Abstract
Testicular yolk sac tumor (YST) of infants is biologically distinct from its adult counterpart. Cytogenetically, YSTs in infants generally lack i(12p), which is highly characteristic of adult germ cell tumors (GCTs), whereas they frequently show a deletion of 1p36, indicating that the loss of a certain gene(s) in this region is an important event in the pathogenesis of infantile YSTs. In the present study, we examined 10 testicular YSTs from infants for promoter methylation status of the RUNX3 gene, localizing in 1p36.1, and loss of heterozygosity (LOH) in this region, on the presumption that RUNX3 acts as a tumor suppressor. Methylation of RUNX3 and LOH at 1p36.1 were detected in 8 of 10 (80%) and 6 of 8 (75%) infantile YSTs examined, respectively. All six cases harboring LOH showed RUNX3 methylation. In contrast, 0 of 12 adult GCTs showed RUNX3 methylation, and LOH at 1p36.1 was less frequent (1 of 6 cases: 16%) in adult GCTs. There is a significant difference in RUNX3 methylation between these 2 groups (P < 0.001). In normal testes of the young group, RUNX3 methylation was not detected. These results strongly suggest that RUNX3 is one of the tumor suppressors involved in the pathogenesis of testicular YSTs in infants.
Yolk sac tumor (YST) is the most common germ cell tumor (GCT) occurring in the testes of infants. 1 The YST occurring in infants differs in many aspects from that in adults; in infants, it is almost always a pure neoplasm, whereas in adults it is, with rare exceptions, one component of a mixed germ cell tumor. 1 In contrast to adult GCTs, infantile YST is usually associated with a favorable prognosis. 1 Cytogenetically, isochromosome i(12p) is a highly specific abnormality in adult GCTs 2-5 whereas it is rare, 6,7 but a deletion of the distal 1p spanning 1p36 is more common in infantile YST. 7-11 It has been strongly suggested that the pathogenesis of infantile YSTs is different from that of adult GCTs, and that there is a certain gene(s) in 1p36, the loss of which is responsible for the development of infantile YST.
In the present study, we focused on the runt-related transcription factor 3 (RUNX3) gene, which is localized to 1p36.1, and decreased expression of which is known to be responsible for the occurrence of gastric cancer. 12 In infantile YSTs, hypermethylation of the RUNX3 gene promoter and loss of heterozygosity (LOH) at 1p36.1 were highly frequent events.
Materials and Methods
Materials
Ten testicular pure YSTs from infants were examined. Materials were obtained during surgery and collected from several hospitals. For comparative studies, we used 12 testicular nonseminomatous germ cell tumors of adulthood, and 10 testes of young individuals, which were considered essentially normal. Most of the 12 adult tumors were mixed GCTs, and the breakdown was as follows: YST + embryonal carcinoma (EC) (5 cases), YST + immature teratoma (Ti) (1case), EC + Ti (3 cases), YST + EC + Ti (1 case), EC + mature teratoma (1 case), and EC (1 case). The breakdown of the 10 normal testes was as follows: 1-, 3-, 7-, 8-, and 14-year-old boy’s testis, and 17-, 17-, 21-, 34-, and 44-year-old adult testes obtained from various urologic surgeries or autopsies. All tissues were fixed in 10% formalin and embedded in paraffin. DNA was extracted from tumors and normal tissue, as described by Goelz et al, 13 from 10-μm-thick paraffin-embedded serial sections.
Methylation-Specific PCR (MSP)
The promoter methylation status of the RUNX3 gene was determined as described previously. 14 Briefly, genomic DNA denatured by NaOH was treated with sodium bisulfite and purified, which was subjected to PCR. The primer sequences used were: nucleotides 64917 to 64940, 5′-ATA ATA GCG GTC GTT AGG GCG TCG-3′; nucleotides 65008 to 65031, 5′-GCT TCT ACT TTC CCG CTT CTC GCG-3′ for detecting the methylated RUNX3 gene promoter; nucleotides 64917 to 64940, 5′-ATA ATA GTG GTT GTT AGG GTG TTG-3′; and nucleotides 65008 to 65031, 5′-ACT TCT ACT TTC CCA CTT CTC ACA-3′ for detecting the unmethylated RUNX3 gene promoter (accession number AL023096). 15 The size of the PCR amplification product for both the methylated and the unmethylated reaction was 115 bp. As a positive control, Sss-I methylase (New England BioLabs, Inc., Beverly, MA) was used to methylate 100 μg of blood-derived DNA. The PCR products were analyzed by electrophoresis on a 6% polyacrylamide gel.
