Abstract
Early local invasion by astrocytoma cells results in tumor recurrence even after apparent total surgical resection, leading to the poor prognosis associated with malignant astrocytomas. Proteolytic enzymes have been implicated in facilitating tumor cell invasion and the current study was designed to characterize the expression of the cysteine proteinase cathepsin S (CatS) in astrocytomas and examine its potential role in invasion. Immunohistochemical analysis of biopsies demonstrated that CatS was expressed in astrocytoma cells but absent from normal astrocytes, oligodendrocytes, neurones and endothelial cells. Microglial cells and macrophages were also positive. Assays of specific activity in 59 astrocytoma biopsies confirmed CatS expression and in addition demonstrated that the highest levels of activity were expressed in grade IV tumors. CatS activity was also present in astrocytoma cells in vitro and the extracellular levels of activity were highest in cultures derived from grade IV tumors. In vitro invasion assays were carried out using the U251MG cell line and the invasion rate was reduced by up to 61% in the presence of the selective CatS inhibitor 4-Morpholineurea-Leu-HomoPhe-vinylsulphone. We conclude that CatS expression is up-regulated in astrocytoma cells and provide evidence for a potential role for CatS in invasion.
Astrocytomas are the commonest primary brain tumors and within this group the malignant grade III and IV tumors predominate (anaplastic astrocytomas and glioblastomas). Despite their non-metastatic nature, prognosis is poor because tumor infiltration of surrounding brain leads to recurrence even after apparently radical surgery. Multiple stereotactic biopsies of astrocytomas have demonstrated isolated tumor cells at considerable distances from the main tumor mass, 1 and recent investigations 2 have shown that tumor cells may be cultured from histologically normal brain at a distance greater than 4 cm from the gross tumor. Astrocytoma invasion is an active process that involves interactions with the host extracellular matrix (ECM), proteolytic modification of the ECM, and migration of the tumor cells into the modified matrix. 3 Members of the metalloproteinase, serine proteinase, and cysteine proteinase superfamilies have been linked to glioma invasion. 4
The cysteine proteinase cathepsin B (CatB) is expressed in gliomas in vitro 5 and immunohistochemical studies have demonstrated its presence in astrocytomas and glioblastomas in contrast to its absence from astrocytes in normal brain. 6,7 The expression and activity of CatB is higher in anaplastic astrocytomas and glioblastomas compared with low-grade tumors and normal brain 8 and high levels of CatB are positively correlated with poor survival. 9 Human glioblastoma cell lines also exhibit higher levels of CatB expression than lower grade astrocytomas 10 and the functional relevance of the enzyme is confirmed by a marked reduction in in vitro invasiveness of a glioblastoma cell line transfected with antisense CatB cDNA. 11 There is evidence of altered trafficking of CatB in glioblastomas, with a redistribution of enzyme to the cell periphery. 12 In addition, a potential role in invasion has been suggested by high levels at the tumor periphery and in infiltrating cells. 6 The closely related cysteine proteinase cathepsin L (CatL) has also been implicated in glioblastoma invasion in an in vitro model. 13
Although much of the attention on cysteine proteinases in cancer has focused on CatB and CatL, another member of the family, cathepsin S (CatS), is also capable of degrading ECM macromolecules such as laminin, collagens, elastin, and chondroitin sulphate proteoglycan (CSPG). 14 Furthermore, it is markedly more stable than either CatB or CatL at extracellular pH, 15 a property that is compatible with a role in extracellular proteolysis. Cathepsin S expression has predominantly been associated with cells of monocyte-macrophage lineage. Functions ascribed to CatS include degradation of the MHC class II chaperone II before peptide loading in the endosomal compartment 16,17,18 and processing of the amyloid precursor protein. 19 Secreted macrophage-derived cysteine proteinases (including CatS) are implicated in ECM remodeling in both pathological 20 and physiological conditions. 14 Inflammatory mediators increase CatS secretion from macrophages leading to ECM protein degradation. 14 Growth factors such as basic fibroblast growth factor (bFGF) have been shown to up-regulate CatS expression. 21 These properties of inducibility, capacity to degrade extracellular macromolecules, and stability at neutral pH are consistent with a role in tumor invasion. To date, expression of CatS has not been characterized in brain tumors and the current project is designed to assess the expression of CatS in human astrocytoma biopsies and cell cultures.
Materials and Methods
Cell Culture
The G-CCM cell line was initiated from a human anaplastic astrocytoma. 22 The CB 109 23 and the U251MG cell lines (American Type Culture Collection, Rockville, MD) were derived from human glioblastomas multiforme. All other cell cultures were derived from brain tumor biopsy specimens from the Regional Neurosurgery Unit of the Royal Victoria Hospital and were used experimentally within 5 to 10 passages. Cultures were tested regularly for mycoplasma using the Hoechst dye-binding method 24 and were found to be negative. Cultures were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20 mmol/L glutamine and 10% fetal calf serum. All cell culture media and reagents were purchased from Gibco BRL, Paisley, Scotland.
