Abstract
Previously, we have shown that ethanol (EtOH) stimulates a rapid increase in the ciliary beat frequency (CBF) of bovine bronchial epithelial cells (BBECs) via the activation of PKA. We have also shown that inhibitors of nitric oxide synthase block EtOH-stimulated increases in CBF. We hypothesize that EtOH acutely stimulates CBF via the activation of both PKA and PKG pathways. Using chemiluminescence detection of nitric oxide (NO), we directly measured increases in NO production in BBECs treated with 100 mmol/L of EtOH beginning at 25 minutes. Pretreatment of BBECs with guanylyl cyclase inhibitors, ODQ or LY83583, resulted in the inhibition of EtOH-stimulated CBF. Low concentrations (1 nmol/L) of cyclic nucleotide analogues do not stimulate CBF increases. However, a combination of both 1 nmol/L of 8Br-cAMP and 8Br-cGMP stimulates a significant increase over baseline CBF. This effect could be blocked by pretreating BBECs with inhibitors of either PKA or PKG. Very high concentrations of either 8Br-cAMP or 8Br-cGMP (≥100 μmol/L) were required to cross-activate both PKA and PKG. This suggests that cross-activation of PKA by cGMP is not occurring at the concentrations (1 nmol/L) capable of stimulating CBF. 8-pCPT-cGMPS, an antagonist analogue to cGMP, blocked EtOH-stimulated PKA activity increases. These data support that EtOH-stimulated increases in CBF require the dual activation of both PKA (via cAMP) and PKG (via NO).
In the lung, the mucociliary apparatus of the airways maintains host protection from various pathogens such as inhaled particles, substances, and microbes. 1 Potential injurious agents can be trapped by the mucus lining the airways and propelled out of the lungs via the orchestrated beating of ciliated epithelial cells. The beating frequency of such cells can vary depending on temperature; age; disease state; or exposure to chemical, pharmacological, and environmental agents. 2,3 Thus, the action of the cilia represents a regulatable host defense. The mechanisms that regulate the stimulation of ciliary beating have been studied extensively in both mammalian and nonmammalian systems. Many of these studies have focused on cyclic nucleotide-dependent regulation of ciliary beat frequency (CBF).
Cyclic nucleotides were first implicated in the ciliary beating of protozoans and other single-celled organisms. 4 Such studies have consistently demonstrated that cAMP-elevating agents stimulate increased CBF. The mechanism of this stimulated cilia beating involves the activation of the cAMP-dependent protein kinase (PKA). PKA has been identified on Tetrahymena thermophila, 5 Paramecium tetraurelia, 6 Chlamydomonas, 7 bovine, 8 ovine, 9 rabbit, 10 and human 11 cilia. A-kinase anchoring proteins have recently been demonstrated to exist on the apical surface of mouse 12 and human 13 airway epithelial cells. Specific dynein-associated substrates for PKA have been identified from ciliary axonemes. Additionally, agents that elevate cGMP can also stimulate increased CBF. This has been shown to occur through the activation of the cGMP-dependent protein kinase (PKG). Although less is known about cGMP regulation of cilia beating compared to cAMP, stimulation of CBF by cGMP has been reported in human, 14 rabbit, bovine, 8 and Paramecium 15 cilia. Specific cGMP-dependent substrate phosphorylation 16,17 and the enriched concentration of PKG on cilia 18 has been demonstrated in Paramecium.
We originally reported that elevations in nitric oxide (NO) were responsible for stimulated CBF. 19 Our studies suggested that NO inhibitors could block the stimulation of bovine cilia by cytokines such as tumor necrosis factor-α and interleukin-1β. 20 Subsequent studies have confirmed an association between NO and cilia beating. 21-25 In the rabbit, a calcium-mediated rise in NO stimulates the production of cGMP and subsequent increases in CBF. 24 In the rat, nitric oxide synthase (NOS) and PKG Iβ have been identified on the cilia by immunohistochemical methods. 26 In bovine ciliated bronchial epithelial cells, NO regulates the ethanol (EtOH)-mediated rapid stimulation of CBF. 27
In EtOH-mediated signaling, NO appears to have an upstream regulatory control of cAMP-dependent processes. We have shown that acute EtOH exposure stimulates bovine bronchial CBF via a PKA-mediated mechanism and chronic EtOH exposure induces an uncoupling of this pathway resulting in a desensitization of the cilia to cAMP-stimulated increases in cilia beating. 28 NOS inhibitors are capable of blocking the EtOH stimulation of PKA and CBF. 29 EtOH has been reported to diminish depolarized backward swimming in Paramecium via a reduction in cGMP levels. 30 EtOH stimulates a specific isoform of adenylyl cyclase (AC7) to produce elevated cAMP levels. 31 Therefore, it is evident that EtOH has the unique capability to elevate two distinct second messengers capable of elevating CBF.
Two distinct pathways (NO or cAMP) have clearly been established to individually be capable of signaling an increase in CBF. Because EtOH has been shown to activate either NO or cAMP, we hypothesize that EtOH-stimulated increases in CBF are mediated via the activation of both PKA and PKG. Such a tandem signaling mechanism required by EtOH might explain the potential for chronic EtOH exposure to uncouple the cAMP-dependent CBF pathway to further stimulation. This may represent a potential mechanistic model for the increased airways diseases associated with excessive and chronic alcohol consumption.
Materials and Methods
Cell Preparation
As previously described 32 the cells were prepared from bovine lung obtained fresh from a local abattoir. Bronchi were necropsied from the lung, cleaned of adjoining lung tissue, and incubated overnight at 4°C in 0.1% bacterial protease (type IV) in minimum essential media. After the overnight incubation, the bronchi were rinsed in Dulbecco’s modified Eagle’s medium with 10% fetal calf serum repeatedly to collect the cells lining the lumen. This technique typically produces a high-viability cell preparation of >95% epithelial cells. 33 The cells were then washed in Dulbecco’s modified Eagle’s medium, counted with a hemacytometer, and plated in 1% type I collagen-coated (Vitrogen; Cohesion, Palo Alto, CA) 100-mm polystyrene Petri dishes at a density of 1 × 104 cells/cm2 in a 1:1 media mixture of LHC-9 and RPMI. 34 Cell incubations were performed at 37°C in humidified 95% air/5% CO2. Confluent monolayers of cells were obtained every 3 days. At this time, each 60-mm dish contained ∼2 mg of total cellular protein. Primary cultures of BBECs were used for these studies because it has been suggested that tissue culture artifact may induce the down-regulation of certain enzyme activity in the late-passaged cell. 35
Determination of Cyclic Nucleotide Levels
Cyclic nucleotide levels were determined using a protein kinase activation assay. 36 The type I cAMP-dependent protein kinase used was partially purified from bovine lung through a DEAE-cellulose chromatography step. 37 Cell monolayers were flash-frozen in liquid nitrogen after addition of 1 ml of KPEM per dish. The dishes were stored at −70°C until assayed. Monolayers were thawed, scraped, added to microfuge tubes, and boiled at 95°C for 5 minutes. Tubes were spun at 10,000 × g for 30 minutes and supernatants collected. Samples were diluted 1:10 in KP buffer with 0.9 mg/ml of bovine serum albumin and 20 μl added to a 50-μl stock reaction mixture consisting of 40 mmol/L Tris-HCl (pH 7.4), 20 mmol/L magnesium-acetate, 130 μmol/L Kemptide (LRRASLG), 0.2 mmol/L IBMX, and 0.2 mmol/L [γ-32P] ATP. Reactions were initiated by the addition of 10 μl of PKA diluted to 0.4 nmol/L with KPEM and 0.9 mg/ml of bovine serum albumin. After incubation at 4°C for 16 to 20 hours, 50 μl aliquots were spotted onto phosphocellulose paper (Whatman P-81) and placed immediately into 75 mmol/L of phosphoric acid. The papers were then washed five times manually for 1 minute each, rinsed 1 minute in EtOH, dried, and counted in nonaqueous scintillant. 38
The assay for cGMP levels was performed similarly to that for cAMP. 36 The PKG used was partially purified from bovine lung as previously described. 39 Samples (10 μl) diluted 1:10 in KP buffer with 0.9 mg/ml of bovine serum albumin were added to 20-μl stock reaction mixtures as above, except that 150 μmol/L of heptapeptide substrate (RKRSRAE) specific for PKG was substituted for Kemptide. Protein kinase inhibitor 5-24 (15 μmol/L) was also added to the reaction mixture. Reactions were initiated with the addition of 10 μl of PKG (10 nmol/L), incubated, and halted as described for the cAMP assay. All incubations were performed in duplicate, and each experiment was repeated three or more times. Cyclic nucleotide concentrations (pmol/mg protein) were determined by comparison to a standard curve of cyclic nucleotide-activated kinase activities (pmol/min/ml) that was performed concurrently with each experiment. Protein in each sample was measured by the technique of Bradford 40 and used to standardize for each experiment. Data were analyzed for statistical significance using one-way analysis of variance. For the experimental design, significance would be achieved at P ≤ 0.05.
