Abstract
Hepatocellular carcinoma (HCC) is one of the most fatal human malignancies, but the molecular mechanisms of hepatocarcinogenesis remain unclear. Although p53 mutations are frequently observed in Asian HCC, it is not a common event in Western HCC. Recent studies suggest that tumor suppressor genes (TSGs) can also be silenced through epigenetic disruption, such as promoter CpG island methylation, during carcinogenesis. To further understand the molecular mechanism of hepatocarcinogenesis, we have investigated the promoter methylation status of nine TSGs (SOCS-1, GSTP, APC, E-cadherin, RAR-β, p14, p15, p16, and p73) in 51 cases of HCC using methylation-specific polymerase chain reaction. We found that 82% of HCCs had methylation of at least one TSG promoter. The most frequently methylated TSGs in HCC were: SOCS-1 (65%), GSTP (54%), APC (53%), E-cadherin (49%), and p15 (49%). Methylation of SOCS-1, GSTP, APC, E-cadherin, and p15 was more frequent in HCC than in nontumor liver (P < 0.05). Methylation of SOCS-1, GSTP, and p15 was also significantly more frequent in HCC than cirrhotic liver (P < 0.05). Although methylation of one or two genes could be seen in both nontumor and cirrhotic livers, 53% of the HCC cases had three or more TSG promoters methylated, in comparison to 0% in nontumor liver and 13% in cirrhosis (P = 0.001). Methylation of SOCS-1, APC, and p15 was more frequently seen in hepatitis C virus-positive HCC than hepatitis C virus/hepatitis B virus-negative HCC. Our data suggest that promoter hypermethylation of TSGs is a common event in HCC and may play an important role in hepatocarcinogenesis.
Hepatocellular carcinoma (HCC) is one of the most common malignancies in the world and among the most fatal of human neoplasms, but the molecular mechanisms of hepatocarcinogenesis are primarily unknown. Recent genetic studies have indicated that both the p53 and pRb pathways may be involved in hepatocarcinogenesis. It has been shown that point mutations of the p53 tumor suppressor gene (TSG) were frequently seen in HCCs in Chinese and African populations. 1-3 However, p53 mutation is not a frequent event in American and European HCCs. 4-6 This discrepancy may result from different ethic backgrounds and/or different etiologies, such as hepatitis B virus (HBV) and hepatitis C virus (HCV) infections or toxin exposure. Both HBV and HCV infections are thought to be involved in hepatocarcinogenesis because chronic hepatitis and cirrhosis associated with either HBV or HCV infection precede most HCCs. It has been shown that HBV encodes a X-gene product, HBx, that not only can activate the JAK/STAT signaling pathway, 7 but can also interact with p53 and impair the function of wild-type p53. 8 The core protein of HCV has also been shown to modulate gene transcription, cell proliferation, and cell death. 9 However, the exact mechanisms underlying virus-associated hepatocarcinogenesis are still unclear.
It has recently been proposed that aberrant methylation of CpG islands, which are CpG dinucleotide rich areas located mainly in the promoter regions of many genes, serves as an alternative mechanism for inactivation of TSGs in cancer. 10-14 It has been demonstrated that many TSGs can be functionally silenced through such aberrant promoter methylation. Recent studies have revealed that aberrant methylation of TSG promoters is frequently found in several common malignancies, such as colon, lung, breast, and prostate cancers. 15-17 Frequent promoter methylation of p16, p15, and GSTP genes has also been observed in the majority of HCCs in Chinese and Japanese populations. 18-20 However, the frequency of TSG methylation in Western HCC has not been studied as extensively. To elucidate the molecular mechanisms of hepatocarcinogenesis in this population, we have studied methylation of nine TSGs involved in multiple cellular signaling transduction pathways in 51 cases of HCC using a multiplex methylation-specific polymerase chain reaction (MSP) method, and have compared methylation of these genes in HCC to nonmalignant liver and cirrhotic liver. Our study indicates that TSG promoter methylation is a frequent event in Western HCC.
