Abstract
Recent studies show CXC chemokine elevations after hepatic resection; blockade of epithelial neutrophil-activating protein (ENA-78), a CXC chemokine, retards hepatic regeneration after resection. Additional studies demonstrate that exogenous macrophage inflammatory protein (MIP)-2, another CXC chemokine, is therapeutic in a murine acetaminophen toxicity model when other therapies fail. The current investigations study MIP-2’s effects on the cellular mechanisms involved in liver regeneration in mice after 70% hepatectomy. Administration of exogenous MIP-2 after 70% hepatectomy dramatically increased hepatocyte proliferation as measured by 5-bromo-2′-deoxyuridine staining. Signal transducer and activator of transcription-3 (stat-3) was also detected in greater abundance and persisted in hepatic nuclear extracts from MIP-2-treated mice compared with control mice after hepatic resection. Further, inhibition of the MIP-2 receptor, CXCR2, decreased baseline hepatocyte proliferation and stat-3 expression in the setting of partial hepatectomy. These data show that MIP-2 is important for hepatocyte proliferation after partial hepatectomy and that pharmacological MIP-2 doses after hepatic injury accelerate hepatic regeneration.
The liver is the only vital organ, aside from the brain, for which we have no pharmacological, mechanical, or extra corporeal means of support for a failing organ. In contrast, we have mechanical ventilation to support patients with pulmonary failure, dialysis to support failing kidneys, and a variety of mechanical and pharmacological interventions to maintain the failing heart. The liver is also unique in that it is the only mammalian organ that can regenerate its biologically functional parenchymal mass after resection or injury, instead of healing with biologically nonfunctional scar. A patient’s ability to restore his or her preoperative hepatic mass after major liver resection is well known. 1 A multitude of mediators that are hepatic mitogens, both in vitro and in vivo, have been identified, but the precise mechanisms involved in liver regeneration remain to be defined. 2
In the quest to elucidate the mechanisms responsible for this unique feature of the liver, extensive studies have been conducted in various clinically relevant models of hepatic injury, including partial hepatectomy, ischemia-reperfusion injury, pathogenic infection, and drug toxicity. Novel treatments for liver injury because of hepatic resection may stem from recent studies demonstrating that cytokines, such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α, and CXC chemokines, such as IL-8, epithelial neutrophil activating protein (ENA-78), and macrophage inflammatory protein-2 (MIP-2), are important priming factors for hepatocyte proliferation and hepatic regeneration. 3-5 It is now well established that TNF and IL-6, important inflammatory cytokines, have mitogenic effects on the liver. 3,6-8 Recent studies illustrate that at least some of TNF’s proliferative effects are mediated via TNF-induced IL-6 up-regulation and/or TNF-induced up-regulation of MIP-2 and ENA-78, emphasizing the overlapping and complex regulatory system that is involved in this process. 4,5,9 In the case of IL-6, hepatocyte proliferation is initiated through the nuclear translocation of signal transducer and activator of transcription-3 (stat-3), 3 whereas TNF regulates nuclear levels of at least two families of transcription factors in proliferating hepatocytes. 10 Nuclear translocation of stat-3 is impaired in IL-6 knockout mice, in which hepatocyte proliferation and liver regeneration is delayed. 3 Further, Mellado and colleagues 11 have recently demonstrated that CXC chemokine receptors also signal through the JAK/stat pathway.
The CXC chemokines are a group of molecules that have both inflammatory and reparative properties. 12,13 These are basic heparin-binding proteins that have four conserved cysteine amino acids, with the first two cysteines separated by one nonconserved amino acid, hence the designation CXC. 12,13 These molecules are best known for their neutrophil chemotactic properties. 12,13 Many members of this family have been described and include ENA-78, MIP-2, IL-8, interferon-γ-inducible protein (IP-10), monokine induced by interferon-γ (MIG), and platelet factor (PF)-4. 12,13 IL-8 is the most well-studied CXC chemokine and is produced by many cell types in response to TNF and IL-1, including monocytes, neutrophils, keratinocytes, mesangial cells, epithelial cells, hepatocytes, fibroblasts, and endothelial cells. 12-16 ENA-78 and MIP-2 are also produced by many cell types including hepatocytes, in response to TNF. 16-19
We have recently documented that hepatic MIP-2 and ENA-78 levels are increased after partial hepatectomy in rats and that ENA-78 blockade after hepatic resection retards hepatic regrowth after this injury, as measured by liver weight to total body weight ratios. 5 These investigations also illustrated that ENA-78 and MIP-2 induced hepatocyte proliferation in vitro in a dose-dependent manner, with MIP-2 being the more potent agent. 5 These early studies did not investigate the cellular mechanisms involved in MIP-2’s effects; the current study expands on our previous knowledge of MIP-2’s effects in the setting of partial hepatectomy in that it outlines the kinetics involved in MIP-2-initiated hepatocyte proliferation in vivo, documents that this is mediated at least in part by the CXCR2 receptor, and suggests that MIP-2 functions in this system via a stat-3-mediated signal transduction pathway. The CXCR2 receptor is the cell surface receptor for the majority of the CXC chemokines; CXCR1 is specific for IL-8, whereas CXCR2 is responsive to most of the CXC chemokines, including MIP-2. In addition, this study also illustrates that pharmacological MIP-2 administration accelerates hepatocyte proliferation after 70% hepatectomy in vivo, suggesting a possible clinical application for this molecule.
Materials and Methods
Mouse Model of 70% Hepatectomy
Female CBAJ mice (6 to 8 weeks of age) were purchased from Charles River Laboratories (Portage, MI) and maintained under specific pathogen-free conditions with free access to water and food before each experiment. Additional experiments were performed in genetically altered CXCR2 knockout mice and the appropriate wild-type controls. These animals were a generous gift from Dr. Peter Henke, University of Michigan Department of Surgery. All experiments were performed in compliance with the standards for animal use and care set by the University of Michigan’s Committee for the Use and Care of Animals. These standards are in compliance with the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health.
