Abstract
We have used spectral karyotyping to assess potential roles of three different components of the nonhomologous DNA end-joining pathway in the maintenance of genomic stability in mouse embryonic fibroblasts (MEFs). MEFs homozygous for mutations that inactivate either DNA ligase IV (Lig4) or Ku70 display dramatic genomic instability, even in the absence of exogenous DNA damaging agents. These aberrant events range from chromosomal fragmentation to nonreciprocal translocations that can involve several chromosomes. DNA-dependent protein kinase catalytic subunit deficiency also promotes genome instability. Deficiency for the p53 cell cycle checkpoint protein has little effect on spontaneous levels of chromosomal instability in Lig4-deficient fibroblasts. However, in the context of ionizing radiation treatment, p53 deficiency allowed visualization of massive acute chromosomal destruction in Lig4-deficient MEFs, which in surviving cells manifested as frequent nonreciprocal translocations. We conclude that nonhomologous DNA end-joining plays a crucial role as a caretaker of the mammalian genome, and that an alternative repair pathway exists that often leads to nonreciprocal translocations.
In mammalian cells, DNA double-strand breaks (DSBs) may result from normal metabolic processes, DNA damaging agents such as ionizing radiation (IR), or specific enzymes such as that which initiates V(D)J recombination in developing lymphocytes (1, 2). Unrepaired DSBs can have severe cellular consequences ranging from death to neoplastic transformation (3, 4). Mammalian cells generally repair DSBs either by the homologous recombination (HR) or by the nonhomologous DNA end-joining (NHEJ) pathways. There are five known components of the NHEJ pathway. Three are subunits of the DNA-dependent protein kinase (DNA-PK), including the Ku70 and Ku80 proteins, which form a DNA end-binding complex (Ku) and the catalytic subunit (DNA-PKcs). The other two components are the DNA ligase IV (Lig4) and XRCC4 proteins, which likely act as a complex to catalyze the ligation step in the reaction (5).
Cells deficient in Ku70, Ku80, Lig4, and XRCC4 exhibit premature senescence, severely impaired V(D)J recombination, and sensitivity to DNA damaging agents that induce DSBs (6–12). Inactivation of any of these four genes in mice leads to multiple defects including growth deficiency, severe combined immunodeficiency, and massively increased apoptosis of newly derived, postmitotic neurons (12–14). DNA-PKcs deficiency also results in sensitivity to DNA damaging agents and impaired V(D)J recombination, but its absence does not lead to as severe a spectrum of defects in mice as deficiencies in the other four known NHEJ components. The less severe consequences of DNA-PKcs deficiency compared with Ku70 or Ku80 deficiency suggests that the latter proteins have functions independent of the DNA-PK holoenzyme (13, 15, 16).
NHEJ deficiencies can lead to increased rates of neoplastic transformation. T cell lymphomas and increased fibroblast transformation have been reported in the context of Ku70 deficiency (8, 17). In addition, a Lig4 mutation has been implicated in a human leukemia patient who died from radiation sensitivity (18). Finally, introduction of the DNA-PKcs or XRCC4-deficient background onto a p53-deficient background led to the appearance, at very high frequency, of pro-B cell lymphomas (3, 4). In the XRCC4-deficient, p53-deficient background, these lymphomas frequently harbored translocations involving the Ig heavy chain locus and the c-myc locus. Previous studies have demonstrated that the HR pathway plays an important role in maintaining chromosomal stability in both murine and avian systems (19, 20). However, the frequent translocations in the XRCC4-deficient, p53-deficient lymphomas raised the possibility that the NHEJ also may contribute to the general maintenance of genomic stability.
To further elucidate the role of NHEJ in the prevention of karyotypic instability that may contribute to malignant transformation, we have used spectral karyotyping (SKY) technology to analyze metaphase chromosomes of normally growing mouse embryonic fibroblasts (MEFs) deficient in either DNA-PKcs (DNA-PKcs−/−), Ku70 (Ku70−/−), or Lig4 (Lig4−/−). SKY allows for the unambiguous identification of all mouse chromosomes (21). In addition, the process involves high-resolution color and gray-scale digital photography and software-based enhancement that may permit identification of chromosomal anomalies not observed with traditional microscopy and photography (22). These studies demonstrate that the NHEJ pathway plays a major role in the suppression of genomic instability including translocations.
