Abstract
Large granular lymphocyte (LGL) leukemia is a well-recognized disease of mature T-CD8+ or less frequently natural killer cells; in contrast, monoclonal expansions of CD4+ T-LGL have only been sporadically reported in the literature. In the present article we have explored throughout a period of 56 months the incidence of monoclonal expansions of CD4+ T-LGL in a population of 2.2 million inhabitants and analyzed the immunophenotype and the pattern of cytokine production of clonal CD4+ T cells of a series of 34 consecutive cases. Like CD8+ T-LGL leukemias, CD4+ T-LGL leukemia patients have an indolent disease; however, in contrast to CD8+ T-LGL leukemias, they do not show cytopenias and autoimmune phenomena and they frequently have associated neoplasias, which is usually determining the clinical course of the disease. Monoclonal CD4+ T-LGLshowed expression of TCRαβ, variable levels of CD8 (CD8−/+dim) and a homogeneous typical cytotoxic (granzyme B+, CD56+, CD57+, CD11b+/−) and activated/memory T cell (CD2+bright, CD7−/+dim, CD11a+bright, CD28−, CD62L− HLA-DR+) immunophenotype. In addition, they exhibited a Th1 pattern of cytokine production [interferon-γ++, tumor necrosis factor-α++, interleukin (IL-2)−/+, IL-4−, IL-10−, IL-13−]. Phenotypic analysis of the TCR-Vβ repertoire revealed large monoclonal TCR-Vβ expansions; only a restricted number of TCR-Vβ families were represented in the 34 cases analyzed. These findings suggest that monoclonal TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL represent a subgroup of monoclonal LGL lymphoproliferative disorders different from both CD8+ T-LGL and natural killer cell-type LGL leukemias. Longer follow-up periods are necessary to determine the exact significance of this clonal disorder.
Large granular lymphocyte (LGL) leukemia is a well-recognized disorder of clonal mature lymphocytes of undetermined significance that commonly displays an indolent course; occasionally it may behave more aggressively. 1-3 Two different subgroups have been reported: a T-cell and a natural killer (NK)-cell type. 3,4 In T-LGL leukemia, clonal cells are reported to display a mature effector T-CD8+ cytotoxic phenotype, CD3+/CD8+/CD45RA+/CD28−/CD27−/CD94+, 5 with variable expression of CD57. 5,6 CD8+ T-LGL is associated to a mild to moderate stable lymphocytosis, neutropenia, splenomegaly, and to a less extent, anemia; 1 in addition, it shows a strong association with autoimmune diseases, especially rheumatoid arthritis. 5,6 The diagnostic category for T-LGL leukemia has been restricted to CD8+ cases and CD4+ T-LGL cases are not usually considered within this disease group. However, recently there have been rare reports of CD4+ T-LGL processes that look like the CD8+ LGL leukemia, but have a different immunophenotype. 7-10 Typically, the few cases of CD4+ T-LGL reported display monoclonal expansions of TCRαβ+/CD4+ T-LGL that co-express the CD56 and CD57 NK-associated antigens (NKa+) and variable levels of CD8 (CD8−/+dim). 7-10
A subset of circulating CD4+ T cells expressing NKa+ and CD8 has been already recognized for a long time both in mice (NK1.1+ T cells) 11 and in humans (TCRαβ+/CD4+/NKa+ T cells). 7,12-14 Although CD4+/CD8+ T cells were initially thought to represent recently produced T lymphocytes, accumulating evidence supports the notion that CD4+/CD8+ lymphocytes are CD4+ T cells that have acquired the CD8 α chain after being activated, and thereafter maintain the CD4+/CD8+ phenotype. 15,16 As murine NK1.1+ T cells, human CD4+/NKa+/CD8−/+d cells are now believed to be a specialized subset of TCRαβ+ T cells with different functional roles, including tumor rejection. 17-19 In this sense, TCRαβ+/CD4+/NKa+ T cells have been found at increased proportions in humans in various pathological conditions, including neoplasias, chronic viral infections, autoimmune disorders, and allografts. 20-30 Previous reports have suggested that, as in mice NK1.1+ T lymphocytes, 31 human CD4+/NKa+/CD8−/+d T cells would express a restricted TCR-Vβ repertoire in different disease conditions in which these cells are expanded. 32-35 As mentioned above, only a few cases displaying monoclonality have been reported so far. Therefore, information currently available on their incidence and characteristics is very limited.
The aim of the present study is to analyze the incidence and the clinical and laboratory features of monoclonal expansions of TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL and to explore both the immunophenotype and the pattern of cytokine production of these cells. Our results indicate that in these patients the expanded CD4+ T-LGL are both phenotypically and functionally homogeneous and monoclonal CD4+ T-LGL cases show an indolent course in the absence of cytopenias, arthritis, and symptoms directly attributed to the lymphoproliferative disorder itself; however, they frequently have an associated second neoplasia, that is usually determining the clinical course of the disease.
Materials and Methods
Patients and Samples
From September 1997 to April 2002 (both included), samples from a total of 810 adults with monoclonal expansions of mature lymphocytosis were consecutively diagnosed at the Hematology Laboratory of the University Hospital of Salamanca (Salamanca, Spain). This laboratory acts as the reference laboratory for a region of 2.2 million inhabitants in the central-western part of Spain (Castilla y León).
From the 810 monoclonal lymphoproliferative cases, 17 corresponded to monoclonal expansions of CD4+/NKa+/CD8−/+d T-cell LGL. Apart from these consecutively received unselected patients, another 17 monoclonal cases suspected of having an expansion of CD4+/NKa+/CD8−/+d T-cell LGL were referred from hospitals outside the Castilla y León region, either to the Laboratory of the University Hospital of Salamanca (n = 5) or to a reference laboratory in Portugal (the Hematology Department of the Santo Antonio Hospital, Porto, Portugal) (n = 12) for clonality studies, and included in this study. Accordingly, a total of 34 patients (14 males, 20 females, with a mean age of 65 ± 11 years; range, 40 to 81 years) with monoclonal proliferations of TCRαβ+/CD4+/NKa+/CD8−/+dim T cells were included in this study. At the moment of closing this study, median follow-up was of 12 months (range, 1 to 89 months).
In all cases, the studies described below were performed in peripheral blood (PB) samples collected into tubes containing either K3-ethylenediaminetetraacetic acid for immunophenotypic and molecular studies or sodium-heparin for functional analyses of cytokine production.
Immunophenotypic and Cytokine Production Studies
Cell Surface and Cytoplasmic Staining
Cell staining was performed using a whole blood stain-and-then-lyse method (FACS lysing solution; Becton/Dickinson Biosciences, San Jose, CA) and a direct immunofluorescence technique as previously reported in detail. 36 Cytoplasmic staining was performed using the Fix & Perm reagent kit (Caltag Laboratories, San Francisco, CA).
The initial screening was performed with a four-color panel of monoclonal antibodies (mAbs) directed against T- and NK-associated antigens conjugated with fluorescein isothiocyanate, phycoerythrin, phycoerythrin-cyanine 5, or peridin chlorophyll protein and allophycocyanin, which include the CD2/CD7/CD56/CD3, TCRαβ/CD5/CD3/TCRγδ, and CD8/CD28/CD4/CD3 stainings. Once the lineage of the expanded LGL was identified as being TCRαβ+/CD4+, these cells were further characterized using the following four-color panels of mAb: CD2/CD7/CD4/CD8, CD5/CD7/CD4/CD8, CD38/CD11b/CD4/CD8, CD57/CD11c/CD4/CD8, CD16/CD56/CD4/CD8, CD122/CD25/CD4/CD8, CD45RA/CD45RO/CD4/CD8, CD62L/CD28/CD4/CD8, CD11a/HLA-DR/CD4/CD8, CD16/NKB1/CD4/CD8, CD158a/CD161/CD4/CD8, CD57/CD8/CD56/CD4, and CD57/cytoplasmic (cyt) granzyme B/CD56/CD4. The source and specificity of each mAb reagent used is shown in Table 1 ▶ .