Materials and Methods
Tumor Samples
Fifty-one cases of HCC, including 25 cases of radical or partial hepatectomy and 26 cases of fine needle aspiration biopsy (FNA), were collected from The Johns Hopkins Hospital pathology archives. Fifteen cases of cirrhotic liver tissues remote from HCC lesions and 14 cases of nontumor liver tissues adjacent to either a hepatic adenoma or a focal nodular hyperplasia were also obtained from surgical resection specimens. The HBV and HCV infection status was tested serologically by enzyme immunoassay before this study and was documented through the pathology laboratory database. This study was reviewed by the Johns Hopkins Institutional Review Board and found to be exempt. No chemotherapy or radiation therapy was instituted before tumor excision or FNA procedure. The case distribution in different clinicopathological categories is summarized in Table 1 ▶ . The tissue was fixed in buffered formalin for surgical specimens or in ethanol-based fixative for FNA specimens. Consecutive 4-μm sections were cut from paraffin-embedded tissue blocks and mounted for histopathological evaluation using conventional hematoxylin and eosin (H&E) staining. H&E-stained sections also served as a guide for tissue selection for DNA analysis. For DNA extraction, consecutive 10-μm sections were directly collected into sterile Eppendorff tubes. Genomic DNA was isolated by digestion with 100 μg/ml of proteinase K and followed by conventional phenol/chloroform (1:1) extraction and ethanol precipitation.
Table 1.
Clinicopathological Data of HCC Cases
| Clinicopathologic characteristics | Number of cases |
|---|---|
| Total case number | 51 |
| FNA specimens | 26 (51%) |
| Resection specimens | 25 (49%) |
| Median age (range) | 58 (27–81) |
| Sex | |
| Male | 30 (59%) |
| Female | 21 (41%) |
| Association with cirrhosis | |
| With cirrhosis | 36 (71%) |
| Without cirrhosis | 15 (29%) |
| Viral status | |
| HBV-positive | 9 (18%) |
| HCV-positive | 18 (36%) |
| Non-HBV, non-HCV | 24 (46%) |
| Tumor differentiation | |
| Well | 25 (49%) |
| Moderately | 18 (35%) |
| Poorly | 8 (16%) |
Bilsulfite Modification and MSP
Bisulfite modification and MSP were conducted based on the principle that bisulfite treatment of DNA converts unmethylated cytosine residues into uracil, whereas methylated cytosine residues remain unmodified. Thus, after bisulfite conversion, methylated and unmethylated DNA sequences can be distinguished by sequence-specific primers. DNA was treated with sodium bisulfite as described previously. 21 Briefly, 1 μg of genomic DNA was denatured by incubation with 0.2 mol/L of NaOH for 10 minutes at 37°C. Aliquots of 10 mmol/L of hydroquinone (30 μl; Sigma Chemical Co., St. Louis, MO) and 3 mol/L of sodium bisulfite (pH 5.0, 520 μl; Sigma Chemical Co.) were added and the solution was incubated at 50°C for 16 hours. Treated DNA was purified by use of the Wizard DNA purification system (Promega Corp., Madison, WI), desulfonated with 0.3 mol/L of NaOH, precipitated with ethanol, and resuspended in water. Modified DNA was stored at −70°C until used. DNA sequences containing promoter regions of SOCS-1, APC, E-cadherin, GSTP, p15, p16, RAR-β, p14, and p73 genes were first amplified in a single PCR run with 30 cycles using flanking primer sets as described previously. 22 DNA methylation of CpG islands was then determined by PCR using specific primers for both methylated and unmethylated DNA. 22 Two sets of primers were used to amplify each region of interest: one pair recognized a sequence in which CpG sites are unmethylated (bisulfite modified to UpG), and the other recognized a sequence in which CpG sites are methylated (unmodified by bisulfite treatment). Negative control samples without DNA were included for each set of PCR. PCR products were analyzed on 1% polyacrylamide gels. Detection of DNA methylation of all TSGs, except SOCS-1, was informative in all 51 cases of HCC. Because of the large size (210 bp) of the flanking PCR products of the SOCS-1 gene, the informative rate of SOCS-1 was relatively low in formalin-fixed surgical resection specimens of HCC, but 100% in alcohol-fixed cytological specimens. Therefore, only data of promoter methylation of SOCS-1 in 26 HCC cases of cytological specimens was reported in the Results section.
Statistical Analysis
Chi-square or Fisher exact tests, depending on the absolute numbers included in the analysis, were used to analyze the association of concurrent TSG methylation in HCC, cirrhosis, and nontumor liver. Chi-square test or Fisher exact test were also applied to the correlation between TSG methylation profiles and clinicopathological data, such as viral status and gender. A logistic regression analysis was used to analyze the correlation between TSG methylation profiles and the degree of tumor differentiation.