Materials and Methods
Generation of Lig4/p53-Deficient Mice.
The generation of Lig4-deficient mice has been reported (11). Lig4 heterozygous mice were crossed to p53-deficient mice (Taconic Farms; TSG-p53) to obtain Lig4+/−p53+/− mice. The double heterozygous mice were intercrossed to obtain embryos of all relevant genotypes for analyses of chromosomal stability and growth of cells in culture.
SKY Analyses.
MEFs were generated from 13.5-day-old embryos by using standard methods. Passage 3–5 MEFs (1 × 106) were plated onto gelatinized 10-cm dishes and cultured for 16 h. Colcemid (GIBCO/BRL; KaryoMAX Colcemid solution) was added to the cultures (100 ng/ml), and the cultures were incubated for 3 h to arrest proliferating cells at metaphase. To examine the effects of IR, MEFs were exposed to 500 rads of IR and allowed to recover at 37°C for 24 h before fixation. The block of internal organs from 13.5-day-old embryos was physically dissociated by using nylon mesh, and the cell suspension was immediately fixed for preparation of metaphases. Chromosomal aberrations were quantified by using a Nikon Eclipse microscope equipped with an Applied Spectral Imaging interferometer and 40× and 63× objectives.
Recovery of Growth After Irradiation.
Passage 2 MEFs (2 × 105) were treated with 300 rads of γ-irradiation from a 137Cr source and plated onto gelatinized 6-cm dishes. The cells were trypsinized and processed as described above.
Results
Chromosomal Instablity in NHEJ-Deficient Embryonic Cells.
We found that Ku70−/− and Lig4−/− MEFs had dramatic chromosomal instability compared with wild-type controls (Table 1). The unstable karyotypes of NHEJ-deficient cells featured chromosome and chromatid fragmentation, as well as the presence of acentric marker chromosomes (Table 1 and Fig. 1). Parallel studies using traditional techniques also have suggested frequent occurrence of such fragmentation in cells lacking Ku80 or DNA-PKcs (23, 24). Translocations are well documented to be contributory to many malignancies, with reciprocal translocations predominating in lymphoid tumors (25) and nonreciprocal translocations predominating in many solid tumors (26). With SKY technology we have been able to ask whether spontaneous DNA lesions in NHEJ-deficient cells are substrates for alternative DSB repair mechanisms that lead to translocations. Both Lig4 and Ku70 deficiency indeed led to translocations (Table 1 and Fig. 2). These aberrant events included both simple translocations involving two chromosomes, as well as complex translocations involving more than two chromosomes (Figs. 1 and 2). All of the translocations are nonreciprocal and present in either one or two copies.
Table 1.
Chromosomal instability in NHEJ-deficient fibroblasts
| Events | Genotypes of MEFs
|
||||||
|---|---|---|---|---|---|---|---|
| WT | Lig4−/−‡ | Lig4−/−p53−/− | p53−/− | Ku70−/−‡ | DNA-PKcs−/−‡ | NHEJ mutants combined† | |
| Metaphases karyotyped | 27 | 14 | 30 | 22 | 16 | 18 | 48 |
| Fragmented chromosomes & chromatids | 0 | 4 | 7 | 0 | 6 | 3 | 13 |
| Detached centromeres | 0 | 4 | 13 | 0 | 1 | 0 | 5 |
| Translocations | 0 | 2 | 5 | 0 | 1 | 0 | 3 |
| Robertsonian translocations (fusions) | 0 | 1 | 4 | 0 | 0 | 0 | 1 |
| Metaphases with structural abnormality,* % | 0 | 64 | 66 | 0 | 44 | 17 | 41 |
The numbers to the right of the categories of events are the total events of that type. Some metaphases had more than one event of a particular type and others a mixture of types of events. Two embryos each for wild type (WT), Lig4−/−, and Lig4−/− p53−/− were analyzed, with duplicates yielding similar results. One each of the remaining genotypes were analyzed.
The percentage of metaphases with any structural abnormality. Aneuploidy was not considered because all genotypes of midpassage MEFs tend to become near tetraploid.
Sum of events arising in the three NHEJ mutant cell lines (denoted by ‡).
