Abstract
A major obstacle in the study of angiogenesis and the testing of new agents with anti-angiogenic potential has been the lack of experimental models with predictive in vivo value. We describe here the combined use of in vitro and in vivo angiogenesis models that are based on endochondral bone development. This approach led to the identification of a new inhibitor of matrix metalloprotease (MMP) activity that inhibits neovascularization in vitro and in vivo while osteoclast invasion, which occurs simultaneously during bone development, remained unaffected. In contrast, the broad-spectrum MMP-inhibitor marimastat inhibited both in vitro angiogenesis and osteoclastogenesis dose-dependently but displayed severe toxic side effects in vivo. The combined use of these experimental models may, therefore, facilitate the discovery of mechanisms underlying angiogenesis and lead to identification of new pharmacological compounds with clinical efficacy and appropriate selectivity in the treatment of angiogenesis-dependent disorders like arthritis and cancer.
Acquisition of a blood supply is a fundamental requirement for organ development and differentiation during embryogenesis and postnatal development. During endochondral bone formation the avascular calcified cartilaginous matrix is replaced by bone via the simultaneous invasion of osteoclasts and capillary endothelial cells that direct osteoblast progenitors to the cartilaginous scaffolds to synthesize bone. 1-8
Invasive processes during osteoclastogenesis and angiogenesis share several similarities. For example, both cell types express matrix metalloproteases (MMPs) that are required for invasion, migration, and degradation of extracellular matrices and the release of growth factors and inhibitors from the matrix. 4,8,9-20 MMPs are a family of Zn-dependent endopeptidases that are able to cleave extracellular matrix molecules during normal tissue (re) modeling (eg, embryonic growth) and remodeling of pathological tissues (eg, rheumatoid arthritis and cancer). 1,21-26 MMPs may also directly facilitate angiogenesis by stimulating matrix degradation and, indirectly, by releasing matrix-associated endothelial growth factors and inhibitors. 27,28
There is compelling evidence that MMPs are involved in skeletal development. Before vascular invasion of calcified cartilage various MMPs are expressed in late hypertrophic chondrocytes, osteoclasts, endothelial cells and osteoblasts. 4,11-13 MMP knockout mice (MMP−9−/− and MT1-MMP−/−) show disturbances in skeletal development, which include defects in vascularization, osteoclast migration and resorption of the growth plate. 9,11,29,30 The use of MMP inhibitors (MMPIs) provided further evidence that MMPs are involved in the migration of osteoclasts and endothelial cells in vitro and in vivo. 12,26,31,32
To date, a major impediment to the study of angiogenesis and the testing of new agents with anti-angiogenic potential has been the lack of experimental models that are predictive of in vivo responses. Here we describe the combined use of an in vitro and an in vivo angiogenesis model that are based on endochondral bone development. The strength of this approach is illustrated by the identification of a new inhibitor of MMP activity that selectively inhibits neovascularization in vitro and in vivo while osteoclast invasion remains unaffected.
Materials and Methods
Compound Preparation
For in vitro assays, MMPIs were suspended in dimethyl sulfoxide (DMSO; 2 × 10−2 M stock) and stored at −20°C. 33 MMPIs were diluted in culture medium and 0.05% DMSO in culture medium was used as vehicle control. For in vivo studies, MMPIs were prepared daily in 50% DMSO + 50% PBS at ambient temperature. MMPIs or vehicle (10 μl) were subcutaneously administered to neonatal mice for 4 consecutive days.
Enzymatic Assays and in Vitro Activities of Tested MMPIs
Human recombinant MMPs were expressed in Escherichia coli as inclusion bodies and purified as described. 34 CH5902 and CH3921 are small synthetic MMPIs which were rationally designed to inhibit a broad range of MMP activities while sparing those of the sheddases. 33 The IC50 values, which are depicted in Table 1 ▶ , were determined in vitro under reducing conditions using purified human MMPs as described previously. 34
Table 1.
