Abstract
Information on oncogenetic events accompanying salivary gland mucoepidermoid carcinoma is so far limited. Activation of extracellular signal-regulated kinases ERK-1 and ERK-2 is strongly correlated to cancer. Using an antibody specific for phosphorylated (active) ERK-1/ERK-2, we examined human salivary gland mucoepidermoid carcinoma samples by immunohistochemistry. The comparison in paired tumor and normal tissue samples showed that phosphorylated ERK-1/ERK-2 immunoreactivity was higher in tumor cells as compared to surrounding normal salivary parenchyma. ERK-1/ERK-2 phosphorylation was observed in ∼39% of mucoepidermoid carcinomas. Those tumors where the ERK-1/ERK-2 pathway was activated had a more aggressive tumor behavior as compared to the group where this pathway was inactive. The association of ERK-1/ERK-2 phosphorylation to a worse prognosis was independent of histological grade. ERK-1/ERK-2 phosphorylation was associated with increased Ki67 and cyclin A indexes, which indicated that ERK-1/ERK-2 pathway activation increased tumor cell proliferation. There was no relationship between ERK-1/ERK-2 phosphorylation and HER-2/neu or p16/INK4a protein expression. In conclusion, ERK-1/ERK-2 pathway is active in salivary gland mucoepidermoid carcinoma and this activation is associated to a more aggressive tumor behavior and a higher proliferative activity. These data suggest that deregulation of ERK-1/ERK-2 pathway contributes to mucoepidermoid carcinoma phenotype and, possibly, represents a target for new anticancer drugs.
Mucoepidermoid carcinoma is one of the most frequent salivary gland malignant tumors. 1 It is more common in women and peaks in the fifth decade. However, it is also the most common salivary gland malignant tumor to arise in children and adolescents under 20 years of age. Among salivary gland malignant tumors, mucoepidermoid carcinoma is unique in that it demonstrates a broad spectrum of aggressiveness, which can be predicted by microscopic grading. 2 High-grade mucoepidermoid carcinoma is a highly aggressive tumor, while its low-grade counterpart usually demonstrates a favorable outcome. However, metastasis also occurs in low-grade mucoepidermoid carcinomas and can be lethal. 3,4 The frequency of this unpredictable event is probably not recognized by analyzing short-term survival results in a disease that often has a long natural history.
Mucoepidermoid carcinoma is composed of varying proportions of mucous, epidermoid, columnar, intermediate, and clear cells. 1 It is thought to arise from the salivary excretory duct. However, some authors have shown that there are essentially two basic types of cells, luminal and intermediate, and that the intermediate cell exhibits characteristics of modified salivary gland myoepithelial cells. 5 Uncertainties regarding mucoepidermoid carcinoma histogenesis is contributed by the lack of known precursor lesions. Information on molecular events causing or accompanying malignant transformation is also very limited. Several studies have reported the occurrence of 6q deletions, a common change in salivary gland carcinomas, which suggests inactivation of tumor suppressor genes. 6,7 Characterization of genotypic features of mucoepidermoid carcinoma is reported in only a few cases, however. 7,8 Press et al 9 have reported the amplification and/or overexpression of HER-2/neu in approximately one-third of mucoepidermoid carcinomas of salivary glands, and have suggested that HER-2/neu overexpression or amplification is important in these tumors. In addition H-Ras gene mutations have been shown by Yoo et al 10 in approximately one-fifth of salivary gland mucoepidermoid carcinomas.
Many oncogenic proteins, such as HER-2/neu and Ras, are members of or interact with cytoplasmic signaling cascades, and transformation is often a direct result of the deregulation of a cytoplasmic signal transduction pathway. 11 Mitogen-activated protein kinases (MAPKs) constitute an evolutionary conserved family of protein kinases. 12-14 In multicellular organisms, there are three well-characterized subfamilies of MAPKs. These MAPKs include the extracellular signal-regulated kinases, ERK1 and ERK2, the c-Jun NH2-terminal kinases, and the four p38 enzymes. Each MAPK subfamily is part of a cascade involving activation of several membrane receptors followed by the sequential activation of two upstream regulators. The high selectivity of the upstream regulators for their substrates is such that cells can respond to different stimuli with the activation of a specific MAPK pathway. The ERK-1 and ERK-2 pathway is one of the best characterized and the more strongly related to human cancer among MAPK pathways. 12,14 ERK-1 and ERK-2 are ubiquitously expressed, respond to a variety of stimuli, including transforming agents and carcinogens, and are involved in cell proliferation, apoptosis, differentiation, angiogenesis, and cell motility. Oncogenic Ras persistently activates the ERK-1/ERK-2 pathway, which contributes to the increased proliferative rate of tumor cells. 15 For this reason, inhibitors of the ERK pathways are entering clinical trials as potential anticancer agents. 16 Raf-1, which is located downstream from Ras and regulates ERK-1/ERK-2 kinases, can also become activated in a Ras-independent manner. 17
Elevated levels of dually phosphorylated (active) ERK-1/ERK-2 have been reported in several human cancers, including colon, prostate, renal cell, and breast adenocarcinomas, as well as in head and neck squamous-cell carcinoma, glial neoplasm, and melanoma. 18-25 Immunohistochemical detection using antibodies specific for phosphorylated ERK-1/ERK-2 has permitted visualization of spatially discrete cellular patterns of ERK-1/ERK-2 activation in several of these tumors, and has generally suggested that ERK-1/ERK-2 pathway activation may contribute to the neoplastic phenotype. 21-25 The activation state of the ERK-1/ERK-2 pathway is not known in human salivary gland mucoepidermoid carcinoma, although it has been shown that activation of this pathway occurs in rat salivary epithelial cells in response to oncogenic Raf-1 and is required for malignant transformation. 26 In this paper, we demonstrate by immunohistochemistry that ERK-1/ERK-2 pathway is active in salivary gland mucoepidermoid carcinoma and that this activation is associated to a more aggressive tumor behavior and a higher proliferative activity. These data suggest that deregulation of ERK-1/ERK-2 pathway contributes to mucoepidermoid carcinoma phenotype and, possibly, represents a target for new anticancer drugs.
Materials and Methods
Case Selection
A total of 57 mucoepidermoid carcinoma cases were retrieved from the archival files of the Service d’Anatomie et de Cytologie Pathologiques, Groupe Hospitalier Pitié-Salpêtrière, Paris, France. All cases for which formalin-fixed, paraffin-embedded tumor specimens from the primary biopsy or resection were available were consecutively included in this study. Clinical data were obtained from medical records. Slides were independently reviewed by two pathologists (A. H.-L. and P.F.) and graded according to Armed Forces Institute of Pathology criteria. 1 In addition, predominant cell type and presence of tumor-associated lymphoid proliferation were noted.
