Abstract
Epstein-Barr virus (EBV) is a ubiquitous viral agent, well known to be associated with lymphoid, epithelial, and smooth-muscle malignancies in immunocompromised individuals. This report describes a 10-year-old patient with an EBV-related liver tumor occurring after kidney transplantation. The neoplasm presented a phenotypic spectrum, ranging from a smooth-muscle tumor to an inflammatory pseudotumor (IPT). The neoplastic cells failed to disclose CD21, CD35, or ALK expression, the latter confirmed by reverse-transcription polymerase chain reaction. Cytogenetic analysis revealed a single clonal cell population showing 46,XY,del (2)(p23),der(3)t (2;3)(p23;q29),der(21) t(Y;21)(q12;p13) karyotype. By metaphase FISH analysis, the neoplastic cells demonstrated the presence of two molecularly different but related aberrant clones, one with the loss of one ALK allele and the second with translocation of the 3′end of ALK kinase domain on the der(3) chromosome. Using FISH with an EBV-specific and 3′end ALK DNA probes, a co-localization of the viral DNA and the ALK sequences was found on the der(3) chromosome. Metaphases with loss of rearranged ALK did not show integrated virus; instead, viral particles together with an associated 3′end ALK domain formed an ex-chromosomal, episomal-like type configuration. The interphase study, using dual-color 5′/3′ end ALK FISH assay, revealed 30% of nuclei with only one fused signal, confirming the total loss of one ALK allele in the subset of tumor cells. A combined immunofluorescence and FISH study indicated this separate clonal variant to correspond to desmin-positive smooth-muscle cells. In contrast, desmin-negative myofibroblasts showed the presence of both normal and rearranged ALK alleles. Our results indicate that ALK locus may be a target of EBV integration, a hitherto unreported finding. Although the sustained clonal expansion in EBV-related smooth-muscle tumors/IPTs may depend on functions provided by the EBV oncogenic proteins, the tumor phenotype may be further modified by the secondary genomic rearrangements imposed by the virus during and/or after the integration event. In this respect, the observed phenotypic heterogeneity most likely reflects divergence during neoplastic progression, with the subsequent expansion of morphologically and molecularly distinct but cytogenetically related clones.
Smooth-muscle tumors are increasingly recognized in children with various immunodeficiencies occurring after organ transplantation. 1-5 Since clonal Epstein-Barr virus (EBV) is consistently demonstrated in these tumors but not in sporadic smooth-muscle tumors, the virus probably plays a pivotal role in tumorigenesis. EBV, a common and the causative agent of infectious mononucleosis, is known to be associated also with other malignancies including lymphomas, nasopharyngeal carcinomas, human immunodeficiency virus-associated and posttransplant lymphoproliferative disorders, and inflammatory pseudotumors (IPTs)/IPT-like follicular dendritic cell tumors. 6-9 EBV is a double-stranded DNA-enveloped virus with a variety of gene products. 10 EBV-associated smooth-muscle tumors exhibit EBV type III latency, characterized by expression of immortalizing Epstein-Barr nuclear antigen (EBNA)-2 in addition to the oncogenic, latent membrane protein (LMP)-1. 11 The latent proteins are the main targets of cytotoxic T cells, and their expression is better tolerated in cases of profound immunodeficiency.
Anaplastic lymphoma kinase (ALK) is a member of the insulin receptor family of receptor tyrosine kinases and its expression is normally restricted to the nervous system. 12 ALK gene fusion was first identified in anaplastic large-cell lymphoma (ALCL), in which most commonly a t(2;5) chromosomal translocation generates an NPM-ALK gene fusion and constitutively activated chimeric protein. 13 Activation of ALK by variant ALK fusions have also been described in a subset of inflammatory myofibroblastic tumors (IMTs) occurring in children and young adults. 14-17 These fusions are associated with striking ALK expression in the myofibroblastic spindle cells in a significant (up to 40%) subset of tumors. 18 Genetic changes that underlie ALK-negative IPTs remain unclear. It is feasible that ALK-negative IPTs might represent a spectrum of entities with overlapping histological features but different underlying transforming mechanisms. 15,18 Some investigators have proposed a role for viruses in the pathogenesis of IPTs, including EBV and human herpesvirus-8. 6,19-21
This report describes a 10-year-old patient who developed a post-transplant EBV-related liver tumor with histologically combined smooth-muscle and IPT characteristics. Tumor cells revealed a spectrum of serial genomic rearrangements, involving the ALK gene and integrated EBV sequences, with the subsequent expansion of morphologically and molecularly distinct but cytogenetically related clones. Our findings indicate that the ALK loci may be a site of EBV virus integration. Virus integration could facilitate secondary genome rearrangements, leading consequently to the development of tumors with morphological features resembling IPTs and to related smooth-muscle tumors.