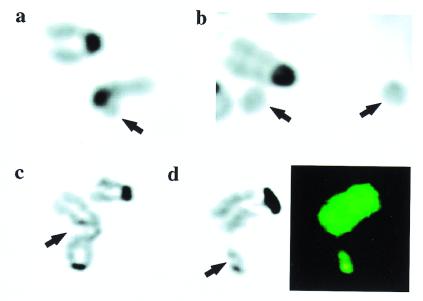
Figure 1.
Spontaneous chromosomal fragmentation in NHEJ-deficient MEFs. Arrows indicate the aberration. (a–c) Images of 4′,6-diamidino-2-phenylindole (DAPI)-stained metaphase chromosomes. (d) Both DAPI staining and SKY analysis. (a) Lig4−/−; broken chromatid. (b) DNA-PKcs−/−; two marker chromosomes (acentric fragments). (c) Ku70−/−; single chromatid gap. (d) Ku70−/−; double chromatid break. (Left) DAPI stain; (Right) SKY analysis. The fragment and the adjacent chromosome show identical staining by SKY, suggesting that the fragment arose from the termini of the adjacent chromosome 7.
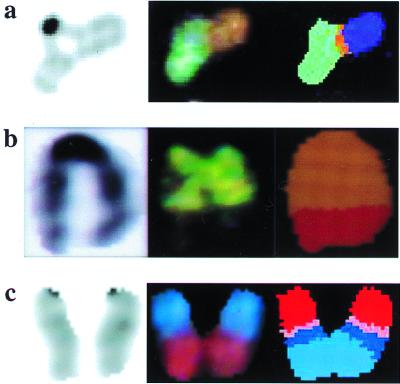
Figure 2.
Spontaneous nonreciprocal translocations arising in NHEJ-deficient cells. (a) Lig4−/− MEF. An unusual chromatid interaction is seen by DAPI staining (Left), which is comprised of at least two chromosomes visible after hybridization with the SKY probe mixture (Center). Spectral analysis yields classified colors (Right), indicating that this is a complex translocation involving material from three different chromosomes. From left to right, the chromosomal material is 15, 8, and X. (b) The cell of origin was from a dispersed abdominal organ block of a midgestation Lig4−/− embryo. The cell was not cultured or exposed to colcemid. The exact cell type is not known. DAPI staining (Left), visible colors after SKY probe hybridization (Center), and spectral analysis (Right) are shown. The chromosome arose from a simple t(5:6) translocation. (c) Lig4−/−p53−/− MEF. Complex translocation involving material from as many as four different chromosomes. DAPI staining (Left), visible colors after SKY hybridization (Center), and classified colors after spectral analysis (Right) are shown. The chromosomal material, from top to bottom, is 13, 7, 17, and 11.
We also found an increased level of chromosomal anomalies in DNA-PKcs−/− MEFs (Table 1). Although our current studies were not intended to define subtle differences among the mutant phenotypes, it is notable that no translocations were found in the DNA-PKcs mutant MEFs and that the percentage of metaphases with fragments was lower than those of other mutant lines (Table 1). A recent study also has reported increased levels of chromosomal anomalies in fibroblasts derived from adult mice that were heterozygous for Lig4 or Ku80 inactivating mutations (24). In our analyses, we did not find increased levels of abnormalities in either Lig4+/− or XRCC4+/− MEFs (data not shown). Additional analyses will be required to determine whether this apparent difference from the previous findings with adult fibroblasts represents accumulation of chromosomal defects over time, a differential requirement for these proteins for chromosomal stability in adult versus embryonic fibroblasts, or some other factor.
In combination, the total number of NHEJ mutant karyotypes that we have analyzed by SKY is highly significant and definitively demonstrates a role for this pathway in maintaining chromosomal stability (Table 1). In addition, we have shown that XRCC4 deficiency similarly leads to chromosomal instability in parallel studies (4). However, an important question was whether the dramatic requirement for NHEJ components to maintain chromosome stability was limited to cultured cells. To address this issue, we prepared metaphases from abdominal organ blocks of mid gestation embryos without culture or the use of colcemid. Of 10 metaphases from a wild-type and 10 from a Lig4−/− embryo, all wild-type metaphases were numerically and structurally normal (data not shown); whereas two Lig4−/− metaphases had one chromosomal fragment each, and a third had a simple nonreciprocal translocation (Fig. 2b). These studies confirm that NHEJ is required for the maintenance of genomic stability in cells that have never been exposed to in vitro culture conditions.
