Abstract
To examine the roles of cytokines in muscle regeneration, we injected cardiotoxin into mouse tibialis anterior muscle and examined the expression profiles of cytokines and related genes in the regeneration process. Expression of 40, 64, and 7 genes among 522 genes spotted on a cytokine expression array were increased more than fivefold at 48 hours, 96 hours, and 7 days after toxin injection, respectively, when compared with those of the control muscle. Especially the levels of mRNA for chemokines and chemokine receptors, many of which are potent regulators of macrophages, were highly elevated 48 hours after injury. The expression of osteopontin (OPN), a versatile regulator of inflammation and tissue repair, was up-regulated more than 118-fold in regenerating muscle at 48 hours after injury. Northern blotting confirmed that the expression of OPN was highest at 48 hours after cardiotoxin injection and declined sharply thereafter. Immunohistochemistry showed that OPN was detected both in the cytoplasm of macrophages and in necrotic muscle infiltrated with macrophages. Our studies suggest OPN may serve as an adhesion molecule that promotes macrophage binding to necrotic fibers and may be an important mediator in the early phase of muscle regeneration.
Skeletal muscle has the ability to regenerate in response to various injuries, including disease processes such as inherited muscular dystrophies. 1-4 In muscle regeneration, the muscle satellite cells, which reside between the basal lamina and the plasma membrane of muscle fibers, play the leading role. Satellite cells are in the G0 phase of the cell cycle in noninjured conditions, while the cells are activated, leave the G0 stage, proliferate, and fuse to form multinucleated myotubes when a muscle is damaged. The resulting myotubes subsequently replace the damaged muscle fibers as regenerating muscle fibers. 1-5
The muscle regeneration process is very similar to myogenesis during development except for the infiltration of inflammatory cells into the damaged muscle. It is widely accepted that the inflammatory cells, particularly macrophages, have important roles in muscle regeneration. 6-8 Macrophages serve as a local source of cytokines, which act on various cells such as the inflammatory cells and satellite cells. To determine which cytokines dominate the complex but highly coordinated muscle regeneration process, and to clarify which cytokines regulate the activation, proliferation, and differentiation of the satellite cells, we examined the expression of cytokines and their related genes during muscle regeneration. We injected a snake venom, cardiotoxin, into mouse tibialis anterior (TA) muscle to induce muscle regeneration, and examined it at different stages of regeneration by using a cDNA macroarray. We found that a number of genes, especially chemokines and their receptors, were increased at 48 hours, 96 hours, and 7 days after cardiotoxin injection. Among them, the gene expression of osteopontin (OPN), a versatile regulator of inflammation, 9-11 was drastically increased at 48 hours and 96 hours after cardiotoxin injection. We confirmed the expression of the gene by Northern blotting. We also detected OPN expression in both the cytoplasm of macrophages and necrotic muscle fibers with macrophage infiltration by immunohistochemistry using an anti-OPN antibody. Our studies suggest that OPN may be an important mediator in the early phase of muscle regeneration.
Materials and Methods
Animals and Cardiotoxin Injection
ICR male mice (8 to 9 weeks old) were purchased from Nihon CLEA (Tokyo, Japan). Mice were anesthetized by inhalation of diethylether (Wako Chemicals, Osaka, Japan), and 100 μl of cardiotoxin 12 of Naja naja atra venom (10 μmol/L in saline, Wako Chemicals) was injected into the right TA muscle with a 27-gauge needle. The concentration of cardiotoxin was determined by a previous study. 13 Our conditions ensure minimal damage to satellite cells and also to the nerves and blood vessels of the original muscles. 13 The contralateral untreated TA muscle served as the control.
Tissue Preparation
For cDNA array and histochemical analysis, mice were sacrificed at 24, 48, or 96 hours, and 7, 14, or 28 days after cardiotoxin injection. After cervical dislocation, bilateral TA muscles were rapidly dissected out and frozen in liquid nitrogen. Some tissues were fixed with 10% formalin and embedded in paraffin.
cDNA Array
The mouse cytokine array purchased from R&D Systems (Minneapolis, MN) provided a pair of charged nylon membranes. Each of them contained 514 different cytokine and cytokine-related genes. According to the manufacturer’s instructions, they were classified as 1) adhesion molecules, 2) angiogenic factors, 3) apoptosis-related factors, 4) binding proteins, 5) cell surface proteins, 6) chemokines and their receptors, 7) cytokines and their receptors, 8) developmental factors, 9) epidermal growth factor family genes, 10) ephrins and their receptors, 11) fibroblast growth factor (FGF) family genes, 12) integrins, 13) interleukins (ILs) and their receptors, 14) neurotrophic factors, 15) nitric oxide metabolism genes, 16) proteases or related factors, 17) signal transduction-related genes, 18) telomerase-related genes, 19) transforming growth factor-β superfamily genes, 20) genes of tumor necrosis factor-α superfamilies, and 21) weight regulation-related genes. The array also contained 8 housekeeping genes and 16 spots of mouse genomic DNA.
Total RNA was isolated from 10 pooled TA muscles with RNAzol B (Tel-Test Inc., Friendswood, TX). Poly(A)+ RNAs were then purified from total RNAs with an Oligotex-DT mRNA purification kit according to the manufacturer’s protocol (TaKaRa, Shiga, Japan). 32P-Radiolabeled cDNA probes for array hybridization were generated by reverse transcriptase reaction. In brief, 500 ng of poly(A)+ RNAs were annealed with the provided mouse-specific primer (R&D Systems), incubated for 2 minutes at 90°C, and gradually cooled down to 42°C. Then, radiolabeling was performed in a reverse transcription buffer containing 333 μmol/L of dATP, dTTP, dGTP; 1.67 μmol/L of dCTP; 20 μCi [32P]-dCTP (1000 to 3000 Ci/mmol/L; Amersham Pharmacia, Piscataway, NJ); 20 U of RNase inhibitor (Ambion, Austin, TX); and 25 U of AMV reverse transcriptase (Life Technologies, Inc., Grand Island, NY). After 3 hours of incubation at 42°C, they were cleared by passage through CHROMA Spin TE-10 (Clontech, Palo Alto, CA). The cDNA array membranes were prehybridized for more than 1 hour and incubated with radiolabeled cDNA probes at 65°C overnight in a buffer containing 5× saline-sodium phosphate-EDTA (SSPE), 2% (W/V) sodium dodecyl sulfate (SDS), 5× Denhardt’s solution, and 100 μg/ml sonicated salmon testes DNA. The membranes were then washed with washing buffers (0.5× SSPE and 1% SDS and 0.1× SSPE and 1% SDS) at 65°C for more than 30 minutes. The signals (pixel intensity/mm2) were quantified using a Phosphorimager (BAS-2500; Fuji Film, Tokyo, Japan) and BAStation (Fuji Film) and analyzed with Microsoft Excel 98 (Microsoft, Redmond, WA).
Data Quantification and Analysis
cDNA array experiments were performed three times with three independent samples from each time point. To compare the injected and control muscles, we used the following data corrections. cDNAs were spotted in duplicate at 10 ng per spot of each gene. First, the average signal intensity (pixel intensity/mm2) of two spots of each gene was calculated (A). The background signal (B) was determined by measuring the signals of spots of negative controls on the array membranes. The corrected signal (C) was obtained from C = A − B. Next, the corrected signals of mouse genomic DNAs on the two sheets were calculated, and the ratio (C.injection of genomic DNA/C.normal of genomic DNA) was used to normalize the corrected signals of the two membranes. We did not use the housekeeping genes to normalize the corrected signals because their expressions change greatly during the muscle regeneration process. The normalized signal of each gene in injected muscle is expressed as A.injection − B.injection (=C.injection), and that of normal muscle is expressed as C.normal × (C.injection of genomic DNA/C.normal of genomic DNA). After normalization, the induction ratio was calculated as the ratio of the normalized signal of the injected control side to that of nontreated side. These results were expressed as -fold increase or decrease. The fold inductions in Tables 1, 3, 4, and 6 ▶ are averages of three independent experiments.
Table 1.
