Abstract
Hypoxia is an important pathogenic factor in ischemic disease and tumorigenesis. Under hypoxia, some cells are irreversibly damaged, whereas others adapt to the stress and may become more resistant to injury. The mechanism underlying such adaptive responses is unclear. Our recent study showed hypoxic induction of inhibitor of apoptosis protein-2 (IAP-2). Here we have investigated the critical steps in the apoptotic cascade that are affected by hypoxia and have identified a role for IAP-2 in apoptosis resistance of hypoxic cells. The results show that cells cultured in hypoxia became resistant to staurosporine-induced apoptosis. Apoptosis resistance of these cells took place at the mitochondria and in the cytosol. At the mitochondrial level, membrane accumulation of the proapoptotic molecule Bax was suppressed. This was accompanied by less cytochrome c (cyt. c) release from the organelles. In the cytosol, hypoxia induced IAP-2; the cytosol with IAP-2 was resistant to cyt. c-stimulated caspase activation. Of significance, immunodepletion of IAP-2 from the hypoxic cytosol restored its competence for caspase activation. Thus, death resistance of hypoxic cells involves multiple factors targeting different stages of apoptosis, with IAP-2 suppressing caspases in the cytosol.
Hypoxia, a condition of decreased availability of oxygen, poses stresses to mammalian cells and is an important pathogenic factor in several types of devastating diseases. 1,2 For example, hypoxia during organ ischemia leads to cell injury and death, which contributes to the development of myocardial infarction, acute renal failure and stroke in the brain. 3 In solid tumors, malformation and malfunction of blood vessels result in poorly oxygenated regions, where cancerous cells are constantly subjected to hypoxic pressure and the selection for death resistance. 4-6 Cells that become adaptive to hypoxia are more resistant to apoptosis and less responsive to cancer therapy, resulting in relapse of the disease, metastasis, and transformation into more aggressive phenotypes. 6
In general, cells may adapt to hypoxic microenvironments by several strategies. 7-9 First, in response to hypoxia, cellular activities including that of ion channels are significantly decreased. Such a decrease conserves energy to maintain the essential elements for cell viability. Second, expression of genes that are involved in oxygen delivery and anaerobic ATP production are drastically up-regulated. A major transcription factor governing the expression of these genes is hypoxia-inducible factor-1 (HIF-1). 10 Finally, while hypoxia induces cell injury and death, it also activates pathways for cell survival. Hypoxic activation of the PI3K/Akt survival pathway has been shown in PC-12 cells. 11 Moreover, our recent studies have documented a striking induction by hypoxia of IAP-2, an apoptosis inhibitory protein. 12,13 Despite these observations, it is unclear how the pathways and genes regulate the apoptosis sensitivity of hypoxic cells.
IAP-2 is a member of the family of inhibitor of apoptosis proteins (IAPs). 14-16 IAPs antagonize apoptosis in a variety of experimental models, probably through their interaction and inhibition of caspases. Regulation of IAPs has been implicated in the development of tissue pathology during neoplasia, neurodegenerative disorders, and brain ischemia. 14-16 Our recent studies showed that IAP-2 was induced under hypoxia through gene transcription, whereas by HIF-1-independent mechanisms. 12,13 Of significance, hypoxic cells expressing IAP-2 became resistant to apoptosis. 12,13 The current study was designed to identify the critical steps in the apoptotic cascade that are suppressed by hypoxia and to determine the role of IAP-2 in death resistance of hypoxic cells. The results show that cells cultured under hypoxia became resistant to staurosporine-induced apoptosis. Such resistance took place not only at caspase activation but also at upstream levels of the mitochondria. The movement of the pro-apoptotic molecule Bax to mitochondria was significantly suppressed in hypoxic cells, which was accompanied by reduced cyt. c release from the organelles. IAP-2 appeared to be a major inhibitory regulator of caspases in hypoxic cells. The cytosol isolated from hypoxic cells contained high levels of IAP-2 and exhibited a significantly lower capacity for caspase activation. Immunodepletion of IAP-2 from the hypoxic cytosol restored its ability for caspase activation. Thus, while death resistance of hypoxic cells takes place at multiple levels, IAP-2 induction plays an important role in the resistance at the level of caspase activation.
Materials and Methods
Materials
The cells used in this study were derived from an immortalized cell line of rat kidney proximal tubular epithelium kindly provided by U. Hopfer (Case Western Reserve University, Cleveland, OH). The cells were cultured and plated for experiments as previously described. 12,17 Antibodies were from the following sources: monoclonal anti-Bax (clone 1D1) from NeoMarkers (Fremont, CA); monoclonal anti-cyt. c (clone 7H8.2C12) from BD Pharmingen (San Diego, CA); polyclonal anti-Bax, anti-IAP-2, anti-lamin B, and anti-gelsolin from Santa Cruz Biotechnology Inc. (Santa Cruz, CA); monoclonal anti-Cox IV (clone 20E8) from Molecular Probes (Eugene, OR); all secondary antibodies from Jackson ImmunoResearch (West Grove, PA). Protein A/G sepharose was obtained from Santa Cruz Biotechnology Inc. and DEVD.AFC was from Enzyme Systems Products (Dublin, CA). Unless indicated, all other reagents were purchased from Sigma Chemical Co. (St. Louis, MO).
Hypoxic Incubation and Staurosporine Treatment
Hypoxic incubation was conducted as previously described. 12,13,17,18 Briefly, cells were washed with phosphate-buffered saline (PBS), transferred to an anaerobic chamber with 95% N2/5% CO2, and incubated in Krebs-Ringer bicarbonate buffer. This buffer was pre-equilibrated with 95% N2/5% CO2. EC Oxyrase, a biocatalytic oxygen reducing agent, was added at 1.2 units/ml to the incubation medium to consume residual O2 and maximize the degree of hypoxia. To minimize cell injury by hypoxic incubation per se and reveal staurosporine-induced apoptosis, 5.5 mmol/L glucose was included in the incubation buffer to facilitate glycolytic ATP production. 12,13,17 For reoxygenation, cells after hypoxic incubation were transferred back to full culture medium in 95% air/5% CO2. Staurosporine was added at 1 μmol/L to hypoxic cells or to normally oxygenated cells in Krebs-Ringer bicarbonate buffer in the presence of 5.5 mmol/L glucose.
Analysis of Apoptosis
Morphological examination of apoptotic cells was described in our previous studies. 12 Typical apoptotic morphology examined included cellular shrinkage, nuclear condensation and fragmentation, and formation of apoptotic bodies. To reveal nuclear changes, cells were exposed to 5 μg/ml of Hoechst 33342 in PBS for 2 to 5 minutes at room temperature. For each condition, apoptosis was monitored in five fields with ∼200 cells per field. The experiments were repeated at least 4 times with duplicate dishes for each condition in every experiment.
