Abstract
The disorganization of E-cadherin/catenin complexes and the overexpression of matrix metalloproteinases (MMPs) are frequently involved in the capacity of epithelial cells to acquire an invasive phenotype. The functional link between E-cadherin and MMPs was studied by transfecting invasive bronchial BZR tumor cells with human E-cadherin cDNA. Using different in vitro (cell dispersion, modified Boyden chamber) and in vivo assays (human airway epithelial xenograft), we showed that E-cadherin-positive clones displayed a decrease of invasive abilities. As shown by immunoprecipitation, the re-expressed E-cadherin was able to sequestrate one part of free cytoplasmic β-catenin in BZR cells. The decrease of β-catenin transcriptional activity in E-cadherin-transfected clones was demonstrated using the TOP-FLASH reporter construct. Finally, we observed a decrease of MMP-1, MMP-3, MMP-9, and MT1-MMP, both at the mRNA and at the protein levels, in E-cadherin-positive clones whereas no changes in MMP-2, TIMP-1, or TIMP-2 were observed when compared with control clones. Moreover, zymography analysis revealed a loss of MMP-2 activation ability in E-cadherin-positive clones treated with the concanavalin A lectin. These data demonstrate a direct role of E-cadherin/catenin complex organization in the regulation of MMPs and suggest an implication of this regulation in the expression of an invasive phenotype by bronchial tumor cells.
The loss of intercellular adhesion and the acquisition of degradative properties are prerequisites for tumor cells to become fully invasive. E-cadherin-mediated cell-cell adhesion largely contributes to the maintenance of epithelial tissue integrity. E-cadherin is linked to the actin cytoskeleton through binding to its cytoplasmic partners: α-, β-, and γ-catenins. 1-5 Loss or dysfunction of E-cadherin/catenin adhesion is largely implicated in malignancy. In particular, E-cadherin deficiency in tumor cells leads to changes in cell morphology and in vitro motility. 2,6-10 In vivo, the loss of E-cadherin expression at the cell membrane is correlated with dedifferentiation, aggressiveness, metastasis, and poor prognosis in different cancer types. 1,3,5,11-13 E-cadherin is therefore considered a tumor/invasion suppressor.
The degradation of basement membrane and extracellular matrix (ECM) involves the participation of proteolytic enzymes. Matrix metalloproteinases (MMPs) constitute a multi-gene family of over 25 secreted and cell surface enzymes that are able to degrade almost all ECM components. MMP activity is controlled by specific inhibitors named TIMPs (tissue inhibitors of matrix metalloproteinases). 14-16 The overexpression of MMPs, leading to a disruption of the balance between MMPs and TIMPs, has been extensively reported in various carcinoma types. In that way, MMPs have been implicated in early as well as in late stages of cancer progression, in particular in cell growth, invasion, angiogenesis, and metastasis. 17-22 In vitro, MMP overexpression is also associated with the acquisition of invasiveness by tumor cells. 23-27
Since the loss of E-cadherin and the overexpression of MMPs are common features of an invasive phenotype, we searched for a functional link between E-cadherin and MMPs. To study the role of E-cadherin on the regulation of MMPs, we performed human E-cadherin cDNA stable transfection in highly invasive bronchial BZR tumor cells lacking E-cadherin expression and we examined the consequences of E-cadherin transfection on cell behavior and MMP production.
Materials and Methods
Cell Line
The human bronchial epithelial cell line BZR was kindly provided by Dr. C. C. Harris (National Institutes of Health, Bethesda, MD). BZR cells were derived from normal bronchial cells immortalized after transfection with the SV40 large T-antigen and infected with the v-Ha-ras oncogene. These cells were grown at 37°C and 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with penicillin, streptomycin, and 10% fetal calf serum (FCS). All chemicals and culture media were purchased from Sigma (St. Louis, MO) and Gibco Invitrogen Corporation (Paisley, UK).
Stable Transfection
The E-cadherin-negative cell line BZR was stably co-transfected with the pJ3ECAD plasmid, derived from pJ3Ω (American Type Culture Collection) and expressing the human full length E-cadherin cDNA, and the pIRES-EGFP vector (Clontech, Palo Alto, CA), containing the EGFP gene and the puromycin resistance gene allowing puromycin selection. As controls, BZR cells were co-transfected with the empty pJ3 plasmid and the pIRES-EGFP vector. Co-transfections were performed in serum-free medium at 200 V and 960 μF using a gene pulser system (Bio-Rad, Richmond, CA). The transfected populations were subjected to a selective pressure of puromycin. Several clones were isolated and characterized.
Western Blotting
For E-cadherin, β-catenin, and MT1-MMP detection, cells were rinsed twice in phosphate-buffered saline (PBS) and extracted in RIPA buffer (50 mmol/L Tris (pH 7.4), 150 mmol/L NaCl, 1% (v/v) Igepal, 1% (w/v) sodium deoxycholate, 5 mmol/L iodoacetamide, and 0.1% (w/v) sodium dodecyl sulfate (SDS)) containing a complete protease inhibitor cocktail (Roche Diagnostics GmbH, Mannheim, Germany). Twenty μg of total protein was separated either on 8% or 12% SDS-PAGE gels for E-cadherin or MT1-MMP detection, respectively. For MMP-1 and MMP-3 detection, 10-fold concentrated serum-free conditioned media were used on 12% SDS-PAGE gels. Separated proteins were transferred to a nitrocellulose membrane (Amersham Pharmacia Biotech, Buckinghamshire, UK). The blots were then blocked with 5% (w/v) nonfat dry milk in PBS containing 0.1% Tween-20 (w/v), before exposure to primary antibodies [anti-E-cadherin (1/2500; Transduction Laboratories, Lexington, KY); anti-β-catenin (1/500; Transduction Laboratories); anti-MT1-MMP (1/10; Oncogene Science, Cambridge, MA); anti-MMP-1 (1/100, Oncogene Science); anti-MMP-3 (1/100; Oncogene Science); anti-actin (1/1000; Sigma)]. The blots were then incubated with a horseradish peroxidase-conjugated goat anti-mouse or swine anti-rabbit antibody (1/1000; Dako, Glostrup, Denmark). The signal was revealed with an ECL+ detection kit (Amersham Pharmacia Biotech).
Immunoprecipitation
Cells were lysed in a buffer containing 1% Triton X-100, 1% Nonidet P-40, and a complete protease inhibitor cocktail (Roche Diagnostics). Five-hundred μg of each protein sample was incubated for 3 hours with HECD-1 antibody at 4°C, followed by incubation with Protein G-Sepharose 4 fast-flow beads (Sigma) for 1 hour. Protein G-Sepharose beads were washed three times in diluted lysis buffer and boiled in 50 μl of Laemmli sample buffer. Immunoprecipitates were resolved by 8% SDS-PAGE and transferred to a nitrocellulose membrane (Amersham Pharmacia Biotech). After blocking with 5% (w/v) nonfat dry milk in PBS containing 0.1% (w/v) Tween-20, the blot was incubated overnight with primary mouse anti-β-catenin antibody (1/500; Transduction Laboratories), followed by three 5-minute washes and incubation with a horseradish peroxidase-conjugated goat anti-mouse antibody (1/1000; Dako). The signal was revealed with an ECL+ detection kit (Amersham Pharmacia Biotech).