The NHEJ Pathway Plays a Major Role in the Repair of IR-Induced Chromsomal Damage.
NHEJ mutant cells are highly sensitive to IR and chemical agents that cause DSBs. To examine the cytogenetic consequences of IR in NHEJ-deficient cells, we sought to examine chromosomes by SKY 24 h after exposure of wild-type and Lig4−/− MEFs to 500 rads of IR. However, no metaphases could be obtained from Lig4−/− MEFs after irradiation; whereas wild-type metaphases were readily formed, albeit with a reduced mitotic index (data not shown). IR likely induces a cell cycle arrest that prevents progression to M phase in Lig4−/− MEFs because of its inability to repair DNA damage (11). In hopes of circumventing this arrest and allowing irradiated Lig4−/− cells to proceed into metaphase, we similarly assayed MEFs deficient for both Lig4, as well as for the p53 cell cycle checkpoint protein (27). Notably, p53 deficiency had no obvious effect on the level of increased spontaneous karyotypic instability observed in Lig4−/− MEFs; in addition, there was no difference in the types of aberrations found in the Lig4−/−p53−/− versus Lig4−/−p53+/+ cells (Fig. 2c; Table 1). However, p53 deficiency did allow us to obtain metaphases, at reduced numbers compared with controls, from the irradiated Lig4−/−p53−/− MEFs (data not shown). Strikingly, 24 h after irradiation Lig4−/−p53−/− MEF metaphases displayed massive chromosomal fragmentation (Fig. 3b), in contrast to those of wild-type (Fig. 3a) or p53−/− MEFs (data not shown), in which an average of only two aberrations per metaphase was observed.
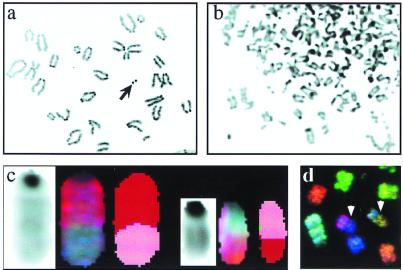
Figure 3.
Genomic instability induced by IR. (a) Portion of a metaphase spread from a wild-type MEF 24 h after a 500-rad dose of IR. The arrow indicates an example of a fragment. (b) Portion of a metaphase from a Lig4p53 double mutant MEF 24 h after a 500-rad dose of IR. Innumerable chromosomal aberrations are evident. (c) Reciprocal translocation involving chromosome 2 (red) and chromosome 7 (pink) found in a wild-type metaphase 12 days after irradiation. DAPI (Left), spectral (Center), and classified (Right) displays are shown for both translocated chromosomes. (d) Portion of a metaphase (spectral colors) from an irradiated Lig4−/−p53−/− culture 12 days after irradiation. Two nonreciprocal translocations are denoted by the arrowheads.
A population of Lig4−/−p53−/− MEFs apparently recover and begin to proliferate 8 days after IR, implying a different repair pathway can be used in the absence of NHEJ and appropriate cell cycle checkpoints (data not shown). To examine the cytogenetic events that have occurred in this population, metaphases were examined by SKY 12 days after IR. Nine of 18 Lig4−/−p53−/− double mutant cells harbored at least one nonreciprocal translocation, with as many as three occurring in a single metaphase. Numerous small dicentric fragments and chromatid breaks also were observed (Table 2). Only two of 18 cells had no cytogenetic aberrations as assayed by SKY. Nineteen wild-type IR survivors also were analyzed. Fifteen had no identifiable aberration, whereas one had three chromosomal fusions, one had a dicentric fragment, and one had a chromatid break. The remaining metaphase contained a reciprocal translocation, unlike all of the IR-induced and spontaneous translocations in NHEJ-deficient cells, which were nonreciprocal (Fig. 3 c and d). Thus, in proliferating Lig4−/−p53−/− cells, IR exposure raised the frequency of nonreciprocal translocations from 14% to 50% and increased the overall percentage of cells with a chromosomal aberration from 64% to 89%. In contrast, only 21% of wild-type survivors of IR treatment had chromosomal aberrations as assayed by SKY, demonstrating the vital role of NHEJ in restoring karyotypic integrity after extensive radiation-induced DNA damage.
