Abstract
Human telomerase reverse transcriptase (hTERT) is a catalytic subunit of telomerase and is a potentially useful diagnostic marker for cancers. There have been few studies in which immunological detection of hTERT has been attempted and its subcellular localization has not been precisely defined. In the present study, we re-evaluated expression and localization of hTERT in cancer and normal cells using a newly developed antibody. Immunohistochemistry revealed that hTERT is expressed in ∼80% of gynecological cancers, but some premalignant lesions exhibited weak expression of hTERT. Interestingly, not only nuclei but also cytoplasm of cancer cells were positive for hTERT staining. This finding was supported by the results of Western blot analysis of cell lines, in which both nuclear and cytoplasmic extracts exhibited significant hTERT bands. Cytoplasmic hTERT in cancer cells may be functional because the telomeric repeat amplification protocol assay of cytoplasmic extracts showed high levels of telomerase activity. Unexpectedly, not all normal primary cells and telomerase-negative cancer cell lines lacked hTERT expression; some exhibited weak TERT signals. In Western analysis, hTERT signals did not always correlate with telomerase activity of the various cell types. These findings suggest that functional hTERT is expressed in both the nucleus and cytoplasm of cancer cells and that hTERT expression does not strictly reflect telomerase activity. Further analysis is needed to clarify the biological significance of cytoplasmic hTERT.
The maintenance of telomeres, specialized nucleoprotein structures at the ends of chromosomes, is essential for chromosome stability. 1,2 Without new synthesis, telomeres undergo progressive shortening with each cell division, leading to replicative senescence of cells. 3 Telomerase is a ribonucleoprotein complex that extends and maintains the telomeres. Activation of this enzyme is therefore required for cells to overcome replicative senescence and be able to divide indefinitely. 4,5 This hypothesis is supported by observations that telomerase is frequently activated in the vast majority of cancers or cancer cell lines but not in most normal tissues. 6 Studies of the telomerase enzyme complex have revealed the presence of two major subunits that contribute to in vitro enzymatic activity: an intrinsic RNA component (hTERC) containing a template region that binds to TTAGGG repeats in telomeres; 7 and a catalytic subunit with reverse transcriptase activity (hTERT). 8,9 Minimal essential components for in vitro reconstitution of telomerase activity is thought to be hTERC and hTERT. 10 Whereas hTERC is constitutively present in normal and cancer cells, expression of hTERT is almost exclusively limited to cancer cells. 8,9 Introduction of the hTERT gene into telomerase-negative normal cells is sufficient to induce telomerase activity and to immortalize cells that would otherwise enter telomere-based replicative senescence. 11-13 Telomerase activity and hTERT mRNA expression are closely associated in human cancers. 14,15 In vitro transformation of telomerase-negative normal cells using defined genetic elements almost usually requires hTERT expression. 16 These findings indicate that hTERT expression is the rate-limiting step in telomerase activity and cellular immortalization.
As a diagnostic marker for cancer, hTERT is potentially useful. In several studies, immunohistochemical detection of hTERT has been attempted in a variety of tumors. 17-23 In most of these studies, nuclei of cancer cells stained positive for hTERT, and staining closely correlated with telomerase activity. However, there has sometimes been unexpected hTERT-positive staining in normal cells and in cytoplasm of cancer cells, depending on the antibodies used. Such unexpected staining has often been dismissed as nonspecific, because most researchers believe that hTERT is exclusively localized to nuclei in cancer cells. Strict evaluation by immunohistochemistry and Western blot analysis, using well-characterized antibodies, is needed to precisely define the expression and localization of hTERT.
The purpose of the present study was to address the above issues using a newly developed antibody against hTERT. Interestingly, the present immunohistochemical results show that hTERT is expressed not only in nuclei but also cytoplasm in cancer cells. This finding was supported by Western blot analysis. Furthermore, some normal or telomerase-negative cancer cells were found to express weak TERT signals. Present telomeric repeat amplification protocol (TRAP) assays showed significant telomerase activity in both nuclei and cytoplasm of cancer cells. These findings appear to support the novel hypothesis that hTERT expression does not always reflect telomerase activity and that functional hTERT is expressed in both nuclei and cytoplasm.
Materials and Methods
Cell Lines
The following cell lines were obtained from the American Type Culture Collection (Rockville, MD): HeLa, C33A, ME180 (cervical cancer), MCF-7 (breast cancer), JEG-3 (choriocarcinoma), and SaOS2 (osteosarcoma). HEC1 and Ishikawa cells (endometrial cancer) were donated by Dr. Hiroyuki Kuramoto (Kitasato University, Tokyo, Japan) and Dr. Masato Nishida (Kasumigaura National Hospital, Ibaragi, Japan), respectively. A-431 cells (vulvar cancer) were provided by Dr. Wen-Chang Chang, National Cheng Kung University, Taiwan. SUSM-1 cells were obtained from Riken (Tsukuba, Japan). The above cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum in an atmosphere of 5% CO2 at 37°C. Human renal corticoepithelial cells (HRCEs) and normal foreskin fibroblasts (NHFs) were purchased from Clonetics (San Diego, CA) and were cultured according to the Clonetics protocol.