In Vitro Cell Dispersion Assay
Time-Lapse Videomicroscopy
Two hours after seeding at a density of 2 × 10 5 cells/ml into 35-mm dishes, cultures were transferred to the environmental chamber (37°C, 5% CO2) of a Zeiss IM35 inverted microscope (Zeiss, Oberkochen, Germany), equipped with a Panasonic WVCD51 camera (Osaka, Japan) and controlled by a Sparc 2 Sun workstation (Sun Microsystems, Mountain View, CA) provided with a parallax video board (Parallax Graphics, Santa Clara, CA). A phase-contrast image was recorded every 15 minutes for 24 hours at a 10-fold magnification to analyze at least 100 cells per field of view.
Cellular Sociology Software
The spatial distribution of cells was characterized and quantified using an algorithmic program of cellular sociology based on the use of three previously described geometrical models, namely Voronoi’s partition, Delaunay’s graph, and minimum spanning tree (MST). 28-30 Results obtained by these three methods are constructed from the set of points locating the position of the cell nuclei, and allow to quantify the disorder and the neighborhood relationship of cells. From each of these methods, two parameters were deduced, namely AD (area disorder) and RFH (roundness factor homogeneity) for Voronoi’s partition, m (average length) and σ (SD) for both Delaunay’s graph and MST. Experimental values of these parameters were compared to theoretical values, obtained by computer simulations of characteristic spatial distributions, to determine the type of spatial distribution of the cell population previously described. 31
Modified Boyden Chamber Invasion Assay
The in vitro invasiveness of transfected cells was assessed using a modified Boyden chamber assay. Cells (105) were suspended in 800 μl of serum-free medium supplemented with 0.2% bovine serum albumin (BSA) and placed in the upper compartment of the chamber (Nucleopore, Pleasanton, CA). For MMP inhibition experiments, the broad-spectrum synthetic hydroxamate MMP inhibitor BB-94 (Batimastat) (kindly provided by British Biotech, Oxford, UK), with potent and specific activity against most of the major MMPs (MMP-1, -2, -3, -7, -9, -14), was added to the cells at 5 × 10−6 M. The lower compartment of the chamber was filled with 200 μl of medium supplemented with 10% FCS and 2% BSA. The two compartments were separated by a porous filter (8-μm pore; Nucleopore) coated with matrigel (50 μg/filter). The matrigel was obtained from the Engelbrecht-Holm-Swarm (EHS) tumor as described by Kleinman et al 32 The chambers were incubated for 5 hours at 37°C. The filters were then fixed in methanol for 10 minutes and stained with hematoxylin for 2 minutes. The cells at the upper surface of the filters were wiped away with a cotton swab. Quantitation of the invasion assay was performed by counting the number of cells at the lower surface of the filters (30 fields at 400-fold magnification).
In Vivo Human Airway Epithelial Xenograft Model
Tracheal xenografts, two per mouse, were prepared as previously described. 33 Briefly, tracheae of male Wistar rats (weighing 220 to 250 grams; Charles River France, Saint-Aubin-les-Elbeuf, France) were frozen at −80°C and thawed two times to remove the surface epithelium. The rat tracheae were aseptically tied at their distal end to sterile polyethylene tubing. The tracheae were then stored at −80°C until inoculation with transfected cells (2 × 10 6 cells in 50 μl of culture medium) and subcutaneous implantation on the back of female recipient nude mice anesthesized with an intraperitoneal injection of pentobarbital sodium (40 mg/kg). The mice were housed under pathogen-free conditions and used for experimentation after 8 weeks of age. To remove tracheal xenografts at various times after implantation, the mice were killed with an injection of an overdose of pentobarbital sodium. Freshly removed tracheal xenografts were fixed in formalin and embedded in paraffin. Sections were stained with hematoxylin, eosin, and saffron. Four tracheae per clone for each time of graft (3 days, 1 week, 3 weeks, 4 weeks, and 8 weeks after implantation) were prepared.
Transient Transfection and Reporter Assay
To determine the transcriptional activity of β-catenin, transient transfections were performed with Fugene transfection reagent (Roche Diagnostics) of the TOP-FLASH plasmid containing three copies of the β-catenin/T-cell factor (TCF)-binding sites upstream of a minimal herpesvirus thymidine kinase promoter driving the firefly luciferase expression. Thirty-thousand cells were plated in 24-well plates 30 minutes before the addition of a mixture containing, for each well, 20 μl of serum-free DMEM, 0.6 μl of Fugene, 0.4 μg of the TOP-FLASH reporter construct, and 0.8 ng of the Renilla luciferase vector phRG-TK (Promega, Madison, WI). Twenty-four hours after transfection, the cells were lysed in 50 μl of passive lysis buffer and the luciferase activity was determined with a luminometer using the Dual Luciferase Assay system (Promega) on 20 μl of lysate. The firefly luciferase activity was normalized to the activity of the renilla luciferase used as internal control. The activity of the TOP-FLASH reporter construct was expressed as normalized relative light units (RLUs).
RT-PCR Analysis
RNA extraction was performed with a High Pure RNA isolation kit as recommended by the manufacturer (Roche Diagnostics). RT-PCR was performed with 10 ng of total RNA using the GeneAmp Thermostable RNA PCR kit (Perkin Elmer, Foster City, CA) and nine pairs of oligonucleotides (Eurogentec, Seraing, Belgium). Forward and reverse primers for human MMP-1, MMP-2, MMP-3, MMP-7, MMP-9, MT1-MMP, TIMP-1, TIMP-2 and 28S were designed as follows: MMP-1 primers [forward 5′-GAGCAAACACATCTGAGGTACAGGA-3′, reverse 5′-TTGTCCCGATGATCTCCCCTGACA-3′], MMP-2 primers [forward 5′-GGCTGGTCAGTGGCTTGGGGTA-3′, reverse5′-AGATCTTCTTCTTC AAGGACCGGTT-3′], MMP-3 primers [forward 5′-GATCTCTTCATTTTGGCCATCTCTTC-3′, reverse 5′-CTCCAGTATTTGTCCTCTACAAAGAA-3′], MMP-7 primers [forward 5′-CCCCCTGCATTTCAGGAA-3′, reverse 5′-TCCTGGCCCATCAAATGG-3′], MMP-9 primers [forward 5′-GCGGAGATTGGGAACCAGCTGTA-3′, reverse 5′-GACGCGCCTGTGTACACCCACA-3′], MT1-MMP primers [forward 5′-CCAT TGGGCATCCAGAAGAGAGC-3′, reverse 5′-GGATACCCAATGCCCATTGGCCA-3′], TIMP-1 primers [forward 5′-CATCCTGTTGTTGCTGTGGCTGAT-3′, reverse 5′-GTC ATCTTGATCTCATAACGCTGG-3′], TIMP-2 primers [forward 5′-GTCATCTTGATCTCATAACGCTGG-3′, reverse 5′-AGCCCATCTGTACCTGTGGTTCA-3′], and 28 S primers [forward 5′-GTTCACCCACTAATAGGGAA-CGTGA-3′, reverse 5′-GGATTCTGACTTAGAGGCGTTC-AGT-3′]. RT-PCR products were separated by acrylamide gel electrophoresis, stained with SYBR Gold (Molecular Probes, Eugene, OR) and quantified by fluorimetric scanning (LAS-1000, Fuji). The expected sizes of the RT-PCR products of MMP-1, MMP-2, MMP-3, MMP-7, MMP-9, MT1-MMP, TIMP-1, TIMP-2, and 28S are 185 bp, 225 bp, 269 bp, 102 bp, 313 bp, 221 bp, 143 bp, 161 bp, and 212 bp, respectively.
