Abstract
Glomerular endothelial injury plays an important role in the pathogenesis of renal diseases and is centrally involved in renal disease progression. Glomerular endothelial repair may help maintain renal function. We examined whether bone-marrow (BM)-derived cells contribute to glomerular repair. A rat allogenic BM transplant model was used to allow tracing of BM-derived cells using a donor major histocompatibility complex class-I specific mAb. In glomeruli of chimeric rats we identified a small number of donor-BM-derived endothelial and mesangial cells, which increased in a time-dependent manner. Induction of anti-Thy-1.1-glomerulonephritis (transient mesangial and secondary glomerular endothelial injury) caused a significant, more than fourfold increase in the number of BM-derived glomerular endothelial cells at day 7 after anti-Thy-1.1 injection compared to chimeric rats without glomerular injury. The level of BM-derived endothelial cells remained high at day 28. We also observed a more than sevenfold increase in the number of BM-derived mesangial cells at day 28. BM-derived endothelial and mesangial cells were fully integrated in the glomerular structure. Our data show that BM-derived cells participate in glomerular endothelial and mesangial cell turnover and contribute to microvascular repair. These findings provide novel insights into the pathogenesis of renal disease and suggest a potential role for stem cell therapy.
Glomerular endothelial injury is an early event in various renal diseases, including glomerulonephritides and vasculitides, thrombotic microangiopathies and renal transplant rejection, and is viewed as a crucial factor in the progression of renal disease, regardless of the initial cause. 1,2 Interestingly, under certain circumstances, spontaneous recovery from glomerular disease may occur. 3,4 In experimental reversible-glomerular-injury models capillary repair was observed, characterized by endothelial cell proliferation, enhanced expression of angiogenic factors and morphological changes consistent with angiogenesis. 5 These data suggest that progressive renal failure may involve not only loss of glomerular cells but also a defective repair response. Indeed, impaired glomerular capillary repair was found to be associated with the development of glomerulosclerosis and renal failure. 6,7 Consistently, progressive renal disease is associated with reduced expression of angiogenic growth factors and enhanced expression of antiangiogenic factors. 8-10 Moreover, administration of the proangiogenic growth factor vascular endothelial growth factor (VEGF) has recently been shown to enhance glomerular capillary repair and accelerate renal recovery or prevent progression of renal disease in several experimental models. 11,12 Insight into the “healing” process of the glomerular microvasculature may enhance our understanding of the pathophysiology of progressive renal failure and provide novel approaches for treatment of renal disease.
During embryonic life, glomerular microvascular development involves not only angiogenic processes such as migration and proliferation of resident glomerular endothelial cells, but also vasculogenesis, ie, de novo assembly of endothelial progenitor cells into vessels, which is followed by recruitment of pericyte-like mesangial cells. 13,14 Recently, several investigators have established the presence of bone-marrow-derived endothelial progenitor cells in the adult circulation 15,16 and demonstrated homing of these cells to sites of neovascularization and differentiation into endothelial cells in situ in experimental animal models for hindlimb ischemia, myocardial infarction, or tumor growth, 17 consistent with “adult vasculogenesis”.
We hypothesized that even in the highly specialized adult glomerular microvasculature, repair may not only involve migration and proliferation of resident cells but also, analogous to embryonic renal development, recruitment and homing of vascular progenitors from the bone marrow. To test our hypothesis we investigated the origin of glomerular cells after induction of reversible nephritis in a rat allogenic bone-marrow-transplant model.
Materials and Methods
Animals
Male 11-week-old WAG/RijHsd (RT-1Au) (WR) and Brown Norway/RijHsd (RT-1An) (BN) rats, weighing 200 to 250 g, were purchased from Harlan (Horst, The Netherlands). The animals were kept in filter-top cages and received sterilized food and acidified water ad libitum. All protocols were approved by the ethics committee of our institution.
Bone Marrow Transplantation
Allogenic bone marrow transplantation (BMT) was performed in BN rats with WR rats as bone marrow (BM) donors to generate rat BM chimeras (WRBM→BN rats), as described previously. 18 In short, donor BM cells were obtained by flushing the cavity of WR rat tibias and femurs with RPMI 1640 (Invitrogen, Carlsbad, CA). A single-cell suspension was obtained by filtering the BM through a cell strainer (100-μm filter, Falcon; Becton Dickinson Labware, Franklin Lakes, NJ). Recipient BN underwent whole-body γ irradiation with 720 cGy, administered by linear accelerator, to ablate BM. Approximately 5 hours after irradiation they received 5 × 107 donor BM cells intravenously.
BM chimerism was evaluated in peripheral blood samples by flow cytometry. The membrane expression of WR-specific and BN-specific major histocompatibility complex class-I (MHC-I) haplotype on peripheral blood mononuclear cells was measured by direct and indirect immunofluorescence on a Calibur flow cytometer (BD Pharmingen, San Diego, CA). The following primary antibodies were used: fluorescein isothiocyanate (FITC)-labeled anti-BN-MHC-I (CL027F; Cedarlane, Hornby, Ontario, Canada), anti-WR-MHC-I (U9F4 19 ; kindly provided by J. Rozing, Groningen, the Netherlands), and secondary antibody goat anti-mouse-phycoerythrin (PE) (Southern Biotechnologies, Birmingham, AL).
Experimental Design
To investigate the involvement of BM-derived cells in glomerular repair the reversible mesangiolytic anti-Thy-1.1 glomerulonephritis model was used. Anti-Thy-1.1 nephritis is a well-known model to study transient immune-mediated mesangial and secondary glomerular endothelial injury, 20 most often described in Wistar rats. Because several authors have reported strain differences in response to anti-Thy-1.1, 21,22 we first performed a series of experiments to determine optimal dose of anti-Thy-1.1 and course of anti-Thy-1.1-induced glomerulonephritis in our WRBM→BN rats. We found that in our rats the optimal dose of anti-Thy-1.1 mAb was 1 mg/kg body weight, which is similar to the dose most often used in Wistar rats. Development of proteinuria was slower in our rats as compared to Wistar rats and did not reach similar levels. Based on the pilot data we chose day 7 as time-point for early glomerular injury. Glomerular sections obtained at this time-point showed mesangiolysis, capillary ballooning, influx of monocytes/macrophages and microaneurysm formation, similar to the glomerular injury reported in Wistar rats.