Table 1.
Monoclonal Antibody Reagents Used in the Present Study
| Surface/cytoplasmatic antigens | TCR Vβ regions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Specificity | Clone | Ig isotype* | Fluorochrome conjugate | Source† | Specificity | Clone | Ig isotype* | Fluorochrome conjugate | Source† | |||
| CD2 | SFCI3Pt2H9 | IgG1 (m) | FITC | BC | Vβ1 | BL37.2 | IgG1 (r) | FITC, PE | IOT | |||
| CD3 | SK7 | IgG1 (m) | PerCP | BDB | Vβ2.1 | MPB2D5 | IgG1 (m) | FITC | IOT/BioD | |||
| CD3 | SK7 | IgG1 (m) | APC | BDB | Vβ3.1 | CH92/8F10 | IgM/IgG1 (m) | FITC | IOT/END | |||
| CD4 | SK3 | IgG1 (m) | PerCP | BDB | Vβ4 | WJF24 | IgM(r) | FITC, PE | IOT | |||
| CD4 | SK3 | IgG1 (m) | APC | BDB | Vβ5.1 | IMMU157 | IgG2a (m) | FITC, PE | IOT | |||
| CD5 | L17F12 | IgG2a (m) | PE | BDB | Vβ5.2 | 36213 | IgG1 (m) | FITC, PE | IOT | |||
| CD7 | 3A1/1,7F3 | IgG2a (m) | FITC | CLB | Vβ5.3 | 3D11 | IgG1 (m) | PE | IOT | |||
| CD7 | 3A1E-12H7 | IgG2b (m) | PE | BC | Vβ6.1 | CRI304.3 | IgM (m) | FITC | IOT | |||
| CD8 | DK25 | IgG1 (m) | FITC | DK | Vβ6.7 | T145 | IgG1 (m) | FITC | END | |||
| CD8 | DK25 | IgG1 (m) | PE | DK | Vβ7.1 | ZOE | IgG2a (m) | FITC, PE | IOT | |||
| CD8 | SK1 | IgG1 (m) | APC | BDB | Vβ7.2 | ZIZOU4 | IgG2a (m) | FITC | IOT | |||
| CD11a | CLB-LFA-1/2 | IgG2a (m) | FITC | CLB | Vβ8.1 + 8.2 | 56C5.2 | IgG2a (m) | FITC | IOT | |||
| CD11b | D12 | IgG2a (m) | PE | BDB | Vβ9.1 | FIN9 | IgG2a (m) | PE | IOT | |||
| CD11c | S-HCL-3 | IgG2b (m) | PE | BDB | Vβ11.1 | C21 | IgG2a (m) | FITC, PE | IOT | |||
| CD16 | 3G8 | IgG1 (m) | FITC | BC | Vβ12.2 | VER2.32 | IgG2a (m) | FITC | IOT | |||
| CD25 | 2A3 | IgG1 (m) | PE | BDB | Vβ13.1 | IMMV222 | IgG2b (m) | PE | IOT | |||
| CD28 | L293 | IgG1 (m) | PE | BDB | Vβ13.2 | H132 | IgG1 (m) | PE | IOT | |||
| CD38 | LD38 | IgG1 (m) | FITC | CYT | Vβ13.6 | JU74.3 | IgG1 (m) | FITC, PE | IOT | |||
| CD44 | L178 | IgG1 (m) | FITC | BDB | Vβ14.1 | CAS 1.1.3 | IgG1 (m) | FITC | IOT | |||
| CD45RA | L48 | IgG1 (m) | FITC | BDB | Vβ16.1 | TAMAYA1.2 | IgG1 (m) | FITC | IOT | |||
| CD45RO | UCHL-1 | IgG2a (m) | PE | BDB | Vβ17.1 | E17.5F3 | IgG1 (m) | FITC, PE | IOT | |||
| CD56 | NCAM16.2 | IgG2b (m) | PE | BDB | Vβ18.1 | BA62.6 | IgG1 (m) | PE | IOT | |||
| CD56 | N901 | IgG1 (m) | PC5 | BC | Vβ20.1 | ELL1.4 | IgG (m) | FITC | IOT | |||
| CD57 | HNK-1 | IgM (m) | FITC | BDB | Vβ21.3 | IG125 | IgG2a (m) | FITC | IOT | |||
| CD62L | SK11 | IgG2a (m) | FITC | BDB | Vβ22.1 | IMMU546 | IgG1 (m) | FITC, PE | IOT | |||
| CD94 | HP-309 | IgG1 (m) | FITC | PHA | Vβ23.1 | AF23 | IgG1 (m) | PE | IOT | |||
| CD122 | MIK-b | IgG2a (m) | FITC | CLB | Cytokines | |||||||
| CD158a | HP-3E4 | IgM (m) | FITC | BDB | IL2 | MQ1-17H12 | IgG2a (r) | PE | PHA | |||
| CD161 | DX12 | IgG1 (m) | PE | BDB | IL4 | 8D4-8 | IgG1 (m) | PE | PHA | |||
| HLA-DR | L243 | IgG2a (m) | PE | BDB | IL10 | JES3-19F1 | IgG2a (r) | PE | PHA | |||
| NKB1 | DX9 | IgG1 (m) | PE | BDB | IL13 | JES-5A2 | IgG1 (r) | PE | PHA | |||
| TCR-α/β | WT31 | IgG1 (m) | FITC | PHA | IFNγ | B27 | IgG1 (m) | PE | PHA | |||
| TCR-γ/δ | 11F2 | IgG1 (m) | PE | BDB | TNFα | MAB11 | IgG1 (m) | PE | PHA | |||
| Granzyme B | CLB-GB11 | IgG1 (m) | PE | CLB | TNFβ | 359-81-11 | IgG1 (m) | PE | PHA | |||
*(m), Mouse immunoglobulins; (r), rat immunoglobulins.
†BC, Beckmann Coulter, Miami, FL; IOT, Immunotech, Marseille, France; BDB, Becton Dickinson Biosciences, San José, CA; CLB, Amsterdam, The Netherlands; DK, Dako A/S, Glostrup, Denmark; CYT, Cytognos, Salamanca, Spain; PHA, Pharmingen, San Diego, CA; BioD, Biodesign International, Saco, ME; END, Endogen, Wobum, MAs.
For the analysis of the TCR-Vβ repertoire of CD4+ T lymphocytes an extensive panel of 26 mAbs directed against 20 different TCR-Vβ families was used (Table 1) ▶ . These reagents covered 60 ± 4% of the TCR-Vβ repertoire of CD4+ T cells present in the PB of normal age- and sex-matched controls. 37 Diagnosis of a TCR-Vβ expansion was based on previously defined criteria: 37 whenever a given TCR-Vβ family was over-represented within the CD4+ T-cell compartment (ie, it exceeded the mean value + 2 standard deviations of normal individuals); or if a dilution pattern was observed (ie, the fraction of CD4+ T cells that were recognized was less than or equal to 85% of the mean value observed in normal individuals with the same panel of anti-TCR-Vβ mAb).