Gelatin Zymography Analysis
Cells were grown with or without 25 μg/ml concanavalin A (Con A) (Boehringer Mannheim, Mannheim, Germany) for 48 hours in serum-free conditions. Supernatants were collected and centrifuged. Samples were separated on a 10% polyacrylamide SDS gel containing 0.1% (w/v) gelatin. The gel was washed for 1 hour at room temperature in a 2% (v/v) Triton X-100 solution, transferred to a 50 mmol/L Tris-HCl/10 mmol/L CaCl2 (pH 7.6) buffer and incubated overnight at 37°C. The gel was stained for 30 minutes in a 0.1% (w/v) Coomassie blue (G250)/45% (v/v) methanol/10% (v/v) acetic acid solution and de-stained in 10% (v/v) acetic acid/20% (v/v) methanol.
Immunohistochemistry
The tracheal xenograft sections were deparaffinized, rehydrated, and treated with 0.3% hydrogen peroxyde for 5 minutes to quench endogenous peroxidase activity. Non-specific binding was blocked with 3% BSA for 20 minutes. Slides were incubated overnight at 4°C with anti-MMP-1 (Oncogene Science), anti-MMP-2 (Oncogene Science), anti-MMP-3 (Oncogene Science), anti-MMP-9 (Oncologix, Gaithersburg, MD), or anti-MT1-MMP (Oncogene Science) monoclonal antibody (10 μg/ml). After three 5-minute washes in PBS, subsequent steps were performed with the peroxidase LSAB kit (labeled-streptavidin biotin method, Dako, Carpinteria, CA). Peroxidase activity was revealed with AEC (3-amino-9-ethylcarbazole) chromogen, which generated a red-brown product. All slides were briefly counterstained with Mayer’s hematoxylin, mounted, and observed under a Zeiss Axiophot microscope.
Statistical Analysis
All experiments were performed three times in triplicate. Data are expressed as mean ± SD. The non-parametric Mann-Whitney test was used for statistical analyses. P < 0.05 was considered significant.
Results
Expression of E-Cadherin in Transfected BZR Cells
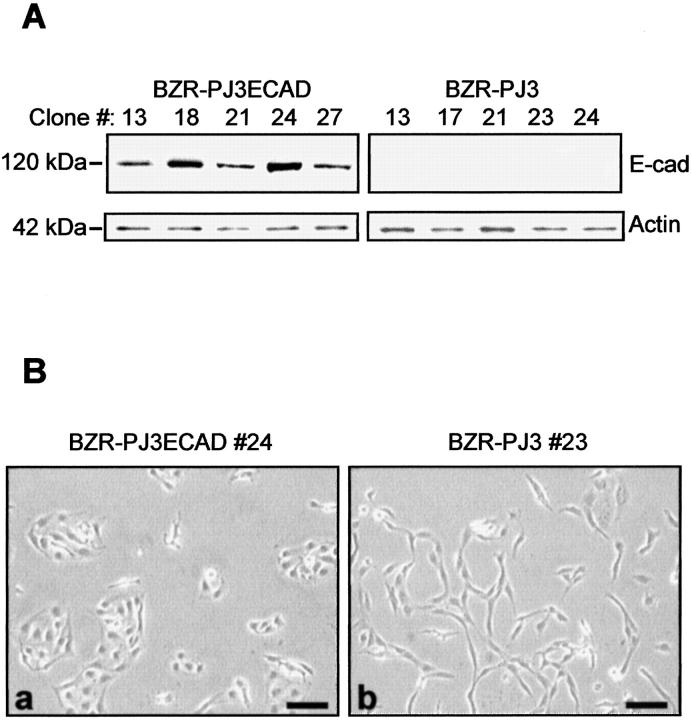
The invasive E-cadherin-negative bronchial tumor cell line BZR was co-transfected with the human E-cadherin cDNA under the control of the SV40 promoter (pJ3ECAD plasmid) and a plasmid encoding puromycin resistance (pIRES-EGFP plasmid). As controls, BZR cells were also co-transfected with empty pJ3 plasmid and pIRES-EGFP plasmid. E-cadherin-transfected clones and control vector-transfected clones were screened for E-cadherin expression by Western blot analysis. Five clones of each were selected for further study (Figure 1A) ▶ . Examining the phenotype of transfected cells, we observed that E-cadherin-transfected clones displayed an epithelioid morphology, whereas control clones showed a spindle fibroblastoid shape similar to parental BZR cells (Figure 1B) ▶ .
Figure 1.
Expression of E-cadherin in transfected BZR cells. A: Screening by Western blot analysis of E-cadherin (E-cad) expression in E-cadherin-transfected clones (BZR-PJ3ECAD) and vector-transfected clones (BZR-PJ3). Anti-actin staining indicates equivalent protein loading. B: Phase-contrast photographies showing the phenotype of E-cadherin-transfected clone 24 (a) and vector-transfected clone 23 (b). Clones 24 and 23 are shown as representative of E-cadherin or control transfectants, respectively. Bars, 100 μm.
E-Cadherin Expression Modifies in Vitro and in Vivo Cell Behavior
Since E-cadherin is known to play a pivotal role in cell-cell adhesion, E-cadherin transfectants and control vector transfectants were first tested in a cell dispersion assay allowing to characterize cell repartition according to three geometrical methods: Voronoi’s partition, Delaunay’s graph, and MST. 31 During the first hours of culture, control cells and E-cadherin-transfected cells displayed the same pattern, ie, quite homogeneous graphs of Voronoi, Delaunay, and MST corresponding to a random distribution (data not shown). However, we observed the progressive appearance of differences of homogeneity of Voronoi area as well as Delaunay and MST segment lengths corresponding to behavior differences between E-cadherin-positive and -negative cells. Comparison of the partitions and graphs obtained by the cellular sociology methods after 24 hours of culture revealed that the E-cadherin-transfected clones displayed more heterogeneity of Voronoi areas and a high variability of Delaunay and MST segment lengths (Figure 2) ▶ . This corresponds to a more cohesive cluster spatial distribution than the control clones that exhibited quite homogeneous Voronoi areas or Delaunay and MST segments representative of a dispersed and random spatial distribution.