Results
Frequency of Individual TSG Methylation in HCC, Cirrhosis, and Nontumor Liver
The frequency of promoter methylation of nine genes in 51 cases of HCC, 15 cirrhotic livers, and 14 nontumor liver tissues was determined using MSP and is shown in Table 2 ▶ . Forty-two (82%) cases of HCC had methylation of at least one TSG. The most frequently methylated TSG promoters in HCC were: SOCS-1 (65%), GSTP (54%), APC (53%), E-cadherin (49%), and p15 (47%). Each of the genes, except APC, has been previously reported to be methylated frequently in HCC. 18-20,23 The four other genes studied demonstrated much less frequent methylation: p14 (6%), p73 (6%), RAR-β (12%), and p16 (16%). The frequency of methylation of E-cadherin, GSTP, APC, p15, SOCS-1, p16, and RAR-β promoters was much lower in both cirrhotic and nontumor livers (Table 2) ▶ . There was no methylation of p14, p15, or p73 in either nontumor or cirrhotic livers. Methylation of SOCS-1, GSTP, APC, E-cadherin, and p15 was significantly more frequent in HCC than in nontumor livers (P = 0.04, P = 0.002, P = 0.01, P = 0.005, and P = 0.001, respectively). Methylation of SOCS-1, GSTP, and p15 was also more frequently seen in HCC than cirrhosis (P = 0.03, P = 0.01, and P = 0.001, respectively). Methylation was also more frequent in HCC than cirrhosis for APC (53% versus 26%) and E-cadherin (49% versus 20%), but these changes were not statistically significant (P = 0.09 and P = 0.07, respectively). There was no significant difference in the methylation frequency of any tested TSGs between nontumor liver and cirrhosis (P > 0.05).
Table 2.
Frequency of TSG Promoter Methylation in HCC and Control Tissues
| Gene | HCC (n = 51) % (n) | Cirrhosis (n = 15) % (n) | Normal (n = 14) % (n) |
|---|---|---|---|
| SOCS-1 | 65 (17)* | 7 (1) | 7 (1) |
| GSTP | 54 (28) | 13 (2) | 7 (1) |
| APC | 53 (27) | 27 (4) | 14 (2) |
| E-cadherin | 49 (25) | 20 (3) | 7 (1) |
| P15 | 47 (24) | 0 (0) | 0 (0) |
| P16 | 16 (8) | 13 (2) | 7 (1) |
| RAR-beta | 12 (6) | 13 (2) | 7 (1) |
| P14 | 6 (3) | 0 (0) | 0 (0) |
| P73 | 6 (3) | 0 (0) | 0 (0) |
*Total number of cases for SOCS-1 analysis is 26.
Cumulative Methylation Patterns in HCC, Cirrhosis, and Nontumor Liver
Methylation status of eight TSGs (GSTP, APC, p15, p14, p73, p16, and RAR-β) was included in the analysis of cumulative methylation patterns. Methylation of SOCS-1 was not included because of the low informative rate in formalin-fixed surgical specimens (see Material and Methods). Methylation of multiple TSGs was commonly seen in HCC (Figure 1) ▶ . Fifty-three percent of the HCC cases had three or more TSG promoters methylated in comparison to 0% in nontumor livers and 13% in cirrhotic livers (P = 0.001). Methylation of four or more TSG promoters was only observed in HCC cases (Figure 1 ▶ and Table 3 ▶ ). Methylation of two or three TSGs was not specifically associated with either HCC or cirrhosis. Single TSG methylation was more frequently seen in nontumor liver (36%) than in HCC (5%) (P = 0.04). Eighteen percent of HCCs showed no methylation of any TSGs tested, in comparison to 47% in cirrhosis and 57% in nontumor liver.
Figure 1.
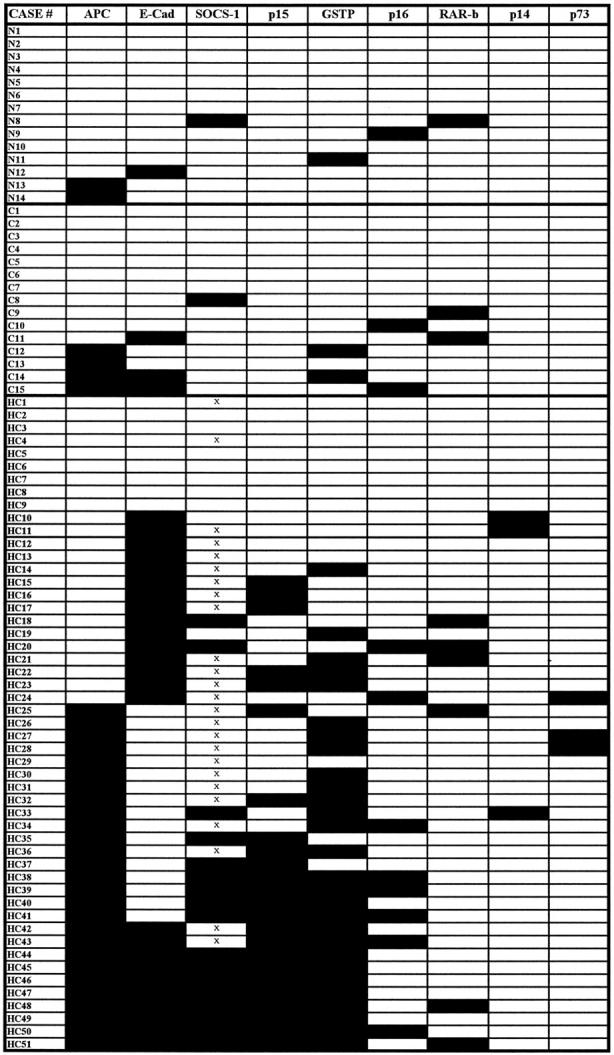
Methylation profiles of nine TSGs in HCC, cirrhosis, and nontumor livers. Cases were labeled as N1 to N14 for nontumor liver; C1 to C15 for cirrhosis; and HC1 to HC51 for HCC. A filled box indicates that promoter methylation was detected by MSP; an open box indicates that no methylation was detected; × indicates a noninformative specimen.