Determination of Cyclic Nucleotide-Dependent Kinase Activity
PKA activity was determined in both DEAE fractions as well as crude whole-cell fractions of bronchial epithelial cells. The assay used is a modification of procedures previously described 41 using 130 μmol/L PKA substrate heptapeptide (LRRASLG), 10 μmol/L cAMP, 0.2 mmol/L IBMX, 20 mmol/L magnesium acetate, and 0.2 mmol/L [γ-32P] ATP in a 40-mmol/L Tris-HCl buffer (pH 7.5). PKG activity was assayed in a similar manner to PKA, with the substitution of the peptide RKRSRAE for the heptapeptide substrate, the addition of 10 μmol/L of cGMP, and the presence of PKI. Samples (20 μl) were added to 50 μl of the above reaction mixture and incubated for 15 minutes at 30°C. Reactions were initiated by the addition of 10-μl cell fraction diluted 1:10 with KPEM and 0.9 mg/ml of bovine serum albumin. Incubations were halted by spotting 50 μl of each sample onto P-81 phosphocellulose papers. Papers were then washed five times for 5 minutes each in phosphoric acid (75 mmol/L), washed once in EtOH, dried, and counted in nonaqueous scintillant as previously described. 38 Negative controls consisted of similar assay samples with or without the appropriate substrate peptide or cyclic nucleotide. A positive control of 0.4 ng/ml of purified catalytic subunit from type I bovine PKA (Promega, Madison, WI) was included as a sample. Kinase activity was expressed in relationship to total cellular protein assayed and calculated in pmol/min/mg. All samples were assayed in triplicate and no less than three separate experiments were performed per unique parameter. Data were analyzed for statistical significance using one-way analysis of variance.
CBF Measurements
Actively beating ciliated cells were observed and their motion quantified by measuring CBF using phase contrast microscopy, videotape analysis, and computerized frequency spectrum analysis. Ciliated cells in culture were maintained at a constant temperature (24 ± 0.5°C) by a thermostatically controlled heated stage. The cells were maintained at room temperature during the time course of the CBF measurements, as the temperature gradient is known to affect CBF. 42 All observations were recorded for analysis using a Panasonic WV-D5000 video camera and a Panasonic AG-1950 videotape recorder. Beat frequency analysis was performed on videotaped experiments using customized software written in LabView (National Instruments, Austin, TX) running on a Macintosh G3 computer. The predominant frequency of a cilium or small group of cilia is determined by collecting data sampled at 40 Hz from 512 samples (12.8 seconds) and performing frequency spectrum analysis. The CBF determined in this manner is deemed acceptable when a single dominant frequency was obtained using this technique. All frequencies represent the mean ± one SEM from six separate cell groups or fields.
NO Analysis
Bronchial epithelial cell NO production was monitored via the detection of NO by a gas-phase chemiluminescent reaction between NO and ozone (Sievers Instruments, Boulder, CO; model 280i). Monolayers of airway epithelial cells were treated with various concentrations of EtOH for various times. The cell medium was separated from the cells and both fractions flash-frozen to halt metabolic reactions. The proteins were precipitated in equal volumes of 0.5 N of NaOH and 10% ZnSO4 for 15 minutes before being centrifuged at 14,000 × g for 5 minutes at 4°C. Supernatants (10 μl) were injected into a reflux column containing 0.1 mol/L of VCl3 in 1 mol/L of HCl at 80°C to reduce any nitrates and nitrites into NO. NO then combines with O3 produced by the analyzer to form NO2. The resulting emission from the excited NO2 was detected by a photomultiplier tube and recorded digitally (mV). The values were then interpolated to a standard curve of NaNO2 concentrations concurrently determined. Sample measurements were made in triplicate for each cell treatment with each sample being injected a minimum of three times for a total of nine readings per data point. Significance was determined by analysis of variance.
Cell Viability Assay
Cell viability was determined by cell media assay of lactate dehydrogenase (LDH) release using a commercially available kit (Sigma, St. Louis, MO).
Materials
LHC basal medium was purchased from Biofluids (Rockville, MD). RPMI 1640, Dulbecco’s modified Eagle’s medium, minimal essential medium, streptomycin-penicillin, and fungizone were purchased from Life Technologies, Inc. (Chagrin Falls, OH). Extraction of frozen bovine pituitaries from Pel Freez (Rogers, AR) was performed as previously described and yielded an extract containing 10 mg/ml of protein. 34 [γ32P]-ATP (ICN, Irvine, CA), phosphocellulose P-81 paper (Whatman, Clifton, NJ), heptapeptide substrates for PKA and PKG (Peninsula Laboratories, Belmont, CA), and absolute EtOH (McCormick Distilleries, Weston, MO) were obtained from the indicated sources. All other reagents not specified were purchased from Sigma Chemical Co (St. Louis, MO).
Results
EtOH Elevates NO Directly
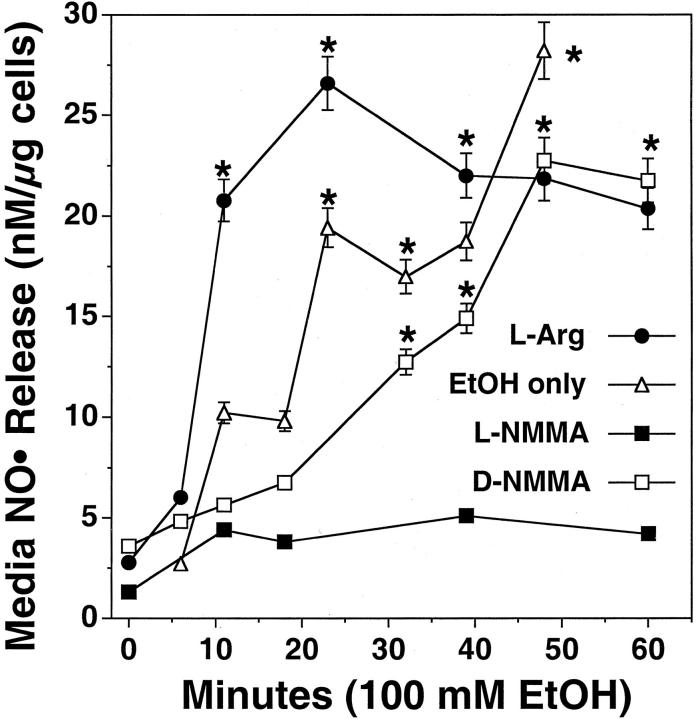
Previous results have shown that either cAMP or cGMP can stimulate CBF 8 and that inhibitors of NOS block EtOH-stimulated CBF. 27 To determine whether EtOH stimulates NOS activity, we directly measured NO release in BBECs exposed to EtOH. Cells were incubated in Ham’s F-12 media in the presence or absence of 100 mmol/L of EtOH from 0 to 60 minutes and media were collected. EtOH stimulated a significant release of NO from 30 to 60 minutes (Figure 1) ▶ . No significant release of NO was measured in the media of control Ham’s F-12-only treated cells or in the media of cells exposed to less than 10 mmol/L of EtOH (not shown). The majority of NO produced by the cells was measured in the cell media with little detectable changes in cell lysate NO observed. When the cells are preincubated with 100 μmol/L of l-arginine, a precursor substrate for NO synthesis, EtOH-stimulated NO production occurs more rapidly (beginning at 10 minutes), but at the same magnitude of release. As expected, preincubation of the cells with 10 μmol/L of NG-monomethyl-l-arginine (L-NMMA), an inhibitor of NOS, blocked EtOH-stimulated NO production. As a control, the inactive isomer, NG-monomethyl-d-arginine (D-NMMA), did not block EtOH-stimulated NO production. These observations suggest that cilia-stimulatory concentrations of EtOH can directly and rapidly elevate the production of NO in the BBECs before the time observed for increased cilia beating.
