Abstract
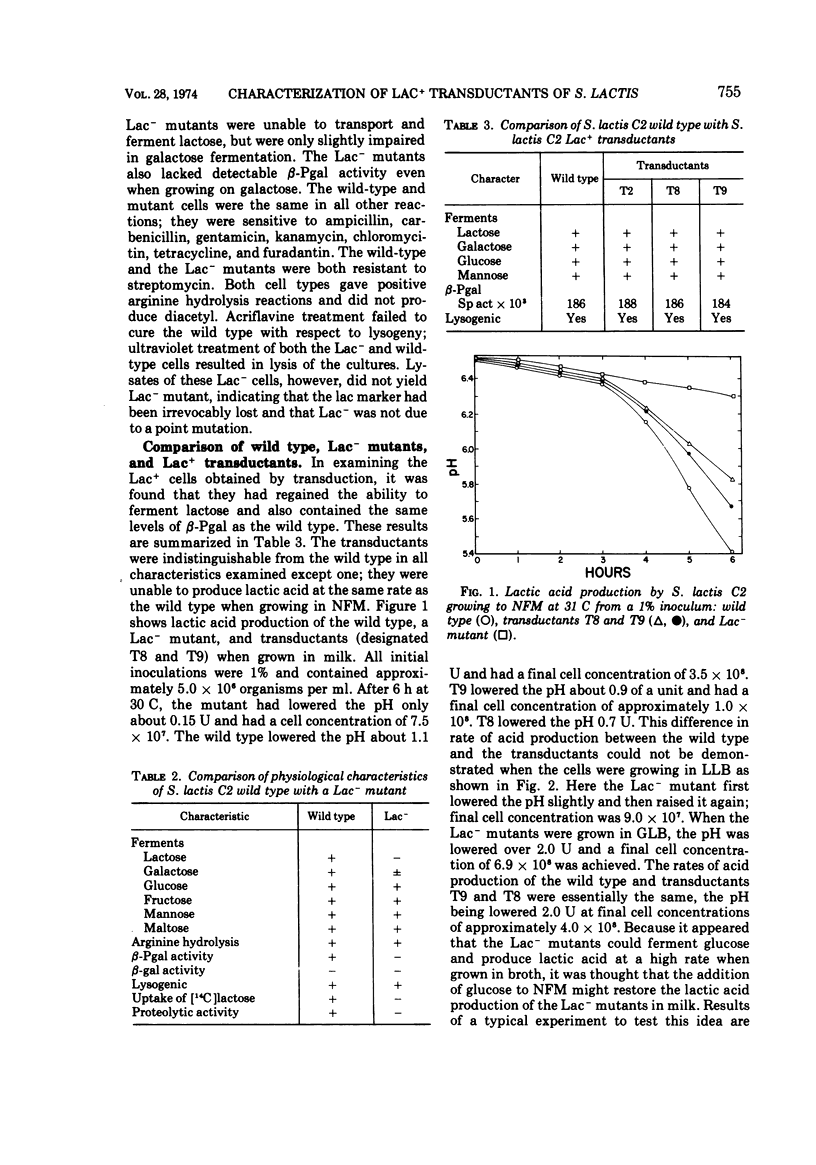
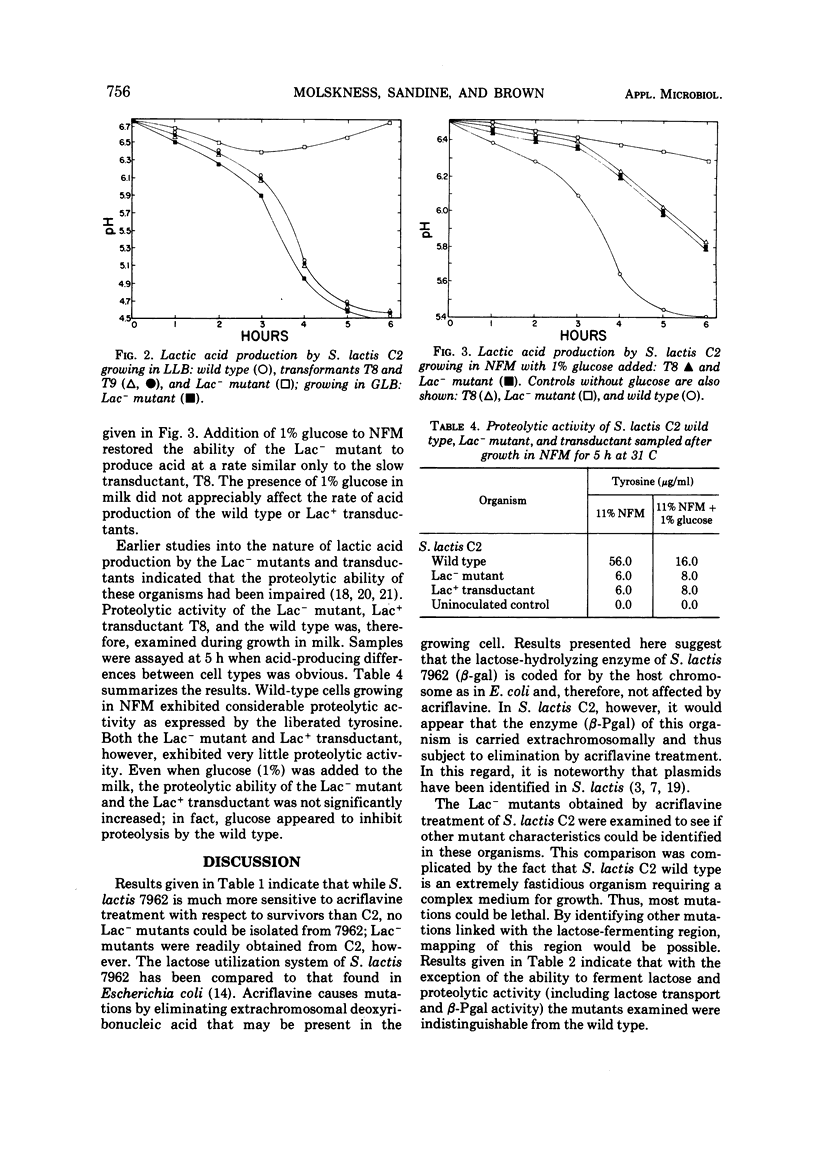
A phage-mediated transducing system was used in studying certain physiological characteristics of S. lactis C2 wild type, lactose-negative mutants, and lactose-positive transductants. Lac- mutants, obtained by acriflavine treatment of the wild type, were similar to the wild type in all characteristics tested except they lacked β-D-phosphogalactoside galactohydrolase (β-Pgal) and could not transport [14C]lactose; they also had approximately 10% of the proteolytic ability than wild-type cells. The lactose-fermenting characteristic was transduced from the wild type to Lac- mutants. The Lac+ transductants obtained were similar to the wild-type parent with respect to lactose fermentation and level of β-Pgal activity (0.186 U of protein per mg). These transductants, however, had not regained full proteolytic ability and were similar to the Lac- mutant in this respect. Lactic acid production of the transductants in milk was approximately two-thirds that of the wild type. Data suggest that both the lactose-fermenting and proteolytic characters are carried on extrachromasomal particles (plasmids).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenchley J. E., Magasanik B. Klebsiella aerogenes strain carrying drug-resistance determinants and a lac plasmid. J Bacteriol. 1972 Oct;112(1):200–205. doi: 10.1128/jb.112.1.200-205.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CITTI J. E., SANDINE W. E., ELLIKER P. R. BETA-GALACTOSIDASE OF STREPTOCOCCUS LACTIS. J Bacteriol. 1965 Apr;89:937–942. doi: 10.1128/jb.89.4.937-942.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cords B. R., McKay L. L., Guerry P. Extrachromosomal elements in group N streptococci. J Bacteriol. 