Case Report
A 10-year-old boy presented in March 2002 with a mass in the right liver lobe. He had a history of a single renal transplantation in February 2000 due to chronic renal insufficiency and bilateral nephrectomy, subsequent to two successive hemolytic uremic syndromes in 1996 and 1997. An immunosuppressive agent, FK 506, was administrated in subtoxic serum levels. Annual follow-up echography and nuclear magnetic resonance scan of the abdomen revealed a heterogeneous and T1-hypodense well-circumscribed nodular solid lesion, 5.3 cm in diameter, in the right lobe of the liver. The transplant kidney and spleen were normal. Laboratory tests revealed serum conversion for EBV, Hepatitis A virus and Polyomavirus but not for Cytomegalovirus, Hepatitis B and C viruses, or Toxoplasma. The mass was removed by a right lobotomy of the liver. In May 2002, the boy was admitted to the hospital following a supracondylar fracture of the humerus. Physical examination and imaging techniques could not reveal new or residual tumor activity.
Materials and Methods
Histopathology and Immunohistochemistry
After sampling for frozen material, tumor samples were fixed in 6% buffered formalin, embedded in paraffin, and stained with hematoxylin and eosin (H&E) for routine histological examination. Indirect immunohistochemistry was performed, either on paraffin or frozen sections, using monoclonal desmin (dilution 1:20; ICN-Cappel, Gent, Belgium), α-smooth-muscle actin (α-SMA, dilution 1:40; DAKO, Glostrup, Denmark), cytokeratin (dilution 1:50; Biodesign, Saco, ME), CD21 (dilution 1:10; DAKO, Merelbek, Belgium), CD35 (dilution 1:10; DAKO), myogenin (dilution 1:30, Novocastra, Gent, Belgium) and ALK-1 (dilution 1:10, DAKO) antibodies.
Electron Microscopy
Small fragments of the biopsy were immediately fixed in 2.5% glutaraldehyde, 0.1 mol/L phosphate buffer. After postfixation in 1% osmiumtetroxide 0.1 mol/L phosphate buffer, the samples were dehydrated in graded series of alcohol and embedded in epoxy resin. Ultrathin sections were cut, stained with uranyl acetate and lead citrate, and examined using a Zeiss EM10 electron microscope.
Cytogenetic and FISH Analysis
Chromosome metaphases were obtained after short-term culture of a primary tumor sample, using standard procedures. G-banded chromosomes were evaluated and classified according to the International System of Human Cytogenetic Nomenclature. 22
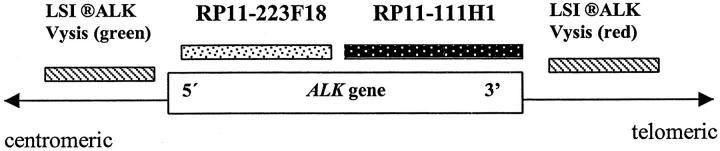
FISH was performed on either metaphase chromosomes or interphase nuclei using probes homologous to 3′ or 5′ regions of ALK gene, as shown in Figure 1 ▶ . The ALK locus was evaluated with the LSI ALK Dual-Color Rearrangement Probe (Vysis, Inc., Downer’s Grove, IL), composed of a mixture of two differentially labeled DNAs that map either 3′ telomeric (SpectrumOrange) or 5′centromeric (SpectrumGreen) to ALK. For subsequent study, the digoxigenin-labeled ALK P1 (PAC RP11–1111H1) (specific for 3′end of ALK catalytic domain) or PAC RP11–223F18 (specific for 5′ end of ALK) clones were used. 23 Additional FISH experiments used the EcoRI-B fragment of EBV DNA probe (kindly provided by Dr. John Arrand from the Paterson Institute for Cancer Research, Manchester, UK) and a probe specific for the subtelomeric sequences of chromosome 3q. FISH was performed according to the protocol previously described. 24 The FISH data were obtained on a Leica DMRB (Leica, Wetzlar, Germany) fluorescence microscope equipped with a cooled black and white charged couple device camera (Photometrics, Tuscon, AZ), run by Quips SmartCapture FISH Imaging Software (Vysis, Bergisch-Gladbach, Germany).
Figure 1.
Map of ALK gene on 2p23 with localization of the FISH probes used in the study.
For simultaneous ascertainment of cytogenetic and immunophenotypic features in individual cells, a sequential imunocytological/FISH analysis was performed on cytological touch preparations of the frozen tumor specimen, using primary monoclonal anti-desmin antibody (dilution 1:20; ICN-Cappel), according to procedure previously described. 25 Thirty randomly chosen immunonegative or positive cells were photographed using an Axioplan 2-fluorescence microscope. Subsequently, FISH was performed on the same slide with the LSI ALK probe described above, and the same cells were re-photographed.