IC50 Values (nM) of MMP-Inhibitors for Various MMPs (MMP Enzyme Assays)
| MMPI | MMP-1 | MMP-2 | MMP-3 | MMP-8 | MMP-9 | MMP-13 | MMP-14 |
|---|---|---|---|---|---|---|---|
| marimastat | 2 | 5 | 20 | 3 | 7 | 3 | 4 |
| CH5902 | 2061 | 15 | 133 | 16 | 545 | 58 | 305 |
| CH3921 | 13 | 24 | 89 | 153 | 449 | 338 | 877 |
Additionally, CH5902 only weakly inhibits TACE (IC50>10,000 nmol/L) and does not inhibit release of tumor necrosis factor (TNF)-α, TNF-RII, L-selectin, interleukin (IL)-1-RII and IL-6-R in cell-based in vitro assays (thought to be mediated by tumor necrosis factor-alpha converting enzyme (TACE)). CH5902 also does not inhibit (IC50 values >30,000 nmol/L) the in vitro activity of serine or cysteine proteinases, which include chymotrypsin, elastase, plasmin, cathepsin B, trypsin, thrombin, and urokinase plasminogen activator (data not shown). CH3921 does not inhibit the release of TNF-α in a cell-based assay but has not been tested in the other systems. These results, therefore, demonstrate that CH5902 and CH3921 are selective, potent nanomolar inhibitors of MMP in vitro activities. Importantly, they do not inhibit closely-related sheddase activities responsible for the release of cell-associated molecules.
In Vitro Angiogenesis Assay
17-day-old fetuses were removed from pregnant Swiss albino mice and metatarsals were dissected as described previously. 10 The isolated metatarsals were cultured for 10 days in 24-well plates in the presence or absence of MMPIs, and fixed and stained for platelet/endothelial cell adhesion molecule 1 (PECAM-1). 10 Total area of PECAM-1-positive tubular structures was determined by image analysis. 12 Data are depicted as treatment control ratios from triplicate experiments.
In Vitro Osteoclast Invasion and Bone Resorption Assay
Pregnant Swiss albino mice were injected with 30 μCi 45Ca (1Ci/mmol) at day 16 of gestation and were sacrificed at day 17. 35 45Ca prelabeled metatarsals were dissected and cultured as described earlier in the presence or absence of MMPIs. 35 Metatarsals were cultured for 6 days and medium was replaced after 3 days. The %45Ca release was used as a measure of bone resorption and osteoclast-precursors and osteoclasts were identified by TRAcP staining as described earlier. 35
Angiogenesis and Osteoclastogenesis in Vivo
All animal procedures were approved by the Leiden University Committee on Animal Experiments. In the neonatal mouse tail primary centers of endochondral ossification are still developing in the most distal vertebrae and provide a suitable model to study bone angiogenesis and osteoclastogenesis in vivo. 8,36 At birth, capillaries and osteoclasts have not yet invaded the 28 neonatal tail vertebrae. Between day 1 and day 6 primary ossification centers appear with a distal progression in all caudal vertebrae, 10,33 and at day 7 these are all remodeled. 10 Neonatal Swiss albino mice were treated with the MMPIs marimastat (6, 12, or 60 μg/day), CH5902 (60 μg/day), or CH3921 (60 μg/day) or vehicle (1:1 DMSO in PBS) for 4 consecutive days, starting at the second day after birth. Every group contained 5 littermates and the experiment was repeated twice. At the start of each experiment, 2 days after birth, the tails of two non-treated mice were fixed in Zinc Macrodex formalin (ZnMF) as described previously. 8 After 4 consecutive days of treatment the animals were sacrificed, total tail-length was determined and tails were processed histochemically. Dolichus biflorus agglutinin (DBA) histochemistry was used to identify endothelial cells. 8 For double staining, TRAcP staining was followed by lectin staining as described. 8