Up-Regulated Genes in Regenerating Skeletal Muscle at 48 Hours after Cardiotoxin Injection
| Category | Description | Average -fold increase | Accession no. |
|---|---|---|---|
| Chemokines and receptors | JE/MCP-1/CCL2 | 73.2 | NM_011333 |
| MIP-1β/CCL4 | 46.6 | M35590 | |
| C10/CCL6 | 38.0 | NM_009139 | |
| Interleukins and receptors | IL-10 receptor-α | 12.8 | NM_008348 |
| IL-2 receptor-γ | 12.4 | L20048 | |
| IL-6 receptor-α | 10.2 | X51975 | |
| IL-10 receptor-β | 5.0 | NM_008349 | |
| Leukocyte markers and functions | M-CSF receptor | 12.9 | NM_007779 |
| Mannose receptor | 9.9 | NM_008625 | |
| G-CSF receptor | 6.0 | NM_007782 | |
| Mer | 5.2 | U21301 | |
| Other cytokines and receptors | Osteopontin | 118.5 | NM_009263 |
| Galectin-9 | 8.4 | NM_010708 | |
| LPS-binding protein | 7.1 | NM_011029 | |
| TNF-α | 6.7 | M13049 | |
| Flt3/Flk-2 ligand (FL) | 5.3 | U04807 | |
| Angiogenesis and vascular response | Endothelin-β receptor | 7.7 | NM_007904 |
| Integrins | Integrin-β2 | 15.6 | NM_008404 |
| Integrin-α5 | 6.7 | NM_010577 | |
| ECM and ECM processing | MMP-15 | 6.5 | NM_008609 |
| Energy metabolism | UCP-3 | 43.7 | NM_009464 |
| UCP-2 | 5.3 | NM_011671 | |
| Apoptosis | Survivin | 7.6 | NM_009689 |
| FasL/TNF SF6 | 7.2 | NM_010177 |
Because many genes show extremely low or no expression in normal skeletal muscle, the determination of the induction ratio results in quite large values (eg, dividing by zero). Further, the corrected signals of some genes were occasionally less than zero. In the cases of those genes, we only selected the genes whose corrected signals in regenerating muscle reach levels similar to those of the genes listed in Table 1 ▶ . We listed those genes in Tables 2, 5, and 7 ▶ .
Table 2.
List of Genes that Show Little or No Expression in Normal Muscle but Are Highly Induced at 48 Hours after Cardiotoxin Injection
| Category | Description | Accession no. |
|---|---|---|
| Chemokines and receptors | MIP-1α/CCL3 | NM_011337 |
| MIP-1γ/CCL9 | NM_011338 | |
| MARC/MCP-3/CCL7 | NM_013654 | |
| MCP-5/CCL12 | NM_011331 | |
| CCR1 | NM_009912 | |
| CCR2 | NM_009915 | |
| CCR5 | NM_009917 | |
| CXCR4 | NM_009911 | |
| Interleukins and receptors | IL-7 receptor α | NM_008372 |
| IL-1 receptor antagonist | M57525 | |
| Leukocyte markers and functions | C5a receptor/CD88 | NM_007577 |
| RP105 | NM_008533 | |
| Integrins | Integrin-β7 | M95632 |
| ECM and ECM processing | MMP-12 | NM_008605 |
| TIMP-1 | NM_011593 | |
| Energy metabolism | UCP-1 | NM_009463 |
Immunohistochemistry
Frozen TA muscles were cut at 10 μm, air-dried, and fixed with acetone at −20°C for 10 minutes. Paraffin sections were cut at 6 μm, deparaffinized with xylene, hydrated with decreasing concentrations of ethanol, and washed twice with 0.01 mol/L of phosphate-buffered saline (pH. 7.4) containing 0.1% Triton X-100 (PBS-T). Sections were treated with 0.03% hydrogen peroxide to deplete endogenous peroxidase activity and washed twice with PBS-T. For immunolabeling of OPN, sections were microwaved in 10 mmol/L of citric acid buffer (pH 6.0) for 10 minutes. After incubation with PBS-T containing 5% bovine serum albumin and 10% goat serum for 1 hour, the sections were probed with either rabbit anti-mouse OPN polyclonal antibody (1:100 dilution; IBL, Gunma, Japan) or rat anti-F4/80 antibody (1:20; OBM, Tokyo, Japan) overnight. After several washings with PBS-T, the sections were incubated with appropriate second antibodies for 1 hour. The second antibodies were biotinylated goat anti-rabbit immunoglobulin (1:200; BD Phar-mingen, La Jolla, CA), biotinylated rabbit anti-rat immunoglobulin (1:300; DAKO, Glostrup, Denmark), Alexa Fluor 594-labeled goat anti-rat IgG or 488-labeled anti-rabbit IgG (1:600; Molecular Probes, Eugene, OR). The sections were then rinsed with PBS-T twice. Some sections were incubated with Vectastain ABC reagents (Vector Laboratories, Burlingame, CA) for 1 hour. After three washings with PBS, the sections were incubated with 0.02% solution of 3,3-diaminobenzidine tetrahydrochloride, 0.01% H2O2 in Tris-buffered saline. Light counterstaining of nuclei using methyl green or TOTO-3 (Molecular Probes) was performed. As negative controls, the primary antibody was substituted with 5% bovine serum albumin.
Northern Blotting
Ten μg of total RNAs were separated on a 1.5% denaturing agarose gel, blotted onto Hybond-N+ membrane (Amersham Pharmacia Biotech, Buckinghamshire, UK) for 6 hours, and then probed with either 32P-dCTP-labeled cDNA of OPN or 18s RNA. The cDNA fragment of mouse OPN was generated by reverse transcriptase-polymerase chain reaction with the following primers: forward primer, 5′-CGA CCA TGA GAT TGG CAG TGA TTT G, and reverse primer, 5′-CCA CTG AAC TGA GAA ATG AGC. The primer designs were based on the sequences of Gene Bank accession no. NM-009263. The expected 996-bp polymerase chain reaction products were cloned into a TA cloning vector (Invitrogen, Carlsbad, CA), sequenced, and labeled with 32P-dCTP using a Random Priming DNA Labeling kit (TaKaRa). The hybridization was performed overnight at 42°C in ULTRAhybe solution (Ambion). Subsequently membranes were washed three times in 2× standard saline citrate and 0.1% SDS at 42°C for 20 minutes and in 0.1× standard saline citrate and 0.1% SDS at 42°C for 20 minutes. To normalize the signals, the membranes were subsequently rehybridized with a 32P-labeled cDNA probe of 18s RNA (Ambion). The images were visualized and quantified by a BAS2500 Phosphorimager (Fuji Film).
Results
Histological Analysis of Muscle Regeneration after Cardiotoxin Injection
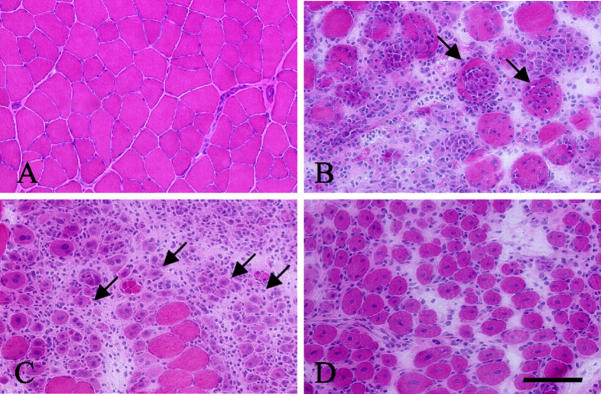
To examine the expression of cytokine and related genes during muscle regeneration, cardiotoxin was injected into right TA muscles of 8- to 9-week-old ICR male mice. Cardiotoxin is a snake venom 12 that selectively injures myofibers but leaves nerves, blood vessels, and satellite cells morphologically intact. 13 Hematoxylin and eosin (H&E) staining revealed that many mononucleated cells had infiltrated the necrotic area 48 hours after toxin treatment (Figure 1B) ▶ , and that they had also penetrated swollen necrotic muscle fibers (Figure 1B ▶ , arrows). After 96 hours, mononucleated cells still actively infiltrated the necrotic area, but newly regenerating myotubes with central nuclei were also observed (Figure 1C ▶ , arrow). Seven days after injury, the numbers of infiltrating cells and necrotic fibers were greatly reduced and the diameter of regenerating muscle fibers with central nuclei had become considerably larger (Figure 1D) ▶ .
Figure 1.
Histological changes of TA muscle after cardiotoxin injection. H&E sections of normal (A) and injected TA muscles of 8- to 9-week-old ICR mice at 48 hours (B), 96 hours (C), and 7 days (D) after cardiotoxin injection. Arrows in B show infiltration of mononucleated cells into necrotic muscle fibers. Arrows in C indicate immature myotubes with central nuclei and basophilic cytoplasm. At 7 days after treatment, the regenerating muscle fibers are larger caliber and the number of inflammatory cells is greatly reduced (D). Scale bar, 100 μm.