Five weeks after BMT, experimental mesangioproliferative glomerulonephritis was induced in 10 WRBM→BN rats by a single intravenous injection of the monoclonal antibody (mAb) anti-rat Thy-1.1 (ER4, mouse IgG2a, 1 mg per kg body weight) (t = day 0). Groups of five rats each were sacrificed at day 7 and at day 28 after anti-Thy-1.1 injection. Five chimeric rats served as controls and were sacrificed at day 28. Additionally, 3 control WRBM→BN rats were sacrificed 6 months after BMT to study the presence of BM-derived cells in the kidney over time.
At the end of the experimental protocol rats were anesthetized with sodium pentobarbital (intraperitoneal injection of 60 mg/kg) and sacrificed after perfusion and excision of the kidneys. Blood was collected from the vena cava for determination of plasma creatinine. The kidneys were perfused in situ at 120 mmHg with 4°C PBS for 3 minutes to remove circulating cells from the renal vasculature. The kidneys were processed for routine histology, immunohistochemistry, and immunofluorescence double-staining. Kidney specimens were cut transversely into 3 slices. Two parts were embedded in Tissue-Tek ornithine carbamyl transferase compound (Sakura Finetek Europe BV, Zoeterwoude, The Netherlands) and snap-frozen in liquid nitrogen. One slice was fixed in 4% buffered formalin and embedded in paraffin for morphological studies.
Renal Function
Urine was collected for determination of urinary protein and creatinine excretion twice weekly between day 0 and day 28 and in all rats before sacrifice. Rats were weighed and placed in metabolic cages, with free access to food and water. Twenty-four-hour urinary protein-loss was determined by Bio-Rad Protein Assay (Bio-Rad Laboratories GmbH, München, Germany). Plasma and urinary creatinine levels were determined colorimetrically (Sigma Diagnostics Inc., St. Louis, MO). Creatinine clearance, calculated by standard formula, was used as an estimate of glomerular filtration rate.
Renal Morphology
Renal morphology was evaluated in periodic acid-Schiff (PAS)-stained sections (3 μm) by normal light microscopy (Leitz Laborlux 12; Ernst Leitz GmbH, Wetzlar, Germany). Glomerular injury was determined by assessing the percentage of glomerular sections containing one or more microaneurysms. For each kidney, cross-sections of 100 glomeruli were examined by normal light microscopy, using 250-fold magnification. Glomerular cross-sections containing a minor portion of the glomerular tuft were excluded from analysis. All slides were evaluated by a blinded observer.
Immunoperoxidase Staining
Donor-derived (WR) cells were detected by immunoperoxidase in 5-μm cryostat sections of WRBM→BN kidneys, with a specific mAb (U9F4) against MHC-I expressed on WR but not BN cells. WR specificity was confirmed in control WR and BN kidney sections. Cryostat sections were fixed in acetone for 10 minutes and air-dried at room temperature. Endogenous peroxidase activity was blocked with 5% hydrogen peroxide. Sections were incubated with 100 μl U9F4, diluted in PBS (1:10), for 1 hour, followed by incubation with 100 μl PowerVision goat anti-mouse poly-horseradish peroxidase-conjugates (ImmunoVision Technologies, Springdale, AZ) for 30 minutes at room temperature. To prevent non-specific binding to rat immunoglobulins, 3% normal rat serum was added. Diaminobenzidine (DAB; σ-Aldrich, Zwijndrecht, The Netherlands) was added as coloring substrate. Sections were counterstained with hematoxylin and mounted with pertex. Negative controls included substitution of the primary antibody with PBS or with an irrelevant murine IgG of the same subclass. Sections were analyzed by light microscopy.
Immunofluorescence Double-Staining
To identify the cell type of BM-derived glomerular cells, immunofluorescent double-staining was performed on 5-μm cryostat sections. The following primary antibodies were used in combination with U9F4 to identify, respectively, donor-derived endothelial cells, mesangial cells, and monocytes or macrophages: RECA-1, a murine IgG1 mAb against a surface antigen presented on all rat endothelial cells 23 (Serotec Ltd., Oxford, England); OX-7, a mesangial-cell-specific murine IgG1 mAb (Serotec Ltd.); ED-1, a murine IgG1 mAb to a cytoplasmic antigen present in monocytes and macrophages (kindly provided by Ed Dub, Department of Cell Biology, Free University, Amsterdam, the Netherlands). We used a biotin-streptavidin detection system to prevent cross-reactivity of the secondary antibodies with the anti-rat Thy1.1 mAb ER4. Primary antibodies U9F4, RECA-1, and ED-1 were biotinylated using the biotinylation reagent in the DAKO Animal Research Kit for mouse primary antibodies (DAKO, Glostrup, Denmark). OX-7 was biotinylated using Biotin (Long Arm) NHS, water soluble (NHSws; Vector Laboratories, Burlingame, CA).
After blocking of endogenous biotin activity (biotin blocking kit, Vector Laboratories), acetone-fixed sections were incubated with 100 μl of the first biotinylated primary antibody (ED-1 or U9F4) for 60 minutes, followed by incubation with 100 μl FITC-conjugated streptavidin (1:100; fluorescent streptavidin kit, Vector Laboratories) for 30 minutes at room temperature. To augment the signal, the tissue sections were subsequently incubated with 100 μl anti-streptavidin-FITC (1:100; Vector Laboratories) for 30 minutes. Remaining biotin binding sites were blocked (biotin blocking kit, Vector Laboratories) and sections were incubated with 100 μl of the second biotinylated primary antibody (U9F4, OX-7, or RECA-1) for 60 minutes followed by incubation with 100 μl tetramethylrhodamine isothiocynate (TRITC)-conjugated streptavidin (1:100; fluorescent streptavidin kit; Vector Laboratories) for 30 minutes at room temperature. Sections were air-dried and mounted with Vectashield (Vector Laboratories). To exclude the possibility that secondary antibodies were picking up the wrong primary, controls were included in which the procedure was performed with substitution of the first primary antibody with a different biotinylated control antibody or with substitution of the second primary antibody with a different biotinylated control antibody.