Primary Antibodies
The rabbit anti-human polyclonal antibody specific for dually phosphorylated (Thr 202/Tyr 204) forms of the MAPK isoforms, ERK-1, and ERK-2 (p44/42), was purchased from Cell Signaling Technology (Phospho-p44/42 MAP Kinase Antibody; IHC-specific; Cell Signaling Technology, Beverly, MA). 27 This antibody was previously used by several authors to visualize phosphorylated ERK-1/ERK-2 in paraffin-embedded specimens. 21,22,24,25 Preliminary experiments on human prostate cancer specimens were performed to determine the optimal incubation conditions which gave the strongest nuclear-specific staining. The reactivity on human prostate cancer tissues was consistent with immunohistochemical results reported by Malik et al. 25
Total ERK-1/ERK-2 expression was checked with the rabbit polyclonal ERK-1 antibody (K-23; Santa-Cruz Biotechnology, Santa-Cruz, CA), which reacts with both ERK-1 p44 and ERK-2 p42. 25
The Ki67 (clone MIB-1; Dako, Carpinteria, CA) and the cyclin A (clone BF683; Santa-Cruz Biotechnology) mouse monoclonal antibodies were used to determine the proliferative index in tumor cells. 28,29
The CB11 (Novocastra, Newcastle-on-Tyne, United Kingdom) and the 16P04 (Neomarkers, Fremont, CA) mouse monoclonal antibodies were used to detect HER-2/neu and p16, respectively. 30,31
Immunohistochemistry
The hematoxylin and eosin-stained slides from each of the 57 cases were screened by light microscopy for representative samples containing mucoepidermoid carcinoma. Adjacent normal salivary gland tissue was also included in the analysis in 39 samples. Four-μm thick sections were cut, mounted on silanized slides, dried overnight at 45°C, dewaxed in xylene, and rehydrated in graded alcohols. Antigen retrieval was performed by bain-marie heating for 30 minutes at 98°C in 10 mmol/L citrate buffer, pH 6.0 (BioGenex; San Ramon, CA). Slides were allowed to cool for 20 minutes, then washed in distilled water. Endogenous peroxidase activity was blocked by incubation in 3% H2O2 for 15 minutes. After rinsing in distilled water, the slides were placed for 5 minutes in phosphate-buffered saline (PBS) containing 0.1% Tween 20 (Amresco, Solon, OH), followed by overnight incubation with the primary antibody for phosphorylated ERK-1/ERK-2 at a 1:100 dilution at 4°C. Slides were washed 2 × 5 minutes in PBS-Tween.The detection step was performed using LSAB+ kit and 3,3′-diaminobenzidine (Dako) as chromogen. Slides were counterstained with hematoxylin, rinsed in tap water, dehydrated, placed in xylene, and mounted. The same immunohistochemical procedure was used for HER-2/neu staining except that the CB11 antibody was applied at room temperature for 1 hour at a 1:800 dilution.
The ERK-1, Ki67, and cyclin A antibodies were applied at room temperature at a 1:100 dilution for 1 hour. The p16 antibody was applied at room temperature at a 1:100 dilution for 90 minutes. The detection step in the ERK-1, Ki67, cyclin A, and p16 analyses was performed using the Vectastain Universal ABC-AP kit (Burlingame, CA) and fast red (Dako) as a substrate.
Controls
A positive control for phosphorylated ERK-1/ERK-2 (human prostate cancer tissue) was included in each analysis. Endothelial cells which displayed moderate nuclear immunoreactivity of phosphorylated ERK-1/ERK2 also served as a positive internal control in each of the sections. As a negative control, the polyclonal primary antibody was substituted for normal rabbit serum (Dako).
We also used breast cancer, colonic mucosa, lymph node, and colon cancer specimens as positive controls for HER-2/neu, Ki67, cyclin A, and p16 staining, respectively. The specificity of staining was controlled by substituting the primary monoclonal antibodies with a mouse monoclonal antibody of the same subclass (IgG1).
Immunohistochemical Evaluation
The immunohistochemical evaluation was independently performed by two of the authors (A.H-L and P.F.) without knowledge of the clinical data.
The phosphorylated ERK-1/ERK2 immunoreactivity levels of each case were assessed semi-quantitatively under light microscope by assessing the average signal intensity (on scale of 0 to 3) and the proportion of cells showing a positive nuclear stain (0, none; 0.1, less than one tenth; 0.5, less than one half; and 1, greater than one half). The intensity and proportion scores were then multiplied to give the H-score. 32
The Ki67 and cyclin A indexes were calculated as the number of positive nuclei per 1000 nuclei. At least 1000 nuclei (range, 1000 to 9000) were counted in 10 high-power fields at a ×400 magnification for each case. 28,29 The median number of total nuclei was 5625 and 6800 for Ki67 and cyclin A, respectively.
For HER-2/neu protein expression, only cases with complete membranous staining were considered positive. 30 For p16/INK4a protein expression, nuclear staining as well as cytoplasmic staining in at least 1% of tumor cells was considered positive. 31
Digital images were acquired with a Axioplan 2 Imaging microscope (Zeiss, Jena, Germany) using an Axiovision software version 3.0 with standard adjustments in image brightness and contrast.
Statistical Analysis
The Wilcoxon test was used to compare two paired groups. Fisher exact test and the χ2 test were used to analyze association between two categorical variables. Trends in proportions were assessed using the Mann-Whitney U-test. Spearman’s correlation was made between Ki67 and cyclin A labeling indexes. Univariate analyses of time to disease-specific progression were performed using the product-limit procedure (Kaplan-Meier method) and tested using the Cox-Mantel log-rank test. Event-free survival accounted for locoregional recurrence, metastasis, and death because of cancer as events. The influence of covariates on the patient event-free survival was analyzed by the Cox’s proportional hazard method. All analyses were carried out with Statistica Software, Version 5.0. A level of 0.05 was chosen to indicate statistical significance. All reported P values are two-sided.
Results
Phosphorylated ERK-1/ERK-2 Immunoreactivity in Normal and Mucoepidermoid Carcinoma Salivary Gland Tissues
Of 57 mucoepidermoid carcinoma samples, 39 contained adjacent normal salivary gland tissue. In 23 of these 39 samples (59%), normal salivary gland tissue was totally unreactive with the polyclonal phosphorylated ERK-1/ERK-2 antibody, although endothelial cell nuclei were reactive. Nuclear staining was observed in a few normal epithelial duct cells (less than 10% of glandular cells) with mild or moderate intensity in 15 cases (38%), while normal acinar cells were completely unreactive in these cases (Figure 1A) ▶ . Only one case exhibited a moderate and diffuse reactivity in both duct and acinar cells. Therefore, using a semi-quantitative method 39 as described in Materials and Methods to evaluate phosphorylated ERK-1/ERK-2 immunoreactivity levels, 23 (59%) unreactive normal salivary gland tissues had a H-score equal to 0, and 15 (38%) had a score between 0.1 and 0.5 (Table 1) ▶ .
Figure 1.
