Abstract
Previous studies have suggested that surface components of papillary thyroid carcinoma (PTC) cells may be aberrantly glycanated, but the precise nature of these molecules has not been unveiled nor documented to be of clinical relevance. A monoclonal antibody was raised against a unique keratan sulfate (KS) determinant and used to differentially screen benign and malignant thyroid tissue for the expression of components carrying these moieties. In a total of 349 cases of benign and malignant thyroid lesions, 100% of the 115 PTC cases examined (including various histological subtypes) were found to contain KS-bearing molecules, whereas these were virtually absent from benign tissues and other thyroid tumors, with the exception of 21% of the follicular carcinoma cases analyzed. A composite immunoaffinity chromatography, immunochemistry, and mass spectrometric approach revealed that the PTC-specific KS-bearing macromolecules were unique glycoforms of thyroglobulin and transferrin. Combined, reciprocal immunoprecipitation and Western blotting further indicated that the former glycoform predominated and that most of the transferrin produced by PTC was glycanated with KS moieties. Fluorescent keratanase II-based fingerprinting of the KS moieties bound to these isoforms further demonstrated several PTC-specific peculiarities: 1) that a considerable portion of the moieties was covalently attached via a novel core protein linkage structure; 2) they had an unusual extended average length; 3) an unusual relative ratio of highly sulfated disaccharides terminating with α (2-3)-linked N-acetylneuraminic acid capping residues; and 4) a novel unidentified oligosaccharide moiety at the nonreducing terminus. Comparative analysis of the relative distribution of transferrin in benign versus PTC tissues highlighted a marked malignancy-associated abundance of the molecule, with a >75% frequency in expression in PTC. These findings demonstrate that PTC cells synthesize unique post-translationally modified thyroglobulin and transferrin variants in situ that may be directly exploitable for diagnosis, through histological and noninvasive cytological procedures; for devising novel strategies for antibody-guided imaging of this tumor in vivo; and for postsurgery follow-up of PTC patients.
Papillary thyroid carcinoma (PTC) is by far the most frequent malignant tumor of the thyroid with a threefold prevalence in females. 1 Early diagnosis is pivotal because this tumor can effectively be treated with surgery assuring in most cases a disease-free survival. 2 Although a combination of clinical evaluation, ultrasonography, and scintigraphy may be suggestive of malignancy, cytological presurgery (by fine-needle aspiration) and histological postsurgery examinations remain the most reliable diagnostic tools. 2 However, in some circumstances, a distinction between benign and malignant lesions, based solely on morphological features, is ambiguous and may result in diagnostic pitfalls. Therefore, despite the relatively facile clinical management of this tumor entity, there has been a considerable interest in searching for more reliable genetic and molecular markers. The advent of powerful genetic and molecular methods, such as those based on global gene screens through differential display, SAGE, and cDNA microarray have recently identified a number of putative genetic signatures and single markers of certain relevance. 3-6
Apart from these recent genetic/molecular approaches, studies of the past 2 decades have lead to the identification of at least 50 putative PTC markers, which include genetic anomalies such as loss of heterozygosity and microsatellite instability, and posttranslational aberrations such as perturbed glycosylation of certain epithelial components. Previous studies on thyroid neoplasms have identified one of these latter types of PTC-associated abnormalities, which consists in the formation of poly-N-acetyllactosamines glycans carrying 6-O-sulfations on certain of their galactose or N-acetylglucosamine units and sialic and/or fucosyl groups at their nonreducing terminal ends. 7,8 However, the precise composition of these glycan moieties, the nature of their backbone proteins and the clinical implication of these transformation-dependent, posttranslational modifications have not been clarified.
We have produced a monoclonal antibody denoted 373E1 and directed against a unique sialylation/conformational-dependent KS epitope that detects a novel glycoprotein complex produced almost exclusively by PTC cells. In this study, combined immunochemical, proteomic, and postproteomic techniques were therefore used to unravel the identity of the constituents of this KS complex, as well as to define the fine structure of the KS moieties bound to these components in PTC.
Materials and Methods
Production and Characterization of Antibody 373E1
Monoclonal antibody (mAb) 373E1 was produced according to standard protocols by immunization of BALB/c female mice with a preparation of embryonic chick proteoglycans (PGs) extracted with 4 mol/L of GuHCl in the presence of protease inhibitors and purified by sequential ion-exchange and gel permeation chromatography according to a procedure described elsewhere. 9 Initial selection of hybridoma clones was performed by conventional enzyme-linked immunosorbent assay (ELISA) using the immunogen and analogous PG preparations from adult chick gizzard. Selected clones were then tested for their ability to recognize a number of KS-bearing cartilage PGs (see below), such as to ascertain their reactivity against this type of glycan structure. Of ∼35 clones originally identified, 3 showed a high and specific reactivity against all of the above types of PGs and one denoted 373E1 was chosen for further subcloning and characterization. The antibody was determined to be of IgM class and was used, depending on the type of immunochemical assay to be performed, in the form of hybridoma culture supernatant, purified immunoglobulins obtained after caprylic acid-precipitation, or ascites fluid produced in nude mice according to standard protocols. Characteristics of mAb 373E1 were determined by direct and competitive ELISA and Western blotting, using a series of KS-bearing and KS-free PGs and mucins as screening antigens. These reactivities were additionally compared to those of previously described anti-KS mAbs, such as 1B4 (mouse IgG), 3D2 (mouse IgM), 4D1 (mouse IgM), and 2D3 (mouse IgM; obtained from Michael Sorrell, Department of Cell and Developmental Biology, Case Western University, Cleveland, OH), 5D4 (mouse IgG; ICN Pharmaceuticals Inc., Costa Mesa, CA), as well as various plant lectins. PGs carrying KS chains and used for antibody characterization included bovine nasal cartilage aggrecan, human articular cartilage aggrecan, chick embryonic cartilage aggrecan, and bovine and human tendon fibromodulin provided by Dick Heinegård (Department of Cell and Molecular Biology, University of Lund, Lund, Sweden). PGs lacking KS chains and used as controls included the rat chondrosarcoma aggrecan (ICN Pharmaceuticals Inc.), mouse EHS perlecan, and bovine tendon decorin and biglycan. MUC2 from porcine intestine and a mixture of MUC5AC, MUC5B, and MUC7 from bovine submaxillary glands were obtained from Sigma and used as negative controls. PGs and mucins were immobilized onto 96-microwell plates (Greiner Labortechnik, Germany) by coating with 0.5 to 1 μg/well of the molecules overnight at 4°C in 0.05 mol/L of bicarbonate buffer, pH 9.6. In some cases, immobilized PGs were chemically desulfated by treatment with 50 mmol/L of HCl in 100% methanol for 4 hours at room temperature before being incubated with the 373E1 antibody. Coatings with the PGs and mucins was routinely followed by blocking with 1% bovine serum albumin in phosphate-buffered saline (PBS) for 1 hour at room temperature, extensive washings, and in some cases digested with the various deglycosylating enzymes described below in their appropriate buffers for 1 hour at 37°C. Digested and nondigested model antigens were then incubated for 1 hour at room temperature with various concentrations of the 373E1 antibody (ie, 1:10 to 1:100,000, depending on the form in which the antibody was used). In a number of experiments, incubation with 373E1 antibody was performed in the presence of a number of molecules to be tested as putative competitors for epitope binding. These were used either in the intact or enzyme digested form. In all cases, excess unbound antibody was washed away and detection of the bound antibody was accomplished by a further 1-hour incubation of the plates with horseradish peroxidase-conjugated goat anti-mouse secondary antibodies (Zymed Immunochemicals, San Francisco, CA) diluted 1:1000 in PBS with 1% bovine serum albumin followed by incubation with 2,2′-azino-di[3-ethyl-benzthiazolinsulfonate].
Patients and Tissue Samples
Tissue samples were collected from patients that underwent surgical removal of one or both thyroid lobes during the years 1989 to 2001 and were classified according to the recently recommended reporting format for thyroid carcinomas. 10 Thyroid tumors and nonneoplastic lesions (a total 349 specimens) were diagnosed at either the Section of Anatomical Pathology, University of Catania, or at the analogous Section at the Institute “Casa Sollievo della Sofferenza” of San Giovanni Rotondo. Neoplastic specimens included 60 follicular adenomas, of which 14 were predominantly composed of Hurthle cells; 115 PTCs (91 females and 24 males in ages from 22 to 83 years), including 78 of the classical type, 10 follicular variants, 7 cases of the encapsulated variant, 15 cases of microcarcinomas, ie, presenting a lesion size <1.0 cm in width, 2 cases of the Hurthle cell variant, 2 cases of the macrofollicular variant, and 1 case of a Warthin’s tumor-like variant; 52 follicular carcinomas, including 29 of the widely invasive and 23 cases of the minimally invasive type of which 5 cases of the former and 4 of the latter type were Hurthle cell variants; 10 medullary carcinomas and 5 anaplastic carcinomas. Local lymphonodal metastases were found in 18 patients affected by PTC. Nonneoplastic lesions included 48 nodular goiters, 20 Grave’s disease cases, and 11 cases of Hashimoto’s thyroiditis. Focal to more widespread papillary hyperplasia was found in 19 nodular goiters, 7 Grave’s disease cases, and 12 of the follicular adenoma patients. Control tissues considered as healthy were represented by the morphologically normal areas surrounding the PTC lesions and by five additional histologically normal thyroids from patients that had undergone total thyroidectomy after diagnosis of PTC in the contralateral lobe.
