Abstract
During October and November 2001, public health authorities investigated 11 patients with inhalational anthrax related to a bioterrorism attack in the United States. Formalin-fixed samples from 8 patients were available for pathological and immunohistochemical (IHC) study using monoclonal antibodies against the Bacillus anthracis cell wall and capsule. Prominent serosanguinous pleural effusions and hemorrhagic mediastinitis were found in 5 patients who died. Pulmonary infiltrates seen on chest radiographs corresponded to intraalveolar edema and hyaline membranes. IHC assays demonstrated abundant intra- and extracellular bacilli, bacillary fragments, and granular antigen-staining in mediastinal lymph nodes, surrounding soft tissues, and pleura. IHC staining in lung, liver, spleen, and intestine was present primarily inside blood vessels and sinusoids. Gram’s staining of tissues was not consistently positive. In 3 surviving patients, IHC of pleural samples demonstrated abundant granular antigen-staining and rare bacilli while transbronchial biopsies showed granular antigen-staining in interstitial cells. In surviving patients, bacilli were not observed with gram’s stains. Pathological and IHC studies of patients who died of bioterrorism-related inhalational anthrax confirmed the route of infection. IHC was indispensable for diagnosis of surviving anthrax cases. The presence of B. anthracis antigens in the pleurae could explain the prominent and persistent hemorrhagic pleural effusions.
During the months of October and November 2001, public health authorities in the United States investigated cases of inhalational and cutaneous anthrax related to bioterrorism. 1-6 The investigation started when Bacillus anthracis was isolated from the cerebrospinal fluid of a patient who worked as a photo editor for a media company in Florida. For the investigation, a confirmed case of anthrax was defined as a clinically compatible case of cutaneous, inhalational, or gastrointestinal illness that was laboratory-confirmed by isolation of B. anthracis from an affected tissue or site or by other laboratory evidence of B. anthracis based on at least 2 supportive laboratory tests, including ELISA serology, immunohistochemistry (IHC), and polymerase chain reaction (PCR). 1 Despite antibiotic therapy, the index patient died, and autopsy results confirmed the cause of death as inhalational anthrax. A suspect second inhalational case of this bioterrorism attack was considered when a co-worker of the index patient presented with pneumonia. This patient had received antibiotics, and bacterial cultures of blood samples showed no growth. For this second patient, inhalational anthrax was confirmed by use of supportive laboratory tests, which primarily included IHC and PCR of pleural fluid. 1,6 In total, 11 patients were identified to have inhalational anthrax during the attack. The Infectious Diseases Pathology Activity at the Centers for Disease Control and Prevention (CDC) received an array of tissue samples on 8 of these patients, of whom 5 died and 3 survived.
Previous pathological studies of fatal human cases of inhalational anthrax have described the presence and distribution of B. anthracis in formalin-fixed tissues by using special stains. 7-12 During the bioterrorism outbreak, special stains and novel B. anthracis cell wall and capsule-specific IHC assays were used to study tissue samples from surviving and non-surviving patients who had received multiple antibiotics. Knowledge of the consequences of effective treatment for B. anthracis on tissues has been limited. This report describes the pathology observed in 8 patients with bioterrorism-related inhalational anthrax and demonstrates how B. anthracis IHC assays can be successfully used for diagnosis and for better understanding the pathogenesis of this disease.
Materials and Methods
Epidemiological, demographic, and clinical information for the 8 patients who contracted inhalational anthrax during the 2001 US bioterrorism attack has been reported previously. 1-6,13-17 The tissues received for IHC study from each case are listed in Table 1 ▶ . Sections from all tissues were stained by using hematoxylin and eosin (H&E) and IHC. Gram’s staining (Brown-Hopps and Brown and Brenn stains) and Steiner silver staining were done on selected blocks from the mediastinum, lungs, liver, and spleen of the 5 patients who died, and on all tissues from the 3 patients who survived.
Table 1.
Case Numbers for 8 Inhalational Anthrax Patients and Tissues Studied by Immunohistochemistry for Each Patient
| Case* | Tissues |
|---|---|
| Fatal | |
| 1 | Mediastinal lymph nodes, lung, heart, liver, spleen, kidney, gastrointestinal tract, skin, conjunctiva |
| 5 | Mediastinal lymph nodes, lung, liver, spleen, gastrointestinal tract, skin |
| 6 | Mediastinal lymph nodes, lung, liver, spleen |
| 10 | Mediastinal lymph nodes, lung |
| 11 | Mediastinal lymph nodes, lung, heart, liver, spleen, kidney, gastrointestinal tract, skin, conjunctiva, nasal mucosa, pleural fluid, central nervous system biopsy |
| Non-fatal | |
| 2 | Bronchial biopsy, pleural biopsy |
| 8 | Pleural fluid cell block |
| 9 | Bronchial biopsy, pleural fluid cell block |
IHC was performed on 3-μm sections of formalin-fixed, paraffin-embedded tissues. Tissue sections were deparaffinized, rehydrated, and placed in a DAKO autostainer (DAKO Corporation, Carpinteria, CA). Sections were incubated for 1 hour using two mouse monoclonal antibodies: 1) an anti-B. anthracis cell-wall (IgM) antibody (slides were previously digested in 0.1 mg/ml Proteinase K) (Boehringer-Mannheim Corporation, Indianapolis, IN) and 2) an anti-B. anthracis capsule (IgM) antibody (slides were not digested before incubation) (USAMRIID, Frederick, MD). 18,19 Optimal dilutions of the antibodies had been determined by previous experiments on positive control tissue samples (cell-wall and capsule antibodies were used at 1:200 and 1:1000 dilutions, respectively). A biotinylated anti-mouse IgM antibody, streptavidin-alkaline phosphatase complex, and naphthol/fast red substrate (DAKO Corporation) were used for detection. Sections were counterstained with Mayer’s hematoxylin (Fisher Scientific, Pittsburgh, PA).
