterized invading “inflammatory” cells in the cornea. 30,31 In fact, we have preliminary evidence (based on collaboration with Claus Cursiefen and Wayne Streilein), based on RT-PCR and Western blotting that corneal DC express both the VEGF-C gene and protein in inflamed, but not normal, corneas. Neuropilin-2 that has been recently identified as a VEGF-C co-receptor, 27 has been suggested to be required for the formation of small lymphatic vessels and capillaries. 81 However, neuropilin-2 was not expressed on corneal DC or skin lymphatic endothelium. Previously, neuropilin-2 has been detected as a VEGF-C co-receptor in intestinal lymphatic vessels, but not in the skin. 27 In the aggregate, our findings complement the published data, suggesting that there are tissue-specific differences in VEGF-C-mediated signaling.
VEGF-C and VEGF-D induce migration of endothelial cells 5,17,21,82,83 and Kaposi sarcoma cells 84 by ligating VEGFR-3. Recently VEGF-C has also been shown to be chemotactic for tumor-associated macrophages through VEGFR-3. 40 Interestingly, the cytokines interleukin-1 (α and β) and tumor necrosis factor-α, 85 as well as transforming growth factor-β (TGF-β), 86 all amply expressed in corneal inflammation, 87 are known to up-regulate VEGF-C. The up-regulation of VEGF-C through proinflammatory cytokines could explain the expression of VEGF-C on stromal DC in inflammation, and their absence in normal tissue, and could provide a molecular basis for the relationship between lymphangiogenesis and inflammation, similar to what has been described for angiogenesis and inflammation through VEGFR-1. 88 Blockade of VEGFR-3 signaling has shown to inhibit lymphangiogenesis in the mouse cornea. 69 Presence of VEGF-C alone however, is apparently not always sufficient to induce the formation of functional lymphatic vessels, since in the cautery model used herein we observe corneal stromal inflammation without accompanying induction of new vessels.
The lymphatic system which serves as an important pathway for APC trafficking is a critical component of the immune system, and the critical relevance of the eye-lymphatic axis in the induction of corneal immunity has been shown recently by Yamagami and Dana. 34,35 Very recently we have shown that the normal cornea, in contrast to longstanding dogma, is indeed endowed with a heterogenous population of APC, including DC, that are able to migrate to draining LN after corneal transplantation. 41,42,44,46 After induction of inflammation, these resident DC are able to undergo maturation and up-regulate expression of MHC class II, B7 costimulatory markers (CD80, CD86), and CD40, as they acquire T cell stimulatory capacity. 43,44 The total numbers of corneal DC also increase in inflammation as the resident population is supplemented by newly recruited DC that infiltrate the cornea from the surrounding conjunctival and limbal vasculature. 43,44 Interestingly, however, the trafficking mechanisms of ocular DC to the draining LN remain so far unknown, but the present findings, together with the fact that VEGF-C is chemotactic for VEGFR-3+ cells, suggest a potential role for signaling through VEGFR-3 in ocular surface APC trafficking. In fact, it is tempting to speculate that our data may hint at a potential link between lymphangiogenesis and immunity. This model would propose that increased secretion of proinflammatory cytokines during inflammation leads to increased expression of VEGFR-3 and VEGF-C, with signaling through VEGFR-3 leading to concurrent lymphangiogenesis and DC recruitment. VEGF-C could also promote the molecular interactions of APC with LE cells, thereby facilitating APC entry into the lymphatics, similar to the promotion of the metastatic spread of tumor cells via lymphatics as shown recently. 2,3 Supporting this hypothesis is the fact that VEGF-A is chemotactic for monocytes, which have been shown capable of expressing VEGFR-1. 89 The expression of VEGFR-3 on blood monocytes and tumor-associated macrophages, and the lack of expression of VEGFR-3 on skin leukocytes, however, suggest that the function of VEGFR-3 ligation, apart from inducing lymphangiogenesis, varies from tissue to tissue. Since the cornea is normally devoid of blood or lymph vessels, and yet corneal DC need to perform their critical function as “sentinels” of the immune system, it is indeed possible that these DC have acquired unique mechanisms to allow their egress from the ocular surface to lymphoid reservoirs.