Results
In 17-day-old fetal mouse metatarsals, endothelial and osteoclast precursors are still confined to the perichondrium. At this stage of development invasion of the avascular calcified cartilage by capillary endothelial cells and osteoclasts occurs simultaneously in these long bone explants. 10,35 When cultured for more than 7 days capillaries will grow out of the bone explant and the extent of capillary outgrowth can be used to test the antiangiogenic potential of novel compounds. 10 Due to these properties the same bone explants can be used as in vitro models of angiogenesis and osteoclastic bone resorption. 10,35
Effect of MMPIs on Angiogenesis in Vitro
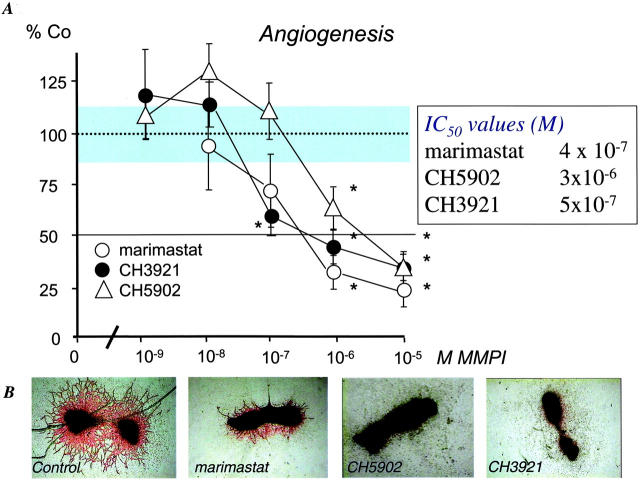
Continuous treatment of the fetal bone explants for ten days with various doses of CH3921, CH5902, and marimastat prevented the outgrowth of PECAM-1-positive tube-like structures in a dose-dependent manner (Figure 1A) ▶ . As shown in Figure 1B ▶ , only scattered endothelial cells were present in the cultures treated with MMPIs. Strikingly, degradation of the calcified matrix (central part of the explant) was not inhibited by CH3921 (Figure 1B) ▶ , whereas the other MMPIs strongly prevented the breakdown of the mineralized matrix. Therefore, we tested whether MMPIs can differentially inhibit osteoclast development and subsequent resorption of mineralized matrix in similar bone explants.
Figure 1.
MMPIs inhibit angiogenesis in vitro. 17-day-old fetal mouse metatarsals were treated with marimastat (open circle), CH3921 (filled circle), CH5902 (open triangle), or vehicle for 10 days. After 10 days, cultures were stained for PECAM-1. A: The total area of PECAM-1-positive capillary-like structures was determined by computerized image analysis. Data are expressed as treatment control of sextuple cultures ± SEM. The experiment was repeated three times. B: Representative images of control cultures, marimastat-, CH5902-, and CH3921 (10−6M)-treated cultures are shown after staining for PECAM-1. Original magnification, ×40.
Effect of MMPIs on Osteoclast Invasion in Vitro
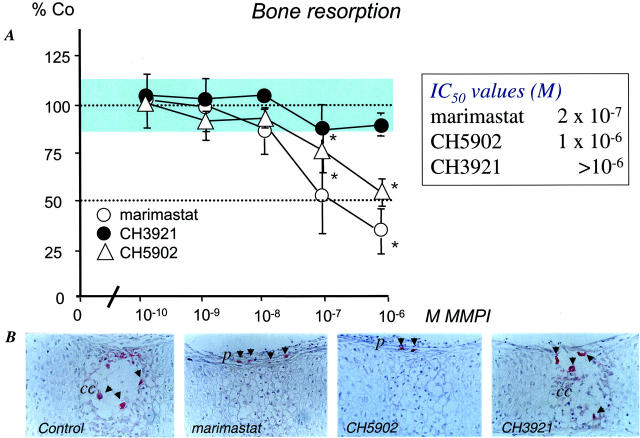
Osteoclastic bone resorption in (45Ca-prelabeled) fetal bones entirely depends on differentiation of osteoclast-precursors that are present in the perichondrium at the start of the experiment, and subsequent invasion and resorption of the calcified matrix. 35 Treatment of bone explants with marimastat and CH5902 dose-dependently inhibited bone resorption in fetal long bones (Figure 2A) ▶ . In contrast, CH3921 did not appear to have an effect on osteoclastic bone resorption (Figure 2A) ▶ . In control sections, mature resorbing osteoclasts were identified in the mineralized cartilaginous matrix (Figure 2B) ▶ . In marimastat- and CH5902-treated long bones, however, osteoclast-precursors were still confined to the perichondrium, whereas there was no inhibitory effect with CH3921 (Figure 2B) ▶ .
Figure 2.