Cathepsin S Assay
Cells for CatS assay were harvested by scraping from confluent T25 flasks, resuspended in 1 ml of Hanks’ balanced salt solution (HBSS) and sedimented at 1000 × g for 5 minutes. The cell pellet was made up to 250 μl in MES buffer (50 mmol/L, pH 6.0) containing 0.1% TritonX-100. Complete cell disruption was ensured by sonication (MSE Soniprep150, Sanyo-Gallen-Kamp, Loughboro, Leicestershire, UK). The protein concentration of the homogenate was determined using the BCA reagent kit (Pierce, Rockford, IL, USA) and aliquots were diluted to a concentration of 25 to 50 mg/L for CatS assay.
Tissue samples used in this study were derived from diagnostic biopsies received from the Regional Neuropathology Service, Royal Victoria Hospital, with the approval of the local ethics committee. All surgical samples were snap-frozen immediately after removal and stored at −70°C. Tissue samples of astrocytomas (7 grade I, 4 grade II, 15 grade III, 33 grade IV) were homogenized in MES lysis buffer (0.2 mol/L, pH 6.0) containing 0.1% TritonX-100 and sonicated (MSE Soniprep 150). The protein concentration of the homogenate was determined using the BCA reagent kit (Pierce) and aliquots were diluted to a concentration of 2 to 5 mg/ml and the supernatants were used for CatS assay.
CatS activity was assayed using carbobenzoxy-valyl-valyl-arginyl-N-methylcoumarylamide (VVR) as substrate. Before assay, homogenates were incubated in 100 mmol/L phosphate buffer (pH 7.5) for 1 hour at 37°C to inactivate cathepsins B and L, after which the pH was returned to pH 6.0 using 0.5 mol/L of MES buffer. Assays were performed in 96-well microtitre plates in the presence of 1 mmol/L dithiothreitol, 200 mmol/L ethylenediaminetetraacetate, and 100 μmol/L VVR in 200 mmol/L MES buffer, pH 6.0. Incubation at 37°C was continued for 90 minutes and the reaction was terminated by addition of 100 mmol/L of acetate buffer, pH 4.3. Fluorescence generated by release of methylcoumarin (nMec) from the substrate was read in Perkin Elmer LS50B luminescence spectrophotometer (Perkin Elmer, Shelton, CT, USA) with excitation and emission wavelengths of 370 nm and 460 nm, respectively. Each assay was performed on triplicate samples and controls were included with substrate omitted from the assay. A calibration line was prepared using nMec, and CatS activity was calculated as nmol nMec produced/mg protein hour−1. 1 There was a linear relationship between relative fluorescence activity and nMec concentration and the enzyme assay was linear with respect to reaction time and protein content of the cell homogenate.
For assay of extracellular CatS activity, confluent cultures were maintained for 24 hours in serum-free medium to obviate the effect of endogenous cysteine proteinase inhibitors in fetal calf serum. The medium was then removed from the cells and centrifuged at 1000 × g for 5 minutes to remove any detached cells before assay. Aliquots of medium (30 μl) were assayed for CatS activity using the same procedure used for cell homogenates. Extracellular CatS activity is expressed as nmol nMec produced/mg cellular protein hour−1. 1
Reverse Transcription-Polymerase Chain Reaction and Sequencing
RNA was prepared from confluent astrocytoma cultures and normal brain using RNAstat 60 (Biogenesis Ltd., Poole, Dorset, UK), reverse-transcribed with Maloney murine leukemia virus (MMLV) reverse transcriptase (Advanced Biotechnologies, Columbia, MD) and cDNA amplified by polymerase chain reaction (PCR). The CatS-specific primers used for PCR were designed to flank the CatS coding region and generate a 1002-bp amplicon. They correspond to positions 158–177 and 1123–1153, respectively. 25
CatS forward primer: 5′-atgaaacggctggtttgtgt-3′
CatS reverse primer: 5′-ctagatttctgggtaagagggaaagctagc-3′
The 1001-bp DNA fragment was purified (Gibco) from the 1% agarose gel and ligated into pIND/V5-His-TOPO using TA Cloning (InVitrogen, UK). The resulting construct was used to transform TOP10 cells. Positive clones were selected and plasmid DNA was prepared (Concert MaxiPrep; Gibco). The fragment was sequenced using the dideoxy chain termination method to confirm its identity with CatS.
Immunocytochemistry
Acetone-fixed cells on coverslips were rehydrated with PBS for 5 minutes and incubated for 1 hour at room temperature in goat polyclonal anti-CatS antibody (Santa Cruz Biotechnologies, Santa Cruz, CA) diluted 1:50 in PBS. An isotype-matched rabbit IgG negative control antibody (Dako, Denmark) was used at the same concentration as the primary antibody. The coverslips were washed three times for 5 minutes in PBS before incubation in fluorescein-isothiocyanate-conjugated anti-goat antibody diluted 1:50 in PBS for 1 hour and after three 5-minute washes in PBS were mounted in Citifluor (0.25 g of 1,4 diazobicyclo-2,2,2-octane, 5 ml of PBS, 5 ml of glycerol). Nuclei were stained by incubation in 1.3 μg/ml of propidium iodide in PBS for 10 seconds, and washed in distilled water before mounting in Citifluor.