An Overview of Changes of Cytokines and Related Gene Expression
A part of the raw images of cDNA array analyses of damaged and control muscles at 48 hours after cardiotoxin injection is shown (Figure 2A) ▶ . Each spot of the injected side is clearly recognizable, whereas some genes in the control side are not. The scattergraphs of all genes examined at 48 hours and 96 hours after muscle damage induced by cardiotoxin are also shown (Figure 2B) ▶ . The signal of each gene and the number of up-regulated genes tended to be greater 96 hours after treatment when compared with those of 48 hours, although expressions of many genes were changed within a cutoff point (fivefold). In Figure 2C ▶ , scattergraphs show induction or reduction of expression of several relevant gene families. Based on the induction pattern, they could be divided into approximately three groups. The first group included the genes whose expressions were increased at both 48 hours and 96 hours. These gene families were chemokines and their receptors, ILs and their receptors, and integrins (Figure 2C) ▶ . The second group included genes whose expressions were increased at 96 hours but not at 48 hours after injection. These included the FGF family, neurotrophic factors (Figure 2C) ▶ , and ephrins and their receptors. The third group included the genes that were induced slightly at both time points. This group was composed of transcription factors and angiogenic factors (Figure 2C) ▶ .
Figure 2.
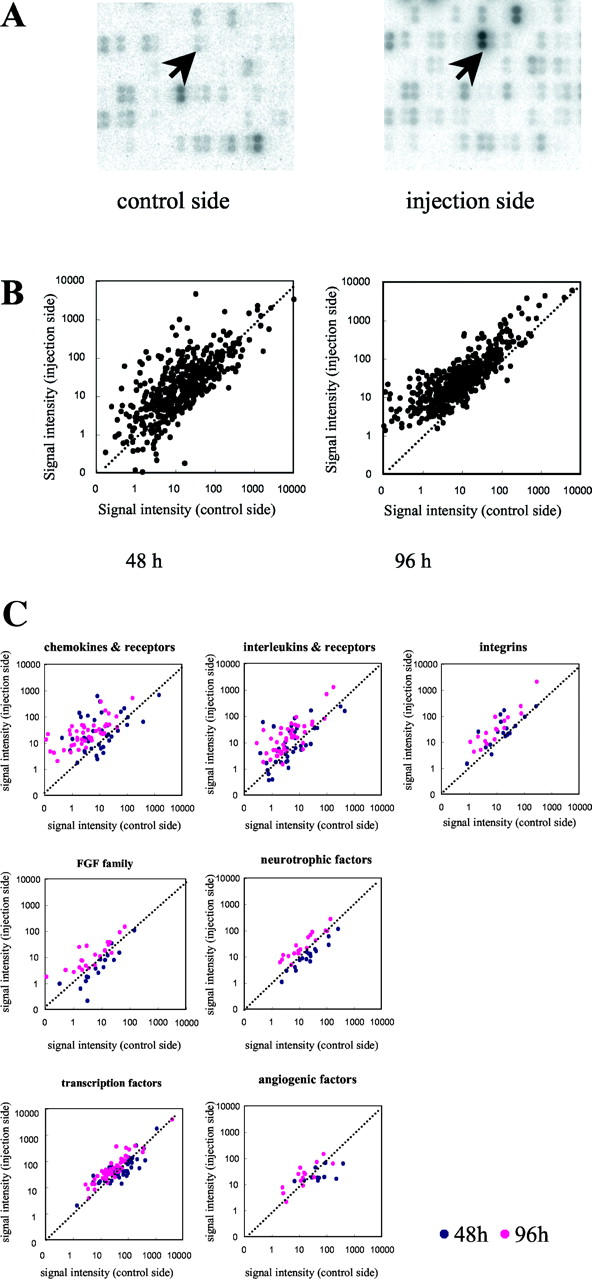
Cytokine cDNA array analysis. A: Partial image of cytokine expression array of untreated (control side) and cardiotoxin-injected (injection side) TA muscles 48 hours after treatment. Arrows indicate the spots of OPN cDNA. B: The scattergraphs show the expression levels (pixel/mm2) of each gene 48 hours and 96 hours after cardiotoxin injection. The transverse axis represents the normalized signal intensities obtained from control muscles, and the longitudinal axis represents those from injected muscles. The data are averages of three independent experiments. One dot corresponds to one gene. C: The scattergraph analysis of representative cytokines and related gene families. The axes are as in B. Blue dots and pink dots are the results at 48 hours and 96 hours, respectively.
Cytokine cDNA Array Analysis 48 Hours after Cardiotoxin Injection
We first analyzed the cytokine gene expression in injured and control TA muscles 48 hours after cardiotoxin injection. As shown in Figure 1 ▶ , mononucleated cells were densely infiltrating into the TA muscle 48 hours after injury. At this stage, numerous inflammation-related cytokines and chemokines were up-regulated (see scattergraphs in Figure 2 ▶ ). In Table 1 ▶ , we listed the genes that increased more than fivefold when compared with the contralateral muscle. Table 2 ▶ is a list of genes that were up-regulated during muscle regeneration despite negligible expression in the control muscle; the induction ratio was not calculated for these genes. The total number of genes listed in Tables 1 and 2 ▶ is 40, which corresponds to 8.7% of the 522 examined genes. Many chemokines and their receptors were up-regulated at this early inflammatory stage of muscle regeneration. The majority of these genes belong to the C-C chemokine/receptor subfamily. 14 JE/MCP-1/CCL2, MIP-1β/CCL4, and C10/CCL6 increased more than 30-fold (Table 1) ▶ , and other C-C chemokines (MIP-1α/CCL3, MIP-1γ/CCL9, MCP-3/CCL7, and MCP-5/CCL12) were also up-regulated (Table 2) ▶ . The expression of their corresponding receptors (CCR1, CCR2, and CCR5) was also increased. Genes encoding ILs and their receptors were up-regulated at this stage (Figure 2C) ▶ .
Several genes of other categories were also up-regulated. Mannose receptors and Mer are related to macrophage phagocytosis. 15 Matrix metalloproteinase-12 (MMP-12) is required when macrophages penetrate the basement membrane, which surrounds muscle fibers. 16 Tissue inhibitor of matrix metalloproteinase-1 (TIMP-1), a natural inhibitor of MMPs, was also increased. Notably, the expression of OPN was most highly up-regulated among the 522 genes on the array (Table 1) ▶ .
The leukemia inhibitory factor (LIF), and hepatocyte growth factor (HGF) which can regulate proliferation of the satellite cells in vitro and/or in vivo 1-6,17-21 were modestly increased: LIF, 2.8-fold; HGF, ∼3.5-fold.
At this time point, FGF family genes (eg, FGF-2 and FGF-6) and transforming growth factor-β superfamily genes were not greatly up-regulated. Similarly, growth factors such as platelet-derived growth factors, insulin-like growth factor (IGF)-1, and IGF-2, which could be potent regulators of satellite cells, 1-6,20,21 were not highly up-regulated.
A list of the down-regulated genes (less than one-fifth) at 48 hours after cardiotoxin treatment is shown in Table 3 ▶ . Nine genes were decreased to less than one-fifth when compared to control muscle. IGF-binding protein (IGFBP)-5 was reported to regulate the effect of IGFs on myogenic cells in culture. 22 Some genes that regulate angiogenesis [eg, vascular endothelial growth factor (VEGF)-A, VEGF-B, and TIE-2] were reduced at this stage. The reduction of erbB3, which is normally expressed in the neuromuscular junction, 23 may reflect reorganization of the neuromuscular junction after muscle necrosis.
Table 3.
Down-Regulated Genes in Regenerating Skeletal Muscle at 48 Hours after Cardiotoxin Injection
| Category | Description | Average -fold decrease | Accession no. |
|---|---|---|---|
| Other cytokines and receptors | Inhibin α subunit | 13.1 | NM_010564 |
| IGF binding protein 5 | 10.1 | NM_010518 | |
| CNTF receptor | 5.9 | L35281 | |
| Angiogenesis and vascular response | VEGF-A | 13.8 | NM_009505 |
| VEGF-B | 6.7 | NM_011697 | |
| TIE-2 | 6.1 | X71426 | |
| ECM and ECM processing | EMMPRIN | 8.4 | Y16256 |
| BMP-1 | 5.3 | L35281 | |
| Muscle regeneration and NMJ | erbB3 | 8.2 | Y16256 |
Cytokine cDNA Array Analysis 96 Hours after Cardiotoxin Injection
We next analyzed the expression of the genes at 96 hours after cardiotoxin injection. Highly up-regulated genes at 96 hours after cardiotoxin injection are shown in Tables 4 and 5 ▶ . Genes that were up-regulated during muscle regeneration despite the nearly complete lack of expression in the control muscle are shown in Table 5 ▶ . The majority of highly up-regulated genes 96 hours after cardiotoxin injection are chemokines and their receptors, as at 48 hours after injection. For instance, the expression of Mig/CXCL9 and RANTES/CCL5 was increased. The expression of MCP-1 and MIP-1β was also elevated at 96 hours, but their induction ratios were decreased when compared with those at 48 hours after injection. ILs and their receptors and integrins were also found in the up-regulated genes.