For the hepatectomy experiments, animals were anesthetized with subcutaneous ketamine hydrochloride (100 mg/kg) and inhalation of isoflurane. All animals received intraperitoneal lactated Ringer’s solution (40 ml/kg) to replace operative fluid and blood losses. Partial (70%) hepatectomy was performed as previously described. 20 Briefly, 3-0 silk suture ligatures were secured around the base of the median and left lateral hepatic lobes and the lobes resected. Sham-operated control animals were treated in an identical manner with the omission of hepatectomy. Previous studies have demonstrated that the mouse liver will regenerate within ∼7 days of 70% hepatectomy. 20
MIP-2, vehicle, anti-CXCR2, or control antibodies were administered by intraperitoneal (IP) injection before 70% hepatectomy or sham laparotomy as follows. Murine recombinant MIP-2 (Peprotech, Rocky Hill, NJ) was reconstituted in sterile phosphate-buffered saline (PBS) and administered 2 hours before hepatectomy or sham laparotomy in a dose of 1 or 10 μg/kg. An identical volume of sterile PBS was used as a vehicle control. For the antibody experiments, mice were injected with 0.5 mg/mouse anti-CXCR2 or control IgG antibodies 10 hours before hepatectomy and again immediately after hepatectomy. Control antibodies consisted of polyclonal rabbit serum without CXCR2-blocking properties.
For the liver regeneration studies, antibodies, MIP-2, or vehicle were administered as described above, animals were sacrificed at 12, 24, 36, 48, 60, 72, and 96 hours after hepatectomy, and liver weight/total body weight ratios determined. Additional investigations at these same time points measured hepatocyte proliferation via 5-bromo-2′-deoxyuridine (BrdU) staining for proliferating hepatocytes; these methods are further described below.
For the investigations involving Western blot analysis, animals were treated as described above with MIP-2, vehicle, or antibodies and were sacrificed at 1, 2, or 3 hours after hepatectomy or sham laparotomy. Liver samples were obtained, snap-frozen in liquid nitrogen, and stored for later preparation of whole cell lysates or nuclear extraction and subsequent Western blot analysis.
BrdU Staining and Analysis
Three hours before sacrifice, animals were injected intraperitoneally with 30 μg BrdU per gram of body weight. Animals were then sacrificed and liver specimens obtained. Liver tissues were fixed in 4% paraformaldehyde for 24 hours, processed for histological analysis, and stained using the Amersham cell proliferation kit (Amersham Pharmacia Biotech Limited, United Kingdom); histological processing and staining is further described below.
Immunohistochemical Staining
Slides containing unstained liver sections were used for immunohistochemical analysis. These slides were first deparaffinized by sequential treatment with xylene, 100% ethanol (EtOH), 90% EtOH, 70% EtOH, 50% EtOH, distilled water, and PBS. To reveal BrdU labeling, these slides were then incubated in 1 N HCl at 37°C for 1 hour, washed three times in PBS, and incubated for 20 minutes in 1% H2O2 in methanol and washed. All slides were then blocked using a 1:2 dilution of normal rabbit serum for 1 hour. Tissue sections were treated with monoclonal anti-BrdU antibody at 1:100 with PBS containing blocking solution for 2 hours at 37°C in a humidified chamber. After incubation, each slide was washed three times with PBS. A 1:300 dilution of horseradish peroxidase-labeled goat anti-mouse antibody (Pierce, Rockford, IL) was placed on the slides for 2 hours at 37°C in a humidified chamber. Slides were again washed twice in PBS. Slides were developed using a diaminobenzidine kit (Vector, Burlington, CA) and counterstained with Mayer’s hematoxylin (0.1%; Sigma Chemical Co., St. Louis, MO). For analysis of proliferation in hepatectomized or sham-operated animals, three animals were used per treatment group per time point and three separate low-power fields were assessed per animal. The number of cells staining positively for BrdU per low-power field were counted and expressed as the mean ± the SEM for each group.
Preparation of Whole Cell Lysates
Frozen liver samples were thawed in prechilled lysis buffer (100 mmol/L Tris, 0.1% sodium dodecyl sulfate, 0.1% Triton X-100, and 15% glycerol), minced, homogenized, and sonicated. All tubes were maintained at 4°C and gently rotated on a rotator for 30 minutes and subsequently clarified through centrifugation at 14,000 × g for 15 minutes at 4°C. The supernatants were removed and centrifuged again at 14,000 × g for 15 minutes at 4°C. The resulting supernatants contained the total cell lysate proteins. Protein quantification was performed using the BCA protein assay kit (Pierce, Rockford, IL).
Preparation of Nuclear Extracts
Preparation of hepatic nuclear extracts was conducted as follows. Liver samples were rapidly homogenized in PBS containing Complete protease inhibitor (10 mg/ml; Boehringer Mannheim, Mannheim, Germany) on ice and washed with fresh PBS. Homogenates were suspended in buffer A (10 mmol/L Hepes, 10 mmol/L KCl, 0.5 mmol/L dithiothreitol, 1% Nonidet P-40) and mixed for 10 minutes on a rotator, followed by centrifugation for 10 minutes at 14,000 × g at 4°C. The resulting supernatant containing the cytoplasmic components was removed. The pellets, containing the cell nuclei, were suspended in buffer C (20 mmol/L Hepes, 20% glycerol, 500 mmol/L KCl, 0.2 mmol/L ethylenediaminetetraacetic acid, 0.5 mmol/L phenylmethyl sulfonyl fluoride, 0.5 mmol/L dithiothreitol, 1.5 mmol/L MgCl2), mixed for 15 minutes on a rotator, and centrifuged at 7000 × g for 10 minutes at 4°C. The resulting supernatants containing the nuclear proteins were removed for Western blot analysis. Nuclear protein concentrations were measured using a Bradford assay (Bio-Rad Laboratories, Inc., Hercules, CA).