Table 3.
Cumulative Methylation Patterns of Eight TSG Promoters in HCC and Control Tissues
| TSG methylation | HCC (n = 51) % (n) | Cirrhosis (n = 15) % (n) | Nontumor (n = 14) % (n) |
|---|---|---|---|
| 0 gene | 18 (9) | 47 (7) | 57 (8) |
| 1 gene | 6 (3) | 27 (4) | 36 (5) |
| 2 genes | 23 (12) | 13 (2) | 7 (1) |
| 3 genes | 25 (13) | 13 (2) | 0 (0) |
| ≥4 genes | 28 (14) | 0 (0) | 0 (0) |
Subgrouping HCC Based on TSG Methylation Profiles
Based on the methylation profiles shown in Figure 1 ▶ , our 51 cases of HCC can be divided into three groups: nonmethylation group, scattered methylation group, and dense methylation group. Nine cases (18%) of HCC showed no methylation of any nine TSGs tested; the scattered methylation group contained 24 cases (54%), which showed methylation of one to three TSG promoters; the dense methylation group included 14 cases (28%), which had methylation of more than four TSG promoters. The most frequently methylated TSGs in the dense methylation group were APC, E-cadherin, SOCS-1, p15, and GSTP. Forty-two cases (82%) of HCC showed TSG methylation of either APC or E-cadherin or both. Interestingly, 76% of the methylated HCC cases exhibited a mutually exclusive methylation pattern between APC and E-cadherin.
Correlation between TSG Methylation Profile and Clinicopathological Data
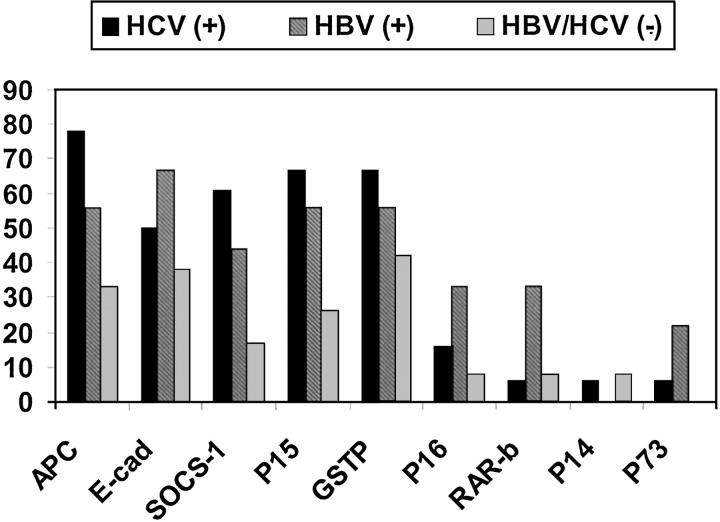
Correlation of HCC methylation profiles to clinicopathological data revealed several interesting associations. Overall, TSG methylation was more common among HBV-positive or HCV-positive HCC than HBV/HCV-negative HCC (Figure 2) ▶ . Methylation of SOCS-1, APC, and p15 was associated with HCV-positive HCC relative to HBV/HCV-negative HCC (P = 0.004, P = 0.006, and P = 0.03, respectively). Although not statistically significant, there seems to be a trend toward methylation of p16, p73, and RAR-β in HBV-positive HCC (Figure 2) ▶ . There was no significant difference between HCV-positive and HBV-positive HCC in the frequency of methylation of any tested TSGs (P > 0.05). There was no association between the frequency of TSG promoter methylation and gender or the histopathological differentiation of the tumor (P > 0.05).