Figure 2.

Effect of E-cadherin expression on cell dispersion. A: Comparison of partitions obtained by the three geometrical models, namely Voronoi’s partition (Voronoi), Delaunay’s graph (Delaunay), and minimum spanning tree (MST) (a, b, c, respectively), of the E-cadherin-transfected clone 24 and the vector-transfected clone 23 (d, e, f, respectively) after 24 hours of culture. B: Comparison of parameters calculated from each of the three graphs (a, b, c) for the E-cadherin-transfected clone 24 and the vector-transfected clone 23. The three geometrical models showing that the RFH (roundness factor homogeneity) value decreases while the AD (area disorder) value increases and that the m (average length) value decreases while the σ (SD) value increases, indicate that the E-cadherin-transfected clone 24 displayed a more cohesive cluster spatial distribution than vector-transfected clone 23 (P < 0.05).
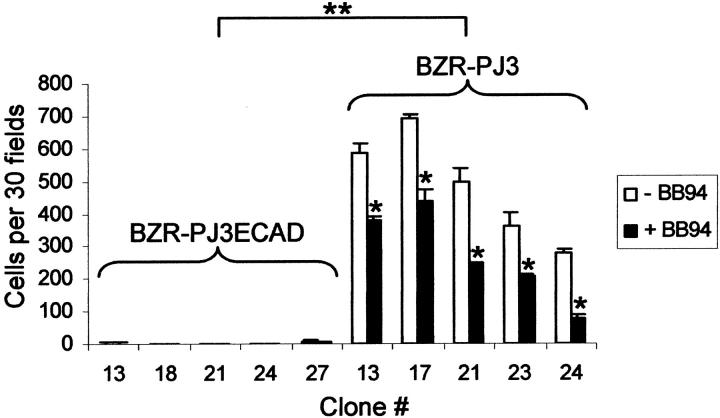
To examine the in vitro invasive abilities of the different clones, we also performed an invasion assay in a modified Boyden chamber. We observed that the five E-cadherin-transfected clones exhibited decreased in vitro invasive abilities (2.5 ± 1.9, 0.5 ± 0.4, 0.5 ± 0.58, 0.7 ± 0.5, and 6.2 ± 3.2 cells per 30 fields) compared to the five clones transfected with the control vector (585 ± 29, 696 ± 12, 498 ± 41, 360 ± 42, and 279 ± 9 cells per 30 fields) (P < 0.01) (Figure 3) ▶ . Moreover, invasiveness of the five clones transfected with the control vector was significantly decreased in the presence of the synthetic MMP inhibitor BB-94 (381 ± 8, 437 ± 40, 248 ± 3, 209 ± 2, and 79 ± 12 cells per 30 fields) (P < 0.05) demonstrating that MMPs were implicated in invasiveness of BZR cells (Figure 3) ▶ .
Figure 3.
Effect of E-cadherin expression on in vitro cell invasion. Analysis of the invasive properties of E-cadherin-transfected clones and vector-transfected clones in a modified Boyden chamber invasion assay. The specific synthetic MMP inhibitor BB-94 (BB94) was used to demonstrate that invasiveness of vector-transfected clones was MMP dependent. Results are expressed as the mean of three different experiments ± SD. Thirty fields (×400) were counted on each filter (**, P < 0.01; *, P < 0.05).
To mimic the process of bronchial tumor invasion, we used an in vivo human airway epithelial xenograft model in which denuded tracheae, inoculated with various clones, are implanted on the backs of nude mice. Observation of paraffin sections of tracheae removed 3 days after the graft revealed that E-cadherin- or control vector-transfected clones had repopulated the denuded tracheae (Figure 4, a and b) ▶ . After 1 week, all transfected cells had proliferated and reformed a multi-layer epithelium (Figure 4, c and d) ▶ . Three weeks after the graft, cells transfected either with E-cadherin or empty vector had completely filled the lumen to form a tumor mass. As in situ carcinomas, these tumor formations were outlined by the basement membrane (Figure 4, e and f) ▶ . After 4 weeks, whereas E-cadherin-transfected cells were still bordered with the basement membrane, control vector-transfected cells showed a highly invasive behavior. Cells had penetrated through the basement membrane and colonized the submucosal space before they invaded the surrounding host tissue beneath the cartilage to form tumor islands surrounded by reactive connective tissue (Figure 4, g and h) ▶ . During the following weeks, highly invasive tumors formed by control vector-transfected cells continued to spread and reached the mouse skin, whereas the tumor mass formed by E-cadherin-transfected cells remained localized to the tracheal area (Figure 4, i and j) ▶ .
Figure 4.
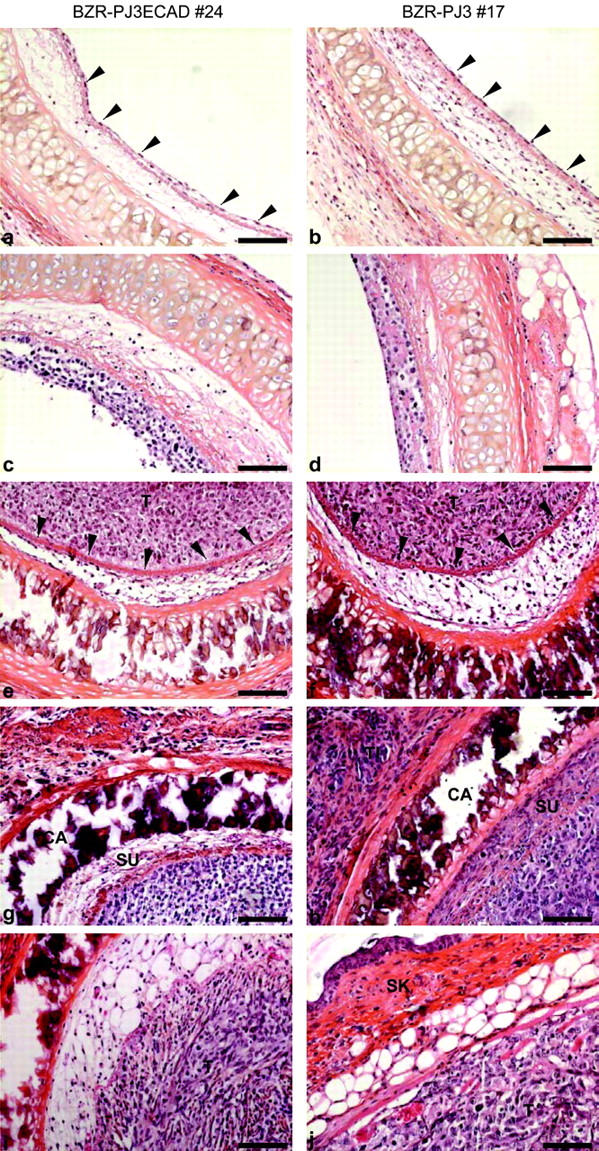
Effect of E-cadherin expression on in vivo cell invasion. A human airway epithelial xenograft model was used to mimic bronchial tumor invasion. HPS staining of paraffin sections of tracheae removed 3 days (a and b), 1 week (c and d), 3 weeks (e and f), 4 weeks (g and h), and 8 weeks (i and j) after the graft was performed. The E-cadherin-transfected clone 24 and the vector-transfected clone 17 are shown as example of E-cadherin and of control transfectants, respectively. After cells had repopulated the denuded tracheae (arrowheads) (a and b), they proliferated (c and d) and formed a tumor mass (T) well delimited by the basement membrane (arrowheads) (e and f). Only control vector-transfected cells gave rise to an aggressive tumor that colonized the submucosal space (SU) and infiltrated beneath the cartilage (CA) to form tumor islands (TI) into the surrounding host tissue (g and h) up to the mouse skin (SK) (i and j). Bars, 84 μm.