ALK RT-PCR Analysis
Total RNA was extracted from the frozen tissue of the present case and from an IMT with ALK-ATIC fusion (positive control) using Trizol (Invitrogen, Carlsbad, CA), according to manufacturer’s protocol. The RNA was reverse-transcribed using MMLV reverse transcriptase. A positive control reverse transcription-polymerase chain reaction (RT-PCR) for the quality of the cDNA synthesis, using the primers 5′-CCCATTTCGGCTCCCATGATT and 5′-GGTCTCGGTGTGTGACTTGGA, was performed to amplify the CIZ gene. To analyze ALK transcripts, two primer sets were developed. The first, ALK3f (GCAACATCAGCCTGAAGACA) and ALK3r (GCCTGTTGAGAGACCAGGAG), amplifies a 192-bp fragment derived from the 3′ end of the ALK transcript. The second set, ALK5f (CTCAGCGAGCTGTTCAGTTG) and ALK5r (GGAGAAGGCATGTTTGTTGG), amplifies a 200-bp fragment derived from the 5′ end of the ALK transcript.
Results
Histopathologic Findings
Generally, the tumor was relatively well delineated from the surrounding parenchyma, although nodular extensions were occasionally seen (Figure 2b) ▶ . A striking phenotypic variability was present in the lesion (Figure 2a) ▶ . Part of the tumor consisted of a typical smooth-muscle tumor, showing fascicles of eosinophilic cells with cigar-like shaped nuclei and strong desmin and α-SMA expression (Figure 2, c and e) ▶ . Some mononuclear inflammatory cells were present between the smooth-muscle cells. Other parts of the tumor revealed an inflammatory, loosely textured background, in which α-SMA-positive and desmin-negative myofibroblasts were present (Figure 2, d and f) ▶ . The inflammatory component consisted mainly of lymphocytes and plasma cells (Figure 2d) ▶ . This part of the tumor showed characteristics of an IPT. Both phenotypes were easily discriminated in most parts of the tumor, albeit that both components were intermingled in some areas (Figure 2) ▶ . Quantitatively, the IPT component outnumbered the smooth-muscle component and the latter was mainly present at the edge of the lesion. Mitotic figures were occasionally present, mainly in the smooth-muscle component. Necrosis was focally seen. Immunostaining on frozen tumor sections using the ALK-1antibody showed no reactivity in the IPT component or in the smooth-muscle compartment. Frozen sections as well as paraffin sections were repeatedly negative on CD21 and CD35 staining. No expression of cytokeratin ormyogenin was found.
Figure 2.
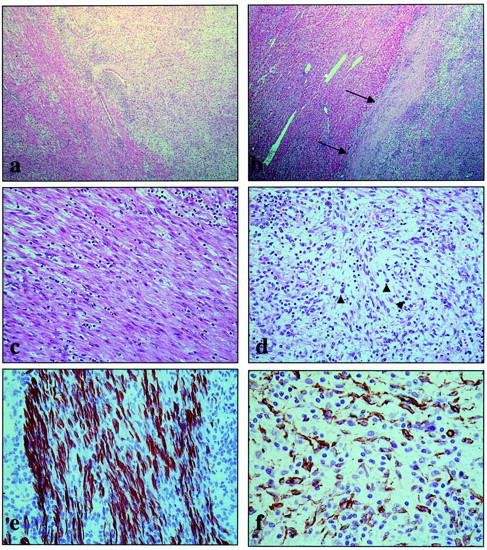
Overview of the histopathological and immunohistochemical findings. In many areas, the transition between the smooth-muscle component (left) and the IPT component (right) is easily seen (H&E stain, ×25) (a). The tumor is relatively well delineated from the surrounding parenchyma (arrows) (H&E stain, ×25) (b). Higher magnification of the smooth-muscle component shows bundles of eosinophilic spindle cells with cigar-shaped nuclei (H&E stain, ×160) (c). Higher power of the IPT component discloses vessels, lymphocytes, plasma cells (arrowheads), and spindle cells in a myxoid background (H&E stain, ×160) (d). The smooth-muscle component is strongly desmin-positive (indirect immunoperoxidase stain, ×400) (e). The spindle cells of the IPT component only express α-SMA (indirect immunoperoxidase stain, ×400) (f).
Electron Microscopic Findings
The tumor consisted of scattered spindle-shaped cells lying in an inconspicuous stroma with some collagen fibers and amorphous matrix components. Two types of spindle-shaped tumor cells could be distinguished. The most frequent type in our sections presented characteristics of myofibroblastic cells. The cells had a large nucleocytoplasmic ratio with an oval nucleus and prominent nucleolus.
The cytoplasm contained some rough endoplasmatic reticulum (RER) cisternae, numerous free ribosomes and polysomes, a small fat droplet and sometimes bundles of peripherally located actin microfilaments (Figure 3) ▶ . The other cell type was more oblong and presented with a more voluminous cytoplasm almost completely filled with bundles of actin microfilaments and densifications. Plasmalemmal attachment plaques became obvious on the peripheral cell membrane as well as caveolae (Figure 3) ▶ . These cells presented characteristics of smooth-muscle cells.