Taken together, these studies contribute to the emerging picture that in addition to its role in lymphangiogenesis, VEGF-C and VEGFR-3 expression may be involved in other processes including immunity. Targeting this pathway by selective targeting of VEGFR-3-ligand interactions in the eye may provide a novel mechanism for regulating antigen-presenting cell trafficking in the eye.
Acknowledgments
We thank our colleagues at the Schepens Eye Research Institute: Drs. Nancy Joyce and Wayne Streilein for helpful discussions, Mr. Don Pottle of the Confocal Microscopy Unit for excellent technical assistance. In addition, we thank Dr. David Jackson, Institute of Molecular Medicine Oxford, Oxford, UK for provision of LYVE-1 antibodies and Hajime Kubo, University of Helsinki, Helsinki, Finland for provision of VEGFR-3 antibodies.
Footnotes
Address reprint requests to Dr. Hamrah, Department of Ophthalmology & Visual Sciences, University of Louisville, 301 E. Mohammed Ali Blvd., Louisville, KY 40202. E-mail: pedram.hamrah@louisville.edu.
Supported by grant EY-12963 from the National Institutes of Health, a research grant from the Massachusetts Lions Eye Research Fund, and a Special Scholar Award from Research to Prevent Blindness (M.R.D.).
Present address for P.H. is the Department of Ophthalmology and Visual Sciences, University of Louisville, The Kentucky Lions Eye Center, 301 E. Muhammad Ali Blvd., Louisville, KY 40202.
References
- 1.Witte MH, Witte CL: Lymphangiogenesis and lymphologic syndromes. Lymphology 1986, 19:21-28 [PubMed] [Google Scholar]
- 2.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG: VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 2001, 7:186-191 [DOI] [PubMed] [Google Scholar]
- 3.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M: Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 2001, 7:192-198 [DOI] [PubMed] [Google Scholar]
- 4.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VWM, Fang GH, Dumont DJ, Breitman M, Alitalo K: Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA 1995, 92:3566-3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh SJ, Jeltsch MM, Birkenhäger R, McCarthy JEG, Weich HA, Christ B, Alitalo K, Wilting J: VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev Biol 1997, 188:96-109 [DOI] [PubMed] [Google Scholar]
- 6.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D: Angiosarcoma express mixed endothelial phenotype of blood lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 1999, 154:385-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG: LYVE-1, a new homologue of the CD44 glycoprotein is a lymph-specific receptor for hyaluronan. J Cell Biol 1999, 144:789-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prevo R, Banerji S, Ferguson DJ, Clasper S, Jackson DG: Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem 2001, 276:19420-19430 [DOI] [PubMed] [Google Scholar]
- 9.Jackson DG: New molecular markers for the study of tumor lymphangiogenesis. Anticancer Res 2001, 21:4279-4283 [PubMed] [Google Scholar]
- 10.Karkkainen MJ, Alitalo K: Lymphatic endothelial regulation, lymphedema, and lymph node metastasis. Semin Cell Dev Biol 2002, 13:9-18 [DOI] [PubMed] [Google Scholar]
- 11.Karkkainen MJ, Mäkinen T, Alitalo K: Lymphatic endothelium: a new frontier of metastasis research. Nature Cell Biol 2002, 4:2-5 [DOI] [PubMed] [Google Scholar]
- 12.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF: Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983, 219:983-985 [DOI] [PubMed] [Google Scholar]
- 13.Ferrera N, Davis-Smyth T: The biology of vascular endothelial growth factor. Endocr Rev 1997, 18:4-25 [DOI] [PubMed] [Google Scholar]
- 14.Dvorak HF, Brown LF, Detmar M, Dvorak AM: Vascular permeability factor/vascular endothelial growth factor, microvascular permeability, and angiogenesis. Am J Pathol 1995, 146:1029-1039 [PMC free article] [PubMed] [Google Scholar]