Immunohistochemistry
An affinity-purified monoclonal rat antibody against hTERT (KM2604) was raised against a GST-fused truncated recombinant protein (corresponding to amino acids 549 to 831 of hTERT) expressed by an Escherichia coli expression system (Kyowa Hakko Kogyo, Tokyo, Japan). Immunohistochemical assays for hTERT were performed on formalin-fixed, paraffin-embedded specimens of a variety of gynecological tissues, using the histofine SAB-PO kit (Nichirei Corporation, Tokyo, Japan). Antigen retrieval was performed for 10 minutes in 1× antigen retrieval solution (Biogenex, San Ramon, CA), and endogenous peroxidase was quenched in 3% H2O2. Then, the tissue sections were incubated for 16 hours at 4°C with anti-hTERT antibody at a dilution of 1:1300 or with nonimmune whole rat serum. After washing, the sections were incubated with a biotinylated secondary antibody, followed by detection by sequential reaction with a streptavidin-biotin-horseradish peroxidase complex, biotinylated tyramide, streptavidin peroxidase, and 3,3′-diaminobenzidine (chromogenic visualization). Sections were lightly counterstained with Ayer’s hematoxylin, and then mounted. Staining was evaluated as + (positive in >25% of the cells at the region of interest), − (<5%), or weak + (positive cells in 5 to 25% or staining intensity was extremely low). The results were judged by two independent pathologists and the cases with discordant results were excluded from the analyses.
Western Blot Analysis
Nuclear and cytoplasmic extracts from a variety of cell lines were prepared using the method of Schreiber and colleagues. 24 Briefly, 1 × 106 cells were collected; washed with phosphate-buffered saline; and resuspended in 400 μl of buffer A containing 10 mmol/L HEPES (pH 7.9), 10 mmol/L KCl, 0.1 mmol/L EDTA, 0.1 mmol/L EGTA, 1 mmol/L dithiothreitol, and 0.5 mmol/L phenylmethyl sulfonyl fluoride and allowed to swell for 15 minutes, after which 25 μl of a 10% solution of Nonidet P-40 (Fluka, Chemie AG, Buchs, Switzerland) is added and the tube is vigorously vortex for 10 seconds. The homogenate is centrifuged at 12,000 rpm for 30 seconds. The supernatant is recovered as cytoplasmic extracts. The nuclear pellet is resuspended in 50 μl of lysis buffer C containing 20 mmol/L HEPES (pH 7.9), 0.4 mol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L dithiothreitol, and 1 mmol/L phenylmethyl sulfonyl fluoride and the tube is vigorously rocked at 4°C for 15 minutes on a shaking platform and then centrifuged at 12,000 rpm for 5 minutes at 4°C and the supernatant is recovered as nuclear extracts. Then, 100 μg of nuclear or cytoplasmic extracts were electrophoresed on a sodium dodecyl sulfate-polyacrylamide gel with a gradient of 5 to 10% (Readygels J161-J351; Bio-Rad, Hercules, CA), and transferred to polyvinylidene difluoride membranes. Membranes were blocked in TBST (150 mmol/L NaCl, 20 mmol/L Tris-Cl, pH 7.5, 0.1% Tween) containing 5% nonfat dried milk, and then incubated with specific antibody against hTERT (KM2604; Kyowa Hakko Kogyo) or Sp1 (PEP2; Santa Cruz Biotechnology, Santa Cruz, CA), followed by reaction with horseradish peroxidase-linked anti-rat IgG for hTERT or anti-rabbit IgG for Sp1. Immunoreactive bands were visualized using the ECL detection system (Amersham, Biosciences, Piscataway, NJ), as suggested by the manufacturer.
TRAP Assay
TRAP assays were performed using the TRAPeze telomerase detection kit (Serologicals Corporation, Norcross, GA), according to the manufacturer’s protocols.
Results
Characterization of hTERT Antibody
We used a newly developed anti-hTERT antibody, KM2604, which was raised against a purified recombinant hTERT protein corresponding to amino acids 549 to 831 of hTERT. To verify the specificity of this antibody, Western blot analyses were performed using purified insect-expressed full-length hTERT. The recombinant hTERT was tagged with FLAG epitope at the amino terminal, and retained its catalytic activity. 10 Immunoblotting with KM2604 yielded significant bands with predicted molecular weight of 127 kd (Figure 1A) ▶ . This signal was absorbed by excess purified hTERT, and the position was confirmed with M2 FLAG-antibody (Figure 1B) ▶ . We thus confirmed that this antibody recognizes hTERT protein.