Functional Analysis of Cytokine Production
In 23 of the 34 patients with monoclonal expansions of TCRαβ+/CD4+/NKa+/CD8−/+dim T cells the cytokine production by the expanded TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL was analyzed by flow cytometry. Briefly, 500 μl of heparinized PB were placed into a tube containing 500 μl of RPMI 1640 culture medium (BioWhittaker, Walkersville, MD) supplemented with l-glutamine (2 mmol/L). Cells were cultured for 4 hours at 37°C in a 5% CO2 and a 95% humidity sterile environment in the presence of phorbol-12 myristate 13-acetate (25 ng/ml), ionomycin (1 μg/ml), and brefeldin A (10 μg/ml) (stimulated samples) or only brefeldin A (nonstimulated samples). Once this incubation period was completed, each sample was aliquoted in different vials of 100 μl and sequentially stained for surface antigens (CD4 and CD8) and intracellular cytokines [interleukin (IL), IL-4, IL-10, IL-13, interferon-γ, tumor necrosis factor (TNF)-α, and TNF-β] (Table 1) ▶ , as previously described in detail. 36
Flow Cytometry Data Acquisition and Analysis
Flow cytometry data acquisition for the immunophenotypic and cytokine production studies was systematically performed in dual-laser FACSCalibur flow cytometers (Becton/Dickinson Biosciences), using the Cell QUEST software program (Becton/Dickinson Biosciences). For each staining, information on a minimum of 2 × 10 5 nongated events was acquired and stored as list mode data. For data analysis the Paint-a-Gate PRO software program (Becton/Dickinson Biosciences) was used. TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL were first identified based on their flow cytometric characteristics—high sideward (SSC) and forward (FSC) light scatter profile, expression of NKa antigens, and negative/low levels of CD8. Once identified, cells fulfilling these criteria were selected (gated cells), their percentage from total lymphocytes was calculated and they were analyzed forthe expression of the surface antigens recognized by the mAb referred above. For each antigen analyzed, the following characteristics were recorded: 1) percentage of positive cells, evaluated as the proportion of gated cells stained above the isotype control value; 2) intensity of expression (absent, dim, moderate, strong, very strong), evaluated by the mean fluorescence intensity, expressed in arbitrary relative linear units scaled from 0 to 10.000; 3) relative intensity of expression (increased, normal, or decreased) in TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL as compared to the residual TCRαβ+/CD4+/NKa−/CD8− non-LGL lymphocytes present in the same sample; and 4) pattern of antigen expression (homogeneous versus heterogeneous), evaluated by the coefficient of variation.
Molecular Analysis of T-Cell Clonality
Rearrangements of the TCR-Vβ genes were evaluated by conventional Southern blotting. 38,39 Briefly, mononuclear cells were obtained after fractionation on a Lymphoprep (Nycomed Pharma AS, Oslo, Norway) density gradient, washed twice in phosphate-buffered saline, and cryopreserved. DNA was extracted using the phenol/chloroform method and digested with EcoRI and HindIII restriction enzymes. DNA fragments were separated by 0.8% agarose gel electrophoresis and transferred to nitrocellulose membranes by vacuum blotting, UV fixed, and hybridized with 32P-labeled probes for the TCR-β gene region (Cβ, TCRBC, and TCRBJ2; DAKO A/S, Glostrup, Denmark).
Other Laboratory Studies
Serum was tested for the presence of antibodies to cytomegalovirus, human hepatitis B and C, type 1 and 2 herpes simplex, rubella, human T-cell lymphotrophic type I and II, and human immunodeficiency type 1 and 2 viruses. Rheumatoid factor, anti-nuclear antibodies, and serum immunoglobulin levels were also determined using conventional techniques.
Results
Incidence of Monoclonal Expansions of TCRαβ+/ CD4+/NKa+/CD8−/+dim T-LGL
Monoclonal expansions of TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL were detected in 17 of the 810 consecutiveunselected individuals who had a monoclonal proliferation of B-, T-, or NK-cell mature lymphocytes (2% of total).Throughout a period of 56 months, this represents an incidence of 3.64 new cases/year for a region of 2.2 million inhabitants (1.65 new cases/1 million inhabitants).
Clinical and Laboratory Features of Individuals with Monoclonal Expansions of TCRαβ+/ CD4+/NKa+/CD8−/+dim T-LGL
From the clinical point of view, individuals with monoclonal expansions of CD4+/NKa+/CD8−/+dim T-LGL showed a relative indolent disease course. At diagnosis, all except six patients (82%) showed normal physical examination. From the six patients displaying an altered physical examination, three presented organomegalies that were attributed to a concomitant B-cell neoplasia and one patient displayed skin lesions compatible with disseminated granuloma annulare based on clinical and histological findings. The other two patients showed a single small (<1 cm) adenopathy each; in these two patients, as well as in the remaining cases, diagnosis was made in a routine blood cell analysis (87%). In Table 2 ▶ the most relevant clinical and laboratory features of these patients at presentation, are summarized.
Table 2.
Clinical and Laboratory Features of Individuals with Monoclonal Expansions of PB TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL
| Age (years) | 65 ± 11 (40–81) |
| Sex (m/f) | 14/20 (41%/59%) |
| Reason for consulting | |
| Routine blood analysis | 87% |
| Abdominal distension | 6.6%* |
| Skin lesions | 3.3%† |
| Fever | 3.3%‡ |
| Physical examination | |
| Splenomegaly | 6.7%* |
| Adenophaties | 13%§ |
| Skin lesions | 3.3%† |
| Laboratory parameters | |
| Hemoglobin (g/L) | 144 ± 16 (122–184) |
| WBC count (× 109/L) | 11.5 ± 6.8 (3–37.1) |
| Lymphocytes (× 109/L) | 7.1 ± 4.5 (1.4–17.3) |
| Neutrophils (× 109/L) | 3.7 ± 3.4 (0.8–19.1) |
| Platelets (× 109/L) | 214 ± 65 (68–346) |
Continuous variables shown as mean ± standard deviation (range).
*These cases presented abdominal distention because of splenomegaly, related to a co-existing B-cell neoplasia.
†Diagnosed as disseminated granuloma annulare.
‡B-CLL co-existing with the T-cell expansion.
§Half of these cases had an associated B-cell lymphoproliferative disorder, one of which also presented splenomegaly.
Interestingly, co-existence of a second neoplasia was found in 10 patients (29%). In six cases these tumors corresponded to hematological disorders, in another threepatients to solid tumors, and in the remaining case, ahematological and a solid malignancy co-existed. As shown in Table 3 ▶ , the hematological disorders corresponded to a splenic marginal zone B-cell non-Hodgkin’s lymphoma (SMZL) (n = 2), a B-cell chronic lymphocytic leukemia (B-CLL) (n = 2), a lymphoplasmacytic lymphoma (LPL) (n = 1) and a leukemia of TCRgd+ T-LGL coexisting with a monoclonal gammopathy of undetermined significance (MGUS) (n = 1). The nonhematological malignancies were a thyroid carcinoma (n = 1), gastric adenocarcinoma (n = 1), and a leiomyosarcoma co-existing with a squamous cell carcinoma (n = 1). In the patient in whom two hematological and nonhematological malignancies co-existed, the diagnoses corresponded to a B-CLL and a colorectal adenocarcinoma, respectively. Diagnosis of the clonal TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL expansion was made either simultaneously (n = 6 cases) or before (n = 2) that of the associated disease; in only two cases the diagnosis of the associated tumor preceded that of the monoclonal expansion of PB T cells (Table 3) ▶ .
Table 3.