Figure 3.
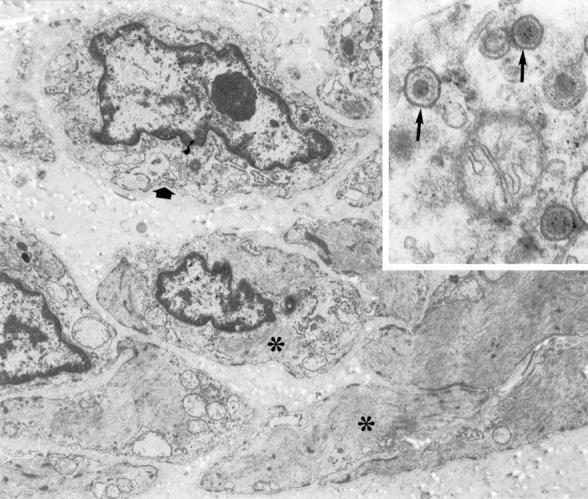
Electron micrograph showing tumor cells with smooth muscle (asterisks) and fibroblast-like (wide arrow) characteristic (×9.200). Inset: detail of EBV virus particles in a cytoplasmic cell fragment (arrow) (×72.500).
Some tumor cells revealed in their cytoplasm the presence of enveloped viral particles or virions. Identical viral particles could be seen in necrotic cell fragments.
Cytogenetic Analysis
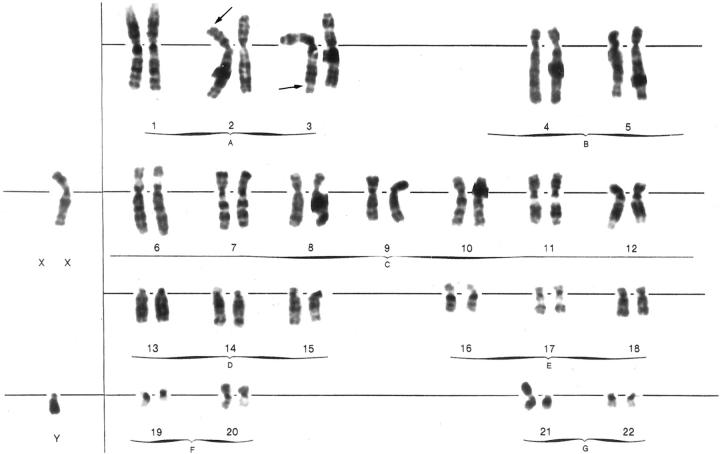
A total of 13 metaphase spreads were analyzed. Six metaphases revealed an abnormal karyotype: 46,XY,del(2)(p2;3),der(3)t(2,3)(p23;q29),der(21)t(Y;21)(q12;p13) (Figure 4) ▶ . Seven cells confirmed a constitutionally normal karyotype.
Figure 4.
G-banded karyogram of abnormal tumor cells showing 46,XY,del(2)(p23),der(3)t(2;3)(p23;q29),der(21)t(Y;21)(q12;p13) karyotype. Arrows point to the breakpoints.
FISH Findings
Dual-color FISH analysis using LSI ALK assay showed two different patterns of hybridization in cells with abnormal karyotype. Sixteen out of 25 analyzed metaphases revealed only one fused (orange/green) signal on normal chromosome 2p23 (Figure 5A) ▶ , while the remaining nine spreads, besides the normal (fused) signal on chromosome 2, disclosed the presence of the 3′end ALK signal (orange) on derivative chromosome 3 (Figure 5B) ▶ . This result indicated the presence of two major clones, both showing a common loss of sequences 5′end to ALK (green). However, in the first clone the region 3′end to ALK (orange) was lost, leading to the loss of the entire ALK allele, while in the second clone it was translocated on 3q29. The existence of two clones was supported also by the results of interphase FISH, using the same dual-color LSI ALK probe. The touch preparation of the tumor specimen showed normal, adjacent (orange/green) signals (two fusions) in 15% of cells. The remaining 85% were abnormal, with 30% of nuclei showing only one fused (orange/green) signal, 39% of nuclei having one fused signal and one orange signal, and 16% of cells with variable number of fused and orange signals (Figure 5C) ▶ .
Figure 5.