Immunohistochemical Staining
Tissue samples to be processed for indirect immunohistochemistry were fixed in 10% buffered formalin and routinely embedded in paraffin or OCT medium for cryosectioning. Cryosectioned specimens included 10 PTC lesions, 10 nodular goiters, 5 follicular adenomas, and 3 widely invasive follicular carcinomas. Before incubation with primary antibodies, cryosections were air-dried for 20 minutes, fixed in cold acetone for 10 minutes at room temperature, further air-dried for 10 minutes, and then incubated with primary and secondary antibodies. Indirect immunohistochemical stainings were performed by the standard streptavidin-biotin-labeling technique using the LSAB kit (DAKO, Glostrup, Denmark). Sections derived from paraffin-embedded specimens were deparaffinized in xylene for 15 minutes, rehydrated, and treated with 3% H2O2 for 10 minutes to block endogenous peroxidase activity, followed by extensive rinsing in double-distilled water and further rinsing for 15 minutes in 0.01 mol/L of phosphate-buffered saline (PBS), pH 7.4. Deparaffinized sections were incubated with either of the primary antibodies, 373E1 (Biogenex, San Ramon, CA), anti-transferrin (clone HT1–13.6.3; ICN Biochemicals, Inc.), and anti-HBME-1 (Dakopatts Carpinteria, CA), or control antibodies 7D1 and 2C12 (mouse IgM) directed against human articular cartilage aggrecan and vascular PG-M/versicans, at 4°C overnight. This was followed by incubation with biotinylated anti-mouse IgM or IgG secondary antibodies for 15 minutes and peroxidase-conjugated streptavidin for 15 minutes at room temperature. In several cases of immunolabeling with antibody 373E1 and in all cases of stainings with the anti-transferrin antibody, sections were pretreated with citrate buffer (pH 6.0) and exposed to radiation in a microwave oven. Superior antigen retrieval was observed for anti-transferrin antibodies, but not for the antigens recognized by antibody 373E1. Similarly, pretreatments of the sections with 0.1% trypsin in PBS did not alter the staining pattern observed with antibody 373E1. To reduce the commonly seen nonspecific immunoreactivity in Hurthle cells, which are frequently present in both benign and malignant thyroid tissues, tissue sections were pretreated with 10 mg/ml of ovalbumin in PBS followed by 0.2% biotin in PBS, each for 15 minutes at room temperature. Bound antibody was revealed by incubation with 3,3′-diaminobenzidine (Sigma Chemical Co.) in 0.01% H2O2 for 5 minutes at room temperature. Sections were then counterstained with hematoxylin, dehydrated, and mounted. The percentage of positively stained cells was assessed semiquantitatively according to a four-tiered-system (<1% positive cells, negative staining; 1 to 10% positive cells, focal staining; 11 to 50% positive cells, heterogeneous staining; >50%, widespread staining). Sections from five cases of classical type PTC and four cases of nodular goiters were treated with: endo-β-galactosidase, having the following specificity:
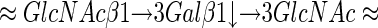 |
keratanase I, having the following specificity:
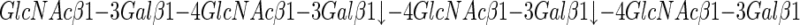 |
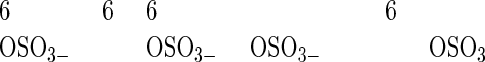 |
keratanase II, having the following specificity:
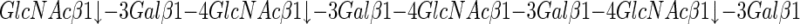 |
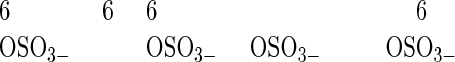 |
three different neuraminidases, having the following specificities:
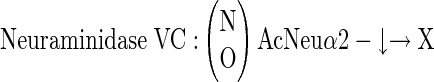 |
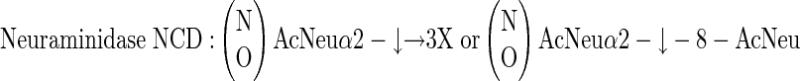 |
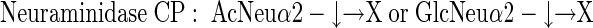 |
Fucosidase or chondroitinase ABC (samples of the enzymes were purchased from three companies: ICN Biochemicals, Gwine, CA; Roche, Basel, Switzerland; and Seikagaku Corporation, Tokyo, Japan) for 2 hours at 37°C, with the enzymes diluted in the appropriate buffers (see below), before being incubated with primary antibodies.
Extraction and Purification of the KS-Bearing Thyroid Components
Surgical material was homogenized and extracted for 20 hours at 4°C in 50 mmol/L of Tris-HCl buffer, pH 8.0, containing 7 mol/L urea, 0.2 mol/L NaCl, 5 mmol/L ethylenediaminetetraacetic acid, 0.1% Triton X-100, 1 mmol/L phenylmethyl sulfonyl fluoride, 10 mmol/L N-ethylmaleimide, 10 mmol/L para-aminobenzamidine, and 100 mmol/L caproic acid (Sigma Chemical Co., St Louis, MO), at a tissue-to-buffer ratio of 1:30. The extracted material was centrifuged at 14,000 rpm for 1 hour at 4°C and the supernatant collected and either extensively dialyzed against immunoprecipitation buffer (see below) or further separated by high performance liquid chromatography. In this case, the material was loaded onto an ion-exchange Mono-Q column, which was equilibrated and eluted at a flow rate of 0.3 ml/min with a linear 0 to 0.5 mol/L gradient of LiCl4 in 10 mmol/L Tris-HCl, pH 8.0, containing 5 mmol/L of ethylenediaminetetraacetic acid and 0.5% Brij 35. Chromatographic runs were monitored by adsorbance at 260 and 232 nm and the collected fractions were analyzed by ELISA using antibody 373E1 or dot-blotting using Alcian blue staining. 10,11 Positive fractions were pooled and either processed for immunoprecipitation or directly resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotted.
Immunoprecipitation
Benign and malignant tissue extracts corresponding to 1 to 3 mg of total protein, or pooled high performance liquid chromatography fractions, were solubilized/dialyzed against immunoprecipitation buffer composed of PBS with 1% (v/v) Igepal CA 630 (Sigma), 0.1% (w/v) SDS, 5 mmol/L ethylenediaminetetraacetic acid, 5 mmol/L N-ethylmaleimide, and 2 mmol/L phenylmethyl sulfonyl fluoride and incubated 2 hours at 4°C with 2 μl of normal mouse serum and 100 μl of protein A-Sepharose CL-4B (Amersham-Pharmacia Biotech), which had previously been diluted 1:1 in immunoprecipitation buffer. The sample was centrifuged for 15 minutes at 10,000 rpm and further incubated overnight at 4°C with 200 μl of hybridoma culture supernatant of antibody 373E1, containing ∼15 μg/ml of the specific immunoglobulin, or 0.5 to .5 μg of anti-thyroglobulin, anti-transferrin antisera, or anti-MUC1 antibody (clone E29; DAKO), used as reference antibody. Samples were centrifuged and the immunoprecipitates extensively washed in immunoprecipitation buffer, followed by resuspension in sample buffer: 0.25 mmol/L Tris-HCl, pH 6.8, 2% (w/s) SDS, 10% (w/s) glycerol, and 0.01% (w/v) bromphenol blue. Immunoprecipitated samples were either tested in ELISA, dot-blotting, or resolved by SDS-PAGE, with and without subsequent electrotransfer onto nitrocellulose membranes and immunoblotting (see below). These experiments also involved reciprocal immunoprecipitations with antibody 373E1, anti-thyroglobulin, or anti-transferrin antibodies, followed by immunoblotting of the precipitated material with the heterologous antibody, or depletion of the material immunoprecipitated by these antibodies and SDS-PAGE separation and immunoblotting of the remaining material.
SDS-PAGE, SDS-Agarose Electrophoresis, and Immunoblotting
The material immunoprecipitated with antibody 373E1 was resolved by SDS-PAGE on 3 to 8% gradient gels or SDS-agarose gel (1%) electrophoresis under reducing and nonreducing conditions, with and without previous enzymatic digestion for 1 to 3 hours at 37°C. Enzyme (Roche, Seikagaku Corporation, and ICN Biochemicals), concentrations and treatment conditions were as follows: keratanase I, 0.01 U/ml in 50 mmol/L Tris-HCl buffer, pH 7.6; chondroitinase ABC and ACII, 0.3 U/ml in 50 mmol/L Tris-HCl buffer, pH 7.6; keratanase II, 0.01 U/ml in Na-acetate buffer, pH 6.5; endo-β-galactosidase 0.01 U/ml in 50 mmol/L Na-acetate buffer, pH 5.8; β-galactosidase, 0.01 U/ml in 100 mmol/L Na-citrate buffer with 10% glycerol, pH 4.3; neuraminidases from Vibrio cholerae, Clostridium perfringens, Athrobacter ureafaciens, and Newcastle disease virus, 0.05 U/ml in Na-acetate buffer, pH 5.5, 5.0, and 5.8, respectively; α-l-fucosidase, 0.05 U/ml in Na-acetate, pH 6.3; N-glycosidase A and F, 0.01 U/ml 50 mmol/L Na-citrate buffer with 10% glycerol, pH 5.0; O-glycosidase, 0.01 U/ml in PBS; α-1,3/4-l-fucosidase 1:100 dilution in 50 mmol/L Na-citrate, pH 6.3; N-acetyl-β-d-glucosaminidase 1:10,000 dilution in 50 mmol/L Na-acetate, pH 5.0; endoglycosidases H/F 0.01 U/ml in 50 mmol/L Na-sodium acetate buffer, pH 5.8. Resolved material was either stained by using a combined silver- and Alcian blue-staining procedure 9,11,12 or electrotransferred onto nitrocellulose membranes (Sartorius, Goettingen, Germany). These were saturated with 2% α-casein (Sigma) in PBS for 2 hours at room temperature and incubated overnight at 4°C with antibody 373E1 (undiluted supernatant or ascites fluid), a rabbit anti-transferrin antiserum (BioTrend Chemikalien GmbH, Cologne, Germany; Sigma-Aldrich; Chemicon International, Temecula, CA), or a mouse monoclonal antibody kit to thyroglobulin (clones 2H11 and 6E1; NeoMarkers, Labvision Corp., Fremont, CA), all diluted in PBS containing 0.5% α-casein. After extensive washing with PBS with 0.2% α-casein and 0.1% Tween-20, the membranes were incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies, diluted 1:1000 in the above buffer, for 1 hour at room temperature. Membranes were extensively washed and developed using the ECL chemiluminescence detection kit (Amersham-Pharmacia Biotech). Densitometric assessment of the intensity of the bands detected in analytical SDS-PAGE runs stained by combined silver-Alcian Blue procedure described above were performed with a GS300 transmittance/reflectance densitometer from Hoefer Scientific Instruments (San Francisco, CA) and evaluated with the SigmaScan 2.0 software.