Tumors Associated to Monoclonal Expansions of TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL
| Type of neoplasia | Number of cases | Time between diagnosis |
|---|---|---|
| Hematological malignancies | 6/33 | |
| B-chronic lymphoproliferative disorders | ||
| SMZL | 2/33 | Simultaneous |
| Lymphoplasmacytic lymphoma | 1/33 | Simultaneous |
| Typical B-CLL | 1/33 | 4 years before |
| Atypical B-CLL | 1/33 | Simultaneous |
| Tgamma-delta leukemia + MGUS | 1/33 | Simultaneous |
| Nonhematological malignancies | 3/33 | |
| Thyroid carcinoma | 1/33 | 2 years before |
| Gastric adenocarcinoma | 1/33 | 4 years later |
| Leiomyosarcoma + squamous cell carcinoma | 1/33 | 4 and 1 year later |
| Two co-existing malignancies | 1/33 | |
| B-CLL + colorectal adenocarcinoma | 1/33 | Simultaneous |
Overall, 10 of 34 cases (29%), showed one or two associated neoplasias.
SMZL; splenic marginal zone B-cell non-Hodgkin lymphoma; B-CLL, B-cell chronic lymphocytic leukemia; MGUS; monoclonal gammopathy of undetermined significance.
From the hematological point of view, both the relative and absolute PB lymphocyte counts were increased (7.1 ± 4.5 × 109 lymphocytes/L, corresponding to 60 ± 17% of all PB leukocytes). Morphologically recognizable LGLs were observed in all cases and they constantly accounted for more than half of all PB lymphocytes (mean percentage of LGL of: 73.8 ± 12%; range, 53 to 91%). The hemoglobin (144 ± 16 g/L), platelet (214 ± 65 × 109/L), and neutrophil (3.7 ± 3.4 × 109/L) counts were within the normal range in all except three cases. Moreover, in 9 of 34 patients (six males and three females) hemoglobin levels were ≥150 g/L.
Serum lactate dehydrogenase levels were within the normal range in all cases, and β2-microglobulin levels were within the normal range in all but one case (3.9 mg/L), corresponding to a patient with an associated B-cell lymphoproliferative disorder. There was no serological evidence for recent or persistent viral infection. Increased IgE serum levels were found in one-third of cases (942 ± 1044 U/L; range, 123 to 2119; normal values <100 U/L). Rheumatoid factor and anti-nuclear antibodies were negative in all patients and only one patient had an associated joint disease documented as osteoarthritis.
Immunophenotype of clonal TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL
TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL comprised 78 ± 18% of the total CD4+ T cells in the 34 patients analyzed and the mean (± SD) absolute number of PB TCRαβ+/CD4+/NKa+/CD8−/+dim T lymphocytes was 7.06 ± 7.7 × 109/L.
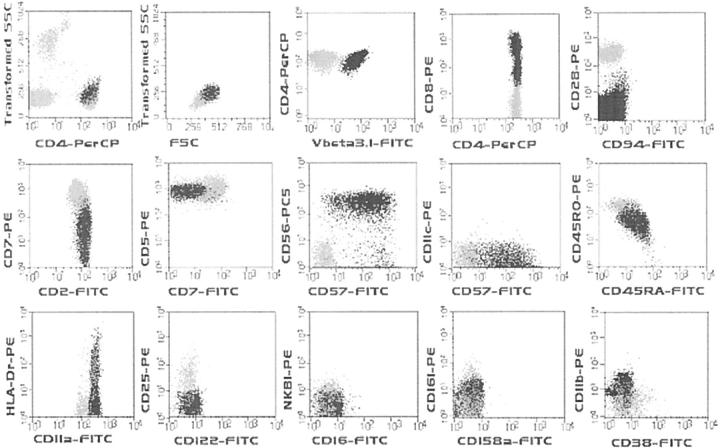
Clonal TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL showed unique immunophenotypic features (Figure 1 ▶ and Table 4 ▶ ), including relatively high light scatter characteristics (FSC and SSC) as compared to normal residual TCRαβ+/CD4+/NKa−/CD8− T-LGL (ratio between FSC and SSC values of CD4+ T-LGL and CD4+ non-LGL T cells of 1.2 ± 0.1 and 1.3 ± 0.1, respectively) and co-expression of cytoplasmic granzyme B as well as of the CD56 and CD57 NKa antigens; reactivity for CD11b was variable, with both positive (47%) and negative (53%) cases (mean number of CD11b+ cells, 52 ± 48%; range, 0 to 100%). Other NKa markers, including CD11c, CD16, CD94, CD158a, CD161, and NKB1 were constantly absent on the clonal T cells.
Figure 1.
Immunophenotypic features of monoclonal TCRαβ+/CD4/NKa+/CD8−/+dim T-LGL. Representative dot plots illustrating the phenotypic patterns observed in monoclonal TCRαβ+/CD4/NKa+/CD8−/+dim T-LGL. Black dots correspond to monoclonal TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL whereas gray dots correspond to residual TCRαβ+/CD4+/NKa−/CD8− non-LGL T cells in all panels except the one in the top left corner in which PB leukocytes other than CD4+ T cells are also displayed as gray events.
Table 4.
Immunophenotypical Features of Monoclonal Expansions of TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL
| No. | TCR Vβ family | % CD4+/NKa+/CD8−/d+ | CD2* | CD5* | CD7† | CD8† | CD11b† | CD56* | CD57† | CD45RA | CD45RO | CD11a* | HLA-DR† |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Vβ2.1 | 87.5 | ++ | +++ | +d | +d (22%) | − | + (83%) | + (98%) | + | + | ++ | +d (23%) |
| 2 | Vβ13.1 | 91.9 | ++ | ++/+++ | +d | − | +d | + (100%) | + (86%) | + | + | ++ | +d (62%) |
| 3 | N.I. | 84.1 | ++ | ++/+++ | +d | +d (97%) | − | + (92%) | + (95%) | − | + | ++ | +d (24%) |
| 4 | Vβ 17.1 | 82.4 | ++ | ++/+++ | +d | +d (100%) | +d | + (98%) | + (96%) | + | − | ++ | +d (66%) |
| 5 | N.I. | 67.6 | ++ | ++/+++ | +d | +d (100%) | +d | + (80%) | + (100%) | + | + | ++ | +d (15%) |
| 6 | N.I. | 71.8 | ++ | ++/+++ | −/+d | +d (96%) | − | + (98%) | + (61%) | + | − | ++ | +d (44%) |
| 7 | Vβ13.1 | 64.1 | ++ | ++ | −/+d | +d (22%) | − | + (96%) | + (99%) | + | + | ++ | +d (43%) |
| 8 | N.I. | 57.0 | ++ | ++ | −/+d | +d (14%) | − | + (97%) | + (81%) | + | + | ++ | +d (96%) |
| 9 | N.I. | 78.2 | ++ | ++/+++ | +d | +d (55%) | − | + (37%) | + (99%) | + | + | ++ | +d (24%) |
| 10 | Vβ13.1 | 27.8 | ++ | ++/+++ | +d | +d (86%) | +d | + (93%) | + (100%) | − | + | ++ | +d (69%) |
| 11 | N.I. | 1.87 | ++ | ++/+++ | +d | − | +d | + (96%) | + (94%) | + | + | ++ | +d (51%) |
| 12 | N.I. | 53.4 | ++ | ++ | +d | +d (56%) | +d | + (72%) | + (76%) | − | + | ++ | +d (71%) |
| 13 | Vβ3.1 | 69.3 | ++ | ++ | −/+d | +d (71%) | +d | + (92%) | + (94%) | + | − | ++ | +d (69%) |