- 15.Mustonen T, Alitalo K: Endothelial receptor tyrosine kinases involved in angiogenesis. J Cell Biol 1995, 129:895-898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olofsson B, Jeltsch M, Eriksson U, Alitalo K: Current biology of VEGF-B and VEGF-C. Curr Opin Biotechnol 1999, 10:528-535 [DOI] [PubMed] [Google Scholar]
- 17.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K: A novel vascular endothelial growth factor, VEGF-C, is a ligand for the FLT4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 1996, 15:290-298 [PMC free article] [PubMed] [Google Scholar]
- 18.Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, Alitalo K: VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development 1996, 122:3829-3837 [DOI] [PubMed] [Google Scholar]
- 19.Achen MG, Jeltsch M, Kukk E, Mäkinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA: Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (FLT4). Proc Natl Acad Sci USA 1998, 95:548-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer M, Clauss M, Lepple-Wienhues A, Waltenberger J, Augustin HG, Ziche M, Lanz C, Buttner M, Rziha HJ, Dehio C: A novel vascular endothelial growth factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signaling through VEGFR-2 (KDR) but not VEGFR-1 (Flt-1) receptor tyrosine kinases. EMBO J 1999, 18:363-374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marconcini L, Marchio S, Morbidelli L, Cartocci E, Albini A, Ziche M, Bussolino F, Oliviero S: c-fos-induced growth factor/vascular endothelial growth factor D induces angiogenesis in vivo and in vitro. Proc Natl Acad Sci USA 1999, 96:9671-9676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K: Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 1997, 276:1423-1425 [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Chedotal A, He Z, Goodman CS, Tessier-Lavigne M: Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins sema E and sema IV but not sema III. Neuron 1997, 19:547-559 [DOI] [PubMed] [Google Scholar]
- 24.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD: Neuropilin is a semaphorin III receptor. Cell 1997, 90:753-762 [DOI] [PubMed] [Google Scholar]
- 25.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M: Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998, 92:735-745 [DOI] [PubMed] [Google Scholar]
- 26.Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G: Neuropilin-2 and neuropilin-1 are receptors for the 165-amino acid form of vascular endothelial growth factor (VEGF) and of placenta growth factor-2, but not neuropilin-2 functions as a receptor for the 145-amino acid form of VEGF. J Biol Chem 2000, 275:18040-18045 [DOI] [PubMed] [Google Scholar]
- 27.Karkkainen MJ, Saaristo A, Jussila L, Karila KA, Lawrence EC, Pajusola K, Bueler H, Eichmann A, Kauppinen R, Kettunen MI, Yla-Herttuala S, Finegold DN, Ferrell RE, Alitalo K: A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci USA 2001, 98:12677-12682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinman RM, Hoffman L, Pope M: Maturation and migration of cutaneous dendritic cells. J Invest Dermatol 1995, 105:2S-7S [DOI] [PubMed] [Google Scholar]
- 29.Hamrah P, Zhang Q, Dana MR: Expression of vascular endothelial growth factor receptor-3 (VEGFR-3) in the conjunctiva: a potential link between lymphangiogenesis and leukocyte trafficking on the ocular surface. Adv Exp Med Biol 2002, 506:851-858 [DOI] [PubMed] [Google Scholar]
- 30.Cursiefen C, Schlötzer-Schrehardt U, Küchle M, Sorokin L, Breiteneder-Geleff S, Alitalo K, Jackson D: Lymphatic vessels in vascularized human corneas: immunohistochemical investigation using LYVE-1 and podoplanin. Invest Ophthalmol Vis Sci 2002, 43:2127-2135 [PubMed] [Google Scholar]