Measurement of Caspase Activity
The enzymatic activity of caspases was measured using an exogenous fluorogenic peptide substrates DEVD.AFC as described in our previous publications. 17,18 DEVD.AFC is a substrate with specificity for caspase-3, -6, and -7. 17,18 Cells were extracted with 1% Triton X-100. The lysates of 25 μg of protein were added to the enzymatic reactions containing 50 μmol/L DEVD.AFC. After 60 minutes of reaction at 37°C, fluorescence at Excitation 360 nm/Emission 530 nm was monitored. For each measurement, a standard curve was constructed using free AFC. Based on the standard curve, the fluorescence reading from each enzymatic reaction was translated into the molar amount of liberated AFC to indicate caspase activity.
Cellular Fractionation Using Digitonin
To analyze the redistribution of Bax and cyt. c during apoptosis, cells were fractionated into cytosolic and membrane-bound fractions using low concentrations of digitonin. Selective permeabilization of plasma membranes by digitonin was monitored by microscopy. This method of cellular fractionation for examination of Bax and cyt. c translocation has been used successfully in recent studies. 17-21 Briefly, cells were exposed to 0.05% digitonin in isotonic sucrose buffer (in mM: 250 sucrose, 10 Hepes, 10 KCl, 1.5 MgCl2, 1 ethylenediaminetetraacetate, and 1 EGTA; pH 7.1) for 2 minutes at room temperature to collect the soluble fraction as cytosolic extracts. Digitonin insoluble fraction was dissolved in 2% sodium dodecyl sulfate (SDS) buffer to collect the membrane-bound part. Since Bax/cyt. c redistribution mainly takes place between the cytosol and mitochondria, immunoblot analysis of the membrane-bound part is expected to reveal mainly the mitochondrial content of these molecules.
Immunoblot Analysis
Proteins were analyzed by immunoblotting using NuPAGE Gel Systems as described previously. 12,17,18,21 To analyze Bax and cyt. c translocations, cytosolic and membrane fractions extracted from the same amounts (∼1 × 105) of cells were subjected to electrophoresis. For other immunoblots, 50-μg proteins were loaded for each lane. After electrophoresis on 10% or 12% Bis-Tris gels in MES running buffer, the proteins were electroblotted onto polyvinylidene difluoride membranes. The membranes were subsequently blocked with 5% milk or bovine serum albumin and exposed to primary antibodies overnight at 4°C. After extensive wash in PBS containing Tween-20, the membranes were exposed to the horseradish-peroxidase-conjugated secondary antibodies. Antigens on the blots were revealed by exposure to chemiluminescent substrates (Pierce, Rockford, IL).
Immunofluorescence Analysis of Bax and Cox IV
Cells were grown on collagen-coated glass coverslips. After experimental incubation, cells were fixed with 4% paraformaldehyde in PBS for 1 hour at room temperature. Cells were then permeabilized for 5 minutes with 0.1% SDS, followed by 1 hour blocking in 5% normal goat serum. The cells were subsequently exposed to a mixture of primary antibodies (rabbit anti-Bax and mouse anti-Cox IV) for 1 hour and blocked again in normal goat serum. Finally, the cells were exposed to a mixture of secondary antibodies (Cy-3-labeled goat anti-rabbit IgG and FITC-labeled goat anti-mouse IgG) for 1 hour. The same cells were examined for fluorescence of Cy-3 (red: Bax signal) and fluorescein isothiocyanate (FITC) (green: Cox signal) by laser-scanning confocal microscopy.
Determination of Cytosolic Capacity for Caspase Activation
Cytosolic capacity for caspase activation was determined by in vitro reconstitution assays as described in our previous study. 18 Cytosols were extracted from normoxic or hypoxic cells with 0.05% digitonin as described above. The cytosols were concentrated to 4 to 5 mg/ml with 3K cutoff microconcentrators. For reconstitution, 1 μl of 0.5 mg/ml rat heart cyt. c and 1 μl of 10 mmol/L dATP were added to 7.5 μl cytosolic extracts with 25 μg protein, and incubated for 1 hour at 30°C. After incubation, 5 μl of the reconstitution mixture was transferred to 200 μl enzymatic reaction buffer with 50 μmol/L DEVD.AFC. The cleavage of DEVD.AFC was monitored as described above to determine reconstituted caspase activity.
Immunodepletion of IAP-2
Cells were subjected to 3 hours of hypoxia in the presence of glucose. Cytosol was extracted with 0.05% digitonin as described in Cellular Fractionation. Protein concentration of the cytosolic extracts was adjusted to 0.5 μg/ml. For immunodepletion, a polyclonal antibody against IAP-2 was added to 200 μg cytosol and incubated at 4°C for 1 hour by mixing on a rocker. Protein A/G sepharose of 30 μl was then added for overnight mixing at 4°C. The mixture was centrifuged to collect the supernatant for immunoblot analysis of IAP-2 to determine the efficacy of immunodepletion. The IAP-2-depleted samples were also analyzed for their capacity of caspase activation by in vitro reconstitutions using exogenous cyt. c.
Chemical Analyses
To measure ATP, cells were extracted with trichloroacetic acid. ATP in cell extracts was measured by luminometry of the luciferin firefly luciferase reaction. 22 ATP values were expressed as nmole per mg cell protein. Protein was quantified with the bicinchoninic acid (BCA) reagent purchased from Pierce.
Statistics
Data were expressed as means ± SE (n ≥4). Statistical differences between means were determined using analysis of variance by two-tailed tests. P < 0.05 was considered to reflect significant differences.
Results
Attenuation of Acute Hypoxic Cell Injury by Facilitating Glycolysis with Glucose
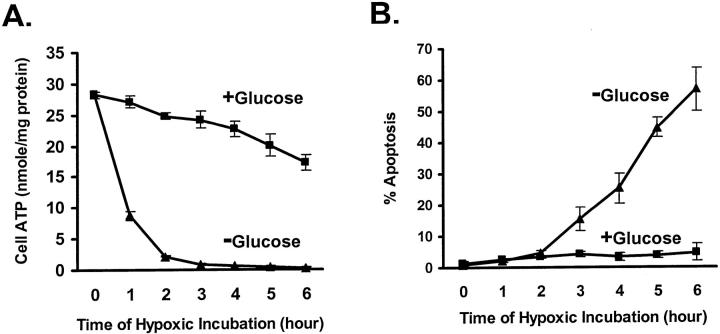
An important consideration for study of apoptosis sensitivity of hypoxic cells was to minimize cell injury caused by hypoxic incubation per se. Removal of hypoxic injury was necessary for establishing a “clean” model to reveal apoptosis that was specifically induced by other insults. Because energy deprivation is a main cause of acute cell damage under hypoxia, we reasoned that facilitation of anaerobic ATP generation via glycolysis might assist the cells to maintain viability. To facilitate glycolysis, we provided 5.5 mmol/L glucose in the hypoxic incubation buffer. As shown in Figure 1A ▶ , in the absence of glucose, hypoxia led to rapid decreases in cellular ATP. At the end of 2 hours of hypoxic incubation, ATP levels were less than 10% of control (Figure 1A ▶ , −Glucose). When glucose was provided, cellular ATP was maintained at substantial levels. As a result, cellular ATP was 65% of control after 6 hours of hypoxic incubation (Figure 1A ▶ , +Glucose). Of significance, provision of glucose diminished cell injury and death caused by hypoxic incubation for a period of time. As shown in Figure 1B ▶ , less than 5% apoptosis was triggered by 6 hours of hypoxic incubation, when glucose was present (+Glucose). In sharp contrast, in the absence of glucose, over 50% of cells underwent apoptosis (Figure 1B ▶ , −Glucose). Amelioration of acute hypoxic injury by glucose provided us a relatively clean model to compare staurosporine-induced apoptosis under hypoxia and under normal oxygen in subsequent experiments.