1974 Mar;117(3):1149–1152. doi: 10.1128/jb.117.3.1149-1152.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman R. A., Speck M. L. Proteinase enzyme system of lactic streptococci. I. Isolation and partial characterization. Appl Microbiol. 1967 Jul;15(4):851–856. doi: 10.1128/am.15.4.851-856.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler A. V. High-level production of -galactosidase by Escherichia coli merodiploids. J Bacteriol. 1972 Nov;112(2):856–860. doi: 10.1128/jb.112.2.856-860.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R., Molskness T., Sandine W. E., Elliker P. R. Carbohydrate metabolism in lactic streptococci: fate of galactose supplied in free or disaccharide form. Appl Microbiol. 1973 Dec;26(6):951–958. doi: 10.1128/am.26.6.951-958.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORSE M. L., ALIRE M. L. An agar medium indicating acid production. J Bacteriol. 1958 Sep;76(3):270–271. doi: 10.1128/jb.76.3.270-271.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Balbinder E. Genetic Characterization of a Stable F' lac Plasmid. J Bacteriol. 1972 Oct;112(1):503–512. doi: 10.1128/jb.112.1.503-512.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Induction of prophage in Streptococcus lactis C2 by ultraviolet irradiation. Appl Microbiol. 1973 Apr;25(4):682–684. doi: 10.1128/am.25.4.682-684.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Zottola E. A. Loss of lactose metabolism in lactic streptococci. Appl Microbiol. 1972 Jun;23(6):1090–1096. doi: 10.1128/am.23.6.1090-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Cords B. R., Baldwin K. A. Transduction of lactose metabolism in Streptococcus lactis C2. J Bacteriol. 1973 Sep;115(3):810–815. doi: 10.1128/jb.115.3.810-815.