Positive controls included tissue sections of formalin-fixed, paraffin-embedded cultures of B. anthracis minced with normal human tissues and tissue samples from veterinary and other human confirmed cases. Negative controls consisted of a sequential tissue section from each block incubated with an IgM isotype antibody (a monoclonal anti-Rhizomucor antibody, catalog number M3565; DAKO Corporation). Controls also included formalin-fixed tissue specimens from autopsies and pleural effusion cell blocks of non-anthrax patients received during the 2001 investigation. Other negative tissue controls tested with the cell-wall and capsule anthrax antibodies included cases of Group A streptococcus, Streptocococcus pneumoniae, Francisella tularensis, Legionellla pneumophila, Yersinia pestis, Clostridium novyi, Clostridium perfringens, Rickettsia rickettsii, Rickettsia akari, lassa fever virus, yellow fever virus, Ebola hemorrhagic fever virus, and human herpesvirus type 1 and 3.
Results
Table 2 ▶ summarizes the demographic and clinical characteristics of the 8 inhalational anthrax patients and indicates the primary laboratory confirmation of B. anthracis. Most patients had occupations that involved handling of mail and presented with a febrile disease that included malaise, cough, and gastrointestinal symptoms. Pleural effusions were demonstrated on the initial chest radiograph of 7 patients. CT scans of 5 patients showed pleural effusions (including the patient with normal initial chest radiograph) and 4 of 5 demonstrated mediastinal lymphadenopathy. Cerebrospinal fluid was obtained only from the index case and showed abundant neutrophils and bacilli. In all fatal cases, anthrax sepsis was confirmed either before (patients 1, 10, and 11) or after death (patients 5 and 6) by culture of blood samples obtained at the time of hospitalization. Although the diagnosis of anthrax was already known in fatal cases, autopsies and IHC assays were performed primarily to establish or confirm the route of entry of B. anthracis. In comparison, pathology and IHC assays of pleural samples from the 3 surviving patients played a pivotal role in diagnosis and clinical management.
Table 2.
Demographic and Clinical Features of Eight Inhalation Anthrax Patients
| Case | Age (years)/sex | Occupation | Symptoms before admission | Findings on first chest radiograph | Findings from chest CT scan | Primary laboratory confirmation of B. anthracis infection |
|---|---|---|---|---|---|---|
| Fatal | ||||||
| 1 | 63/male | Media company (photo editor) | Fever, malaise, vomiting, altered mental status | Widened upper mediastinum, pleural effusion | Not obtained | Cerebro-spinal fluid culture |
| 5 | 55/male | Postal worker | Fever, fatigue myalgias, cough, shortness of breath | Lung infiltrates, perihilar tissue fullness, pleural effusion | Not obtained | Blood culture |
| 6 | 47/male | Postal worker | Cough, nausea, vomiting, syncope | Initially read as normal, upon review: perihilar lung infiltrates | Large bilateral pleural effusions, mediastinal lymphadenophathy and hemorrhage, perihilar infiltrates | Blood culture |
| 10 | 61/female | Hospital worker | Malaise, myalgias, dyspnea, chest pain, cough | Pleural effusions, pulmonary venous congestion | Bilateral pleural effusions, mediastinal lymphadenopathy and hemorrhage | Blood and pleural fluid cultures |
| 11 | 94/female | Retired | Fever, loss of appetite, fatigue, cough | Small pleural effusion, possible hilar enlargement | Not obtained | Blood culture |
| Non-fatal | ||||||
| 2 | 73/male | Media company (mail handler) | Fever, fatigue, cough, conjunctivitis, abdominal pain, vomiting | Multilobar pneumonia, pleural effusion, no mediastinal widening | Bilateral pleural effusions, pulmonary consolidation, no mediastinal lymphadenopathy | Pleural biopsy and bronchial biopsy IHC serology, and pleural fluid PCR |
| 8 | 56/female | Postal worker | Vomiting, diarrhea, fever, cough, shortness of breath, chest pain | Right pleural effusion, bibasilar infiltrates, no mediastinal widening | Large left pleural effusion, mediastinal lymphadenopathy, bibasilar pulmonary infiltrates | Pleural fluid IHC, blood PCR, and serology |
| 9 | 43/female | Postal worker | Fever, cough, shortness of breath, chest discomfort, myalgias, vomiting | Right pleural effusion, right hilar consolidation | Pleural effusion, mediastinal lymphadenopathy, perihilar consolidation | Pleural fluid and bronchial biopsy IHC, and serology |
Significant postmortem findings have been reported for individual cases. 13-16 The major pathological and histopathological findings in patients with fatal and non-fatal inhalational anthrax are presented in Tables 3 and 4 ▶ , respectively. In all patients who died, the most prominent gross autopsy findings included presence of large amounts of serosanguinous fluid in pleural cavities and edema and hemorrhage of mediastinal lymph nodes and surrounding soft tissues (Figure 1A) ▶ . Cutaneous lesions, clinically not suggestive of cutaneous anthrax, were noted in patient 5 (dermal hematoma) and patient 8 (chronic erosion on neck). Multiple focal hemorrhages in the small intestine were documented in patients 1 and 6, but no significant ulceration or mesenteric lymphadenopathy was reported.