Figure 1.
Suppression of acute hypoxic injury and cell death by glucose. A: Cell ATP. B: Apoptosis. Cells were subjected to hypoxic incubation in the presence or absence of 5.5 mmol/L glucose. For ATP measurement, the cells were extracted with trichloroacetic acid at the end of hypoxic incubation. To monitor apoptosis, after hypoxia the cells were transferred back to oxygenated full culture medium for 1 hour of reoxygenation and then stained with Hoechst 33342. Apoptotic cells were identified by cellular and nuclear morphology. Data are expressed as means ± SE (n = 5). The provision of glucose during hypoxic incubation maintained cellular ATP and prevented acute cell injury.
Suppression of Staurosporine-Induced Apoptosis by Hypoxia
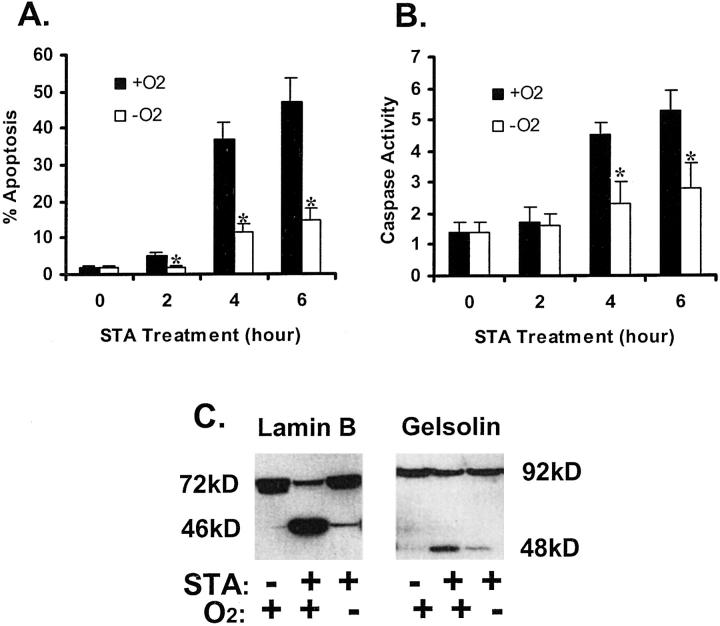
Staurosporine (STA) is a broad-spectrum inhibitor of protein kinases, which is also a potent inducer of apoptosis. Apoptosis induced by STA has been widely documented in diverse types of cells. Thus, we chose STA as the apoptosis inducer to examine the effects of hypoxia on cellular sensitivity to apoptosis. The kinetics of apoptosis development was monitored following the addition of STA, under normal oxygen tension (21% O2) or severe hypoxia (near 0% O2) (Figure 2A) ▶ . In cells with normal oxygen (Figure 2A ▶ , +O2), significant amounts of apoptosis were induced after 4 hours of STA incubation. The rate of apoptosis increased to ∼50% by the end of 6 hours of STA exposure. In sharp contrast, apoptosis was significantly less when STA was added to hypoxic cells. For example, only 11% and 15% apoptosis was induced by 4 and 6 hours of STA exposure, respectively (Figure 2A ▶ , −O2). These morphological observations were consistent with biochemical analyses. As shown in Figure 2B ▶ , STA activated caspases in cells under normal oxygen tensions and this activation was significantly suppressed in hypoxic cells. We further examined the breakdown of endogenous caspase substrates during STA treatment. As shown in Figure 2C ▶ , STA induced proteolysis of lamin B and gelsolin, releasing characteristic fragments of apoptosis. 23 Again, fragmentation of these proteins was significantly ameliorated under hypoxia. Together, these results indicate clearly that hypoxic cells, compared with normoxic ones, were more resistant to apoptosis.
Figure 2.
Inhibition of staurosporine-induced apoptosis by hypoxia. A: % apoptosis estimated by cell morphology. B: Caspase activity. C: Proteolytic processing of lamin B and gelsolin. For measurements of apoptosis and caspase activity, cells were incubated for 0 to 6 hours with 1 μmol/L STA under normal 21% oxygen (+O2) or under severe hypoxia (−O2). Apoptotic cells were identified by cellular and nuclear morphology. Caspase activity was measured using the fluorogenic peptide substrate DEVD.AFC, as described in Materials and Methods. Data are expressed as means ± SE (n = 4). *, significantly different from the results of the normoxic groups (P < 0.05). To analyze lamin B and gelsolin, cells were incubated for 4 hours with 1 μmol/L STA under normal 21% oxygen or under severe hypoxia. Control cells were incubated under normal oxygen without STA exposure. Whole cell lysates were collected for immunoblot analysis. STA-induced apoptotic morphology, caspase activation, and cleavage of lamin B and gelsolin under normal oxygen tensions; development of these apoptotic features was significantly diminished under hypoxia.