Immunohistochemistry
Tissue samples used in this study were derived from diagnostic biopsies received from the Regional Neuropathology Service. Tissue was fixed in 10% formalin before embedding in paraffin wax with a Tissue Tek VIP (Miles Scientific) automated processor. The paraffin blocks were sectioned at a thickness of 6 μm, sections were dewaxed, rehydrated, and endogenous peroxidase activity blocked by a 10-minute incubation in 3% H2O2 in methanol, followed by a rinse in distilled water. Sections were subjected to microwave antigen retrieval as previously described. 26 After rinsing in running distilled water, non-specific binding of secondary antibody was blocked by incubating in 5% normal rabbit serum for 10 minutes. Sections were subsequently incubated overnight at 4°C in anti-CatS antibody at 1:50 dilution. Isotype-matched rabbit negative-control antibody raised to Aspergillus niger glucose oxidase was used at 1:200 dilution to achieve an equivalent primary antibody concentration. Bound antibodies were detected with biotinylated rabbit anti-goat IgG and streptavidin-biotin-peroxidase complex as previously described. 26
Dual-Labeled Immunofluorescence
Paraffin sections of brain tumor biopsies were incubated overnight at 4°C in anti-CatS antibody at 1:50 dilution. This was then detected by incubating with biotin-conjugated rabbit anti-goat IgG (1:400 dilution, 30 minutes, RT) followed by 30-minute incubation in streptavidin peroxidase (1:500 dilution, RT; NEN Sciences, USA) with TBS washes between each step. Sections were then incubated for 20 minutes at RT in tetramethylrhodamine tyramide reagent, diluted 1:50 in amplification reagent (tetramethyl-thodamine tyramide reagent (TTR); NEN Life Sciences, Boston, MA, USA). Following further washes in TBS, excess peroxidase sites were quenched with 1% hydrogen peroxide in TBS for 15 minutes. The sections were incubated overnight at 4°C in monoclonal anti-CD68 antibody (Dako) at 1:50 dilution or in monoclonal anti-glial fibrillary acidic protein (GFAP) antibody (Dako) at 1:100 dilution. Sections were then incubated in peroxidase-conjugated rabbit anti-mouse IgG (1:50 dilution) for 30 minutes followed by 20 minutes incubation in fluorescein tyramide reagent, diluted 1:50 in amplification reagent (fluorescein tyramide reagent (FTR); NEN Life Sciences) both at RT. Following final washes in TBS, sections were mounted in Citifluor and viewed with a Leica TCS/NT confocal microscope equipped with a krypton/argon laser.
In Vitro Invasion Assay
Invasion assays were performed as previously described, 27 with variations, using a modified Boyden chamber (Costar Transwell plates) with 12-μm pore membranes. The membranes were coated with Matrigel (100 μg/cm2) and allowed to dry overnight in a tissue culture hood; 5 × 10 U251MG glioblastoma cells were added to the well in 500 μl serum-free medium.
Experiments testing the effect of the CatS inhibitor, 4-Morpholineurea-Leu-HomoPhe-vinylsulphone (LHVS), a gift from Axys Pharmaceuticals, used medium containing LHVS at concentrations of 10 nmol/L and 50 nmol/L. 1.5 ml of homologous conditioned growth medium was added to the lower chamber and triplicate invasion plates were incubated at 37°C and 5% CO2 for 24 hours. Cells remaining on the upper surface of the membrane were removed by wiping and the invaded cells were fixed in Carnoy’s fixative for 15 minutes.
After drying, the nuclei of the invaded cells on the lower surface of the membrane were stained with Hoechst 33258 (50 ng/ml) in HBSS for 30 minutes at room temperature. The insert was washed twice in PBS and mounted in Citifluor for microscopic examination. Invaded cells were viewed with a Leica DMLB fluorescence microscope. Four digital images of representative fields from each of the triplicate membranes were taken at a magnification of ×200. Results were expressed as the mean number of cells present per field.
Results
RT-PCR
mRNA isolated from a range of astrocytoma cell lines was reverse transcribed and subjected to PCR using primers specific for sequences flanking the coding region of CatS. Agarose gel electrophoresis (Figure 1) ▶ showed a single amplicon of a size consistent with CatS (996 bp). After purification the 996 bp amplicon was cloned, sequenced, and shown to have complete identity with the sequence of CatS (GenBank Accession Number M90696).
Figure 1.