Table 3.
Major Pathologic Findings for Five Inhalational Anthrax Patients Who Died
| Pathology | Patient 1 | Patient 5 | Patient 6 | Patient 10* | Patient 11 |
|---|---|---|---|---|---|
| Duration of antibiotic treatment in relation to time of death | 82 hours | 11 hours | 3 hours | 55 hours | 95 hours |
| Skin lesions | None | Dermal hematoma | None | None | None |
| Pleural fluid† | R: >1000 cc | R: 1300 cc | R: 250 cc | R: 2,500 cc | R: 1,000 cc |
| L: >1000 cc | L: 700 cc | L: 500 cc | L: 1,000 cc | L: 800 cc | |
| Mediastinal lymph nodes | Hemorrhage, necrosis, effacement | Hemorrhage, necrosis, immunoblasts, neutrophils | Hemorrhage, necrosis, immunoblasts, neutrophils | Hemorrhage | Hemorrhage, necrosis, immunoblasts, neutrophils |
| Pleurae and interhilar septae | Hemorrhage | Hemorrhage, inflammation | Hemorrhage | Inflammation | |
| Lung parenchyma | Focal hyaline membranes | Unremarkable | Prominent intra-alveolar macrophages | Prominent intra-alveolar macrophages | Peribronchial inflammation, intra-alveolar edema |
| Spleen | Necrosis | Congestion, immunoblasts | Congestion, immunoblasts, neutrophils | Congestion | |
| Intestine | Focal hemorrhage | Unremarkable | Focal hemorrhage and inflammation | Unremarkable | |
| Gram-positive bacilli | None | Present in all tissues | Present in all tissues | Present in lymph node | None |
| Location of bacilli with Steiner stain | Thoracic and abdominal tissues | Thoracic and abdominal tissues | Thoracic and abdominal tissues | Mediastinal lymph node | Mediastinal tissues, lung |
| IHC staining in thoracic tissues‡ | Abundant | Abundant | Abundant | Abundant | Abundant |
| IHC staining in liver and spleen | Present | Present | Present | Negative |
*Limited amounts of tissue available for study.
†, Amount of pleural fluid on right (R) and left (L) pleural cavity.
‡, Thoracic tissues include mediastinal lymph nodes and soft tissues and pleurae.
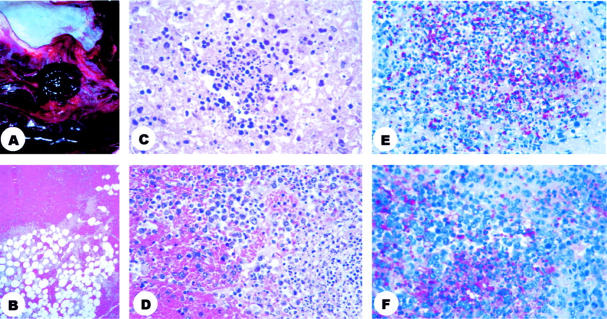
Figure 1.
Pathology and immunohistochemisty of mediastinal lymph nodes. A: Photograph of mediastinal compartment showing an enlarged, hemorrhagic lymph node and edematous, hemorrhagic soft tissues. B: Photomicrograph of mediastinal soft tissues showing hemorrhage. C: Photomicrograph of mediastinal lymph node showing necrosis and pyknotic nuclei. D: Photomicrograph of mediastinal lymph node showing hemorrhage, immunoblasts, and pyknotic nuclei. E: Photomicrograph of mediastinal lymph node showing abundant granular antigen-staining and bacillary fragments (B. anthracis cell-wall antibody). F: Photomicrograph of the same mediastinal lymph node, note the different pattern of granular antigen-staining when using the B. anthracis capsule antibody. H&E stain (B, C, D); immunohistochemical assay using naphthol/fast red substrate with hematoxylin counterstain (E, F). Original magnifications: B, ×5; C, D, E, and F ×40.
Histopathology
Samples from all patients who died showed hemorrhage (Figure 1B) ▶ and various amounts of necrosis of mediastinal lymph nodes (Figure 1C) ▶ . In 3 of these 5 patients, mediastinal lymph nodes also showed infiltration by neutrophils and prominent immunoblasts (Figure 1D) ▶ . Focal hemorrhage and edema of pleura and interhilar septa were found in all 5 non-surviving patients but were prominent in 3. In 2 of the 5 patients, these findings were accompanied by various degrees of mononuclear inflammation (Figure 2A) ▶ . Other findings in the lungs included variable intraalveolar edema and focal areas of hyaline membrane formation (Figure 2B) ▶ ; interstitial mononuclear inflammation was present in 1 patient (Figure 2B) ▶ . Intraalveolar inflammatory infiltrate was not observed in any of the non-surviving inhalational anthrax patients.
Figure 2.
Photomicrograph showing pathology and immunohistochemistry of lung. A: Lung showing thickened pleura with inflammation and fibrin. B: Lung showing intraalveolar edema and inflammation in the alveolar septa. C: Pleura showing gram-positive bacilli. D: Pleura showing silver staining bacilli. E: Lung showing abundant granular antigen-staining in the pleura, interalveolar septa, and intra alveolar macrophages. F: Lung showing granular antigen-staining of intraalveolar macrophages. H&E stain (A and B); gram’s stain (C); Steiner stain (D); immunohistochemical assay using the B. anthracis capsule antibody and naphthol/fast red substrate with hematoxylin counterstain (E and F). Original magnifications: A, ×10; B, ×20; C, ×100; D, E, and F, ×40.