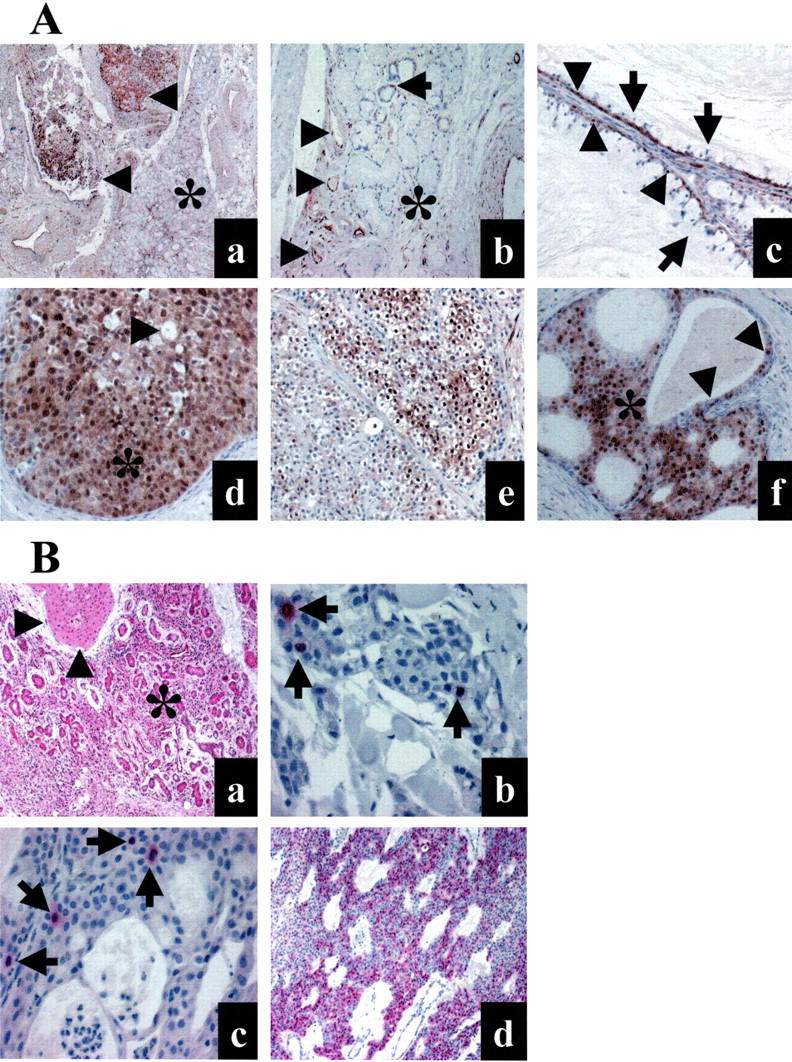
Representative examples of salivary gland mucoepidermoid carcinoma and surrounding tissue samples analyzed by immunohistochemistry for phosphorylated ERK-1/ERK-2, total ERK-1/ERK-2, P16, Ki67, and cyclin A. A: Immunoperoxydase visualization of phosphorylated ERK-1/ERK-2. a: The nodular tumor tissue (arrowheads) is strongly reactive, while the normal adjacent parenchyma (asterisk) is mainly unreactive. b: Same sample as in A. Reactive endothelial nuclei (arrowheads) and normal salivary parenchyma with few reactive ductal cells (arrow) and unreactive acini (asterisk). c: Low-grade mucoepidermoid carcinoma. The basal myoepithelial cells (arrowheads) are consistently reactive, while the mucous cells (arrows) are unreactive. d: High-grade mucoepidermoid carcinoma. Intermediate cells (asterisk) with nuclear anaplasia are diffusely and strongly reactive. Note a few unreactive mucous cells (arrowhead). e: Intermediate-grade mucoepidermoid carcinoma. Reactive clear cells. f: A majority of intermediate cells are reactive (asterisk). Some glandular nonspecific cells are also reactive (arrowheads). Original magnification, ×25 (a); ×100 (b); ×400 (c); ×200 (d); ×200 (e); ×200 (f). B: Total ERK-1/ERK-2, Ki67, cyclin A, and P16 expression analyzed with phosphatase alkaline staining (red). a: Total ERK-1/ERK-2 expression in tumor (arrowheads) and surrounding salivary gland tissue (asterisk). b: Ki67 expression in one intermediate-grade phospho-ERK-positive mucoepidermoid carcinoma (6.6 positive nuclei per 1000 nuclei). Three positive nuclei (arrows) are present among 77 total tumor nuclei in this field. c: Cyclin A expression in one low-grade phospho-ERK-positive mucoepidermoid carcinoma (5.9 positive nuclei per 1000 nuclei). Four positive nuclei (arrows) are present among 191 total tumor nuclei in this field. d: Diffuse immunostaining for P16 in a phospho-ERK-positive mucoepidermoid carcinoma. Original magnification, ×100 (a); ×400 (b); ×400 (c); ×100 (d).
Table 1.
Phosphorylated ERK-1/ERK2 Immunoreactivity in Human Salivary Glands and Mucoepidermoid Carcinomas
| Phosphorylated ERK-1/ERK-2 H-Score* No (%) | Normal salivary gland (N = 39) | Tumor (N = 57) |
|---|---|---|
| 0 | 23 (59) | 14 (25) |
| 0.1–0.5 | 15 (38) | 21 (37) |
| 1–2 | 1 (3) | 15 (26) |
| 3 | 0 | 7 (12) |
*Phosphorylated ERK-1/ERK-2 H-score was determined by multiplying the proportion of positive cells by the intensity of staining with the polyclonal antibody specific for phosphorylated ERK-1/ERK-2.
In contrast, tumor cells displayed phosphorylated ERK-1/ERK-2 in their nuclei in 43 of the 57 mucoepidermoid carcinoma samples (75%), and this immunoreactivity was strong in 15 cases (26%) or diffuse (more than 50% cells) in 14 cases (25%). Using the same semi-quantitative H-score method, the median immunoreactivity level for all tumor tissues was 0.5 (interquartile range, 0.1 to 1.5). The median immunoreactivity level for reactive tumors was 1.0 (interquartile range, 0.1 to 1.5). This staining was mainly observed in intermediate cells. Columnar cells were occasionally reactive. Mucous cells were usually unreactive. Representative examples are shown in Figure 1A ▶ .
Using the H-score method, phosphorylated ERK-1/ERK-2 immunoreactivity levels in tumor cells were directly compared to those observed in adjacent normal salivary gland tissue in 39 samples. The data were analyzed using a Wilcoxon matched pairs statistical test. The levels in the normal salivary gland tissue (median 0, interquartile range, 0 to 0.1) was significantly (P = 0.0005) lower than levels in tumor tissues (median, 0.2 interquartile range, 0 to 1.0). This result implied ERK-1/ERK-2 pathway to be active in mucoepidermoid carcinoma as compared to normal salivary gland tissue.
Of 57 tumor samples, 22 cases (39%) displayed an elevated phosphorylated ERK-1/ERK-2 immunoreactivity that was equal or more than 1 (Table 1) ▶ , meaning moderate or strong staining in at least 10% of tumor cells. Although a direct comparison with normal salivary gland tissue could not be made in all these samples, a level of 1 was observed in only 1 (3%) normal salivary gland tissue. This phosphorylated ERK-1/ERK-2 immunoreactivity level was thus chosen as a cutoff to identify tumor samples where the ERK-1/ERK-2 pathway was considered to be active.
We checked expression of total ERK-1/ERK-2 using an antibody that reacts with both ERK-1 p44 and ERK-2 p42. 25 The staining for total ERK-1/ERK-2 was moderate or strong in a majority of epithelial cells in both normal and tumor tissues, thus excluding overexpression of ERK families in tumor cells (Figure 1B) ▶ . The presence of ERK-1-ERK-2 in tumor cells that were non-reactive with the phosphorylated ERK-1/ERK-2 antibody also demonstrated that lack of the phosphorylated protein was not a consequence of a lack of total ERK-1/ERK-2 family expression.