Microsatellite Analysis
DNAs from tumors and adjacent normal testicular tissues were analyzed for loss of heterozygosity (LOH) by amplification of repeat sequences using PCR. Two polymorphic microsatellite markers, including D1S199 and D1S507, spanning chromosomal band 1p36.1, were used. Both primers were obtained from MapPairs (Research Genetics, Huntsville, AL). The PCR products were analyzed by electrophoresis on a 6% polyacrylamide gel.
Reverse Transcription-PCR (RT-PCR)
Total RNA was extracted, by use of Trizol reagent (Invitrogen, Carlsbad, CA), from two normal testes (17- and 34-year-old adult testes) obtained from autopsy. One microgram of total RNA was used as the template for RT-PCR, to amplify a 152-bp fragment of RUNX3. The primer sequences used were: 5′-AGG CAT TGC GCA GCT CAG CGG AGT A-3′ (sense) and 5′-TCT GCT CCG TGC TGC CCT CGC ACT G-3′ (antisense) (accession number Z38104) and a 587-bp fragment of β-actin. The reverse transcription was performed at 50°C for 30 minutes, and followed by a PCR protocol including 38 cycles at an annealing temperature of 55°C. The PCR products were analyzed by electrophoresis on a 6% polyacrylamide gel.
Statistical Analysis
Statistical comparisons were performed using Fisher’s exact test.
Results
Promoter Methylation Status of the RUNX3 Gene
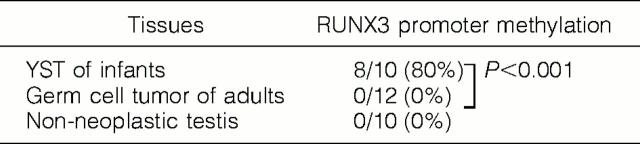
A summary of the MSP analysis is shown in Table 1 ▶ . The RUNX3 promoter was methylated in 8 of the 10 (80%) infantile YSTs (Figure 1a) ▶ . In contrast, 0 of the 12 adult GCTs showed methylation of RUNX3 (Figure 1b) ▶ . RUNX3 promoter methylation was significantly more frequent in infantile YSTs than in adult GCTs (P < 0.001). All of the corresponding normal testicular tissues obtained from 6 infantile cases, which were available for MSP, showed no methylation of RUNX3. In all of the 10 control normal testes, methylation of RUNX3 was not detected (Figure 1c) ▶ .
Table 1.
Methylation Status of RUNX3 in Testicular Yolk Sac Tumors (YSTs) of Infants, Testicular Nonseminomatous Germ Cell Tumors of Adults, and Nonneoplastic Testicular Tissue
Figure 1.