The most frequent changes in the abdominal cavity were present in the spleen, where congestion (3 of 4 patients), presence of immunoblasts (2 of 4) and neutrophils (1 of 4), and necrosis (1 of 4) were observed. Two of 3 patients showed focal hemorrhages and inflammation in the small intestine. Findings not related to anthrax included centrilobular hepatocyte degeneration and fatty change in the liver and acute tubular necrosis in kidneys in 1 patient; another patient had scarred kidneys with cyst formation.
All 3 surviving inhalational anthrax patients showed reactive mesothelial cells admixed with inflammatory elements in pleural samples (Figure 3A) ▶ . Transbronchial biopsies of 2 patients demonstrated soft tissue edema, various amounts of hemorrhage, and minimal inflammatory infiltrate.
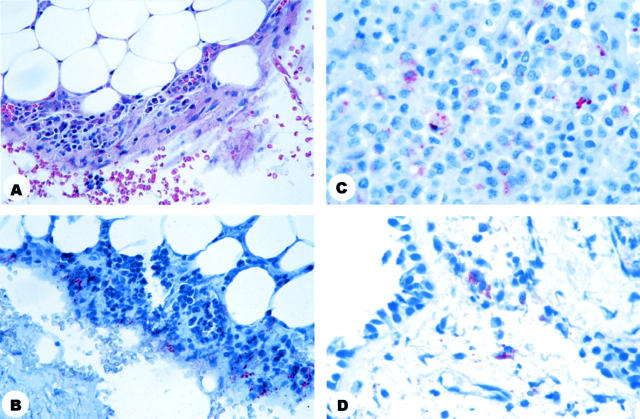
Figure 3.
Pathology and immunohistochemistry of biopsy specimens obtained from surviving patients. A: Photomicrograph of parietal pleural biopsy specimen showing reactive mesothelium and mixed inflammation. B: Photomicrograph of parietal pleural biopsy specimen showing abundant granular antigen-staining. C: Photomicrograph of pleural effusion cell block showing bacillary fragments and granular antigen-staining. D: Photomicrograph of transbronchial biopsy showing granular antigen-staining in histiocytes of the submucosa. H&E stain (A); immunohistochemical assay using the B. anthracis capsule antibody (B) or the B. anthracis cell-wall antibody (C, D) naphthol/fast red substrate with hematoxylin counterstain. Original magnifications: A and B, ×40; C and D, ×63.
Bacilli were identified, by use of H&E stain, in a mediastinal lymph node in only 1 patient. Demonstration of bacilli by use of gram’s stain varied and depended on the duration of antibiotic treatment. Three patients with fatal anthrax who were treated for less than 55 hours before they died showed abundant gram-positive bacilli in mediastinal tissues and pleura (Figure 2C) ▶ . In contrast, gram-positive bacilli were not identified in the 2 fatalities treated for more than 72 hours. Steiner silver stain demonstrated bacilli in all non-surviving and in l surviving patient. They were easily observed in pleural fluid, pleurae (Figure 2D) ▶ , mediastinal soft tissues, and spleen of the patients with treatment lasting less than 55 hours; patients treated for more than 72 hours showed various amounts of bacilli that primarily appeared “ghost-like,” folded, and knobbed.
IHC Assays
Overall, IHC using cell-wall antibody demonstrated bacilli, bacillary fragments, and lesser amounts of granular antigen-staining. Using the B. anthracis capsule antibody, the IHC pattern showed mostly bacterial granular antigens, while intact bacilli were less frequently identified. Bacilli were observed most often in extracellular spaces, while bacterial granular antigen-staining and bacillary fragments were seen intracellularly (in mononuclear inflammatory cells and interstitial fibroblastic cells) and extracellularly. Positive tissue sections always showed staining with both the capsule and cell-wall antibody; however, the amounts varied in each tissue. Negative tissue controls did not react with the cell-wall or capsule anthrax antibodies.
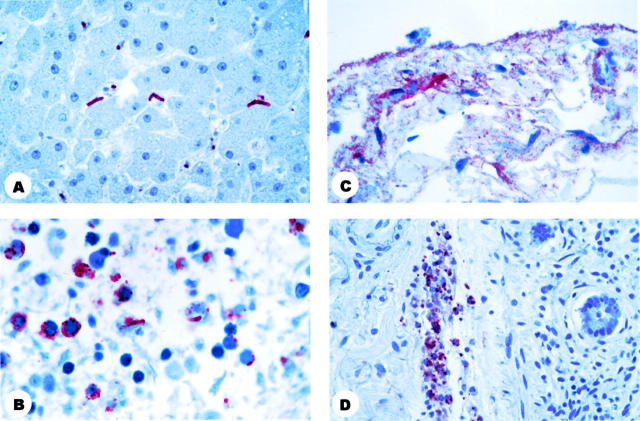
Abundant granular antigen-staining and bacillary fragments were present in necrotic areas of mediastinal lymph nodes and surrounding soft tissues of all 5 patients who died (Figure 1, E and F) ▶ and in the pleurae (Figure 2E) ▶ and interhilar lung septae of 4 of the 5 patients. Granular antigen-staining was present but in lower amounts in intraalveolar macrophages (3 of 5 patients) (Figure 2F) ▶ , and interalveolar septa (3 of 5), around small bronchi (2 of 5), and on the surface of bronchial epithelial cells (1 of 5). Cell blocks prepared from pleural fluid from 1 of the non-surviving patients showed granular antigen-staining in mononuclear inflammatory cells. In the abdominal cavity, granular antigen-staining and bacillary fragments were present in Kupffer’s cells of the liver (3 of 4 patients) (Figure 4A) ▶ , spleen (3 of 4) (Figure 4B) ▶ , necrotic hepatocytes (1 of 4), and focally in the serosa (Figure 4C) ▶ and blood vessels (Figure 4D) ▶ of the intestine (2 of 3). Sections from the nasal mucosa were available from 1 patient and showed granular antigen-staining in the submucosa. Sections from a sample of the dermal hematoma showed granular antigen-staining in macrophages of dermis. Other tissues available for study (conjunctiva, oral mucosa, central nervous system) did not demonstrate IHC staining.