Western Blot Analysis
Fifty μg of hepatic nuclear protein or total cell lysate protein were electrophoresed on a 12% polyacrylamide gel and then transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories Inc., Hercules, CA). Equal protein loading was confirmed by Coomassie blue staining of the gel after transfer. Membranes were blocked for 1 hour at room temperature in 5% dry milk and were then incubated with primary antibodies at the following dilutions (all reagents were purchased from Santa Cruz Biotechnology, Inc., Santa Cruz, CA): phosphotyrosine stat-3 (p-stat-3), 1:100 and stat-3, 1:1000. The antibodies were diluted in 5% dry milk in Tris-buffered saline with 0.1% Tween 20 and the membranes were incubated with the antibodies overnight at 4°C. The horseradish peroxidase-linked secondary antibody (Pierce) was then added at a 1:3000 dilution for 2 hours at room temperature, and protein bands were visualized by chemiluminescence (Bio-Rad). Some blots were also stripped and reanalyzed using anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase) monoclonal antibodies (Chemicon International, Inc., Temecula, CA) as an internal protein loading control.
Statistical Analysis
For all investigations, five mice were used per group per time point, unless otherwise noted. For Western blot analysis, each experiment was repeated a minimum of three times and representative gels are illustrated; the densitometry that accompanies the gel represents the mean densitometry values for three gels from three separate animal groups. Groups of data were evaluated by analysis of variance by the methods of Student-Newman-Keuls to indicate groups with significant differences. Differences were considered significant if P values were less than 0.05. Results are presented as means ± SEM. Data were analyzed using a PowerPC 7100 computer using the Statview II statistical software package (Abacus Concepts, Inc., Berkley, CA).
Results
The CXCR2 Receptor Is Required for Hepatic Recovery after Partial Hepatectomy
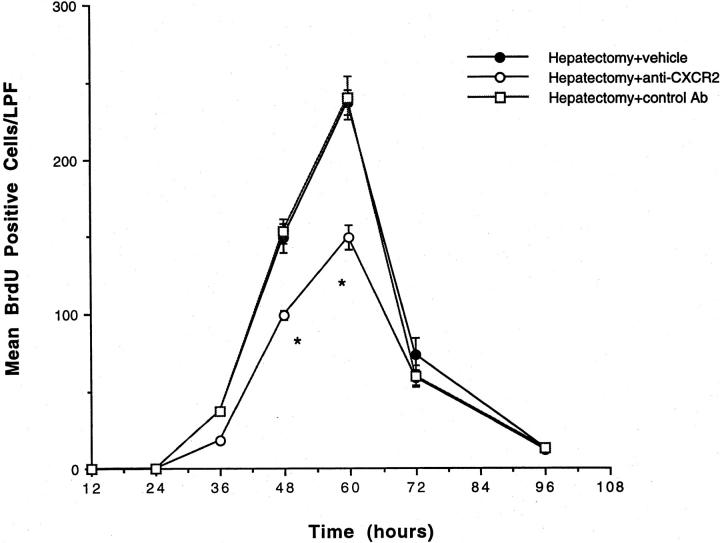
Neutralization of MIP-2 through the use of anti-MIP-2 antibodies in the setting of partial hepatectomy did not significantly decrease liver weight/body weight ratios (data not shown). Previous studies have shown that more than one chemokine is up-regulated after partial hepatectomy; 5 because our previous studies in an acetaminophen toxicity model of liver injury suggest that the CXC chemokines function in this system via the CXCR2 receptor, 5,21 initial experiments were performed in which mice were treated with anti-CXCR2 antibodies during hepatectomy to block the effects of all of the CXC chemokines. Mice were treated with anti-CXCR2 or control antibody and underwent 70% hepatectomy. As illustrated in Figures 1 and 2 ▶ , animals treated with anti-CXCR2 antibodies demonstrated a significant decrease in liver weight/body weight ratios and BrdU staining after 70% hepatectomy. Similar decreases were not seen in mice treated with control antibodies, suggesting that the CXC chemokines are important for hepatic recovery after 70% hepatectomy and that these molecules are functioning via the CXCR2 receptor.
Figure 1.
Liver weight/body weight ratios in mice undergoing partial hepatectomy and treatment with anti-CXCR2 antibody. Mice were treated with anti-CXCR2 antibody or control antibody and underwent 70% hepatectomy. Liver weight/total body weight ratios were measured at 24, 48, 60, 72, and 96 hours after resection. There was a significant decrease in liver weight/total body weight ratios in the mice treated with anti-CXCR2 antibody at 72 and 96 hours after resection, as compared to mice receiving control antibodies. *, P < 0.05 versus control Ab + Hep.