Table 4.
Up-Regulated Genes in Regenerating Skeletal Muscle at 96 Hours after Cardiotoxin Injection
| Category | Description | Average -fold increase | Accession no. |
|---|---|---|---|
| Chemokines and receptors | C10/CCL6 | 31.3 | NM_009139 |
| JE/MCP-1/CCL2 | 12.7 | NM_011333 | |
| MIP-1β/CCL4 | 6.5 | M35590 | |
| Interleukins and receptors | IL-1 receptor 1 | 12.1 | NM_008362 |
| IL-3 receptor β | 8.6 | NM_007781 | |
| IL-2 receptor γ | 8.4 | L20048 | |
| IL-4 receptor α | 7.9 | NM_010557 | |
| IL-10 receptor β | 7.6 | NM_008349 | |
| NO metabolism | iNOS | 5.5 | NM_010927 |
| Leukocyte markers and functions | Mannose receptor | 12.8 | NM_008625 |
| C5a receptor | 10.3 | NM_007577 | |
| M-CSF receptor | 9.8 | NM_007779 | |
| CD30/TNFRSF8 | 8.2 | NM_011671 | |
| Mer | 7.1 | U21301 | |
| MD-1 | 5.4 | NM_010745 | |
| Other cytokines and receptors | Osteopontin | 37.4 | NM_009263 |
| IGF-2 | 16.5 | NM_010514 | |
| Dhh | 9.9 | NM_007857 | |
| BMP-9 | 9.7 | AF188286 | |
| FGF-11 | 9.6 | NM_010198 | |
| LTBP-1 | 8.1 | AF022889 | |
| Galectin-9 | 7.1 | NM_010708 | |
| Plasminogen | 6.4 | NM_008877 | |
| IGF-binding protein 7 | 6.3 | AB012886 | |
| Flt-3/Flk-2 ligand (FL) | 6.1 | U04807 | |
| IGF-1 | 5.9 | NM_010512 | |
| WNT-16 | 5.9 | AF172064 | |
| Integrins | Integrin-β2 | 8.4 | NM_008404 |
| Integrin-α4 | 7.8 | NM_010576 | |
| Integrin-α6 | 6.5 | X69902 | |
| Integrin-β5A | 5.2 | NM_010580 | |
| ECM and ECM processing | Biglycan | 12.1 | NM_007542 |
| MMP-3 | 8.9 | NM_010809 | |
| ADAM-10 | 5.0 | NM_007399 | |
| Transcription factors | STAT3 | 6.6 | U06922 |
| Energy metabolism | UCP-2 | 19.6 | NM_011671 |
| Muscle regeneration and NMJ | SFRP2/SARP-1 | 10.4 | NM_009144 |
| Ephrin-A2 | 5.4 | NM_007909 | |
| Housekeeping genes | β2-Microglobulin | 12.2 | NM_009735 |
| α-Tubulin | 5.2 | M13446 |
Table 5.
List of Genes that Show Little or No Expression in Normal Muscle but Are Highly Induced at 96 Hours after Cardiotoxin Injection
| Category | Description | Accession no. |
|---|---|---|
| Chemokines and receptors | Mig/CXCL9 | NM_008599 |
| MIP-1γ/CCL9 | NM_011338 | |
| MCP-3/CCL7 | Z12297 | |
| MCP-5/CCL12 | NM_011331 | |
| RANTES/CCL5 | M77747 | |
| CCR1 | NM_009912 | |
| CCR2 | NM_009915 | |
| CCR5 | NM_009917 | |
| CXCR4 | NM_009911 | |
| Interleukins and receptors | IL-1β | NM_008361 |
| IL-18 | NM_008360 | |
| IL-13 receptor α1 | S80963 | |
| IL-1 receptor antagonist | M57525 | |
| Leukocyte markers and functions | CD45/PTPRC | NM_011210 |
| RP105 | NM_008533 | |
| TNFR2/TNFRSF1B | NM_011610 | |
| GITR/TNFRSF18 | NM_009400 | |
| Other cytokines and receptors | HGF | X84046 |
| FGF-12 | U66201 | |
| ECM and ECM processing | MMP-12 | NM_008605 |
| TIMP-1 | NM_011593 | |
| Apoptosis | A1 | L16462 |
| Caspase-1 | NM_009807 | |
| Caspase-3 | NM_009810 |
Several genes of other categories were also up-regulated. OPN still showed high levels of expression, as it had at 48 hours. The expression levels of potent activators of muscle satellite cells, such as IGI-1, IGF-2, and HGF, were increased. IGFBP-7, which was reported to regulate the effect of IGFs on myogenic cells in culture, 24 was also increased. SFRP2/SARP-1 have recently been shown to be increased in muscle regeneration, 25 and ephrin-A2 is expressed in the neuromuscular junction during muscle development. 26 One of the substrates of MMP-3 is OPN. 27 It has been suggested that increased expression of MMP-2 is associated with new myotube formation in muscle regeneration. 28,29 We observed that the expression of MMP-2 was increased with a time course similar to those of previous reports: 28,29 2.5-fold at 96 hours and fourfold at 7 days after the cardiotoxin injection. LIF, FGF-2, FGF-6, and platelet-derived growth factor, which regulate muscle regeneration, were modestly increased: LIF, 2.5-fold; FGF-2, 3.4-fold; FGF-6, 1.8-fold; platelet-derived growth factor-a, 4.1-fold. 1-6,20,21,30,31 At this time point, genes that were down-regulated to less than one-fifth were not found.
Cytokine cDNA Array Analysis 7 Days after Cardiotoxin Injection
Tables 6 and 7 ▶ show up-regulated genes at 7 days after muscle injury. A much smaller number of genes was up-regulated more than fivefold at this stage. Most of them are also recognized to be increased at 96 hours after muscle injury and are related to maturation of regenerating myofibers or tissue remodeling.
Table 6.
Up-Regulated Genes in Regenerating Skeletal Muscle at 7 Days after Cardiotoxin Injection
| Category | Description | Average -fold increase | Accession no. |
|---|---|---|---|
| Other cytokines and receptors | IGF-2 | 17.5 | NM_010514 |
| IGF-1 | 5.2 | NM_010512 | |
| Angiogenesis and vascular response | VAP-1 | 5.4 | NM_009675 |
| ECM and ECM processing | Biglycan | 10.5 | NM_007542 |
| Energy metabolism | UCP-2 | 7.7 | NM_011671 |
| Muscle regeneration and NMJ | SARP-1/SFRP-2 | 5.8 | NM_009144 |
| Housekeeping genes | β2-Microglobulin | 12.7 | NM_009735 |
Throughout the period examined, the gene expressions of epidermal growth factor, transforming growth factor-β1, and FGF-2, which correlate with fibrotic changes of diseased muscle, 32-34 were not highly up-regulated.
OPN Expression in Regenerating Muscle
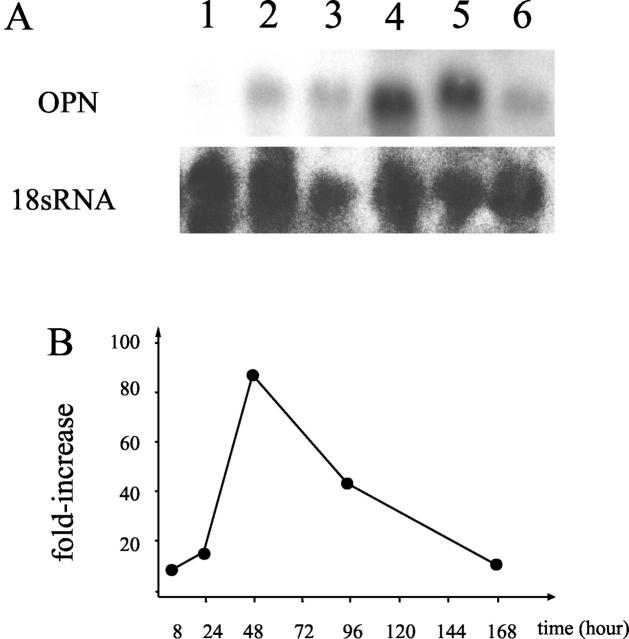
Our array experiments showed that the OPN gene was one of the most prominently up-regulated genes (Tables 1 and 4) ▶ . Northern blotting confirmed that expression of the OPN gene occurred at 8 hours after cardiotoxin injection. The expression level of the OPN gene was greatly up-regulated 48 hours after injection but was slightly down-regulated at 96 hours, as shown in Figure 3 ▶ . The OPN signals in control muscles were quite low. When the 18s RNA signal was used as an internal control, the induction ratio of OPN was calculated as 84-fold at 48 hours and 45-fold at 96 hours after injection, respectively. Seven days after injection, expression of OPN had declined greatly. These results indicate that the expression level of the OPN gene was elevated during the early phases of inflammation and decreased rapidly thereafter.