Figure 1.
Western blot analysis to verify specificity of hTERT antibody. A: FLAG-tagged purified recombinant hTERT was electrophoresed on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a membrane, and probed with hTERT antibody (KM2604). For absorption test, the antibody was preincubated with (+) or without (−) purified hTERT. A significant band is detected with predicted molecular weight of 127 kd. B: Western blotting with FLAG M2 antibody to verify the position of recombinant protein.
Immunohistochemistry Reveals both Nuclear and Cytoplasmic Expression of hTERT in Gynecological Cancers
To investigate the expression and localization of hTERT, a variety of gynecological malignancies were examined by immunohistochemistry: four cervical cancers, seven endometrial cancers, eight ovarian cancers, and one uterine leiomyosarcoma. All four cervical cancers, five of the seven endometrial cancers, six of the eight ovarian cancers, and the uterine sarcoma were significantly positive for hTERT staining; additionally, two endometrial cancers and one ovarian cancer exhibited faint signals. One ovarian cancer exhibited no hTERT staining. TRAP assay showed that all of the above tumors were telomerase-positive (data not shown). In some hTERT-positive tumors, hTERT signals were mainly observed in the nucleus, with granular or scattered patterns (Figure 2A) ▶ . However, other tumors also exhibited cytoplasmic hTERT staining (Figure 2B) ▶ . Cervical cancers exhibited particularly predominant cytoplasmic hTERT staining (Figure 3) ▶ . We also examined pelvic lymph nodes of patients with cervical cancer. Lymphocytes present in pelvic lymph nodes were generally hTERT-negative. However, significant hTERT staining was clearly observed in metastatic foci of cervical cancers (Figure 3) ▶ . To verify the specificity of the signals, an absorption test was performed, in which excess full-length purified FLAG-hTERT protein or antigen peptide was preincubated with primary antibody. Positive signals were completely eliminated by excess purified hTERT protein (Figure 4) ▶ or antigen peptide (data not shown), verifying the specificity of the signals.
Figure 2.

Immunohistochemistry of gynecological tumors with hTERT antibody. A: Predominantly nuclear hTERT signals in endometrial cancer (case 30). B: Both nuclear and cytoplasmic signals in ovarian cancer (case 47). Original magnifications: ×200 (A); ×400 (B).
Figure 3.
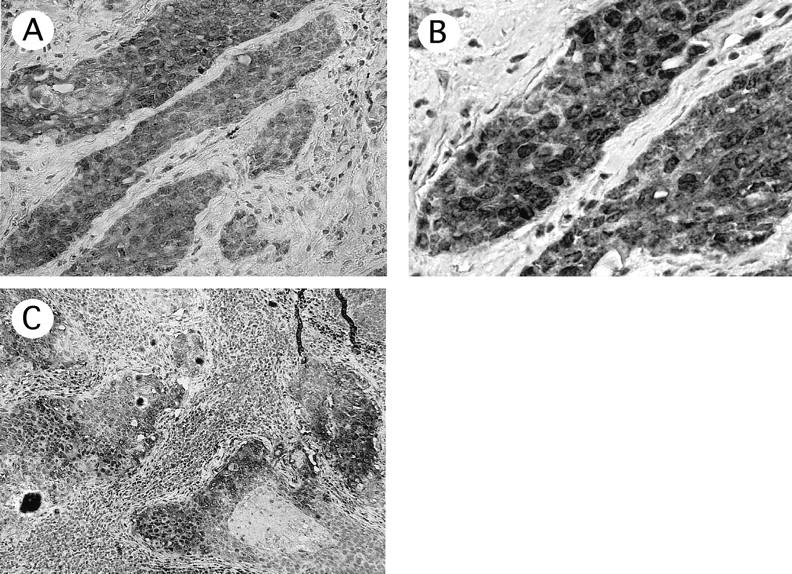
Immunohistochemistry of cervical cancers with hTERT antibody. A and B: Predominantly cytoplasmic signals in cervical cancer (case 13). C: hTERT signals in metastatic foci of pelvic lymph node in this patient. Original magnifications: ×200 (A); ×400 (B); ×100 (C).
Figure 4.
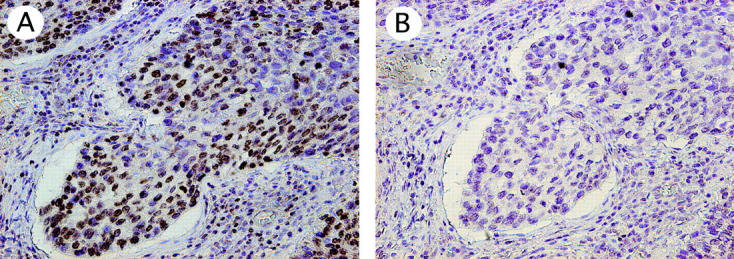
Absorption test for hTERT signals. Nuclear signals detected by hTERT antibody (KM2604) (case 12) (A) were completely eliminated by preincubation with full-length purified recombinant hTERT (B). Original magnifications, ×200 (A and B).