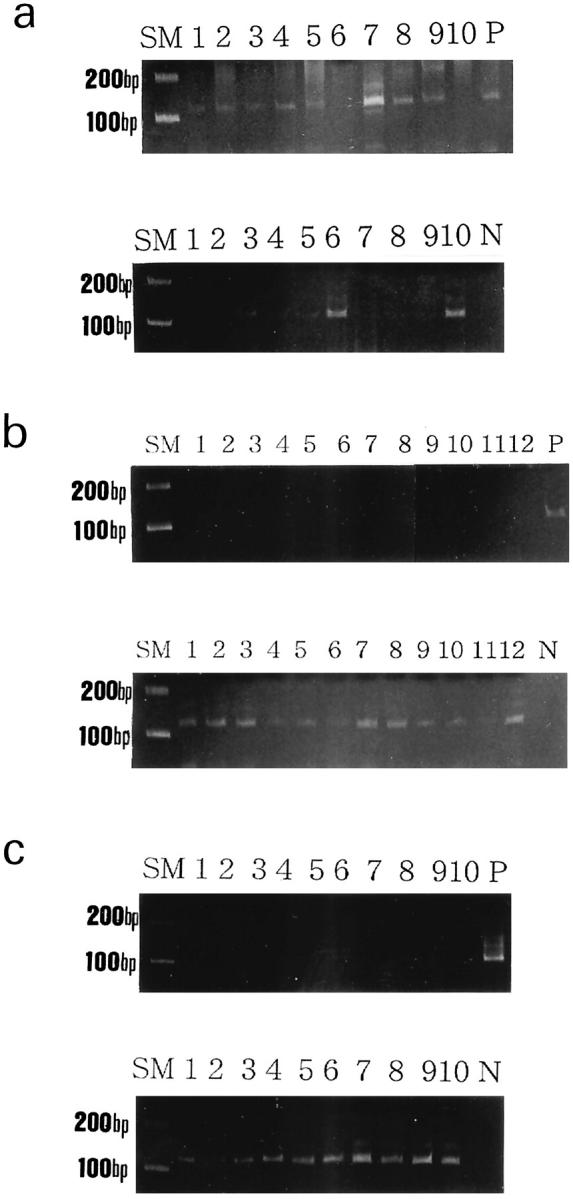
a: MSP analysis of the RUNX3 gene promoter in 10 yolk sac tumors (YSTs) from infants. Upper, methylated sequence-specific PCR for RUNX3; lower, unmethylated sequence-specific PCR for RUNX3. Methylated PCR products were present in 8 of 10 YSTs of infants. b: MSP analysis of the RUNX3 gene promoter in 12 germ cell tumors (GCTs) of adults. Upper, methylated sequence-specific PCR for RUNX3; lower, unmethylated sequence-specific PCR for RUNX3. No methylated PCR products were present in 12 adult GCTs. Unmethylated PCR products were present in all of the samples. c: MSP analysis of the RUNX3 gene promoter in 10 normal testes. Cases 1 to 10 correspond to 1-, 3-, 6-, 7-, 8-, 17-, 17-, 21-, 34-, and 44-year-old human testes, respectively. Upper, methylated sequence-specific PCR for RUNX3; lower, unmethylated sequence-specific PCR for RUNX3. No methylated PCR products were detected in any of the samples. Unmethylated PCR products were present in all 10 samples. P, positive control; N, distilled water as a negative control; SM, size marker.
LOH at 1p36.1
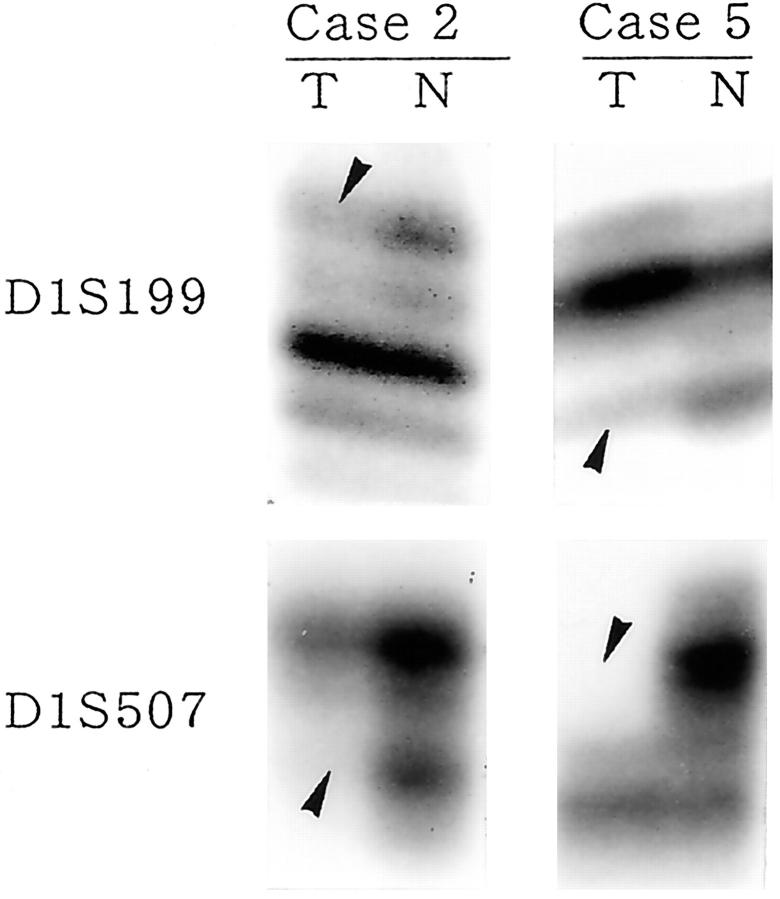
LOH at the RUNX3 locus (D1S199 and/or D1S507) was observed in 6 of the 8 (75%) infantile YSTs examined (Table 2 ▶ ; Figure 2 ▶ ). On the other hand, among 6 adult GCTs available for this analysis, only 1 (16%) showed LOH. LOH at the RUNX3 locus was significantly more frequent in infantile YSTs than in adult GCTs (P < 0.05).
Table 2.
Methylation Status of RUNX3 and Patterns of Allelic Loss on Chromosome at 1p36.1 in Testicular Yolk Sac Tumors of Infants
| Case no. | Age | Methylation status of RUNX3* | LOH analysis† | |
|---|---|---|---|---|
| D1S199 | D1S507 | |||