Figure 2.
Association of TSG promoter methylation with the viral status of HCC. The percentage of HCC cases with TSG methylation is indicated for groups of patients that are positive for HBV, HCV, and neither HBV nor HCV.
Discussion
HCC, the major type of primary liver cancer, is one of the most common cancers worldwide and a leading cause of death in many countries. Although many of the major viral and environmental risk factors for HCC development have been unraveled, the genetic and epigenetic pathways leading to malignant transformation of liver cells have remained obscure. The heterogeneous geographical distribution of HCC further complicates efforts to identify the common genetic and epigenetic events responsible for hepatocarcinogenesis. One recent study indicated that expression of two crucial DNA methyltransferases, DNMT1 and DNMT3a, was significantly higher in HCC compared to nontumor liver, 24 suggesting that epigenetic mechanisms may be important. It will be important to identify the genes silenced through promoter CpG island hypermethylation during hepatocarcinogenesis.
We have studied methylation profiles of nine TSGs involved in several signal transduction pathways in 51 cases of Western HCC. We found that methylation of TSG promoters was a frequent event in HCC, as 82% cases of HCC had at least one TSG promoter methylated. Among nine TSGs tested, the most frequently methylated were SOCS-1, GSTP, APC, E-cadherin, and p15. In comparison to nontumor liver tissues, methylation of these five TSGs was specifically associated with HCC. Methylation of SOCS-1, GSTP, and p15 was also significantly more frequent in HCC than in cirrhotic liver. Other recent studies have also shown frequent methylation of SOCS-1, p15, and E-cadherin genes in Chinese and Japanese HCC. 18,19,25 It seems that silencing of TSGs through promoter CpG island methylation may play an important role during hepatocarcinogenesis.
It should be noted that our comparison tissues were not normal liver, but rather cirrhotic liver from patients with HCC and nontumor liver from patients with benign liver abnormalities, such as adenomas. Previous studies have found no methylation in normal liver from patients without disease. 19 However, to assess the utility of methylation profiling in diagnostic pathology, it is important to study hepatic tissue surrounding lesions. Our data suggest that gene methylation profiling may be clinically useful in distinguishing HCC from nonmalignant liver.
The most striking methylation pattern in HCC is the concurrent methylation of multiple TSGs. Although single or double TSG methylation can be seen in nontumor and cirrhotic liver tissue, the majority of our HCC cases harbored three or more methylated TSGs. In fact, methylation of four or more TSG promoters was only seen in HCC. Such multiple TSG methylation patterns were also observed in HCC by others using a set of genes involved in cell-cycle regulation. 26 These results emphasize the importance of analyzing multiple TSGs rather than a single gene. Also, this suggests that disruption of multiple signal transduction pathways is biologically required during hepatocarcinogenesis.
Previous studies have suggested that the Wnt signal transduction pathway may be important in hepatocarcinogenesis. In this pathway, the oncoprotein β-catenin normally interacts with E-cadherin at the plasma membrane. Its turnover is mediated by phosphorylation and ubiquitin-mediated degradation via the APC-Axin_GSK3b complex. 27-31 In many human neoplasms, this normal regulation is disrupted, resulting in the cytoplasmic and nuclear accumulation of β-catenin. Such an accumulation of β-catenin subsequently turns on the transcription factor TCF-1, which in turn enhances the expression of a set of genes involved in cell-cycle control and cell proliferation. This disregulation may result from mutations in APC, E-cadherin, Axin, or β-catenin genes. Immunohistochemical analyses of HCCs indicate abnormal nuclear accumulation of β-catenin in many cases. 32-34 However, mutations in the APC, β-catenin, or Axin genes appear to be uncommon in HCC, 35-42 suggesting that other gene-silencing mechanisms may be important. We found methylation of APC, E-cadherin, or both in 82% of HCCs. Interestingly, ∼76% of methylated HCC cases showed mutually exclusive APC and E-cadherin methylation, suggesting that inactivation of either APC or E-cadherin may lead to the accumulation of β-catenin in HCC.