Nanoeletrospray Tandem Quadruple/Time-Of-Flight (Q/TOF) MS/MS Spectrometry
The immunoaffinity-purified KS-bearing complex was sequentially digested with endoglycosydase H/F, N-glycosidase F, and endo-β-galactosidase, 50 mmol/L Na-acetate buffer, pH 5.8, and 50 mmol/L Tris-HCl buffer, pH 7.3, for 2 hours each at 37°C and resolved by SDS-PAGE in preparative 6% or 3 to 8% gradient gels under nonreducing conditions, and subsequently stained according to modifications of the Blum’s silver-staining procedure. 13-15 Stained gels were arbitrary divided into 10 different zones covering the Mr range of 40 to >900 kd and the polypeptides contained within each zone were sliced out from the gel and digested with trypsin as previously described. 16 Briefly, gel slices were washed with a mixture of 200 mmol/L of Tris-HCl buffer, pH 8.5, containing acetonitrile 1:1 (v/v) for 30 minutes at 30°C under shaking. The wash solution was removed and the dried gel pieces were rehydrated with 100 mmol/L of Tris-HCl buffer, pH 8.0, incubated in the same buffer containing 1 mmol/L CaCl2, 10% acetonitrile, and 0.2 to 0.5 μg trypsin, and finally incubated for 15 hours at 36°C to obtain in-gel digestion of the polypeptides. Thereafter, slices were incubated with an excess of 2% aqueous trifluoracetic acid (TFA) at 60°C for 1 hour under shaking and further processed by tandem nanospray ESI-Q/TOF MS/MS fragmentation analysis according to a previously detailed protocol. 17-19 For this purpose, the peptide mixtures obtained after enzyme treatment were lyophilized and resuspended in 5 μl of a solution containing methanol/water/formic acid at a 50:49:1 ratio (v/v/v) and introduced into nanoelectrospray needles that were pulled and operated as previously described. 16-18 Tandem mass spectra of the peptides was obtained with >50 ppm mass accuracy. MS/MS measurements performed using the Q-TOF apparatus (Micromass, Manchester, UK) on 39 of a total of 65 peptides examined by mass spectrometry yielded the relative masses and charges of the peptides for which the amino acid sequence was derived using a specifically dedicated sequence tag software based on AppleScript (Apple, Cupertino, CA) and BioMultiView (Sciex, Toronto, Canada). For database identification, the mass fragments with highest m/z in the Y ion series in the MS/MS spectra of tryptic peptides were joined into a short sequence stretch. When combined with the molecular mass information, this stretch was assembled into approximately three-amino acid sequence tags, with the residual mass N-terminal and C-terminal of the interpreted region and the peptide mass, and searched in a nonredundant protein sequence database containing >230,000 entries using PeptideSearch version 2.9 (available via www.mann.embl-heidelberg.de) as previously described. 20,21
Determination of KS Structure
Structural traits of the KS chains contained by the 373E1 immunocomplex were analyzed by a recently devised fluorescent keratanase II-based 22,23 fingerprinting method, 24 providing a valid alternative to the high-field 1H- and 13C-NMR spectroscopical techniques. 22,23,25,26 The KS-bearing antigen complex was eluted from 373E1-coated affinity beads by the addition of 0.5 ml of 4 mol/L guanidine hydrochloride/50 mmol/L Tris-HCl/0.1% CHAPS, pH 6.8. The sample was then diluted by the addition of 1.5 ml of 5 mmol/L Tris-HCl, pH 8.0, and then loaded onto a column of Source 15Q (10 × 1 cm) equilibrated in 5 mmol/L Tris-HCl, pH 8.0, and eluted with a linear gradient of 5 mmol/L Tris-HCl2 mol/L NaCl, pH 8.0, from 15 to 45 minutes. 373E1-positive fractions detected by ELISA were pooled and lyophilized before desalting of thesample on a column of Bio-Gel P2. A sample of the KS-bearing complex was re-dissolved in 200 μl double-distilled H2O and applied to a Microcon concentrator (30,000 Mr cutoff) followed by centrifugation at 10,000 × g for 1 hour. The retained material was washed twice with 100 μl of 10 mmol/L sodium acetate, pH 6.5, and then resuspended in 20 μl of sodium acetate buffer. The sample was then prepared for keratanase II fingerprinting by digestion with 1.0 mU of keratanase II for 16 hours at 37°C followed by lyophilization to remove all traces of water and labeling of the oligosaccharides with the fluorophore 2-aminobenzoic acid using the 2-AA labeling kit from Oxford Glycosciences Ltd. according to the manufacturer’s instructions. This was done by incubation of the KS oligosaccharides with 2 μl of a solution containing dimethyl sulfoxide, glacial acetic acid, 2-aminobenzoic acid, and sodium cyanoborohydride for 2 hours at 65°C. The labeled sample was diluted to 50 μl with distilled water and 10 μl applied to a Dionex AS4A-SC column (250 × 4 mm) equilibrated with 2 ml/min 150 mmol/L NaOH and eluted with a linear gradient of 2 ml/min 0 to 600 mmol/L NaCl/150 mmol/L NaOH from 5 to 65 minutes. The column was maintained at a constant temperature of 50°C and the eluate monitored on line using a fluorescence detector. The excitation and emission wavelengths were 315 nm and 400 nm, respectively.
Results
Specificity of Antibody 373E1
To ascertain its KS specificity, antibody 373E1 was initially tested in ELISA on a number of model antigens, including KS-bearing and KS-lacking PGs used as model antigens; compositionally well-characterized KSs isolated from two distinct tissue sources; and mucins carrying abundant poly-O-acetyllactosamine moieties. The antibody strongly reacted with all KS-containing model antigens, but failed to recognize control PGs carrying exclusively other types of glycosaminoglycans, including the rat chondrosarcoma aggrecan, perlecan, decorin, and biglycan (not shown). Concentration-dependent enzymatic predigestions of the KS-containing antigens with endo-β-galactosidase and keratanase II caused a strong to complete elimination of the immunoreactivity, whereas keratanase I digestion was somewhat less efficient (Table 1) ▶ . The use of other lyases, such as chondroitinase ABC; heparitinase III; endoglycosidases H and F; β-galactosidase; O-glycosidase; N-acetyl-β-d-glucosaminidase; α-1,3/4-l-fucosidase; N-glycosidases A, F, and N, did not significantly alter antibody binding, confirming that the specificity of the antibody was against KS.
Table 1.
Characteristics of the 373E1 Immunoreactivity as Determined by Combined Direct and Competitive ELISA and Dot-Blotting Using Human Articular Cartilage Aggrecan as a Model Antigen
| Treatment of model antigen | Added competitor | Treatment of competitor | Relative level of immunoreactivity* |
|---|---|---|---|
| None | – | – | 3 |
| Endo-β-galactosidase | – | – | 0 |
| Keratanase I | – | – | 1/(2) |
| Keratanase II | – | – | 1 |
| Chondroitinase ABC | – | – | 3 |
| Chondroitinase ACII | – | – | 3 |
| O-glycosidase | – | – | 3 |
| β-galactosidase | – | – | 3 |
| Endoglycosidase H/F | – | – | 2/(3) |
| N-glycosidase A† | – | – | 3 |
| Neuraminidase CP | – | – | >3 |
| Neuraminidase VC | – | – | >3 |
| Neuraminidase NDV | – | – | >3 |
| N-acetylglucosaminidase | – | – | 3 |
| N-acetyl-β-d-glucosaminidase | – | – | 3 |
| Fucosidase | – | – | 3 |
| α-1,3/4-l-fucosidase | – | – | 3 |
| Chemical desulfation | – | – | 3 |
| – | Fucosyl-lactose | – | 3 |
| – | 3′-N-Ac-Neu-lac | – | 3 |
| – | 6′-N-Ac-Neu-lac | – | 3 |
| – | N-Ac-Neu-lac-N-neo-tetraose | – | 3 |
| – | N-Ac-NeuA | – | 3 |
| – | Dermatan sulfate | – | 3 |
| – | Chondroitin sulfates | – | 3 |
| – | Heparan sulfate | – | 3 |
| – | Hyaluronan | – | 3 |
| – | Keratan sulfates‡ | – | 0 |
| – | Keratan sulfates | Keratanase I | 1 |
| – | Keratan sulfates | Keratanase II | 3 |
| – | Keratan sulfates | Endo-β-galactosidase | 3 |
| – | Aggrecan§ | Papain | 1 |
| – | Aggrecan§ | Trypsin | 1 |
* Arbritary units for relative immunoreactivity are: 3, strong; 2, moderate; 1, weak; 0, no reactivity.
† Similar results were obtained with N-glycosidase A and F;
‡ Different results were obtained with keratan sulfates purified from cornea and cartilage indicating a further specificity of the 373E1 for certain keratan sulfate structures.
§ Human articular and bovine nasal cartilage aggrecans were extensively digested with the indicated enzyme and the dialysed digest was subsequently used as a competitor.