MMPIs and osteoclastic resorption of 45Ca-prelabeled 17-day-old fetal mouse metatarsals in vitro. A: The fetal bone explants were incubated with various doses of marimastat (open circle), CH3921 (filled circle), CH5902 (open triangle) or vehicle (DMSO). B: MMPIs marimastat and CH5902 inhibit the invasion of osteoclast-precursors from the perichondrium into the calcified cartilage. Fetal bones were incubated with 10−6M marimastat, CH5902, or vehicle for 4 consecutive days and processed for paraffin embedding and TRAcP staining. 39 TRAcP-positive multinuclear cells were identified in calcified cartilage (ml), while in the marimastat- and CH5902-treated metatarsal TRAcP-positive cells resided in the perichondrium (p). CH3921 did not affect the invasion of the calcified cartilage by osteoclasts. Original magnification, ×200.
Effect of MMPIs on Angiogenesis and Osteoclastogenesis in Vivo
We further tested the effects of these MMPIs in the in vivo model of endochondral bone formation. 8 At the start of the experiment (day 2 after birth) the 28 caudal vertebrae of the mouse tail can be divided into different developmental stages (Figure 3A) ▶ . From proximal to distal vertebrae are composed of developing trabecular bone with a primitive bone marrow cavity (caudal vertebrae 1 to 10), calcified avascular cartilage 11-21 and non-mineralized avascular cartilage (vertebrae 22 to 28). 8,36 After double-staining for TRAcP and lectin (DBA), TRAcP-positive osteoclasts were closely associated to DBA-positive endothelial cells in the primitive bone marrow cavity (vertebrae 1 to 10). More proximal lectin-positive endothelial cells were identified in the perichondrium or surrounding mesenchyme (vertebrae 10 to 28) in close proximity to mononuclear TRAcP-positive osteoclast-precursors. After 4 days, the invasion front progressed to the distal end and was located at approximately vertebra 18 (Figure 3A) ▶ .
Figure 3.
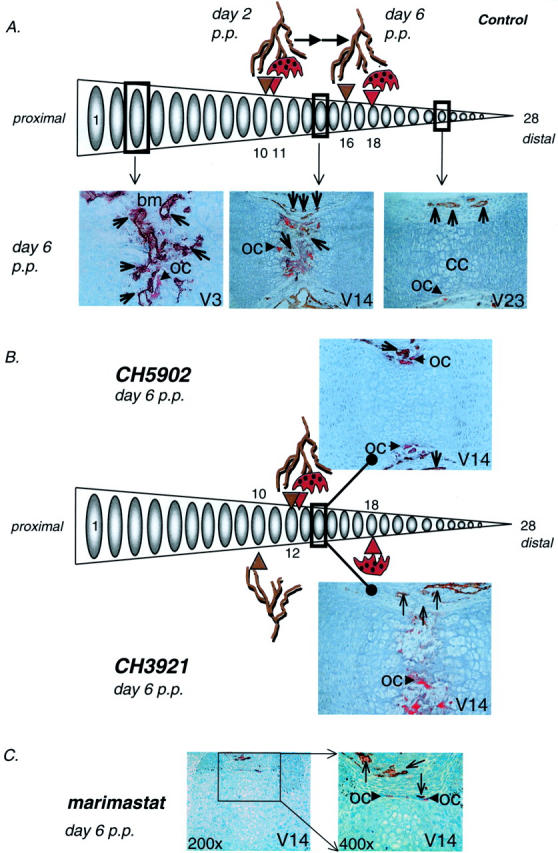
The effect of MMPIs on angiogenesis and osteoclast invasion of caudal vertebrae of neonatal mice in vivo. A: Schematical representation of angiogenesis and osteoclastogenesis in the caudal vertebrae in the neonatal mouse tails under control conditions (vehicle). Tails were processed and double-stained for TRAcP (red) and lectin (brown). At day 2 post partem (p.p.) osteoclast-precursors and blood capillaries have started to invade the bone collar at caudal vertebrae 11 and 10 respectively (V11, V10). Four days later, at day 6 p.p., osteoclast-precursors and capillaries have progressed in a distal manner and their invasion fronts can now be identified at caudal vertebrae 16 (capillaries, V16) and 18 (osteoclasts, V18). Representative images of vertebra 3, 14, and 23 are shown. Original magnifications, ×200 (V14, 23) and ×400 (V3). Open arrowheads denote lectin-positive capillaries. oc, osteoclast; bm, bone marrow; ml, calcified cartilage. B: Distal progression of the invasion front of blood capillaries and osteoclast-precursors in neonatal mouse tails after 4 days of treatment (day 6 p.p.) with CH5902 or CH3921. Angiogenesis was significantly inhibited by both MMPIs (V12 and V10 for CH5902 and CH3921, respectively). In contrast to CH5902, CH3921 did not affect osteoclastogenesis in vivo. Representative images of vertebrae 14 (V14) are shown. Original magnification, ×200. Open arrowheads denote lectin-positive capillaries. oc, osteoclast. C: Administration of various doses of marimastat was found to be cytotoxic and lead to severe side effects such as growth retardation and, in most cases, death (see text). Morphological changes of osteoclasts and sinusoids after four days of treatment with 6 mg/kg/day marimastat in caudal vertebra 14 (V14; C). Neither osteoclasts nor blood capillaries have invaded the calcified cartilage and show signs of severe side effects. Original magnifications, ×200 and ×400. Open arrowheads denote lectin-positive capillaries. oc, osteoclast.