| 1 | 1 year 7 months | M | — | LOH |
| 2 | 1 year | M | LOH | LOH |
| 3 | 2 years | M | ND | ND |
| 4 | 11 months | M | — | LOH |
| 5 | 1 year 7 months | M | LOH | LOH |
| 6 | 1 year | — | ND | ND |
| 7 | 1 year | M | — | — |
| 8 | 1 year 8 months | M | LOH | NI |
| 9 | 3 years | M | LOH | NI |
| 10 | 2 months | — | — | NI |
*M, methylated; —, unmethylated.
†—, heterozygosity retained; NI, not informative; ND, not done.
Figure 2.
LOH analysis at 1p36.1 in infantile YSTs. LOH of both D1S199 and D1S507 markers was detected in case 2 and case 5. Arrowheads indicate LOH. T, tumor; N, normal testicular tissue.
Correlation Between RUNX3 Methylation and LOH at 1p36.1
All 6 infantile YSTs harboring LOH at 1p36.1 showed RUNX3 methylation (Table 2) ▶ .
RT-PCR of RUNX3
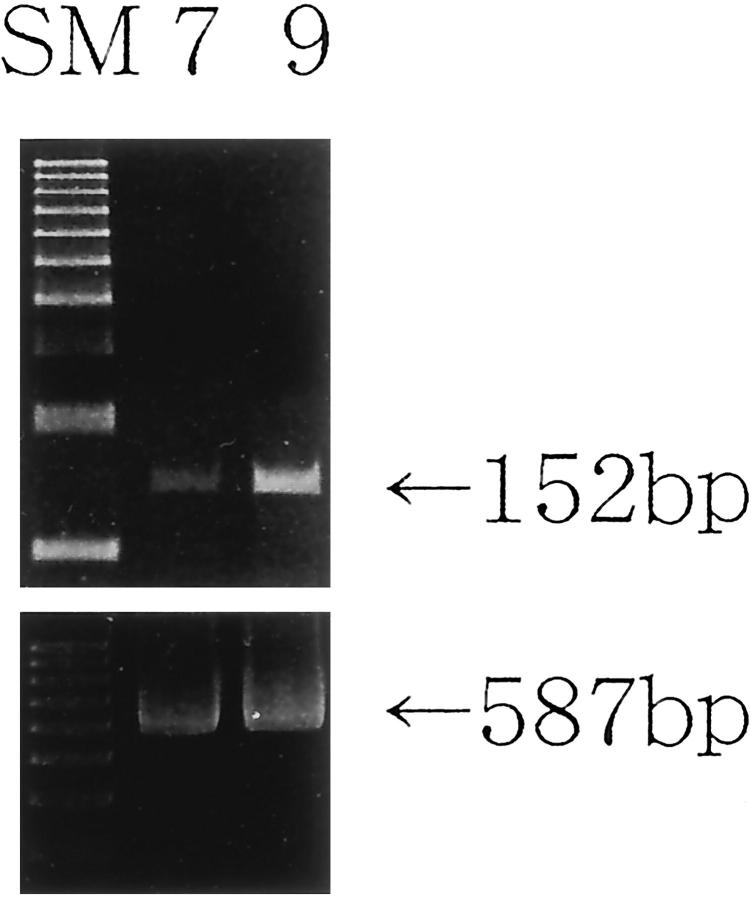
RUNX3 mRNA was expressed in both 17- and 34-year-old human normal testes examined, which had no methylated RUNX3 promoter (Figure 3) ▶ .
Figure 3.
RT-PCR of RUNX3 in 17-year-old (Case 7) and 34-year-old (Case 9) human normal testes (Cases 7 and 9 correspond to those in Figure 1c ▶ ). RUNX3 mRNA (152 bp) was detected in both. β-actin (587 bp) served as an internal control. SM, size marker.
Discussion
The present study showed that hypermethylation of the RUNX3 gene promoter frequently occurred in testicular YSTs of infants. Furthermore, LOH at 1p36.1, where the RUNX3 gene localizes, was detected in the most hypermethylated cases. In human gastric cancers it has been shown that promoter hypermethylation and hemizygous deletion of the RUNX3 gene correlated with a significant reduction in expression, and the tumorigenicity of cell lines in nude mice was inversely related to their level of RUNX3 expression, indicating that RUNX3 is a tumor suppressor involved in the development of gastric cancers. 