Abbreviations: CP, Clostridium perfringens; VC, Vibro cholerea; NDV, Newcastle disease virus; 3′-N-Ac-Neu-lac, 3′-N-acetylneuramin-lactose; 6′-N-Ac-Neu-lac, 6′-N-acetylneuramin-lactose; N-Ac-Neu-lac-N-neo-tetraose, N-acetylneurami-lacto-N-neo-tetraose; N-Ac-NeuA, N-acetylneuraminic acid.
Similarly, in competition ELISA involving various glycosaminoglycans, only KSs (from either articular cartilage or cornea) effectively competed off antibody binding (Table 1) ▶ . However, if these KSs were predigested with KS-degrading enzymes, they completely lost their ability to interfere with antibody-antigen binding, with the exception of predigestion with keratanase I, which produced KS disaccharide units retaining competitive activity (ie, preserved the intact epitope). This observation and a number of other experiments involving other previously described anti-KS antibodies (of both IgG and IgM immunoglobulin subtype) strongly indicated that antibody 373E1 recognized a complex KS epitope. Dose-dependent digestion with three of four different neuraminidases, significantly incremented the binding of 373E1 to human articular cartilage aggrecan, chosen as a primary model antigen, with the most pronounced effect seen with neuraminidase from Newcastle disease virus. This effect was completely inhibited by the neuraminidase-specific inhibitor 2,3-dehydroxy-2-deoxy-N-acetylneuraminic acid, whereas various neuraminic lactoses were ineffective as antibody-antigen-binding competitors (Table 1) ▶ . These findings demonstrated that terminal sialic acid residues of KS moieties partly occluded the 373E1 epitope, but did not themselves constitute the antigenic determinant, and further highlighted the unique reactivity of the antibody against defined KS structures.
Specific KS-reactivity of the antibody was also asserted by Western blotting involving predigestion of the model antigens with either endo-β-galactosidase or chondroitinase ABC (not shown). In accordance with the enzyme digestion results, treatment of the above antigens with NH4OH under both mild and harsh conditions entirely abolished the antibody reactivity (Table 1) ▶ . On the other hand, antibody recognition was not found to be dependent on the sulfation degree of the KS chains because chemical desulfation of human cartilage aggrecan failed to alter immunoreactivity (Table 1) ▶ .
Elective Expression of 373E1-Reactive Components in PTC
Healthy, hyperplastic, and benign neoplastic thyroidal tissues did not express, or only occasionally and focally manifested detectable amounts of the KS-bearing components recognized by antibody 373E1 (Table 2 ▶ and Figure 1 ▶ ; B to D). In contrast, irrespectively of histological classification, lesion size, or presence or absence of metastatic formations, all PTC cases (115 of 115) contained up to 75% of the malignant cells expressing the KS components (Table 2 ▶ ; Figure 1, A and C ▶ ; Figure 2, B and D ▶ ). In these tumors, coincident intracellular, apical cell-surface and extracellular (luminal) staining patterns were observed, indicating the presence of both cell-retained and secreted forms of these molecules (Figure 1, A and C ▶ ; Figure 2, B and D ▶ ). PTC-selectivity of the KS components recognized by antibody 373E1 was further demonstrated by several criteria: 1) positive staining of individual neoplastic follicles that intermingled with healthy follicles in the periphery of the main tumor lesion; 2) positive immunolabeling of single neoplastic cells or clusters of cells embedded in a fibrosclerotic tumor stroma; 3) positive staining of cells invading the fibrous tumor capsule (encapsulated variant of PTC); and 4) positive staining of cells that had invaded lymphatic and/or venous vessels (Figure 1E) ▶ . This latter observation also implied that all lymphnodal PTC metastases (ie, 18 of 18) analyzed showed positive immunoreactivity (Table 2 ▶ ; Figure 1F ▶ ). Among the other malignant thyroid tumors analyzed, only 21% of follicular carcinomas (both of the widely invasive and minimally invasive types) contained scattered positively stained cells (Table 2 ▶ ; Figure 2C ▶ ). Conversely, no immunoreactivity was seen in any of the cells of anaplastic and medullary carcinomas (Table 2) ▶ .
Table 2.
Expression Pattern of Transferrin and KS-Bearing Thyroglobulin/Transferrin Isoforms in Healthy and Diseased Thyroid Tissues*
| Tissue | Component | |
|---|---|---|
| KS-bearing thyroglobulin/transferrin isoforms | Pan-transferrin | |
| Healthy thyroids | 0/10 | 0/10 |
| Nodular goiters | 1/48 (F-1)† | 3/20 (H-3) |
| Graves’ disease | 0/20 | 0/10 |
| Hashimoto’s thyroiditis | 2/11 (F-2) | 2/11 (H-2) |
| Follicular adenoma | 2/60 (F-2) | 3/26 (F-1, H-2) |
| PTC | 115/115 (F-8, H-20, W-87) | 35/45 (W-29, H-4, F-2) |
| PTC metastases‡ | 18/18 (F-1, H-2, W-15) | N.D. |
| Follicular carcinoma | 11/52 (F-7; H-4) | N.D. |
| Medullary carcinoma | 0/10 | N.D. |
| Anaplastic carcinoma | 0/5 | N.D. |
* The data refer to number of positive cases/out of the total analysed.
† The letter code and the adjacent number indicated within brackets refer to how many cases, out of the total analysed for each tissue type, exhibited the three patterns adopted for the semi-quantitative evaluation of the immunohistochemical stainings (ie, F, focal staining; H, heterogeneous staining; W, widespread staining; see Materials and Methods), which are based upon the relative number of positive cells detected in each lesion.
‡ Refers to lymphonodal metastases disclosed in PTC patients.
Figure 1.
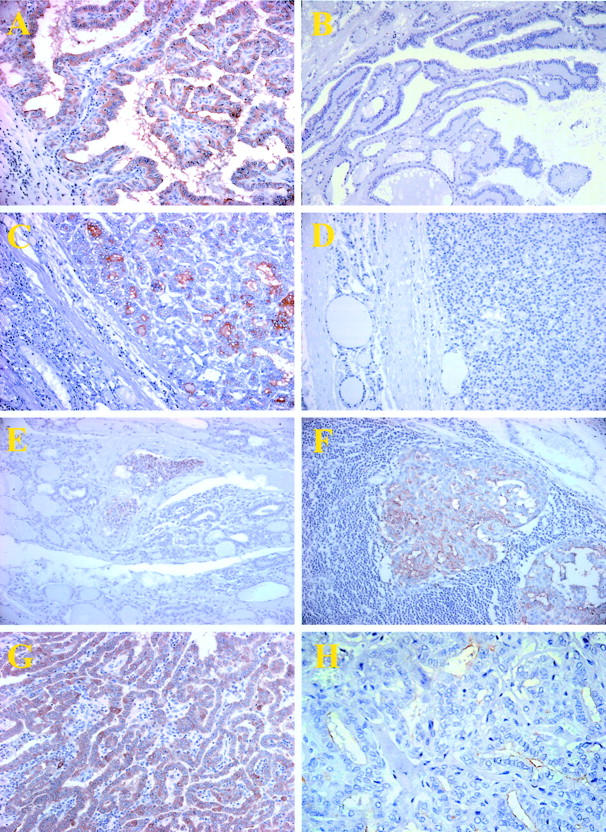
Immunostaining of benign and PTC lesions with antibody 373E1. A: A representative staining pattern observed in a classical type PTC lesion that shows coincident expression of the KS-bearing molecules in different cellular/tissue compartments, ie, cytoplasm, apical cell surface, and intraluminal space. B: In contrast, no staining was noted in hyperplastic papillae of nodular goiters. 373E1 immunoreactivity was also detected in a follicular variant of PTC (C), but not in the normal surrounding tissue (left), or in follicular adenomas (D). Positively stained PTC cells were also observed in neoplastic emboli within venules (E) located at the periphery of the main tumor lesions and in lymphonodal metastatic formations (F). G: A representative overexpression of transferrin in a PTC lesion. H: A representative markedly decreased 373E1 immunostaining in a follicular variant of PTC after endo-β-galactosidase pretreatment of the section.
Figure 2.
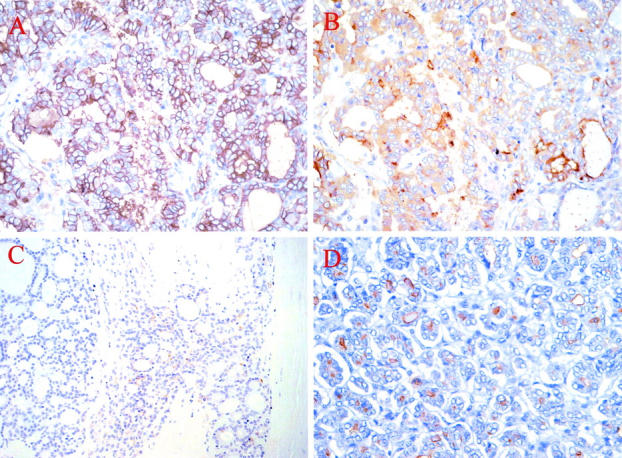
A and B: Consecutive serial sections of a follicular variant of PTC stained with mAbs HBME-1 (A) and 373E1 (B), respectively. Note how the former shows a cell membrane-associated staining contrasting the diffuse cytoplasmic and apical ones observed with mAb 373E1. C and D: Direct comparison of the staining patterns observed with 373E1 in a follicular carcinoma (C), showing tumor capsule penetration, and in a follicular variant of PTC (D).