E-Cadherin Expression Decreases β-Catenin Transcriptional Activity
Because β-catenin is known to play a role of transcription co-factor when it is dissociated from the E-cadherin complex and stabilized, 34 we examined the effect of E-cadherin transfection on β-catenin expression and transcriptional activity. We first observed by Western blot analysis that the overall level of β-catenin remained unchanged after transfection of E-cadherin in BZR cells (Figure 5A) ▶ . Moreover, immunoprecipitation experiments with an E-cadherin antibody demonstrated that E-cadherin was able to sequestrate free cytoplasmic β-catenin in E-cadherin transfectants (Figure 5B) ▶ . Finally, we performed transient transfections of the TOP-FLASH luciferase reporter construct that clearly showed a significant decrease of transcriptional activity of β-catenin/TCF in E-cadherin-transfected clones (Figure 5C) ▶ .
Figure 5.

Effect of E-cadherin expression on β-catenin transcriptional activity. A: Western blot analysis of overall expression of β-catenin (β-cat) in E-cadherin-transfected clones and vector-transfected clones. Anti-actin staining indicates equivalent protein loading. B: Analysis of interaction between E-cadherin and β-catenin in E-cadherin-transfected clones by E-cadherin immunoprecipitation coupled to β-catenin Western blot. C: Measure of β-catenin/TCF activity in E-cadherin-transfected clones and vector-transfected clones by transient transfection of the TOP-FLASH firefly luciferase reporter construct. Data are expressed as RLUs (relative light units) normalized to the co-transfected Renilla luciferase-encoding phRG-TK plasmid (**, P < 0.01).
E-Cadherin Expression Down-Regulates MMP Production
Since invasiveness of control vector-tansfected cells was MMP-dependent and since in vitro and in vivo invasive abilities of E-cadherin-transfected cells were reduced, we investigated the effect of E-cadherin transfection on MMP production. Comparing by RT-PCR the five clones of E-cadherin transfectants and the five clones of vector-transfected cells, we found a significant decrease of MMP-1, MMP-3, MMP-9, and MT1-MMP (32, 18, 2, and twofold, respectively) in the E-cadherin transfectants. The levels of MMP-2 and of the inhibitors TIMP-1 and TIMP-2 remained unaffected. MMP-7 was not detected (Figure 6A) ▶ . Western blot analysis showed that MMP-1, MMP-3, and MT1-MMP were active in control cells (42 kd, 45 kd, and 60/43 kd, respectively) and that these MMPs were dramatically decreased in E-cadherin-positive cells (Figure 6B) ▶ . Zymography analysis confirmed the decrease of MMP-9 (92 kd) at the protein level in E-cadherin transfectants (Figure 6C) ▶ . To study the ability of the clones to activate MMP-2, transfected cells were treated with Con A, a lectin known to stimulate MMP-2 activation in several systems. Analysis by zymography showed activation of MMP-2 (62/59 kd) only in control-transfected cells. The comparison of MT1-MMP mRNA levels in the absence or presence of Con A by RT-PCR proved that MMP-2 activation was dependent on the level of MT1-MMP (Figure 6C) ▶ .
Figure 6.
Effect of E-cadherin expression on in vitro MMP production. A: RT-PCR analysis of levels of MMP and TIMP mRNA in E-cadherin-transfected clones and vector-transfected clones. The levels were normalized to the control 28S and results are expressed as the mean of three different experiments ± SD (*, P < 0.05; **, P < 0.01). B: Western blot analysis of conditioned media or cellular extracts of E-cadherin-transfected clones and vector-transfected clones using monoclonal antibodies directed against MMP-1, MMP-3, and MT1-MMP. HT1080 cells were used as a control showing the main forms (pro-form and active form, respectively) of MMP-1 (52 and 42 kd), MMP-3 (57 and 45 kd), and MT1-MMP (63, 60, and 43 kd). For MMP-1 and MMP-3 detection, conditioned medium from equal cell numbers was used. For MT1-MMP detection, anti-actin staining indicates equivalent protein loading. C: Analysis by zymography of gelatinolytic activities (92 kd, pro-MMP-9; 72 kd, pro-MMP-2; 62/59 kd, active MMP-2) in conditioned media of E-cadherin-transfected clones and vector-transfected clones treated (+) or not (−) with Con A. An MT1-MMP RT-PCR was performed on corresponding cells to demonstrate its involvement in MMP-2 activation. MT1-MMP levels were normalized to the control 28S.
To verify the E-cadherin-dependent down-regulation of MMP expression under in vivo conditions, MMP expression profile of the E-cadherin- and control vector-transfected cells was examined by immunohistochemistry using monoclonal antibodies directed against human MMPs on tracheal xenograft paraffin sections. The cytoplasm of E-cadherin-transfected cells implanted in vivo was more intensely stained by MMP-1, MMP-3, MMP-9, and MT1-MMP antibodies compared with that of empty vector-transfected cells. As demonstrated in vitro, no change of staining was observed for MMP-2 in the E-cadherin-transfected and control cells in the in vivo airway epithelial xenograft model (Figure 7) ▶ .
Figure 7.

Effect of E-cadherin expression on in vivo MMP production. Immunohistochemistry with monoclonal antibodies directed against human MMP-1 (a and b, respectively), MMP-2 (c and d, respectively), MMP-3 (e and f, respectively), MMP-9 (g and h, respectively), and MT1-MMP (i and j, respectively) was performed to compare the MMP expression profile of E-cadherin- and vector-transfected clones in the in vivo human airway epithelial xenograft model. Bars, 21 μm.