Figure 3.
OPN transcripts are abundant in cardiotoxin-injured muscle. A: Top: Northern blotting of OPN mRNA in normal (lane 1) and cardiotoxin-injected TA muscle at 8 hours (lane 2), 24 hours (lane 3), 48 hours (lane 4), 96 hours (lane 5), and 7 days (lane 6) after treatment. The level of OPN mRNA is considerably up-regulated at 48 hours after cardiotoxin injection. Bottom: The expression of 18s RNA. B: Time course of the relative expression of OPN mRNA during the skeletal muscle regeneration is shown.
To examine the temporal and spatial expression of OPN protein, skeletal muscles from 8 hours to 14 days after cardiotoxin injection were stained with anti-OPN antibody. At 24 hours after the injection, the expression of OPN was detected in the most severely damaged area (Figure 4A) ▶ . At 48 hours after the injection, OPN expression was observed in the necrotic fibers with invasion of mononucleated cells. OPN expression was also detected in the cytoplasm of some, but not all, mononucleated cells (Figure 4, C and D) ▶ . Intense expression of OPN was found at the boundary between the necrotic (Figure 4B ▶ , indicated as “necrotic”) and intact areas (Figure 4B ▶ , indicated as “intact”); importantly, necrotic fibers in which mononucleated cells have not yet accumulated, lack OPN expression (asterisks), suggesting that OPN was produced and secreted by mononucleated cells but not by the damaged fibers themselves. On days 7 and 14 after treatment, staining for OPN was not detected in the regenerating muscles. As a positive control, we used sections of kidney from the mice challenged with cardiotoxin injection. OPN was expressed in the long loop of Henle and in the distal tubule of the kidney (Figure 4G) ▶ . On the other hand, when the primary antibody was substituted with 5% bovine serum albumin, there was no apparent staining (Figure 4H) ▶ . Thus, the expression of OPN protein was increased at the early phase of muscle inflammation and then rapidly decreased; the results are consistent with those of cDNA array and Northern blotting.
Figure 4.
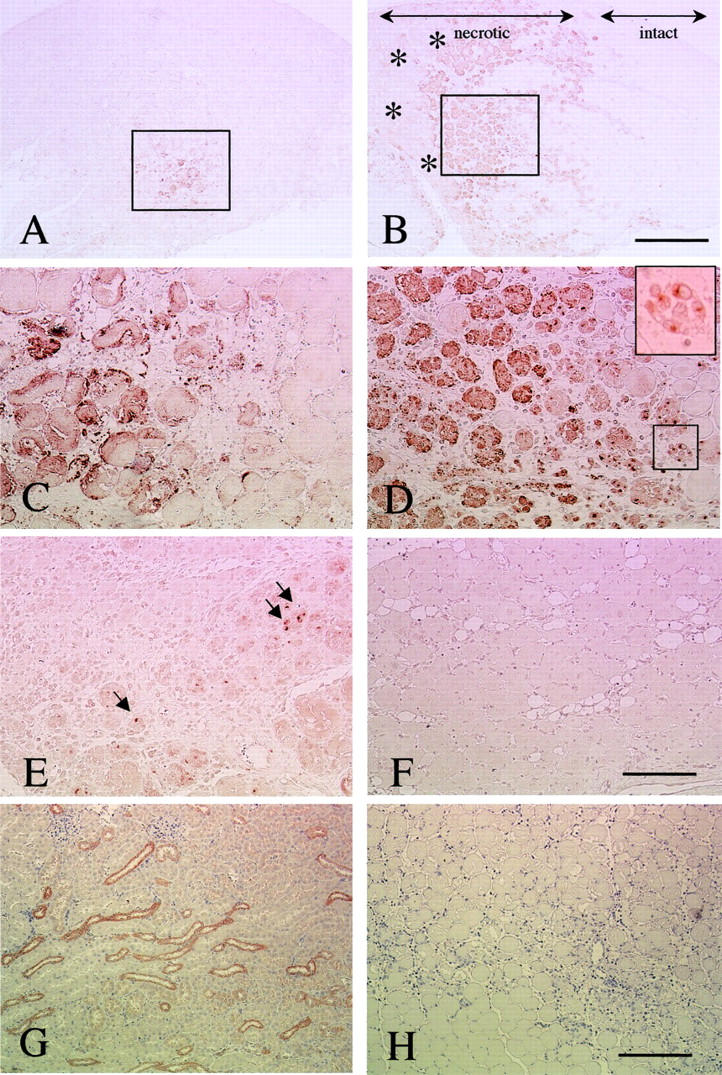
Immunohistochemical analysis of OPN in cardiotoxin-treated muscle. Transverse sections at 24 hours (A, C), 48 hours (B, D), 96 hours (E), and 7 days (F) after cardiotoxin injection were stained with anti-OPN antibody. C and D are high-power magnifications of the areas indicated by rectangles in A and B, respectively. The immunoreactivity is observed in mononucleated cells (inset in D) and necrotic fibers. The intact area, which escaped cardiotoxin injury, is indicated in B. Note that necrotic areas where mononucleated cells have not infiltrated yet were negative for OPN staining (asterisks in B). At 96 hours after treatment, OPN expression was considerably decreased when compared to that at 48 hours, but some OPN-positive mononucleated cells were observed (arrows in E). F: OPN staining was not detected at 7 days after the treatment. G: Positive control section from kidney of ICR mice. OPN was expressed in the long loop of Henle and in the distal tubule of the kidney. H: The negative control section of muscle at 2 days after cardiotoxin treatment showed no apparent staining. All sections were stained by the immunoperoxidase (ABC) method with methyl green (A-F) or hematoxylin (G and H). Scale bars: 400 μm (A, B), 200 μm (C, D, E, and B), 100 μm (G and H).
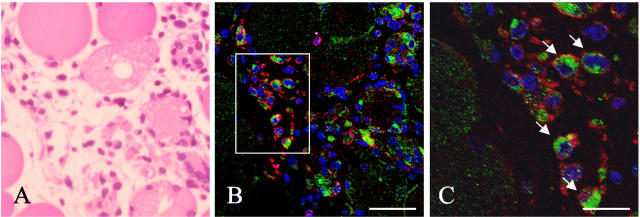
To determine whether macrophages produce OPN, double immunostaining was performed with anti-OPN antibody and anti-F4/80 antibody, which can specifically recognize macrophages (Figure 5) ▶ . OPN-positive mononucleated cells were also positive for F4/80, while there were F4/80-positive cells without OPN expression. OPN expression in mononucleated cells was found in the cytoplasm and often polarized to the perinuclear area. Murry and colleagues 35 reported OPN mRNA in satellite cell-like cells from injured skeletal muscle by in situ hybridization; we, however, failed to detected OPN signals from satellite cells by the double immunostaining with anti-OPN and anti-desmin antibodies. The latter antibody can recognize activated satellite cells (data not shown). In addition, Western blotting showed no OPN protein in cultured primary myoblasts (data not shown). These observations suggest that myoblasts in vivo would express limited amounts, if any, of OPN.
Figure 5.
F4/80-positive cells produce OPN. A: H&E sections of TA muscles at 48 hours after injection. After immunofluorescence observation, the section was washed and restained with H&E. A and B are identical fields. B: Sections of cardiotoxin-injected muscle were immunostained for OPN (green) and F4/80, a macrophage marker (red). Nuclei were stained with TOTO-3 (blue). C: High-power magnification of indicated area in B. Arrows show doubly stained cells. Immunofluorescence with TOTO-3. Scale bars: 40 μm (B); 16 μm (C).
Discussion
In the present study, we examined the expression of 522 genes encoding cytokines and related molecules during the regeneration process of skeletal muscle by using a cytokine cDNA array. We identified several genes, such as OPN, that are highly up-regulated in skeletal muscle regeneration but have not been studied in the context of muscle regeneration.