Pretreatment of sections from PTC lesions with either endo-β-galactosidase or keratanase II markedly reduced or completely abrogated 373E1 immunoreactivity, with the former enzyme being generally more effective in reducing staining intensity and distribution (Figure 1H) ▶ . In contrast, digestion with chondroitinase ABC, keratanase I, or fucosidase did not alter the staining pattern (not shown). Consistently with the determined specificity of antibody 373E1 reported here and elsewhere (Perissinotto et al, in preparation), predigestion of sections with neuraminidase slightly augmented the relative intensity of staining without, however, perturbing the overall distribution of the staining (not shown). When performed on benign lesions, these enzymatic treatments did not unveil 373E1 immunoreactivities and, similarly, equivalent staining patterns were observed in routine formalin-fixed and paraffin-embedded material and cryosectioned specimens indicating that the lack of the positive reaction in these tissues was not because of masking effects.
Because a number of previous and more recent reports have documented a certain value of antibody HBME-1 in discriminating malignant thyroid tissues, 27-30 but the antigen recognized by this antibody remains unknown, we have compared its reactivity pattern with that observed with our 373E1 antibody. For this purpose, consecutive serial sections from 10 randomly chosen PTC cases (eight of the classical type and two of the follicular variant) were stained in a parallel or competitive manner (ie, using biotinylated mAb 373E1 detected with streptavidin-horseradish peroxidase and untagged HBME-1 detected with a secondary antibody). No competition was observed (not shown) and, although immunolabeling with mAb HBME-1 was as diffuse as that of mAb 373E1 in neoplastic PTC cells, the cellular localization of the immunostaining was clearly different: antibody HBME-1 stained exclusively the cell surface (Figure 2, A and B) ▶ .
Identification of Thyroglobulin and Transferrin as the Primary KS Substituted Glycoproteins in PTC
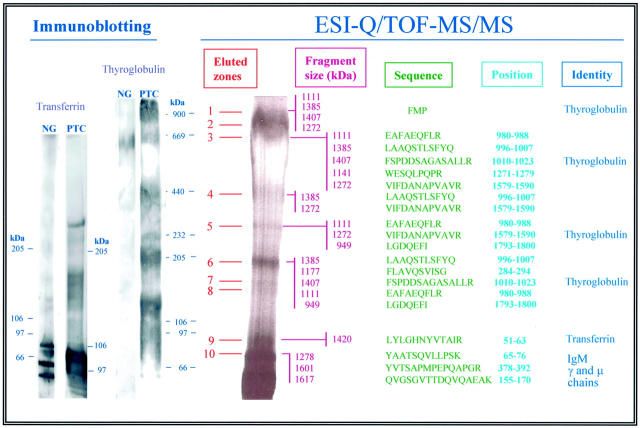
Because of the excessive glycosylation of the immunopurified 373E1-binding PTC components, conventional immunoaffinity-MALDI-TOF procedures 31,32 could not be successfully used to obtain reliable information on their constituent polypeptides. Therefore, the immunoaffinity-purified PTC material was analyzed by nanospray ESI-Q/TOF in co-axial MS/MS mode. Of a total of 65 tryptic peptides that were examined, the primary 23 from which sequence was successfully determined were found to correspond to thyroglobulin and transferrin, indicating that these glycoproteins were the prevailing proteins carrying KS moieties (Figure 3) ▶ . The analysis was performed by dividing the SDS-PAGE preparative separation of the 373E1-immunoprecipitated PTC material into 10 arbitrary chosen zones and electroeluting each individual zone for tryptic peptide generation. No reliable sequences were obtained from peptides derived from zones 7 and 8, whereas the transferrin sequence was found at the border of zones 9 and 10 (Figure 3) ▶ . An additional transferrin peptide of Mr 1423 d was also found in zone 5 (probably deriving from transferrin multimers), but the precise sequence of this peptide was not further defined. Three peptides having the respective Mr of 1468, 1290, and 1061 d and the putative amino acid sequences (I)TFD(I)SSNIGSA(SR), VTEF(I/L)E(VNPN)K, and T(I/L)(I/L)DSN(Q/K)NR (identity of amino acids within parentheses were not fully asserted) were identified in the material derived from zone 4. The first one shows 34% homology with CFTCR, whereas the others do not match with currently known proteins. These peptides and their derivation were not further investigated here.
Figure 3.
Proteomic and immunochemical analysis of the KS-bearing PTC antigen complex. For Q-TOF-MS/MS involving nanoelectrospraying and isotopic end labeling of the tryptic peptides (right), these were derived from preparative SDS-PAGE silver-stained gels in which the lane was subdivided into 10 arbitrarily selected zones (eluted zones; see Materials and Methods). Size (fragment size) and primary structure (sequence) data are reported from the 23 major peptides identified. The position column indicates the relative position of the determined amino acid residues in the corresponding identified (identity) polypeptides. Peptides derived from zone 10 also encompassed residual portions of the 373E1 IgM used for the immunoaffinity purification of the complex. Immunoblotting (left) of both benign nodular goiters (NG) and PTC was performed on surgical specimens treated as for proteomic analyses, electrotransferred onto nitrocellulose membranes, and detected with anti-thyroglobulin and anti-transferrin antibodies. Both naturally occurring degradations and putative multimeric formations (the latter only in PTC) were observed for transferrin, whereas an extensive electrophoretic mobility difference and proteolysis susceptibility was seen for KS-thyroglobulin in PTC, but not benign tissues.
The relative electrophoretic mobility of the PTC-specific KS-substituted transferrin highlighted an apparently significantly larger Mr for this glycoform, when compared to previously described serum transferrins (Figure 3) ▶ . This discrepancy was likely to have been entirely accounted by the KS moieties of this specific glycoform. The KS-bearing thyroglobulin isoform found in PTC also appeared larger in size (ie, ≥900 kd), when compared to both the heterologous one detected in nodular goiters and the 670-kd bovine homologue used as molecular weight reference. The KS glycanation variant of this protein also seemed to be diversely susceptible to proteolytic processing in situ in the malignant, but not in the benign, tissue (Figure 3) ▶ . This observation suggested that KS substitution of the backbone incremented the proteolysis susceptibility of this glycoprotein.
The immunohistochemical data indicated to us that, as may have been expected, thyroglobulin was substantially more expressed than transferrin (see below) in PTC lesions. To attempt an assessment of the relative ratio of the KS-substituted glycoforms of thyroglobulin and transferrin, we undertook a comparative densitometric analysis of bands visualized in the zones corresponding to thyroglobulin and transferrin (Figure 3) ▶ , after analytical separation by SDS-PAGE and staining with the combined silver-Alcian blue procedure, and by performing reciprocal immunoprecipitations and Western blottings with the antibody 373E1 and antibodies to thyroglobulin and transferrin. For the first type of analysis, we made two assumptions: that the silver-Alcian blue staining method labeled the two glycoforms in a comparable manner and that the thyroglobulin bands below 700 kd all represented naturally occurring degradation products of the original 800- to 900-kd macromolecule. Density measurements of zones 1 to 3, containing intact KS-thyroglobulin, versus zone 9, encompassing the KS-bearing transferrin in optimally stained gels indicated that the ratio between the two molecules was 11.8:1. On the other hand, if lower bands identified as pertaining to KS-thyroglobulin were considered, the difference was ∼20-fold in favor of KS-thyroglobulin.
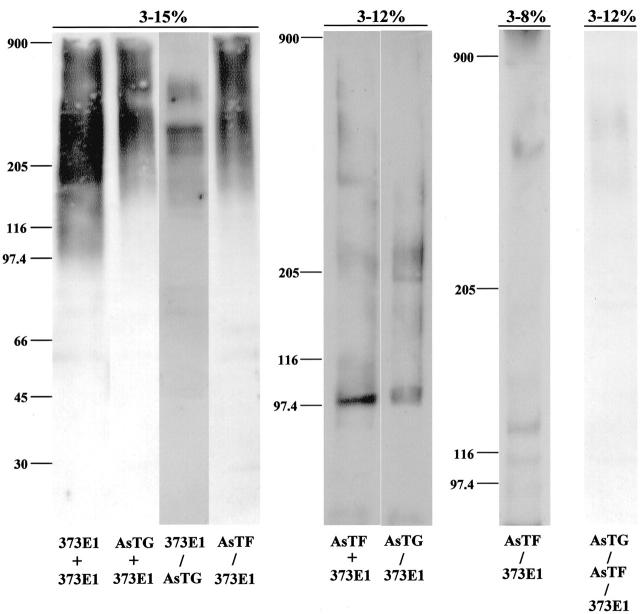
In another set of experiments, PTC extracts were either immunoprecipitated with 373E1, anti-thyroglobulin, or anti-transferrin antibodies resolved by SDS-PAGE under nonreducing conditions and then blotted with the heterologous antibodies or depleted of 373E1-reactive material, and similarly resolved by SDS-PAGE under nonreducing conditions and immunoblotted with antibodies to either thyroglobulin or transferrin (Figure 4) ▶ . These experiments highlighted KS chains attached to PTC thyroglobulin accounted for the size polydispersity of this glycoform, whereas the relatively homogenous bands of PTC transferrin (Figure 4) ▶ suggested that it contained less prominent KS chains (presumably both in length and size). Furthermore, most, if not all, of the transferrin produced by PTC seemed to glycanate with KS, whereas the tumor synthesized some KS-free thyroglobulin (Figure 4) ▶ . The relative enrichment of both KS-bearing and KS-free thyroglobulin and transferrin through the use of the specific antibodies also supported the substantially higher levels of (KS)-thyroglobulin in PTC. Finally, in several occasions, an unidentified 220- to 240-kd band recognized by anti-transferrin antibodies could be specifically detected in PTC extracts. It is presently unclear whether it represents oligomeric forms of the protein or an alternative transferrin-like glycoprotein.
Figure 4.