- 31.Mimura T, Amano S, Usui T, Kaji Y, Oshika T, Ishii Y: Expression of vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 in corneal lymphangiogenesis. Exp Eye Res 2001, 72:71-78 [DOI] [PubMed] [Google Scholar]
- 32.Collin HB: Lymphatic drainage of 131I-albumin from the vascularized cornea. Invest Ophthalmol Vis Sci 1970, 9:146-155 [PubMed] [Google Scholar]
- 33.Fine M, Stein M: The role of corneal vascularization in human graft rejection. Corneal Graft Failure (Ciba Foundation Symposium; 15). 1973:pp 193-204 Associated Scientific Publishers, Amsterdam, New York
- 34.Yamagami S, Dana MR: The critical role of draining lymph nodes in corneal allosensitization. Invest Ophthalmol Vis Sci 2001, 42:1293-1298 [PubMed] [Google Scholar]
- 35.Yamagami S, Dana MR, Tsuru T: Draining lymph nodes play an essential role in alloimmunity generated in response to high-risk corneal transplantation. Cornea 2002, 21:405-409 [DOI] [PubMed] [Google Scholar]
- 36.Collin HB: Corneal lymphatics in alloxan vascularized rabbit eyes. Invest Ophthalmol Vis Sci 1966, 5:1-13 [PubMed] [Google Scholar]
- 37.Collin HB: Endothelial cell lined lymphatics in vascularized rabbit cornea. Invest Ophthalmol Vis Sci 1966, 5:337-354 [PubMed] [Google Scholar]
- 38.Collin HB: The fine structure of growing corneal lymphatic vessels. J Pathol 1971, 104:99-113 [DOI] [PubMed] [Google Scholar]
- 39.Schoppman SF, Birner P, Stockl J, Kalt R, Ullricj R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D: Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol 2002, 161:947-956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, Detmar M: Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol 2001, 159:893-903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamrah P, Zhang Q, Liu Y, Dana MR: Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Invest Ophthalmol Vis Sci 2002, 43:639-646 [PubMed] [Google Scholar]
- 42.Hamrah P, Liu Y, Zhang Q, Dana MR: The corneal stroma is endowed with a significant number of resident dendritic cells. Invest Ophthalmol Vis Sci 2003, 44:581-589 [DOI] [PubMed] [Google Scholar]
- 43.Hamrah P, Liu Y, Zhang Q, Dana MR: Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation. Arch Ophthalmol 2003, in press [DOI] [PubMed]
- 44.Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR: Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukoc Biol 2003, in press [DOI] [PubMed]
- 45.Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL: Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci 2002, 43:2264-2271 [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Hamrah P, Zhang Q, Taylor AW, Dana MR: Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J Exp Med 2002, 195:259-268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Hamrah P, Taylor AW, Dana MR: Resident corneal dendritic cells that migrate from corneal explants may mediate alloreactivity. Invest Ophthalmol Vis Sci 2002, 43(Suppl):S2264 [Google Scholar]
- 48.Huq SO, Liu Y, Illigens B, Qian Y, Benichou G, Dana MR: Direct pathway of allosensitization plays a significant role in high risk corneal transplantation. Invest Ophthalmol Vis Sci 2002, 43(Suppl):S2275 [Google Scholar]
- 49.Schanzlin DJ, Cryr RJ, Friedlaender MH: Histopathology of corneal neovascularization. Arch Ophthalmol 1983, 101:472-474 [DOI] [PubMed] [Google Scholar]
- 50.Dekaris I, Zhu SN, Dana MR: TNF-α regulates corneal Langerhans cell migration. J Immunol 1999, 162:4235-4239 [PubMed] [Google Scholar]
- 51.Williamson JSP, Dimarco S, Streilein JW: Immunobiology of Langerhans cells on the ocular surface:: I. Langerhans cells within the central cornea interfere with induction of anterior chamber associated immune deviation. Invest Ophthalmol Vis Sci 1987, 21:759-765 [PubMed] [Google Scholar]
- 52.Bacci S, Alard P, Dai R, Nakamura T, Streilein JW: High and low doses of haptens dictate whether dermal or epidermal antigen-presenting cells promote contact hypersensitivity. Eur J Immunol 1997, 27:442-448 [DOI] [PubMed] [Google Scholar]