RT-PCR of mRNA extracted from cultures derived from astrocytomas of World Health Organization grade I (1 to 3), World Health Organization grade II (4 to 7), World Health Organization grade III (8 and 9), and World Health Organization grade IV. 10 and 11: RT-PCR of mRNA extracted from a normal brain sample. 12: PCR, without RT step, of mRNA extracted from World Health Organization grade IV cell culture to demonstrate the absence of DNA contamination in the mRNA preparation. 13: Positive control mRNA sample of 500 bp. 14: Water (negative control). 15: Size markers. 16: CatS is represented by the fraction of 996 bp.
Immunohistochemistry/Cytochemistry
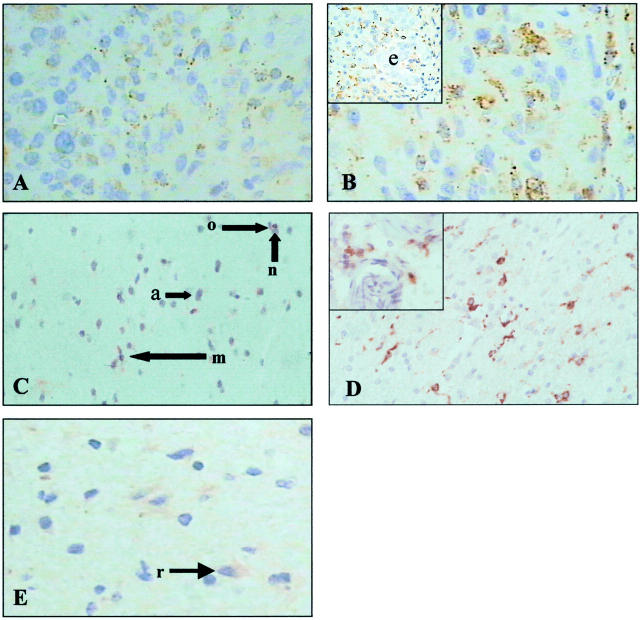
Thirty-one brain tumor biopsy specimens were examined, representing all four World Health Organization grades. 28 (5 grade I, 10 grade II, 5 grade III, 11 grade IV). In all cases tumor cells were positive for CatS expression (Figure 2,A and B) ▶ . Normal astrocytes and other glial cells (Figure 2C) ▶ , neurones (Figure 2C) ▶ and endothelial cells (Figure 2B) ▶ were CatS-negative while reactive astrocytes in zones of gliosis were faintly CatS-positive (Figure 2E) ▶ . The intensity of neoplastic astrocyte staining within and between grades was variable and there was no correlation between level of expression and tumor grade.
Figure 2.
A: CatS immunostaining of World Health Organization grade II astrocytoma. B: CatS immunostaining of World Health Organization grade IV astrocytoma (inset shows area of endothelial hyperplasia (e) with CatS-negative endothelial cells). C: CatS immunostaining of normal brain. n, neurone; a, astrocyte; o, oligodendrocyte; m, macrophage. D: CD68 staining of tumor-associated microglia/macrophages in World Health Organization grade IV astrocytoma (inset shows perivascular macrophages). E: CatS immunostaining of brain with gliosis from a World Health Organization grade II astrocytoma (r, reactive astrocyte). Original magnification, ×400.
CD68 immunostaining confirmed the presence of cells of microglia/monocytic origin within tumors (Figure 2D) ▶ and in surrounding brain. Double immunohistochemical staining for CatS and CD68 revealed expression of CatS in the CD68-positive microglia/monocytic cells as well as in CD68-negative tumor cells (Figure 3, A and B) ▶ . Double immunohistochemical staining for CatS and GFAP revealed distinct localization of CatS and GFAP within astrocytoma cells (Figure 3C) ▶ . Twenty-one astrocytoma cell cultures examined were CatS-positive and immunostaining showed a punctate cytoplasmic distribution characteristic of lysosomal localization (Figure 3D) ▶ . There was no correlation between the degree of immunostaining and grade of tumor.
Figure 3.
A: Immunostaining of grade IV astrocytoma to show CD68-positive microglia/macrophages (arrows). B: CatS/CD68 dual labeling. Microglia/macrophages are positive for CatS and CD68 (arrows) whereas astrocytoma cells (arrowheads) are only positive for CatS. C: CatS (red)/GFAP (green) dual labeling. The lysosomal localization of CatS immunostaining (I) is distinct from that of GFAP which is predominantly within tumor cell foot processes (p). m, mitotic figure; n, outline of nucleus; bv, blood vessel. D: CatS immunostaining of astrocytoma cells in vitro. Nuclei are counterstained with propidium iodide. Original magnification, ×400.
Cathepsin S Activity
Biopsies: CatS activity in tumor lysates varied considerably between tumors (Figure 4) ▶ . Astrocytoma grades I to III had similar ranges of activity; however, while some grade IV tumors expressed activity levels similar to the lower grades, almost half of them had markedly higher levels.
Figure 4.
Specific activity of CatS in lysates derived from biopsies of astrocytomas I-IV.
Cell Cultures
Activity of CatS was measured in 31 astrocytoma cultures. Intracellular activity was present in all cultures and within each grade there was considerable variation in specific activity (Figure 5A) ▶ . There was no correlation between CatS activity and grade of the tumor of origin.