Combined immunoprecipitation and immunoblotting analysis of KS-bearing and KS-free glycoforms of thyroglobulin and transferrin produced by PTC. Material extracted from PTC lesions as described for Figure 2 ▶ was immunoprecipitated by the first listed antibody, resolved by SDS-PAGE under nonreducing conditions in gradient gels, as indicated on the top (ie, 3 to 15%, 3 to 12%, and 3 to 8% gradients), electrotransferred onto nitrocellulose or PDVF membranes, and immunoblotted with the second antibody (this procedure referred to with a +). Alternatively, the material was immunoprecipitated with as above and the supernatant collected, concentrated, resolved by SDS-PAGE, and blotted with the heterologous antibody. The 373E1 IgM served as the 900-kd Mr marker. AsTG, anti-thyroglobulin antiserum; AsTF, anti-transferrin antiserum.
Differential Overexpression of Transferrin in PTC Lesions
The identification of transferrin as one of the KS glycanated components specifically produced by PTC was unexpected and prompted us to examine whether this glycoprotein was also differentially expressed in this tumor versus benign thyroid lesions. Indeed, immunostaining of tissue section with anti-transferrin antibodies revealed that the molecule was overexpressed in PTC, with a preponderant (78%) frequency of expression in the cases analyzed (Figure 1G) ▶ and a mere 11 to 18% frequency in benign tissues (Table 1) ▶ . This observation indicated that the transferrin gene product was generally overtranscribed in PTC and was consistent with the outcome of the combined immunoprecipitation/immunoblotting experiments.
Composition of the KS Moieties Linked to the PTC Thyroglobulin and Transferrin Isoforms
KS was found to be the prevalent glycosaminoglycan associated with the thyroglobulin and transferrin produced by PTC, as shown by dot-blotting with 11 different plant lectins (not shown) and Western blotting (Figure 5A) ▶ , involving differential enzyme digestions with a number of endo- and exoglycosydases. These experiments also showed that the specific KS glycans were only partly degraded by keratanase I (Figure 5A) ▶ and, accordingly, were likely to embody a certain amount of α(1-3)-linked fucose residues. Furthermore, in accordance with the apparent specificity of antibody 373E1, which seems to react with a KS epitope located adjacently to N-acetylneuraminic acid residues, neuraminidase digestion significantly augmented the reactivity levels and coincidentally altered the mobility pattern of the macromolecules (Figure 5A) ▶ . Even in this case, neuraminidase from Newcastle disease virus was by far the most efficient in producing this effect, which was entirely abrogated in the presence of 5 mmol/L of the neuraminidase-specific inhibitor 2,3-dehydro-2-deoxy-N-acetylneuraminic acid (not shown). Similar results were also obtained in ELISA and dot-blot assays in which, before antibody binding, the immunoprecipitates were treated with increasing concentrations of various neuraminidases. These findings indicated that the KS moieties of these thyroglobulin and transferrin isoforms were heterogeneously and highly sialylated in a prevalent α(2-3), and possibly α(2-8), linkage.
Figure 5.
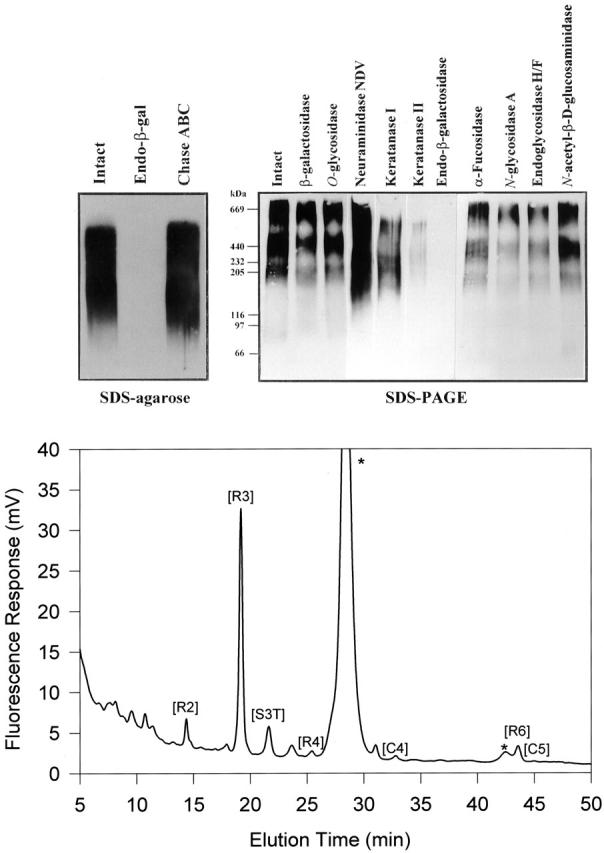
A: Analytical Western blotting of the affinity-purified 373E1 antigen isolated from PTC lesions. The material was treated with the indicated enzymes and resolved by SDS-agarose under nonreducing conditions (left) or SDS-PAGE under reducing conditions (right). Arrows highlight the major polypeptides, whereas minor smeared bands are noticeable below 100 kd. High Mr standards run in parallel under nonreducing conditions were: bovine thyroglobulin (669 kd), ferritin (440 kd), catalase (232 kd), myosin (205 kd), lactate dehydrogenase (140 kd), β-galactosidase (119 kd), phosphorylase B (97 kd), bovine serum albumin (67 kd), and ovalbumin (45 kd). B: A Dionex AS4A-SC ion-exchange chromatogram of oligosaccharides produced by keratanase II digestion/2-AA labeling of KS isolated from the thyroglobulin-transferrin-immunoprecipitated complex. Peak captions refer to the oligosaccharide identity: [R2], Galβ1–4GlcNAc(6S) β1–3Galβ1–4GlcNAc(6S)-2AA; [R3], Gal(6S) β1–4GlcNAc(6S)-2AA; [S3T], NeuAcα 2-3 Gal(6S) β1–4GlcNAc(6S)-2AA; [R4], Gal(6S) β1–4GlcNAc(6S) β1–3Galβ1–4GlcNAc(6S)-2AA; [C4], NeuAcα 2-3 Galβ1–4GlcNAc(6S) β1–3Gal(6S) β1–4GlcNAc(6S)-2AA; [R6], Gal(6S) β1–4GlcNAc(6S) β1–3Gal(6S) β1–4GlcNAc(6S)-2AA; [C5], NeuAcα 2-3 Gal(6S) β1–4GlcNAc(6S) β1–3Gal(6S) β1–4GlcNAc(6S)-2AA, where 2-AA is 2-aminobenzoic acid. Peaks labeled with an asterisk derive from contaminants. Analysis of the areas under each oligosaccharide peak was used to calculate the average chain length.
KS chains bound to the PTC-specific thyroglobulin and transferrin glycoforms were not released from the protein core by alkali treatment (50 mmol/L NaOH at 45°C for 24 hours) suggesting that they are not O-linked through N-acetylgalactosamine to serine or threonine as in type II KS. 33 In contrast, a proportion of the chains was released by treatment with peptide N-glycosidase F indicating that certain chains were attached through a KS type I-characteristic N-linkage via N-acetylglucosamine to asparagine residues. Analysis of the keratanase II-generated profiles of the purified KS chains (Figure 5B) ▶ demonstrated a marked preponderance of the disulfated disaccharide unit [R3], Gal(6S)β(1-4)GlcNAc(6S), which eluted at 19.2 minutes. In contrast, there was no evidence for the presence of significant amounts of the monosulfated disaccharide species, Galβ(1-4)GlcNAc(6S), which would have eluted at ∼7 minutes. This finding further indicates that the PTC thyroglobulin/transferrin KS chains were almost fully sulfated on their galactose residues. However, the small peak detected at 14.4 minutes was identifiable as the disulfated tetrasaccharide [R2], Galβ(1-4)GlcNAc(6S)β(1-3)Galβ(1-4)GlcNAc(6S), showing that they also contained some unsulfated galactose residues.
The second most abundant oligosaccharide peak in the KS profile eluted at 21.6 minutes, which is compatible with the nonreducing terminal trisaccharide unit NeuAcα(2-3)Gal(6S)β(1-4)GlcNAc(6S). This assignment was corroborated by treatment of the digested sample with neuraminidase, which caused disappearance of this peak and a concomitant increase in the disulfated disaccharide peak, [R2]. A further oligosaccharide moiety derived from the nonreducing terminus of the KS glycans and corresponding to [C5], NeuAcα(2-3)Gal(6S)β(1-4)GlcNAc(6S)β(1-3)Galβ(1-4)GlcNAc(6S), was weakly noticeable in the peak eluting at 45.0 minutes. This observation, and the detected binding of the Sambucus nigra agglutinin to the complex (not shown), collectively supported the idea that the majority, if not all, of the KS chains terminated with a α(2-3)-linked N-acetylneuraminic acid-capping residue. Thus, this finding was in perfect accord with the antibody specificity data and the observations on the tumor tissues in situ. However, a small sialidase resistant peak was observed at 23.7 minutes and found to not be compatible with any of the previously identified KS oligosaccharides. The paucity of material contained in this peak did not allow the determination as to whether it represents a genuine oligosaccharide or a contaminant but, if real, it may correspond to an additional nonreducing terminal structure. The elution position of this peak suggests that it may be a trisulfated trisaccharide and it could possibly be one having the composition of GalNAc(6S)β(1-3)Gal(6S)β(1-4)GlcNAc(6S) or GlcNAc(6S)β(1-3)Gal(6S)β(1-4)GlcNAc(6S), which have previously been identified as nonreducing termini in corneal (but not skeletal) KSs. 34,35 Unfortunately, because the profiling method use here is incapable of resolving these two oligosaccharides and alternative more complex and laborious methods, as well as large amounts of material would be required, the precise composition can require a larger quantity of sample that was unavailable in this study.