Activated ERK-1/ERK-2 Pathway in Mucoepidermoid Carcinomas and Baseline Clinico-Pathological Parameters
The median age of the patients was 51.5 years (interquartile range, 37 to 63 years). The patients included 17 males and 40 females. Sixteen cases (28%) were located in the parotid, 3 (5%) in the submandibular gland, 7 (12%) in the sublingual gland, and 31 (54%) in the minor salivary glands. Tumor size varied between 0.3 and 4 cm (median, 1.3 cm). Lymph node metastasis was identified in four cases (7%). Of the 57 mucoepidermoid carcinomas, 33 (58%) were classified as low grade, 9 (16%) as intermediate grade, and 15 (26%) as high grade. Tumor-associated lymphoid proliferation and predominance of intermediate cells were noted in 31 of 57 cases (54%) and 36 of 57 cases (63%, respectively).
Activated ERK-1/ERK-2 pathway (immunoreactivity level equal or more than 1) did not correlate with any of the baseline clinical characteristics, including age, sex, location, tumor size, and node stage (P > 0.20 for all comparisons). The trend of a positive correlation between ERK-1/ERK-2 pathway activation and increasing histological grade was not significant (P = 0.34). Similarly, there was no difference in the individual grading criteria, including intracystic component, neural invasion, necrosis, mitosis index more than 4 per 1.7 square mm, and anaplasia, according to whether ERK-1/ERK-2 pathway was activated or not (Table 2) ▶ . Tumor-associated lymphoid proliferation was not associated with elevated phosphorylated ERK-1/ERK-2. However, there was a trend for poorly differentiated tumors, in which intermediate cells formed the bulk of tumor tissue, to display high phosphorylated ERK-1/ERK-2 levels (P = 0.08).
Table 2.
Pathological Features of 57 Mucoepidermoid Carcinomas According to Phosphorylated ERK-1/ERK2 Immunoreactivity
| Pathological feature-N | Phosphorylated ERK-1/ERK-2 | P Value* | ||
|---|---|---|---|---|
| H-score† less than 1 (N = 35) | H-score equal or more than 1 (N = 22) | |||
| Tumor grade‡ | 0.34 | |||
| Low | 22 | 11 | ||
| Intermediate | 5 | 4 | ||
| High | 8 | 7 | ||
| Intracystic component§ | 0.72 | |||
| Yes | 19 | 13 | ||
| No | 16 | 9 | ||
| Neural invasion | 0.72 | |||
| Yes | 7 | 3 | ||
| No | 28 | 19 | ||
| Necrosis | 0.79 | |||
| Yes | 9 | 5 | ||
| No | 26 | 17 | ||
| Mitosis¶ | 0.36 | |||
| Yes | 2 | 3 | ||
| No | 33 | 19 | ||
| Anaplasia | 0.12 | |||
| Yes | 9 | 10 | ||
| No | 26 | 12 | ||
| Tumor-associated lymphoid proliferation | 0.59 | |||
| Yes | 20 | 11 | ||
| No | 15 | 11 | ||
| Predominance of intermediate cells | 0.08 | |||
| Yes | 19 | 17 | ||
| No | 16 | 5 | ||
*, The trend in increasing proportions of phosphorylated ERK-1/ERK-2 immunoreactivity with tumor grade was assessed using the Mann-Whitney U test. For two-by-two comparisons P values were determined using the Fisher’s exact test or the χ2 test.
†, H-score, product of the intensity of staining by the proportion of positive cells.
‡, Tumor grade was established using AFIP criteria.
§, Intracystic component was more than 20% of tumor.
¶, More than 4 mitoses per 1.7 square mm was measured.
Activated ERK-1/ERK-2 Pathway in Mucoepidermoid Carcinomas and Progression of Disease
Follow-up data were available for 35 patients (median follow-up, 48 months). Ten patients showed local recurrences and one patient had lung, liver, and bone metastases. Three of the patients died, two because of local extension of disease, one due to aplasia induced by chemotherapy.
Kaplan-Meier curves were constructed to evaluate the predictive value of the various clinicopathological parameters on progression-free survival, which accounted for local recurrence, metastasis, or cancer-related death, excluding chemotherapy-related death. As expected, 3,4,28 tumor grade was significantly correlated with progression of disease (P = 0.001; Figure 2 A ▶ ). The survival curves for low and intermediate grades were nearly identical. The group with high-grade tumors had a clearly worse prognosis than the two other groups analyzed separately or combined.
Figure 2.
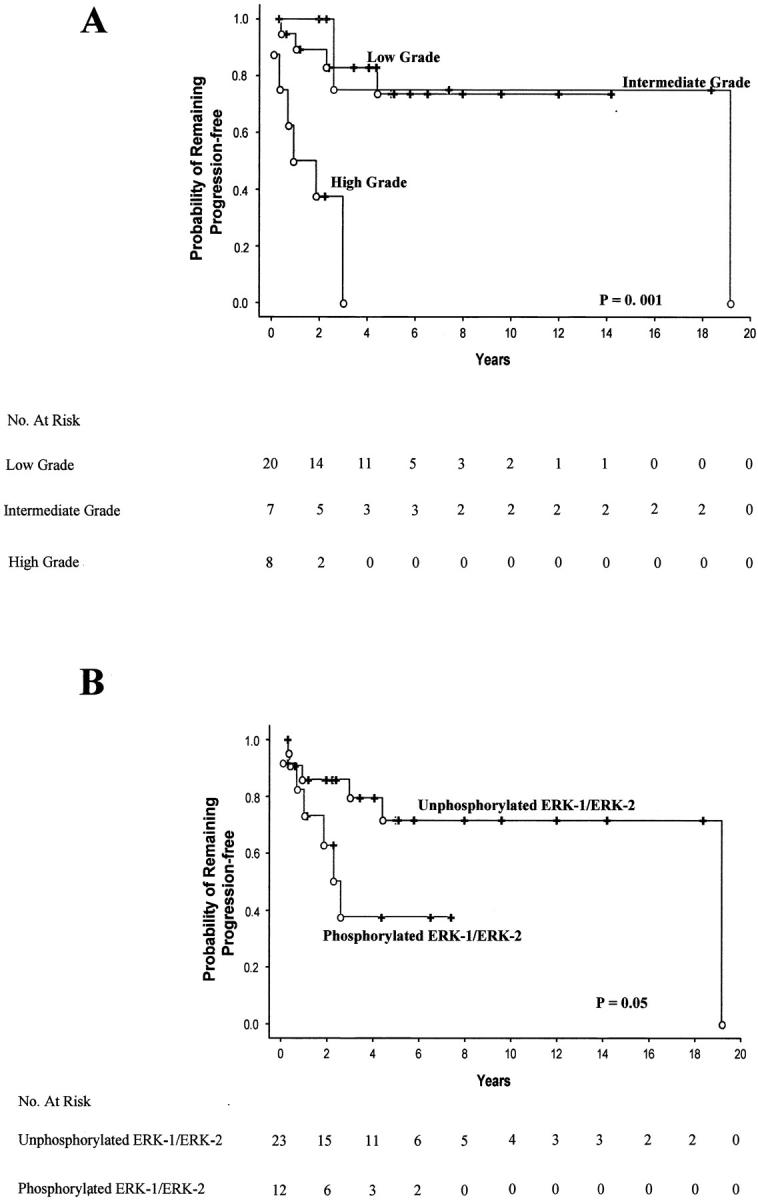
Kaplan-Meier curves showing progression-free survival according to histological grade (A) or phosphorylated ERK-1/ERK-2 immunoreactivity (B). P values were determined using the two-sided Cox-Mantel log-rank test. Events (open circles) account for local recurrence, metastasis, and cancer-related death.
The Kaplan-Meier curves also showed a marginally significant difference (P = 0.05) in the probability that patients would remain free of disease between the group with an activated ERK-1/ERK-2 pathway and the group in which this pathway was inactive (Figure 2B) ▶ .