12 Our recent study on RUNX3 methylation in gastric cancer cell lines also showed that the hypermethylation was associated with the loss of RUNX3 expression. 15 In the present study, we confirmed the expression of RUNX3 mRNA in normal control testes. All these results indicate that the RUNX3 gene acts as a tumor suppressor in testicular YSTs of infants, as well as in gastric cancers. In fact, the presence of a certain tumor suppressor gene(s) at 1p36 has been pointed out in recent years, because a deletion that encompasses this region has been frequently detected in infantile YSTs. 7-11
In contrast, 0 of 12 adult GCTs showed hypermethylation of the RUNX3 gene promoter, and LOH at 1p36.1 was less frequent in adult GCTs than in infantile YSTs. It is less likely that RUNX3 is involved in the pathogenesis of adult GCTs. Previous cytogenetic studies had revealed that hyperploidy and structural abnormality of the chromosomes, such as isochromosome i(12p), are common in testicular GCTs of adults, 2-5 but rare in infantile YSTs. 6,7 The fact that YSTs in infants are usually not associated with intratubular germ-cell neoplasia, 16-19 which is common in adult GCTs, 16,18,20 also indicates that the pathogenesis is fundamentally different between infantile YST and adult GCT. Our results also support this idea. It is well known that YSTs in infants rarely metastasize or recur, so orchiectomy alone provides a favorable prognosis, whereas adult GCTs usually show an aggressive biological behavior despite intense chemotherapy. Differences in genetic or cytogenetic background possibly reflect the contrasting biological behavior of these two groups.