Table 2.
Cytogenetic effects of IR in Lig4 p53 double mutant MEFs
| Events | Wild type | Lig4−/− p53−/− |
|---|---|---|
| Metaphases karyotyped | 19 | 18 |
| Fragmented chromosomes & chromatids | 2 | 5 |
| Detached centromeres | 0 | 0 |
| Translocations | 1 | 15 |
| Robertsonian translocations (fusions) | 3 | 0 |
| Metaphases with structural abnormality, %* | 21 | 89 |
The numbers to the right of the categories of events are the total events of that type. Some metaphases had more than one event of a particular type and others, a mixture of types of events.
The percentage of metaphases with any structural abnormality. Aneuploidy was not considered because all genotypes of midpassage MEFs tend to become near tetraploid.
Discussion
We have found that deficiency for Lig4 or Ku70 leads to a striking chromosomal instability, even in the absence of exogenous DNA damaging agents. DNA-PKcs deficiency also leads to chromosomal instability, although possibly at reduced levels. In separate studies, we also have shown that deficiency for XRCC4 similarly leads to chromosomal anomalies in MEFs (4). The presence of various forms of chromosomal fragments in these different NHEJ-deficient cells supports a role of the NHEJ pathway in the direct religation of broken chromosomes. Similar conclusions also were reached by parallel studies that used traditional techniques (23, 24). However, with SKY we have further demonstrated that the fate of DSBs in NHEJ-deficient fibroblasts is not limited to the mere formation of chromosomal fragments. Thus, we show that DSBs also can lead to nonreciprocal translocations. This outcome holds both for spontaneous and IR-induced damage.
In eukaryotes, the maintenance of chromosomal stability in the face of spontaneous or exogenously induced DSBs is ensured both by NHEJ and HR (28). Several studies have established a role for HR in maintaining chromosomal stability in both murine and avian systems. Repressed expression of Rad51, a key component of the HR machinery, in chicken DT40 cells lead to rapid cessation of cell division accompanied by chromosomal fragmentation (20). In this same system, Ku70 depletion had little effect on chromosomal stability (29). This finding, combined with experiments that showed end joining in murine cells sometimes is accompanied by deletions, led some to speculate that the NHEJ pathway is mutagenic, and its potential activity for maintaining chromosomal stability may be suppressed in favor of a more accurate HR pathway (19). However, our results suggest that the NHEJ pathway is not indiscriminant and, in fact, strongly favors religation to the appropriate partner after a DSB, a notion supported by our finding that translocations are infrequent in irradiated wild-type cells after IR sufficient to generate numerous DSBs.
Our findings show that a mechanism must exist that is capable of repairing breaks in the absence of NHEJ, but that this mechanism often leads to promiscuous interchromosomal interactions, resulting in nonreciprocal translocations. Although such a pathway may involve a second end-joining mechanism, HR also is a likely candidate. In yeast, it has been demonstrated that the HR pathway can catalyze the formation of nonreciprocal translocations (30). These events were initiated through invasion of an intact chromosome by a free DSB created by endonuclease cleavage. Invasion of one free end into a region of homologous sequence on an intact heterologous chromosome initiated break-induced replication, that in some cases spanned the entire length of the chromosome arm, resulting in a nonreciprocal translocation. If HR is indeed responsible for the nonreciprocal translocations that we observe, they are likely formed by a mechanism that avoids formation of Holliday junctions (31), which often would lead to reciprocal translocations via crossover events. The predominance of nonreciprocal translocations favors models for the generation of translocations in NHEJ-deficient cells that involve a close association between strand exchange and replication fork movement, without formation of Holliday intermediates (32–34).
We conclude that the NHEJ pathway of DSB repair is a crucial caretaker of the mammalian genome, acting on spontaneous as well as exogenously induced chromosomal damage. NHEJ deficiency can lead to the frequent formation of potentially oncogenic nonreciprocal translocations that apparently are generated by an alternative DSB repair pathway that is prone to errors that lead to interchromosomal recombination events.