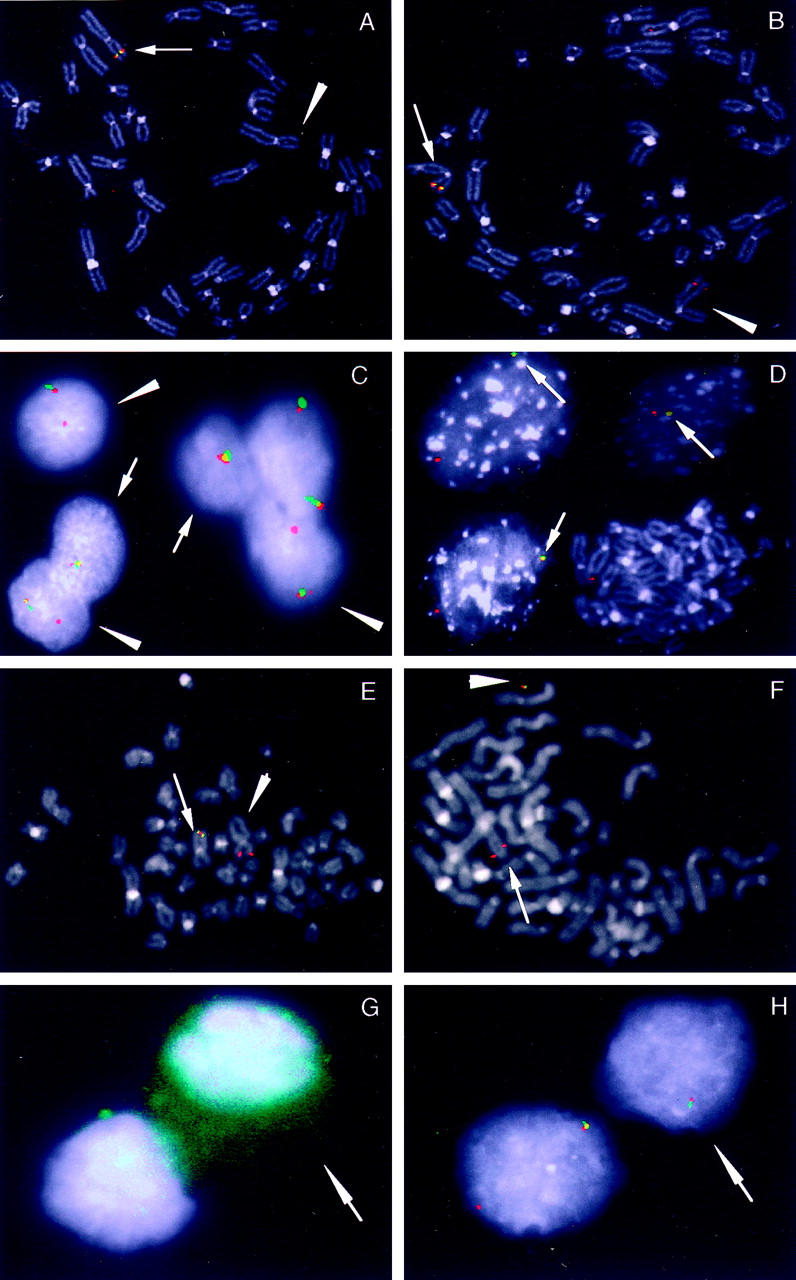
Molecular cytogenetic analysis of abnormal tumor cells carrying del(2)(p23) and der(3)t(2;3)(p23;q29) chromosomes. A: Metaphase FISH using LSI ALK (Vysis) probe: dual-color signals are present only on normal chromosome 2p23 (arrow), indicating clone 1 with the loss of ALK allele (arrowhead). B: Metaphase FISH using LSI ALK probe: dual-color signal is present on normal chromosome 2p23 (arrow) and the red signal is present on der(3) chromosome, indicating clone 2 with the rearranged ALK gene (arrowhead). C: Dual-color hybridization to interphase nuclei from tumor specimen using LSI ALK probe: two separate clones, one with loss (arrow) and the second with rearranged ALK allele (arrowheads) are evident. D: Co-hybridization of digoxigenin-labeled ALKP1 and biotin-labeled EBV probes: a single red and overlapping red/green (arrows) signals in interphase nuclei indicate the integration of EBV at the site of ALK in subset of cells; metaphase cell reveals only red signal on normal chromosome 2. E: Co-hybridization of digoxigenin-labeled ALKP1 and biotin-labeled EBV probes: metaphase cell showing single red signal on normal chromosome 2p23 (arrowhead) and fused green/red signal on der(3) chromosome (arrow). F: Co-hybridization of digoxigenin-labeled ALKP1 and biotin-labeled EBV probes: metaphase cell showing single red signal on normal chromosome 2p23 (arrow) and fused red/green signal outside chromosome, indicating the presence of EBV together with 3′ end ALK sequences in the episomal form (arrowhead). G: Identification of desmin-positive cells by immunostaining (arrow). Nuclei are counterstained with DAPI. H: Double-color interphase FISH analysis of cells corresponding to E, using double-color LSI ALK probe. Loss of rearranged ALK allele was revealed only in the nuclei that corresponded to desmin-positive cells (arrow).