Discussion
In accordance with the role of E-cadherin in cell-cell adhesion and cell differentiation, we first showed that transfection of E-cadherin in highly invasive bronchial tumor cells results in an epithelioid phenotype and in changes in cell migratory and invasive behavior. Indeed, we observed that E-cadherin-transfected cells displayed a greater cluster distribution than control cells that had a random distribution. These results match our previous findings that a random distribution is related to invasive cells and that a cluster distribution is related to noninvasive cells. 31 These data were confirmed by the observation of a significant decrease of in vitro invasive abilities of BZR cells after E-cadherin transfection in a modified Boyden chamber assay. Moreover, using an in vivo human airway epithelial xenograft model in nude mice, we showed that E-cadherin-transfected cells were less invasive than control vector-transfected cells. These data are in agreement with previous studies on other types of tumor cells, 6,7,35 highlighting the role of E-cadherin as an invasion suppressor. In vivo models of bronchial tumors are, nevertheless, not well documented. Klein-Szanto et al 36 have reported the capacity of immortalized human bronchial epithelial cell lines (BEAS-2B cells), grown in deepithelialized rat tracheae subcutaneously transplanted into nude mice, to induce invasive neocarcinoma after a 6-month cigarette-smoke exposure. Nevertheless, the molecular mechanisms involved in this in vivo invasion model have not been analyzed. The human xenograft model that we used in our work has the advantage to be an “air-opened” model that mimics the environmental conditions encountered in the airways. The xenograft provides a tissue structure containing E-cadherin- or control vector-transfected epithelial cells and a stromal host-tissue. The observation that the in vivo invasive ability of E-cadherin-negative bronchial cell line BZR occurs within a short period (4 weeks) after implantation highlights the major role of E-cadherin cell-cell interactions in the bronchial tumor progression.
Concomitantly with a decrease of in vitro and in vivo invasive abilities, we here demonstrate that transfection of E-cadherin induced a decrease of MMP-1, MMP-3, MMP-9, and MT1-MMP production in in vitro as well as in in vivo conditions. It has been previously shown that MMP-9 and MT1-MMP were regulated by E-cadherin in mouse skin carcinoma cells and in human tongue squamous cell carcinoma cells, respectively. 37-38 Moreover, in agreement with the studies of Miyaki et al 35 and Ara et al, 38 the ability of MMP-2 to be activated was dramatically reduced in E-cadherin-transfected cells, without modification of its production. In our experiments, MMP-2 activation was only seen in control E-cadherin-negative clones after Con A treatment. In agreement with its well-described role in MMP-2 activation, MT1-MMP was concomitantly induced by Con A treatment. 39 Other research has nevertheless shown an E-cadherin-induced decrease of MMP-2 expression. 40 These contradictory results may be attributed to the varying cell differentiation state and to the tissue origin of the cells used. Our data showed that E-cadherin is able to regulate the expression of different types of MMPs known to degrade distinct substrates and to play distinct roles in the early and late stages of tumor progression. 41-45 These results, demonstrating a regulation of MMP expression by E-cadherin, are supported by in vivo studies showing inverse correlations between MMP and E-cadherin expression in cancers. 46,47 No change in expression of the MMP inhibitors TIMP-1 and TIMP-2 was observed, so that an MMP/TIMP balance could be restored in E-cadherin-transfected clones. Taken together, these results suggest that E-cadherin is able to regulate the expression of different MMPs and thus to maintain an MMP/TIMP balance favoring the inhibition of cancer progression.
The signal transduction pathway through which E-cadherin regulates MMP gene expression is not well known. One way of MMP regulation by E-cadherin may engage the β-catenin/TCF pathway. When β-catenin is disconnected from E-cadherin, it may accumulate in the cytoplasm and translocate into the nucleus where it complexes with a transcription factor of the Lef/TCF family to induce transactivation of genes involved in tumor progression, such as c-myc, cyclin D1, fibronectin, slug, CD44, γ2 chain of laminin-5, uPAR, MMP-7, and MMP-26. 34,48-57 Accordingly, we observed that E-cadherin expression was sufficient to decrease the transcriptional activity of β-catenin by recruiting it and consequently reducing its cytoplasmic pool. Among MMPs regulated by E-cadherin in this study, MT1-MMP has been recently described as a target of β-catenin/TCF transactivation. 58 Moreover, MMP-1, MMP-3, and MMP-9 constitute potential targets of β-catenin/TCF, because their promoters contain TCF-binding sites. 55,57 Interestingly, the MMP-2 promoter, which does not contain any TCF-binding site, is not regulated by E-cadherin transfection. The fact that only MMPs, which are described as potential targets of β-catenin/TCF, are regulated by E-cadherin transfection and that a significant decrease of β-catenin transcriptional activity is found in E-cadherin-transfected cells, plead in favor of the β-catenin/TCF pathway as a major way to regulate MMPs by E-cadherin. However, we cannot exclude that other signaling pathways are involved. In particular, other transcription factors, such as PEA3 or AP-1, could also regulate (either alone or in synergy with the β-catenin/TCF complex) the expression of MMPs, as previously shown for MMP-7. 59
In conclusion, we demonstrate that E-cadherin transfection down-regulates MMP expression, potentially by a diminution of β-catenin/TCF transcriptional activity. Our data, showing a decrease of invasiveness by E-cadherin transfection, further suggest an implication of this regulation in the expression of an invasive phenotype by bronchial tumor cells.
Acknowledgments
We thank Dr. C. C. Harris (National Institutes of Health, Bethesda, MD) for BZR cells, and Drs. L. A. Huber (Department of Histology and Molecular Cell Biology, Institute of Anatomy, Histology and Embryology, University of Innsbruck, Austria) and H. C. Clevers (University Hospital, Utrecht, The Netherlands) for the TOP-FLASH reporter construct.
Footnotes
Address reprint requests to Béatrice Nawrocki-Raby, INSERM UMRS 514, Institut Fédératif de Recherche (IFR) 53, Centre Hospitalier Universitaire (CHU) Maison Blanche, 45 rue Cognacq-Jay, 51092 Reims Cedex, France. E-mail: beatrice.raby@univ-reims.fr.
Supported by grants from the Société de Pneumologie de Langue Française (SPLF), the Association Régionale pour l’Enseignement et la Recherche Scientifique et Technologique (ARERS), the Ligue contre le Cancer (Comité de la Marne), the Lions Club of Soissons, and the Commissariat Général aux Relations Internationales (CGRI) de la Communauté française Wallonie-Bruxelles/INSERM.