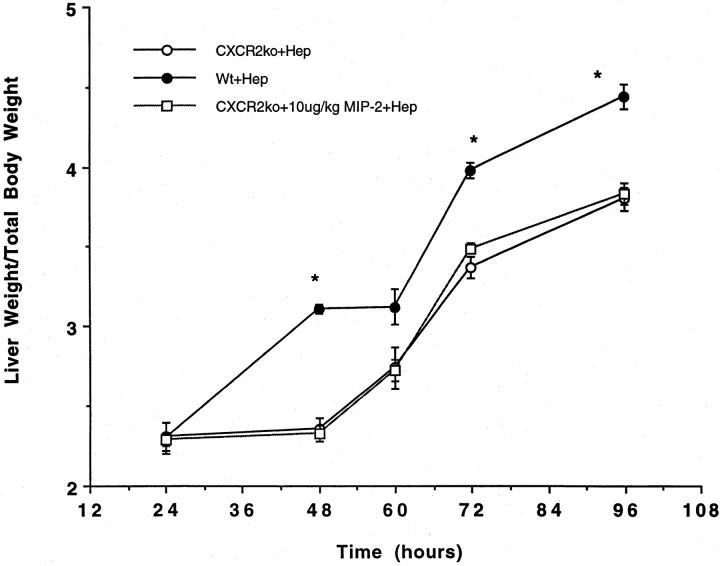
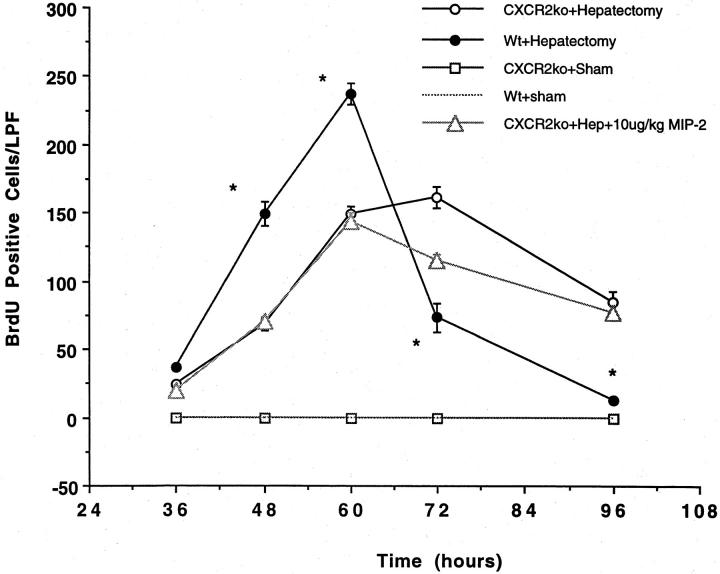
Because our initial experiments neutralizing CXCR2 with antibodies demonstrated a significant decrease in liver weight/body weight ratios and BrdU staining after partial hepatectomy, additional experiments were performed in CXCR2 knockout mice. These mice were subjected to 70% hepatectomy or sham laparotomy and liver weight/body weight ratios and BrdU staining measured kinetically. As illustrated in Figures 3 and 4 ▶ , significant decreases in liver weight/body weight ratios and BrdU staining are seen in the genetically altered animals, as compared to wild-type animals. Further experiments were performed in which the CXCR2 knockout mice were treated with 10 μg/kg of MIP-2, to document that MIP-2’s effects were occurring through the CXCR2 receptor. No increase in liver weight/body weight ratios or BrdU staining were observed in CXCR2 knockout mice treated with MIP-2 (Figure 3 and 4) ▶ .
Figure 3.
Liver weight/body weight ratios in CXCR2 knockout mice or wild-type controls undergoing partial hepatectomy. Partial hepatectomy was performed in CXCR2 knockout (CXCR2ko) or wild-type (WT) control mice. An additional group of CXCR2 knockout mice were treated with 10 μg/kg of MIP-2 and underwent hepatectomy. Liver weight/total body weight ratios were measured at 24, 48, 60, 72, and 96 hours after resection. There was a significant decrease in liver weight/total body weight ratios in the CXCR2 knockout mice at 48, 72, and 96 hours after resection, as compared to wild-type mice; this difference was not corrected by administration of exogenous MIP-2, suggesting that MIP-2 functions in this system via the CXCR2 receptor. *, P < 0.05 versus CXCR2ko + Hep and CXCR2ko + 10 μg/kg MIP-2.
Pharmacological Doses of MIP-2 Promote Rapid Liver Recovery after Partial Hepatectomy
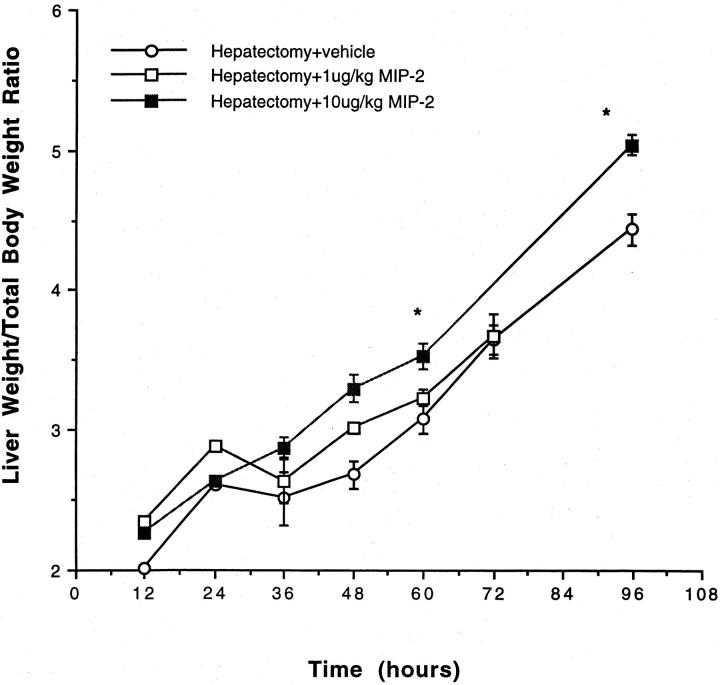
Mice received exogenous MIP-2 (1 or 10 μg/kg) before partial hepatectomy. Kinetic analysis of the effects of exogenous MIP-2 on hepatic regeneration demonstrates that exogenous MIP-2 administration increases hepatic regeneration, as compared to animals treated with vehicle. A significant increase in liver weight/body weight ratios was seen in animals treated with exogenous MIP-2, as compared to animals treated with vehicle, beginning 60 hours after hepatectomy and persisting up to 96 hours after hepatectomy (Figure 5) ▶ . This was particularly notable at the higher MIP-2 doses. Similarly, there was a significant increase in BrdU staining in animals undergoing partial hepatectomy and treatment with MIP-2, as compared to animals undergoing partial hepatectomy and treatment with vehicle (Figure 6) ▶ . MIP-2 treatment resulted in an earlier initiation of hepatocyte proliferation, as well as a significant increase in overall proliferation. Further, both doses of MIP-2 appeared to have similar effects on proliferation, as measured by BrdU staining. There was no increase in BrdU staining in animals undergoing sham laparotomy and treatment with exogenous MIP-2, as compared with mice undergoing sham laparotomy and treatment with vehicle (data not shown).