We next examined expression of hTERT in normal, benign, and premalignant lesions. No significant signals were observed in normal cervical mucosa. Faint or weak signals were observed in severe dysplasia of uterine cervix and carcinoma in situ; whole layers of stratified atypical cells were positive for hTERT expression (Figure 5) ▶ . These premalignant lesions exhibited both nuclear and cytoplasmic staining. Neither of the two uterine myomas and none of the six benign ovarian tumors was positive for hTERT staining. Thirteen normal endometria were also examined. None of the eight atrophic endometrial glands in postmenopausal women were positive for hTERT expression. One of the three endometrial glands in proliferative phase was strongly positive for hTERT staining in both nuclei and cytoplasm, whereas neither of the two glands in secretory phase were significantly positive (Figure 5) ▶ . These results are summarized in Table 1 ▶ . Thus, both nuclear and cytoplasmic hTERT staining was observed in most cancers as well as some telomerase-positive normal tissues, such as proliferative endometrium.
Figure 5.

Immunohistochemistry of premalignant and normal tissues. Weak but consistent signals were observed in carcinoma in situ of the uterine cervix (case 10) with hTERT antibody (A) but not with control serum (B). hTERT staining was positive in normal proliferative-phase endometrium (case 16) (C), but not in atrophic endometrium of postmenopausal woman (case 21) (D). Original magnifications, ×200 (A–D).
Table 1.
Results of the Immunohistochemistry
| Case | Organ | Lesion | Signal | Pattern | Comment |
|---|---|---|---|---|---|
| 1 | Cervix | Normal | − | ||
| 2 | Cervix | Normal | − | ||
| 3 | Cervix | Normal | − | ||
| 4 | Cervix | Normal | − | ||
| 5 | Cervix | Mild dysplasia | − | ||
| 6 | Cervix | Mild dysplasia | − | ||
| 7 | Cervix | Moderate dysplasia | − | ||
| 8 | Cervix | Moderate dysplasia | − | ||
| 9 | Cervix | Severe dysplasia | w+ | ||
| 10 | Cervix | CIS | w+ | ||
| 11 | Cervix | CIS | w+ | ||
| 12 | Cervix | Ca (SCC) | + | N<C | |
| 13 | Cervix | Ca (SCC) | + | N<C | LN + (#1) |
| 14 | Cervix | Ca (clear cell) | + | N<C | |
| 15 | Cervix | Ca (mucinous) | + | N<C | LN + (#1) |
| 16 | Endometrium | Normal proliferative | + | N<C | |
| 17 | Endometrium | Normal proliferative | − | ||
| 18 | Endometrium | Normal proliferative | − | ||
| 19 | Endometrium | Normal secretory | − | ||
| 20 | Endometrium | Normal secretory | − | ||
| 21 | Endometrium | Atrophic | − | ||
| 22 | Endometrium | Atrophic | − | ||
| 23 | Endometrium | Atrophic | − | ||
| 24 | Endometrium | Atrophic | − | ||
| 25 | Endometrium | Atrophic | − | ||
| 26 | Endometrium | Atrophic | − | ||
| 27 | Endometrium | Atrophic | − | ||
| 28 | Endometrium | Atrophic | − | ||
| 29 | Endometrium | Ca (serous) | + | N>C | |
| 30 | Endometrium | Ca (EMoid G1) | + | N>C | |
| 31 | Endometrium | Ca (EMoid G1) | w+ | ||
| 32 | Endometrium | Ca (EMoid G2) | + | N<C | |
| 33 | Endometrium | Ca (EMoid G1) | w+ | ||
| 34 | Endometrium | Ca (EMoid G1) | + | N<C | |
| 35 | Endometrium | Ca (carcinosarcoma) | + | N<C(Ca) N>C(Sar) | |
| 36 | Myometrium | Leiomyosarcoma | + | N>C | |
| 37 | Myometrium | Cellular leiomyoma | − | ||
| 38 | Myometrium | Leiomayoma | − | ||
| 39 | Ovary | Surface epithelium | − | ||
| 40 | Ovary | Surface epithelium | − | ||
| 41 | Ovary | Surface epithelium | − | ||
| 42 | Ovary | Dermoid cyct | − | ||
| 43 | Ovary | Endometriosis | − | ||
| 44 | Ovary | Mucinous cyct | − | ||
| 45 | Ovary | Ca (serous) | + | N<C | |
| 46 | Ovary | Ca (SSPC) | w+ | ||
| 47 | Ovary | Ca (Endometrioid) | + | N=C | |
| 48 | Ovary | Ca (Endometrioid) | − | ||
| 49 | Ovary | Ca (mucinous) | + | N>C | |
| 50 | Ovary | Ca (mucinous) | w+ | N<C | |
| 51 | Ovary | Ca (clear cell) | + | N<C | |
| 52 | Ovary | Ca (undifferentiated) | + | N=C |
W, Weak staining; Ca, cancer; Sar, sarcoma; N, nucleus; C, cytoplasm; Emoid, endometrioid; LPM, low potential malignancy; SSPC, serous surface papillary carcinoma.