Staurosporine-Induced Bax Translocation to Mitochondria is Suppressed by Hypoxia
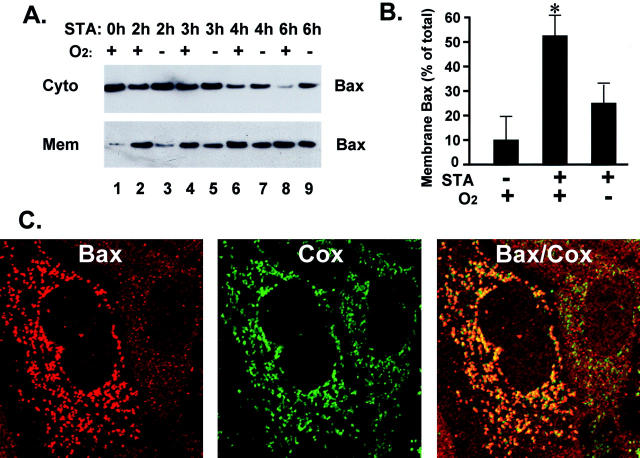
Our results have demonstrated apoptosis resistance of hypoxic cells (Figure 2) ▶ . Although our previous studies showed IAP-2 induction in these cells and suggested a role for IAP-2 in apoptosis resistance, hypoxia also activates other pathways for cell survival. For example, a recent study has documented hypoxic activation of the survival pathway of Akt/PI3K. 11 Thus, to further examine the molecular basis of the acquired apoptosis resistance, we determined the critical steps in the apoptotic cascade that were affected by hypoxia. Our first experiments analyzed the translocation of Bax to mitochondria by immunoblotting. Bax is a pro-apoptotic member of Bcl-2 family proteins. Accumulation of Bax in mitochondria has been recognized as an early event of apoptosis, which leads to permeabilization of the mitochondrial outer membrane, followed by the release of apoptogenic factors including cyt. c. 24-28 As shown in Figure 3A ▶ , in control cells the majority of Bax was detected in the cytosolic fraction, with limited amounts in the membrane-bound organellar fraction (lane 1). After 2 hours of STA incubation, significant amounts of Bax accumulated in the membrane fraction, which was accompanied by the loss of Bax from the cytosol (lane 2). Bax translocation increased as STA incubation was prolonged (lanes 1, 2, 4, 6, and 8). Of significance, accumulation of Bax in the membrane fraction was suppressed under hypoxia (lanes 3, 5, 7, and 9). After 2 hours of STA, Bax translocation to the membrane fraction was very limited in hypoxic cells (lane 3), whereas significant Bax translocation already took place in normoxic cells (lane 2). To quantify the results, Bax immunoblots from four separate experiments were subjected to densitometry, and the results are shown in Figure 3B ▶ . In control cells, ∼11% Bax was membrane-associated. STA incubation for 2 hours led to 52% Bax in the membrane fraction. When STA incubation was conducted under hypoxia, the association of Bax to the membranes was decreased to 26% (Figure 3B) ▶ . To determine which membranes or organelles Bax was associated with, we examined the intracellular localization of this molecule by immunofluorescence (Figure 3C) ▶ . The cells were incubated for 2 hours with STA. To facilitate immunofluorescence analysis, the general caspase inhibitor VAD was provided during STA treatment to prevent the development of apoptotic morphology. Our previous study showed that VAD did not significantly affect mitochondrial events of apoptosis including Bax translocation or cyt. c release ( 17 and unpublished data). Shown in Figure 3C ▶ are images of two neighboring cells. The one on the right side showed punctuate organellar staining of Bax, while the one on the left side displayed much finer cytoplasmic staining (Figure 3C ▶ , left). The same cells were co-immunostained for Cox IV, an integral mitochondrial membrane protein (Figure 3C ▶ , middle). Clearly, Bax staining in the right-hand cell showed a significant overlap with the mitochondrial Cox signal. On the contrary, the cell on the left did not exhibit such co-localizations. This conclusion was further supported by examination of the superimposed images (Figure 3C ▶ , left). Due to the overlap of red Bax and green Cox signals, the right-hand cell displayed punctuate orange mitochondrial staining, whereas in the left-hand cell the Bax and Cox signals were segregated. The results indicate that a major intracellular site for Bax translocation was the mitochondria. Together with the immunoblot analyses, it is suggested that a critical step in apoptosis inhibited by hypoxia might be Bax movement to mitochondria.
Figure 3.
Staurosporine-induced Bax translocation from the cytosol to mitochondria is suppressed under hypoxia. A: Immunoblot analysis of Bax translocation. B: Densitometric analysis of Bax immunoblots. C: Dual-immunofluorescence staining of Bax and Cox in STA-treated cells. For immunoblot analysis of Bax, cells were incubated under normal oxygen tension or under severe hypoxia. STA was added to the cells for 2, 3, 4, or 6 hours of incubation. After incubation, cells were fractionated into cytosolic fractions (Cyto) and membrane-bound organellar fractions (Mem) by digitonin, as described in Material and Methods. Bax in these fractions was analyzed by immunoblotting (A). Bax immunoblots from four separate experiments were subjected to densitometry. For each blot, membrane-associated Bax was calculated as a percentage of total Bax. The results of three conditions (control, 2 hours of STA under normoxia, and 2 hours of STA under hypoxia) are summarized in (B). Data are expressed as mean ± SE (n = 4); *, significantly higher than the other two values. The results indicate that STA-induced Bax translocation from the cytosol to the membrane-bound organellar fraction, and the translocation was significantly suppressed under hypoxia. To localize Bax within the cells, dual-immunofluorescence staining of Bax and the integral mitochondrial protein Cox IV was examined (C). The cells were incubated for 2 hours with STA and then were fixed and processed for dual-immunofluorescence staining as described in Materials and Methods. The image on the left is immunofluorescence of Bax (red), the image in the middle is Cox IV signal (green) and the right panel represents a superimposed image. The results show that, after translocation, the majority of Bax colocalized with Cox or the mitochondria.
Staurosporine-Induced Cytochrome C Release from Mitochondria is Inhibited under Hypoxia
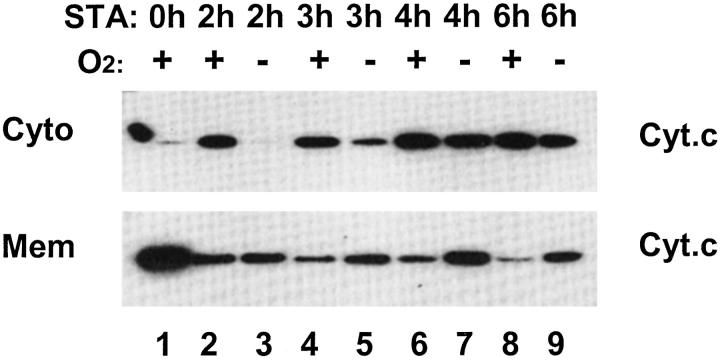
In the intrinsic pathway of apoptosis that are often activated by cellular stress, targeting of mitochondria by pro-apoptotic molecules including Bax leads to the release of apoptogenic factors. 26 Among these factors, cyt. c has been recognized as one of the most important. Thus, following the examination of Bax, we went on to analyze the release of cyt. c and the effects of hypoxia. These results are shown in Figure 4 ▶ . As expected, in control cells without STA exposure, cyt. c was shown in the membrane fraction containing mitochondria, with little in the cytosol (lane 1). STA incubation led to the appearance of cyt. c in the cytosol, accompanied by the loss of cyt. c from the membrane fraction. Mitochondrial release of cyt. c depended on the durations of STA exposure; more release was detected as STA incubation was prolonged (lanes 2, 4, 6, and 8). However, when STA was added to hypoxic cells, significantly less cyt. c release was detected. For example, after 2 hours of STA incubation, cyt. c release into the cytosol was very limited in hypoxic cells (lane 3), while normoxic cells already showed significant cyt. c leakage (lane 2). As STA incubation was prolonged, more cyt. c was released into the cytosol, regardless the presence or absence of oxygen. Together with the results of Bax translocation (Figure 2) ▶ , these observations indicate that apoptosis resistance of hypoxic cells may take place at least in part at the mitochondrial level.