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Walter L. A., Sandine W. E., Elliker P. R. Involvement of phosphoenolpyruvate in lactose utilization by group N streptococci. J Bacteriol. 1969 Aug;99(2):603–610. doi: 10.1128/jb.99.2.603-610.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molskness T. A., Lee D. R., Sandine W. E., Elliker P. R. -D-phosphogalactoside galactohydrolase of lactic streptococci. Appl Microbiol. 1973 Mar;25(3):373–380. doi: 10.1128/am.25.3.373-380.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse M. L., Hill K. L., Egan J. B., Hengstenberg W. Metabolism of lactose by Staphylococcus aureus and its genetic basis. J Bacteriol. 1968 Jun;95(6):2270–2274. doi: 10.1128/jb.95.6.2270-2274.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce L. E., Skipper N. A., Jarvis B. D. Proteinase activity in slow lactic acid-producing variants of Streptococcus lactis. Appl Microbiol. 1974 May;27(5):933–937. doi: 10.1128/am.27.5.933-937.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff D. C., Cowman R. A., Speck M. L. Isolation and partial characterization of a particulate proteinase from a slow acid producing mutant of Streptococcus lactis. J Dairy Sci. 1971 Sep;54(9):1253–1258. doi: 10.3168/jds.S0022-0302(71)86016-2. [DOI] [PubMed] [Google Scholar]
- Westhoff D. C., Cowman R. A. Substrate specificity of the intracellular proteinase from a slow acid producing mutant of Streptococcus lactis. J Dairy Sci. 1971 Sep;54(9):1265–1269. doi: 10.3168/jds.S0022-0302(71)86018-6. [DOI] [PubMed] [Google Scholar]
- Westhoff D. C., Cowman R. A., Swaisgood H. E. Characterization of an intracellular proteinase of a slow acid producing mutant of Streptococcus lactis. J Dairy Sci. 1971 Sep;54(9):1259–1264. doi: 10.3168/jds.S0022-0302(71)86017-4. [DOI] [PubMed] [Google Scholar]