#1, Metastatic lesions in the lymph nodes are positive.
Western Blot Analysis Identifies Subcellular Localization of hTERT Expression
Based on the findings of immunohistochemistry, we further examined the subcellular localization of hTERT by Western blot analysis using various telomerase-positive and telomerase-negative cell lines and primary normal cells. Nuclear and cytoplasmic extracts were separately collected and subjected to Western blot analysis. hTERT was detected at the predicted size (127 kd) in both nuclear and cytoplasmic extracts from cancer cells (Figure 3) ▶ . The band intensity varied among cell lines: cervical cancer C33A and HeLa, Ishikawa (endometrial cancer), and A-431 (vulvar cancer) cells exhibited strong bands; ME180 (cervical cancer), HEC1 (endometrial cancer), and MCF-7 cells (breast cancer) showed rather weak bands; JEG3 cells did not show definite bands. In contrast, no significant signals were detected in nuclear and cytoplasmic extracts from normal renal cells (HRCEs). NHFs as well as SaOS2 and SUSUM-1 cells, cell lines that use the ALT (alternative lengthening of telomere) mechanism of telomere elongation without detectable levels of telomerase activity, exhibited faint or weak signals in nuclear extracts, and exhibited no signal in cytoplasmic extracts. We considered the possibility that some of our cytoplasmic extracts were contaminated by nuclear fractions, and that this contamination was responsible for the cytoplasmic signals that we detected. To confirm that nuclear and cytoplasmic extracts were properly separated from each other, Western blot analysis was performed using the nuclear protein Sp1. Significant Sp1 expression was detected in nuclear extracts, whereas no or only weak bands were detected in cytoplasmic extracts, indicating that our cytoplasmic extracts were not significantly contaminated by nuclear fractions (Figure 6) ▶ . These findings suggest that both nuclear and cytoplasmic extracts of cancer cells contain hTERT protein, supporting the results of immunohistochemistry.
Figure 6.
Western blot analysis of hTERT expression in cell lines. Nuclear or cytoplasmic extracts (100 μg) from each cell line were electrophoresed on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to membranes, and probed with specific antibodies against hTERT (KM2604) or Sp1. Immunoreactive bands were visualized by the ECL detection system. Specific signals of the expected size (127 kd) were observed in both nucleic and cytoplasmic extracts from cancer cell lines. In contrast, this signal was faint or absent in telomerase-negative SaOs2, SUSUMU-1, HRCEs, and NHFs; additional bands of lower molecular weight (∼110 kd) were observed in these cells. N, nuclear extract; C, cytoplasmic extract.
An additional band with a molecular mass of ∼100 kd was observed in ALT, SaOs2, SUSUMU-1, HRCE, and NHF cells, all of which are TRAP-negative. This band was detected in both nuclear and cytoplasmic extracts of these cells, except SaOs2, which only exhibited the band in nuclear extracts. This band was also observed in some cancer cells, including ME180.
Telomerase Activity in Nuclear and Cytoplasmic Fractions
To examine the subcellular localization of telomerase activity, we subjected nuclear and cytoplasmic extracts to TRAP assays. Strong telomerase activity was observed with nuclear extracts from C33A, ME180, and HeLa cell lines (Figure 7) ▶ . However, telomerase activity was also observed in cytoplasmic extracts from these cells. Interestingly, telomerase activity appeared to be stronger in cytoplasmic extracts than in nuclear extracts. In contrast, significant telomerase activity was not detected in nuclear or cytoplasmic extracts from normal cells (HRCEs and NHFs) or the ALT cells (SUSUMU-1 and SaOS2).
Figure 7.
A: Telomerase activity in nuclear and cytoplasmic extracts from each cell line. B: Assay to examine whether composition of lysis buffers affect levels of the TRAP activity. Buffer A and C mean lysis buffers with which cytoplasmic and nuclear extracts were extracted, respectively. Nuclear extracts of C33A were diluted with an equal amounts of cytoplasmic lysis buffer (buffer A), and cytoplasmic extracts of C33A were diluted with an equal amounts of nuclear lysis buffer (buffer C). This manipulation equalizes the composition of each extraction buffer. IC, internal control to verify amplification efficiency of PCR.