Figure 4.
Photomicrographs showing immunohistochemistry of abdominal organs from patients who died. A: Liver showing bacilli in the sinusoids and bacillary fragments in Kupffer’s cells. B: Spleen showing bacillary fragments and granular antigen-staining. C: Intestinal serosa showing granular antigen-staining. D: Intestinal submocosal blood vessel showing granular antigen-staining. Immunohistochemical assay using the B. anthracis capsule antibody (B, C, and D) or the B. anthracis cell wall antibody (A) naphthol/fast red substrate with hematoxylin counterstain. Original magnifications: A and B, ×100; C, ×63; D, ×40.
Intact bacilli were identified by using IHC stains in mediastinal lymph nodes and surrounding soft tissues (5 of 5 patients), lung interstitium (3 of 5), around smaller bronchi (2 of 5), pleural surface (3 of 4), liver sinusoids (3 of 4) (Figure 4A) ▶ , spleen (3 of 4) (Figure 4B) ▶ , and focally in the serosa and vessels of the intestine (2 of 3). Rare bacilli were noted circulating in blood vessels of the heart and kidneys. In general, bacilli were more abundant on the pleural surface. Relatively fewer numbers were present in the other tissues. There was also variability, depending on the duration of antibiotic treatment, regarding the number of intact bacilli. Bacilli were abundant in patients who had received less than 55 hours of treatment, while specimens from patients who had received more than 72 hours of antibiotic treatment showed comparatively fewer bacilli.
IHC staining of tissues from surviving patients demonstrated abundant granular antigen-staining and bacillary fragments in pleural specimens (pleural tissue fragments, Figure 3B ▶ ; pleural effusion cell blocks, Figure 3C ▶ ). Bacilli were abundant in the patient who received less than 55 hours of antibiotic treatment, while the patients with more than 72 hours of treatment showed rare bacillary fragments. The transbronchial biopsies of 2 patients who survived showed rare interstitial histiocytes with granular antigen-staining (Figure 3D) ▶ .
Discussion
The patients presented here represent the first human cases of fatal and non-fatal inhalation anthrax related to a bioterrorism attack that have been studied using newly developed IHC assays. Pleural effusions that were small on presentation progressively enlarged and persisted even with effective antibiotic treatment were the most significant clinicopathologic feature. 6 The compromised respiratory function present in inhalational anthrax cases has been attributed, in part, to pleural effusions. 7 Bacilli and abundant B. anthracis cell wall and capsule antigens were demonstrated in the pleura of these patients by use of IHC. In addition, PCR amplification of B. anthracis was most successful from the pleural fluid of these patients. 20 Bacilli were abundant in the non-surviving patients who received less than 55 hours of antibiotic treatment. Granular cell wall and capsule antigen-staining and bacillary fragments were prominent in cell blocks prepared from pleural effusions of non-surviving and surviving patients who had received antibiotics for more than 72 hours. Our findings suggest that during the initial phase of inhalational anthrax, the pleura became infected by large numbers of B. anthracis, leading to the development of pleural effusions. In later phases of the infection, the large, persistent pleural effusions that occurred even after effective treatment may be explained by a continued inflammatory reaction to residual bacterial antigens and possibly the effect of toxins. The prominent finding of B. anthracis bacilli, bacillary fragments, and antigens in pleural effusions emphasizes the importance of IHC diagnostic assays in pleural fluid specimens of suspected anthrax patients.
The fatal inhalational anthrax cases related to this bioterrorism attack demonstrated hemorrhagic mediastinitis without pneumonia; the pulmonary infiltrates that were noted on chest radiographs corresponded to pulmonary edema and hyaline membrane formation. Animal models have demonstrated that inhaled B. anthracis spores are taken up by the alveolar macrophages in the lungs and transported to the mediastinal lymph nodes within hours, leaving no specific pulmonary lesions. 21-23 In the lymph nodes, the anthrax spores germinate, forming vegetative bacilli, which multiply intra- and extracellularly and release toxins. The edema and lethal-factor anthrax toxins bind to the protective anthrax antigen, allowing entry to the cell cytosol. 24 The edema toxin induces cyclic AMP, which results in damage to water homeostasis. The lethal toxin cleaves MAP kinases, releasing oxygen radicals and proinflammatory cytokines that result in cell death. Our IHC findings are consistent with these animal models; all patients who died had large numbers of bacilli, bacillary fragments, and B. anthracis cell wall and capsule antigens in the mediastinal lymph nodes, surrounding soft tissues, and pleura, with lesser numbers found in the lung parenchyma and organs in the abdominal cavity. Since the highest burden of B. anthracis bacilli and antigens was found in the mediastinal tissues and pleura, B. anthracis bacilli in the mediastinal lymph nodes may have directly infected the adjoining pleural space and rapidly spread in the thoracic cavity covering the lungs. Alternatively, some spore-containing macrophages may have disseminated to the pleural spaces where they germinated forming bacilli.