Treatment of 2-day-old neonatal mice with the MMPIs CH5902 and CH3921 (60 μg/day for 4 days) did not affect the body weight of the animals (not shown). The total length of the tails after 4 days of treatment, however, was significantly inhibited from 2.00 cm ± 0.06 in control animals to 1.70 cm ± 0.04 (P < 0.001) in CH5902-treated animals and from 2.24 cm ± 0.06 in control animals to 2.00 cm ± 0.05 (P < 0.05) in CH3921-treated mice. In contrast, administration of marimastat at various doses (6, 12, and 60 μg/day) to neonatal mice led to severe cytotoxic effects, such as severe tail deformities and significant decreases in body weight, and eventually to death. In most cases the young growing animals died after 2 consecutive injections with marimastat; few animals survived after 4 days of treatment with all tested doses of marimastat (<5% after 4 days).
Longitudinal sections of neonatal mouse tails revealed that administration of the MMPIs CH3921 and CH5902 (60 μg/day for 4 consecutive days) resulted in a significant delay in angiogenesis (Figure 3B ▶ and Table 2 ▶ ). The invasion front was determined as the first sign of vascular invasion and/or osteoclast invasion of a developing caudal vertebra (Figure 3A) ▶ . In line with our in vitro observations, CH3921 did not affect invasion of osteoclasts in vivo, while vascular invasion was significantly delayed (P < 0.001, Figure 3B ▶ and Table 2 ▶ ). As depicted in Figure 3B ▶ and Table 2 ▶ the vascular invasion front in CH3921-treated animals did not progress and was retained at the level of day 2. In contrast, CH5902 significantly delayed both the formation of a primitive marrow cavity by osteoclasts and vascular invasion (Figure 3B ▶ and Table 2 ▶ ). These data again show that CH3921 selectively inhibits angiogenesis without affecting osteoclastogenesis. In contrast, histological evaluation of the tail vertebrae of the marimastat-treated animals revealed severely affected vertebrae with poorly developed blood vessels and osteoclasts (Figure 3C) ▶ .
Table 2.
Caudal Vertebra Number Displaying First Signs of Blood Vessel or Osteoclast Invasion
| MMPI | Angiogenesis | Osteoclastogenesis |
|---|---|---|
| Control (vehicle) | 16 ± 0.3† | 18 ± 0.1 |
| CH5902 | 12 ± 1.2* | 12 ± 1.0* |
| CH3921 | 10 ± 0.9*† | 18 ± 0.7 |
Data show angiogenesis and osteoclastogenesis, respectively, after 4 days of treatment with MMPIs (60 μg/mouse/day).
*P ≤ 0.001 (vs. control animals).
†, P ≤ 0.005 (angiogenesis vs. osteoclastogenesis in same animal), n = 6.
Discussion
Invasion of endothelial cells and osteoclasts into the avascular calcified cartilage has been recognized for a long time but only recently the factors that modulate these processes have been identified. 3-10 Knowledge about the regulation of these events is limited, which is mainly due to the lack of suitable in vivo models in which both invasive processes can be studied simultaneously. In the present study we describe the combined use of in vitro and in vivo models of angiogenesis and osteoclast invasion that allow rapid screening for putative pharmacological agents (drug discovery), and provide information about underlying mechanisms and assessment of in vivo toxicities.