The precise composition of this structure was not further investigated here, but it would represent the first tumor-specific KS structure to be ever identified. Finally, if we disregard from this novel structure and assume that all KS units produced by PTC were fully capped by α(2-3)-linked N-acetylneuraminic acid residues, then an approximate size for the PTC-specific thyroglobulin- and transferrin-associated KS glycans can be approximated to 9 to 10 disaccharides in length, which would translate into an average Mr for the KS chains of 7600 d (considering that a classical KS linkage region would have a Mr of 1900 d). This size estimation is in accordance with the one that we independently established by gel-permeation chromatography on a Bio-Gel TSK-30 column calibrated with KS chains of 6900 d (data not shown).
Discussion
It is well known that on malignant transformation, epithelial cells modulate the glycosylation profile of their secretion products and that these posttranslational changes may represent exploitable diagnostic/prognostic markers, as well as important tools for the elucidation of the molecular mechanisms responsible for the progression of these tumors. Bearing this in mind, we have used a unique anti-KS antibody to address the possibility, previously alluded to, 7 that the elaboration of KS chains could represent a specific trait of PTC. Indeed, we found that expression of KS-bearing molecules occurred in 100% of the PTC cases investigated, with a widespread cellular distribution in the majority of the lesions analyzed. In contrast, molecules with a similar glycanation pattern were absent from benign tissues and other thyroid tumor types, with the exception of a rather limited expression in follicular carcinomas. Parallel immunohistochemical stainings also demonstrated that the KS components identified in situ by antibody 373E1 were associated with both the metastatic and nonmetastatic PTC cells, as well as occurring in the form of a secreted product(s) of tumor. This apparent specificity of the KS-carrying molecules for malignant PTC cells incited us to devise suitable protocols for the isolation and structural characterization of the components, such as to set the basis for their full clinical exploitation.
By combining biochemical, immunochemical, and chromatographic purification schemes we were able to enrich for the KS-containing complex and, to overcome the technical obstacle given by its high glycan content, we adopted an ESI-Q-TOF MS/MS procedure involving analysis of tryptic peptides derived from the immunoaffinity-purified components. Sequence determination achieved by this method identified thyroglobulin and transferrin as the prevalent glycoproteins carrying the PTC-produced KS moieties. These data further showed that the former glycoprotein, by virtue of its unusual glycosylation, had a significantly incremented Mr and exhibited a diverse in vivo proteolysis pattern, when compared to thyroglobulin from normal thyroid. In fact, our preliminary evidence is that the KS substitution of PTC thyroglobulin markedly protects the molecule from cleavage by cathepsin-K, clostripain, thermolysin, and thrombin, reduces its susceptibility to plasmin, whereas it has no effect on degradation of the glycoform by V8 protease, subtilisin, bacterial elastase, trypsin, and chymotrypsin (data not shown). Notable was also that some of the naturally occurring proteolytic fragments of the KS-bearing thyroglobulin isoform identified here shared some size similarities with previously described PTC-specific oncofetal fibronectin fragments recognized by antibody JT-95 and found circulating in the peripheral blood of PTC patients. 36 However, we do not find any evidence that this PTC fibronectin would carry KS moieties.
KS-containing thyroglobulin seemed to prevail over KS-lacking thyroglobulin in PTC and KS-bearing transferrin seemed to be rather exclusive for this tumor type. The former glycoform was also substantially more abundant than the latter and also seemed to contain more KS moieties. Interestingly, structural determination of KS chains attached to thyroglobulin and transferrin, which was accomplished through a fluorescent fingerprinting protocol involving analysis of keratanase II-derived oligosaccharide fragments, highlighted the uniqueness of the PTC glycan chains and the modes by which these were associated to these backbone polypeptides. In fact, a considerable portion of these moieties was not bound through the classical N- and O-linkages previously characterized for type I and II KS chains. PTC-associated KS moieties were further found to be unusually long and have a prevalence of neuraminic acid residues bound through an α(2-6)-linkage and the nonreducing termini also embodied novel oligosaccharide moieties. To our knowledge, this is the second detailed characterization of KS chains isolated from a tumor lesion, 24 which, in addition, highlights unique structural traits of this glycosaminoglycan produced by a malignant tissue. The findings also provide the grounds for approaching the structural characterizations of KS as a potential means to identify malignancy-associated structural patterns in glycosaminoglycan synthesis.
We believe that the elaboration of the unorthodox KS glycoforms of thyroglobulin and transferrin discovered here constitute an elective trait of PTC that can be exploitable for different practical applications. From a surgical pathological point of view, it may represent a reliable ancillary tool in the differential diagnosis of PTC versus benign conditions, such as nodular goiter, Graves’ disease, Hashimoto’s thyroiditis, and follicular adenoma, which may exhibit PTC-like cyto-architectural alterations. In fact, the PTC-specificity of mAb 373E1 seems to be even higher than that reported for mAb HBME-1, which may be frequently used in the disclosure of malignant thyroid conditions and the specific detection of PTC. 27-30 Although variations in the glycosylation pattern of thyroglobulin have been reported in thyroid tumors, 37,38 they have not been deemed to be sufficiently significant for biological or clinical applications. The present study opens the possibility that the long-term, postoperative follow-up involving the monitoring of serum thyroglobulin levels, which is routinely adopted in surgically treated PTC patients, may be further improved by a more specific serological assessment of the levels of KS-bearing thyroglobulin.
Transferrin is known to be diversely posttranslationally modified in hepatocellular carcinomas, 39-42 but no information has previously been reported with regard to its compositional traits in thyroid pathologies. Comparative evaluation of transferrin expression in PTC versus benign thyroid tissues revealed augmented levels of the glycoprotein in PTC lesions. This indicates that the alteration of transferrin in PTC occurred at all levels, ie, transcriptional, translational, and posttranslational, and was to some extent corresponded by the analogous alteration affecting the thyroglobulin gene. Interestingly, changes in the glycosylation profile of transferrin have been noted during pregnancy, 43 linking thereby the putative hormone-associated prevalence of PTC in females with aberrant glycosylations traits of the protein in neoplasia. Finally, the restricted anatomical distribution of KS in the neck region and the specific appearance of these hitherto described glycoforms in PTC also opens the avenues for the possible exploitation of antibody 373E1 and radiolabeled transferrin (normally used for gallium-67-based imaging of lymphomas) for PET-based in vivo imaging of malignant thyroid lesions.
Acknowledgments
We thank Dr. Mauro Melato, University of Trieste, Italy, for providing a number of tumor samples; and Maria Teresa Mucignat and Antonella Corsaro for invaluable technical assistance.
Footnotes
Address reprint requests to Roberto Perris, Department of Evolutionary and Functional Biology, University of Parma, Viale delle Scienze 11/A, Parma, Italy I-43100. E-mail: rperris@cro.it.
Supported by the Italian Ministry of Health (Fondo Sanitario Nazionale RF99 and RF00), the Associazione Italiana Ricerca sul Cancro, and intramural funds from the University of Parma.