Acknowledgments
This work was supported in part by National Institutes of Health Grants A.I.35714 (F.W.A.), A.I.01428 (K.M.F.), R01HD34880 (R.A.D.), and R01HD28317 (R.A.D.). J.S. is the Richard D. Frisbee III Foundation Fellow of the Leukemia Society of America. Y.G. is an associate of the Howard Hughes Medical Institute. R.A.D. is an American Cancer Society Research Professor. F.W.A. is an Investigator of the Howard Hughes Medical Institute. SKY was performed at the Arthur and Rochelle Belfer Cancer Genomics Center at the Dana Farber Cancer Institute.
Abbreviations
- NHEJ
nonhomologous end joining
- HR
homologous recombination
- DSB
double-strand break
- IR
ionizing radiation
- MEF
mouse embryonic fibroblast
- SKY
spectral karyotyping
- Lig4
ligase IV
- DAPI
4′,6-diamidino-2-phenylindole
- DNA-PK
DNA-dependent protein kinase
- DNA-PKcs
DNA-PK catalytic subunit
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.110152897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.110152897
References
- 1.Jeggo P A. Radiat Res. 1998;150:S80–S91. [PubMed] [Google Scholar]
- 2.Gellert M. Adv Immunol. 1997;64:39–64. doi: 10.1016/s0065-2776(08)60886-x. [DOI] [PubMed] [Google Scholar]
- 3.Vanasse G J, Halbrook J, Thomas S, Burgess A, Hoekstra M F, Disteche C M, Willerford D M. J Clin Invest. 1999;103:1669–1675. doi: 10.1172/JCI6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao Y, Ferguson D O, Xie W, Manis J, Sekiguchi J, Frank K M, Chaudhuri J, Horner J, DePinho R A, Alt F W. Nature (London) 2000;404:897–900. doi: 10.1038/35009138. [DOI] [PubMed] [Google Scholar]
- 5.Lieber M R. Genes Cells. 1999;4:77–85. doi: 10.1046/j.1365-2443.1999.00245.x. [DOI] [PubMed] [Google Scholar]
- 6.Nussenzweig A, Chen C, da Costa Soares V, Sanchez M, Sokol K, Nussenzweig M C, Li G C. Nature (London) 1996;382:551–555. doi: 10.1038/382551a0. [DOI] [PubMed] [Google Scholar]
- 7.Zhu C, Bogue M A, Lim D S, Hasty P, Roth D B. Cell. 1996;86:379–389. doi: 10.1016/s0092-8674(00)80111-7. [DOI] [PubMed] [Google Scholar]
- 8.Gu Y, Jin S, Gao Y, Weaver D T, Alt F W. Proc Natl Acad Sci USA. 1997;94:8076–8081. doi: 10.1073/pnas.94.15.8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu Y, Seidl K J, Rathbun G A, Zhu C, Manis J P, van der Stoep N, Davidson L, Cheng H L, Sekiguchi J M, Frank K, et al. Immunity. 1997;7:653–665. doi: 10.1016/s1074-7613(00)80386-6. [DOI] [PubMed] [Google Scholar]
- 10.Ouyang H, Nussenzweig A, Kurimasa A, Soares V C, Li X, Cordon-Cardo C, Li W, Cheong N, Nussenzweig M, Iliakis G, et al. J Exp Med. 1997;186:921–929. doi: 10.1084/jem.186.6.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank K M, Sekiguchi J M, Seidl K J, Swat W, Rathbun G A, Cheng H L, Davidson L, Kangaloo L, Alt F W. Nature (London) 1998;396:173–177. doi: 10.1038/24172. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y, Sun Y, Frank K M, Dikkes P, Fujiwara Y, Seidl K J, Sekiguchi J M, Rathbun G A, Swat W, Wang J, et al. Cell. 1998;95:891–902. doi: 10.1016/s0092-8674(00)81714-6. [DOI] [PubMed] [Google Scholar]
- 13.Gu Y, Sekiguchi J, Gao Y, Dikkes P, Frank K, Ferguson D, Hasty P, Chun J, Alt F W. Proc Natl Acad Sci USA. 2000;97:2668–2673. doi: 10.1073/pnas.97.6.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes D E, Stamp G, Rosewell I, Denzel A, Lindahl T. Curr Biol. 1998;8:1395–1398. doi: 10.1016/s0960-9822(98)00021-9. [DOI] [PubMed] [Google Scholar]