Altered expression of MMP activity constitutes part of the pathogenic mechanism associated with a wide range of diseases. These include the destruction of cartilage and bone in rheumatoid and osteoarthritis, 25 tissue breakdown, and remodeling during invasive tumor growth and tumor angiogenesis. 19,26 Given the crucial role of various MMPs in pathological tissue remodeling, targeting MMP activity through synthetic MMPIs has become an attractive strategy. 34,37-41 The redundancy and overlap in function of MMPs requires the development of compounds with improved oral bioavailability that selectively inhibit the MMP-driven process of angiogenesis without affecting normal remodeling of extracellular matrices. 27 We have, therefore, designed selective MMPIs that potently inhibit MMP activities while minimally affecting those of other metalloproteinases (eg, sheddases) involved in the release of cell-associated molecules.
All tested MMPIs inhibited the outgrowth of capillary structures from fetal mouse bone explants in a dose-dependent manner, indicating that all tested MMPIs inhibited the activities of MMP that are required for neovascularization. A different picture emerged when these MMPIs were tested in similar bone explants for their ability to interfere with osteoclast invasion and subsequent osteoclastic resorption. Both marimastat and CH5902 inhibited osteoclastic resorption in a dose-dependent manner. Strikingly, however, continuous treatment of fetal metatarsals with the CH3921 did not inhibit osteoclast invasion and osteoclastic bone resorption. The in vitro assays described here may therefore provide the necessary tools to prescreen for active compounds that selectively inhibit angiogenesis or osteoclastogenesis in vivo. In addition, an in vivo assay (developing mouse tail vertebrae) is essential to monitor toxic side effects. In line with the in vitro observations, CH5902 inhibited angiogenesis and osteoclastogenesis during endochondral bone formation in vivo. Again, CH3921 selectively inhibited angiogenesis and did not affect osteoclastogenesis in the same vertebrae in vivo, which is in agreement with our in vitro observations.
Some of the general shortcomings of broad-spectrum MMPIs, despite their favorable effects on tumor progression, are the musculo-skeletal side effects that often occur, which are most likely due to inhibition of MMP activity at those physiological remodeling sites. 37,39-41 Because fast-growing neonatal mice exhibit extensive tissue remodeling, these animals can be of invaluable help to rapidly identify compounds that affect tissue remodeling and to establish potential side effects. This is best illustrated by marimastat, which displayed severe toxic effects characterized by stunted growth and decreased body weight, eventually leading to death. The more selective compounds did not show this general toxicity but still affected tail-length. These effects are compatible with the observation that MMPIs inhibit angiogenesis in vitro and in vivo.
Taken together, the combined use of these in vitro and in vivo experimental models may, therefore, greatly facilitate the discovery of mechanisms underlying angiogenesis and lead to identification of pharmacological compounds with potential clinical efficacy and appropriate selectivity in the treatment of angiogenesis-dependent disorders. The identification of the selective MMPI CH3921 may be illustrative for this approach.
Footnotes
Address reprint requests to Gabri van der Pluijm, Ph.D., Leiden University Medical Center, Department of Endocrinology C4–86, Albinusdreef 2, 2333 ZA Leiden, The Netherlands. E-mail: g.van_der_pluijm@lumc.nl.