Figure 5.

Specific activity of CatS in astrocytoma cultures (grades I to IV). a: Intracellular activity. b: Extracellular activity. c: Plot of extracellular CatS versus intracellular CatS activity for each culture.
All cultures were shown to secrete CatS into the medium and there was a clear trend toward high levels of secretion in cultures derived from grade IV tumors (Figure 5B) ▶ . There was no correlation between secreted CatS activity and cell-associated activity (Figure 5C) ▶ .
Invasion Assay
The effect of the CatS inhibitor LHVS29 was assessed using the U251MG glioblastoma cell line in the Matrigel invasion assay. Incorporation of 10 nmol/L and 50 nmol/L LHVS in the medium produced significant decreases (P < 0.0001) in invasion of 49% and 61%, respectively (Figure 6) ▶ .
Figure 6.
In vitro invasion assay. Photomicrographs (magnification, ×200) show Hoechst-stained nuclei of U251MG glioblastoma cells which have invaded to the lower side of the Matrigel-coated microporous membrane. a: Control assay. b: 10 nmol/L LHVS incorporated in the invasion assay. c: 50 nmol/L LHVS incorporated in the assay. The histogram is a quantitative summary of the data. Each assay was performed in triplicate and four fields were counted in each assay.
Discussion
Biochemical and immunohistochemical studies of the cysteine proteinases CatB and CatL have reported their presence in astrocytomas in contrast to their absence from astrocytes in normal brain. 7 The present investigation has demonstrated for the first time that the related cysteine proteinase CatS is also expressed in astrocytomas in vivo and in vitro. Normal astrocytes, however, were negative for CatS immunoreactivity in agreement with other studies of CatS in normal brain. Neurones, oligodendrocytes, and endothelial cells were also CatS-negative. Petanceska 30 used in situ hybridization on rat brain to show that in contrast to the wide distribution of CatB and CatL, the expression of CatS mRNA is restricted to monocyte-derived microglial cells. In human brain also, CatS is not widely distributed. 31 In an immunohistochemical study, Lemere et al 32 demonstrated the absence of CatS from normal astrocytes, but in the brain of patients with Down syndrome and Alzheimer’s disease CatS was up-regulated in a subset of astrocytes and in neurones with neurofibrillary tangles.
Few studies of CatS in tumors have been carried out, but in an immunohistochemical survey of soft tissue sarcomas Wurl et al 33 showed that 65% of cases expressed CatS. CatS expression was associated with poor prognosis and local recurrence. Fernandez et al 34 demonstrated CatS expression at all stages of prostatic carcinoma progression with no correlation between expression levels and tumor grade. In a study of lung cancer, Kos et al 35 found higher levels of CatS protein in tumor tissue when compared with adjacent control tissue, but in this case the risk of death was higher in patients with low levels of CatS in the tumor and parenchyma. Nissler et al 36 demonstrated that intracellular pro-CatS in a lung tumor cell line has twice the half-life of pro-CatS expressed in human macrophages and that substantial amounts of pro-CatS were secreted in vitro. The present immunohistochemical investigation of CatS protein found up-regulated CatS expression in astrocytoma cells but did not suggest a positive correlation between CatS expression level and astrocytoma grade. However, direct measurement of CatS activity in astrocytoma homogenates indicated highest activity levels in grade IV tumors. Interpretation of CatS expression data from tumor lysates is problematic because of the cellular complexity of the tumor environment, which contains host normal and inflammatory cells in addition to tumor cells. We have shown that normal glial cells, neurones, and endothelial cells do not express CatS. However, macrophages and microglia may make significant contributions to the apparent CatS expression of the tumor. CD68/CatS dual labeling in the current investigation confirms the presence of considerable numbers of CatS-positive microglia/macrophages. Astrocytomas characteristically have a high content of cells of microglial/monocytic origin, 37 with a higher density present in high-grade astrocytomas relative to low-grade tumors. A positive correlation has been reported between microglia/monocyte content and astrocytoma grade and this may explain the high CatS-specific activity that we found in many grade IV tumors. 38,39 It seems possible that brain microglia and macrophages may play an active role in tumor invasion rather than non-specific reaction to tissue injury. This is suggested by Bettinger et al 40 who have shown that microglial cells promote astrocytoma migration in vitro. The secreted migration-stimulating factors were not specifically identified, although growth factors such as transforming growth factor-β were suggested as potential candidates. However, microglia/macrophages express high levels of a range of proteolytic enzymes including CatS which may augment the proteolytic activity of the tumor cells and promote invasion by modifying the extracellular matrix. 41-43
To assess tumor cell expression and a potential role in invasion in the absence of other cell types, we have used astrocytoma cultures. We found no correlation between intracellular CatS activity and grade of the tumor of origin. In contrast, extracellular activity, which is likely to be most relevant to proteolysis associated with the invasive process, was significantly elevated in grade IV tumors. The absence of up-regulation of intracellular enzyme in the presence of increased secretion may be unexpected, but a similar situation has been described during macrophage activation. Liuzzo et al 14 found that lipopolysaccharide and other inflammatory mediators induced an increase in CatS secretion while expression of intracellular CatS mRNA and protein was down-regulated.