B. N.-R. and C. G. contributed equally to this research.
References
- 1.Takeichi M: Cadherin cell adhesion receptors as a morphogenetic regulator. Science 1991, 251:1451-1455 [DOI] [PubMed] [Google Scholar]
- 2.Aberle H, Schwartz H, Kemler R: Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem 1996, 61:514-523 [DOI] [PubMed] [Google Scholar]
- 3.Wijnhoven BPL, Dinjens WNM, Pignatelli M: E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg 2000, 87:992-1005 [DOI] [PubMed] [Google Scholar]
- 4.Gumbiner BM: Regulation of cadherin adhesive activity. J Cell Biol 2000, 148:399-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bremnes RM, Veve R, Hirsch FR, Franklin WA: The E-cadherin cell-cell adhesion complex and lung cancer invasion, metastasis, and prognosis. Lung Cancer 2002, 36:115-124 [DOI] [PubMed] [Google Scholar]
- 6.Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Lochner D, Birchmeier W: E-cadherin mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol 1991, 113:173-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vleminckx K, Vakaet L, Jr, Mareel M, Fiers W, Van Roy F: Genetic manipulation with E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 1991, 66:107-119 [DOI] [PubMed] [Google Scholar]
- 8.Mareel M, Berx G, Van Roy F, Bracke M: Cadherin/catenin complex: a target for anti-invasive therapy? J Cell Biochem 1996, 61:524-530 [DOI] [PubMed] [Google Scholar]
- 9.Simard D, Nabi IR: Inverse relation of autocrine motility factor receptor and E-cadherin expression following MDCK epithelial cell transformation. Biochem Biophys Res Commun 1996, 219:122-127 [DOI] [PubMed] [Google Scholar]
- 10.Handschuh G, Candidus S, Luber B, Reich U, Schott C, Oswald S, Becke H, Hutzler P, Birchmeier W, Hofler H, Becker KF: Tumour-associated E-cadherin mutations alter cellular morphology, decrease cellular adhesion, and increase cellular motility. Oncogene 1999, 18:4301-4312 [DOI] [PubMed] [Google Scholar]
- 11.Zschiesche W, Schönborn I, Behrens J, Herrenknecht K, Hartveit F, Lilleng P, Birchmeier W: Expression of E-cadherin and catenins in invasive mammary carcinomas. Anticancer Res 1997, 17:561-568 [PubMed] [Google Scholar]
- 12.Nawrocki B, Polette M, Van Hengel J, Tournier JM, Van Roy F, Birembaut P: Cytoplasmic redistribution of E-cadherin-catenin adhesion complex is associated with down-regulated tyrosine phosphorylation of E-cadherin in human bronchopulmonary carcinomas. Am J Pathol 1998, 153:1521-1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirohashi S: Inactiavtion of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol 1998, 153:333-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sternlicht MD, Werb Z: How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 2001, 17:463-516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curran S, Murray GI: Matrix metalloproteinases: molecular aspects of their roles in tumor invasion and metastasis. Eur J Cancer 2000, 36:1621-1630 [DOI] [PubMed] [Google Scholar]
- 16.Chang C, Werb Z: The many faces of metalloproteases: cell growth, invasion, angiogenesis, and metastasis. Trends Cell Biol 2001, 11:37-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egeblad M, Werb Z: New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002, 2:161-174 [DOI] [PubMed] [Google Scholar]
- 18.Monteagudo C, Merino M, San-Juan J, Liotta L, Stetler-Stevenson WG: Immunohistochemical distribution of type IV collagenase in normal, benign, and malignant breast tissue. Am J Pathol 1990, 136:585-592 [PMC free article] [PubMed] [Google Scholar]
- 19.Okada A, Bellocq JP, Rouyer N, Chenard MP, Rio MC, Chambon P, Basset P: Membrane-type matrix metalloproteinase (MT-MMP) gene is expressed in stromal cells of human colon, breast, and head and neck carcinomas. Proc Natl Acad Sci USA 1995, 92:730-734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nawrocki B, Polette M, Marchand V, Monteau M, Gillery P, Tournier JM, Birembaut P: Expression of matrix metalloproteinases and their inhibitors in human bronchopulmonary carcinomas: quantitative and morphological analyses. Int J Cancer 1997, 72:556-567 [DOI] [PubMed] [Google Scholar]
- 21.Chambers AF, Matrisian LM: Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst 1997, 89:1260-1270 [DOI] [PubMed] [Google Scholar]
- 22.Curran S, Murray GI: Matrix metalloproteinases in tumor invasion and metastasis. J Pathol 1999, 189:300-308 [DOI] [PubMed] [Google Scholar]
- 23.Gilles C, Polette M, Pierre J, Birembaut P, Foidart JM: Epithelial-to-mesenchymal transition in HPV-33-transfected cervical keratinocytes is associated with increased invasiveness and expression of gelatinase A. Int J Cancer 1994, 59:661-666 [DOI] [PubMed] [Google Scholar]
- 24.Polette M, Birembaut P: Matrix metalloproteinases in breast cancer. Breast J 1996, 2:209-220 [Google Scholar]
- 25.Polette M, Gilles C, de Bentzmann S, Gruenert D, Tournier JM, Birembaut P: Association of fibroblastoid features with the invasive phenotype in human bronchial cancer cell lines. Clin Exp Metastasis 1998, 16:105-112 [DOI] [PubMed] [Google Scholar]
- 26.Balduick M, Zerimech F, Gouyer V, Lemaire R, Hemon B, Grard G, Thiebaut C, Lemaire V, Dacquembronne E, Duhem T, Lebrun A, Dejonghe MJ, Huet G: Specific expression of matrix metalloproteinases 1, 3, 9, and 13 associated with invasiveness of breast cancer cells in vitro. Clin Exp Metastasis 2000, 18:171-178 [DOI] [PubMed] [Google Scholar]
- 27.Ntayi C, Lorimier S, Berthier-Vergnes O, Hornebeck W, Bernard P: Cumulative influence of matrix metalloproteinase-1 and -2 in the migration of melanoma cells within three-dimensional type I collagen lattices. Exp Cell Res 2001, 270:110-118 [DOI] [PubMed] [Google Scholar]
- 28.Dussert C, Rasigni M, Palmari J, Rasigni G, Llebaria A, Marty F: Minimal spanning tree analysis of biological structures. J Theor Biol 1987, 125:317-323 [DOI] [PubMed] [Google Scholar]
- 29.Marcelpoil R, Usson Y: Methods for the study of cellular sociology: Voronoi diagrams and parametrization of the spatial relationships. J Theor Biol 1992, 154:359-369 [Google Scholar]
- 30.Bertram M, Wendrock H: Characterization of planar local arrangement by means of the Delaunay neighborhood. J Microsc 1996, 181:45-53 [Google Scholar]
- 31.Nawrocki Raby B, Polette M, Gilles C, Clavel C, Strumane K, Matos M, Zahm JM, Van Roy F, Bonnet N, Birembaut P: Quantitative cell dispersion analysis: new test to measure tumor cell aggressiveness Int J Cancer 2001, 93:644-652 [DOI] [PubMed] [Google Scholar]