A Large Number of Cytokines Were Up-Regulated in Injured Muscle
Our results showed that many cytokine genes and cytokine-related genes are up-regulated at the inflammatory stage (48 hours), at the stage of differentiation into myotubes (96 hours), and at the stage of maturation of myofibers (7 days). The results indicate that migration and adhesion of inflammatory cells, activation of satellite cells, reorganization of the extracellular matrix, and removal of necrotic debris actively and almost simultaneously occur throughout the process of muscle regeneration. Among the genes examined, up-regulation of several chemokines and growth factors for satellite cells, was found, and OPN was the one most characteristically changed in this study. In turn, the expression of angiogenic factors (VEGF and VEGF receptor, and TIE) were reduced at 48 hours after cardiotoxin injection. These findings contrasted sharply to those of ischemic injury, which induced the expression of VEGF and receptor 2 in skeletal muscle tissue. 36 Recently, Paoni and colleagues 37 also reported induction of hypoxia-induced factor-1α, which is an important regulator of VEGF production under different oxygen concentrations, 38 in femoral artery ligation models by using a cDNA array. 37 The unique characteristics of cardiotoxin, which selectively damages muscle fibers and leaves satellite cells, nerves, and vascular endothelial cells morphologically intact, 13 may account for these differences.
The limitations of cDNA array are that the changes of mRNA level do not always correlate with the changes in protein level and that changes in mRNA levels do not reveal the functional role(s) of the molecules. For example, FGF-2 mRNA was not highly up-regulated after cardiotoxin treatment although FGF-2 is a potent stimulator of the proliferation of myogenic cells in vitro. In contrast, previous studies showed elevated levels of FGF-2 protein in regenerating muscle after crush injury. 39 Furthermore, a rapid increase of expression of FGF-2 expression was detected in crush-injured muscle by immunohistochemistry, implying exposure of the epitope of pre-existing growth factor because of muscle necrosis rather than new translation of FGF-2. 39 In addition, administration of exogenous FGF-2 has little additional effect on normal regeneration 30,40 indicating that FGF-2 is not normally a limiting factor. 3,4 Instead, the availability of specific receptors and proteoglycans that facilitate binding of growth factor to their receptors is more likely the crucial factor. Thus, careful consideration is required for the interpretation of the data obtained by cDNA array in light of these complex relationships among the mRNA level, protein level, and functional roles of each molecule during muscle regeneration.
Comparison of the Cytokine Expression Profile in the Regeneration Model with Those of Dystrophic Muscle and Inflammatory Myopathies
cDNA array technologies are now extensively used to elucidate the molecular pathogenesis of human muscle diseases. 41-43 In muscular dystrophies such as Duchenne muscular dystrophy, however, muscle regeneration and inflammatory responses are usually limited. Indeed, the levels of inflammatory cytokines in human muscular dystrophies seem much lower than in the cardiotoxin-induced regeneration model. 41,42 Very interestingly, expression profiles of mdx mice, an animal model of Duchenne muscular dystrophy, indicated an active inflammatory response in the limb muscles. 44,45 Further, expression profiles of mdx mice are qualitatively similar to those of cardiotoxin-induced muscle regeneration. For example, up-regulation of several chemokines (MCP-1, C10, MIP-1γ, MCP-3, CCR2, and CXCR4), IL receptors (IL-10 receptor and IL-1ra), MMPs (MMP-3 and MMP-12), and several cytokines (OPN and IGF-2) were observed in both the mdx and cardiotoxin-induced regeneration models. 44 Many features of dystrophin-deficient muscle pathology are not clearly related to the loss of mechanical support of muscle membrane by dystrophin, and some changes could be ascribed to chronic inflammatory responses. 46 Taken together, active inflammation, associated with active regeneration, may in part explain the phenotypic differences between mdx mice and Duchenne muscular dystrophy.
Human idiopathic myositis is characterized by many infiltrating leukocytes, which produced large amounts of inflammatory cytokines in skeletal muscle. 43 Importantly, however, different sets of cytokines are overexpressed in human myositis compared with cardiotoxin-induced muscle regeneration. For example, many immune-related cytokines and their receptors (eg, IL-1α, IL-2, IL-4, IL-6, tumor necrosis factor-β, interferon-γ, and their receptors) are expressed in inflammatory myopathies, 43,47 but are not extensively expressed in the cardiotoxin-injection model. Active muscle regeneration exists beside muscle necrosis in polymyositis/dermatomyositis, but the qualitative difference between cardiotoxin models and polymyositis/dermatomyositis may be ascribed to the immune response in the latter condition.
Up-Regulation of Chemokines
Previous studies have demonstrated that infiltration of macrophages triggers the regeneration process, 7,8 and molecules that recruit macrophages are expressed in the injured muscle tissue. 6,48 Several cytokines such as platelet-derived growth factor, transforming growth factor-β, FGF-2, LIF, and HGF have been shown to promote chemotaxis of macrophages or muscle satellite cells. 48,49 In the present study, we found that C-C chemokines/receptors, which regulate the infiltration of monocytes/macrophages, were highly up-regulated: eg, MIP-1α and MCP-1 are ligands for CCR1, MCP-1 and MCP-3 for CCR2, and MIP-1α and MIP-1β for CCR5. 14 Monocytes/macrophages have expressed CCR1, CCR2, and CCR5. 50 Although the precise cellular sources of these chemokines remain to be determined, the parallel expression patterns between ligands and receptors and the close relationship between gene expressions and histological changes suggest that these chemokines may participate in the infiltration of monocytes/macrophages into the injured muscle. Furthermore, it is interesting that the expression levels of C10/CCL6 were high at both 48 and 96 hours after injury, whereas those of MCP-1/CCL2 and MIP-1β/CCL4 had rapidly declined by 96 hours. The expression of chemokines might be strictly controlled throughout the time course of inflammation associated with muscle regeneration.
Growth Factors that Activate Satellite Cells
Satellite cells modulate their cell-cycle state in response to growth factors. 1-6 In fact, there are many reports that growth factors (eg, HGF, FGFs, IGFs) stimulate proliferation of satellite cells in vitro; however, which cytokines activate dormant satellite cells and promote the proliferation of satellite cells during muscle regeneration in vivo is not well established. It is also unclear which molecules regulate the timing of differentiation. At 48 hours after cardiotoxin injection, we detected modest induction of HGF and LIF. At 96 hours after the cardiotoxin injection, HGF, IGF-1, and IGF-2 mRNAs were found to be considerably elevated. High levels of IGF-1 and IGF-2 mRNA expression are still observed 7 days after injury. Up-regulation of IGF-1 and IGH-2 is consistent with previous findings and further supports the idea that IGF-1 and IGF-2 are the major factors that promote both the proliferation of satellite cells and muscle growth during muscle regeneration. 51,52
IGF-1 and IGF-2 can stimulate both proliferation and differentiation of myoblasts through the same type 1 IGF receptor. 53 The use of binding proteins such as IGFBPs and vitronectin is essential to their biological activity, 54 and IGFBPs are suggested to determine whether myoblasts respond to IGFs by proliferation or differentiation. 53,55 Therefore, it is interesting to note that IGFBP-5 was decreased at 48 hours and then recovered at 96 hours after injection. IGFBP-5 can inhibit both proliferative and differentiative effects of IGF-2 in cultured myogenic cells. 22 On the other hand, IGFBP-7, which was up-regulated at 96 hours, has been recently shown to inhibit differentiation without affecting the proliferative effects of IGFs in myogenic cell culture. 24 These changes of IGFBP expression may finely regulate proliferation and differentiation of myoblasts.
Role of OPN in Skeletal Muscle Regeneration
OPN (also called secreted phosphoprotein-1, minopontin, or Eta-1) is a phosphorylated glycoprotein that contains an arginine-glycine-aspartate (RGD) sequence, is present in mineralized tissues as extracellular matrices, and is implicated in many pathological conditions as a multifunctional cytokine. 9-11,56,57 In this study, we showed that OPN was highly up-regulated during the muscle regeneration process. In the context of inflammation and the tissue repair/remodeling process, OPN has been implicated as a chemoattractant for monocytes/macrophages and an adhesive molecule for various cells. 9-11 But what is the most important role of OPN in the muscle regeneration process?
OPN may not be directly involved in the macrophage influx process of muscle regeneration. Indeed, the role of OPN as a macrophage guide is less than conclusive, 11 and the results differ according to the experimental model. 35,58-62 In our series of cDNA array experiments, the expressions of not only OPN but of several chemokines for monocytes/macrophages, such as MCP-1, were highly increased in regenerating muscle. Further, we observed that gene expression of MCP-1 was much greater than that of OPN at 8 hours after cardiotoxin injection (unpublished observations).
Alternatively, OPN secreted by macrophages may serve as an adhesion molecule for macrophages to promote phagocytosis. 63 This study revealed that a major source of OPN was the macrophages themselves, and protein localization of OPN was associated with the necrotic fibers under invasion by macrophages. It has been shown that macrophages can produce integrin ανβ3, a receptor for OPN, which plays a role in macrophage phagocytosis. 64 Indeed, supporting this idea, one of the most striking differences between mice lacking OPN and the wild type was decreased clearance of tissue debris and infectious agents. 61,62
Finally, OPN might have accessory roles in muscle regeneration, because this molecule is a multifunctional cytokine. In particular, OPN affects vascularization 65 and collagen synthesis and fibrosis 61,66 during muscle regeneration. 44,67
In conclusion, our cDNA array experiment contributes to the understanding of the muscle regeneration phenomenon, a highly coordinated, dynamic event that results from an interplay of numerous inflammatory and myogenic regulators. In particular, further investigation into the role for OPN in muscle regeneration could provide a novel intervention for muscular dystrophy.