Tumor grade and phosphorylated ERK-1/ERK-2 immunoreactivity were then entered in a multivariate analysis of progression-free survival (Table 3) ▶ using a Cox’s proportional-hazard model. This analysis showed that high tumor grade (P = 0.0005) and ERK-1/ERK-2 pathway activation (P = 0.03) were independent prognostic factors.
Table 3.
Independent Factors of Cancer Progression
| Factor | Cancer progression | P value | |
|---|---|---|---|
| Categories | Hazard ratio* (95% CI) | ||
| Phosphorylated ERK-1/ERK-2 | |||
| H-Score† | 0.03 | ||
| Less than 1 | 1 | ||
| Equal or more than 1 | 4.0 (1.1–13.7) | ||
| Grade | 0.0005 | ||
| 1 or 2 | 1 | ||
| 3 | 12.3 (3.0–50.1) | ||
*, Hazard ratios were determined using a Cox’s proportional-hazard model. CI denotes confidence interval.
†H-score, product of the proportion of positive tumor cells by the intensity of staining with the phosphorylated ERK-1/ERK2 antibody.
Activated ERK-1/ERK-2 Pathway and Tumor Cell Proliferation
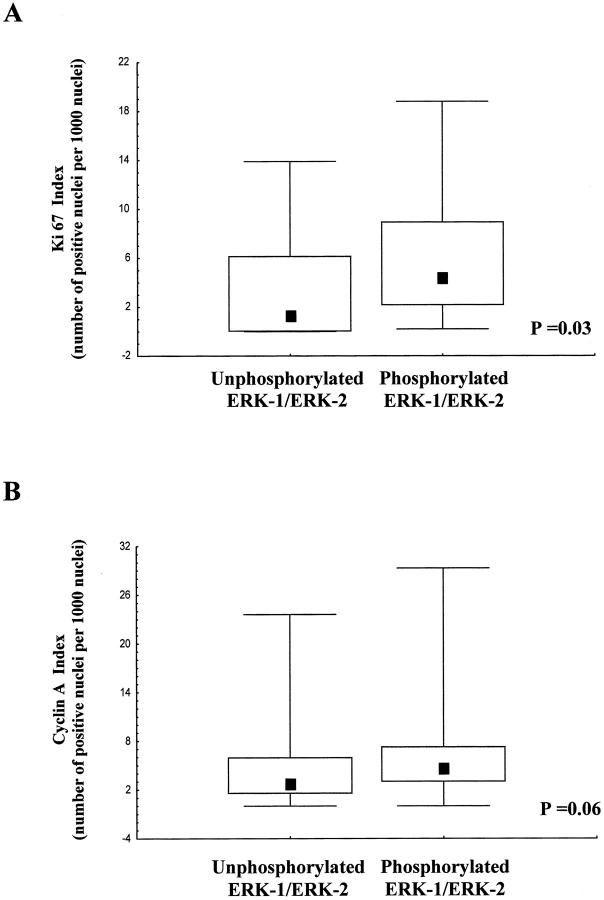
As already stated, there was no correlation between mitosis index equal or more than 4 per 10 high-power fields (1.7 square mm) and activated ERK-1/ERK-2. However, a likely cellular response to activated ERK-1/ERK-2 was increased proliferative activity. We thus stained mucoepidermoid carcinoma samples with the Ki67 antibody, which reacts with cells in G1 to M phases of the cell cycle 29 (Figure 1B) ▶ . The Ki67 index was higher (P = 0.03; Figure 3 A ▶ ) in the group of tumors with activated ERK-1/ERK-2 pathway (median 4, interquartile range, 2 to 8) than in the group in which this pathway was not activated (median 1.5, interquartile range, 0 to 6).
Figure 3.
Box plots of Ki67 (A) and cyclin A (B) index values according to phosphorylated ERK-1/ERK-2 immunoreactivity. Lower and upper limits of boxes indicate the 25th and 75th percentiles; squares within boxes indicate the median values; the lines extending from the boxes indicate the range of non-outlying values. Outliers were not plotted, but included in the statistical analysis.
Since the difference in Ki67 staining was small, we sought to strengthen our data by analyzing cyclin A labeling index (Figure 1B) ▶ . Although cyclin A expression defines a different window of cell cycle progression compared to that identified by anti-Ki67 antibodies, cyclin A expression levels are usually correlated with those of Ki67 expression. 29 As expected, we found a significant correlation between cyclin A and Ki67 labeling values (r = 0.48; P < 0.001), indicating that both markers are co-regulated. Importantly, similar to Ki67 index, the cyclin A labeling index was higher (Figure 3B) ▶ in the group of tumors with activated ERK-1/ERK-2 pathway (median 4.5, interquartile range, 3 to 7) than in the group in which this pathway was not activated (median 2.5, interquartile range, 1.5 to 6), although the difference was only close to significance (P = 0.06).
Activated ERK-1/ERK-2 Pathway and p16/INK4a or HER-2/neu Protein Expression
We stained mucoepidermoid carcinoma samples with the HER-2/neu CB11 monoclonal antibody (not shown). Of the 57 samples, nine cases (16%) exhibited a complete membranous staining, mainly in intermediate and squamous cells. There was no relationship between HER-2/neu membranous staining and ERK-1/ERK-2 activation (P = 0.46) as only two tumors with activated ERK-1/ERK-2 also demonstrated HER-2/neu staining. However, by comparing adjacent sections, in both tumors the foci of HER-2/neu reactive intermediate cells seemed also to express phosphorylated ERK-1/ERK-2 in their nuclei.
Lastly, we evaluated p16/INK4a loss by staining samples with a monoclonal antibody to p16 in the subset of tumors demonstrating activated ERK-1/ERK-2 (Figure 1B) ▶ . Five cases failed to react with the p16 antibody even in stromal or normal salivary gland duct cells. As these cells usually express p16, 31,33 lack of p16 immunoreactivity in these samples was likely caused by alteration or loss of the corresponding epitope. Therefore, these five cases were considered as inappropriate and were excluded from the immunohistochemical analysis. The 17 remaining cases displayed p16 reactive tumor cells in each case and were considered p16-positive.
Discussion
Using a polyclonal antibody specific for the dually phosphorylated (active) ERK-1/ERK-2 isoforms, we have examined by immunohistochemistry phosphorylated ERK-1/ERK-2 immunoreactivity levels in mucoepidermoid carcinomas and their adjacent normal salivary gland tissues. The direct comparison of immunohistochemical reactivity between normal and tumor salivary gland tissue shows that mucoepidermoid carcinomas display higher levels of phosphorylated ERK-1/ERK-2 than their surrounding normal salivary parenchyma. Activation of signals upstream of ERK in tumor cells is actually occurring rather than overexpression of ERK families since total ERK-1/ERK-2 is expressed at similar levels in normal and tumor tissues. By using as a cutoff the immunoreactivity level below which >95% of normal salivary gland tissue samples express phosphorylated ERK-1/ERK-2, we show that ∼39% of mucoepidermoid carcinomas display high levels of phosphorylated ERK-1/ERK-2. The data presented here suggest that ERK-1/ERK-2 pathway activation occurs during tumorigenesis and, possibly, plays a role in the oncogenic process. An additional interesting finding is that patients whose tumors display increased levels of phosphorylated ERK-1/ERK-2 have a worse prognosis, ie, are more likely to experience disease-related events such as local recurrence, metastasis, or death, than patients with tumors in which this MAPK pathway is inactive. This finding suggests that ERK-1/ERK-2 pathway activation is associated with a more aggressive tumor behavior and plays a role in mucoepidermoid carcinoma progression as well as in tumorigenesis. Since little is known regarding oncogenic molecular events occurring in mucoepidermoid carcinoma, we believe that our report is a significant advance in the knowledge of these neoplasms, which account for one of the most frequent malignant salivary gland tumor in adults and children.