Figure 1.
EtOH stimulates the production of NO in BBECs. Ciliated BBECs were cultured as primary cells from a bovine bronchus and analyzed for NO production. Cells were incubated in Ham’s F-12 media in the presence or absence of 100 mmol/L EtOH from 0 to 60 minutes and media were collected. EtOH stimulated a significant release of NO from 30 to 60 minutes. The addition of 100 μmol/L of l-arginine (L-arg) to the treatment media augmented the time of maximal NO release. Pretreatment of the cells for 1 hour with 10 μmol/L of NG-monomethyl-l-arginine (L-NMMA) blocked EtOH-stimulated NO release. Similar pretreatment with NG-monomethyl-d-arginine (D-NMMA) had no effect on EtOH-stimulated NO release. No significant release of media NO was observed in media control-exposed cells at any time point (not shown). Media NO was calculated as nmol/L NO released per μg total cellular protein assayed. Bars represent SEM of separate experiments performed in triplicate (n = 9). Significance (P ≤ 0.05) indicated by an asterisk.
Guanylyl Cyclase Inhibition Blocks EtOH-Stimulated CBF
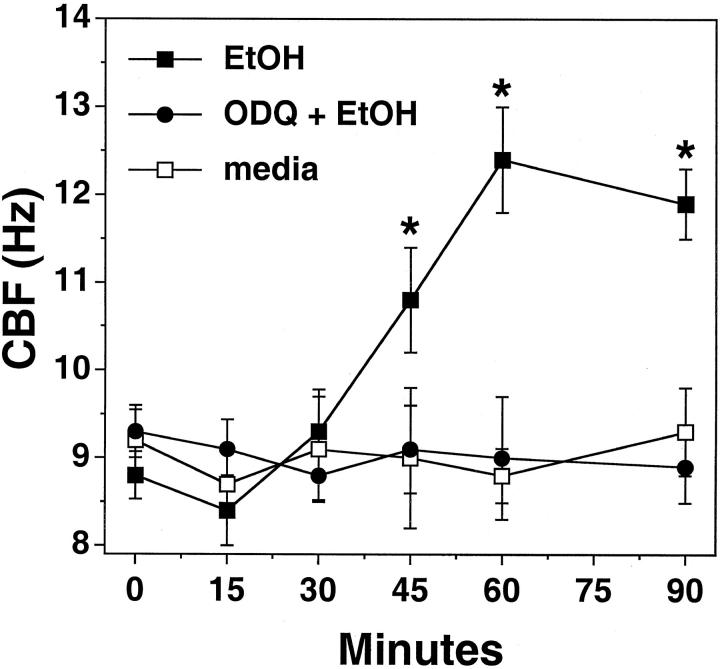
Increases in NO are associated with the activation of guanylyl cyclase in many cell types. To determine whether the NO-mediated component of EtOH-stimulated increases in CBF involves the activation of guanylyl cyclase, BBECs were stimulated with EtOH in the presence or absence of guanylyl cyclase inhibitors and CBF was measured. Ciliated BBECs were pretreated for 30 minutes with or without 1 μmol/L of 1H-[1,2,4] oxadiazole [4,3-a] quinoxalin-1-one (ODQ), an inhibitor of guanylyl cyclase. BBECs were then stimulated with or without 100 mmol/L of EtOH for up to 90 minutes and CBF measured. Pretreatment of BBECs with ODQ resulted in the inhibition of EtOH-stimulated increases in CBF (Figure 2) ▶ . Although EtOH treatment alone stimulated a significant increase in CBF (∼3 Hz), no change over baseline media control CBF levels were observed in cells treated with ODQ alone. No significant decrease in cell viability was observed in response to 1 μmol/L of ODQ (data not shown). Likewise, when BBECs were pretreated with an alternative guanylyl cyclase inhibitor, LY83583, EtOH-stimulated increases in CBF were also blocked (data not shown). These data suggest that EtOH-stimulated CBF requires the activation of guanylyl cyclase by EtOH-stimulated increases in NO.
Figure 2.
EtOH-stimulated CBF requires the activation of guanylyl cyclase in BBECs. Cells were treated with or without 100 mmol/L EtOH in the presence or absence of 1 μmol/L of ODQ and CBF measured. EtOH stimulated significant increases in CBF by 1 hour. Pretreatment of cells with ODQ for 30 minutes blocked any EtOH-stimulated increases in CBF. No change in CBF was observed with ODQ alone (not shown) or media control-treated cells. CBF was expressed as cycles per second (Hz). Bars represent SEM of separate experiments performed in triplicate (n = 9). Significance (P ≤ 0.05) indicated by an asterisk.
EtOH Stimulates Cyclic Nucleotide Production
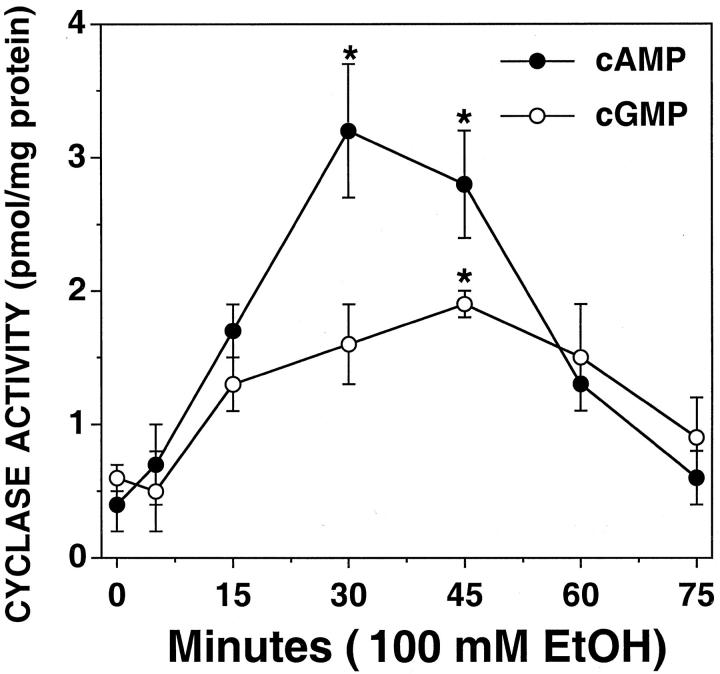
We have previously shown that cellular elevations in cAMP or cGMP concentration regulate increased CBF in BBECs. 8 EtOH is already known to stimulate a specific isoform of adenylyl cyclase in certain cell types 43 including BBECs. 28 Because EtOH-stimulated increases in CBF require an increase in NO and the activation of guanylyl cyclase, EtOH could be stimulating increases in both cAMP and cGMP. To test this, BBECs were stimulated with 100 mmol/L of EtOH for up to 2 hours and cell cyclase activity determined as a function of cyclic nucleotide production. EtOH significantly elevated cAMP levels in BBECs with maximal concentrations detected between 30 to 60 minutes (Figure 3) ▶ . A lower concentration of cGMP was stimulated by EtOH during the same time course. Elevations in cGMP were temporally correlated with the increased production of NO in the BBECs. These data suggest that EtOH elevates both cAMP and cGMP in the BBECs.