To further characterize the BM-donor-derived cells in the tubulo-interstitium we performed an additional immunofluorescent double-staining with U9F4 and polyclonal mouse anti-desmin (specific for myofibroblasts, activated mesangium, and podocytes) (Biogenex, Duiven, the Netherlands). Acetone-fixed cryostat sections were incubated with polyclonal mouse anti-desmin for 60 minutes at room temperature, followed sequentially by FITC-conjugated goat-anti-mouse Igs (1:200), and FITC-conjugated rabbit-anti-goat Igs (1:40) in PBS containing 10% normal rat serum. Endogenous biotin was blocked followed by incubation with biotinylated U9F4 and TRITC-conjugated streptavidin as described above.
Sections were numbered and evaluated by two blinded investigators. To assess the number of different cell types in the glomeruli, the amount of cells in 20 glomeruli was averaged. Cells were counted using a Leica DMR microscope (Leica GmbH, Wetzlar, Germany) at a 630-fold magnification. Cells were identified as BM-derived endothelial cells, mesangial cells, or monocytes/macrophages based on immunofluorescent double-staining with U9F4/RECA-1, U9F4/OX-7, or U9F4/ED-1, respectively, as well as morphological characteristics. For more detailed analysis confocal laser-scanning microscopy was performed (Leica DM IRB/TCS NT; Leica GmbH).
Statistics
All values are expressed as mean ± SD. Statistical significance (defined as P < 0.05) was evaluated using Student’s t-test or analysis of variance where appropriate.
Results
WagRij Bone-Marrow Cells Reconstitute Lethally Irradiated Brown Norway Rat Bone Marrow
Flow cytometry of peripheral blood samples showed that at 4 weeks after BMT the BM of lethally irradiated recipient BN rats was reconstituted with WR BM cells (>75%) (Table 1) ▶ . BM chimerism showed a gradual increase over the study period to >90% at 6 months after BMT.
Table 1.
Strain-Specific MHC-I Expression on Peripheral Blood Leukocytes of WR, BN, and WRBM → BN Rats
| Control WR | Control BN | WRBM → BN | |
|---|---|---|---|
| U9F4 | 99.7% | 0.0% | 79.0 ± 6.0% |
| OX-27 | 0.3% | 100.0% | 21.0 ± 6.0% |
The percentages of U9F4 (WR MHC-I-specific)-positive and OX-27 (BN MHC-I-specific)-positive peripheral blood leukocytes in control WR and BN rats and WRBM → BN rats at 4 weeks after BMT.
WRBM→BN Rats Display No Evidence of Radiation-Induced Glomerular Damage
Baseline glomerular morphology in 3-μm PAS-stained tissue sections was similar in WRBM→BN and BN rats (Figure 1,A and B) ▶ . PAS-stained glomerular sections remained unchanged in control WRBM→BN rats at 6 months after bone marrow transplantation (Figure 1C) ▶ . We also found no differences between control WRBM→BN and control BN in glomerular endothelial (RECA-1-positive), mesangial (OX-7-positive), or macrophage/monocyte (ED-1-positive) populations (data not shown). We did observe an increased tubulo-interstitial cell infiltrate in WRBM→BN compared to non-transplanted BN rats, which remained unchanged from 2 to 6 months after BMT. Baseline creatinine clearance (1.98 ± 0.15 ml/min) and 24-hour protein excretion (16 ± 5 mg/24 hours) in WRBM→BN rats were at levels similar to those observed in control BN rats (1.96 ± 0.36 ml/min and 13 ± 3 mg/24 hours, respectively; both P = not significant). Over the study period there were also no changes in creatinine clearance (1.98 ± 0.30 ml/min at 6 months after BMT; P = not significant compared to controls at baseline) and proteinuria (at 6 months: 12 ± 1 mg/24 hours; P = not significant compared to controls at baseline).
Figure 1.

Histological changes in anti-Thy-1.1 nephritis in WRBM→BN rats. A: Renal morphology in control BN rats. B: Renal morphology in control WRBM→BN rats. C: in WRBM→BN rats at 6 months after bone marrow transplantation no significant changes in renal morphology have occurred. D: At day 7 after injection of anti-Thy-1.1 there is marked loss of mesangial cells with dissolution of the mesangial matrix and formation of microaneurysms. E: At day 28 after anti-Thy-1.1 injection glomerular morphology has partially recovered. (PAS, ×400).
Injection with Anti-Thy-1 Antibody Causes Reversible Glomerular Endothelial and Mesangial Injury in WRBM→BN Rats
Injection of anti-Thy-1.1 in WRBM→BN rats caused acute mesangiolysis, capillary ballooning, and microaneurysm formation, followed by a (partial) resolution phase (Figure 1) ▶ . Glomerular morphological changes in response to anti-Thy-1.1 injection in WRBM→BN rats were very similar to those previously reported in Wistar rats. 20 At day 7 after anti-Thy-1.1 administration we observed significant increases in the percentage of glomerular sections containing microaneurysms and in the number of ED-1 positive monocytes and macrophages in glomeruli. On day 28, glomerular morphology had partially recovered with significant decreases in the number of microaneurysms and with reduction of the number of monocytes and macrophages (Table 2) ▶ . No obsolescent glomeruli were observed. Urinary protein-loss was less than previously reported in Wistar rats but showed a significant increase after anti-Thy-1.1 administration from 13 ± 4 (versus 16 ± 5 in controls) to a maximum of 110 ± 19 mg/24 hours (P < 0.05 versus controls), followed by a gradual decline to 72 ± 8 mg/24 hours at 28 days after anti-Thy-1.1 injection. There was a decrease in creatinine clearance at day 7 after induction of anti-Thy-1.1 nephritis (1.06 ± 0.31 versus 1.98 ± 0.15 ml/min in controls; P < 0.05), followed by partial recovery at day 28 (1.31 ± 0.14 ml/min).