Unexpectedly, levels of telomerase activity in cytoplasmic extracts were higher than those in nuclear extracts. Care should be taken when the levels of telomerase activity are compared in extracts containing different detergents, because the intensity of TRAP activity may be dependent of the nature of the detergents. To examine this possibility, composition of each buffer was equalized by diluting nuclear extracts of C33A with an equal amounts of cytoplasmic lysis buffer (buffer A) or diluting cytoplasmic extracts of C33A with an equal amounts of nuclear lysis buffer (buffer C). Then, diluted extracts were examined by the TRAP assay. As shown in Figure 7B ▶ , levels of the TRAP activity were not altered by this dilution and lysis buffer A or C alone did not produce any telomerase activity, suggesting that detergents or other reagents contained in each extraction buffer did not significantly affect the levels of the TRAP activity in our system.
Discussion
The key to success in immunological detection is undoubtedly selection of the antibody. Specificity and suitability of the antibody must be carefully examined using purified recombinant proteins. We first characterized the monoclonal antibody KM2604 using purified recombinant hTERT and confirmed that this antibody recognized full-length hTERT. Immunohistochemistry using this antibody showed significant positive staining in most of the gynecological cancers we examined. hTERT staining was also observed in a subset of precursor lesions (severe dysplasia or carcinoma in situ) of uterine cervix. In several studies, weak but consistent telomerase activity has been detected in these lesions, and this is consistent with the present results. 25-27 Strong hTERT staining was also observed in normal proliferative endometrium in the present study, and this is consistent with the significant telomerase activity we detected in this tissue in previous studies. 28 Also in the present study, we found significant expression of hTERT in metastatic foci of pelvic lymph nodes in patients with cervical cancer. Previous findings suggest the presence of telomerase activity in progenitors of hematopoietic cells. 29,30 However, in the present immunohistochemical examinations, peripheral lymphocytes in lymph nodes were not significantly positive for hTERT, whereas metastatic foci of cancer cells exhibited significant staining. These findings suggest that hTERT has diagnostic value as a potential marker for lymph node metastasis.
Western blot analysis revealed several important findings. Unexpectedly, there was no strong correlation between hTERT expression level and telomerase activity level, as determined by conventional TRAP assay. C33A, HeLa, Ishikawa, and A431 cells exhibited high levels of hTERT expression, whereas ME180, HEC1, and MCF-7 cells showed rather weak expression, and JEG3 cells showed no clear expression, despite the fact that all of these cells exhibited considerable TRAP activity. These discrepancies are consistent with the lack of hTERT staining in some cancers that exhibit telomerase activity, as shown in the present immunohistochemical assays. In contrast, normal human fibroblasts and ALT, SaOS2, and SUSUM-1 cells exhibited weak hTERT bands in their nuclear extracts, despite the lack of detectable TRAP activity. Thus, hTERT expression levels did not always correlate with telomerase activity. Weak hTERT expression in ME180, MCF-7, and JEG3 cells, despite significant telomerase activity, suggests that telomerase activity levels are determined by complex mechanisms, not simply by hTERT expression levels. Qualitative modifications of hTERT, such as protein phosphorylation, undoubtedly have effects on telomerase activity levels. Detection of hTERT expression without significant telomerase activity in ALT, SaOs2, and SUSUMU-1 cells or normal human fibroblasts may be because of hTERT expression in these cells not reaching threshold levels required for enzymatic activity of telomerase. More sensitive assays may enable detection of telomerase activity in these cells. Alternatively, additional factors important for full enzymatic activity may be deficient in these cells, or inhibitory factors that block TERT functions may be present in these cells.
An additional band with a molecular mass of ∼100 kd was observed in Western blots of telomerase-negative ALT, SaOs2, SUSUMU-1, HRCE, and NHF cells. Detection of this band correlates with negative TRAP assay results. This band may represent splice variants of hTERT, although there is no evidence to support this hypothesis. 31,32 Further biochemical analysis is needed to characterize this band.
The most interesting finding of the present study is the subcellular localization of hTERT. The immunohistochemistry clearly demonstrated that hTERT is expressed not only in nuclei but also in cytoplasm of cancer cells. Western blot analyses supported this finding, showing significant bands in both nuclear and cytoplasmic extracts. These findings contradict the idea that hTERT is localized to nuclei. 17-23 This raises the question of the biological function of cytoplasmic hTERT. Perhaps it is only present in cytoplasm before its translocation to the nucleus. Alternatively, it may have some unknown function in cytoplasm. In a recent study, induction of telomerase expression in resting CD4+ T cells by anti-CD3 stimulation was followed by phosphorylation of hTERT, which correlated with translocation from the cytoplasm to the nucleus. 33 This suggests that cytoplasmic hTERT is unphosphorylated and inactive. However, the present findings showed significant TRAP activity in cytoplasm. The TRAP assay reflects the in vitro activity and does not necessarily prove telomerase activity in vivo. Therefore, further assessment of in vivo activity of cytoplasmic TERT will be needed to clarify its biological significance. We are currently examining hTERT phosphorylation in cytoplasm and nuclei.