The evidence that ERK-1/ERK-2 pathway is active in mucoepidermoid carcinoma is based on the specific reactivity of the polyclonal antibody for phosphorylated ERK-1/ERK-2, which has been previously established by Kharitonenkov et al 27 using Western blots. In a study of gliomas, Mandell et al 23 have been the first authors to demonstrate the practicability and usefulness of using immunohistochemical detection to visualize phosphorylated ERK-1/ERK-2 patterns. Thereafter, similar to our study, several immunohistochemical studies have used the same polyclonal antibody to determine phosphorylated ERK-1/ERK-2 immunoreactivity in various cancers, including squamous-cell carcinomas by Albanell et al, 22 prostate cancers by Malik et al, 25 and melanomas by Cohen et al. 24 In an immunohistochemical study of breast cancer progression, Adeyinka et al 21 have also demonstrated the validity of using this polyclonal antibody to establish levels of phosphorylated ERK-1/ERK-2 immunoreactivity in paraffin-embedded breast cancer cells. Our report, using the semi-quantitative scoring method (H-score) described by Al-Haddad et al 32 and used by Adeyinka et al, 21 is the first to document increased levels of phosphorylated ERK-1/ERK-2 immunoreactivity in salivary gland mucoepidermoid carcinomas. The advantage of immunohistochemistry is the ability to visualize at the cellular level MAPK activation patterns. It is particularly useful to distinguish MAPK activation in tumor cells and in stromal cells, as many endothelial cells also display phosphorylated ERK-1/ERK-2 and can be a confounding factor in Western blot studies. In immunohistochemical assays, endothelial cells serve as a convenient internal control to detect possible heterogeneity in tissue immunoreactivity related to differences in fixation procedure. The nuclear localization of the immunohistochemical staining is a further microscopic feature to confirm the state of activation of ERK-1/ERK2, although it is known that activated ERK-1/ERK2 forms can also activate cytosolic proteins, such as the membrane-bound phospholipase A2. 34
Since there is a good correlation between extracellular agents that lead to cell proliferation and stimulation of the MAPK cascade, the most likely impact of ERK-1/ERK-2 activation in tumor cells is to increase cellular proliferation. 12-14 A weak association between activated ERK-1/ERK-2 and proliferative index has been reported in head and neck squamous-cell carcinoma. 22 Mucoepidermoid carcinomas are usually slow-growing tumors with proliferative rates lower than those observed in more aggressive carcinomas such as head and neck squamous-cell carcinomas. Therefore, it is not unexpected that we observed low levels of both Ki67 and cyclin A expression in most mucoepidermoid carcinomas. Our data are validated by the clear correlation between Ki67 and cyclin A index values. Our report indicates that these markers are co-regulated and thus truly reflect proliferative activity in tumor cells. However, cyclin A expression is involved in both S phase and in the G2/M transition and defines a different window in the cell cycle progression compared to that identified by anti-Ki67 antibodies. This may explain why the relation of ERK activation to cell cycle appears stronger in the analysis using Ki67 as compared to cyclin A as cell cycle markers. More importantly, the differences in Ki67 and cyclin A indexes between phospho-ERK-negative and -positive tumors are perfectly consistent. Together, the data presented here strongly support that ERK-1/ERK-2 activity in mucoepidermoid carcinoma is one of the factors contributing to increased cell proliferation. Nevertheless, it seems very likely that deregulation of cell cycle in mucoepidermoid carcinoma depends on additional genetic changes, which together with ERK-1/ERK-2 activation explain the full proliferative activity of tumor cells. We tested whether loss of p16 cooperates with ERK-1/ERK-2 activation, as recently described. 35 Our results do not support this hypothesis, although a more extensive immunohistochemical study with several antibodies and a genetic study may be needed to definitively exclude such cooperation. With regard to the contribution of ERK-1/ERK-2 activation to mucoepidermoid carcinoma aggressiveness, it is likely that the aggressive behavior of these tumors do not depend on tumor cell proliferative activity alone. Other cellular ERK-dependent responses including angiogenesis and altered cell motility can play a role in a more aggressive tumor behavior. Some of these phenotypic transformations could be mediated by altered transcription of ERK-1/ERK-2 target genes, such as vascular endothelial growth factor and tissue factor, as demonstrated for invasive phase melanoma. 24 Other important cell modifications could involve transcription-independent suppression of integrin activation, which reduces cell adhesion to the extracellular matrix and enhances cell mobility. 36 As previously noted in mucoepidermoid carcinomas, phosphorylated ERK-1/ERK-2 immunoreactivity is mainly observed in intermediate cells, which are thought to be modified myoepithelial cells. These cells are located around luminal cells and have basal lamina partially lining their surface and, thus, interact via integrin receptors with extracellular matrix. 1 It would be interesting to know whether phosphorylated ERK-1/ERK-2 immunoreactivity in intermediate cells correlates to their integrin activation state.
Deregulation of ERK pathway is often associated with oncogenic transformation. 12 Phosphorylated ERK-1/ERK-2 immunoreactivity has been related to tumor progression in oligodendrogliomas, 23 to progression of dysplastic naevi to melanomas, 24 and to metastasis in head and neck squamous-cell carcinoma 22 and in breast cancer. 21 Thus, in several cancers as well as in salivary gland mucoepidermoid carcinoma, ERK-1/ERK-2 pathway activation is associated with more advanced stages of disease and/or a worse prognosis. However, ERK-1/ERK-2 does not seem to possess any oncogenic potential by itself, and no naturally occurring ERK-1/ERK-2 kinase oncogenes have been found. 12 ERK-1/ERK-2 phosphorylation may reflect the oncogenic potential of upstream activators such as Ras, Raf-1, Mos, and the EGF receptor. H-Ras mutations have been detected in approximately one-fifth of mucoepidermoid carcinomas, a prevalence which appears less than the proportion of cases showing ERK-1/ERK-2 pathway activation. 10 Li et al 26 have reported that oncogenic Raf-1 activates the ERK-1/ERK-2-signaling pathway, which is required for the transformation of the rat salivary epithelial cell line, Pa-4. However, the status of Raf in human mucoepidermoid carcinoma is yet to be determined. Expression of EGF receptor is mainly localized to epidermoid cells in mucoepidermoid carcinomas, while our report indicates that phosphorylated forms of ERK-1/ERK-2 are mainly found in intermediate cells. 37 Press et al 9 have reported HER-2/neu amplification and/or overexpression in about a third of mucoepidermoid carcinomas. We report HER-2/neu membranous expression in 16% of cases in our series. Admittedly, the CB11 monoclonal antibody is thought to be less sensitive than the polyclonal antibody used by Press and al. 38 Other authors have reported that, in breast cancer samples, discrepancies between polyclonal and monoclonal antibodies are represented by tumors weakly positive (2+) with polyclonal antibodies and that this weak reactivity with polyclonal antibodies correlates poorly with gene amplification. 30 Our data clearly show that overexpression of HER-2/neu is not a necessary factor in ERK-1/ERK-2 pathway activation in mucoepidermoid carcinoma. However, our understanding of the connections within the MAPK pathways is incomplete, and we cannot exclude that oncogenic activation of ERK-1/ERK-2 pathway occurs at different levels in mucoepidermoid carcinoma, including HER-2/neu in some cases. Alternatively, ERK-1/ERK-2 pathway activation may reflect the phenotypic alterations induced by other oncogenic events that occur as tumor cells acquire a more aggressive potential. For example, it can be speculated that, at an invasive stage, destruction of the adjacent conjunctive tissue can unmask matrix-embedded growth factors, which in turn can be responsible for the activation of ERK-1/ERK-2 pathway.