The translocation of the ALK catalytic domain in a subset of metaphases was further confirmed by FISH analysis with the ALK P1 probe (specific for ALK 3′ end), which revealed seven out of 11 metaphases with only one signal on the normal chromosome 2p23, while the remaining spreads showed signals both on the normal 2p23 and der(3). By contrast, hybridization of abnormal metaphases with the PAC 223F18 probe (specific for ALK 5′ end) showed only one signal, at the normal 2p23, in all analyzed cells. Altogether, rearrangement of ALK associated with loss of the 5′ end of the gene was shown. FISH results are summarized in Table 1 ▶ and Table 2 ▶ .
Table 1.
Results of FISH Analysis
| Probe (localization)/specificity (fluorochrome*) | FISH results† (% of interphase nuclei) | Conclusion |
|---|---|---|
| LSI ALK dual-color (2p23)/3′telomeric to ALK (O) 5′centromeric to ALK (G) | Metaphase FISH‡: | |
| Chr 2 GO [16]§ | Clone 1: loss of one ALK allele | |
| Chr 2 GO, der(3) O [9] | Clone 2: loss of sequences centromeric to ALK 5′end and translocation of sequences telomeric to ALK 3′end on 3q29 | |
| Interphase FISH [200]: | ||
| 2× GO (15%) | Normal cells: 15% cells with two normal chr 2 | |
| 1× GO (30%) | Clone 1: loss of one ALK allele (30% of cells) | |
| 1× GO, 1× O (39%) | Clone 2: loss of the sequences centromeric to ALK 5′end (39% of cells) | |
| Other (16%) | ||
| ALKP1 RP11-111H1 (2p23)/3′end ALK (O) | Metaphase FISH: | |
| Chr 2 O [7] | Confirms loss of 3′end ALK sequences in clone 1 | |
| Chr 2 O, der(3) O [4] | Confirms translocation of 3′end ALK to 3q29 in clone 2 | |
| RP11-223F18 (2p23)/5′end ALK (G) | Metaphase FISH: | |
| Chr 2 G [10] | Confirms loss of ALK 5′end in all abnormal cells | |
| ALKP1 (2p23)/3′end ALK (O) | Metaphase FISH: | |
| Chr 2 O [9] | Cells of clone 1: without EBV in metaphase | |
| EBV/EcoRI-B (G) | Chr 2 O, der(3) GO [5] | Cells of clone 2: EBV DNA associated with 3′end ALK on der(3) or in episomal form |
| Chr 2 O, episomal GO [5] | ||
| Interphase FISH [100]: | ||
| 1× G, 1× O (35%) | Cells of clone 1: with EBV not associated with 3′end ALK | |
| 1× GO, 1× O (43%) | Cells of clone 2: with EBV sequences associated with 3′end ALK | |
| 1× GO (5%) | ||
| 2× O (17%) | Normal cells: two chr 2 and without EBV |
*O, orange; G, green.
†Only abnormal metaphases were selected.
‡GO, fused green/orange; G, green; O, orange signals.
§Number in square brackets is number of analyzed cells.
Table 2.
The Hybridization Signals in Interphase Nuclei by FISH Using LSI ALK Dual-Color (Vysis) Probe in Desmin-Positive and Desmin-Negative Cells
| Cells phenotype by immunostaining | Number of cells with signals/total number of cells (%) | |||
|---|---|---|---|---|
| Two adjacent 5′/3′ (green/orange) signal* | One adjacent 5′/3′ (green/orange) signal† | One adjacent 5′/3′ (green/orange) + one 3′ (orange) signal‡ | Other | |
| Desmin-positive | 2/30 (7%) | 12/30 (40%) | 0 | 0 |
| Desmin-negative | 1/30 (3%) | 0 | 11/30 (37%) | 4/30 (13%) |
*Normal, diploid cells.
†Clone 1, with the loss of one ALK allele.
‡Clone 2, with the loss of 5′ALK end only.
Hybridization with the probe specific for subtelomeric 3q sequences disclosed signals only on the terminal end of normal chromosome 3. This result confirmed the presence of an unbalanced der(3)t(2;3) translocation (data not shown).
Co-hybridization of digoxigenin-labeled ALKP1 together with biotin-labeled EBV DNA probes with the interphase nuclei of tumor cells revealed one red and one fused green/red (yellow) signal in 43% (Figure 5D) ▶ , one red and one green signal in 35% and two red signals in 17% of cells. While the abnormal chromosome spreads were evaluated, a single red signal at normal chromosome 2p23 and overlapping red/green hybridization signal at the breakpoint of a der(3) was present (Figure 5E) ▶ . This result indicated the integration of EBV DNA in the translocated 3′end ALK region in the molecularly distinctive clone. In contrast, the metaphases that failed to show translocated 3′ end ALK did not show chromosomal integration of the virus DNA. In five of 14 of these metaphases, however, the overlapping green/red signal was found outside the counterstained chromosomal chromatin borders, pointing the presence of co-localized EBV and ALK sequences in an autonomous (non-integrated) episomal form (Figure 5F) ▶ . Normal metaphases showed only red signals from ALK P1 probe on two normal homologous chromosomes 2p23 and no green signals from EBV probe.