To date, little has been elucidated about the expression site or function of RUNX3 in normal human tissue. In mouse fetus, RUNX3 expression was found in the gastrointestinal tract, trigeminal or dorsal root ganglia, and the cartilage in limbs and the nasal cavity. 12 In the RUNX3 null mouse, the gastric mucosa exhibits hyperplasia due to stimulated proliferation and suppressed apoptosis of epithelial cells, and the cells are resistant to the growth-inhibitory and apoptosis-inducing action of TGF-β, indicating that RUNX3 is a major growth regulator of gastric epithelial cells. 12 In humans, RUNX3 expression has been confirmed in hematopoietic cells. 21-23 In both mice and humans, however, little mention has been made of its expression in the gonads, including the testes. We confirmed the presence of RUNX3 mRNA in 17- and 34-year-old normal human testes. RUNX3 might be also related to cell proliferation or apoptosis, especially of germ cells, in human testes. Further study on the intratesticular localization (germ cells and/or somatic cells) and function of RUNX3 is needed to better interpret the abnormalities of RUNX3 detected in the present study.
In conclusion, both hypermethylation of the RUNX3 gene promoter and LOH at its locus, 1p36.1, are highly frequent events in testicular infantile YST. RUNX3 is probably one of the tumor suppressors in the pathogenesis of infantile YSTs.
Footnotes
Address reprint requests to Noriko Kato, Department of Pathology, Yamagata University School of Medicine, Yamagata, 990-9585, Japan. E-mail: nkato@med.id.yamagata-u.ac.jp.
References
- 1.Ulbright TM, Amin MB, Young RH: Germ cell tumors: nonseminomatous. Atlas of Tumor Pathology. Tumors of the Testis, Adnexa, Spermatic Cord, and Scrotum. 1997:103-174 Armed Forces Institute of Pathology, Washington DC
- 2.Atkin NB, Baker MC: Specific chromosome change, i(12p) in testicular tumours? Lancet 1982, 2:1349. [DOI] [PubMed] [Google Scholar]
- 3.Castedo SMMJ, De Jong B, Oosterhuis JW, Seruca R, Meerman GJ, Dam A, Koops HS: Cytogenetic analysis of ten human seminomas. Cancer Res 1989, 49:439-443 [PubMed] [Google Scholar]
- 4.Castedo SMMJ, De Jong B, Oosterhuis JW, Seruca R, Idenburg VJS, Dam A, Meerman G, Koops HS, Sleijfer DT: Chromosomal changes in human primary testicular nonseminomatous germ cell tumors. Cancer Res 1989, 49:5696-5701 [PubMed] [Google Scholar]
- 5.De Jong B, Oosterhuis JW, Castedo SMMJ, Vos A, Meerman GJ: Pathogenesis of adult testicular germ cell tumors: a cytogenetic model. Cancer Genet Cytogenet 1990, 48:143-167 [DOI] [PubMed] [Google Scholar]
- 6.Oosterhuis JW, Castedo SMMJ, Jong B, Seruca R, Buist J, Koops HS, Leeuw JB: Karyotyping and DNA flow cytometry of an orchidoblastoma. Cancer Genet Cytogenet 1988, 36:7-11 [DOI] [PubMed] [Google Scholar]
- 7.Perlman EJ, Cushing B, Hawkins E, Griffin CA: Cytogenetic analysis of childhood endodermal sinus tumors: a pediatric oncology group study. Pediatr Pathol 1994, 14:695-708 [DOI] [PubMed] [Google Scholar]
- 8.Jenderny J, Koster E, Meyer A, Borchers O, Grote W, Harms D, Janig U: Detection of chromosome aberrations in paraffin sections of seven gonadal yolk sac tumors of childhood. Hum Genet 1995, 96:644-650 [DOI] [PubMed] [Google Scholar]
- 9.Perlman EJ, Valentine MB, Griffin CA, Look AT: Deletion of 1p36 in childhood endodermal sinus tumors by two-color fluorescence in situ hybridization: a pediatric oncology group study. Genes Chromosomes Cancer 1996, 16:15-20 [DOI] [PubMed] [Google Scholar]