Figure 5.
Liver weight/body weight ratios in mice undergoing partial hepatectomy and treatment with exogenous MIP-2. Mice were treated with vehicle or 1 or 10 μg/kg of MIP-2 and underwent 70% hepatectomy. Liver weight/total body weight ratios were measured at 12, 24, 36, 48, 60, 72, and 96 hours after resection. There was a significant increase in liver weight/total body weight ratios in the mice treated with 10 μg/kg of MIP-2 at 60 and 96 hours after resection, as compared to mice receiving vehicle. *, P < 0.05 versus hepatectomy + vehicle.
Figure 6.
BrdU staining in mice undergoing partial hepatectomy and treatment with exogenous MIP-2. Mice were treated with vehicle or 1 or 10 μg/kg of MIP-2 and underwent 70% hepatectomy. BrdU staining was performed on liver tissue obtained at 12, 24, 36, 48, 60, 72, and 96 hours after resection and is expressed as number of BrdU-positive cells per low-power field (LPF). There was a significant increase in the number of BrdU-positive cells in animals treated with either dose of MIP-2 at 36, 48, 60, and 72 hours after resection. *, P < 0.05 versus hepatectomy + 1 μg/kg MIP-2 and hepatectomy + 10 μg/kg MIP-2.
Effects of MIP-2 and Anti-CXCR2 Treatment on Stat-3 Levels after Partial Hepatectomy
Nuclear translocation of stat-3 is a critical early event during liver regeneration. 22,23 Stat-3 binding activity increases significantly within 30 minutes of partial hepatectomy and peaks at more than 30 times baseline 3 hours after hepatectomy; this is not observed in sham-operated animals. 23 Stat-3 activation is somewhat unusual as compared to other immediate early genes in that it extends beyond the immediate-early period and remains near peak levels 5 hours after hepatectomy. 23
Our next experiments investigated the effects of exogenous MIP-2 therapy on the nuclear translocation of stat-3. After partial hepatectomy, baseline increases in nuclear stat-3 expression are seen at 1, 2, and 3 hours after hepatectomy (Figure 7) ▶ . Further, nuclear stat-3 levels are increased by pretreatment with MIP-2 and decreased by pretreatment with anti-CXCR2 (Figure 7) ▶ . The MIP-2-induced increases in nuclear stat-3 are most notable at 1 and 2 hours after hepatectomy; in contrast, the effects of anti-CXCR2 are most notable at 2 and 3 hours after hepatectomy.
Figure 7.
Hepatic nuclear extract stat-3 levels after partial hepatectomy plus vehicle, MIP-2, or anti-CXCR2, as measured by Western blot analysis. Nuclear stat-3 levels increase after partial hepatectomy as compared to shams. Nuclear stat-3 levels are further increased in animals treated with exogenous MIP-2 before partial hepatectomy, particularly at the 1- and 2-hour time points. Stat-3 levels are decreased after partial hepatectomy in animals treated with exogenous anti-CXCR2 antibody, particularly at the 2- and 3-hour time points.
Because stat-3 must be phosphorylated in the cytosol to become activated and transported into the nucleus, whole cell liver lysate p-stat-3 levels were also measured by Western blot analysis. Tissue samples were obtained 1, 2, and 3 hours postoperatively from mice undergoing sham laparotomy alone, sham laparotomy plus vehicle, sham laparotomy plus 1 μg/kg MIP-2, sham laparotomy plus 10 μg/kg MIP-2, 70% hepatectomy plus vehicle, 70% hepatectomy plus 1 μg/kg MIP-2, 70% hepatectomy plus 10 μg/kg MIP-2, 70% hepatectomy plus anti-CXCR2 antibody, and 70% hepatectomy plus control antibody. Throughout the 3-hour time period studied, p-stat-3 levels gradually increased after partial hepatectomy (Figure 8) ▶ . A 1-hour sham animal is included for reference; p-stat-3 levels for 1-hour sham are significantly less than the p-stat-3 levels at 1 hour for animals undergoing hepatectomy plus vehicle. P-stat-3 levels are further increased after partial hepatectomy in animals treated with exogenous MIP-2 before hepatectomy; this was statistically significance at the 1- and 2-hour time points. Treatment with anti-CXCR2 resulted in a decrease in p-stat-3 levels after partial hepatectomy (Figure 8) ▶ . This reached statistical significance at the 1- and 3-hour time points. MIP-2 administration to sham-operated animals and administration of control antibodies to animals undergoing partial hepatectomy had no effects. This data has not been presented for clarity.
Figure 8.