The present immunological assays for hTERT produced the following novel findings: 1) some cancer cells express hTERT in both the nucleus and cytoplasm; 2) weak hTERT expression detected by Western blotting does not always correlate with detectable telomerase activity and that hTERT expression is not always detectable in telomerase-positive samples; and 3) some ALT cell lines and normal cells exhibit weak levels of TERT expression, despite the lack of detectable telomerase activity. Although, in previous immunohistochemical analyses, cytoplasmic hTERT staining either has not been detected or has been dismissed as nonspecific, the present findings suggest that such signals should be carefully evaluated. It would be interesting to know whether hTERT-staining patterns are associated with clinicopathological characteristics or prognosis of tumors. A greater understanding of such issues may lead to the use of immunological detection of hTERT as a diagnostic and prognostic marker of cancers.
Footnotes
Address reprint requests to Satoru Kyo, Department of Obstetrics and Gynecology, Kanazawa University, 13-1 Takaramachi, Kanazawa, Ishikawa 920-8641, Japan. E-mail: satoruky@med.kanazawa-u.ac.jp.
Supported in part by a Grant-in Aid for the Second Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health and Welfare, Japan.
References
- 1.Harley CB, Futcher AB, Greider CW: Telomeres shorten during ageing of human fibroblasts. Nature 1990, 345:458-460 [DOI] [PubMed] [Google Scholar]
- 2.Greider CW: Chromosome first aid. Cell 1991, 67:645-647 [DOI] [PubMed] [Google Scholar]
- 3.Watson JD: Origin of concatemeric T7 DNA. Nature (Lond) 1972, 239:197-201 [DOI] [PubMed] [Google Scholar]
- 4.Harley CB: Telomeres and telomerase in aging and cancer. Curr Opin Genet Dev 1995, 5:249-255 [DOI] [PubMed] [Google Scholar]
- 5.Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S: Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J 1992, 11:1921-1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW: Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266:2011-2015 [DOI] [PubMed] [Google Scholar]
- 7.Feng J, Funk WD, Wang SS, Weinrich AAA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J, Le S, West MD, Harley CB, Andrew WH, Greider CW, Villeponteau B: The RNA component of human telomerase. Science (Washington DC) 1995, 269:1236-1241 [DOI] [PubMed] [Google Scholar]
- 8.Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q, Bacchetti S, Haber DA, Weinberg RA: hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell 1997, 90:785-795 [DOI] [PubMed] [Google Scholar]
- 9.Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR: Telomerase catalytic subunit homologs from fission yeast and human. Science 1997, 277:955-959 [DOI] [PubMed] [Google Scholar]
- 10.Masutomi K, Kaneko S, Hayashi N, Yamashita T, Shirota Y, Kobayashi K, Murakami S: Telomerase activity reconstituted in vitro with purified human telomerase reverse transcriptase and human telomerase RNA component. J Biol Chem 2000, 275:22568-22573 [DOI] [PubMed] [Google Scholar]
- 11.Weinrich SL, Pruzan R, Ma L, Ouellette M, Tesmer VM, Holt SE, Bodnar AG, Lichtsteiner S, Kim NW, Trager JB, Taylor RD, Carlos R, Andrews WH, Wright WE, Shay JW, Harley CB, Morin GB: Reconstitution of human telomerase with template RNA component hTERC and the catalytic protein subunit hTERT. Nat Genet 1997, 17:498-502 [DOI] [PubMed] [Google Scholar]
- 12.Nakayama J, Tahara H, Tahara E, Saito M, Ito K, Nakamura H, Nakanishi T, Tahara E, Ide T, Ishikawa F: Telomerase activation by hTRT in human normal fibroblasts and hepatocellular carcinomas. Nat Genet 1998, 18:65-68 [DOI] [PubMed] [Google Scholar]
- 13.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE: Extension of life-span by introduction of telomerase into normal human cells. Science 1998, 279:349-352 [DOI] [PubMed] [Google Scholar]
- 14.Takakura M, Kyo S, Kanaya T, Tanaka M, Inoue M: Expression of human telomerase subunits and correlation with telomerase activity in cervical cancer. Cancer Res 1998, 58:1558-1561 [PubMed] [Google Scholar]
- 15.Kyo S, Kanaya T, Takakura M, Tanaka M, Yamashita A, Inoue H, Inoue M: Expression of human telomerase subunits in ovarian malignant, borderline and benign tumors. Int J Cancer 1999, 80:804-809 [DOI] [PubMed] [Google Scholar]