| 14 | N.I. | 70.9 | ++ | ++ | −/+d | +d (44%) | +d | + (100%) | + (98%) | + | + | ++ | +d (43%) |
| 15 | N.I. | 26.6 | ++ | ++/+++ | +d | +d (86%) | +d | + (66%) | + (94%) | − | + | ++ | +d (59%) |
| 16 | Vβ13.1 | 50.7 | ++ | ++ | +d | +d (52%) | − | + (82%) | + (84%) | − | + | ++ | +d (84%) |
| 17 | Vβ3.1 | 32.9 | ++ | ++/+++ | +d | +d (89%) | +d | + (86%) | + (98%) | + | + | ++ | +d (20%) |
| 18 | N.I. | 38.0 | ++ | ++/+++ | +d | +d (39%) | +d | + (92%) | + (94%) | + | + | ++ | +d (15%) |
| 19 | N.I. | 26.0 | ++ | ++ | −/+d | +d (47%) | − | + (71%) | + (26%) | + | + | ++ | +d (31%) |
| 20 | Vβ2.1 | 7.3 | ++ | ++/+++ | +d | +d (76%) | − | + (60%) | + (92%) | + | + | ++ | +d (25%) |
| 21 | Vβ13.1 | 82.6 | ++ | ++/+++ | −/+d | +d (20%) | +d | + (20%) | + (16%) | − | + | ++ | +d (14%) |
| 22 | Vβ13.1 | 14.7 | ++ | +++ | −/+d | − | − | + (98%) | + (100%) | − | + | ++ | +d (31%) |
| 23 | Vβ13.1 | 40.5 | ++ | ++ | −/+d | +d (100%) | − | + (61%) | + (25%) | + | − | ++ | +d (35%) |
| 24 | Vβ2.1 | 23.0 | ++ | ++/+++ | + | +d (28%) | − | + (100%) | + (100%) | + | − | +++ | +d (57%) |
| 25 | Vβ13.1 | 31.7 | ++ | +++ | −/+d | +d (100%) | − | − | + (100%) | + | − | +++ | +d (20%) |
| 26 | N I | 51.1 | ++ | ++ | +d | +d (49%) | +d | + (100%) | + (100%) | + | + | +++ | +d (84%) |
| 27 | Vβ13.1 | 84.0 | ++ | ++ | +d | +d (65%) | −/+d | + (100%) | + (100%) | − | + | ++ | +d (20%) |
| 28 | Vβ13.1 | 31.7 | ++ | +++ | +d | +d (100%) | −/+d | + (100%) | + (100%) | − | + | ++ | ND |
| 29 | Vβ13.1 | 11.8 | ++ | +++ | −/+d | − | − | + (100%) | + (100%) | − | + | ++ | ND |
| 30 | Vβ17.1 | 45.8 | ++ | ++ | −/+d | +d (84%) | − | − | + (100%) | + | + | +++ | +d (64%) |
| 31 | Vβ9.1 | 40.1 | ++ | ++ | + | +d (100%) | − | + (100%) | + (100%) | − | + | ++ | − |
| 32 | Vβ13.1 | 54.2 | ++ | ++ | −/+d | +d (90%) | − | + (50%) | + (17%) | − | + | +++ | +d (88%) |
| 33 | N I | 2.0 | ++ | ++ | −/+d | +d (100%) | +d | + (100%) | + (100%) | + | + | ++ | +d |
| 34 | Vβ7.1 | 22.8 | ++ | ++ | −/+d | +d (100%) | − | + (24%) | + (9%) | + | + | ++ | +d |
% CD4+/NKa+/CD8−/d+: percentage of CD4+/NKa+/CD8−/d+ cells from the total PB lymphocytes.
Clonal CD4+ LGL were constantly positive for granzyme B and were negative for CD11c, CD16, CD25, CD28, CD38, CD62L, CD94, CD122, CD158a, CD161, NKB1.
N.I.: not identified. ND: not determined.
*, Homogeneous expression;
†, heterogeneous expression; −, negative; +d; dim expression; +, moderate expression; ++, strong expression; +++; very strong expression.
Data in brackets: percentage of CD4+ LGL expressing that antigen. CD2, CD5, CD45RA, CD45RO, and CD11a once positive were present in 100% of cells.
With regard to T-cell associated antigens, TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL typically co-expressed low and heterogeneous levels of CD8 (88% of cases), the percentage of CD4+/CD8+dim T cells ranging from 14% to 100% (mean of 60 ± 36%). Additionally, TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL showed a bright and homogeneous CD2 expression as compared to the TCRαβ+/CD4+/NKa−/CD8− residual T cells; in a similar way, CD5 was also homogeneously positive in all cases, but its levels were frequently slightly reduced. In turn, reactivity for CD7 was constantly heterogeneous and either dim or nearly absent.
In approximately half of the cases (16 of 34, 47%), TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL were CD45RA+/CD45RO+. In addition, clonal T cells constantly expressed increased and homogeneous levels of CD11a; HLA-DR was usually dimly positive and heterogeneously expressed (Figure 1) ▶ . Other surface markers that have been related to T-cell activation, such as CD25, CD28, CD38, CD62L, and CD122 were constantly absent.
TCR-Vβ Repertoire of Clonal TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL
All patients with monoclonal expansions of TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL had a major TCR-Vβ expansion, which accounted for 74 ± 21% of the total CD4+ T cells. The magnitude of the TCR-Vβ expansion significantly correlated (P < 0.05) with the fraction of TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL. In 20 cases (59%) we were able to identify the expanded TCR-Vβ family: TCR-Vβ2.1 in 4 cases (12%), TCR-Vβ3.1 in 2 (6%), TCR-Vβ13.1 in 12 (35%), and TCR-Vβ17.1 in 2 cases (6%). In the remaining 13 patients the expanded TCR-Vβ family was not represented in the panel of mAbs used. There was no statistical association between the type of TCR-Vβ family expanded and the presence or absence of an associated neoplasia. Molecular studies confirmed the existence of clonal TCR-β chain gene rearrangements in all cases.
Cytokine Production by Clonal TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL
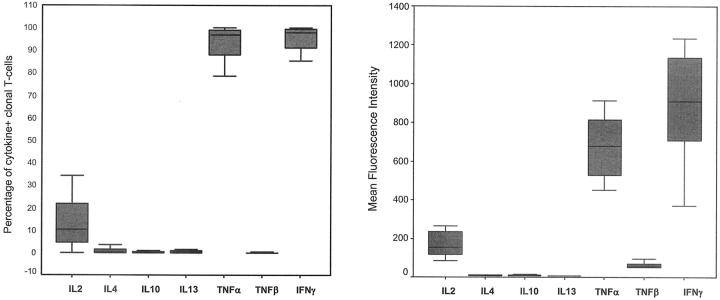
In all cases analyzed (n = 23) clonal TCRαβ+/CD4+/NKa+/CD8−/+dim T cells showed a typical Th1 pattern of cytokine production after stimulation with phorbol-12 myristate 13-acetate plus ionomycin (Figure 2) ▶ . Accordingly, these cells produced large amounts of both interferon-γ and TNF-α. Interestingly, in 70% of these cases, a variable proportion of the TCRαβ+/CD4+/NKa+/CD8−/+dim clonal T-LGL (25 ± 21%, range, 6 to 77%) also produced IL-2. By contrast, under these stimulatory conditions, production of IL-4, IL-10, IL-13, and TNF-β could not be detected in any of the cases analyzed.
Figure 2.