Figure 3.
Both cAMP and cGMP are elevated in BBECs treated with EtOH. Confluent monolayers of BECs were stimulated with 100 mmol/L of EtOH and flash-frozen in cell lysis buffer (see Materials and Methods). Cell homogenates were boiled and assayed for cAMP and cGMP concentrations. EtOH stimulated a significant increase in cAMP concentration at ∼30 minutes of treatment. EtOH also stimulated a measurable increase in cGMP with the maximal cGMP concentration observed at 45 minutes. Cyclic nucleotide levels were expressed as pmol cyclic nucleotide per mg total cellular protein. Bars represent SEM of separate experiments performed in triplicate (n = 6). Significance (P < 0.05) indicated by an asterisk.
CBF Substimulatory Levels of cAMP and cGMP Combine to Increase CBF
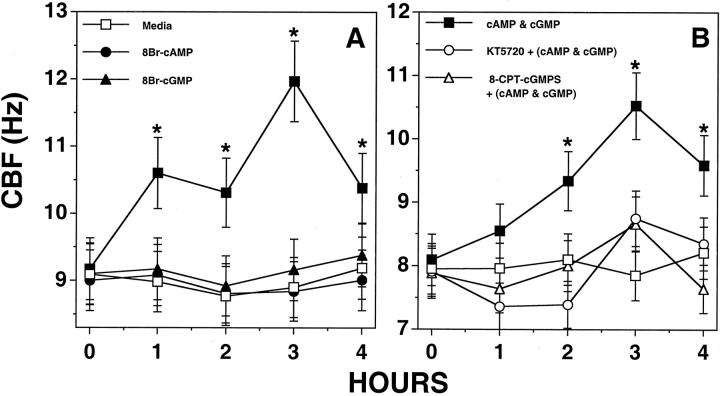
Because EtOH is capable of elevating both cAMP and cGMP levels in BBECs, the combination effect of these cyclic nucleotides on CBF was investigated. High concentrations (≥1 μmol/L) of either cAMP or cGMP alone are capable of stimulating increased CBF in BBECs. 8 To determine whether EtOH-stimulated cAMP and cGMP function in concert to increase CBF, BBECs were treated with substimulatory concentrations of cyclic nucleotides for up to 4 hours and CBF measured. Low concentrations (1 nmol/L) of either 8Br-cAMP or 8Br-cGMP alone failed to stimulate BBEC CBF (Figure 4A) ▶ . However, the combination of both 1 nmol/L of 8Br-cAMP and 1 nmol/L of 8Br-cGMP synergistically stimulate CBF by 1 hour treatment with the maximal increase in CBF measured at 3 hours. This combination effect of cyclic nucleotides on CBF was blocked by pretreating the cells for 1 hour with inhibitors of either PKA (1 μmol/L KT5720) or PKG (0.1 μmol/L 8-pCPT-cGMPS) (Figure 4B) ▶ . These data suggest that the lower concentrations of cyclic nucleotides produced by EtOH stimulation of BBECs can combine together to stimulate CBF.
Figure 4.
Substimulatory concentrations of cAMP and cGMP combine to stimulate CBF increases in BBECs. Confluent monolayers of ciliated BECs were stimulated with 1 nmol/L of 8Br-cAMP, 1 nmol/L of 8Br-cGMP, or both for 1 to 4 hours and CBF measured. At low concentrations (1 nmol/L), neither 8Br-cAMP nor 8Br-cGMP stimulated a significant change in CBF as compared to media controls (A). However, the combination of both 8Br-cAMP and 8Br-cGMP at 1 nmol/L stimulated an ∼2 to 3 Hz increase in CBF during the same incubation period (A and B). Preincubation of the cells with either 1 μmol/L of KT5720 or 100 nmol/L of 8-CPT-cGMPS blocked the CBF stimulatory effect of combined low-level cyclic nucleotides (B). CBF was expressed as cycles per second (Hz). Bars represent SEM of separate experiments performed in triplicate (n = 9). Significance (P ≤ 0.05) indicated by an asterisk.
CBF Substimulatory Levels of Cyclic Nucleotides Do Not Cross-Activate Kinases
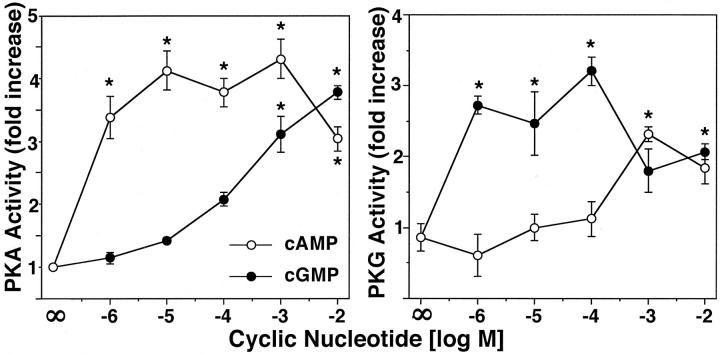
Because of the structural similarities between cyclic nucleotides and their respective protein kinase-binding sites, the cross-activation of PKG by cAMP has been reported. 41 Thus, it might be possible that the EtOH-stimulated accumulation of both cyclic nucleotides might combine to stimulate just PKA or PKG only. To determine whether the combination of substimulatory cyclic nucleotides results in the cross-activation of either PKA or PKG and subsequently in elevated CBF, we directly assayed both PKA and PKG activities in BBECs in response to various concentrations of cell-permeable cyclic nucleotide analogues. Although the cross-activation of BBEC PKA was observed by very high concentrations of 8Br-cGMP (≥100 μmol/L), concentrations of cGMP <100 μmol/L failed to cross-activate PKA (Figure 5) ▶ . Conversely, the concentrations of 8Br-cAMP at or less than 100 μmol/L do not cross activate PKG. These data suggest that although cyclic nucleotide cross-activation can be observed in BBECs exposed to very high doses of cyclic nucleotides, the concentrations required for kinase cross activation (1 mmol/L) is well beyond those cyclic nucleotide combination concentrations (1 nmol/L) causing increased CBF.
Figure 5.
Cross-activation of cyclic nucleotide kinases in BBECs. BBECs were treated with various concentrations of 8Br-cAMP or 8Br-cGMP and cell homogenates assayed for both PKA and PKG activity. Cyclic AMP stimulated PKA activity and cGMP stimulated PKG activity at all concentrations. High concentrations (≥1 mmol/L) of 8Br-cGMP cross-activated PKA and 8Br-cAMP (≥1 mmol/L) cross-activated PKG. However, at lower concentrations of cyclic nucleotide (≤1 μmol/L), no cross-activation was observed. Cyclic nucleotide kinase activity was expressed as fold increase over baseline media control cells. Bars represent SEM of separate experiments performed in triplicate (n = 9). Significance (P < 0.05) indicated by an asterisk.