- 16.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA: Creation of human tumour cells with defined genetic elements. Nature 1999, 400:464-468 [DOI] [PubMed] [Google Scholar]
- 17.Hiyama E, Hiyama K, Yokoyama T, Shay JW: Immunohistochemical detection of telomerase (hTERT) protein in human cancer tissues and a subset of cells in normal tissues. Neoplasia 2000, 3:17-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumaki F, Kawai T, Hiroi S, Shinomiya N, Ozeki Y, Ferrans VJ, Torikata C: Telomerase activity and expression of human telomerase RNA component and human telomerase reverse transcriptase in lung carcinomas. Hum Pathol 2001, 32:188-195 [DOI] [PubMed] [Google Scholar]
- 19.Frost M, Bobak JB, Gianani R, Kim N, Weinrich S, Spalding DC, Cass LG, Thompson LC, Enomoto T, Uribe-Lopez D, Shroyer KR: Localization of telomerase hTERT protein and hTR in benign mucosa, dysplasia, and squamous cell carcinoma of the cervix. Am J Clin Pathol 2000, 114:726-734 [DOI] [PubMed] [Google Scholar]
- 20.Wada H, Enomoto T, Yoshino K, Ozaki K, Kurachi H, Nomura T, Murata Y, Kim N, Weinrich S, Lea-Chou E, Lopez-Uribe D, Shroyer KR: Immunohistochemical localization of telomerase hTERT protein and analysis of clonality in multifocal vulvar intraepithelial neoplasia. Am J Clin Pathol 2000, 114:371-379 [DOI] [PubMed] [Google Scholar]
- 21.Kawakami Y, Kitamoto M, Nakanishi T, Yasui W, Tahara E, Nakayama J, Ishikawa F, Tahara H, Ide T, Kajiyama G: Immuno-histochemical detection of human telomerase reverse transcriptase in human liver tissues. Oncogene 2000, 19:3888-3893 [DOI] [PubMed] [Google Scholar]
- 22.Tahara H, Yasui W, Tahara E, Fujimoto J, Ito K, Tamai K, Nakayama J, Ishikawa F, Tahara E, Ide T: Immuno-histochemical detection of human telomerase catalytic component, hTERT, in human colorectal tumor and non-tumor tissue sections. Oncogene 1999, 18:1561-1567 [DOI] [PubMed] [Google Scholar]
- 23.Iczkowski KA, Pantazis CG, McGregor DH, Wu Y, Tawfik OW: Telomerase reverse transcriptase subunit immunoreactivity. A marker for high-grade prostate carcinoma. Cancer 2002, 95:2487-2493 [DOI] [PubMed] [Google Scholar]
- 24.Schreiber E, Matthias P, Muller MM, Schaffner W: Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res 1989, 17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyo S, Kanaya T, Ishikawa H, Ueno H, Inoue M: Telomerase activity in gynecologic tumor. Clin Cancer Res 1996, 2:2023-2028 [PubMed] [Google Scholar]
- 26.Shroyer KR, Thompson LC, Enomoto T, Eskens JL, Shroyer AL, McGregor JA: Telomerase expression in normal epithelium, reactive atypia, squamous dysplasia, and squamous cell carcinoma of the uterine cervix. Am J Clin Pathol 1998, 109:153-162 [DOI] [PubMed] [Google Scholar]
- 27.Kyo S, Takakura M, Tanaka M, Kanaya T, Inoue M: Telomerase activity in cervical cancer is quantitatively distinct from that in its precursor lesions. Int J Cancer 1998, 79:66-70 [DOI] [PubMed] [Google Scholar]
- 28.Kyo S, Takakura M, Kohama T, Inoue M: Telomerase activity in human endometrium. Cancer Res 1997, 57:610-614 [PubMed] [Google Scholar]
- 29.Hiyama K, Hirai Y, Kyoizumi S, Akiyama M, Hiyama E, Piatyszek MA, Shay JW, Ishioka S, Yamakido M: Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J Immunol 1995, 155:3711-3715 [PubMed] [Google Scholar]
- 30.Counter CM, Gupta J, Harley CB, Leber B, Bacchetti S: Telomerase activity in normal leukocytes and in hematologic malignancies. Blood 1995, 85:2315-2320 [PubMed] [Google Scholar]
- 31.Ulaner GA, Hu JF, Vu TH, Giudice LC, Hoffman AR: Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res 1998, 58:4168-4172 [PubMed] [Google Scholar]
- 32.Ulaner GA, Hu JF, Vu TH, Oruganti H, Giudice LC, Hoffman AR: Regulation of telomerase by alternate splicing of human telomerase reverse transcriptase (hTERT) in normal and neoplastic ovary, endometrium and myometrium. Int J Cancer 2000, 85:330-335 [PubMed] [Google Scholar]
- 33.Liu K, Hodes RJ, Weng NP: Cutting edge: telomerase activation in human T lymphocytes does not require increase in telomerase reverse transcriptase (hTERT) protein but is associated with hTERT phosphorylation and nuclear translocation. J Immunol 2001, 166:4826-4830 [DOI] [PubMed] [Google Scholar]