References
- 1.Murray MJ, Lessey BA: Embryo implantation and tumor metastasis: common pathways of invasion and angiogenesis. Semin Reprod Endocrinol 1999, 17:275-290 [DOI] [PubMed] [Google Scholar]
- 2.Erlebacher A, Filvaroff E, Gitelman SE, Derynck R: Toward a molecular understanding of skeletal development. Cell 1995, 80:371-378 [DOI] [PubMed] [Google Scholar]
- 3.Gerber HP, Ferrara N: Angiogenesis and bone growth. Trends Cardiovasc Med 2000, 10:223-228 [DOI] [PubMed] [Google Scholar]
- 4.Colnot CI, Helms JA: A molecular analysis of matrix remodeling and angiogenesis during long bone development. Mech Dev 2001, 100:245-250 [DOI] [PubMed] [Google Scholar]
- 5.Stevens DA, Williams GR: Hormone regulation of chondrocyte differentiation and endochondral bone formation. Mol Cell Endocrinol 1999, 151:195-204 [DOI] [PubMed] [Google Scholar]
- 6.Karsenty G: Chondrogenesis just ain’t what it used to be. J Clin Invest 2001, 107:405-407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parfitt AM: The mechanism of coupling: a role for the vasculature. Bone 2000, 26:319-323 [DOI] [PubMed] [Google Scholar]
- 8.Deckers MML, van Beek ER, van der Pluijm G, Wetterwald A, van der Wee-Pals LJA, Cecchini MG, Papapoulos SE, Löwik CWGM: Dissociation of angiogenesis and osteoclastogenesis during endochondral bone formation in neonatal mice. J Bone Min Res 2002, 17:998-1007 [DOI] [PubMed] [Google Scholar]
- 9.Engsig MT, Chen QJ, Vu TH, Pedersen AC, Therkidsen B, Lund LR, Henriksen K, Lenhard T, Foged NT, Werb Z, Delaisse JM: Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J Cell Biol 2000, 151:879-890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deckers MML, van der Pluijm G, Dooijewaard S, Kroon ME, van Hinsbergh VW, Papapoulos SE, Löwik CWGM: Effect of angiogenic and antiangiogenic compounds on the outgrowth of capillary structures from fetal mouse explants. Lab Invest 2001, 81:5-15 [DOI] [PubMed] [Google Scholar]
- 11.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z: MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 1998, 93:411-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blavier L, Delaisse JM: Matrix metalloproteinases are obligatory for the migration of preosteoclasts to the developing marrow cavity of primitive long bones. J Cell Sci 1995, 108:3649-3659 [DOI] [PubMed] [Google Scholar]
- 13.Haas TL, Madri JA: Extracellular matrix-driven matrix metalloproteinase production in endothelial cells: implications for angiogenesis. Trends Cardiovasc Med 1999, 9:70-77 [DOI] [PubMed] [Google Scholar]
- 14.Fenwick SA, Gregg PJ, Kumar S, Smith J, Rooney P: Intrinsic control of vascularization in developing cartilage rudiments. Int J Exp Pathol 1997, 7:187-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alini M, Marriott A, Chen T, Abe S, Poole AR: A novel angiogenic molecule produced at the time of chondrocyte hypertrophy during endochondral bone formation. Dev Biol 1996, 176:124-132 [DOI] [PubMed] [Google Scholar]
- 16.Descalzi Cancedda F, Melchiori A, Benelli R, Gentili C, Masiello L, Campanile G, Cancedda R, Albini A: Production of angiogenesis inhibitors and stimulators is modulated by cultured growth plate chondrocytes during in vitro differentiation: dependence on extracellular matrix assembly. Eur J Cell Biol 1995, 66:60-68 [PubMed] [Google Scholar]
- 17.Brown RA, Kayser M, McLaughlin B, Weiss JB: Collagenase and gelatinase production by calcifying growth plate chondrocytes. Exp Cell Res 1993, 208:1-9 [DOI] [PubMed] [Google Scholar]
- 18.DeSimone DP, Reddi AH: Vascularization and endochondral bone development: changes in plasminogen activator activity. J Orthop Res 1992, 10:320-324 [DOI] [PubMed] [Google Scholar]
- 19.Werb Z, Vu TH, Rinkenberger JL, Coussens LM: Matrix-degrading proteases and angiogenesis during development and tumor formation. APMIS 1999, 107:11-18 [DOI] [PubMed] [Google Scholar]
- 20.Pepper MS, Montesano R: Proteolytic balance and capillary morphogenesis. Cell Differ Dev 1990, 32:319-327 [DOI] [PubMed] [Google Scholar]
- 21.Stetler-Stevenson WG: Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest 1999, 103:1237-1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCawley LJ, Matrisian LM: Matrix metalloproteinases: multifunctional contributors to tumor progression. Mol Med Today 2000, 6:149-156 [DOI] [PubMed] [Google Scholar]