Figure 4.
Staurosporine-induced cyt. c release from mitochondria is suppressed under hypoxia. Cells were incubated under normal oxygen tension or under severe hypoxia. STA was added to the cells for 2, 3, 4, or 6 hours. After STA incubation, cells were fractionated into cytosolic fractions (Cyto) and membrane-bound fractions containing mitochondria (Mito), as described in Materials and Methods. Cyt. c in these fractions was analyzed by immunoblotting. In oxygenated cells, STA treatment led to the release of mitochondrial cyt. c into the cytosol as early as 2 hours, and the release increased as STA treatment was prolonged (lane 1, 2, 4, 6, and 8). The translocation was delayed under hypoxia (lanes 3, 5, 7, and 9). Please note that the spot to the far left of the top blot was not a protein band but an accidental stain.
Cytosol of Hypoxic Cells Exhibits Lower Capacity for Caspase Activation
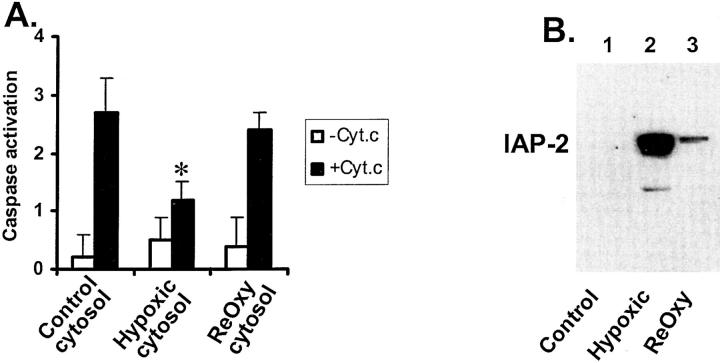
The results shown in the above sections have suggested apoptotic resistance of hypoxic cells at the mitochondrial level. To determine whether downstream apoptotic events are also affected by hypoxia, we compared the cytosols of normoxic and hypoxic cells for their capacity of caspase activation. To this end, cytosols were extracted from normoxic and hypoxic cells. Exogenous cyt. c was added to the cytosols to reconstitute caspase activation. The results are shown in Figure 5A ▶ . Clearly, cyt. c stimulated caspase activation in cytosols from normoxic as well as hypoxic cells; however, the latter showed significantly lower activation. Of interest, the cytosol extracted from hypoxia-reoxygenated cells had caspase activation at levels similar to normoxic cells and was significantly higher than hypoxic cytosol. The cytosolic sensitivity to cyt. c stimulation showed a reverse relationship with the expression of IAP-2 (Figure 5B) ▶ . For example, the hypoxic cytosol had the highest level of IAP-2 and showed the lowest caspase activation on cyt. c stimulation. The results are in support of the hypothesis that IAP-2 may antagonize caspase activation in the cytosol of hypoxic cells. 12
Figure 5.
Cytosol of hypoxic cells is more resistant to cyt. c-induced caspase activation. A: Cyt. c-stimulated caspase activation in the cytosol. B: IAP-2 expression in the cytosol. Cells were incubated for 3 hours under normal oxygen tensions, severe hypoxia, or hypoxia followed by 1 hour of reoxygenation. Cytosols were extracted from these three groups of cells with digitonin. To determine their capacity for caspase activation, the cytosols were incubated with or without cyt. c. Caspase activity after the incubation was measured as described in Materials and Methods (A). Data are expressed as mean ± SE (n = 4). *, significantly different from the results of control cytosol and the reoxygenated cytosol. To examine IAP-2, equal amounts of cytosols from control, hypoxic, and reoxygenated cells were analyzed by immunoblotting (B). Cyt. c-activated caspases in the cytosol of normoxic cells and the activation was significantly less for the cytosol of hypoxic cells. Of interest, the cytosol isolated from hypoxia-reoxygenated cells showed higher caspase activation on cyt.c stimulation, compared with the cytosol from hypoxic cells.
Immunodepletion of IAP-2 from Hypoxic Cytosol Restores its Capacity for Caspase Activation
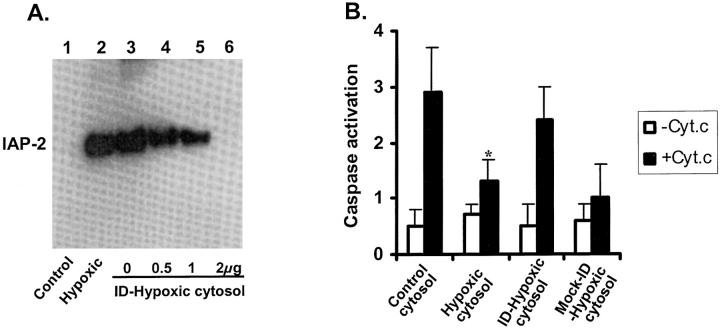
To further determine the role of IAP-2 in apoptotic resistance of hypoxic cells, we depleted this protein from the cytosol using a specific antibody and then examined caspase activation on cyt. c stimulation. The results of immunodepletion are shown in Figure 6A ▶ . IAP-2 in the cytosol of control cells was undetectable (lane 1), while a significant amount of IAP-2 was shown in the cytosol of hypoxic cells (lane 2). IAP-2 in the hypoxic cytosol was depleted by increasing amounts of antibodies (lanes 4 to 6). Removal of IAP-2 was nearly complete, when 200 μg cytosol was depleted with 2 μg antibody (lane 6). “Mock” immunodepletion with non-immune serum was not effective (lane 3). We then tested the cytosols for their capacity of caspase activation; the results are shown in Figure 6B ▶ . Consistent with previous experiments (Figure 5) ▶ , cyt. c activated caspases, and the activation was notably lower for hypoxic cytosol. Importantly, after IAP-2 immunodepletion, the hypoxic cytosol demonstrated significant improvement in its capacity for caspase activation, on cyt. c stimulation. The increased capacity for caspase activation was not shown for the hypoxic cytosol subjected to mock immunodepletion. The results indicate an important role for IAP-2 induction in apoptotic resistance of hypoxic cells, at the caspase activation level.
Figure 6.