Activation of the JAK/STAT pathway has also been implicated in hepatocarcinogenesis. Suppressor of cytokine signaling (SOCS-1, also known as JAB and SSI-1) is a protein that suppresses the JAK/STAT pathway by rendering cells unresponsive to cytokine stimulation. One recent study has indicated that the SOCS-1 gene was methylated in the majority of Chinese HCC cases. 19 The tumor suppressor function of SOCS-1 was further demonstrated by suppression of cell growth and colony formation after restoration of SOCS-1 into HCC cell lines. 19 Using a multiplex MSP approach, we have analyzed the methylation status of SOCS-1 in 26 FNA specimens of Western HCC. Consistent with previous studies, we have found that the SOCS-1 gene is frequently methylated in Western HCC. Methylation of SOCS-1 was more frequent in HBV-positive and HCV-positive HCC than HBV/HCV-negative HCC. It has been shown that activation of the JAK/STAT pathway by phosphorylation of STAT3 was observed both in cells constitutively expressing HBx and in cells that SOCS-1 was inactivated epigenetically. 7,19 Whether methylation of SOCS-1 mediates HBx-induced activation of JAK/STAT signaling pathway in HBV-positive HCC needs to be further characterized. Our study contributes to the thought that an imbalance of cytokine stimulation initiated by viral infection and subsequent silencing of SOCS-1 may play a role in hepatocarcinogenesis.
Another recent study has indicated that the p16 gene is frequently inactivated in HCC through homozygous deletion or promoter hypermethylation. 43 However, the reported frequency of p16 promoter methylation varies greatly in other reports. A high frequency of p16 methylation has been reported in Asian HCCs, 25,44 particularly those associated with viral infections. Recent studies from Korean and Taiwan populations showed a relatively low frequency of p16 promoter methylation (0 to 35%). 43,45 In our samples, we found that only 16% of Western HCC contained p16 promoter methylation. The discrepancies between these studies on p16 methylation may be because of geographic/ethic differences. Alternatively, it could also be because of the different conditions used for MSP analysis.
The p15 gene, which encodes another cyclin-dependent kinase inhibitor, is aberrantly methylated in several human neoplasms, especially hematopoietic malignancies. 17 It has been reported that p15 promoter methylation is present in 64% of Chinese HCC and 25% of HCC patients’ plasma and serum samples. 18 In our study, we found that 47% of HCC harbored p15 promoter methylation. Because methylation of p15 is not commonly seen in other solid carcinomas, except brain tumors, we examined the association of p15 methylation with viral infections in HCC. Interestingly, we found that p15 promoter methylation, along with SOCS-1 and APC, is more frequently seen in HBV-positive and HCV-positive HCC than HBV/HCV-negative HCC. Our study suggests that silencing of p15 through promoter methylation may be involved in virus-induced hepatocarcinogenesis. However, the absence of p15 promoter methylation in cirrhosis in our study argues against the hypothesis that disruption of p15 may be an early event of hepatocarcinogenesis. 26
In summary, we have shown that silencing of TSGs through promoter methylation is a frequent event in HCC. Epigenetic abnormalities in HCC not only play important roles during hepatocarcinogenesis, but also might be useful in the diagnosis of HCC.
Footnotes
Address reprint requests to Douglas P. Clark, M.D., Department of Pathology, Pathology Building, Rm 406, Johns Hopkins Medical Institutions, 600 North Wolfe St., Baltimore, MD 21287. E-mail: dclark@jhmi.edu.
B. Y. and M. G. contributed equally to this study.
References
- 1.Bressac B, Kew M, Wands J, Ozturk M: Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature 1991, 350:429-431 [DOI] [PubMed] [Google Scholar]
- 2.Hollstein MC, Wild CP, Bleicher F, Chutimataewin S, Harris CC, Srivatanakul P, Montesano R: p53 mutations and aflatoxin B1 exposure in hepatocellular carcinoma patients from Thailand. Int J Cancer 1993, 53:51-55 [DOI] [PubMed] [Google Scholar]
- 3.Hsu IC, Metcalf RA, Sun T, Welsh JA, Wang NJ, Harris CC: Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature 1991, 350:427-428 [DOI] [PubMed] [Google Scholar]