- 15.Gao Y, Chaudhuri J, Zhu C, Davidson L, Weaver D T, Alt F W. Immunity. 1998;9:367–376. doi: 10.1016/s1074-7613(00)80619-6. [DOI] [PubMed] [Google Scholar]
- 16.Taccioli G E, Amatucci A G, Beamish H J, Gell D, Xiang X H, Torres Arzayus M I, Priestley A, Jackson S P, Marshak Rothstein A, Jeggo P A, Herrera V L. Immunity. 1998;9:355–366. doi: 10.1016/s1074-7613(00)80618-4. [DOI] [PubMed] [Google Scholar]
- 17.Li G C, Ouyang H, Li X, Nagasawa H, Little J B, Chen D J, Ling C C, Fuks Z, Cordon-Cardo C. Mol Cell. 1998;2:1–8. doi: 10.1016/s1097-2765(00)80108-2. [DOI] [PubMed] [Google Scholar]
- 18.Riballo E, Critchlow S E, Teo S H, Doherty A J, Priestley A, Broughton B, Kysela B, Beamish H, Plowman N, Arlett C F, et al. Curr Biol. 1999;9:699–702. doi: 10.1016/s0960-9822(99)80311-x. [DOI] [PubMed] [Google Scholar]
- 19.Liang F, Han M, Romanienko P J, Jasin M. Proc Natl Acad Sci USA. 1998;95:5172–5177. doi: 10.1073/pnas.95.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonoda E, Sasaki M S, Buerstedde J M, Bezzubova O, Shinohara A, Ogawa H, Takata M, Yamaguchi-Iwai Y, Takeda S. EMBO J. 1998;17:598–608. doi: 10.1093/emboj/17.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liyanage M, Coleman A, du Manoir S, Veldman T, McCormack S, Dickson R B, Barlow C, Wynshaw-Boris A, Janz S, Wienberg J, et al. Nat Genet. 1996;14:312–315. doi: 10.1038/ng1196-312. [DOI] [PubMed] [Google Scholar]
- 22.Kemp L M, Jeggo P A. Mutat Res. 1986;166:255–263. doi: 10.1016/0167-8817(86)90025-8. [DOI] [PubMed] [Google Scholar]
- 23.Bailey S M, Meyne J, Chen D J, Kurimasa A, Li G C, Lehnert B E, Goodwin E H. Proc Natl Acad Sci USA. 1999;96:14899–904. doi: 10.1073/pnas.96.26.14899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karanjawala Z E, Grawunder U, Hsieh C L, Lieber M R. Curr Biol. 1999;9:1501–1504. doi: 10.1016/s0960-9822(00)80123-2. [DOI] [PubMed] [Google Scholar]
- 25.Hilgenfeld E, Padilla-Nash H, Schrock E, Ried T. Curr Top Microbiol Immunol. 1999;246:169–174. doi: 10.1007/978-3-642-60162-0_21. [DOI] [PubMed] [Google Scholar]
- 26.Atkin N B. Cancer Genet Cytogenet. 1986;21:275–278. doi: 10.1016/0165-4608(86)90009-9. [DOI] [PubMed] [Google Scholar]
- 27.Donehower L A, Harvey M, Slagle B L, McArthur M J, Montgomery C A, Jr, Butel J S, Bradley A. Nature (London) 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 28.Haber J E. Nature (London) 1999;398:665–667. doi: 10.1038/19423. [DOI] [PubMed] [Google Scholar]
- 29.Takata M, Sasaki M S, Sonoda E, Morrison C, Hashimoto M, Utsumi H, Yamaguchi-Iwai Y, Shinohara A, Takeda S. EMBO J. 1998;17:5497–5508. doi: 10.1093/emboj/17.18.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bosco G, Haber J E. Genetics. 1998;150:1037–1047. doi: 10.1093/genetics/150.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szostak J W, Orr-Weaver T L, Rothstein R J, Stahl F W. Cell. 1983;33:25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- 32.Nassif N, Penney J, Pal S, Engels W R, Gloor G B. Mol Cell Biol. 1994;14:1613–1625. doi: 10.1128/mcb.14.3.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferguson D O, Holloman W K. Proc Natl Acad Sci USA. 1996;93:5419–5424. doi: 10.1073/pnas.93.11.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paques F, Leung W Y, Haber J E. Mol Cell Biol. 1998;18:2045–2054. doi: 10.1128/mcb.18.4.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]