Inhibition of CatS activity of U251MG cells with LHVS resulted in a 60% reduction in invasion, suggesting a significant role for CatS in the invasive process. Biroc et al 44 observed a comparable 60% reduction in joint tissue/ECM destruction with oral LHVS administration in a rat model of rheumatoid arthritis. Local invasion of tumor cells beyond resection borders is a feature of malignant astrocytomas leading to recurrence which is largely responsible for failure of conventional astrocytoma therapy. Protease inhibitors may therefore offer a method for the control of proteolytic activity associated with ECM degradation and cell invasion. It is likely that successful treatment of malignant astrocytoma will involve the application of combinatorial therapeutic strategies.
CatS retains optimal activity over a wider pH range (5.0 to 7.5) than CatB and CatL (5.5 to 6.5). 15 The broad pH profile of mature CatS indicates that it would remain active in the astrocytoma extracellular environment. We have demonstrated the presence of extracellular CatS activity in vitro which may reflect either secretion of mature enzyme or extracellular activation of secreted proenzyme by other proteinases. Active CatS in the extracellular environment of astrocytomas may contribute to degradation of ECM components thereby facilitating the invasive process.
In summary, this investigation has demonstrated that CatS is expressed and active in astrocytoma cells. In addition, we have provided evidence that CatS plays a role in the invasive process and is therefore a potential target for anti-invasive therapy.
Acknowledgments
We thank Dr. Jim Palmer of Axys Pharmaceuticals, for the gift of 4-Morpholineurea-Leu-HomoPhe-vinylsulphone and Gordon McGregor for his assistance with immunohistochemistry.
Footnotes
Address reprint requests to Patrick G. Johnston, Department of Oncology, Belfast City Hospital, Lisburn Road, Belfast BT9 7AB. E-mail: p.johnson@qub.ac.uk.
Supported by the Brain and Spine Foundation; the Samantha Dickson Research Trust; the Royal Victoria Hospital, Belfast; the Health Research Board, Dublin; the Ulster Cancer Foundation; Action Cancer; the Mason Medical Foundation; the Irish Institute of Clinical Neuroscience; and the Royal College of Surgeons, Edinburgh.
References
- 1.Daumas-Duport C, Scheithauer BW, Kelly T: A histologic and cytologic method for the spatial definition of gliomas. Mayo Clin Proc 1987, 62:435-439 [DOI] [PubMed] [Google Scholar]
- 2.Silbergeld DL, Chicoine MR: Isolation and characterization of human malignant glioma cells from histologically normal brain. J Neurosurg 1997, 86:525-531 [DOI] [PubMed] [Google Scholar]
- 3.Liotta LA: Tumor invasion and metastases role of the extracellular-matrix: Rhoads memorial award lecture. Cancer Res 1986, 46:1-7 [PubMed] [Google Scholar]
- 4.Rooprai HK, McCormick D: Proteases and their inhibitors in human brain tumours: a review. Anticancer Res 1997, 17:4151-4162 [PubMed] [Google Scholar]
- 5.McCormick D: Secretion of Cathepsin B by human gliomas in vitro. Neuropathol Appl Neurobiol 1993, 19:146-151 [DOI] [PubMed] [Google Scholar]
- 6.Mikkelsen T, Yan PS, Ho KL, Sameni M, Sloane BF, Rosenblum ML: Immunolocalization of cathepsin B in human glioma: implications for tumor invasion and angiogenesis. J Neurosurg 1995, 83:285-290 [DOI] [PubMed] [Google Scholar]
- 7.Rempel SA, Rosenblum ML, Mikkelsen T, Yan P-S, Ellis KD, Golembieski WA, Sameni M, Rozhin J, Ziegler G, Sloane BF: Cathepsin B expression and localization in glioma progression and invasion. Cancer Res 1994, 54:6027-6031 [PubMed] [Google Scholar]
- 8.Sivaparvathi M, Sawaya R, Wang SW, Rayford A, Yamamoto M, Liotta LA, Nicolson GL, Rao JS: Overexpression and localization of cathepsin B during the progression of human gliomas. Clin Exp Metastasis 1995, 13:49-56 [DOI] [PubMed] [Google Scholar]
- 9.Strojnik T, Kos J, Zidanik B, Golouh R, Lah T: Cathepsin B immunohistochemical staining in tumor and endothelial cells is a new prognostic factor for survival in patients with brain tumors. Clin Cancer Res 1999, 5:559-567 [PubMed] [Google Scholar]