Table 7.
List of Genes that Show Little or No Expression in Normal Muscle but Are Highly Induced at 7 Days after Cardiotoxin Injection
| Category | Description | Accession no. |
|---|---|---|
| Chemokines and receptors | RANTES/CCL5 | M77747 |
| Mig/CXCL9 | NM_008599 | |
| Other cytokines and receptors | PDGF-b | NM_011057 |
Acknowledgments
We thank all the members of the Department of Molecular Therapy for technical assistance and discussion.
Footnotes
Address reprint requests to Shin’ichi Takeda, M.D., Ph.D., Department of Molecular Therapy, National Institute of Neuroscience, National Center of Neurology and Psychiatry, 4-1-1 Ogawa-Higashi, Kodaira, Tokyo 187-8502, Japan. E-mail: takeda@ncnp.go.jp.
Supported by grants-in-aid for scientific research from the Center of Excellence, Research on Nervous and Mental Disorders (10B-1, 13B-1), health science research grants for research on the human genome and gene therapy (H10-genome-015, H13-genome-001), for research on brain science (H12-brain-028) from the Ministry of Health, Labor, and Welfare; grants-in aid for scientific research (10557065, 11470153, 11170264, and 14657158) from the Ministry of Education, Culture, Sports, Science, and Technology; and a research grant from the Human Frontier Science Project.
References
- 1.Bischoff R: The satellite cell and muscle regeneration. Engel AG Franzini-Armstrong C eds. Myology. 1994:pp 97-118 McGraw-Hill, New York
- 2.Best TM, Hunter KD: Muscle injury and repair. Phys Med Rehabil Clin North Am 2000, 11:251-266 [PubMed] [Google Scholar]
- 3.Grounds MD: Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann NY Acad Sci 1998, 854:78-91 [DOI] [PubMed] [Google Scholar]
- 4.Grounds MD: Muscle regeneration: molecular aspects and therapeutic implications. Curr Opin Neurol 1999, 12:535-543 [DOI] [PubMed] [Google Scholar]
- 5.Seale P, Rudnicki MA: A new look at the origin, function, and “stem cell” status of muscle satellite cells. Dev Biol 2000, 218:115-124 [DOI] [PubMed] [Google Scholar]
- 6.Tidball JG: Inflammatory cell response to acute muscle injury. Med Sci Sports Exerc 1995, 27:1022-1032 [DOI] [PubMed] [Google Scholar]
- 7.Akiyama C, Kobayashi S, Nonaka I: Comparison of behavior in muscle fiber regeneration after bupivacaine hydrochloride- and acid anhydride-induced myonecrosis. Acta Neuropathol 1992, 83:584-589 [DOI] [PubMed] [Google Scholar]
- 8.Lescaudron L, Peltékian E, Fontaine-Pérus J, Paulin D, Zampieri M, Garcia L, Parrish E: Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscul Disord 1999, 9:72-80 [DOI] [PubMed] [Google Scholar]
- 9.Denhardt DT, Guo X: Osteopontin: a protein with diverse functions. EMBO J 1993, 7:1475-1482 [PubMed] [Google Scholar]
- 10.Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS: Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest 2001, 107:1055-1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giachelli CM, Steitz S: Osteopontin: a versatile regulator of inflammation and biomineralization. Matrix Biol 2000, 19:615-622 [DOI] [PubMed] [Google Scholar]
- 12.Fletcher JE, Jiang M-S: Possible mechanisms of action of cobra snake venom cardiotoxins and bee venom melittin. Toxicon 1993, 31:669-695 [DOI] [PubMed] [Google Scholar]
- 13.Couteaux R, Mira J-C, d’Albis A: Regeneration of muscles after cardiotoxin injury. I. Cytological aspects. Biol Cell 1988, 62:171-182 [PubMed] [Google Scholar]
- 14.Zlotnik A, Yoshie O: Chemokines: a new classification system and their role in immunity. Immunity 2000, 12:121-127 [DOI] [PubMed] [Google Scholar]
- 15.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK: Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 2001, 411:207-211 [DOI] [PubMed] [Google Scholar]
- 16.Shipley JM, Wesselschmidt RL, Kobayashi DK, Ley TJ, Shapiro SD: Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc Natl Acad Sci USA 1996, 93:3942-3946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurek JB, Bower JJ, Romanella M, Koentgen F, Murphy M, Austin L: The role of leukemia inhibitory factor in skeletal muscle regeneration. Muscle Nerve 1997, 20:815-822 [DOI] [PubMed] [Google Scholar]
- 18.Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM: Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol 1995, 165:307-312 [DOI] [PubMed] [Google Scholar]
- 19.Tatsumi R, Anderson JE, Nevoret CJ, Halvey O, Allen RE: HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol 1998, 194:114-128 [DOI] [PubMed] [Google Scholar]
- 20.Husmann I, Soulet L, Gautron J, Martelly I, Barritault D: Growth factors in skeletal muscle regeneration. Cytokine Growth Factor Rev 1996, 7:249-258 [DOI] [PubMed] [Google Scholar]
- 21.Cannon JG, St. Pierre BA: Cytokines in exertion-induced skeletal muscle injury. Mol Cell Biochem 1998, 179:159-167 [DOI] [PubMed] [Google Scholar]
- 22.Ewton DZ, Coolican SA, Mohan S, Chernausek SD, Florini JR: Modulation of insulin-like growth factor actions in L6A1 myoblasts by insulin-like growth factor binding protein (IGFBP)-4 and IGFBP-5: a dual role for IGFBP-5. J Cell Physiol 1998, 177:47-57 [DOI] [PubMed] [Google Scholar]
- 23.Zhu X, Lai C, Thomas S, Burden SJ: Neuregulin receptors, erbB3 and erbB4, are localized at neuromuscular synapses. EMBO J 1995, 23:5842-5848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haugk KL, Wilson H-M, Swisshelm K, Quinn LS: Insulin-like growth factor (IGF)-binding protein-related protein-1: an autocrine/paracrine factor that inhibits skeletal myoblast differentiation but permits proliferation in response to IGF. Endocrinology 2000, 141:100-110 [DOI] [PubMed] [Google Scholar]
- 25.Levin JM, Boubaker RA, Andalousi E, Dainat J, Reyne Y, Bacou F: SFRP2 expression in rabbit myogenic progenitor cells and in adult skeletal muscles. J Muscle Res Cell Motil 2001, 22:361-369 [DOI] [PubMed] [Google Scholar]
- 26.Lai K-O, Ip FCP, Cheung J, Fu AKY, Ip NY: Expression of Eph receptors in skeletal muscle and their localization at the neuromuscular junction. Mol Cell Neurosci 2001, 17:1034-1047 [DOI] [PubMed] [Google Scholar]
- 27.Kherif S, Dehaupas M, Lafuma C, Fardeau M, Alameddine HS: Matrix metalloproteinases MMP-2 and MMP-9 in denervated muscle and injured nerve. Neuropathol Appl Neurobiol 1998, 24:309-319 [DOI] [PubMed] [Google Scholar]
- 28.Kherif S, Lafuma C, Dehaupas M, Lachkar S, Fournier J-G, Verdière-Sahuqué M, Fardeau M, Alameddine HS: Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev Biol 1999, 205:158-170 [DOI] [PubMed] [Google Scholar]
- 29.Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L: Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin). J Biol Chem 2001, 276:28261-28267 [DOI] [PubMed] [Google Scholar]
- 30.Lefaucheur JP, Sebille A: Basic fibroblast growth factor promotes in vivo muscle regeneration in murine muscular dystrophy. Neurosci Lett 1995, 202:121-124 [DOI] [PubMed] [Google Scholar]
- 31.Floss T, Arnold H-H, Braun T: A role for FGF-6 in skeletal muscle regeneration. Gene Dev 1997, 11:2040-2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Amore PA, Brown RH, Jr, Ku P-T, Hoffman EP, Watanabe H, Arahata K, Ishihara T, Folkman J: Elevated basic fibroblast growth factor in the serum of patients with Duchenne muscular dystrophy. Ann Neurol 1994, 35:362-365 [DOI] [PubMed] [Google Scholar]
- 33.Iannaccone S, Quattrini A, Smirne S, Sessa M, de Rino F, Ferini-Strambi L, Nemni R: Connective tissue proliferation and growth factors in animal models of Duchenne muscular dystrophy. J Neurol Sci 1995, 128:36-44 [DOI] [PubMed] [Google Scholar]