Histopathological grading of salivary gland mucoepidermoid carcinoma has proved useful to predict prognosis and can be important to make the decision for postoperative radiation therapy. 2 However, some patients with low-grade mucoepidermoid carcinoma do not experience the favorable clinical course anticipated on histopathological findings. 3,4 Numerous grading methods have been proposed to enhance reproducibility and predictability. One of the most reliable grading systems has been described by Goode et al, 3,4 although these authors stress the need for additional markers to improve predictability of low-grade mucoepidermoid carcinoma. Our report, using the AFIP criteria show survival results entirely consistent with data reported by these authors as well as those reported by Brandwein et al 3,4,28 using the same criteria. We show that phosphorylated ERK-1/ERK-2 immunoreactivity levels and histopathological grade are independent prognostic factors of progression-free survival in a multivariate analysis. This finding suggests that immunohistochemical staining for this marker can be useful in the clinical practice. The only morphological characteristic to which phosphorylated ERK-1/ERK-2 immunoreactivity may be associated is predominance of intermediate tumor cells, which form the bulk of poorly differentiated tumors, as defined in the World Health Organization Salivary Gland Tumors Classification. 39
Another very interesting point is that anticancer drugs targeting the MAPK pathway are currently being developed. 16 The detection of phosphorylated ERK-1/ERK-2 immunoreactivity in excised tumor samples could be a straightforward way to select patients for these treatments and to monitor drug response. Using the antibody against phosphorylated ERK-1/ERK-2, in vivo evaluation of MAPK kinase inhibition by new compounds has already been successfully assayed in pre-clinical animal models. 40 Since activation of ERK-1/ERK-2 pathway is associated with a worse prognosis, patients with mucoepidermoid carcinoma displaying increased levels of phosphorylated ERK-1/ERK-2 appear to be good candidates to test these new drugs.
In conclusion, phosphorylated ERK-1/ERK-2 immunoreactivity is increased in mucoepidermoid carcinomas as compared to normal salivary gland tissue, and predicts a worse prognosis independently of tumor grade. An increase in tumor cell proliferation is associated with ERK-1/ERK-2 pathway activation. Our findings suggest that ERK-1/ERK-2 pathway is involved in tumorigenesis and tumor progression of salivary gland mucoepidermoid carcinoma, and is an important target for new anticancer drugs to control these neoplasms.
Acknowledgments
We thank Joelle Jasgchian, Edith Joly, Annette Lesot, and Nicole Vignot for technical assistance.
Footnotes
Address reprint requests to Pierre Fouret. Service d’Anatomie et de Cytologie Pathologiques. Groupe Hospitalier Pitié-Salpêtrière, 83 Boulevard de l’Hôpital, 75634 Paris Cedex 13, France. E-mail: pierre.fouret@psl.ap-hop-paris.fr.
Supported by grant of the Association pour la Recherche contre le Cancer.
References
- 1.Ellis GL, Auclair PL: Mucoepidermoid carcinoma. Rosai J Sobin LH eds. Tumors of the Salivary Glands. 1996:pp 155-172 Armed Forces Institute of Pathology, Washington, DC
- 2.Sessions R, Harrison L, Forastiere A: Tumors of the salivary glands and paragangliomas. De Vita VT Hellman S Rosenberg SA eds. Cancer: Principles and Practice of Oncology, ed 6 2000:pp 886-900 Lippicott Williams and Wilkins, Philadelphia, PA
- 3.Goode RK, Auclair PL, Ellis GL: Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer 1998, 82:1217-1224 [DOI] [PubMed] [Google Scholar]
- 4.Auclair PL, Goode RK, Ellis GL: Mucoepidermoid carcinoma of intraoral salivary glands: evaluation and application of grading criteria in 143 cases. Cancer 1992, 69:2021-2030 [DOI] [PubMed] [Google Scholar]
- 5.Dardick I, Gliniecki MR, Heathcote JG, Burford-Mason A: Comparative histogenesis and morphogenesis of mucoepidermoid carcinoma and pleomorphic adenoma: an ultrastructural study. Virchows Arch A Pathol Anat Histopathol 1990, 417:405-417 [DOI] [PubMed] [Google Scholar]
- 6.Stenman G, Sandros J, Dahlenfors R, Juberg-Ode M, Mark J: 6q- and loss of the Y chromosome: two common deviations in malignant human salivary gland tumors. Cancer Genet Cytogenet 1986, 22:283-293 [DOI] [PubMed] [Google Scholar]
- 7.Jin C, Martins C, Jin Y, Wiegant J, Wennerberg J, Dictor M, Gisselsson D, Strombeck B, Fonseca I, Mitelman F, Tanke HJ, Hoglund M, Mertens F: Characterization of chromosome aberrations in salivary gland tumors by FISH, including multicolor COBRA-FISH. Genes Chromosomes Cancer 2001, 30:161-167 [PubMed] [Google Scholar]
- 8.el-Naggar AK, Lovell M, Killary AM, Batsakis JG: Genotypic characterization of a primary mucoepidermoid carcinoma of the parotid gland by cytogenetic, fluorescence in situ hybridization, and DNA ploidy analysis. Cancer Genet Cytogenet 1996, 89:38-43 [DOI] [PubMed] [Google Scholar]
- 9.Press MF, Pike MC, Hung G, Zhou JY, Ma Y, George J, Dietz-Band J, James W, Slamon DJ, Batsakis JG, El-Naggar AK: Amplification and overexpression of HER-2/neu in carcinomas of the salivary gland: correlation with poor prognosis. Cancer Res 1994, 54:5675-5682 [PubMed] [Google Scholar]
- 10.Yoo J, Robinson RA: H-ras gene mutations in salivary gland mucoepidermoid carcinomas. Cancer 2000, 88:518-523 [DOI] [PubMed] [Google Scholar]
- 11.Cantley LC, Auger KR, Carpenter C, Duckworth B, Graziani A, Kapeller R, Soltoff S: Oncogenes and signal transduction. Cell 1991, 64:281-302 [DOI] [PubMed] [Google Scholar]
- 12.Seger R, Krebs EG: The MAPK signaling cascade. EMBO J 1995, 9:726-735 [PubMed] [Google Scholar]
- 13.Lewis TS, Shapiro PS, Ahn NG: Signal transduction through MAP kinase cascades. Adv Cancer Res 1998, 74:49-139 [DOI] [PubMed] [Google Scholar]
- 14.Johnson GL, Lapadat R: Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298:1911-1912 [DOI] [PubMed] [Google Scholar]
- 15.Leevers SJ, Marshall CJ: Activation of extracellular signal-regulated kinase, ERK2, by p21ras oncoprotein. EMBO J 1992, 11:569-574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sebolt-Leopold JS: Development of anticancer drugs targeting the MAP kinase pathway. Oncogene 2000, 19:6594-6599 [DOI] [PubMed] [Google Scholar]