- 53.Kurimoto I, Grammer SF, Shimizu T, Nakamura T, Streilein JW: Role of F4/80+ cells during induction of hapten-specific hypersensitivity. Immunology 1995, 85:621-629 [PMC free article] [PubMed] [Google Scholar]
- 54.Kurimoto I, Streilein JW: Studies of contact hypersensitivity induction in mice with optimal sensitizing doses of hapten. J Invest Dermatol 1993, 101:132-136 [DOI] [PubMed] [Google Scholar]
- 55.Kubo H, Fujiwara T, Jussila L, Hashi H, Ogawa M, Shimizu K, Awane M, Sakai Y, Takabayashi A, Alitalo K, Yamaoka Y, Nishikawa SI: Involvement of vascular endothelial growth factor receptor-3 in maintenance of integrity of endothelial cell lining during tumor angiogenesis. Blood 2000, 96:546-553 [PubMed] [Google Scholar]
- 56.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K: Immunobiology of dendritic cells. Annu Rev Immunol 2000, 18:767-811 [DOI] [PubMed] [Google Scholar]
- 57.Hart DNJ: Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 1997, 90:3245-3287 [PubMed] [Google Scholar]
- 58.Liu Y-J: Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell 2001, 106:259-262 [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Harada A, Wang J-B, Zhang Y-Y, Hashimoto S-I, Naito M, Matsushima K: Bifurcated dendritic cell differentiation in vitro from murine lineage phenotype-negative c-kit+ bone marrow hematopoietic progenitor cells. Blood 1998, 92:118-128 [PubMed] [Google Scholar]
- 60.Metley JP, Witmer Pack MD, Agger R, Crowley MT, Lawless D, Steinman RM: The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med 1990, 171:1753-1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vremec D, Shortman K: Dendritic cell subtypes in mouse lymphoid organs: cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J Immunol 1997, 159:565-573 [PubMed] [Google Scholar]
- 62.Vremec D, Zorbas M, Scollay R, Saunders DJ, Ardavin CF, Wu L, Shortman K: The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med 1992, 176:47-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ardavin C, Wu L, Li CL, Shortman K: Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature 1993, 362:761-763 [DOI] [PubMed] [Google Scholar]
- 64.Guerriero A, Langmuir PB, Spain LM, Scott EW: PU.1 is required for myeloid-derived but not lymphoid-derived dendritic cells. Blood 2000, 95:879-885 [PubMed] [Google Scholar]
- 65.Anjuere F, Martin P, Ferrero I, Fraga ML, del Hoyo GM, Wright N, Ardavin C: Definition of dendritic cell subpopulations present in the spleen, Peyer’s patches, lymph nodes, and skin of the mouse. Blood 1999, 93:590-598 [PubMed] [Google Scholar]
- 66.Wu L, Li CL, Shortman K: Thymic dendritic cell precursors: relationship to the T lymphocyte lineage and phenotype of the dendritic cell progeny. J Exp Med 1996, 184:903-911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shortman K, Caux C: Dendritic cell development: multiple pathways to nature’s adjuvants. Stem Cells 1997, 15:409-419 [DOI] [PubMed] [Google Scholar]
- 68.Schuler G, Steinman RM: Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med 1985, 161:526-546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kubo H, Cao R, Bräkenhielm E, Cao Y, Alitalo K: Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci USA 2002, 99:8868-8873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jussila L, Alitalo K: Vascular growth factors and lymphangiogenesis. Physiol Rev 2002, 82:673-700 [DOI] [PubMed] [Google Scholar]
- 71.Detmar M, Hirakawa S: The formation of lymphatic vessels and its importance in the setting of malignancy. J Exp Med 2002, 196:713-718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K: Lymphangiogenesis and cancer metastasis. Nat Rev Cancer 2002, 2:573-583 [DOI] [PubMed] [Google Scholar]
- 73.Pepper MS: Lymphangiogenesis and tumor metastasis: myth or reality? Clin Cancer Res 2001, 7:462-468 [PubMed] [Google Scholar]