- 10.Konduri S, Lakka SS, Tasiou A, Yanamandra N, Gondi CS, Dinh DH, Olivero WC, Gujrati M, Rao JS: Elevated levels of cathepsin B in human glioblastoma cell lines. Int J Oncol 2001, 19:519-524 [DOI] [PubMed] [Google Scholar]
- 11.Mohanam S, Jasti SL, Kondraganti SR, Chandrasekar N, Lakka SS, Kin Y, Fuller GN, Chandrasekar N, Lakka SS, Kin Y, Fuller GN, Yung AWK, Kyritsis AP, Dinh DH, Olivero WC, Gujrati M, Ali-Osman F, Rao JS: Down-regulation of cathepsin B expression impairs the invasive and tumorigenic potential of human glioblastoma cells. Oncogene 2001, 20:3665-3673 [DOI] [PubMed] [Google Scholar]
- 12.Sameni M, Elliott E, Ziegler G, Fortgens PH, Dennison C, Sloane BF: Cathepsins B and D are localized at the surface of human breast cancer cells. Pathol Oncol Res 1995, 1:43-53 [DOI] [PubMed] [Google Scholar]
- 13.Sivaparvathi M, Yamamoto M, Nicolson GL, Gokaslan ZL, Fuller GN, Liotta, Sawaya R, Rao JS: Expression and immunohistochemical localization of cathepsin L during the progression of human gliomas. Clin Exp Metastasis 1996, 14:27-34 [DOI] [PubMed] [Google Scholar]
- 14.Liuzzo JP, Petanceska SS, Moscatelli D, Devi LA: Inflammatory mediators regulate cathepsin S in macrophages and microglia: a role in attenuating heparan sulfate interactions. Molecular Med 1999, 5:320-333 [PMC free article] [PubMed] [Google Scholar]
- 15.Bromme D, Bonneau PR, Lachance P, Wiederanders B, Kirschke H, Peters C, Thomas DY, Storer AC, Vernet T: Functional expression of human cathepsin S in saccharomyces cerevisiae: purification and characterization of the recombinant enzyme. J Biol Chem 1993, 268:4832-4838 [PubMed] [Google Scholar]
- 16.Reise RJ, Mitchell RN, Villadangos JA, Shi GP, Palmer JT, Karp ER, De Sanctis GT, Pleogh HL, Chapman HA: Cathepsin S activity regulates antigen presentation and immunity. J Clin Invest 1998, 101:2351-2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Driessen C, Bryant RR, Lennon-Dumenil AM, Villadangos JA, Bryant PW, Shi GP, Chapman HA, Ploegh HL: Cathepsin S controls the trafficking and maturation of MHC class II molecules in dendritic cells. J Cell Biol 1999, 147:775-790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi GP, Bryant RA, Riese R, Verhelst S, Driessen C, Li Z, Bromme D, Ploegh HL, Chapman HA: Role for cathepsin F in invariant chain processing and major histocompatibility complex class II peptide loading by macrophages. J Exp Med 2000, 191:1177-1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munger JS, Haass C, Lemere CA, Shi GP, Wong WSP, Teplow DB, Selkoe DJ, Chapman HA: Lysosomal processing of amyloid precursor protein to A β peptides: a distinct role for cathepsin S. Biochem J 1995, 311:299-305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy VY, Zhang QY, Weiss SJ: Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages. Proc Natl Acad Sci USA 1995, 92:3849-3853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liuzzo JP, Petanceska SS, Devi LA: Neurotrophic factors regulate cathepsin S in macrophages and microglia: a role in the degradation of myelin basic protein and amyloid beta peptide. Molecular Med 1999, 5:334-343 [PMC free article] [PubMed] [Google Scholar]
- 22.Frame MC, Freshney RI, Vaughan PT, Graham DI, Shaw R: Interrelationship between differentiation and malignancy-associated properties in glioma. Br J Cancer 1984, 49:269-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chauzy C, Delpech B, Olivier A, Bastard C, Girard N, Courel MN, Maingonnat C, Frebourg T, Tayot J, Creissard P: Establishment and characterisation of a human glioma cell line. Eur J Cancer 1992, 28A:1129-1134 [DOI] [PubMed] [Google Scholar]
- 24.Chen TR: In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res 1977, 104:255-262 [DOI] [PubMed] [Google Scholar]
- 25.Wiederanders B, Bromme D, Kirschke H, Vonfigura K, Schmidt B, Peters C: Phylogenetic conservation of cysteine proteinases: cloning and expression of a cDNA coding for human cathepsin S. J Biol Chem 1992, 267:13708-13713 [PubMed] [Google Scholar]
- 26.McQuaid S, McConnell, McMahon J, Herron B: Microwave antigen retrieval for immunocytochemistry in formalin-fixed, paraffin-embedded postmortem CNS tissues. J Pathol 1995, 176:207-216 [DOI] [PubMed] [Google Scholar]