- 4.Boix-Ferrero J, Pellin A, Blesa R, Adrados M, Llombart-Bosch A: Absence of p53 gene mutations in hepatocarcinomas from a Mediterranean area of Spain. A study of 129 archival tumour samples. Virchows Arch 1999, 434:497-501 [DOI] [PubMed] [Google Scholar]
- 5.Challen C, Lunec J, Warren W, Collier J, Bassendine MF: Analysis of the p53 tumor-suppressor gene in hepatocellular carcinomas from Britain. Hepatology 1992, 16:1362-1366 [DOI] [PubMed] [Google Scholar]
- 6.Kazachkov Y, Khaoustov V, Yoffe B, Solomon H, Klintmalm GB, Tabor E: p53 abnormalities in hepatocellular carcinoma from United States patients: analysis of all 11 exons. Carcinogenesis 1996, 17:2207-2212 [DOI] [PubMed] [Google Scholar]
- 7.Lee YH, Yun Y: HBx protein of hepatitis B virus activates Jak1-STAT signaling. J Biol Chem 1998, 273:25510-25515 [DOI] [PubMed] [Google Scholar]
- 8.Kew MC: Increasing evidence that hepatitis B virus X gene protein and p53 protein may interact in the pathogenesis of hepatocellular carcinoma. Hepatology 1997, 25:1037-1038 [DOI] [PubMed] [Google Scholar]
- 9.Forrester K, Lupold SE, Ott VL, Chay CH, Band V, Wang XW, Harris CC: Effects of p53 mutants on wild-type p53-mediated transactivation are cell type dependent. Oncogene 1995, 10:2103-2111 [PubMed] [Google Scholar]
- 10.Herman JG, Baylin SB: Promoter-region hypermethylation and gene silencing in human cancer. Curr Top Microbiol Immunol 2000, 249:35-54 [DOI] [PubMed] [Google Scholar]
- 11.Herman JG, Jen J, Merlo A, Baylin SB: Hypermethylation-associated inactivation indicates a tumor suppressor role for p15INK4B. Cancer Res 1996, 56:722-727 [PubMed] [Google Scholar]
- 12.Baylin SB, Belinsky SA, Herman JG: Aberrant methylation of gene promoters in cancer—concepts, misconcepts, and promise. J Natl Cancer Inst 2000, 92:1460-1461 [DOI] [PubMed] [Google Scholar]
- 13.Baylin SB, Herman JG: DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet 2000, 16:168-174 [DOI] [PubMed] [Google Scholar]
- 14.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP: Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res 1998, 72:141-196 [PubMed] [Google Scholar]
- 15.Esteller M, Corn PG, Baylin SB, Herman JG: A gene hypermethylation profile of human cancer. Cancer Res 2001, 61:3225-3229 [PubMed] [Google Scholar]
- 16.Herman JG: p16(INK4): involvement early and often in gastrointestinal malignancies. Gastroenterology 1999, 116:483-485 [DOI] [PubMed] [Google Scholar]
- 17.Herman JG, Civin CI, Issa JP, Collector MI, Sharkis SJ, Baylin SB: Distinct patterns of inactivation of p15INK4B and p16INK4A characterize the major types of hematological malignancies. Cancer Res 1997, 57:837-841 [PubMed] [Google Scholar]
- 18.Wong IH, Lo YM, Yeo W, Lau WY, Johnson PJ: Frequent p15 promoter methylation in tumor and peripheral blood from hepatocellular carcinoma patients. Clin Cancer Res 2000, 6:3516-3521 [PubMed] [Google Scholar]
- 19.Yoshikawa H, Matsubara K, Qian GS, Jackson P, Groopman JD, Manning JE, Harris CC, Herman JG: SOCS-1, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity. Nat Genet 2001, 28:29-35 [DOI] [PubMed] [Google Scholar]
- 20.Tchou JC, Lin X, Freije D, Isaacs WB, Brooks JD, Rashid A, De Marzo AM, Kanai Y, Hirohashi S, Nelson WG: GSTP1 CpG island DNA hypermethylation in hepatocellular carcinomas. Int J Oncol 2000, 16:663-676 [DOI] [PubMed] [Google Scholar]
- 21.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB: Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 1996, 93:9821-9826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.House M, Guo M, Iacobuzio-Donahue C, Herman J: Molecular progression of promoter methylation in intraductal papillary mucinous neoplasms (IPMN) of the pancreas. Carcinogenesis 2003, 24:193-198 [DOI] [PubMed] [Google Scholar]
- 23.Matsumura T, Makino R, Mitamura K: Frequent down-regulation of E-cadherin by genetic and epigenetic changes in the malignant progression of hepatocellular carcinomas. Clin Cancer Res 2001, 7:594-599 [PubMed] [Google Scholar]
- 24.Saito Y, Kanai Y, Sakamoto M, Saito H, Ishii H, Hirohashi S: Expression of mRNA for DNA methyltransferases and methyl-CpG-binding proteins and DNA methylation status on CpG islands and pericentromeric satellite regions during human hepatocarcinogenesis. Hepatology 2001, 33:561-568 [DOI] [PubMed] [Google Scholar]