Non-human primate inhalational anthrax models have shown that hematogenous spread to abdominal organs and central nervous system follows the initial mediastinal lymph node seeding. 25 In a pathological study of human inhalational anthrax after a large outbreak in Sverdlovsk, Russia, hematogenous spread of B. anthracis was found to be associated with capillary and vascular lesions that consist of fibrin deposition and various amounts of neutrophilic infiltrate surrounding the vessel wall. 7 The capillaritis and vasculitis weakened the vessel wall and produced high- and low-pressure hemorrhages. 7 We observed hemorrhages in the 2001 bioterrorism-related cases, but the small vessels and capillaries showed minimal inflammation. The reasons for the difference in pathological findings between these two groups of inhalational anthrax cases are unclear, but potential explanations include earlier recognition and initiation of treatment, treatment with newer antibiotic combinations and better supportive care, dose and characteristics of B. anthracis spores used in the attack, or a combination of the above.
Meningeal spread of B. anthracis has been described in up to 80% of inhalational anthrax cases. 7 The initial patient with inhalational anthrax related to this bioterrorism attack presented with meningitis, and B. anthracis was cultured from cerebrospinal fluid. Other patients did not show meningitis clinically and the 2 patients who had postmortem examination of the central nervous system had no meningeal involvement. 14,16 The decreased meningeal spread in the bioterrorism-related anthrax cases can be explained by the early recognition and effective treatment regimens, and correlates with mortality rates lower than those previously associated with inhalational anthrax. 17
Before it was evident that these anthrax cases were part of a bioterrorism attack, the first autopsy was performed to definitively determine how the patient had acquired anthrax (cutaneous versus gastrointestinal versus inhalational). 1,6 The pathological and IHC features that indicated a respiratory route included mediastinal enlargement and hemorrhage with abundant IHC staining of mediastinal tissues, persistent pleural effusions, IHC evidence of vascular spread to other organs, and lack of an obvious primary cutaneous or gastrointestinal focus. Once it was established that B. anthracis had been acquired through the respiratory tract, public health officials were able to better define potential sources of inhalational anthrax. As new suspect cases appeared in this bioterrorism attack, postmortem and IHC studies were an indispensable factor in determining that the infections were acquired through the respiratory route and thus increased awareness of precautions necessary when performing autopsies on patients with infectious diseases. 1-5 In anthrax patients, most of the bacteria present in tissues during the first hours after death are in the vegetative stage; thus, the risk to personnel performing autopsies is through splashes and splatters to mucous membranes and percutaneous injuries. Procedures such as opening the skull and embalming should be avoided if possible since they can create aerosols with microscopic droplets containing bacilli, which may eventually sporulate. To prevent persistence of B. anthracis (in either vegetative or spore forms) in the environment and the consequent potential for occupational risk of cutaneous anthrax, the autopsy instruments and suite should be decontaminated with 0.5% hypochlorite solution. CDC advises that all clinicians, pathologists, and laboratorians need to be aware of the clinicopathologic features of diseases potentially caused by bioterrorism agents (eg, anthrax, tularemia, plague, botulism, hemorrhagic fevers, smallpox) and precautions necessary to obtain specimens that would aid in the prompt diagnosis so that public health measures are instituted to contain possible casualties. 26-29
IHC performed in formalin-fixed, paraffin-embedded tissues has traditionally offered several advantages, including the specific diagnosis of an agent, use of specimens that pose minimal biohazard for laboratory personnel, provision of a permanent record, and preservation of tissue morphology, which permits localization of the microorganism in specific structures. Interpretation of IHC assays should be done with caution, taking into consideration the clinical and exposure histories, morphological and technical aspects (eg, previously reported cross reactivity of the cell wall antibody with occasional Bacillus cereus strains), and results of other confirmatory tests, such as culture, PCR, and serology. 17,19 In our experience, the IHC diagnosis of anthrax infections should include interpretation of assays with both the cell wall and capsule antibodies. The cell wall antibody stained the bacilli present in tissues of patients who briefly received treatment, while the capsule antibody demonstrated bacillary fragments and granular antigens prominently in tissues of patients who had been treated for up to 14 days. IHC proved extremely valuable in 3 surviving patients who received treatment before collection of specimens and from whom gram’s stains, culture, and PCR failed to detect B. anthracis.
In summary, pathological and B. anthracis-specific IHC studies were performed in 8 inhalational anthrax cases related to the bioterrorism attack of 2001 in the United States. The pathological and IHC studies were indispensable for the investigation since they confirmed the route of infection for the 5 patients who died and contributed to the diagnosis in 3 surviving patients. IHC for B. anthracis is a powerful diagnostic modality since it demonstrates bacilli, bacillary fragments, and granular antigen-staining. We demonstrated for the first time by IHC that large numbers of bacilli and large amounts of cell wall and capsule antigens were present in pleural tissues. IHC was an important diagnostic tool and provided insights into the pathogenesis of the large, persistent pleural effusions present in inhalational anthrax.
Table 4.