Immunodepletion of IAP-2 from hypoxic cytosol restores its capacity for caspase activation. A: Immunodepletion of IAP-2. B: Reconstitution of caspase activation by adding exogenous cyt. c to the cytosols. Cytosols were extracted with digitonin from control and hypoxic cells. Immunodepletion of IAP-2 from the hypoxic cytosol was conducted with 0.5-, 1-, or 2-μg antibodies according to Materials and Methods. Mock immunodepletion was conducted in parallel using non-immune rabbit serum. IAP-2 in the samples was analyzed by immunoblotting (A). To compare their capacity for caspase activation, equal amounts of cyt. c were added to the control cytosol, hypoxic cytosol, IAP-2 immunodepleted (ID) hypoxic cytosol, or mock-immunodepleted hypoxic cytosol (B). Caspase activation by cyt. c in these cytosols was measured using the fluorogenic substrate DEVD.AFC. Data are expressed as mean ± SE (n = 4). *, significantly different from the results of control cytosol and the immunodepleted-hypoxic cytosol. Depletion of IAP-2 from the hypoxic cytosol restored its sensitivity to cyt. c stimulation.
Discussion
Mammalian cells rely on the presence of molecular oxygen for respiration and aerobic ATP production to maintain homeostasis. Under conditions of oxygen deficiency, cells can be irreversibly injured and die, unless they become adaptive to the hypoxic environment. 7-9 Hypoxic cell injury is a key determinant of tissue pathology in ischemic diseases. 3 On the other hand, cellular adaptation to hypoxia is considered to be an important event for tumor formation and the development of resistance to cancer therapy. 4-6 Cells that have adapted to the hypoxic stress may become resistant to subsequent injurious insults. The current study has determined the critical steps in the apoptotic cascade that are suppressed by hypoxia. The results show that both mitochondrial and cytosolic apoptotic events are suppressed in hypoxic cells. Hypoxic induction of IAP-2 may play an important role in antagonizing caspase activation in the cytosol.
Hypoxia poses a direct stress to mammalian cells and leads to cellular damage by itself. Thus, an important consideration for examination of apoptotic sensitivity of hypoxic cells is to minimize cell injury caused by hypoxia per se. The current study took advantage of the fact that acute cell injury by hypoxia is caused by energy deprivation of the cells due to the cessation of aerobic ATP production, and such defects can be corrected at least temporarily through anaerobic ATP generation via glycolysis. 17 The cells used in this study had a kidney proximal tubule origin and were immortalized and cultured in vitro, possessing significant capacity of glycolysis. 29 In the presence of glucose, cellular ATP was maintained at substantial levels. For example, after 6 hours of hypoxia the cells maintained an ATP level of 60 to 70% of control (Figure 1A) ▶ ; such energy status was sufficient to prevent irreversible damage and as a result preserve cell viability. In experiments of the current study, apoptosis induced by up to 6 hours of hypoxia was minimal (Figure 1B) ▶ . Significantly, cell death developed only when hypoxic incubation was prolonged to 12 hours or more (data not shown). Removal of acute hypoxic injury by facilitating glycolysis provided us a relatively clean model to investigate the response of hypoxic cells to apoptotic insults such as that induced by staurosporine.
Staurosporine was chosen in this study because it is a potent inducer of apoptosis and triggers apoptosis in various types of cells within a few hours. Rapid induction of apoptosis was important for the current study to avoid the contamination by cell death caused by hypoxic incubation per se. As discussed above, significant apoptosis would develop after 12 hours or longer periods of hypoxic incubation, even in the absence of additional apoptotic stimuli. Apoptosis induced by staurosporine is considered to be mediated by the mitochondrial pathway. 30,31 Consistent with these studies, results from the current work have demonstrated mitochondrial targeting of Bax, permeabilization of mitochondrial outer membranes and the release of cyt. c (Figures 3 and 4) ▶ . These were followed by caspase activation and the development of apoptotic morphology. Of note, the redistribution of Bax and cyt. c occurred after 2 hours of STA incubation, while significant caspase activation and apoptotic morphology developed later (Figures 1 to 4) ▶ . The sequential events enabled us to determine the critical steps in the apoptotic cascade that were affected by hypoxia.
This study has provided clear-cut evidence that apoptotic resistance of hypoxic cells takes place on at least two levels, on the mitochondrion and in the cytosol. At the mitochondrial level, permeabilization of the outer membrane during STA incubation was significantly suppressed under hypoxia. This was indicated by the amelioration of cyt. c release from the intermembrane space of the organelles. Although it remains unclear how mitochondrial opening is developed during apoptosis, Bcl-2 family proteins may directly participate in and regulate this stage. 24,27,28 Recent studies suggest a direct involvement of Bax/Bak in apoptotic pore formation in the outer membrane of mitochondria, while BH3 domain-only proteins such as Bid act as upstream triggers and Bcl-2/Bcl-XL as inhibitory regulators. 32,33 These studies suggest strongly that lesion development in the mitochondrial outer membrane may depend on the accumulation of pro-apoptotic molecules. In the model of the current study, Bax alone or in cooperation with other molecules might form pathological pores in the outer membrane of mitochondria, resulting in the leakage of cyt. c. For Bax, deletion of its membrane-targeting domain prevented its accumulation in mitochondria and significantly inhibited its apoptotic activity. 34 In our experimental model, STA-induced Bax movement to mitochondria was significantly suppressed under hypoxia. This might be responsible for the lack of mitochondrial lesion and cyt. c release in the hypoxic cells.
The signal directing Bax translocation from the cytosol to mitochondria remains to be clarified. 24,27,28 Structurally, Bax has a hydrophobic transmembrane domain at the C terminus. In normal non-apoptotic cells, this domain is buried by its interaction with the N terminus of the protein. Deletion of the N terminus results in mitochondrial localization of Bax even in the absence of apoptotic stimuli. 34 However, removal of N terminus by proteolysis is not considered as a common mechanism for the exposure of the transmembrane domain in vivo, because Bax remains intact during apoptosis regardless its location within the cells. There are at least two hypotheses on the regulation of Bax movement; each is currently supported by significant evidence. In the first hypothesis, Bax is proposed to interact with a regulatory protein. Modifications of the interaction may lead to conformational changes in Bax and the exposure of the transmembrane domain at the C terminus. A potential Bax-interacting protein might be Bid, a BH3 domain-only member of the Bcl-2 family protein. During apoptosis, Bid interacts with Bax and induces conformational changes to expose the C-terminus for integration of Bax into mitochondrial membranes. 32 Another protein regulating Bax might be 14-3-3, as suggested by a recent study. 35 It was shown that the 14-3-3 protein bound Bax in living cells; on apoptotic stimulation, Bax was released from 14 to 3-3 and translocated to mitochondria. Overexpression of 14-3-3 inhibited Bax-induced apoptosis, but could not inhibit apoptosis induced by a Bax mutant that did not bind 14-3-3. 35 Of interest, protein interactions with 14-3-3 changed as cells were exposed to hypoxia or ischemia. 36 The second hypothesis on the mechanisms of Bax translocation emphasizes a role for changes in the cytosolic environment. In particular, pH alterations in the cytosol might be critical. 37,38 A shift of intracellular pH toward either alkalization or acidification was linked to conformational changes in Bax, followed by insertion of the molecule into mitochondrial membranes. 37,38 Mammalian cells under hypoxia are forced to activate glycolysis for ATP generation, which is usually accompanied by a drop of cellular pH due to the accumulation of acidic metabolites such as lactate. 39 Thus, if Bax translocation during STA incubation depends on cytosolic alkalization as suggested by a recent study, 38 hypoxic glycolysis and concomitant acidification may neutralize STA-induced pH changes and as a result prevent Bax movement to mitochondria. Finally, hypoxia activates the PI3K/Akt pathway in PC-12 cells. 11 Although direct regulation of Bax by PI3K/Akt is unknown, Akt may regulate Bax activation and mitochondrial integrity indirectly, for example, through phosphorylation and regulation of Bad, another pro-apoptotic Bcl-2 family protein. 40,41 These possibilities need to be tested in future investigations to address the question: why is Bax activation diminished in hypoxic cells?