- 32.Kleinman HK, McGarvey ML, Hasel JR, Star VL, Cannon FB, Laurie GW, Martin GR: Basement membrane complexes with biological activity. Biochemistry 1986, 5:312-318 [DOI] [PubMed] [Google Scholar]
- 33.Dupuit F, Gaillard D, Hinnrasky J, Mongodin E, De Bentzmann S, Copreni E, Puchelle E: Differentiated and functional human airway epithelium regeneration in tracheal xenografts. Am J Physiol 2000, 278:165-176 [DOI] [PubMed] [Google Scholar]
- 34.Hecht A, Kemler R: Curbing the nuclear activities of β-catenin. EMBO Rep 2000, 1:24-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyaki M, Tanaka K, Kikuchi-Yanoshita R, Muraoka M, Konishi M, Takeichi M: Increased cell-substratum adhesion, and decreased gelatinase secretion and cell growth, induced by E-cadherin transfection of human colon carcinoma cells. Oncogene 1995, 11:2547-2552 [PubMed] [Google Scholar]
- 36.Kein-Szanto AJP, Iizasa T, Momiki S, Garcia-Palazzo I, Caamano J, Metcalf R, Welsh J, Harris CC: A tobacco-specific N-nitrosamine or cigarette smoke condensate causes neoplastic transformation of xenotransplanted human bronchial epithelial cells. Proc Natl Acad Sci USA 1992, 89:6693-6697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Llorens A, Rodrigo I, Lopez-Barcons L, Gonzales-Garrigues M, Lozano E, Vinyals A, Quintilla M, Cano A, Fabra A: Down-regulation of E-cadherin in mouse skin carcinoma cells enhances a migratory and invasive phenotype linked to matrix metalloproteinase-9 gelatinase expression. Lab Invest 1998, 78:1131-1142 [PubMed] [Google Scholar]
- 38.Ara T, Deyama Y, Yoshimura Y, Higashino F, Shindoh M, Matsumoto A, Fukuda H: Membrane-type 1-matrix metalloproteinase expression is regulated by E-cadherin through the suppression of mitogen-activated protein kinase cascade. Cancer Lett 2000, 157:115-121 [DOI] [PubMed] [Google Scholar]
- 39.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M: A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 1994, 370:61-65 [DOI] [PubMed] [Google Scholar]
- 40.Luo J, Lubaroff DM, Henrix MJC: Suppression of prostate cancer invasive potential and matrix metalloproteinase activity by E-cadherin transfection. Cancer Res 1999, 59:3552-3556 [PubMed] [Google Scholar]
- 41.Wiesen JF, Werb Z: The role of stromelysin-1 in stromal-epithelial interactions and cancer. Enzyme Protein 1996, 49:174-181 [DOI] [PubMed] [Google Scholar]
- 42.Brinckeroff CE, Rutter JL, Benbow U: Interstitial collagenases as markers of tumor progression. Clin Cancer Res 2000, 6:4823-4830 [PubMed] [Google Scholar]
- 43.John A, Tuszynski G: The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res 2001, 7:14-23 [DOI] [PubMed] [Google Scholar]
- 44.Gianelli G, Antonaci S: Gelatinases and their inhibitors in tumor metastasis: from biological research to medical applications. Histol Histopathol 2002, 17:339-345 [DOI] [PubMed] [Google Scholar]
- 45.Yana I, Seiki M: MT-MMPs play pivotal roles in cancer dissemination. Clin Exp Metastasis 2002, 19:209-215 [DOI] [PubMed] [Google Scholar]
- 46.Kuniyasu H, Ellis LM, Evans DB, Abbruzzese JL, Fenoglio CJ, Bucana CD, Cleary KR, Tahara E, Fidler IJ: Relative expression of E-cadherin and type IV collagenase genes predicts disease outcome in patients with respectable pancreatic carcinoma. Clin Cancer Res 1999, 5:25-33 [PubMed] [Google Scholar]
- 47.Herbst RS, Yano S, Kuniyasu H, Khuri FR, Bucana CD, Guo F, Liu D, Kemp B, Lee JL, Hong WK, Fidler IJ: Differential expression of E-cadherin and type IV collagenase genes predicts outcome in patients with stage I non-small cell lung carcinoma. Clin Cancer Res 2000, 6:790-797 [PubMed] [Google Scholar]
- 48.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, Da Costa LT, Morin PJ, Vogelstein B, Kinzler KW: Identification of c-MYC as a target of the APC pathway. Science 1998, 281:1509-1512 [DOI] [PubMed] [Google Scholar]
- 49.Tetsu O, McCormick F: β-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999, 398:422-426 [DOI] [PubMed] [Google Scholar]
- 50.Gradl D, Kuhl M, Wedlich D: The Wnt/wg signal transducer β-catenin controls fibronectin expression. Mol Cell Biol 1999, 19:5576-5587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vallin J, Thuret R, Giacomello E, Faraldo MM, Thiery JP, Broders F: Cloning and characterization of three Xenopus slup promoters reveal direct regulation by Lef/β-catenin signaling. J Biol Chem 2001, 276:30350-30358 [DOI] [PubMed] [Google Scholar]
- 52.Wielenga VJ, Smits R, Korinek V, Smit L, Kielman M, Fodde R, Clevers H, Pals ST: Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J Pathol 1999, 154:515-523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hlubek F, Jung A, Kotzor N, Kirchner T, Brabletz T: Expression of the invasion factor laminin γ 2 in colorectal carcinomas is regulated by β-catenin. Cancer Res 2001, 61:8089-8093 [PubMed] [Google Scholar]
- 54.Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, Bodmer WF, Moyer MP, Riecken EO, Buhr HJ, Hanski C: Target genes of β-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci USA 1999, 96:1603-1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T: B-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol 1999, 155:1033-1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crawford HC, Fingleton BM, Rudolph-Owen LA, Heppner Goss KJ, Rubinfeld B, Polakis P, Matrisian LM: The metalloproteinase matrilysin is a target of β-catenin transactivation in intestinal tumors. Oncogene 1999, 18:2883-2891 [DOI] [PubMed] [Google Scholar]
- 57.Marchenko GN, Marchenko ND, Leng J, Strongin AY: Promoter characterization of the novel human matrix metalloproteinase-26 gene: regulation by the T-cell factor-4 implies specific expression of the gene in cancer cells of epithelial origin. Biochem J 2002, 363:253-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi M, Tsunoda T, Seiki M, Nakamura Y, Furukawa Y: Identification of membrane-type metalloproteinase-1 as a target of the β-catenin/Tcf4 complex in human colorectal cancers. Oncogene 2002, 21:5861-5867 [DOI] [PubMed] [Google Scholar]
- 59.Crawford HC, Fingleton B, Gustavson MD, Kurpios N, Wagenaar RA, Hassell JA, Matrisian LM: The PEA3 subfamily of Ets transcription factors synergizes with β-catenin-LEF-1 to activate matrilysin transcription in intestinal tumors. Mol Cell Biol 2001, 21:1370-1383 [DOI] [PMC free article] [PubMed] [Google Scholar]