- 74.Swartz MA, Skobe M: Lymphatic function, lymphangiogenesis, and cancer metastasis. Microsc Res Tech 2001, 55:92-99 [DOI] [PubMed] [Google Scholar]
- 75.Krishnan J, Kirkin V, Steffen A, Hegen M, Weih D, Tomarev S, Wilting J, Sleeman JP: Differential in vivo and in vitro expression of vascular endothelial growth factor (VEGF)-C and VEGF-D in tumors and its relationship to lymphatic metastasis in immunocompetent rats. Cancer Res 2003, 63:713-722 [PubMed] [Google Scholar]
- 76.Baldwin ME, Stacker SA, Achen MG: Molecular control of lymphangiogenesis. Bioessays 2002, 24:1030-1040 [DOI] [PubMed] [Google Scholar]
- 77.Alitalo K, Carmeliet P: Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 2002, 1:219-227 [DOI] [PubMed] [Google Scholar]
- 78.Partanen TA, Arola J, Saaristo A, Jussila L, Ora A, Miettinen M, Stacker SA, Achen MG, Alitalo K: VEGF-C and VEGF-D expression in neuroendocrine cells and their receptor, VEGFR-3, in fenestrated blood vessels in human tissues. EMBO J 2000, 14:2087-2096 [DOI] [PubMed] [Google Scholar]
- 79.Wilting J, Eichmann A, Christ B: Expression of the avian VEGF receptor homologues Quek1 and Quek2 in blood-vascular and lymphatic endothelial and non-endothelial cells during quail embryonic development. Cell Tissue Res 1997, 288:207-223 [DOI] [PubMed] [Google Scholar]
- 80.Fernandez Pujol B, Lucibello FC, Zuzarte M, Lutjens P, Muller R, Havemann K: Dendritic cells derived from peripheral monocytes express endothelial markers and in the presence of angiogenic growth factors differentiate into endothelial-like cells. Eur J Cell Biol 2001, 80:99-110 [DOI] [PubMed] [Google Scholar]
- 81.Yuan L, Moyon D, Pardanaud L, Breant C, Karkkainen MJ, Alitalo K, Eichmann A: Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development 2002, 129:4797-4806 [DOI] [PubMed] [Google Scholar]
- 82.Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, Qi J-H, Claesson-Welsh L, Alitalo K: Vascular endothelial growth factor C induces angiogenesis in vivo. Proc Natl Acad Sci USA 1998, 95:14389-14394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee J, Gray A, Yuan J, Luoh S-M, Avraham H, Wood WI: Vascular endothelial growth factor-related protein: a ligand and specific activator of the tyrosine kinase receptor Flt4. Proc Natl Acad Sci USA 1996, 93:1988-1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marchiò S, Prima L, Pagano M, Palestro G, Albini A, Veikkola T, Cascone I, Alitalo K, Bussolino F: Vascular endothelial growth factor-C stimulates the migration and proliferation of Kaposi’s sarcoma cells. J Biol Chem 1999, 274:27617-27622 [DOI] [PubMed] [Google Scholar]
- 85.Ristimäki A, Narko K, Enholm B, Joukov V, Alitalo K: Proinflammatory cytokines regulate expression of the lymphatic endothelial mitogen vascular endothelial growth factor-C. J Biol Chem 1998, 273:8413-8418 [DOI] [PubMed] [Google Scholar]
- 86.Engholm B, Paavonen K, Ristimäki A, Kumar V, Gunji Y, Klefstrom J, Kivinen L, Laiho M, Olofsson B, Joukov V, Eriksson U, Alitalo K: Comparison of VEGF, VEGF-B, VEGF-C, and Ang-1 mRNA regulation by serum, growth factors, oncoproteins, and hypoxia. Oncogene 1997, 14:2475-2483 [DOI] [PubMed] [Google Scholar]
- 87.Dana MR, Qian Y, Hamrah P: Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea 2000, 19:625-643 [DOI] [PubMed] [Google Scholar]
- 88.Stechschulte SU, Joussen AM, von Recum HA, Poulaki V, Moromizato Y, Yuan J, D’Amato RJ, Kuo C, Adamis AP: Rapid ocular angiogenic control via naked DNA delivery to cornea. Invest Ophthalmol Vis Sci 2001, 42:1975-1979 [PubMed] [Google Scholar]
- 89.Shen H, Clauss M, Ryan J, Schmidt AM, Tijburg P, Borden L, Connolly D, Stern D, Kao J: Characterization of vascular permeability factor/vascular endothelial growth factor receptors on mononuclear phagocytes. Blood 1993, 81:2767-2773 [PubMed] [Google Scholar]