In addition to mitochondria, hypoxic cells also demonstrated apoptosis resistance in the cytosol, specifically at the level of caspase activation (Figure 5) ▶ . In these experiments, we isolated cytosols from normoxic and hypoxic cells, and compared their capacities for caspase activation after adding exogenous cyt. c. Under these conditions, the added cyt. c was expected to bind Apaf-1, resulting in the recruitment and activation of caspase-9, followed by the activation of downstream caspases. 25 The cytosol from hypoxic cells was shown to be significantly less competent in reconstitution of caspase activation (Figure 5) ▶ . A critical factor responsible for the decreased caspase activation capacity in these cells was identified to be IAP-2. First, significantly higher IAP-2 was detected in the cytosol of hypoxic cells, which exhibited lower caspase activation on cyt. c stimulation. Second, when hypoxic cells were returned to normal oxygen for reoxygenation, IAP-2 expression decreased rapidly to basal levels; cytosol isolated from these reoxygenated cells reconstituted higher caspase activity than hypoxic cytosols. Finally, direct evidence to support a role for IAP-2 was obtained from the immunodepletion experiments. When IAP-2 was depleted from the hypoxic cytosol, its reconstitution capacity for caspase activation was restored (Figure 6) ▶ . These results together have established a role for IAP-2 in apoptosis resistance in the cytosol of hypoxic cells. IAP-2, as a member of the family of apoptosis inhibitory proteins, may directly interact with caspases and inhibit their activation.
The observation that hypoxic cells become resistant to apoptosis at multiple levels may have implications in strategic design of approaches to antagonize this form of cell death. At least for the intrinsic pathway, a good strategy should include protections at the mitochondria and in the cytosol. A primary protection at the mitochondria would drastically reduce the release of apoptogenic factors such as cyt. c. A secondary protection would inhibit caspase activation in the cytosol, providing a grace period for the cell to recover or repair, in case a small amount of apoptogenic factors has been released. These mechanisms in combination are expected to offer a more efficient strategy to diminish apoptosis.
Of note, despite their apoptotic resistance, the hypoxic cells after STA exposure did not show significantly higher long-term survival than those treated under normal oxygen (not shown); however, this does not necessarily suggest that the apoptotic resistance of hypoxic cells is temporary. One possibility is that STA as a general inhibitor of protein kinases may affect other vital processes of the cells (eg, proliferation or adhesion), in addition to inducing apoptosis. Future investigations should test the effects of hypoxia on apoptosis induced by insults that are more specific and readily removable or reversible.
Cell injury induced by hypoxia has been recognized for a long time. On the contrary, survival pathways activated by hypoxia were not revealed until recently. 11,12 At the beginning, it seemed surprising that cells were more resistant to injurious stimuli when challenged under hypoxia, although cytoprotection by hypoxic/ischemic pre-conditioning has been documented by in vitro as well as in vivo studies. For example, in the kidneys, prior ischemic episodes protected the organs from injury by subsequent insults. 42 The key to unveil death resistance of hypoxic cells is to minimize injury by hypoxia per se, as shown by the current study. On the basis of these results, mammalian cells appear to respond to hypoxia in two opposite and yet interrelated directions, injury and adaptation. Hypoxic injury is initiated by depletion of cellular ATP, and depending on the severity of the insults, cell death by apoptosis or necrosis ensues. In the same cells, mechanisms for cell survival are also activated; prominent among them is the induction of IAP-2, a caspase inhibitory protein. This study has also indicated clearly that IAP-2 is not the only factor responsible for the acquired apoptosis resistance. Death-resistance of hypoxic cells takes place on at least two levels, on the mitochondria and in the cytosol. IAP-2, together with the factors that prevent Bax translocation and preserve mitochondrial integrity, may facilitate cell survival under hypoxia, a condition implicated in ischemic injury and solid tumor formation.
Acknowledgments
We thank Qiu Zong for technical assistance.
Footnotes
Address reprint requests to Zheng Dong, Ph.D., Department of Cellular Biology and Anatomy, Medical College of Georgia, 1459 Laney Walker Blvd., Augusta, GA 30912. E-mail: zdong@mail.mcg.edu.
Supported by grants from the National Kidney Foundation, the American Society of Nephrology, and the National Institutes of Health (DK58831).
Zheng Dong is a Carl W. Gottschalk Research Scholar of the American Society of Nephrology.