- 17.Schmidt M, Goebeler M, Posern G, Feller SM, Seitz CS, Brocker EB, Rapp UR, Ludwig S: Ras-independent activation of the Raf/MEK/ERK pathway upon calcium-induced differentiation of keratinocytes. J Biol Chem 2000, 275:41011-41017 [DOI] [PubMed] [Google Scholar]
- 18.Loda M, Capodieci P, Mishra R, Yao H, Corless C, Grigioni W, Wang Y, Magi-Galluzzi C, Stork PJ: Expression of mitogen-activated protein kinase phosphatase-1 in the early phases of human epithelial carcinogenesis. Am J Pathol 1996, 149:1553-1564 [PMC free article] [PubMed] [Google Scholar]
- 19.Magi-Galluzzi C, Mishra R, Fiorentino M, Montironi R, Yao H, Capodieci P, Wishnow K, Kaplan I, Stork PJ, Loda M: Mitogen-activated protein kinase phosphatase 1 is overexpressed in prostate cancers and is inversely related to apoptosis. Lab Invest 1997, 76:37-51 [PubMed] [Google Scholar]
- 20.Oka H, Chatani Y, Hoshino R, Ogawa O, Kakehi Y, Terachi T, Okada Y, Kawaichi M, Kohno M, Yoshida O: Constitutive activation of mitogen-activated protein (MAP) kinases in human renal cell carcinoma. Cancer Res 1995, 55:4182-4187 [PubMed] [Google Scholar]
- 21.Adeyinka A, Nui Y, Cherlet T, Snell L, Watson PH, Murphy LC: Activated mitogen-activated protein kinase expression during human breast tumorigenesis and breast cancer progression. Clin Cancer Res 2002, 8:1747-1753 [PubMed] [Google Scholar]
- 22.Albanell J, Codony-Servat J, Rojo F, Del Campo JM, Sauleda S, Anido J, Raspall G, Giralt J, Rosello J, Nicholson RI, Mendelsohn J, Baselga J: Activated extracellular signal-regulated kinases: association with epidermal growth factor receptor/transforming growth factor α expression in head and neck squamous carcinoma and inhibition by anti-epidermal growth factor receptor treatments. Cancer Res 2001, 61:6500-6510 [PubMed] [Google Scholar]
- 23.Mandell JW, Hussaini IM, Zecevic M, Weber MJ, VandenBerg SR: In situ visualization of intratumor growth factor signaling: immunohistochemical localization of activated ERK/MAP kinase in glial neoplasms. Am J Pathol 1998, 153:1411-1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen C, Zavala-Pompa A, Sequeira JH, Shoji M, Sexton DG, Cotsonis G, Cerimele F, Govindarajan B, Macaron N, Arbiser JL: Mitogen-actived protein kinase activation is an early event in melanoma progression. Clin Cancer Res 2002, 8:3728-3733 [PubMed] [Google Scholar]
- 25.Malik SN, Brattain M, Ghosh PM, Troyer DA, Prihoda T, Bedolla R, Kreisberg JI: Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin Cancer Res 2002, 8:1168-1171 [PubMed] [Google Scholar]
- 26.Li D, Lin HH, McMahon M, Ma H, Ann DK: Oncogenic raf-1 induces the expression of non-histone chromosomal architectural protein HMGI-C via a p44/p42 mitogen-activated protein kinase-dependent pathway in salivary epithelial cells. J Biol Chem 1997, 272:25062-25070 [DOI] [PubMed] [Google Scholar]
- 27.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A: A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature 1997, 386:181-186 [DOI] [PubMed] [Google Scholar]
- 28.Brandwein MS, Ivanov K, Wallace DI, Hille JJ, Wang B, Fahmy A, Bodian C, Urken ML, Gnepp DR, Huvos A, Lumerman H, Mills SE: Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol 2001, 25:835-845 [DOI] [PubMed] [Google Scholar]
- 29.Noguchi T, Dobashi Y, Minehara H, Itoman M, Kameya T: Involvement of cyclins in cell proliferation and their clinical implications in soft tissue smooth muscle tumors. Am J Pathol 2000, 156:2135-2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lebeau A, Deimling D, Kaltz C, Sendelhofert A, Iff A, Luthardt B, Untch M, Lohrs U: Her-2/neu analysis in archival tissue samples of human breast cancer: comparison of immunohistochemistry and fluorescence in situ hybridization. J Clin Oncol 2001, 19:354-363 [DOI] [PubMed] [Google Scholar]
- 31.Klussmann JP, Gultekin E, Weissenborn SJ, Wieland U, Dries V, Dienes HP, Eckel HE, Pfister HJ, Fuchs PG: Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol 2003, 162:747-753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Haddad S, Zhang Z, Leygue E, Snell L, Huang A, Niu Y, Hiller-Hitchcock T, Hole K, Murphy LC, Watson PH: Psoriasin (S100A7) expression and invasive breast cancer. Am J Pathol 1999, 155:2057-2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soria JC, Morat L, Commo F, Dabit D, Perie S, Sabatier L, Fouret P: Telomerase activation cooperates with inactivation of p16 in early head and neck tumorigenesis. Br J Cancer 2001, 84:504-511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Rossum GS, Klooster R, van den Bosch H, Verkleij AJ, Boonstra J: Phosphorylation of p42/44(MAPK) by various signal transduction pathways activates cytosolic phospholipase A(2) to variable degrees. J Biol Chem 2001, 276:28976-28983 [DOI] [PubMed] [Google Scholar]
- 35.Govindarajan B, Klafter R, Miller MS, Mansur C, Mizesko M, Bai X, LaMontagne K, Jr, Arbiser JL: Reactive oxygen-induced carcinogenesis causes hypermethylation of p16(Ink4a) and activation of MAP kinase. Mol Med 2002, 8:1-8 [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes PE, Renshaw MW, Pfaff M, Forsyth J, Keivens VM, Schwartz MA, Ginsberg MH: Suppression of integrin activation: a novel function of a Ras/Raf-initiated MAP kinase pathway. Cell 1997, 88:521-530 [DOI] [PubMed] [Google Scholar]
- 37.Yamada K, Iwai K, Okada Y, Mori M: Immunohistochemical expression of epidermal growth factor receptor in salivary gland tumours. Virchows Arch A Pathol Anat Histopathol 1989, 415:523-531 [DOI] [PubMed] [Google Scholar]
- 38.Press MF, Hung G, Godolphin W, Slamon DJ: Sensitivity of HER-2/neu antibodies in archival tissue samples: potential source of error in immunohistochemical studies of oncogene expression. Cancer Res 1994, 54:2771-2777 [PubMed] [Google Scholar]
- 39.Seifert G, Sobin LH, : Pathologists in 6 countries: Seifert G Sobin LH eds. Histological Typing of Salivary Gland Tumours. 1991:pp 20-21 Springer-Verlag Berlin, Germany
- 40.Sebolt-Leopold JS, Dudley DT, Herrera R, Van Becelaere K, Wiland A, Gowan RC, Tecle H, Barrett SD, Bridges A, Przybranowski S, Leopold WR, Saltiel AR: Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat Med 1999, 5:810-816 [DOI] [PubMed] [Google Scholar]