Table 2.
Characteristics of the Anti-Thy-1.1 Nephritis Model in WRBM → BN Rats
| % of glomerular sections containing microaneurysms | Number of ED-1+ cells per glomerulus | |
|---|---|---|
| Controls | 1.0 ± 0.9% | 1.3 ± 0.3 |
| t = day 7 | 17.3 ± 3.1%* | 14.6 ± 2.8* |
| t = day 28 | 10.8 ± 1.3%† | 10.4 ± 2.2† |
*P < 0.05 as compared to control rats;
†P < 0.05 as compared to day 7.
Bone-Marrow-Derived Cells are Present in the Kidney of WRBM→BN Rats
Using both BN MHC-I specific U9F4-peroxidase as well as U9F4-FITC stains, we observed that all kidneys of WRBM→BN rats contained U9F4-positive donor-derived cells. In glomeruli of untreated chimeric rats at 2 months after BMT we found small numbers of donor-derived cells. In addition, significant amounts of U9F4-positive cells were present in the tubulo-interstitium (Figure 2) ▶ . Using immunofluorescent double-staining we demonstrated that glomeruli contained donor-BM-derived endothelial cells, mesangial cells, and monocytes and macrophages. The number of BM-derived endothelial and mesangial cells in glomeruli showed a time-dependent increase whereas there was no significant increase in the number of donor-derived ED-1-positive cells (Table 3) ▶ .
Figure 2.

A: Immunoperoxidase staining of 5-μm cryostat kidney sections of a control BN rat (no irradiation/no bone marrow transplantation/no anti-Thy-1.1 nephritis), negative staining with U9F4. B: Donor bone-marrow-derived U9F4-positive cells (brown) are present in both glomeruli (arrows) as well as tubulo-interstitium of control WRBM→BN rat kidney, 2 months after irradiation and bone marrow reconstitution (no anti-Thy-1.1 injection) (×200).
Table 3.
Bone-Marrow-Derived Cells in Glomeruli of Control WRBM → BN Rats Over Time
| U9F4/RECA-1 | U9F4/OX-7 | U9F4/ED-1 | |
|---|---|---|---|
| 2 months | 2.46 ± 0.73 | 1.80 ± 1.07 | 1.33 ± 0.31 |
| 6 months | 7.77 ± 3.17* | 6.88 ± 2.60* | 2.93 ± 0.92 |
*P < 0.05 as compared to 2 months after BMT.
The average number (± SD) of bone-marrow-derived endothelial (U9F4/RECA-1+) and mesangial (U9F4/OX-7+) cells and monocytes/macrophages (U9F4/ED-1+) per glomerular section in WRBM → BN rats at 2 and 6 months after BMT.
Further characterization of the U9F4-positive, donor-derived cells in the tubulo-interstitium by morphology, localization and double-staining with RECA-1, ED-1, or desmin revealed that there were low numbers of BM-derived endothelial cells and monocytes/macrophages. There were also some BM-derived myofibroblasts present in the tubulointerstitium. However, the main portion of BM-derived cells could not be characterized and may include leukocytes, antigen-presenting cells, and fibroblasts.
Administration of Anti-Thy-1.1 Causes a Significant Increase in the Number of Glomerular Bone-Marrow-Derived Cells
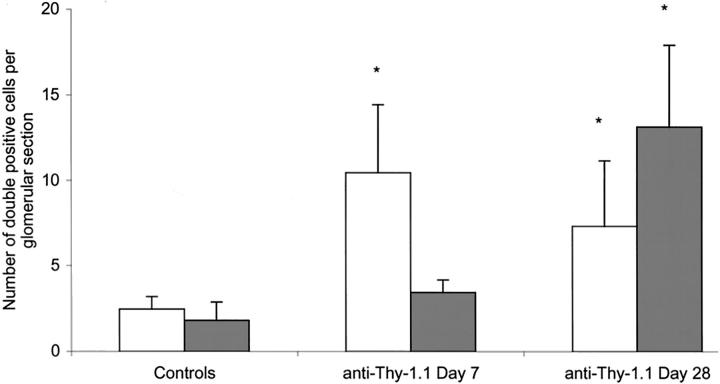
At 1 week after anti-Thy-1.1 injection extensive loss of mesangial cells was observed in most glomeruli. If mesangial cells were visible they occasionally formed small clusters located at the vascular pole of the glomerulus. Although the total number of BM-derived mesangial cells at 1 week after anti-Thy-1.1 injection was small, with no significant increase as compared to control rats, they were often seen in these small cell clusters (Figure 3) ▶ . At 4 weeks after induction of anti-Thy-1.1 nephritis near-normalization of the mesangium had occurred. At this time-point, a more than sevenfold increase in the number of donor-derived mesangial cells compared to control levels was observed (P < 0.05; Figure 4,D to F ▶ ; Figure 5 ▶ ). These U9F4-positive mesangial cells were randomly integrated in the normalized mesangial structure. The number of BM-derived endothelial cells showed a significant, more than fourfold increase at 1 week after injection of anti-Thy-1.1 (P < 0.05) and remained high at day 28 (Figure 4, A to C ▶ ; Figure 5 ▶ ). At both time-points these U9F4/RECA-1 double-positive cells were randomly distributed throughout the glomerulus. Anti-Thy-1.1 injection caused a significant increase in the number of monocytes/macrophages (see above). These ED-1-positive cells were almost all of donor origin (Figure 4, G to I) ▶ . All controls were negative, indicating that the biotin-blocking steps were complete and that no cross-reactivity had occurred (Figure 6,A to F) ▶ .
Figure 3.