References
- 1.Semenza GL, Agani F, Feldser D, Iyer N, Kotch L, Laughner E, Yu A: Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv Exp Med Biol 2000, 475:123-130 [DOI] [PubMed] [Google Scholar]
- 2.Saikumar P, Dong Z, Weinberg JM, Venkatachalam MA: Mechanisms of cell death in hypoxia/reoxygenation injury. Oncogene 1998, 17:3341-3349 [DOI] [PubMed] [Google Scholar]
- 3.Cotran RS, Kumar V, Collins T: Cell injury and cellular death. Cotran RS Kumar V Collins T eds. Robbins Pathologic Basis of Disease. 6th ed. 1999:pp 1-29 W. B. Saunders Co., Philadelphia
- 4.Semenza GL: HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med 2002, 8:S62-S67 [DOI] [PubMed] [Google Scholar]
- 5.Brown JM: The hypoxic cell: a target for selective cancer therapy: eighteenth Bruce F. Cain Memorial Award lecture. Cancer Res 1999, 59:5863-5870 [PubMed] [Google Scholar]
- 6.Harris AL: Hypoxia: a key regulatory factor in tumour growth. Nat Rev Cancer 2002, 2:38-47 [DOI] [PubMed] [Google Scholar]
- 7.Bunn HF, Poyton RO: Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev 1996, 76:839-885 [DOI] [PubMed] [Google Scholar]
- 8.Hochachka PW, Buck LT, Doll CJ, Land SC: Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci USA 1996, 93:9493-9498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Barneo J, Pardal R, Ortega-Saenz P: Cellular mechanism of oxygen sensing. Annu Rev Physiol 2001, 63:259-287 [DOI] [PubMed] [Google Scholar]
- 10.Semenza GL: Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol 1999, 15:551-578 [DOI] [PubMed] [Google Scholar]
- 11.Alvarez-Tejado M, Naranjo-Suarez S, Jimenez C, Carrera AC, Landazuri MO, del Peso L: Hypoxia induces the activation of the phosphatidylinositol 3-kinase/Akt cell survival pathway in PC12 cells: protective role in apoptosis. J Biol Chem 2001, 276:22368-22374 [DOI] [PubMed] [Google Scholar]
- 12.Dong Z, Venkatachalam MA, Wang J, Patel Y, Saikumar P, Semenza GL, Force T, Nishiyama J: Up-regulation of apoptosis inhibitory protein IAP-2 by hypoxia: Hif-1-independent mechanisms. J Biol Chem 2001, 276:18702-18709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong Z, Nishiyama J, Yi X, Venkatachalam MA, Denton M, Gu S, Li S, Qiang M: Gene promoter of apoptosis inhibitory protein IAP2: identification of enhancer elements and activation by severe hypoxia. Biochem J 2002, 364:413-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salvesen GS, Duckett CS: IAP proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol 2002, 3:401-410 [DOI] [PubMed] [Google Scholar]
- 15.LaCasse EC, Baird S, Korneluk RG, MacKenzie AE: The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene 1998, 17:3247-3259 [DOI] [PubMed] [Google Scholar]
- 16.Deveraux QL, Reed JC: IAP family proteins: suppressors of apoptosis. Genes Dev 1999, 13:239-252 [DOI] [PubMed] [Google Scholar]
- 17.Saikumar P, Dong Z, Patel Y, Hall K, Hopfer U, Weinberg JM, Venkatachalam MA: Role of hypoxia-induced Bax translocation and cytochrome c release in reoxygenation injury. Oncogene 1998, 17:3401-3415 [DOI] [PubMed] [Google Scholar]
- 18.Dong Z, Saikumar P, Patel Y, Weinberg JM, Venkatachalam MA: Serine protease inhibitors suppress cytochrome c-mediated caspase-9 activation and apoptosis during hypoxia-reoxygenation. Biochem J 2000, 347:669-677 [PMC free article] [PubMed] [Google Scholar]
- 19.Waterhouse NJ, Goldstein JC, von Ahsen O, Schuler M, Newmeyer DD, Green DR: Cytochrome c maintains mitochondrial transmembrane potential and ATP generation after outer mitochondrial membrane permeabilization during the apoptotic process. J Cell Biol 2001, 153:319-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottlieb RA, Granville DJ: Analyzing mitochondrial changes during apoptosis. Methods 2002, 26:341-347 [DOI] [PubMed] [Google Scholar]
- 21.Mikhailov V, Mikhailova M, Pulkrabek DJ, Dong Z, Venkatachalam MA, Saikumar P: Bcl-2 prevents Bax oligomerization in the mitochondrial outer membrane. J Biol Chem 2001, 276:18361-18374 [DOI] [PubMed] [Google Scholar]
- 22.Dong Z, Venkatachalam MA, Weinberg JM, Saikumar P, Patel Y: Protection of ATP-depleted cells by impermeant strychnine derivatives: implications for glycine cytoprotection. Am J Pathol 2001, 158:1021-1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Earnshaw WC, Martins LM, Kaufmann SH: Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 1999, 68:383-424 [DOI] [PubMed] [Google Scholar]
- 24.Adams JM, Cory S: The Bcl-2 protein family: arbiters of cell survival. Science 1998, 281:1322-1326 [DOI] [PubMed] [Google Scholar]
- 25.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X: Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 1999, 15:269-290 [DOI] [PubMed] [Google Scholar]
- 26.Green DR: Apoptotic pathways: the roads to ruin. Cell 1998, 94:695-698 [DOI] [PubMed] [Google Scholar]
- 27.Gross A, McDonnell JM, Korsmeyer SJ: BCL-2 family members and the mitochondria in apoptosis. Genes Dev 1999, 13:1899-1911 [DOI] [PubMed] [Google Scholar]
- 28.Martinou JC, Green DR: Breaking the mitochondrial barrier. Nat Rev Mol Cell Biol 2001, 2:63-67 [DOI] [PubMed] [Google Scholar]
- 29.Woost PG, Orosz DE, Jin W, Frisa PS, Jacobberger JW, Douglas JG, Hopfer U: Immortalization and characterization of proximal tubule cells derived from kidneys of spontaneously hypertensive and normotensive rats. Kidney Int 1996, 50:125-134 [DOI] [PubMed] [Google Scholar]
- 30.Antonsson B, Montessuit S, Sanchez B, Martinou JC: Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem 2001, 276:11615-11623 [DOI] [PubMed] [Google Scholar]
- 31.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ: Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 2001, 292:727-730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ: BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell 2001, 8:705-711 [DOI] [PubMed] [Google Scholar]
- 33.Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD: Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 2002, 111:331-342 [DOI] [PubMed] [Google Scholar]
- 34.Goping IS, Gross A, Lavoie JN, Nguyen M, Jemmerson R, Roth K, Korsmeyer SJ, Shore GC: Regulated targeting of BAX to mitochondria. J Cell Biol 1998, 143:207-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nomura M, Shimizu S, Sugiyama T, Narita M, Ito T, Matsuda H, Tsujimoto Y: 14–3-3 interacts directly with and negatively regulates pro-apoptotic Bax. J Biol Chem 2003, 278:2058-2065 [DOI] [PubMed] [Google Scholar]
- 36.Chen XQ, Yu AC: The association of 14–3-3γ and actin plays a role in cell division and apoptosis in astrocytes. Biochem Biophys Res Commun 2002, 296:657-663 [DOI] [PubMed] [Google Scholar]
- 37.Khaled AR, Kim K, Hofmeister R, Muegge K, Durum SK: Withdrawal of IL-7 induces Bax translocation from cytosol to mitochondria through a rise in intracellular pH. Proc Natl Acad Sci USA 1999, 96:14476-14481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tafani M, Cohn JA, Karpinich NO, Rothman RJ, Russo MA, Farber JL: Regulation of intracellular pH mediates bax activation in HeLa cells treated with staurosporine or TNF-α. 2002. J Biol Chem [DOI] [PubMed]
- 39.Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA: Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci USA 2002, 99:12825-12830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME: Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997, 91:231-241 [DOI] [PubMed] [Google Scholar]
- 41.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G: Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 1997, 278:687-689 [DOI] [PubMed] [Google Scholar]
- 42.Bonventre JV: Kidney ischemic preconditioning. Curr Opin Nephrol Hypertens 2002, 11:43-48 [DOI] [PubMed] [Google Scholar]