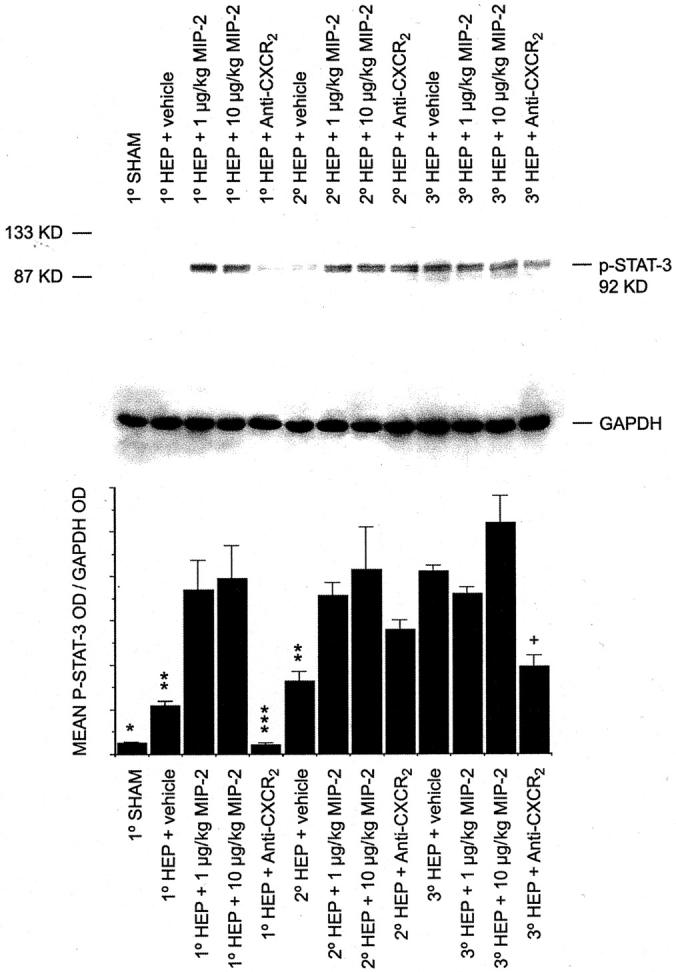
Hepatic p-stat-3 levels in total cellular lysates after partial hepatectomy and treatment with MIP-2 or anti-CXCR2, as measured by Western blot analysis. The illustrated gel is a representative gel from one experiment. The densitometry represents the mean densitometry data from three gels from each of three separate experiments using three separate sets of animals. After Western blot analysis for p-stat-3, each gel was stripped and reanalyzed for GAPDH as an internal protein loading control. A 1-hour sham animal is included for reference. *, P < 0.05 1°sham versus 1°Hep + vehicle. **, P < 0.05 Hep + vehicle versus Hep + 1 μg/kg MIP-2 and versus Hep + 10 μg/kg MIP-2, at each of the respective time points. ***, P < 0.05 1°Hep + anti-CXCR2 versus 1°Hep + vehicle. +, P < 0.05 3°Hep + anti-CXCR2 versus 3°Hep + vehicle.
Discussion
We have recently demonstrated that delayed exogenous MIP-2 administration, working through the CXCR2 receptor, is therapeutic in a murine model of acetaminophen toxicity. 21,24 From this and other studies, it appears that this therapeutic effect may be mediated via a direct proproliferative effect of MIP-2 on hepatocytes. 5,21,24 Thus, the aim of the present study was to explore in greater detail whether alterations in MIP-2 and CXCR2 influence the cellular events necessary for the initiation of hepatocyte proliferation after partial hepatectomy. BrdU staining reveals cells that are in the S phase of the mitotic cycle. 25,26 Previous studies in our laboratory have identified beneficial therapeutic effects for the CXCR2 ligand, MIP-2, in a murine acetaminophen toxicity model when treatment with N-acetyl-cysteine, the standard therapy for acetaminophen overdose, is ineffective. 21 Additional studies in a rat model have shown an increase in hepatic MIP-2 and ENA-78 levels after 70% hepatectomy and a delay in hepatic regeneration in animals treated with anti-ENA-78 antibody in this setting. 5 Although our previous studies suggest that MIP-2’s effects are hepatoregenerative, the cellular mechanisms through which MIP-2 and its receptor, CXCR2, exert this effect are uncharacterized. Thus, the present study addresses the cellular and molecular events in the liver that follow the administration of pharmacological MIP-2 doses or CXCR2 receptor blockade in the setting of hepatic resection in mice. The data presented demonstrate that MIP-2 and CXCR2 are an integral part of the accelerated progression of quiescent hepatocytes into the cell cycle after 70% hepatectomy.
The results presented in this study are significant in that previous studies administering pharmacological doses of other cytokines, such as IL-6 or TNF, in the setting of liver injury or resection failed to demonstrate beneficial effects on hepatic regeneration. Furthermore, hyperstimulation with IL-6 has actually been shown to be detrimental to hepatic regeneration. During fulminant hepatic failure, early and sustained increases in IL-6 blood levels are associated with inhibition of liver regeneration because of up-regulation of the protein inhibitor of activated stat-3. 27 Similarly, IL-6 hyperstimulation in a mouse partial hepatectomy model causes a strong activation of stat-3 inhibitors and a delay and inhibition of cell-cycle progression. 28 Thus, after massive hepatocyte loss, an early and rapid increase in IL-6 may weaken the hepatic regenerative response via up-regulation of stat-3 inhibitors. 27,28 In contrast, our current investigations in a mouse hepatectomy model document a significant beneficial effect of administration of pharmacological MIP-2 doses, resulting in enhanced and accelerated hepatocyte proliferation. More specifically, the BrdU data document an earlier and larger increase in hepatocyte proliferation after 70% hepatectomy in animals treated with MIP-2, suggesting a possible beneficial effect to clinical treatment with this molecule to enhance hepatic regeneration after resection.
Our current investigations suggest that MIP-2 has proliferative effects on hepatocytes in the setting of partial hepatectomy and that these proliferative effects are at least partially mediated through a p-stat-3/stat-3 signal transduction pathway. Although MIP-2 is actively involved in hepatocyte proliferation, this investigation did not definitely define whether these effects were dependent on IL-6 or TNF. Although there were dramatic elevations in cytosolic p-stat-3 and nuclear stat-3 expression in response to MIP-2 treatment, IL-6 was never significantly elevated in either the serum or the liver in response to MIP-2 treatment as compared to controls (data not shown). Previous studies in our laboratory have suggested that at least some of TNF’s proliferative effects are related to up-regulation of MIP-2 and ENA-78. 5 If MIP-2 is up-regulated via TNF or IL-6 in this system, it is logical that MIP-2-mediated effects would occur via the stat-3 signal transduction pathway, because this has already been confirmed for TNF and IL-6. 8,10,23,29 In addition, Mellado and colleagues 11 have recently demonstrated that chemokine receptors for the CXC family do signal through the JAK/stat pathway, further supporting the data presented in this study. Recent studies have also suggested that the CXC chemokine, interferon-γ inducible protein-10 (IP-10), has a role in hepatic regeneration after partial hepatectomy, 30 likely related to IP-10-induced up-regulation of CXCR2, confirming the important role of this family of molecules in the liver’s recovery from injury, as well as the importance of the CXCR2 receptor.