Anti-Thy-1.1 injection causes extensive loss of mesangial cells followed by regeneration. Immunofluorescent double-staining at day 7 with U9F4 (green) and OX-7 (red) shows a few mesangial cells, which occasionally form small clusters located at the vascular pole of the glomerulus (arrow). BM-derived mesangial cells (yellow) are often located in these small cell clusters near the vascular pole. (×630).
Figure 4.
Immunofluorescent double-staining of glomerular sections from WRBM→BN rats at 28 days after induction of anti-Thy-1.1 nephritis. Confocal laser-scanning microscopy (×630, pinhole 2.5). A: Staining for BM donor (WR) MHC-I (U9F4, green). B: Staining for endothelial cells (RECA-1, red). C: U9F4/RECA-1 double-staining identifies bone-marrow-derived endothelial cells, randomly integrated in the glomerular endothelium (U9F4/ED-1 double-positive, yellow). D: Staining for BM donor (WR) MHC-I (U9F4, green). E: Staining for mesangial cells (OX-7, red). F: U9F4/OX-7 double-staining showing donor bone-marrow-derived mesangial cells (U9F4/OX-7 double-positive, yellow) integrated in the glomerulus. G: Staining for BM donor (WR) MHC-I (U9F4, red). H: Staining for monocytes/macrophages (ED-1, green). I: U9F4/ED-1 double-staining shows a BM donor-derived (U9F4/ED-1 double positive, yellow) monocyte/macrophage. ▵▵, U9F4/OX-7, U9F4/RECA-1, U9F4/ED-1 double-positive cells (yellow), ie, donor-derived mesangial and endothelial cells and a donor-derived monocyte/macrophage. ∧∧, single-U9F4 staining.
Figure 5.
The average number of BM-derived endothelial cells (RECA-1/U9F4 double-positive cells, white bars) and bone-marrow-derived mesangial cells (OX-7/U9F4 double-positive cells, shaded bars) per glomerular section in control WRBM→BN rats and in rats at 7 and 28 days after anti-Thy-1.1 injection. Values are expressed as mean ± SD per glomerular section. *, P < 0.05 versus controls.
Figure 6.
Controls of the immunofluorescent double-staining as shown in Figure 4 ▶ . All pictures were taken with the same settings as used for Figure 4 ▶ and were processed by the same procedure. Confocal laser-scanning microscopy (×630, pinhole 2.5). The following controls were included: substitution of the first primary antibody with a different biotinylated control antibody and normal second primary and substitution of the second primary antibody with a different biotinylated control antibody. A: ED-1+/U9F4 control. B: U9F4 control/RECA-1+. C: U9F4 control/OX-7+. D: ED-1 control/U9F4+. E: U9F4+/RECA-1 control. F: U9F4 control/RECA-1 control. A to F: All controls were negative, indicating that the biotin-blocking steps were complete and that no cross-reactivity occurred.
Discussion
Over the last decade it has become recognized that glomerular components, endothelial, mesangial, and epithelial cells, have a regenerative potential. It has also been demonstrated that bone marrow can give rise to mesangial as well as epithelial cells. 24-27 Here, we report for the first time that bone-marrow-derived cells may home to injured glomerular endothelium, differentiate into endothelial cells, and participate in regeneration of the highly specialized glomerular microvasculature. In addition, we confirm previous observations that bone-marrow-derived cells can replace injured mesangial cells. 24
To study the origin of glomerular cells we generated rat hematopoietic chimeras (WRBM→BN rats) by allogenic BMT of WR bone marrow into lethally irradiated BN recipients. At 4 weeks after BMT nearly 80% of peripheral blood leukocytes were donor-derived, an adequate level of chimerism to allow identification and quantification of donor bone-marrow-derived cells using donor WR MHC class-I haplotype-specific immunohistochemistry. In the glomeruli of control WRBM→BN rats we observed low levels of donor-derived endothelial and mesangial cells and a significant increase of these donor-derived cells over time, which could not be explained by an increase in hematopoietic chimerism. Previously, autoradiographic studies have demonstrated low rates of glomerular mesangial and endothelial cell proliferation in control rats, indicating low but constant rates of glomerular cell renewal of about 1% per day. 28 Our data suggest that bone-marrow-derived cells contribute to normal physiological glomerular-endothelial and mesangial cell turnover.
Experimental anti-Thy-1.1 glomerulonephritis is an established model to study mesangial and glomerular endothelial injury and repair in rats. Single injection with the complement-fixing antibody to Thy1 antigen present on the mesangial-cell membrane causes acute mesangial-cell loss and matrix dissolution, leading to ballooning of glomerular capillaries, formation of microaneurysms and loss of endothelial cells, followed by a repair phase that is characterized by mesangial and endothelial cell migration and proliferation, apoptosis, and matrix expansion. 5,20,29,30 In our WRBM→BN hematopoietic chimeras we demonstrated that anti-Thy1.1-induced glomerular injury was associated with a significant influx of bone-marrow-derived cells. Immunofluorescent double-staining with cell-specific markers revealed that these donor-derived cells included not only monocytes and macrophages, as expected, but also endothelial and mesangial cells, which were fully integrated into the glomerular structure and remained present after restoration of glomerular architecture had occurred. Our data indicate that glomerular repair cannot only be attributed to stimulation of migration and proliferation of resident mesangial and endothelial cells but also involves bone-marrow-derived cells.
The contribution of bone-marrow-derived endothelial cells to neovascularization and re-endothelialization has been addressed in various animal BMT models. Bone-marrow-derived cells have been shown to incorporate sparsely in the endothelium of “normal” blood vessels, reflecting a contribution to physiological endothelial cell turnover, significantly in neovasculature associated with hindlimb ischemia, myocardial infarction, corneal injury, retinal ischemia, cutaneous wounds, grafted aorta segments, and tumor growth. 17,31-33 Few studies have addressed the origin of the renal vasculature. In the past there have been sparse and conflicting reports on the presence of host endothelial cells in kidney allografts. 34-37 Recently, Lagaaij et al 38 showed that endothelial cells of human renal transplant recipients could replace those of the donor and that the extent of replacement of endothelial cells lining small renal vessels was related to the severity of vascular injury. They suggested that recipient cells may be recruited from circulating endothelial progenitor cells as a repair response. However, they did not comment on the origin of the glomerular endothelium and from their studies it is not possible to determine whether the replaced cells are bone-marrow-derived or involve mature endothelial cells. We recently identified male donor-derived endothelial cells in the renal vessels of a female patient who developed thrombotic microangiopathy after sex-mismatched bone marrow transplantation, confirming integration of bone-marrow-derived endothelial cells into human renal vasculature. 39 Our current data provide evidence that bone-marrow-derived endothelial cells participate in maintenance and repair of the glomerular endothelium.