GC Inhibitors Block EtOH Stimulation of PKA
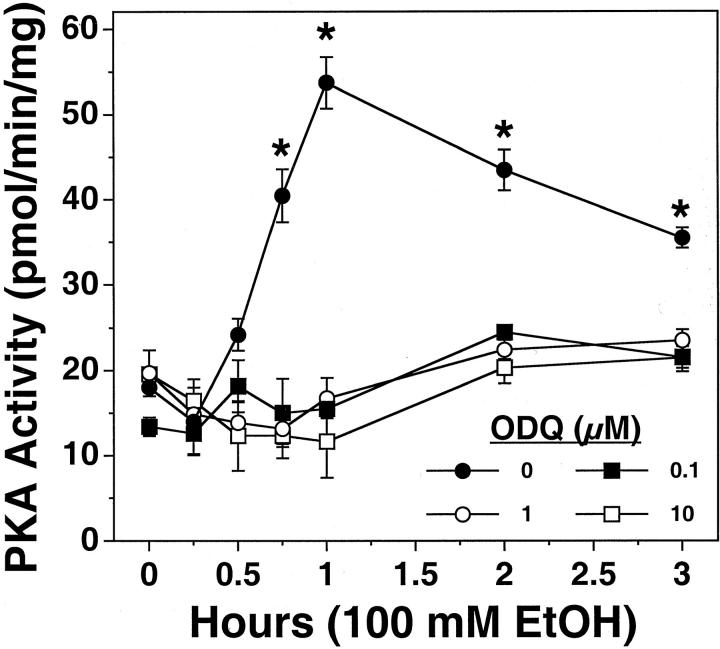
Previously, we indirectly demonstrated that the production of NO was required for EtOH-stimulated PKA activation. 29 The combination of substimulatory cyclic nucleotides collectively stimulating CBF suggests that cGMP might also be necessary for EtOH-stimulated PKA activity as well. To test this, BBECs were stimulated with 100 mmol/L of EtOH in the presence or absence of increasing concentrations of ODQ and PKA activity was assayed. Pretreatment of BBECs for 30 minutes with 0.1 to 10 μmol/L of ODQ blocked EtOH-stimulated increases in PKA activation (Figure 6) ▶ . In the absence of ODQ, EtOH stimulated a twofold to threefold increase in PKA activity. These data suggest that guanylyl cyclase activation is a precedent requirement for EtOH-stimulated PKA activation in BBECs.
Figure 6.
EtOH-stimulated cAMP-dependent protein kinase (PKA) activity requires the activation of guanylyl cyclase in BBECs. Cells were treated with 100 mmol/L of EtOH from 1 to 3 hours in the presence or absence of 0.1 to 10 μmol/L of ODQ and PKA activity assayed. EtOH stimulated significant increases in CBF by 1 hour. Pretreatment of cells for 30 minutes with ODQ blocked EtOH-stimulated increases in PKA. No change in PKA was observed with any concentration of ODQ alone or media control-treated cells. PKA was expressed as pmol ATP transferred per minute per mg total protein assayed. Bars represent SEM of separate experiments performed in triplicate (n = 9). Significance (P ≤ 0.05) indicated by an asterisk.
PKG Inhibition Blocks EtOH Stimulation of PKA
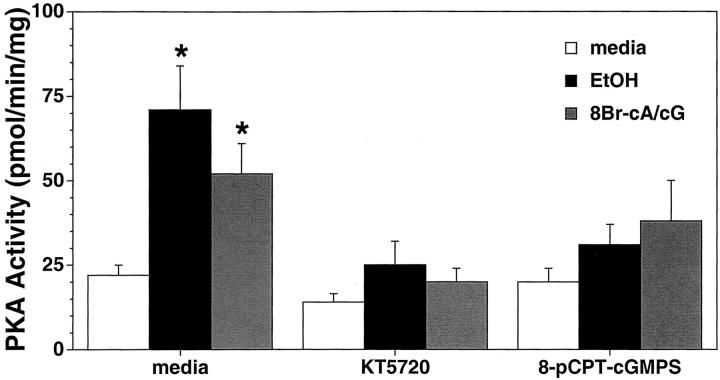
To determine whether PKG is involved in the EtOH-stimulated activation of PKA, BBECs were treated for 1 hour with or without a PKG antagonist analog, 8-pCPT-cGMPS (100 nmol/L) followed by stimulation with 100 mmol/L of EtOH for 1 hour. The antagonist analog to cGMP blocked EtOH-stimulated PKA activity (Figure 7) ▶ . When cilia substimulatory concentrations (1 nmol/L) of 8Br-cAMP and 8Br-cGMP are combined, a smaller, but significantly elevated activation of PKA is observed. This PKA activation can be inhibited by pretreatment of the cells with either 8-pCPT-cGMPS or the PKA inhibitor, KT5720 (1 μmol/L). Throughout all treatments KT5720 was more effective at inhibiting PKA than the cGMP antagonist analog. However, 8-pCPT-cGMPS blocks the significant activation of PKA by either EtOH or the combination of low-dose cyclic nucleotides. These data suggest that EtOH activates PKA via the generation of both cAMP and cGMP and that activatable PKG is necessary for the EtOH activation of PKA.
Figure 7.
EtOH-stimulated cAMP-dependent protein kinase (PKA) activity requires the activation of cGMP-dependent protein kinase (PKG) in BBECs. Cells were treated with media only (white bars), 100 mmol/L of EtOH (black bars), or the combination of both 1 nmol/L of 8Br-cAMP and 1 nmol/L of 8Br-cGMP (gray bars), for 1 hour in the presence or absence of a 1-hour pretreatment with either 1 μmol/L of KT5720, 0.1 μmol/L of 8-pCPT-cGMPS, or media only and PKA activity assayed. EtOH or the combination of cyclic nucleotides stimulated a significant increase in PKA activity. EtOH-stimulated PKA activity was blocked by pretreatment of BBECs with 8-pCPT-cGMPS. PKA was expressed as fold increase over baseline media control cells. Bars represent SEM of separate experiments performed in triplicate (n = 9). Significance (P ≤ 0.05) indicated by an asterisk.
Discussion
Alcohol abuse has long been associated with pulmonary complications related to impaired lung host defenses such as pneumonia, lung abscesses, and bronchitis. 44 Although alcoholism is widely associated with the pathological manifestation of disease, few studies have focused specifically on excessive alcohol abuse and lung disease. The co-morbidity of cigarette smoking has complicated such studies. 45 Because mucociliary clearance is a first-line lung host defense mechanism, we have focused these studies on the signaling mechanisms in airway ciliated cells that control mucociliary clearance and are likely altered by alcohol.