Combined Immunofluorescence and FISH Study
The immunofluorescence and subsequent FISH study was performed to find out if the molecular heterogeneity was in any way associated with the phenotypic diversity of tumor cells. Using anti-desmin antibodies, positive cytoplasmatic immunoreactivity was found in 27% of cells. Immunopositive cells were randomly dispersed among negative ones. Twelve out of 14 desmin immunopositive cells that were evaluated demonstrated loss of one ALK allele using LSI ALK probe (Figure 5, G and H) ▶ ; two cells showed two fused, red and green, signals. Eleven out of 16 desmin immunonegative cells revealed the presence of one fused plus one single red signal. The remaining five cells showed the varying number of fused and/or single red signals.
ALK RT-PCR Analysis
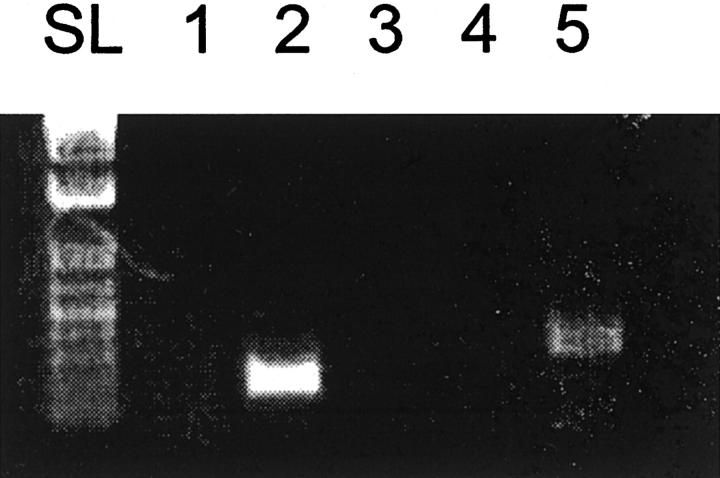
To confirm ALK expression status, we performed RT-PCR analysis of total RNA prepared from the tumor specimen and from control IMT with ATIC-ALK chimeric fusion. As shown in Figure 6 ▶ , the tumor cells of the present case did not show ALK specific transcripts using primers for either the 5′ or the 3′end of the ALK transcript. A control experiment demonstrated RT-PCR detection of cDNAs for an unrelated gene, CIZ, in the same sample. In contrast, when cDNA derived was used from an ATIC-ALK positive control case, a PCR product was obtained with the primers amplifying 3′ ALK but not with 5′ ALK primers. These findings confirm the lack of detectable ALK expression in the tumor under the study.
Figure 6.
RT-PCR analysis of ALK cDNA. RT-PCR was performed with primers designed to amplify respectively the 3′-end (lanes 1 and 2) or the 5′-end (lanes 3 and 4) of ALK. Lanes 1 and 3 show the absence of any product using cDNA of the tumor. Lanes 2 and 4: PCR was performed with cDNA from an ATIC-ALK positive control case. Lane 5 is a positive control for the cDNA synthesis showing amplification of the CIZ gene, starting from cDNA of the tumor.