In vitro cytokine production by monoclonal TCRαβ+/CD4/NKa+/CD8−/+dim T-LGL. Monoclonal TCRαβ+/CD4/NKa+/CD8−/+dim T-LGL constantly showed a typical Th1 pattern of cytokine production: production of large amounts of both TNF-α and interferon-γ, whereas production of IL-4, IL-10, IL-13, and TNF-β by phorbol-12 myristate 13-acetate + ionomycin-stimulated clonal T cells was constantly irrelevant. Results are expressed as percentage of cytokine+ clonal CD4+ T cells (A) and mean fluorescence intensity (MFI) levels of expression of cytoplasmic cytokines in clonal CD4+ T cells (B). Boxes extend from the 25th to the 75th percentiles; the line in the middle and the vertical lines represent median values and 95% confidence intervals, respectively.
Follow-Up and Clinical Outcome
At the moment of closing this study, all 34 patients remained alive and in the absence of clinical manifestations directly related to the monoclonal TCRαβ+/CD4+/NKa+/CD8−/+dim T-cell expansion. Despite this, changes were observed in five cases. One displayed anemia because of gastrointestinal hemorrhage associated with a gastric adenocarcinoma, whereas the other three cases showed pancytopenia because of either bone marrow infiltration by leiomyosarcoma (n = 1) or to treatment for an associated B-cell lymphoproliferative disorder (n = 2). Interestingly, in the fifth case a spontaneous remission of the CD4+ LGL proliferation occurred 4 years after the initial diagnosis, as assessed by immunophenotypic and TCR-Vβ analysis.
Discussion
Recent studies have shown the presence of increased numbers of TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL in small groups of individuals, from which around one-third were found to be monoclonal. 8-10 Because of the low number of cases reported and the design of these studies, analysis of the incidence and the clinical and laboratory features of patients displaying a monoclonal expansion of TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL is still lacking. In the present article we report for the first time on a large series of individuals with monoclonal expansions of TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL. From them, 17 were consecutive unselected individuals referred to a central laboratory covering immunophenotypic and molecular diagnosis of a region with 2.2 million inhabitants during a period of nearly 5 years, confirming the suspected low incidence of this condition. By pooling these cases with another 17 cases referred from other different institutions, a complete characterization of this condition was achieved.
Analysis of the clinical and hematological data established some clear differences between TCRαβ+/CD4+/NKa+/CD8−/+dim and CD4−/CD8+ T-LGL clonal proliferations. Indeed, our results indicate that clonal expansions of TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL do not associate with either neutropenia or splenomegaly. In addition, anemia was not detected in any case, a considerable number of patients even presenting with increased hemoglobin levels. Moreover, there was no clinical evidence of arthritis and rheumatoid factor was negative in all cases, whereas frequently positive in individuals having CD4−/CD8+ T-LGL disorders. 4,5,40 Another remarkable clinical feature of clonal expansions of CD4+ T-LGL is the high incidence of associated malignant diseases. Association of TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL proliferations and other tumors has been sporadically reported. 27,41 However, to the best of our knowledge, until now no other series has provided enough clinical data to speculate about a preferential association between clonal TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL proliferations and other neoplasias. Our results not only provide evidence for this association, but they also show that, in some cases, the associated tumor may clinically develop months, or even years, after the diagnosis of the monoclonal T-cell proliferation. The high frequency of second malignancies would support the relevance of performing a wide cancer survey and a close follow-up of patients with clonal expansions of TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL, even when there is no evidence of an associated disease at diagnosis, and PB lymphocyte counts are stable. According to the present data, apart from lymphocytosis, neither clinical manifestations nor abnormal levels of laboratory parameters were found unless the patient had a concomitant disease. Thus, in these individuals the presence of clinical symptoms, an abnormal physical examination or high levels of lactate dehydrogenase and β2-microglobulin should be considered as an alert for the possibility of an associated neoplasia.
The exact mechanism that could explain the relationship between the TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL lymphocytosis to the associated tumor is not clear. Previous reports have shown that human TCRαβ+/CD4+/NKa+/CD8−/+dim T cells may exhibit cytotoxic activity; 42,43 in a similar way, murine NK1.1+ T cells have been shown to exhibit potent cytotoxicity against B-cell lineage leukemia and lymphoma cells and inhibit tumor growth. 17-19 In the present study, monoclonal TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL showed a remarkably uniform cytotoxic T-cell phenotype, as reflected by a common pattern of expression of NKa markers and killer receptors—CD56+, CD57+, cyt granzyme B+, CD11b−/+dim, CD11c−, CD16−, CD94−, CD158a−, CD161−, NKB1−. At the same time, they displayed an activated T-cell phenotype, similar to that observed in other T-cell activation-associated conditions, 44 consisting of increased levels of CD2, CD11a, and HLA-DR, co-expression of CD8, decreased expression of CD7, and absence of expression of CD28 and CD62L. However, based on previous reports showing that during acute T-cell activation the percentage of normal PB CD4+/CD45RA+/CD45RO+ cells decreases, 44 our results suggest that clonal TCRαβ+/CD4+/NKa+/CD8−/+dim T cells do not show terminal maturation toward an effector cell, because they co-express CD45RA and CD45RO in half of cases. Based on these observations, it could be hypothesized that activated TCRαβ+/CD4+/NKa+/CD8−/+dim T-LGL may proliferate and expand as an effort of the immune system to control tumor growth. This hypothesis is also supported by our findings that these cells actively produce Th1 cytokines, consisting mainly of production of high levels of interferon-γ, which has been considered more effective than Th2 cytokines or priming and supporting or anti-tumor cytotoxic T-cell response, 45 and show a preferential use of specific TCR-Vβ families. In any case, further studies are necessary to demonstrate or rule out such hypotheses.
In conclusion, our results show that a proportion of T-LGL may correspond to monoclonal expansions of cytotoxic/Th1 CD4+ T cells; despite their indolent clinical behavior, clear clinical differences exist between these CD4+ T-LGL and the classical CD8+ T-LGL, especially as regards the absence of neutropenia, anemia, and splenomegaly, and the higher incidence of an associated neoplasia in the former group.
Acknowledgments
We thank our colleagues to whom we are indebted and grateful for sending us the patients: Cristina Gonçalves, Jorge Coutinho, Lucília Marques, Luciana Pinho, and Pinto Ribeiro (Hospital Santo António, Porto, Portugal); Lurdes Rodrigues (Hospital Pedro Hispano, Matosinhos, Portugal); António Parreira and Paulo Lúcio (Instituto Português de Oncologia, Lisboa, Portugal); Maria Jorge Arroz (Hospital Egas Moniz, Lisboa, Portugal), Cristina Menezes (Centro Hospitalar de Coimbra, Coimbra, Portugal); Elisa Granjo and Jorge Candeias (Hospital São João, Porto, Portugal); Mercedes Romero (Hospital Rio Hortega, Valladolid, Spain); Isabel Rodriguez-Salazar (Hospital General Yague de Burgos, Burgos, Spain); Juana Merino (Hospital Virgen del Camino, Pamplona, Spain); Josefina Galende (Hospital del Bierzo, Ponferrada, Spain); Jose Antonio Queizan (Hospital General de Segovia, Segovia, Spain); Marcos Barbón (Hospital de Leon, Leon, Spain); Alejandro Martín (Hospital Virgen de la Concha, Zamora, Spain); Pilar Mayayo (Hospital Miguel Servet, Zaragoza); and Inmaculada Pérez-Fernández (Hospital Virgen de la Victoria, Málaga, Spain); and Beckman/Coulter for providing part of anti-TCR-Vβ reagents used in this study.