The data presented herein demonstrate that MIP-2 therapy increases cytosolic p-stat-3 and accelerates the nuclear translocation of stat-3 compared with saline-treated mice. An increased number of hepatocytes in MIP-2-treated mice entered the S phase of mitosis by 48 hours, as detected by the increases in BrdU incorporation. When MIP-2 activity was targeted by immunoneutralization or genetic ablation of the CXCR2 receptor, cytosolic p-stat-3 levels and nuclear stat-3 expression were both dramatically reduced, concurrent with a significant reduction in BrdU labeling. When MIP-2 was immunoneutralized, similar inhibitory effects on hepatic regeneration were not observed. It is conceivable that other CXCR2 ligands may have contributed to the hepatoregenerative effect in this situation. Previous experiments have shown that other CXCR2 ligands such as IL-8 and ENA-78 can also have proproliferative effects on hepatocytes. 5 This seems to be the case because neutralization of MIP-2 alone in the hepatectomy model was ineffective at significantly reducing liver regeneration (data not shown), whereas neutralization of the CXCR2 after hepatectomy did have a significant effect on hepatic regeneration after partial hepatic resection. Thus, these data suggest that MIP-2, acting through the CXCR2 receptor, supplies a unique mitogenic signal to the liver that permits the rapid recovery of hepatocytes from injury. In summary, this study demonstrates that endogenous and exogenous MIP-2 rapidly up-regulates the nuclear transcription factor, stat-3, resulting in the regeneration of the acutely injured liver. Further experiments examining the role of CXCR2-dependent CXC chemokines after hepatic injury are needed to reveal the tremendous therapeutic potential these chemokines may have in fulminant hepatic failure.
Figure 2.
Hepatic BrdU staining in mice undergoing partial hepatectomy and treatment with anti-CXCR2 antibody. Mice were treated with anti-CXCR2 antibody, control antibody, or vehicle and underwent 70% hepatectomy. BrdU staining was performed on liver tissue obtained at 24, 36, 48, 60, 72, and 96 hours after resection and is expressed as number of BrdU-positive cells per low-power field (LPF). A significant decrease in the number of BrdU-positive cells is noted at 48 and 60 hours after resection in animals treated with anti-CXCR2 antibody, as compared to animals treated with vehicle or control antibody. *, P < 0.05 versus hepatectomy + vehicle and hepatectomy + control Ab.
Figure 4.
BrdU staining in CXCR2 knockout mice or wild-type controls undergoing partial hepatectomy. Partial hepatectomy was performed in CXCR2 knockout or wild-type control mice. An additional group of CXCR2 knockout mice were treated with 10 μg/kg of MIP-2 and underwent hepatectomy. BrdU staining was performed on liver tissue obtained at 36, 48, 60, 72, and 96 hours after resection and is expressed as the number of BrdU-positive cells per low-power field (LPF). There was a significant decrease in the number of BrdU-positive cells in the CXCR2 knockout mice at 48, 60, 72, and 96 hours after resection, as compared to wild-type mice; this difference was not corrected by administration of exogenous MIP-2, suggesting that MIP-2 functions in this system via the CXCR2 receptor. *, P < 0.05 versus CXCR2ko + Hep and CXCR2ko + 10 μg/kg MIP-2.
Footnotes
Address reprint requests to Lisa M. Colletti, M.D., 2922G Taubman Center, Box 0331, 1500 East Medical Center Dr., Ann Arbor, MI 48109-0331. E-mail: colletti@umich.edu.
Supported by the National Institutes of Health (grant R01DK53224) and the American Heart Association (grant-in-aid 9950211N).
References
- 1.Weinbren K, Hadjis NS: Compensatory hyperplasia of the liver. Blumgart LH eds. Surgery of the Liver and Biliary Tract. 1990:pp 51-54 Churchill Livingstone, London
- 2.Fausto N, Laird AD, Webber EM: Role of growth factors and cytokines in hepatic regeneration. EMBO J 1996, 9:1527-1536 [DOI] [PubMed] [Google Scholar]
- 3.Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R: Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 1996, 274:1379-1383 [DOI] [PubMed] [Google Scholar]
- 4.Yamada Y, Kirillova I, Peschon JJ, Fausto N: Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA 1997, 94:1527-1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colletti LM, Green M, Burdick MD, Kunkel SL, Strieter RM: Proliferative effects of CXC chemokines in rat hepatocytes in vitro and in vivo. Shock 1998, 10:248-257 [DOI] [PubMed] [Google Scholar]
- 6.Yamada Y, Webber EM, Kirillova I, Peschon JJ, Fausto N: Analysis of liver regeneration in mice lacking type 1 or type 2 tumor necrosis factor receptor: requirement for type 1 but not type 2. Hepatology 1999, 28:906-913 [DOI] [PubMed] [Google Scholar]
- 7.Webber EM, Bruis J, Pierce RH, Fausto N: Tumor necrosis factor primes hepatocytes for DNA replication in the rat. Hepatology 1998, 28:1226-1234 [DOI] [PubMed] [Google Scholar]