G. M. and D. P. contributed equally to this work.
References
- 1.Greenlee RT, Murray MPH, Golden S, Wingo PA: Cancer statistics, 2000. CA Cancer J Clin 2000, 50:7-33 [DOI] [PubMed] [Google Scholar]
- 2.Bi J, Lu B: Advances in the diagnosis and management of thyroid neoplasms. Curr Opin Oncol 2000, 12:54-59 [DOI] [PubMed] [Google Scholar]
- 3.Takano T, Matsuzuka F, Sumizaki H, Kuma K, Amino N: Rapid detection of specific messenger RNAs in thyroid carcinomas by reverse transcription-PCR with degenerate primers: specific expression of oncofetal fibronectin messenger RNA in papillary carcinoma. Cancer Res 1997, 57:3792-3797 [PubMed] [Google Scholar]
- 4.Gonsky R, Knauf JA, Elisei R, Wang JW, Su S, Fagin JA: Identification of rapid turnover transcripts overexpressed in thyroid tumours and thyroid cancer cell lines: use of a targeted differential RNA display method to select for mRNA subsets. Nucleic Acid Res 1997, 25:3823-3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takano T, Hasegawa Y, Matsuzuka F, Miyauchi A, Yoshida H, Higashiyama T, Kuma K, Amino N: Gene expression profiles in thyroid carcinomas. Br J Cancer 2000, 83:1495-1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Y, Prasad M, Lemon WJ, Hampel H, Kornacker K, LiVolsi V, Frankel W, Kloos RT, Eng C, Pellegata NS, de la Chapelle A: Gene expression in papillary thyroid carcinoma reveals highly consistent profiles. Proc Natl Acad Sci USA 2002, 98:15044-15049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito N, Yokota M, Nagaike C, Morimura Y, Hatake K, Tanaka O, Matsunaga T: Simultaneous expression of keratan sulphate epitope (a sulphated poly-N-acetyllactosamine) and blood group ABH antigens in papillary carcinomas of the human thyroid gland. Histochem J 1996, 28:613-623 [DOI] [PubMed] [Google Scholar]
- 8.Ito N, Yokota M, Kawahara S, Nagaike C, Morimura Y, Hirota T, Matsunaga T: Histochemical demonstration of different types of poly-N-acetyllactosamine structures in human thyroid neoplasms using lectins and endo-β-galactosidase digestion. Histochem J 1995, 27:620-629 [PubMed] [Google Scholar]
- 9.Perissinotto D, Iacopetti P, Pettway Z, Gabriele E, Zambon M, Bellina I, Colombatti A, Pettway Z, Bronner-Fraser M, Shinomura T, Kimata K, Löfberg J, Mörgelin M, Perris R: PG-M/versicans in avian morphogenesis: distributional and structural analysis of splice variants and proposed functional role during neural crest cell movement in vivo. Development 2000, 127:2823-2842 [DOI] [PubMed] [Google Scholar]
- 10.Rosai J, Carcangiu ML, DeLellis RA, Simoes MS: Recommendations for the reporting of thyroid carcinomas. Association of Directors of Anatomic and Surgical Pathology. Hum Pathol 2000, 31:1199-1201 [DOI] [PubMed] [Google Scholar]
- 11.Buee L, Boyle NJ, Zhang L, Delacourte A, Fillit HM: Optimization of an Alcian blue dot-blot assay for the detection of glycosaminoglycans and proteoglycans. Anal Biochem 1991, 195:238-242 [DOI] [PubMed] [Google Scholar]
- 12.Krueger RC, Jr, Schwartz NB: An improved method of sequential Alcian blue and ammoniacal silver staining of chondroitin sulfate proteoglycan in polyacrylamide gels Anal Biochem 1987, 167:295-300 [DOI] [PubMed] [Google Scholar]
- 13.Jay GD, Culp DJ, Jahnke MR: Silver staining of extensively glycosylated proteins on sodium dodecyl sulfate-polyacrylamide gels: enhancement by carbohydrate-binding dyes. Anal Biochem 1990, 185:324-330 [DOI] [PubMed] [Google Scholar]
- 14.Nesterenko MV, Tilley M, Upton SJ: A simple modification of the Blum’s silver stain method allows for a 30 min detection of proteins in polyacrylamide gels. J Biochem Biophys Methods 1994, 28:239-242 [DOI] [PubMed] [Google Scholar]
- 15.Rabilloud T, Carpentier G, Tarroux P: Improvement and simplification of low-background silver staining of proteins by using sodium dithionite. Electrophoresis 1998, 9:288-291 [DOI] [PubMed] [Google Scholar]
- 16.Otto A, Thiede B, Müller E-C, Scheler C, Wittmann-Liebold B, Jungblut P: Identification of human myocardial proteins separated by two-dimensional electrophoresis using an effective sample preparation for mass spectrometry. Electrophoresis 1996, 17:1643-1650 [DOI] [PubMed] [Google Scholar]
- 17.Wilm M, Shevchenco A, Houthaeve T, Breit S, Schweigerer L, Fotsis T, Mann M: Femtomole sequencing of proteins from polyacrylamide gels by nano-electrospray mass spectrometry. Nature 1996, 379:466-469 [DOI] [PubMed] [Google Scholar]
- 18.Shevchenco A, Chernushevich I, Ens W, Standing KG, Thomson B, Wilm M, Mann M: Rapid de novo peptide sequencing by a combination of nanoelectrospray, isotopic labeling and a quadrupole/time-of-flight mass spectrometer. Rapid Commn Mass Spectrom 1997, 11:1015-1024 [DOI] [PubMed] [Google Scholar]
- 19.Shevchenco A, Shevchenco A, Chernushevich I, Wilm M, Mann M: De novo peptide sequencing by nanoelectrospray tandem mass spectrometry using triple and quadrupole and quadrupole/time-of-flight instruments. Methods Mol Biol 2000, 146:1-16 [DOI] [PubMed] [Google Scholar]
- 20.Mann M, Wilm MS: Error tolerant identification of peptides in sequence database by sequence tags. Anal Chem 1994, 66:4390-4399 [DOI] [PubMed] [Google Scholar]
- 21.Mortz E, O’Connor PB, Roepstorff P, Kelleher NL, Wood TD, McLafferty FW, Mann M: Sequence tag identification of intact proteins by matching tandem mass spectral data against sequence data bases. Proc Natl Acad Sci USA 1996, 93:8264-8267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown GM, Huckerby TN, Nieduszynski IA: Oligosaccharides derived by keratanase II digestion of bovine articular cartilage keratan sulphates. Eur J Biochem 1994, 224:281-308 [DOI] [PubMed] [Google Scholar]
- 23.Brown GM, Huckerby TN, Morris HG, Abram BL, Nieduszynski IA: Oligosaccharides derived from bovine articular cartilage keratan sulfates after keratanase II digestion: implication for keratan sulfate structural fingerprinting. Biochemistry 1994, 33:4836-4846 [DOI] [PubMed] [Google Scholar]
- 24.Whitham KM, Hadley JL, Morris HG, Andrew SM, Nieduszynski IA, Brown GM: An improved method for the structural profiling of keratan sulfates: analysis of keratan sulfates from brain and ovarian tumours. Glycobiology 1999, 9:285-291 [DOI] [PubMed] [Google Scholar]
- 25.Brown GM, Huckerby TN, Abram BL, Nieduszynski IA: Characterization of a non-reducing terminal fragment from bovine articular cartilage keratan sulphates containing α(2-3)-linked sialic acid and α(1-3)-linked fucose. Biochem J 1996, 319:137-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huckerby TN, Brown GM, Nieduszynski IA: 13C-NMR spectroscopy of keratan sulphates. Assignment of four poly(N-acetyllactosamine)-repeat-sequence tetrasaccharides derived from bovine articular cartilage keratan sulphate by keratanase II digestion Eur J Biochem 1995, 231:779-783 [DOI] [PubMed] [Google Scholar]
- 27.Sack MJ, Astengo-Osuna C, Lin BT, Battifora H, LiVolsi VA: HBME-1 immunostaining in thyroid fine-needle aspirations: a useful marker in the diagnosis of carcinoma. Mod Pathol 1997, 10:668-674 [PubMed] [Google Scholar]
- 28.van Hoeven KH, Kovatich AJ, Miettinen M: Immunocytochemical evaluation of HBME-1, CA 199, and CD15 (Leu-M1) in fine-needle aspirates of thyroid nodules. Diagn Cytopathol 1998, 18:93-97 [DOI] [PubMed] [Google Scholar]
- 29.Cheung CC, Ezzat S, Freeman JL, Rosen IB, Asa SL: Immunohistochemical diagnosis of papillary thyroid carcinoma. Mod Pathol 2001, 14:338-342 [DOI] [PubMed] [Google Scholar]
- 30.Mai KT, Bokhary R, Yazdi HM, Thomas J, Commons AS: Reduced HBME-1 immunoreactivity of papillary thyroid carcinoma and papillary thyroid carcinoma-related neoplastic lesions with Hurthle cell and/or apocrine-like changes. Histopathology 2002, 40:133-142 [DOI] [PubMed] [Google Scholar]
- 31.Lu J, van Halbeek H: Complete 1H and 13C resonance assignments of a 21-amino acid glycopeptide prepared from human serum transferrin. Carbohydr Res 1996, 296:1-21 [DOI] [PubMed] [Google Scholar]
- 32.Lacey JM, Bergen HR, Magera MJ, Naylor S, O’Brien JF: Rapid determination of transferrin isoforms by immunoaffinity liquid chromatography and electrospray mass spectrometry. Clin Chem 2001, 47:513-518 [PubMed] [Google Scholar]
- 33.Nieduszynski IA, Huckerby TN, Dickenson JM, Brown GM, Tai G-H, Morris HG, Eady S: There are two major types of skeletal keratan sulphates. Biochem J 1990, 271:243-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tai GH, Huckerby TN, Nieduszynski IA: Multiple non-reducing chain termini isolated from bovine corneal keratan sulfates. J Biol Chem 1996, 271:23535-23546 [DOI] [PubMed] [Google Scholar]
- 35.Tai GH, Huckerby TN, Nieduszynski IA: Human corneal keratan sulfates. J Biol Chem 1997, 272:28227-28231 [DOI] [PubMed] [Google Scholar]
- 36.Kimura N, Kurogawa K, Yamamoto K, Narimatsu H, Kimura H, Hosoya T, Takeyama H: Molecular identification of the antigens recognized by monoclonal antibody JT95 specific for thyroid carcinomas. Biochem Biophys Res Commun 1998, 251:449-453 [DOI] [PubMed] [Google Scholar]
- 37.Sinadinovic J, Cvejic D, Savin S, Jancic-Zuguricas M, Micic JV: Altered terminal glycosylation of thyroglobulin in papillary thyroid carcinoma. Exp Clin Endocrinol 1992, 100:124-128 [DOI] [PubMed] [Google Scholar]
- 38.Maruyama M, Kato R, Kobayashi S, Kasuga Y: A method to differentiate between thyroglobulin derived from normal thyroid tissue and from thyroid carcinoma based on analysis of reactivity to lectins. Arch Pathol Lab Med 1998, 122:715-720 [PubMed] [Google Scholar]
- 39.Campion B, Leger D, Wieruszeski JM, Montreuil J, Spik G: Presence of triantennary, tetraantennary and pentaantennary glycans in transferrin synthesized by the human hepatocarcinoma cell line Hep G2. Eur J Biochem 1989, 184:405-413 [DOI] [PubMed] [Google Scholar]
- 40.Hahn TJ, Goochee CF: Growth-associated glycosylation of transferrin secreted by HepG2 cells. J Biol Chem 1992, 267:23982-23987 [PubMed] [Google Scholar]
- 41.Matsumoto K, Maeda Y, Koto S, Yuki H: Alteration of asparagine-linked glycosylation in serum transferrin of patients with hepatocellular carcinoma. Clin Chim Acta 1994, 224:1-18 [DOI] [PubMed] [Google Scholar]
- 42.Yamashita K, Koide N, Endo T, Iwaki Y, Kobata A: Altered glycosylation of serum transferrin of patients with hepatocellular carcinoma. J Biol Chem 1989, 264:2415-2423 [PubMed] [Google Scholar]
- 43.van Rooijen JJ, Jeschke U, Kamerling JP, Vliegenhart JF: Expression of N-linked sialyl Le(x) determinants and O-glycans in the carbohydrate moiety of human amniotic fluid transferrin during pregnancy. Glycobiology 1998, 8:1053-1064 [DOI] [PubMed] [Google Scholar]