Footnotes
Address reprint requests to Julia Almeida M.D. Ph.D., Service of Cytometry, Cancer Research Center (Lab 11) (University of Salamanca-CSIC), Avda Universidad de Coimbra s/n. 37007 Salamanca, Spain. E-mail: jalmeida@usal.es.
Supported by the following grants: Comissão de Fomento da Investigação em Cuidados de Saúde, Ministério da Saúde, Portugal PI 51/99; Acção Integrada Luso-Espanhola E31/99, Conselho de Reitores das Universidades Portuguesas, Ministério da Educação, Lisboa, Portugal; Acción Integrada Hispano-Lusa HP1998-0091, Dirección General de Enseñanza Superior e Investigación Científica, Ministerio de Educación y Cultura, Madrid, Spain, FIS 99/1240; Ministerio de Sanidad y Consumo, Madrid, Spain; FIS 02/1244; Ministerio de Sanidad y Consumo, Madrid, Spain, and SA103/03, Consejería de Educación y Cultura, Junta de Castilla y León, Valladolid, Spain; and the University of Salamanca (grant Reg. N. 430 to P. B.).
References
- 1.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC: A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994, 84:1361-1392 [PubMed] [Google Scholar]
- 2.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD: World Health Organization Classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee Meeting-Airlie House, Virginia, November 1997. J Clin Oncol 1999, 17:3835-3849 [DOI] [PubMed] [Google Scholar]
- 3.Lamy T, Loughran TP: Current concepts: large granular lymphocyte leukemia. Blood Rev 1999, 13:230-240 [DOI] [PubMed] [Google Scholar]
- 4.Loughran T: Clonal diseases of large granular lymphocytes. Blood 1993, 82:1-14 [PubMed] [Google Scholar]
- 5.Bigouret V, Hoffmann T, Arlettaz L, Villard J, Colonna M, Ticheli A, Gratwohl A, Samii K, Chapuis B, Rufer N, Roosnek E: Monoclonal T-cell expansions in asymptomatic individuals and in patients with large granular leukemia consist of cytotoxic effector T cells expressing the activating CD94-NKG2C/E and NKD2D killer cell receptor. Blood 2003, 101:3198-3204 [DOI] [PubMed] [Google Scholar]
- 6.Melenhorst JJ, Sorbara L, Kirby M, Hensel NF, Barrett AJ: Large granular lymphocyte leukemia is characterized by a clonal T-cell receptor rearrangement in both memory and effector CD8+ lymphocyte populations. Br J Haematol 2001, 112:189-194 [DOI] [PubMed] [Google Scholar]
- 7.Richards SJ, Sivakumaran M, Parapia LA, Balfour I, Norfolk DR, Kaeda J, Scott CS: A distinct large granular lymphocytic (LGL)/NK-associated (NKa) abnormality characterized by membrane CD4 and CD8 coexpression. Br J Haematol 1992, 82:494-501 [DOI] [PubMed] [Google Scholar]
- 8.de Totero D, Tazzari PL, DiSanto JP, di Celle PF, Raspadori D, Conte R, Gobbi M, Ferrara GB, Flomenberg N, Lauria F: Heterogeneous immunophenotype of large granular lymphocyte expansions: differential expression of the CD8α and CD8β chains. Blood 1992, 80:1765-1773 [PubMed] [Google Scholar]
- 9.Sala P, Tonutti E, Feruglio C, Florian F, Colombatti A: Persistent expansions of CD4+CD8+ peripheral blood T-cells. Blood 1993, 82:1546-1552 [PubMed] [Google Scholar]
- 10.Tonutti E, Sala P, Feruglio C, Yin Z, Colombatti A: Phenotypic heterogeneity of persistent expansions of CD4+ CD8+ T cells. Clin Immunol Immunopathol 1994, 73:312-320 [DOI] [PubMed] [Google Scholar]
- 11.Vicari AP, Zlotnik A: Mouse NK1.1+ T cells: a new family of T cells. Immunol Today 1996, 17:71-76 [DOI] [PubMed] [Google Scholar]
- 12.Blue ML, Daley JF, Levine H, Schlossman SF: Coexpression of T4 and T8 on peripheral blood T cells demonstrated by two-color fluorescence flow cytometry. J Immunol 1985, 134:2281-2286 [PubMed] [Google Scholar]
- 13.Ortolani C, Forti E, Radin E, Cibin R, Cossarizza A: Cytofluorimetric identification of two populations of double positive (CD4+, CD8+) T lymphocytes in human peripheral blood. Biochem Biophys Res Commun 1993, 191:601-609 [DOI] [PubMed] [Google Scholar]
- 14.Zuckermann FA: Extrathymic CD4/CD8 double positive T cells. Vet Immunol Immunopathol 1999, 72:55-66 [DOI] [PubMed] [Google Scholar]
- 15.Paliard X, Malefijt RW, de Vries JE, Spitz H: Interleukin-4 mediates CD8 induction on human CD4+ T-cell clones. Nature 1988, 335:642-644 [DOI] [PubMed] [Google Scholar]
- 16.Rabinowitz R, Hadar R, Schlesinger M: The appearance of the CD4+CD8+ phenotype on activated T cells: possible role of antigen transfer. Hum Immunol 1995, 55:1-10 [DOI] [PubMed] [Google Scholar]
- 17.Sugie T, Kubota H, Sato M, Nakamura E, Imamura M, Minato N: NK 1+ CD4− CD8− alpha-beta T cells in the peritoneal cavity: specific T cell receptor-mediated cytotoxicity and selective IFN-gamma production against B cell leukemia and myeloma cells. J Immunol 1996, 157:3925-3935 [PubMed] [Google Scholar]
- 18.Emoto M, Emoto Y, Kaufmann SH: TCR-mediated target cell lysis by CD4+NK1+ liver T lymphocytes. Int Immunol 1997, 9:563-571 [DOI] [PubMed] [Google Scholar]
- 19.Tamada K, Harada M, Abe K, Li T, Tada H, Onoe Y, Nomoto K: Immunosuppressive activity of cloned natural killer (NK1.1+) T cells established from murine tumor-infiltrating lymphocytes. J Immunol 1997, 158:4846-4854 [PubMed] [Google Scholar]
- 20.Legendre CM, Forbes RD, Loertscher R, Guttmann RD, Legendre CM: CD4+/Leu-7+ large granular lymphocytes in long-term renal allograft recipients. A subset of atypical T cells. Transplantation 1989, 47:964-971 [DOI] [PubMed] [Google Scholar]
- 21.Gold JE, Louis-Charles A, Ghali V, Babu A, Little JR, Athan E, Knowles DM, Zalusky R: T cell chronic lymphocytic leukemia. Unusual morphologic, phenotypic and karyotypic features in association with light chain amyloidosis. Cancer 1992, 70:86-93 [DOI] [PubMed] [Google Scholar]
- 22.Legac E, Autran B, Merle-Beral H, Katlama C, Debre P: CD4+CD7−CD57+ T cells: a new T-lymphocyte subset expanded during human immunodeficiency virus infection. Blood 1992, 79:1746-1753 [PubMed] [Google Scholar]
- 23.Bachetoni A, Lionetti P, Cinti P, Alo P, Molajoni ER, Di Tondo U, Barnaba V, Alfani D, Cortesini R: Homing of CD4+CD56+ T lymphocytes into kidney allografts during tubular necrosis or rejection. Clin Transplant 1995, 9:433-437 [PubMed] [Google Scholar]