- 25.Matsuda Y, Ichida T, Matsuzawa J, Sugimura K, Asakura H: p16(INK4) is inactivated by extensive CpG methylation in human hepatocellular carcinoma. Gastroenterology 1999, 116:394-400 [DOI] [PubMed] [Google Scholar]
- 26.Roncalli M, Bianchi P, Bruni B, Laghi L, Destro A, Di Gioia S, Gennari L, Tommasini M, Malesci A, Coggi G: Methylation framework of cell cycle gene inhibitors in cirrhosis and associated hepatocellular carcinoma. Hepatology 2002, 36:427-432 [DOI] [PubMed] [Google Scholar]
- 27.Barth AI, Nathke IS, Nelson WJ: Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol 1997, 9:683-690 [DOI] [PubMed] [Google Scholar]
- 28.Hajra KM, Fearon ER: Cadherin and catenin alterations in human cancer. Genes Chromosom Cancer 2002, 34:255-268 [DOI] [PubMed] [Google Scholar]
- 29.Ilyas M, Tomlinson IP: The interactions of APC, E-cadherin and beta-catenin in tumour development and progression. J Pathol 1997, 182:128-137 [DOI] [PubMed] [Google Scholar]
- 30.Novak A, Dedhar S: Signaling through beta-catenin and Lef/Tcf. Cell Mol Life Sci 1999, 56:523-537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Behrens J: Control of beta-catenin signaling in tumor development. Ann NY Acad Sci 2000, 910:21-33 [DOI] [PubMed] [Google Scholar]
- 32.Nhieu JT, Renard CA, Wei Y, Cherqui D, Zafrani ES, Buendia MA: Nuclear accumulation of mutated beta-catenin in hepatocellular carcinoma is associated with increased cell proliferation. Am J Pathol 1999, 155:703-710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki T, Yano H, Nakashima Y, Nakashima O, Kojiro M: Beta-catenin expression in hepatocellular carcinoma: a possible participation of beta-catenin in the dedifferentiation process. J Gastroenterol Hepatol 2002, 17:994-1000 [DOI] [PubMed] [Google Scholar]
- 34.Wong CM, Fan ST, Ng IO: Beta-catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer 2001, 92:136-145 [DOI] [PubMed] [Google Scholar]
- 35.Chen TC, Hsieh LL, Ng KF, Jeng LB, Chen MF: Absence of APC gene mutation in the mutation cluster region in hepatocellular carcinoma. Cancer Lett 1998, 134:23-28 [DOI] [PubMed] [Google Scholar]
- 36.de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A, Perret C: Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA 1998, 95:8847-8851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang H, Fujii H, Sankila A, Mahler-Araujo BM, Matsuda M, Cathomas G, Ohgaki H: Beta-catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis C virus infection. Am J Pathol 1999, 155:1795-1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Legoix P, Bluteau O, Bayer J, Perret C, Balabaud C, Belghiti J, Franco D, Thomas G, Laurent-Puig P, Zucman-Rossi J: Beta-catenin mutations in hepatocellular carcinoma correlate with a low rate of loss of heterozygosity. Oncogene 1999, 18:4044-4046 [DOI] [PubMed] [Google Scholar]
- 39.Hsu HC, Jeng YM, Mao TL, Chu JS, Lai PL, Peng SY: Beta-catenin mutations are associated with a subset of low-stage hepatocellular carcinoma negative for hepatitis B virus and with favorable prognosis. Am J Pathol 2000, 157:763-770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cui J, Zhou X, Liu Y, Tang Z: Mutation and overexpression of the beta-catenin gene may play an important role in primary hepatocellular carcinoma among Chinese people. J Cancer Res Clin Oncol 2001, 127:577-581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujie H, Moriya K, Shintani Y, Tsutsumi T, Takayama T, Makuuchi M, Kimura S, Koike K: Frequent beta-catenin aberration in human hepatocellular carcinoma. Hepatol Res 2001, 20:39-51 [DOI] [PubMed] [Google Scholar]
- 42.Clevers H: Axin and hepatocellular carcinomas. Nat Genet 2000, 24:206-208 [DOI] [PubMed] [Google Scholar]
- 43.Jin M, Piao Z, Kim NG, Park C, Shin EC, Park JH, Jung HJ, Kim CG, Kim H: p16 is a major inactivation target in hepatocellular carcinoma. Cancer 2000, 89:60-68 [DOI] [PubMed] [Google Scholar]
- 44.Gong Y, Deng S, Zhang M, Wang G, Minuk GY, Burczynski F: A cyclin-dependent kinase inhibitor (p21(WAF1/CIP1)) affects thymidine incorporation in human liver cancer cells. Br J Cancer 2002, 86:625-629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin YW, Chen CH, Huang GT, Lee PH, Wang JT, Chen DS, Lu FJ, Sheu JC: Infrequent mutations and no methylation of CDKN2A (P16/MTS1) and CDKN2B (p15/MTS2) in hepatocellular carcinoma in Taiwan. Eur J Cancer 1998, 34:1789-1795 [DOI] [PubMed] [Google Scholar]