In contrast to our observations, Ito et al 24 could not find any bone-marrow-derived glomerular endothelial cells in the anti-Thy-1.1 model. The reasons for the contrasting results are not clear but may include differences in severity of endothelial damage (strain variations in the response to anti-Thy-1.1 have been reported), or reduced expression of the enhanced green fluorescence protein (EGFP) cell marker in repopulating endothelial cells. In line with our results, Cornacchia et al 26 reported that glomerular endothelial cells can be derived from the bone marrow in mice.
In our experiments we observed a more than fourfold increase in the number of bone-marrow-derived endothelial cells at 7 days after induction of anti-Thy-1.1-nephritis as compared to controls, followed by a slight decrease over the next 3 weeks, suggesting a role for the bone-marrow-derived endothelial cells in glomerular repair followed by remodeling and apoptosis. The relative contribution of bone-marrow-derived endothelial cells to glomerular endothelial repair in comparison to local endothelial regeneration cannot be determined from our current data. The extensive endothelial cell loss and proliferation that has previously been reported in the anti-Thy-1.1 nephritis model, in addition to the focal distribution of bone-marrow-derived cells in the glomeruli, suggest that only part of the endothelial cells is bone-marrow-derived. This is consistent with previous findings in various animal neovascularization models. Interestingly, it was recently shown that bone-marrow-derived endothelial cells may not only be incorporated into foci of neovascularization but also secrete potent angiogenic ligands and cytokines. 40 Thus the presence of a relatively small number of cells may stimulate resident endothelial cells to migrate and proliferate. It is important to note that, irrespective of the proportional contribution of bone-marrow-derived cells in physiological glomerular repair, the observation that bone-marrow-derived cells have the capacity to, under certain circumstances, home to injured glomerular endothelium and differentiate into endothelial cells suggests potential new therapeutic strategies for renal disease, such as enhanced mobilization and biological modification of bone-marrow-derived endothelial progenitor cells.
Glomerular mesangial cells have been characterized as pericyte- or smooth-muscle-like cells that provide structural capillary support in the glomerulus. Previously, recovery of the mesangium after mesangial injury was thought to occur through proliferation of surviving resident intraglomerular-mesangial cells. Hugo et al 41 have demonstrated that repopulation of the mesangium in the anti-Thy-1.1 model involves migration of cells from the hilar area and from the extraglomerular mesangium in the juxtaglomerular apparatus. Furthermore, mature vascular endothelial cells may serve as a potential source of mesangial cells, consistent with endothelial-mesenchymal transdifferentiation observed in embryogenesis and in adult endothelium in vitro. 42,43 Our present data show that glomerular mesangial repair in the anti-Thy-1.1 model involves bone-marrow-derived cells, confirming recent observations. 24 The results are also consistent with two recent reports which show that bone-marrow-derived cells have the potential to differentiate into mesangial cells in mice. 26,27 Several routes have been proposed for bone-marrow-derived cells before differentiation into mesangial cells. Circulating mesangial progenitor cells may be recruited to injured mesangium through the glomerular endothelium and differentiate into mesangial cells in situ. Alternatively, bone-marrow-derived mesangial precursor cells may migrate via the juxtaglomerular apparatus into the mesangium in response to injury, consistent with the observations of Hugo et al. 41 The presence of bone-marrow-derived mesangial cells in clusters located at the vascular pole supports their migration from the juxtaglomerular apparatus.
There are some limitations to our observations. Two recent studies have demonstrated that embryonic stem cells could fuse with bone marrow cells 44 or neural cells 45 in vitro to form tetraploid hybrid cells which could adopt some of the phenotypes typical of embryonic stem-cell differentiation. We cannot exclude the possibility that some of the U9F4-positive cells in the glomeruli of our WRBM→BN rats may have resulted from fusion between recipient (Brown Norway) endothelial or mesangial cells and donor (Wag/Rij) bone marrow cells. However, thus far, cell fusion has only been shown in tissue culture systems, not in vivo. Furthermore, the phenomenon has been shown to occur infrequently.
In our model, light microscopical evaluation or renal function tests revealed no evidence of radiation-induced glomerular injury. However, we cannot exclude the possibility that irradiation may have caused discrete glomerular injury or may have influenced turnover rates. “Dysfunction” of the resident mesangial and endothelial cell population may have caused greater bone-marrow-derived cell involvement leading to overestimation of the contribution of bone-marrow-derived cells to glomerular turnover and repair.
Our findings provide proof of principle that bone-marrow-derived cells have the capacity to participate in glomerular endothelial and mesangial repair. Several investigators have established the presence of bone-marrow-derived endothelial progenitor cells in the adult circulation. 15,16 Recent studies also suggest the presence of vascular smooth-muscle progenitor cells. 46 In experimental models cytokine or growth factor therapy to enhance endogenous endothelial progenitor cells or injection of ex vivo expanded endothelial progenitor cells have been shown to promote neovascularization in ischemic tissue. Our data suggest that progenitor-cell-based therapies may constitute novel tools in the treatment of renal disease.
Acknowledgments
We thank B. van Middelaar for excellent technical assistance.
Footnotes
Address reprint requests to Marianne C. Verhaar, M.D., Ph.D., Department of Vascular Medicine, F 02.226, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands. E-mail: m.c.verhaar@azu.nl.