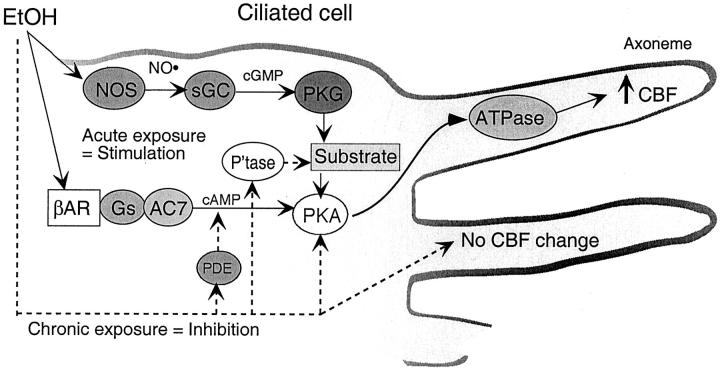
The exact mechanism of EtOH action on airway epithelial cell ciliary beating in vivo is unknown. Because of the increased association of alcoholism with lung disease, including chronic obstructive pulmonary disease, early hypotheses would suggest that EtOH might inhibit cilia beating. In fact, the opposite effects were found in that in vitro acute EtOH treatment of ciliated cells resulted in the stimulation of increased cilia beating. 27 EtOH appears to be poised as a unique regulator of ciliary beating in that it requires an orchestrated cooperation of both cyclic nucleotide pathways. In Figure 8 ▶ , we have proposed a hypothetical model of dual cyclic nucleotide regulation of ciliary beating in response to EtOH. In our model, EtOH acts directly on the cell by increasing NO levels. Others have demonstrated that the ciliated airway epithelium contains the largest localization of NO and NOS. 46 We have previously shown that inhibitors of NOS block stimulated CBF. 29 Likewise, in airway epithelium, NO has been shown to stimulate CBF via cGMP. 46 We report here that EtOH increases cGMP and have previously shown that cGMP elevations are associated with increased CBF. 8 Our model also predicts that EtOH activates an adenylyl cyclase and elevates cellular cAMP levels. EtOH has been shown to stimulate a specific adenylyl cyclase (AC7). 31 We have previously shown that EtOH elevates cellular cAMP concentrations. 47 Studies are underway to determine the mechanism of EtOH activation of AC7 in BBECs. Consequent to cyclic nucleotide elevations, both cAMP or cGMP are capable of individual and distinct up-regulation of ciliary motility via their target protein kinases, PKA and PKG. 8 These distinct pathways appear to converge in that we previously found that NOS inhibitors block EtOH-stimulated activation of PKA. 29 In the present study, we have confirmed this divergent pathway by blocking EtOH-stimulated PKA activity using inhibitors of guanylyl cyclase and PKG. In addition, the novel observation that the combination of both cAMP and cGMP can function at a much lower effective concentration than either individual nucleotide would support our hypothesis that CBF is orchestrated via the joint actions of PKG and PKA. Low concentrations (1 nmol/L) of individual cyclic nucleotide do not stimulate kinase activity nor do they stimulate CBF. We have previously reported that high concentrations (>1 μmol/L) of individual cAMP or cGMP activate kinase and increase CBF. 8 The mechanism of dual cyclic nucleotide orchestration is the subject of current study. EtOH may represent a unique agent functioning to increase CBF via elevation of both cyclic nucleotides.
Figure 8.
Hypothetical signaling mechanism of EtOH-regulated CBF in airway epithelial cells. Acute EtOH exposure to BECs results in the activation of two kinase pathways. EtOH stimulates NOS to produce nitric oxide (NO•). NO• activates a soluble guanylyl cyclase (sGC) that stimulates cGMP production and leads to the activation of PKG. PKG activation and substrate phosphorylation facilitates the activation of PKA, which also requires EtOH-stimulated production of cAMP via an EtOH-sensitive isoform of adenylyl cyclase (AC7). Once activated, PKA then phosphorylates an axonemal substrate resulting in increased dynein ATPase activity leading to increased CBF. Chronic EtOH exposure activates cAMP-phosphodiesterase (PDE4) resulting in a decrease in cAMP throughout time. Chronic EtOH may also activate phosphatases (P’tase) leading to the dephosphorylation of a PKG substrate that facilitates the activation of PKA. This chronic EtOH inactivation results in a desensitized cell that no longer responds to stimulation of CBF.
Cyclic nucleotides can have complimentary or opposing effects. In Paramecium, the interplay between cyclic nucleotides in the regulation of CBF has been reported to be important in the differential regulation of ciliary swimming direction and power stroke. 15,48 This observation parallels the bi-directional control hypothesis of cyclic nucleotide action observed in neutrophils and other cells of myeloid origin. 49 However, cyclic nucleotides often regulate redundant functions in nonmyeloid mammalian cells. Such overlapping characteristics are observed in the regulation of vascular smooth muscle function whereby relaxation can be induced via either cAMP or cGMP. 50 In mammalian airway epithelial cells, distinct cilia beating pathways can be stimulated by either cAMP or cGMP. 8,51 Acetylcholine stimulation of ciliary beating involves both an early calcium-dependent cGMP-dependent pathway and a later calcium-independent cAMP pathway. 52 Either pathway of cilia stimulation requires PKG. Consistent with these findings, EtOH stimulation of PKA and cilia beating requires PKG, although this mechanism does not appear to be calcium-dependent.
Although the significant elevation of PKA activity can be measured after acute EtOH stimulation of ciliated bronchial epithelial cells, 8,29 we have not detected a significant increase in PKG after EtOH exposure. Likewise, the concentration of cGMP elevated by EtOH is significantly lower than EtOH-stimulated cAMP levels. However, this is consistent with the estimations that cellular cGMP concentrations can be up to 200-fold less that those of cAMP. 53 This idea is supported by our findings that very low concentrations of either cAMP or cGMP alone fail to stimulate ciliary beating while the combination of these cyclic nucleotides in substimulatory concentrations results in elevated CBF. Such low concentrations of cGMP may reflect a very small and localized activation of PKG at a specific subcellular target site such as the ciliary axoneme. In fact, NO, PKG, A-kinase anchoring protein (AKAP), and PKA RII have been localized with the ciliary axonemes at the apical surface of the airway epithelial cell. 13,26 In our model of dual cyclic nucleotide regulation by EtOH (Figure 8) ▶ , specific substrate phosphorylation by PKG may be necessary for axonemal targeting or activation of PKA. Such a localization scenario would be required for PKA phosphorylation or activation of a dynein ATPase leading to increased CBF.
The localized compartmentalization of cyclic nucleotide kinases on the axoneme represents a model that could explain cyclic nucleotide regulation of cilia across all concentrations. Such a model supports the previous observations that concentrations in the micromolar range of either cAMP or cGMP can stimulate increased CBF. 8 These data suggest that a threshold saturation concentration of cyclic nucleotide can be reached that can directly stimulate CBF via PKA or PKG. Because cross activation of PKG by cAMP has been well established in other cell types, 41,54,55 cyclic nucleotide cross-talk must be taken into consideration in our model. Indeed, we demonstrate for the first time in airway epithelial cells that very high concentrations (0.1 to 1 mmol/L) of cAMP can activate PKG and high levels of cGMP can activate PKA. However, these pharmacological doses are not likely to occur in vivo, even at highly localized regions of cyclase activity. In fact, treatment concentrations of either cyclic nucleotide can actually reach levels (1 to 10 mmol/L) in which the cyclic nucleotide nonspecifically interferes with ATP binding and begins to decrease kinase activity. Our data would suggest that cyclic nucleotide cross-activation, while possible, does not occur at the concentrations of combined cyclic nucleotides (1 nmol/L) capable of stimulating CBF. Thus, the ability of acute EtOH treatment to stimulate CBF increases appears to be a product of the joint orchestration of PKG and PKA.
A dual CBF regulation by acute EtOH stimulation of both PKG and PKA would explain the observations related to the chronic treatment of ciliated cells with EtOH. Unlike the rapid stimulation of increased cilia beating observed with acute EtOH exposure in vitro, we have observed a chronic EtOH-induced desensitization of ciliated cells to agents that normally would augment cilia beat. 28 We have shown that chronic EtOH exposure does stimulate increased cAMP-phosphodiesterase catalytic activity in airway epithelium, 56 although such action does not explain the desensitization of PKA to phosphodiesterase-resistant analogues of cAMP. 28 If PKG activation via NO/cGMP were a precedent requirement for EtOH-increased PKA and CBF, the observed down-regulation of PKA in response to chronic EtOH could be explained by an uncoupling of the interaction between PKG and PKA. This uncoupling does not appear to be at the level of NOS as chronic EtOH-treated cells can still be stimulated to produce and release NO (data not shown). As our hypothetical model suggests, such an interaction would functionally be regulated by the phosphorylation and dephosphorylation of a targeting protein substrate (Figure 8) ▶ . Indeed, it has been recently suggested in ovine cells that the prolonged maintenance of CBF is not entirely because of PKA and that phosphatases may play a role in the down-regulation response. 57 The identity and location of such substrates remains to be identified.