Pathologic Findings for three Surviving Inhalational Anthrax Patients
| Pathology | Patient 2 | Patient 8 | Patient 9 |
|---|---|---|---|
| Duration of antibiotics in relation to specimen collection | 11 days for bronchial biopsy, 14 days for pleural biopsy | 40 hours | 3 days for pleural fluid, 6 days for bronchial biopsy |
| Skin lesions | None | Chronic erosion on neck* | None |
| Pleural fluid† | Recurrent, L: >500 cc | Progressive, R: 1,400 cc | R: 500 cc |
| Pleural tissue samples‡ | Inflammation, reactive mesothelium | Inflammation, reactive mesothelium | Inflammation, reactive mesothelium |
| Gram-positive bacilli | None | None | None |
| Steiner stain | Non-contributory | Bacilli present | Non-contributory |
| IHC staining in pleural tissues | Abundant granular antigens, rare bacilli | Abundant bacilli and granular antigens | Abundant granular antigens, rare bacilli |
| IHC staining in transbronchial biopsy specimen | Rare granular antigens | Not available | Rare granular antigens |
*The cutaneous lesion on neck was not temporarily or clinically consistent with cutaneous anthrax.
†Amount of pleural fluid on right (R) and left (L) pleural cavity.
‡Pleural tissue samples included cell blocks prepared from pleural fluid and a parietal pleural biopsy specimen.
Acknowledgments
We thank the Medical Examiner from Connecticut, Dr. H. Wayne Carver for inviting us to participate in performing the autopsy; the Medical Examiners from Maryland (Mary Ripple, Jack Titus, and David Fowler); the Medical Examiners from Washington D.C. (Michael Pollanen, Constance DiAngelo, Jacqueline Lee, and Jonathan Arden); and the Medical Examiner from New York (James Hill) for kindly providing tissues for study at the Centers for Disease Control and Prevention. Special thanks to State Health Epidemiologists Marc Traeger, Marcelle Layton, Annie Fine, James Hadler, and Eddy Bresnitz for arranging shipment of specimens and data to IDPA.
Footnotes
Address reprint requests to Sherif R. Zaki, MD, Ph.D., Infectious Disease Pathology Activity, Centers for Disease Control and Prevention, Mailstop G32, 1600 Clifton Rd, NE, Atlanta, GA 30333. E-mail: szaki@cdc.gov.
The research was carried out at the National Center for Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA.
The members of the Inhalational Anthrax Pathology Working Group are Jeanine Bartlett, Patricia Greer, Tara Ferebee-Harris, Jeltley Montague, Tim Morken, Christopher Paddock, and Chalanda Smith (from Infectious Diseases Pathology Activity); Conrad Quinn, Marc Fischer, and Rob Weyant (from the Division of Bacterial and Mycotic Diseases); Richard F. Meyer (from Bioterrorism Preparedness and Response); Michael Bell (from Division of Healthcare and Quality Promotion); and State Health Epidemiologist Steven Wiersma.
References
- 1.CDC Update: investigation of anthrax associated with intentional exposure and interim public health guidelines, October 2001. MMWR Morb Mortal Wkly Rep 2001, 50:889-893 [PubMed] [Google Scholar]
- 2.CDC Update: investigation of bioterrorism-related anthrax and interim guidelines for exposure management and antimicrobial therapy, October 2001. MMWR Morb Mortal Wkly Rep 2001, 50:909-919 [PubMed] [Google Scholar]
- 3.CDC Update: investigation of bioterrorism-related anthrax and interim guidelines for clinical evaluation of persons with possible anthrax. MMWR Morb Mortal Wkly Rep 2001, 50:941-948 [PubMed] [Google Scholar]
- 4.CDC Update: investigation of bioterrorism-related anthrax and adverse events from antimicrobial prophylaxis. MMWR Morb Mortal Wkly Rep 2001, 50:973-976 [PubMed] [Google Scholar]
- 5.CDC Update: Investigation of bioterrorism-related inhalational anthrax, Connecticut, 2001. MMWR Morb Mortal Wkly Rep 2001, 50:1029-1031 [PubMed] [Google Scholar]
- 6.Jernigan JA, Stephens DS, Ashford DA, Omenaca C, Topiel MS, Galbraith M, Tapper M, Fisk TL, Zaki S, Popovic T, Meyer RF, Quinn CP, Harper SA, Fridkin SK, Sejvar JJ, Shepard CW, McConnell J, Guarner J, Shieh WJ, Malecki JM, Gerberding JL, Hughes JM, Perkins BA, : members of the anthrax bioterrorism investigation team: Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg Infect Dis 2001, 7:933-944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grinberg LM, Abramova FA, Yampolskaya OV, Walker DH, Smith JH: Quantitative pathology of inhalational anthrax 1: quantitative microscopic findings. Mod Pathol 2001, 14:482-495 [DOI] [PubMed] [Google Scholar]
- 8.Walker DH, Yampolska O, Grinberg LM: Death at Sverdlovsk: what have we learned? Am J Pathol 1994, 144:1135-1141 [PMC free article] [PubMed] [Google Scholar]
- 9.Abramova FA, Grinberg LM, Yampolskaya OV, Walker DH: Pathology of inhalational anthrax in 42 cases from the Sverdlovsk outbreak of 1979. Proc Natl Acad Sci USA 1993, 90:2291-2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albrink WS, Brooks SM, Biron RE, Kopel M: Human inhalation anthrax, a report of three fatal cases. Am J Pathol 1960, 61:457-471 [PMC free article] [PubMed] [Google Scholar]