Supported by NWO (the Netherlands Organization for Scientific Research, grant 016.036.041 to M.C.V.), the Dutch Kidney Foundation (grant PC 127) and an institutional grant from the University Medical Center Utrecht (Genvlag).
M.B.R. and A.M.S. contributed equally to this manuscript
References
- 1.Kang DH, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, Schreiner GF, Johnson RJ: Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol 2002, 13:806-816 [DOI] [PubMed] [Google Scholar]
- 2.Yamanaka N, Shimizu A: Role of glomerular endothelial damage in progressive renal disease. Kidney Blood Press Res 1999, 22:13-20 [DOI] [PubMed] [Google Scholar]
- 3.Abouna GM, Al Adnani MS, Kremer GD, Kumar SA, Daddah SK, Kusma G: Reversal of diabetic nephropathy in human cadaveric kidneys after transplantation into non-diabetic recipients. Lancet 1983, 2:1274-1276 [DOI] [PubMed] [Google Scholar]
- 4.Clark G, White RH, Glasgow EF, Chantler C, Cameron JS, Gill D, Comley LA: Poststreptococcal glomerulonephritis in children: clinicopathological correlations and long-term prognosis. Pediatr Nephrol 1988, 2:381-388 [DOI] [PubMed] [Google Scholar]
- 5.Iruela-Arispe L, Gordon K, Hugo C, Duijvestijn AM, Claffey KP, Reilly M, Couser WG, Alpers CE, Johnson RJ: Participation of glomerular endothelial cells in the capillary repair of glomerulonephritis. Am J Pathol 1995, 147:1715-1727 [PMC free article] [PubMed] [Google Scholar]
- 6.Ostendorf T, Kunter U, Eitner F, Loos A, Regele H, Kerjaschki D, Henninger DD, Janjic N, Floege J: VEGF (165) mediates glomerular endothelial repair. J Clin Invest 1999, 104:913-923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimizu A, Kitamura H, Masuda Y, Ishizaki M, Sugisaki Y, Yamanaka N: Rare glomerular capillary regeneration and subsequent capillary regression with endothelial cell apoptosis in progressive glomerulonephritis. Am J Pathol 1997, 151:1231-1239 [PMC free article] [PubMed] [Google Scholar]
- 8.Kang DH, Joly AH, Oh SW, Hugo C, Kerjaschki D, Gordon KL, Mazzali M, Jefferson JA, Hughes J, Madsen KM, Schreiner GF, Johnson RJ: Impaired angiogenesis in the remnant kidney model: i. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol 2001, 12:1434-1447 [DOI] [PubMed] [Google Scholar]
- 9.Kang DH, Anderson S, Kim YG, Mazzalli M, Suga S, Jefferson JA, Gordon KL, Oyama TT, Hughes J, Hugo C, Kerjaschki D, Schreiner GF, Johnson RJ: Impaired angiogenesis in the aging kidney: vascular endothelial growth factor and thrombospondin-1 in renal disease. Am J Kidney Dis 2001, 37:601-611 [DOI] [PubMed] [Google Scholar]
- 10.Shulman K, Rosen S, Tognazzi K, Manseau EJ, Brown LF: Expression of vascular permeability factor (VPF/VEGF) is altered in many glomerular diseases. J Am Soc Nephrol 1996, 7:661-666 [DOI] [PubMed] [Google Scholar]
- 11.Masuda Y, Shimizu A, Mori T, Ishiwata T, Kitamura H, Ohashi R, Ishizaki M, Asano G, Sugisaki Y, Yamanaka N: Vascular endothelial growth factor enhances glomerular capillary repair and accelerates resolution of experimentally induced glomerulonephritis. Am J Pathol 2001, 159:599-608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YG, Suga SI, Kang DH, Jefferson JA, Mazzali M, Gordon KL, Matsui K, Breiteneder-Geleff S, Shankland SJ, Hughes J, Kerjaschki D, Schreiner GF, Johnson RJ: Vascular endothelial growth factor accelerates renal recovery in experimental thrombotic microangiopathy. Kidney Int 2000, 58:2390-2399 [DOI] [PubMed] [Google Scholar]
- 13.Takahashi T, Huynh-Do U, Daniel TO: Renal microvascular assembly and repair: power and promise of molecular definition. Kidney Int 1998, 53:826-835 [DOI] [PubMed] [Google Scholar]
- 14.Robert B, St. John PL, Hyink DP, Abrahamson DR: Evidence that embryonic kidney cells expressing flk-1 are intrinsic, vasculogenic angioblasts. Am J Physiol 1996, 271:F744-F753 [DOI] [PubMed] [Google Scholar]
- 15.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Scatteman G, Isner JM: Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275:964-967 [DOI] [PubMed] [Google Scholar]
- 16.Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP: Evidence for circulating bone-marrow-derived endothelial cells. Blood 1998, 92:362-367 [PubMed] [Google Scholar]
- 17.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM: Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999, 85:221-228 [DOI] [PubMed] [Google Scholar]
- 18.Weijtens M, van Spronsen A, Hagenbeek A, Braakman E, Martens A: Reduced graft-versus-host disease-inducing capacity of T cells after activation, culturing, and magnetic cell sorting selection in an allogeneic bone marrow transplantation model in rats. Hum Gene Ther 2002, 13:187-198 [DOI] [PubMed] [Google Scholar]
- 19.Stet RJM, Zantema A, van Laar T, de Waal RMW, Vaessen LMB, Rozing J: U9F4: a monoclonal antibody recognizing a rat polymorphic class I determinant. Transplant Proc 1987, 19:3004-3005 [Google Scholar]
- 20.Bagchus WM, Hoedemaeker PJ, Rozing J, Bakker WW: Glomerulonephritis induced by monoclonal anti-Thy 1.1 antibodies. A sequential histological and ultrastructural study in the rat. Lab Invest 1986, 55:680-687 [PubMed] [Google Scholar]
- 21.Van Dixhoorn MG, Salazar-Exaire D, Sato T, Daha MR, Quigg RJ, Bruijn JA, Couser WG, De Heer E: Anti-vitronectin antibodies enhance anti-Thy-1-induced proteinuria in PVG/c, but not in Wistar rats. J Am Soc Nephrol 1998, 9:994-1007 [DOI] [PubMed] [Google Scholar]