- 27.Radotra B, McCormick D: Glioma invasion in vitro is mediated by CD44-hyaluronan interactions. J Pathol 1997, 181:434-438 [DOI] [PubMed] [Google Scholar]
- 28.Cavenee WK, Furnari FB, Nagane M, Huang HJS, Newcomb EN, Bigner DD, Weller W, Berens ME, Plate KH, Israel MA, Noble MD, Kleihues P: Diffusely infiltrating astrocytomas. Kleihues P Cavenee WK eds. Pathology and Genetics: Tumours of the Nervous System. 2000, :pp 10-21 IARC Press, Lyon [Google Scholar]
- 29.Palmer JT, Rasnick D, Klaus JL, Bromme D: Vinyl sulfones as mechanism-based cysteine protease inhibitors. J Med Chem 1995, 38:3193-3196 [DOI] [PubMed] [Google Scholar]
- 30.Petanceska S, Burke S, Watson SJ, Devi L: Differential distribution of messenger-RNAs for cathepsin B, cathepsin L and cathepsin S in adult rat brain: an in situ hybridization study. Neuroscience 1994, 59:729-738 [DOI] [PubMed] [Google Scholar]
- 31.Shi GP, Webb AC, Foster KE, Knoll JHM, Lemere CA, Munger JS, Chapman HA: Human cathepsin S: chromosomal localization, gene structure, and tissue distribution. J Biol Chem 1994, 269:11530-11536 [PubMed] [Google Scholar]
- 32.Lemere CA, Munger JS, Shi GP, Natkin L, Haass C, Chapman HA, Selkoe DJ: The lysosomal cysteine protease, cathepsin S, is increased in Alzheimer’s disease and Down syndrome brain: an immunocytochemical study. Am J Pathol 1995, 146:848-860 [PMC free article] [PubMed] [Google Scholar]
- 33.Wurl P, Taubert H, Meye A, Dansranjavin T, Weber E, Gunther D, Berger D, Schmidt H, Dralle H, Rath FW: Immunohistochemical and clinical evaluation of cathepsin expression in soft tissue sarcomas. Virchows Arch 1997, 430:221-225 [DOI] [PubMed] [Google Scholar]
- 34.Fernandez PL, Farre X, Nadal A, Fernandez E, Peiro N, Sloane BF, Shi GP, Chapman HA, Campo E, Cardesa A: Expression of cathepsins B and S in the progression of prostate carcinoma. Int J Cancer 2001, 95:51-55 [DOI] [PubMed] [Google Scholar]
- 35.Kos J, Sekirnik A, Kopitar G, Cimerman N, Kayser K, Stremmer A, Fiehn W, Werle B: Cathepsin S in tumours, regional lymph nodes, and sera of patients with lung cancer: relation to prognosis. Br J Cancer 2001, 85:1193-1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nissler K, Strubel W, Kreusch S, Rommerskirch W, Weber E, Wiederanders B: The half-life of human procathepsin S. Eur J Biochem 1999, 263:717-725 [DOI] [PubMed] [Google Scholar]
- 37.Giometto B, Bozza F, Faresin F, Alessio L, Mingrino S, Tavolato B: Immune infiltrates and cytokines in gliomas. Acta Neurochir (Wien) 1996, 138:50-56 [DOI] [PubMed] [Google Scholar]
- 38.Morris CS, Esiri MM: Immunocytochemical study of macrophages and microglial cells and extracellular matrix components in human CNS disease:: 1. gliomas. J Neurol Sci 1991, 101:47-58 [DOI] [PubMed] [Google Scholar]
- 39.Nishie A, Ono M, Shono T, Fukushi J, Otsubo M, Onoue H, Ito Y, Inamura T, Ikezaki K, Fukui M, Iwaki T, Kuwano M: Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res 1999, 5:1107-1113 [PubMed] [Google Scholar]
- 40.Bettinger I, Thanos S, Paulus W: Microglia promote glioma migration. Acta Neuropathol (Berl) 2002, 103:351-355 [DOI] [PubMed] [Google Scholar]
- 41.Hildenbrand R, Dilger I, Horlin A, Stutte HJ: Urokinase and macrophages in tumour angiogenesis. Br J Cancer 1995, 72:818-823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gottschall PE, Yu X, Bing B: Increased production of gelatinase B (matrix metalloproteinase-9) and interleukin-6 by activated rat microglia in culture. J Neurosci Res 1995, 42:335-342 [DOI] [PubMed] [Google Scholar]
- 43.Nakajima K, Honda S, Tohyama Y, Kurihara T, Kohsaka S: Ceramide-enhanced urokinase-type plasminogen activator (uPA) release is mediated by protein kinase C in cultured microglia. Glia 2000, 32:226-233 [DOI] [PubMed] [Google Scholar]
- 44.Biroc SL, Gay S, Hummel K, Magill C, Palmer JT, Spencer DR, Sa S, Klaus JL, Michel BA, Rasnick, Gay RE: Cysteine protease activity is up-regulated in inflamed ankle joints of rats with adjuvant-induced arthritis and decreases with in vivo administration of a vinyl sulfone cysteine protease inhibitor. Arthritis Rheum 2001, 44:703-711 [DOI] [PubMed] [Google Scholar]