- 10.Bussey KJ, Lawce HJ, Olson SB, Arthur DC, Kalousek DK, Krailo M, Giller R, Heifetz S, Womer R, Magenis RE: Chromosome abnormalities of eighty-one pediatric germ cell tumors: sex-, age-, site-, and histopathology-related differences: a Children’s Cancer Group study. Genes Chromosomes Cancer 1999, 25:134-146 [PubMed] [Google Scholar]
- 11.Mostert M, Rosenberg C, Stoop H, Schuyer M, Timmer A, Oosterhuis W, Looijenga L: Comparative genomic and in situ hybridization of germ cell tumors of the infantile testis. Lab Invest 2000, 80:1055-1064 [DOI] [PubMed] [Google Scholar]
- 12.Li QL, Ito K, Sakakura C, Fukamachi H, Inoue K, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB, Kim HM, Kim WJ, Yamamoto H, Yamashita N, Yano T, Ikeda T, Itohara S, Inazawa J, Abe H, Hagiwara A, Yamagishi H, Ooe A, Kaneda A, Sugimura T, Ushijima T, Bae SC, Ito Y: Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell 2002, 109:113-124 [DOI] [PubMed] [Google Scholar]
- 13.Goelz SE, Hamilton SR, Vogelstein B: Purification of DNA from formaldehyde-fixed and paraffin-embedded human tissue. Biochem Biophys Res Commun 1985, 130:118-126 [DOI] [PubMed] [Google Scholar]
- 14.Herman JG, Graff JR, Myohane S, Nelkin BD, Baylin SB: Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 1996, 93:9821-9826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waki T, Tamura G, Sato M, Terashima M, Nishizuka S, Motoyama T: Promoter methylation status of DAP-kinase and RUNX3 genes in neoplastic and non-neoplastic gastric epithelia. Cancer Sci 2003, 94:360-364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koide O, Iwai S, Baba K, Iri H: Identification of testicular atypical germ cells by an immunohistochemical technique for placental alkaline phosphatase. Cancer 1987, 60:1325-1330 [DOI] [PubMed] [Google Scholar]
- 17.Manivel JC, Simonton S, Wold LE, Dehner LP: Absence of intratubular germ cell neoplasia in testicular yolk sac tumors in children. A histochemical and immunohistochemical study. Arch Pathol Lab Med 1988, 112:641-645 [PubMed] [Google Scholar]
- 18.Soosay GN, Bobrow L, Happerfield L, Parkinson MC: Morphology and immunohistochemistry of carcinoma in situ adjacent to testicular germ cell tumours in adults and children: implications for histogenesis. Histopathology 1991, 19:537-544 [DOI] [PubMed] [Google Scholar]
- 19.Hawkins E, Heifetz SA, Giller R, Cushing B: The prepubertal testis (prenatal and postnatal): its relationship to intratubular germ cell neoplasia: a combined pediatric oncology group and children’s cancer study group. Hum Pathol 1997, 28:404-410 [DOI] [PubMed] [Google Scholar]
- 20.Jacobsen GK, Henriksen OB, Maase HVD: Carcinoma in situ of testicular tissue adjacent to malignant germ-cell tumors: a study of 105 cases. Cancer 1981, 47:2660-2662 [DOI] [PubMed] [Google Scholar]
- 21.Levanon D, Negreanu V, Bernstein Y, Bar-Am I, Avivi L, Groner Y: AML1, AML2, and AML3, the human members of the runt domain gene-family: cDNA structure, expression, and chromosomal localization. Genomics 1994, 23:425-432 [DOI] [PubMed] [Google Scholar]
- 22.Meyers S, Lenny N, Sun W, Hiebert SW: AML2 is a potential target for transcriptional regulation by the t(8;21) and t(12;21) fusion proteins in acute leukemia. Oncogene 1996, 13:303-312 [PubMed] [Google Scholar]
- 23.Le XF, Groner Y, Kornblau SM, Gu Y, Hittelman WN, Levanon D, Mehta K, Arlinghaus RB, Change KS: Regulation of AML2/CBFA3 in hematopoietic cells through the retinoic acid receptor-dependent signaling pathway. J Biol Chem 1999, 274:21651-21658 [DOI] [PubMed] [Google Scholar]