- 24.Van den Hove LE, Van Gool SW, Vandenberghe P, Boogaerts MA, Ceuppens JL: CD57+/CD28− T cells in untreated hemato-oncological patients are expanded and display a Th1-type cytokine secretion profile, ex vivo cytolytic activity and enhanced tendency to apoptosis. Leukemia 1998, 12:1573-1582 [DOI] [PubMed] [Google Scholar]
- 25.Mizutani H, Katagiri S, Uejima K, Ohnishi M, Tamaki T, Kanayama Y, Tsubakio T, Kurata Y, Yonezawa T, Tarui S: T-cell abnormalities in patients with idiopathic thrombocytopenic purpura: the presence of OKT4+8+ cells. Scand J Haematol 1985, 35:233-239 [DOI] [PubMed] [Google Scholar]
- 26.Prince HE, Golding J, York J: Characterization of circulating CD4+ CD8+ lymphocytes in healthy individuals prompted by identification of a blood donor with a markedly elevated level of CD4+ CD8+ lymphocytes. Clin Diagn Lab Immunol 1994, 1:597-605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Airo P, Rossi G, Facchetti F, Marocolo D, Garza L, Lanfranchi A, Prati E, Brugnoni D, Malacarne F, Cattaneo R: Monoclonal expansion of large granular lymphocytes with a CD4+ CD8+dim+/− phenotype associated with hairy-cell leukemia. Haematologica 1995, 80:146-149 [PubMed] [Google Scholar]
- 28.Van den Hove LE, Van Gool SW, Van Poppel H, Baert L, Coorevits L, Van Damme B, Dal Cin P, Van den Berghe H, Ceuppens JL: Identification of an enriched CD4+ CD8alpha++ CD8beta+ T-cell subset among tumor-infiltrating lymphocytes in human renal cell carcinoma. Int J Cancer 1997, 71:178-182 [DOI] [PubMed] [Google Scholar]
- 29.Bagot M, Echchakir H, Mami-Chouaib F, Delfau-Larue MH, Charue D, Bernheim A, Chouaib S, Boumsell L, Bensussan A: Isolation of tumor-specific cytotoxic CD4+ and CD4+CD8dim+ T-cell clones infiltrating a cutaneous T-cell lymphoma. Blood 1998, 91:4331-4341 [PubMed] [Google Scholar]
- 30.Hirao J, Sugita K: Circulating CD4+CD8+ T lymphocytes in patients with Kawasaki disease. Clin Exp Immunol 1998, 111:397-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohteki T, MacDonald HR: Stringent V beta requirement for the development of NK1.1+ T cell receptor-alpha/beta+ cells in mouse liver. J Exp Med 1996, 183:1277-1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imberti L, Sottini A, Signorini S, Gorla R, Primi D: Oligoclonal CD4+ CD57+ T-cell expansions contribute to the imbalanced T-cell receptor repertoire of rheumatoid arthritis patients. Blood 1997, 89:2822-2832 [PubMed] [Google Scholar]
- 33.Serrano D, Monteiro J, Allen SL, Kolitz J, Schulman P, Lichtman SM, Buchbinder A, Vinciguerra VP, Chiorazzi N, Gregersen PK: Clonal expansion within the CD4+CD57+ and CD8+CD57+ T cell subsets in chronic lymphocytic leukemia. J Immunol 1997, 158:1482-1489 [PubMed] [Google Scholar]
- 34.Weiss L, Roux A, Garcia S, Demouchy C, Haeffner-Cavaillon N, Kazatchkine MD, Gougeon ML: Persistent expansion, in a human immunodeficiency virus-infected person, of V beta-restricted CD4+CD8+ T lymphocytes that express cytotoxicity-associated molecules and are committed to produce interferon-gamma and tumor necrosis factor-alpha. J Infect Dis 1998, 178:1158-1562 [DOI] [PubMed] [Google Scholar]
- 35.Currier JR, Stevenson KS, Kehn PJ, Zheng K, Kirch VM, Robinson MA: Contributions of CD4+, CD8+, and CD4+CD8+ T cells to skewing within the peripheral T cell receptor beta chain repertoire of healthy macaques. Hum Immunol 1999, 60:209-222 [DOI] [PubMed] [Google Scholar]
- 36.Almeida J, Bueno C, Alguero MC, Sanchez ML, Canizo MC, Fernandez ME, Vaquero JM, Laso FJ, Escribano L, San Miguel JF, Orfao A: Extensive characterization of the immunophenotype and the pattern of cytokine production by distinct subpopulations of normal human peripheral blood dendritic cells. Clin Exp Immunol 1999, 118:392-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lima M, Almeida J, Santos AH, dos Anjos Teixeira M, Alguero MC, Queiros ML, Balanzategui A, Justica B, Gonzalez M, San Miguel JF, Orfao A: Immunophenotypic analysis of the TCR-Vβ repertoire in 98 persistent expansions of CD3+/TCRαβ+ large granular lymphocytes: utility in assessing clonality and insights into the pathogenesis of the disease. Am J Pathol 2001, 159:1861-1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Sanz R, Vargas Montero M, Gonzalez Diaz M, del Carmen M, Santos C, Balanzategui Echevarria A, Flores Corral T, Hernandez Martin JM, Caballero Barrigon MD, San Miguel JF: Action of single and associated lesions on the Bcl-l, Bcl-2, Bcl-6, c-Myc, p53, and p16 genes in B-cell non Hodgkin’s lymphomas. Value of molecular analysis for a better assignment of the histologic subtype. Haematologica 1998, 83:209-216 [PubMed] [Google Scholar]
- 39.Van Dongen JJ, Wolvers-Tettero IL: Analysis of immunoglobulin and T cell receptor genes. Part II: possibilities and limitations in the diagnosis and management of lymphoproliferative diseases and related disorders. Clin Chim Acta 1991, 198:93-174 [DOI] [PubMed] [Google Scholar]
- 40.Semenzato G, Zambello R, Starkebaum G, Oshimi K, Loughran TP, Jr: The lymphoproliferative disease of granular lymphocytes: updated criteria for diagnosis. Blood 1997, 89:256-260 [PubMed] [Google Scholar]
- 41.Claudepierre P, Bergamasco P, Delfau MH, Voisin MC, Farcet JP, Larget-Pied B, Chevalier X: Unusual CD3+ CD4+ large granular lymphocyte expansion associated with a solid tumor. J Rheumatol 1998, 25:1434-1436 [PubMed] [Google Scholar]
- 42.Yasukawa M, Utsunomiya Y, Inoue Y, Kimura N, Fujita S: Monoclonal proliferation of CD4+ large granular lymphocytes with cytolytic activity. Br J Haematol 1995, 91:419-420 [DOI] [PubMed] [Google Scholar]
- 43.Van den Hove LE, Van Gool SW, Van Poppel H, Baert L, Coorevits L, Van Damme B, Ceuppens JL: Phenotype, cytokine production and cytolytic capacity of fresh (uncultured) tumour-infiltrating T lymphocytes in human renal cell carcinoma. Clin Exp Immunol 1997, 109:501-509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lima M, Teixeira MA, Queiros ML, Santos AH, Balanzategui A, Fonseca S, Goncanves C, Correia J, Farinha F, Mendonca F, Soares JMN, Almeida J, Orfao A, Justica B: Immunophenotype and TCR-Vbeta repertoire of peripheral blood T-cells in acute infectious mononucleosis. Blood Cells Mol Dis 2003, 30:1-12 [DOI] [PubMed] [Google Scholar]
- 45.Tagawa M: Cytokine therapy for cancer. Curr Pharm Des 2000, 6:681-699 [DOI] [PubMed] [Google Scholar]