- 34.Bernasconi P, Torchana E, Confalonieri P, Brugnoni R, Barresi R, Mora M, Cornelio F, Morandi L, Mantegazza R: Expression of transforming growth factor-β1 in dystrophic patient muscles correlates with fibrosis: pathogenetic role of a fibrogenic cytokine. J Clin Invest 1995, 96:1137-1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murry CE, Giachelli CM, Schwartz SM, Vracko R: Macrophages express osteopontin during repair of myocardial necrosis. Am J Pathol 1994, 145:1450-1462 [PMC free article] [PubMed] [Google Scholar]
- 36.Rissanen TT, Vajanto I, Hiltunen MO, Rutanen J, Kettunen MI, Niemi M, Leppänen P, Turunen MP, Markkanen JE, Arve K, Alhava E, Kauppinen RA, Ylä-Herttuala S: Expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 (KDR/Flk-1) in ischemic skeletal muscle and its regeneration. Am J Pathol 2002, 160:1393-1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paoni NF, Peale F, Wang F, Errett-Baroncini C, Steinmetz H, Toy K, Bai W, Williams PM, Bunting S, Gerritsen ME, Powell-Braxton L: Time course of skeletal muscle repair and gene expression following acute hind limb ischemia in mice. Physiol Genomics 2002, 11:263-272 [DOI] [PubMed] [Google Scholar]
- 38.Forsythe JA, Jiang B-H, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL: Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 1996, 16:4604-4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson JE, Mitchell CM, McGeachie JK, Grounds MD: The time course of basic fibroblast growth factor expression in crush-injured skeletal muscles of SJL/J and BALB/c mice. Exp Cell Res 1995, 216:325-334 [DOI] [PubMed] [Google Scholar]
- 40.Mitchell CA, McGeachie JK, Grounds MD: The exogenous administration of basic fibroblast growth factor to regenerating skeletal muscle in mice does not enhance the process of regeneration. Growth Factors 1996, 13:37-55 [DOI] [PubMed] [Google Scholar]
- 41.Chen Y-W, Zhao P, Borup R, Hoffman EP: Expression profiling in the muscular dystrophies: identification of novel aspects of molecular pathophysiology. J Cell Biol 2000, 151:1321-1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haslett JN, Sanoudou D, Kho AT, Bennett RR, Greenberg SA, Kohane IS, Beggs AH, Kunkel LM: Gene expression comparison of biopsies from Duchenne muscular dystrophy (DMD) and normal skeletal muscle. Proc Natl Acad Sci USA 2002, 99:15000-15005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenberg SA, Sanoudou D, Haslett JN, Kohane IS, Kunkel LM, Beggs AH, Amato AA: Molecular profiles of inflammatory myopathies. Neurology 2002, 59:1170-1182 [DOI] [PubMed] [Google Scholar]
- 44.Porter JD, Khanna S, Kaminski HJ, Rao JS, Merriam AP, Richmonds CR, Leahy P, Li J, Guo W, Andrade FH: A chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin-deficient mdx mice. Hum Mol Genet 2002, 11:263-272 [DOI] [PubMed] [Google Scholar]
- 45.Rouger K, Le Cunff M, Steenman M, Potier M-C, Gibelin N, Dechesne CA, Leger JJ: Global/temporal gene expression in diaphragm and hindlimb muscles of dystrophin-deficient (mdx) mice. Am J Physiol 2002, 253:C773-C784 [DOI] [PubMed] [Google Scholar]
- 46.Spencer MJ, Tidball JG: Do immune cells promote the pathology of dystrophin-deficient myopathies? Neuromusc Disord 2001, 11:556-564 [DOI] [PubMed] [Google Scholar]
- 47.Tews DS, Goebel HH: Cytokine expression profile in idiopathic inflammatory myopathies. J Neuropathol Exp Neurol 1996, 55:342-347 [DOI] [PubMed] [Google Scholar]
- 48.Robertson TA, Maley MAL, Grounds MD, Papadimitriou JM: The role of macrophages in skeletal muscle regeneration with particular reference to chemotaxis. Exp Cell Res 1993, 207:321-331 [DOI] [PubMed] [Google Scholar]
- 49.Bischoff R: Chemotaxis of skeletal muscle satellite cells. Dev Dyn 1997, 208:505-515 [DOI] [PubMed] [Google Scholar]
- 50.Baggiolini M: Chemokines and leukocyte traffic. Nature 1998, 392:565-568 [DOI] [PubMed] [Google Scholar]
- 51.Marsh DR, Criswell DS, Hamilton MT, Booth FW: Association of insulin-like growth factor mRNA expressions with muscle regeneration in young, adult, and old rats. Am J Physiol 1997, 273:R353-R358 [DOI] [PubMed] [Google Scholar]
- 52.Keller HL, St. Pierre Schneider B, Eppihimer LA, Cannon JG: Association of IGF-É and IGF-Đ with myofiber regeneration in vivo. Muscle Nerve 1999, 22:347-354 [DOI] [PubMed] [Google Scholar]
- 53.Florini JR, Ewton DZ, Coolican SA: Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev 1996, 17:481-517 [DOI] [PubMed] [Google Scholar]
- 54.Upton Z, Webb H, Hale K, Yandell CA, McMurtry JP, Francis GL, Ballard FJ: Identification of vitronectin as a novel insulin-like growth factor-Đ binding protein. Endocrinology 1999, 140:2928-2931 [DOI] [PubMed] [Google Scholar]
- 55.Jennische E, Hall CM: Expression and localisation of IGF-binding protein mRNAs in regenerating rat skeletal muscle. APMIS 2000, 108:747-755 [DOI] [PubMed] [Google Scholar]
- 56.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H: Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 2000, 287:860-864 [DOI] [PubMed] [Google Scholar]
- 57.Chabas D, Baranzini SE, Mitchell D, Bernard CCA, Rittling SR, Denhardt DT, Sobel RA, Lock C, Karpuj M, Pedotti R, Heller R, Oksenberg JR, Steinman L: The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science 2001, 294:1731-1735 [DOI] [PubMed] [Google Scholar]
- 58.Singh RP, Patarca R, Schwartz J, Singh P, Cantor H: Definition of a specific interaction between the early T lymphocyte activation-1 (Eta-1) protein and murine macrophages in vitro and its effect upon macrophages in vivo. J Exp Med 1990, 171:1931-1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giachelli CM, Lombardi D, Johnson RJ, Murry CE, Almeida M: Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am J Pathol 1998, 152:353-358 [PMC free article] [PubMed] [Google Scholar]
- 60.Pichler R, Giachelli CM, Lombardi D, Pippin J, Gordon K, Alpers CE, Schwartz SM, Johnson RJ: Tubulointerstitial disease in glomerulonephritis. Potential role of osteopontin (uropontin). Am J Pathol 1994, 144:915-926 [PMC free article] [PubMed] [Google Scholar]
- 61.Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BLM: Altered wound healing in mice lacking a functional osteopontin gene (spp1). J Clin Invest 1998, 101:1468-1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nau GJ, Liaw L, Chupp GL, Berman JS, Hogan BLM, Young RA: Attenuated host resistance against Mycobacterium bovis BCG infection in mice lacking osteopontin. Infect Immun 1999, 67:4223-4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McKee MD, Nancy A: Secretion of osteopontin by macrophages and its accumulation at tissue surfaces during wound healing in mineralized tissues: a potential requirement for macrophage adhesion and phagocytosis. Anat Rec 1996, 245:394-409 [DOI] [PubMed] [Google Scholar]
- 64.Savill J, Dransfield I, Hogg N, Haslett C: Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature 1990, 343:170-173 [DOI] [PubMed] [Google Scholar]
- 65.Asou Y, Rittling SR, Yoshitake H, Tsuji K, Shinomiya K, Nifuji A, Denhardt DT, Noda M: Osteopontin facilitates angiogenesis, accumulation of osteoclasts, and resorption in ectopic bone. Endocrinology 2001, 142:1325-1332 [DOI] [PubMed] [Google Scholar]
- 66.Trueblood NA, Xie Z, Communal C, Sam F, Ngoy S, Liaw L, Jenkins AW, Wang J, Sawyer DB, Bing OHL, Apstein CS, Colucci WS, Singh K: Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ Res 2001, 88:1080-1087 [DOI] [PubMed] [Google Scholar]
- 67.Grounds MD, McGeachie JK: Myogenic cell replication in minced skeletal muscle isografts of Swiss and Balb/c mice. Muscle Nerve 1990, 13:305-313 [DOI] [PubMed] [Google Scholar]