- 11.Enticknap JB, Galbraith NS, Tomlinson JH, Elias-Jones TF: Pulmonary anthrax caused by contaminated sacks. Br J Industr Med 1968, 25:72-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Severn M: A fatal case of pulmonary anthrax. Br Med J 1976, 1:748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bush LM, Abrams BH, Beall A, Johnson CC: Index case of fatal inhalational anthrax due to bioterrorism in the United States. N Engl J Med 2001, 345:1607-1610 [DOI] [PubMed] [Google Scholar]
- 14.Borio L, Frank D, Mani V, Chiriboga C, Pollanen M, Ripple M, Ali S, DiAngelo C, Lee J, Arden J, Titus J, Fowler D, O’Toole T, Masur H, Bartlett J, Inglesby T: Death due to bioterrorism-related inhalational anthrax. JAMA 2001, 286:2554-2559 [DOI] [PubMed] [Google Scholar]
- 15.Quintiliani R, Jr, Quintiliani R: Fatal case of inhalational anthrax mimicking intra-abdominal sepsis. Conn Med 2002, 66:261-267 [PubMed] [Google Scholar]
- 16.Barakat LA, Quentzel HL, Jernigan JA, Kirschke DL, Griffith K, Spear SM, Kelley K, Barden D, Mayo D, Stephens DS, Popovic T, Martson C, Zaki SR, Guarner J, Shieh WJ, Carver HW, Meyer RF, Swerdlow DL, Mast EE, Hadler JL, : Anthrax Bioterrorism Investigation Team: Fatal inhalational anthrax in a 94-year-old Connecticut woman. JAMA 2002, 287:863-868 [DOI] [PubMed] [Google Scholar]
- 17.Jernigan DB, Raghunathan PL, Bell BP, Brechner R, Bresnitz EA, Butler JC, Cetron M, Cohen M, Doyle T, Fischer M, Green C, Griffith KS, Guarner J, Hadler JL, Hayslett JA, Meyer R, Petersen LR, Phillips M, Pinner R, Popovic T, Quinn CP, Reefhuis J, Reissman D, Rosenstein N, Schuchat A, Shieh WJ, Siegal L, Swerdlow DL, Tenover FC, Traeger M, Ward JW, Weisfuse I, Wiersma S, Yeskey K, Zaki S, Ashford DA, Perkins BA, Ostroff S, Hughes J, Fleming D, Koplan JP, Gerberding JL, : National Anthrax Epidemiologic Investigation Team: Investigation of bioterrorism-related anthrax, United States 2001: epidemiologic findings. Emerg Infect Dis 2002, 8:1019-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezzell JW, Abshire TG, Little SF, Lidgerding BC, Brown C: Identification of Bacillus anthracis by using monoclonal antibody to cell wall galactose-N-acetylglucosamine polysaccharide. J Clin Microbiol 1990, 28:223-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De BK, Bragg SL, Sanden GN, Wilson KE, Diem LA, Marston CK, Hoffmaster AR, Barnett GA, Weyant RS, Abshire TG, Ezzell JW, Popovic T: Two-component direct fluorescent-antibody assay for rapid identification of Bacillus anthracis. Emerg Infect Dis 2002, 8:1060-1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmaster AR, Meyer RF, Bowen MP, Marston CK, Weyant RS, Barnett GA, Sejvar JJ: Jernigan JA, Perkins BA, Popovic T: Evaluation and validation of real-time polymerase chain reaction assay for rapid identification of Bacillus anthracis. Emerg Infect Dis 2002, 8:1178-1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross JM: The pathogenesis of anthrax following the administration of spores by the respiratory route. J Pathol Bact 1957, 73:485-494 [Google Scholar]
- 22.Barnes JM: The development of anthrax following the administration of spores by inhalation. Br J Exp Pathol 1947, 28:385-394 [Google Scholar]
- 23.Hanna PC, Acosta D, Collier RJ: On the role of macrophages in anthrax. Proc Natl Acad Sci USA 1993, 90:10198-10201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixon TC, Meselson M, Guillemini J, Hanna PC: Anthrax. N Engl J Med 1999, 341:815-826 [DOI] [PubMed] [Google Scholar]
- 25.Fritz DL, Jaax NK: Lawrence WB, Davis KJ, Pitt MLM, Ezzell JW, Friedlander AM: Pathology of experimental inhalation anthrax in the Rhesus monkey. Lab Invest 1995, 73:691-702 [PubMed] [Google Scholar]
- 26.CDC: Biological and chemical terrorism: strategic plan for preparedness and response: recommendations of the CDC strategic planning workgroup. MMWR Morb Mortal Wkly Rep 2000, 49:1-14 [PubMed] [Google Scholar]
- 27.Guarner J, Greer PW, Bartlett J, Chu MC, Shieh WJ, Zaki SR: Immunohistochemical detection of Francisella tularensis in formalin-fixed paraffin-embedded tissue. App Immunohisto Molec Morphol 1999, 7:122-126 [Google Scholar]
- 28.Guarner J, Shieh WJ, Greer PW, Gabastou JM, Chu M, Hayes E, Nolte KB, Zaki SR: Immunohistochemical detection of Yersinia pestis in formalin-fixed, paraffin-embedded tissue. Am J Clin Pathol 2002, 117:205-209 [DOI] [PubMed] [Google Scholar]
- 29.Zaki SR, Shieh WJ, Greer PW, Goldsmith CS, Ferebee T, Katshitshi J, Tshioko FK, Bwaka MA, Swanepoel R, Calain P, Khan AS, Lloyd E, Rollin PE, Ksiazek TG, Peters CJ: A novel immunohistochemical assay for the detection of ebola virus in skin: implications for diagnosis, spread, and surveillance of ebola hemorrhagic fever. J Infect Dis 1999, 179(Suppl 1):S36-S47 [DOI] [PubMed] [Google Scholar]