In summary, the data presented here indicate that EtOH functions in a unique manner to stimulate increases in ciliary beating. EtOH directly stimulates NOS leading to the production of NO in the bronchial epithelial cell. This elevation in NO launches a traditional pathway of signal transduction involving the stimulation of guanylyl cyclase, cGMP production, and PKG activation. Concomitantly, EtOH directly stimulates an isoform of adenylyl cyclase resulting in the production of elevated cAMP levels and the potential for PKA activation. However, our data demonstrate that the EtOH-mediated activation of PKA can only proceed if the EtOH-stimulated PKG pathway is intact and active. Thus, dual orchestration of both cGMP and cAMP pathways are essential for the stimulation of increased ciliary beating in response to EtOH. Such a dual regulatory pathway may become uncoupled under conditions of chronic EtOH administration, leading to dysfunctional ciliary beating and the promotion of mucociliary clearance-associated disease.
Footnotes
Address reprint requests to Todd A. Wyatt, Ph.D., Department of Internal Medicine, Pulmonary and Critical Care Medicine Section, University of Nebraska Medical Center, 985300 Nebraska Medical Center, Omaha, NE 68198-5300. E-mail: twyatt@unmc.edu.
Supported by the Department of Veterans Affairs (merit review grant to T. A. W.) and the National Institutes of Health (grant 5 RO1 AA08769-12 to J. H. S.).
T. A. W. is an American Lung Association Career Investigator.
References
- 1.Wanner A, Salathe M, O’Riordan TG: Mucociliary clearance in the airways. Am J Respir Crit Care Med 1996, 154:1868-1902 [DOI] [PubMed] [Google Scholar]
- 2.Puchelle E, Zahm JM, Bertrand A: Influence of age on bronchial mucociliary transport. Scand J Respir Dis 1979, 60:307-313 [PubMed] [Google Scholar]
- 3.Sanderson MJ, Lansley AB, Dirksen ER: Regulation of ciliary beat frequency in respiratory tract cells. Chest 1992, 101:69S-71S [DOI] [PubMed] [Google Scholar]
- 4.Wolfe J: Cell division, ciliary regeneration and cyclic AMP in a unicellular system. J Cell Physiol 1973, 82:39-48 [DOI] [PubMed] [Google Scholar]
- 5.Murofushi H: Protein kinases in Tetrahymena cilia. II. Partial purification and characterization of adenosine 3′,5′-monophosphate-dependent and guanosine 3′,5′-monophosphate-dependent protein kinases. Biochim Biophys Acta 1974, 370:130-139 [DOI] [PubMed] [Google Scholar]
- 6.Mason PA, Nelson DL: Cyclic AMP-dependent protein kinases of Paramecium. II. Catalytic and regulatory properties of type II kinase from cilia. Biochim Biophys Acta 1989, 1010:116-121 [DOI] [PubMed] [Google Scholar]
- 7.Howard DR, Habermacher G, Glass DB, Smith EF, Sale WS: Regulation of Chlamydomonas flagellar dynein by an axonemal protein kinase. J Cell Biol 1994, 127:1683-1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wyatt TA, Spurzem JR, May K, Sisson JH: Regulation of ciliary beat frequency by both PKA and PKG in bovine airway epithelial cells. Am J Physiol 1998, 275:L827-L835 [DOI] [PubMed] [Google Scholar]
- 9.Salathe M, Pratt MM, Wanner A: Cyclic AMP-dependent phosphorylation of a 26 kD axonemal protein in ovine cilia isolated from small tissue pieces. Am J Respir Cell Mol Biol 1993, 9:306-314 [DOI] [PubMed] [Google Scholar]
- 10.Tamaoki J, Kondo M, Takizawa T: Effect of cAMP on ciliary function in rabbit tracheal epithelial cells. J Appl Physiol 1989, 66:1035-1039 [DOI] [PubMed] [Google Scholar]
- 11.Romberger DJ, Heires P, Rennard SI, Wyatt TA: Beta-adrenergic agonist modulation of monocyte adhesion to airway epithelial cells in vitro. Am J Physiol 2000, 278:L139-L147 [DOI] [PubMed] [Google Scholar]
- 12.Dong F, Feldmesser M, Casadevall A, Rubin CS: Molecular characterization of a cDNA that encodes six isoforms of a novel murine A kinase anchor protein. J Biol Chem 1998, 273:6533-6541 [DOI] [PubMed] [Google Scholar]
- 13.Kultgen PL, Byrd SK, Ostrowski LE, Milgram SL: Characterization of an a-kinase anchoring protein in human ciliary axonemes. Mol Biol Cell 2002, 13:4156-4166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geary CA, Davis CW, Paradiso AM, Boucher RC: Role of CNP in human airways: cGMP-mediated stimulation of ciliary beat frequency. Am J Physiol 1995, 268:L1021-L1028 [DOI] [PubMed] [Google Scholar]
- 15.Bonini NM, Nelson DL: Differential regulation of Paramecium ciliary motility by cAMP and cGMP. J Cell Biol 1988, 106:1615-1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eistetter H, Seckler B, Bryniok D, Schultz JE: Phosphorylation of endogenous proteins of cilia from Paramecium tetraurelia in vitro. Eur J Cell Biol 1983, 31:220-226 [PubMed] [Google Scholar]
- 17.Travis SM, Nelson DL: Regulation of axonemal Mg2+-ATPase from Paramecium cilia: effects of Ca2+ and cyclic nucleotides. Biochim Biophys Acta 1988, 966:84-93 [DOI] [PubMed] [Google Scholar]
- 18.Miglietta LA, Nelson DL: A novel cGMP-dependent protein kinase from Paramecium. J Biol Chem 1988, 263:16096-16105 [PubMed] [Google Scholar]
- 19.Jain B, Rubinstein I, Robbins RA, Leise KL, Sisson JH: Modulation of airway epithelial cell ciliary beat frequency by nitric oxide. Biochem Biophys Res Commun 1993, 191:83-87 [DOI] [PubMed] [Google Scholar]
- 20.Jain B, Rubinstein I, Robbins RA, Sisson JH: TNF-α and IL-1β upregulate nitric oxide-dependent ciliary motility in bovine airway epithelium. Am J Physiol 1995, 268:L911-L917 [DOI] [PubMed] [Google Scholar]
- 21.Tamaoki J, Tagaya E, Sakiai N, Kondo A: Role of nitric oxide in ciliary responses to isoproterenol and bradykinin in rabbit tracheal epithelium. Eur Respir J 1994, 7:12s [Google Scholar]
- 22.Salathe M, Bookman R: Is nitric oxide involved in the cholinergic or purinergic modulation of ciliary best frequency? Am J Respir Crit Care Med 1995, 151:A653 [Google Scholar]
- 23.Martin LD, Rochelle LG, Fischer BM, Krunkosky TM, Adler KB: Airway epithelium as an effector of inflammation: molecular regulation of secondary mediators. Eur Respir J 1997, 10:2139-2146 [DOI] [PubMed] [Google Scholar]
- 24.Uzlaner N, Priel Z: Interplay between the NO pathway and elevated [Ca2+]i enhances ciliary activity in rabbit trachea. J Physiol (Lond) 1999, 516:179-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueda T, Takumida M, Takeno S, Tashiro T, Kawamoto H, Yajin K: Functional role of nitric oxide in the nasal mucosa of the guinea pig after instillation with lipopolysaccharide. Acta Otolaryngol 2001, 121:510-516 [PubMed] [Google Scholar]