- 22.Brandt J, Pippin J, Schulze M, Hansch GM, Alpers CE, Johnson RJ, Gordon K, Couser WG: Role of the complement membrane attack complex (C5b-9) in mediating experimental mesangioproliferative glomerulonephritis. Kidney Int 1996, 49:335-343 [DOI] [PubMed] [Google Scholar]
- 23.Duijvestijn AM, van Goor H, Klatter F, Majoor GD, van Bussel E, Breda Vriesman PJ: Antibodies defining rat endothelial cells: rECA-1, a pan-endothelial cell-specific monoclonal antibody. Lab Invest 1992, 66:459-466 [PubMed] [Google Scholar]
- 24.Ito T, Suzuki A, Imai E, Okabe M, Hori M: Bone marrow is a reservoir of repopulating mesangial cells during glomerular remodeling. J Am Soc Nephrol 2001, 12:2625-2635 [DOI] [PubMed] [Google Scholar]
- 25.Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA: Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol 2001, 195:229-235 [DOI] [PubMed] [Google Scholar]
- 26.Cornacchia F, Fornoni A, Plati AR, Thomas A, Wang Y, Inverardi L, Striker LJ, Striker GE: Glomerulosclerosis is transmitted by bone-marrow-derived mesangial cell progenitors. J Clin Invest 2001, 108:1649-1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imasawa T, Utsunomiya Y, Kawamura T, Zhong Y, Nagasawa R, Okabe M, Maruyama N, Hosoya T, Ohno T: The potential of bone-marrow-derived cells to differentiate to glomerular mesangial cells. J Am Soc Nephrol 2001, 12:1401-1409 [DOI] [PubMed] [Google Scholar]
- 28.Pabst R, Sterzel RB: Cell renewal of glomerular cell types in normal rats. An autoradiographic analysis. Kidney Int 1983, 24:626-631 [DOI] [PubMed] [Google Scholar]
- 29.Shimizu A, Kitamura H, Masuda Y, Ishizaki M, Sugisaki Y, Yamanaka N: Apoptosis in the repair process of experimental proliferative glomerulonephritis. Kidney Int 1995, 47:114-121 [DOI] [PubMed] [Google Scholar]
- 30.Floege J, Johnson RJ, Gordon K, Iida H, Pritzl P, Yoshimura A, Campbell C, Alpers CE, Couser WG: Increased synthesis of extracellular matrix in mesangial proliferative nephritis. Kidney Int 1991, 40:477-488 [DOI] [PubMed] [Google Scholar]
- 31.Crosby JR, Kaminski WE, Schatteman G, Martin PJ, Raines EW, Seifert RA, Bowen-Pope DF: Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res 2000, 87:728-730 [DOI] [PubMed] [Google Scholar]
- 32.Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, Mames RN, Byrne BJ, Vaught T, Spoerri PE, Peck AB, Scott EW: Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med 2002, 8:607-612 [DOI] [PubMed] [Google Scholar]
- 33.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA: Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest 2001, 107:1395-1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinclair RA: Origin of endothelium in human renal allografts. Br Med J 1972, 4:15-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams GM, Alvarez CA: Host repopulation of the endothelium in allografts of kidneys and aorta. Surg Forum 1969, 20:293-294293–4. [PubMed] [Google Scholar]
- 36.Randhawa PS, Starzl T, Ramos HC, Nalesnik MA, Demetris J: Allografts surviving for 26 to 29 years following living-related kidney transplantation: analysis by light microscopy, in situ hybridization for the Y chromosome, and anti-HLA antibodies. Am J Kidney Dis 1994, 24:72-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andersen CB, Ladefoged SD, Larsen S: Cellular inflammatory infiltrates and renal cell turnover in kidney allografts: a study using in situ hybridization and combined in situ hybridization and immunohistochemistry with a Y-chromosome-specific DNA probe and monoclonal antibodies. APMIS 1991, 99:645-652 [DOI] [PubMed] [Google Scholar]
- 38.Lagaaij EL, Cramer-Knijnenburg GF, van Kemenade FJ, van Es LA, Bruijn JA, van Krieken JHJM: Endothelial cell chimerism after renal transplantation and vascular rejection. Lancet 2001, 357:33-37 [DOI] [PubMed] [Google Scholar]
- 39.Rookmaaker MB, Tolboom H, Goldschmeding R, Zwaginga JJ, Rabelink TJ, Verhaar MC: Bone-marrow-derived cells contribute to endothelial repair after thrombotic microangiopathy. Blood 2002, 99:1095. [DOI] [PubMed] [Google Scholar]
- 40.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T: Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation 2001, 104:1046-1052 [DOI] [PubMed] [Google Scholar]
- 41.Hugo C, Shankland SJ, Bowen-Pope DF, Couser WG, Johnson RJ: Extraglomerular origin of the mesangial cell after injury: a new role of the juxtaglomerular apparatus. J Clin Invest 1997, 100:786-794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeRuiter MC, Poelmann RE, VanMunsteren JC, Mironov V, Markwald RR, Gittenberger-de Groot AC: Embryonic endothelial cells transdifferentiate into mesenchymal cells expressing smooth muscle actins in vivo and in vitro. Circ Res 1997, 80:444-451 [DOI] [PubMed] [Google Scholar]
- 43.Frid MG, Kale VA, Stenmark KR: Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circ Res 2002, 90:1189-1196 [DOI] [PubMed] [Google Scholar]
- 44.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW: Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 2002, 416:542-545 [DOI] [PubMed] [Google Scholar]
- 45.Ying QL, Nichols J, Evans EP, Smith AG: Changing potency by spontaneous fusion. Nature 2002, 416:545-548 [DOI] [PubMed] [Google Scholar]
- 46.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R: Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med 2002, 8:403-409 [DOI] [PubMed] [Google Scholar]