- 23.Nagase H, Woessner JF: Matrix metalloproteinases. J Biol Chem 1999, 274:21491-21494 [DOI] [PubMed] [Google Scholar]
- 24.Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA: Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha V beta 3. Cell 1996, 31:683-693 [DOI] [PubMed] [Google Scholar]
- 25.Martel-Pelletier J, Welsch DJ, Pelletier JP: Metalloproteases and inhibitors in arthritic diseases. Best Pract Res Clin Rheumatol 2001, 15:805-829 [DOI] [PubMed] [Google Scholar]
- 26.Yu AE, Hewitt RE, Connor EW, Stetler-Stevenson WG: Matrix metalloproteinases: novel targets for directed cancer therapy. Drugs Aging 1997, 11:229-244 [DOI] [PubMed] [Google Scholar]
- 27.Yip D, Ahmad A, Karapetis CS, Hawkins CA, Harper PG: Matrix metalloproteinase inhibitors: applications in oncology. Invest New Drugs 1999, 17:387-399 [DOI] [PubMed] [Google Scholar]
- 28.O’Reilly MS, Wiederschain D, Stetler-Stevenson WG, Folkman J, Moses MA: Regulation of angiostatin production by matrix metalloproteinase-2 in a model of concomitant resistance. J Biol Chem 1999, 274:29568-29571 [DOI] [PubMed] [Google Scholar]
- 29.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H: MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 1999, 99:81-92 [DOI] [PubMed] [Google Scholar]
- 30.Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K: Impaired endochondral ossification and angiogenesis in mice deficient in membrane type matrix metalloproteinase I. Proc Natl Acad Sci USA 2000, 97:4052-4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiraoka N, Allen E, Apel IJ, Gyetko MR, Weiss SJ: Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell 1998, 95:365-77 [DOI] [PubMed] [Google Scholar]
- 32.Hidalgo M, Eckhardt SG: Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst 2001, 93:178-193 [DOI] [PubMed] [Google Scholar]
- 33.Baxter AD, Bird JB, Bannister R, Bhogal R, Manallack DT, Watson R: D1927 and D2163: novel mercaptoamide inhibitors of matrix metalloproteinases. Clendeninn NJ Appelt K eds. Cancer Drug Discovery and Development: Matrix Metalloproteinase Inhibitors in Cancer Therapy. 2000:pp 193-221 Humana Press, Inc., New Jersey
- 34.Naglich JG, Jure-Kunkel M, Gupta E, Fargnoli J, Henderson AJ, Lewin AC, Talbott R, Baxter A, Bird J, Savopoulos R, Wills R, Kramer RA, Trail PA: Inhibition of angiogenesis and metastasis in two murine models by the matrix metalloproteinase inhibitor, BMS-275291. Cancer Res 2001, 61:8480-8455 [PubMed] [Google Scholar]
- 35.van der Pluijm G, Most W, van der Wee-Pals LJA, de Groot H, Papapoulos SE, Löwik CWGM: Two distinct effects of recombinant human tumor necrosis factor-α on osteoclast development and subsequent resorption of mineralized matrix. Endocrinology 1991, 129:1596-1604 [DOI] [PubMed] [Google Scholar]
- 36.van der Pluijm G, Vloedgraven H, Papapoulos SE, Löwik CWGM, Grzesik W, Kerr J, Robey PG: Attachment characteristics and involvement of integrins in adhesion of breast cancer cell lines to extracellular bone matrix components. Lab Invest 1997, 77:665-675 [PubMed] [Google Scholar]
- 37.Brown PD: Ongoing trials with matrix metalloproteinase inhibitors. Expert Opin Investig Drugs 2000, 9:2167-2177 [DOI] [PubMed] [Google Scholar]
- 38.Steward WP, Thomas AL: Marimastat: the clinical development of a matrix metalloproteinase inhibitor. Expert Opin Investig Drugs 2000, 9:2913-2922 [DOI] [PubMed] [Google Scholar]
- 39.Wojtowicz-Praga S, Torri J, Johnson M, Steen V, Marshall J, Ness E, Dickson R, Sale M, Rasmussen HS, Chiodo TA, Hawkins MJ: Phase I trial of Marimastat, a novel matrix metalloproteinase inhibitor, administered orally to patients with advanced lung cancer. J Clin Oncol 1998, 16:2150-2156 [DOI] [PubMed] [Google Scholar]
- 40.Primrose JN, Bleiberg H, Daniel F, Van Belle S, Mansi JL, Seymour M, Johnson PW, Neoptolemos JP, Baillet M, Barker K, Berrington A, Brown PD, Millar AW, Lynch KP: Marimastat in recurrent colorectal cancer: exploratory evaluation of biological activity by measurement of carcinoembryonic antigen. Br J Cancer 1999, 79:509-514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoekstra R, Eskens FALM, Verweij J: Matrix metalloproteinase inhibitors: current developments and future perspectives. The Oncologist 2001, 6:415-427 [DOI] [PubMed] [Google Scholar]