- 8.Debonera F, Aldeguer X, Shen X, Gelman AE, Gao F, Que X, Greenbaum LE, Furth EE, Taub R, Olthoff KM: Activation of interleukin-6/stat3 and liver regeneration following transplantation. J Surg Res 2001, 96:289-295 [DOI] [PubMed] [Google Scholar]
- 9.Yamada Y, Fausto N: Deficient liver regeneration after carbon tetrachloride injury in mice lacking type 1 but not type 2 tumor necrosis factor receptor. Am J Pathol 1998, 152:1577-1589 [PMC free article] [PubMed] [Google Scholar]
- 10.Taub R: Liver regeneration 4: transcriptional control of liver regeneration. EMBO J 1996, 10:413-417 [PubMed] [Google Scholar]
- 11.Mellado M, Rodriguez-Frade JM, Manes S, Martinez-A C: Chemokine signaling and functional responses: the role of receptor dimerization and TK pathway activation. Annu Rev Immunol 2001, 19:397-421 [DOI] [PubMed] [Google Scholar]
- 12.Oppenheim JJ, Zachariae OC, Mukaida N, Matsushima K: Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol 1991, 9:617-648 [DOI] [PubMed] [Google Scholar]
- 13.Miller MD, Krangel MS: Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol 1992, 12:17-46 [PubMed] [Google Scholar]
- 14.Thornton AJ, Strieter RM, Lindley I, Baggiolini M, Kunkel SL: Cytokine-induced gene expression of a neutrophil chemotactic factor/IL-8 in human hepatocytes. J Immunol 1990, 144:2609-2613 [PubMed] [Google Scholar]
- 15.Mawet E, Shiratori Y, Hikiba Y, Takada H, Yoshida H, Okano K, Komatsu Y, Matsumura M, Nowa Y, Omata M: Cytokine-induced neutrophil chemoattractant release from hepatocytes is modulated by Kupffer cells. Hepatology 1996, 23:353-358 [DOI] [PubMed] [Google Scholar]
- 16.Thornton AJ, Ham J, Kunkel SL: Kupffer cell-derived cytokines induce synthesis of a leukocyte chemotactic peptide, interleukin-8, in human hepatoma and primary hepatocyte cultures. Hepatology 1991, 14:1-11 [PubMed] [Google Scholar]
- 17.Walz A, Burgener R, Car B, Baggiolini M, Kunkel SL, Strieter RM: Structure and neutrophil-activating properties of a novel inflammatory peptide (ENA-78) with homology to interleukin-8. J Exp Med 1991, 174:1355-1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strieter RM, Kunkel SL, Burdick MD, Lincoln PM, Walz A: The detection of a novel neutrophil-activating peptide (ENA-78) using a sensitive ELISA. Immunol Invest 1992, 21:589-596 [DOI] [PubMed] [Google Scholar]
- 19.Driscoll KE, Hassenbein DG, Howard BW, Isfort RJ, Cody D, Tindal MH, Suchanek M, Carter JM: Cloning, expression, and functional characterization of rat MIP-2: a neutrophil chemoattractant and epithelial cell mitogen. J Leukoc Biol 1995, 58:359-364 [DOI] [PubMed] [Google Scholar]
- 20.Higgins GM, Anderson RM: Experimental pathology of the liver; restoration of the liver of the white rat following partial surgical removal. Arch Pathol 1931, 12:186-202 [Google Scholar]
- 21.Hogaboam CM, Bone-Larson CL, Steinhauser ML, Lukacs NW, Colletti LM, Simpson KJ, Strieter RM, Kunkel SL: Novel CXCR2-dependent liver regenerative qualities of ELR-containing CXC chemokines. EMBO J 1999, 13:1565-1574 [DOI] [PubMed] [Google Scholar]
- 22.Trautwein C, Rakemann T, Niehof M, Rose-John S, Mannus MP: APRF, increased binding and target gene activation during liver regeneration. Gastroenterology 1996, 110:214-220 [DOI] [PubMed] [Google Scholar]
- 23.Cressman DL, Diamond RH, Taub R: Rapid activation of the Stat3 transcription complex in liver regeneration. Hepatology 1995, 21:1443-1449 [PubMed] [Google Scholar]
- 24.Hogaboam CM, Simpson KJ, Chensue SW, Steinhauser ML, Lukacs NW, Gauldie J, Strieter RM, Kunkel SL: Macrophage inflammatory protein-2 gene therapy attenuates adenovirus- and acetaminophen-mediated hepatic injury. Gene Ther 1999, 6:573-584 [DOI] [PubMed] [Google Scholar]
- 25.Assy N, Minuk GY: Liver regeneration: methods for monitoring and their applications. J Hepatol 1997, 26:945-952 [DOI] [PubMed] [Google Scholar]
- 26.Baratta B, Rizzoli R, Galliani I, Vitale M, Rizzi E, Matteucci A, Galanzi A, Zamai L, Mazzotti G: Early events of liver regeneration in rats: a multiparametric analysis. Histochem Cell Biol 1996, 105:61-69 [DOI] [PubMed] [Google Scholar]
- 27.Kamohara Y, Sugiyama N, Mizuguchi T, Inderbitzin D, Lilja H, Middleton Y, Neuman T, Demetriou AA, Rozga J: Inhibition of signal transducer and activator transcription factor 3 in rats with acute hepatic failure. Biochem Biophys Res Commun 2000, 273:129-135 [DOI] [PubMed] [Google Scholar]
- 28.Wustefeld T, Rakemann T, Kubicka S, Manns MP, Trautwein C: Hyperstimulation with interleukin 6 inhibits cell cycle progression after hepatectomy in mice. Hepatology 2000, 32:514-522 [DOI] [PubMed] [Google Scholar]
- 29.Zhong Z, Wen Z, Darnell JE: Stat3: a Stat family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 1994, 264:95-98 [DOI] [PubMed] [Google Scholar]
- 30.Koniaris LG, Zimmers-Koniaris T, Hsiao EC, Chavin K, Sitzmann JV, Farber JM: Cytokine-responsive gene-2/IFN-inducible protein-10 expression in multiple models of liver and bile duct injury suggests a role in tissue regeneration. J Immunol 2001, 167:399-406 [DOI] [PubMed] [Google Scholar]