- 26.Zhan X, Li D, Johns RA: Immunohistochemical evidence for the NO cGMP signaling pathway in respiratory ciliated epithelia of rat. J Histochem Cytochem 1999, 47:1369-1374 [DOI] [PubMed] [Google Scholar]
- 27.Sisson JH: Ethanol stimulates apparent nitric oxide-dependent ciliary beat frequency in bovine airway epithelial cells. Am J Physiol 1995, 268:L596-L600 [DOI] [PubMed] [Google Scholar]
- 28.Wyatt TA, Sisson JH: Chronic ethanol downregulates PKA activation and ciliary beating in bovine bronchial epithelial cells. Am J Physiol 2001, 281:L575-L581 [DOI] [PubMed] [Google Scholar]
- 29.Sisson JH, May K, Wyatt TA: Nitric oxide-dependent ethanol stimulation of ciliary motility is linked to cAMP-dependent protein kinase (PKA) activation in bovine bronchial epithelium. Alcohol Clin Exp Res 1999, 23:1528-1533 [PubMed] [Google Scholar]
- 30.Schultz JE, Guo Y, Kleefeld G, Volkel H: Hyperpolarization- and depolarization-activated Ca2+ currents in Paramecium trigger behavioral changes and cGMP formation independently. J Membr Biol 1997, 156:251-259 [DOI] [PubMed] [Google Scholar]
- 31.Tabakoff B, Hoffman PL: Adenylyl cyclases and alcohol. Adv Second Messenger Phosphoprotein Res 1998, 32:173-193 [DOI] [PubMed] [Google Scholar]
- 32.Wu R, Smith D: Continuous multiplication of rabbit tracheal epithelial cells in a defined, hormone-supplemented medium. In Vitro 1982, 18:800-812 [DOI] [PubMed] [Google Scholar]
- 33.Shoji S, Rickard KA, Ertl RF, Linder J, Rennard SI: Lung fibroblasts produce chemotactic factors for bronchial epithelial cells. Am J Physiol 1989, 257:L71-L79 [DOI] [PubMed] [Google Scholar]
- 34.Lechner JF, LaVeck MA: A serum-free method for culturing normal human bronchial epithelial cells at clonal density. J Tissue Cult Methods 1985, 9:43-48 [Google Scholar]
- 35.Cornwell TL, Soff GA, Traynor AE, Lincoln TM: Regulation of the expression of cyclic GMP-dependent protein kinase by cell density in vascular smooth muscle cells. J Vasc Res 1994, 31:330-337 [DOI] [PubMed] [Google Scholar]
- 36.Corbin JD, Gettys TW, Blackmore PF, Beebe SJ, Francis SH, Glass DB, Redmon JB, Sheorain VS, Landiss LR: Purification and assay of cAMP, cGMP, and cyclic nucleotide analogs in cells treated with cyclic nucleotide analogs. Methods Enzymol 1988, 159:74-82 [DOI] [PubMed] [Google Scholar]
- 37.Rannels SR, Beasley A, Corbin JD: Regulatory subunits of bovine heart and rabbit skeletal muscle cAMP-dependent protein kinase isozymes. Methods Enzymol 1983, 99:55-62 [DOI] [PubMed] [Google Scholar]
- 38.Roskoski R, Jr: Assays of protein kinase. Methods Enzymol 1983, 99:3-6 [DOI] [PubMed] [Google Scholar]
- 39.Lincoln TM, Flockhart DA, Corbin JD: Studies on the structure and mechanism of activation of the guanosine 3′,5′-monophosphate-dependent protein kinase. J Cell Biol 1978, 253:6002-6009 [PubMed] [Google Scholar]
- 40.Bradford MM: A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976, 72:248-254 [DOI] [PubMed] [Google Scholar]
- 41.Jiang H, Colbran JL, Francis SH, Corbin JD: Direct evidence for cross-activation of cGMP-dependent protein kinase by cAMP in pig coronary arteries. J Biol Chem 1992, 267:1015-1019 [PubMed] [Google Scholar]
- 42.Sanderson MJ, Dirksen ER: Mechanosensitive and beta-adrenergic control of the ciliary beat frequency of mammalian respiratory tract cells in culture. Am Rev Respir Dis 1989, 139:432-440 [DOI] [PubMed] [Google Scholar]
- 43.Yoshimura M, Tabakoff B: Selective effects of ethanol on the generation of cAMP by particular members of the adenylyl cyclase family. Alcohol Clin Exp Res 1995, 19:1435-1440 [DOI] [PubMed] [Google Scholar]
- 44.Krumpe PE, Cummiskey JM, Lillington GA: Alcohol and the respiratory tract. Med Clin North Am 1984, 68:201-219 [DOI] [PubMed] [Google Scholar]
- 45.Rankin JG, Hale GS, Wilkinson P, O’Day DM, Santamaria JN, Babarczy G: Relationship between smoking and pulmonary disease in alcoholism. Med J Aust 1969, 1:730-733 [DOI] [PubMed] [Google Scholar]
- 46.Li D, Shirakami G, Zhan X, Johns RA: Regulation of ciliary beat frequency by the nitric oxide-cyclic guanosine monophosphate signaling pathway in rat airway epithelial cells. Am J Respir Cell Mol Biol 2000, 23:175-181 [DOI] [PubMed] [Google Scholar]
- 47.Lansley AB, Sanderson MJ, Dirksen ER: Control of the beat cycle of respiratory tract cilia by Ca2+ and cAMP. Am J Physiol 1992, 263:L232-L242 [DOI] [PubMed] [Google Scholar]
- 48.Ann KS, Nelson DL: Protein substrates for cGMP-dependent protein phosphorylation in cilia of wild type and atalanta mutants of Paramecium. Cell Motil Cytoskeleton 1995, 30:252-260 [DOI] [PubMed] [Google Scholar]
- 49.Goldberg ND, Haddox MK, Dunham E, Lopez C, Hadden JW: The yin yang hypothesis of biological control: opposing influences of cyclic GMP and cyclic AMP in the regulation of cell proliferation and other biological processes. Clarkson B Baserga R eds. Control of Proliferation in Animal Cells. 1974:pp 609-625 Cold Spring Harbor Laboratory, Cold Spring Harbor
- 50.Lincoln TM, Cornwell TL: Towards an understanding of the mechanism of action of cyclic AMP and cyclic GMP in smooth muscle relaxation. Blood Vessels 1991, 28:129-137 [DOI] [PubMed] [Google Scholar]
- 51.Yang B, Schlosser RJ, McCaffrey TV: Dual signal transduction mechanisms modulate ciliary beat frequency in upper airway epithelium. Am J Physiol 1996, 270:L745-L751 [DOI] [PubMed] [Google Scholar]
- 52.Zagoory O, Braiman A, Priel Z: The mechanism of ciliary stimulation by acetylcholine: roles of calcium PKA, and PKG. J Gen Physiol 2002, 119:329-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cornwell TL, Pryzwansky KB, Wyatt TA, Lincoln TM: Regulation of sarcoplasmic reticulum protein phosphorylation by localized cyclic GMP-dependent protein kinase in vascular smooth muscle cells. Mol Pharmacol 1991, 40:923-931 [PubMed] [Google Scholar]
- 54.Abdel-Latif AA: Cross talk between cyclic nucleotides and polyphosphoinositide hydrolysis, protein kinases, and contraction in smooth muscle. Exp Biol Med (Maywood) 2001, 226:153-163 [DOI] [PubMed] [Google Scholar]
- 55.Pelligrino DA, Wang Q: Cyclic nucleotide crosstalk and the regulation of cerebral vasodilation. Prog Neurobiol 1998, 56:1-18 [DOI] [PubMed] [Google Scholar]
- 56.Forgèt M, Sisson JH, Spurzem JR, Wyatt TA: Ethanol increases phosphodiesterase 4 activity in bovine bronchial epithelial cells. Alcohol (n press) [DOI] [PubMed]
- 57.Lieb T, Frei CW, Frohock JI, Bookman RJ, Salathe M: Prolonged increase in ciliary beat frequency after short-term purinergic stimulation in human airway epithelial cells. J Physiol 2002, 538:633-646 [DOI] [PMC free article] [PubMed] [Google Scholar]