Discussion
Recent studies demonstrate that in addition to inflammatory myofibroblastic tumors, ALK rearrangements and ALK expression are found in other neoplasms, especially neuroblastomas, rhabdomyosarcomas, and malignant peripheral nerve sheet tumors, supporting the concept of ALK involvement in a broader group of malignancies. 26 In this report we present an abnormalities of the ALK gene in a case of hepatic posttransplant smooth-muscle tumor. The unusual and hitherto unreported finding in this tumor is its phenotypic spectrum. One phenotype corresponds to the typical EBV-related smooth-muscle tumor in immunosuppressed patients, based on the histology, immunohistochemistry (desmin), and ultrastructural features. The other phenotype shows features of an inflammatory pseudotumor: a desmin-negative, α-SMA-positive spindle-cell population, admixed with mononuclear inflammatory cells, including plasma cells and vessels, in a myxoid background. IPTs have been described in immunosuppressed patients and IPTs from the liver, spleen, and epidydimis have been associated with EBV. 27-30 A major proportion of EBV-related liver and spleen IPTs correspond to EBV-associated follicular dendritic reticulum cell proliferations, but this is not the case in the tumor we describe, since specific follicular dendritic reticulum cell markers (CD21 and CD35) were negative. In addition, no ultrastructural features of follicular dendritic reticulum cells could be found. In the differential diagnosis of the IPT component, an inflammatory leiomyosarcoma could be considered. However, the latter entity generally does not show the loosely textured stroma as seen in our case and tend to be more cellular. In addition, one would expect desmin to be expressed in an inflammatory leiomyosarcoma. Moreover, these tumors often show foam cells, or even psammoma bodies, which are completely lacking. Finally, inflammatory leiomyosarcoma shows a near haploid karyotype, which is not the case in the tumor we present. 31
Cytogenetic analysis demonstrated an unbalanced translocation involving bands 2p23 (ALK locus region) and 3q29. FISH analysis confirmed that the 2p23 rearrangement was within the ALK gene in a significant proportion of cells. Furthermore, among visually the same abnormal metaphase cells two molecularly distinctive clones were recognized: one with loss and the second with retained 3′ ALK catalytic domain on derivative chromosome 3. The former resulted probably from the latter by a secondary rearrangement during aberrant cell expansion and tumor formation. Using an EBV-specific probe, FISH revealed integration of viral EBV DNA sequences at the 3q29 translocation breakpoint site and EBV co-localization with the translocated 3′ end ALK kinase domain in the latter. The clone that revealed loss of translocated ALK contained viral sequences together with 3′ end ALK in the nuclear episomal form. It is unknown whether the chromosomal integration of EBV sequences occurred before or after the translocation. However, the EBV integration site itself was shown to be a chromosomal region prone to enhanced fragility due to an altered chromatin structure. 32 The loss of the integrated EBV viral genome due to chromosomal breaks proximal to the integration site was documented in BL cells and NPC cells in culture. 33-34 This observation led to the hypothesis that under certain conditions EBV might act by a so-called “hit and run” mechanism during oncogenic transformation. Accordingly, due to increased chromosomal instability and a strong positive selection pressure toward cells retaining only episomal EBV, transient EBV integration into the host genome might be followed by loss of the virus together with the adjacent host cell chromosome fragments. Similarly, the loss of integrated EBV with the adjacent translocated ALK sequences in a subset of cells was observed in our tumor, suggesting the hit-and-run mechanisms of EBV-driven transformation that occurred in vivo.
In this context, the biological implications of EBV infection associated with tumor formation are of special interest. An integration of the viral particles into the human genome in EBV-associated disease has been well documented. 34 The integration of EBV within the vicinity of the ALK receptor tyrosine kinase gene was never reported. As is known from other RNA and DNA tumor viruses, integration of viral DNA might modify the expression of viral as well as of cellular genes and might also cause chromosomal instability. The known mechanism of ALK activation in ALCLs and IMTs is formation of chimeric fusions that result in constitutive, ligand-independent receptor oligomerization. In the present case ALK kinase domain was also involved in the translocation. However, in contrast to the subset of IMTs with known variant chromosomal translocations involving the ALK gene, activation of ALK could not be demonstrated in myofibroblastic cells of the tumor, as shown by immunostaining and as confirmed by RT-PCR analysis. Thus, the IPT component of the present case belongs to the category of ALK-negative IPTs, although the possibility of transient ALK expression during the EBV integration near or within ALK gene or during the subsequent ALK secondary rearrangements cannot be excluded. Alternatively, the clonal expansion of cells harboring the ALK translocation could gain the required proliferative advantage through EBV association, and not by direct ALK activation. This hypothesis is supported by the recent finding that a small proportion of normal lymphoid cells in non-neoplastic lymph nodes are positive for chimeric ALK transcripts, suggesting that ALK gene rearrangement by itself might be insufficient to induce tumor formation. 35 Furthermore, it is known that EBV-associated smooth-muscle tumors contain oncogenic latent membrane proteins that alone may implicate neoplastic cellular transformation. 36 The transforming ability of LMP-1 is well recognized in lymphoid malignancies and LMP-1 might play a role in the pathogenesis of ALK-negative ALCLs. 11,38
The very intriguing relation between distinct cellular genotypes, with respect to ALK changes and desmin-positive/-negative phenotypes that was observed within tumoral cells of the present case, requires further consideration. Both metaphase and interphase FISH confirmed the spectrum of serial genomic changes involving the ALK gene and integrated EBV sequences. Immunofluorescence anti-desmin staining combined with subsequent FISH analysis demonstrated the presence and the loss of translocated 3′ end of ALK tyrosine kinase domain in desmin-negative and -positive cells, respectively. The desmin-negative cells most likely correspond to IPT-related myofibroblasts while the desmin-positive cells represent the smooth-muscle tumor component. Although myofibroblasts can also express desmin, on the histological sections the desmin-positive cells were only seen in the histologically typical smooth-muscle component of the tumor. The combined cytogenetic, immunofluorescence and morphological findings indicate that, although phenotypically different, both components of the tumor shared a common single-cell origin. We believe that this case represents a single immunosuppression-related EBV-driven lesion, which shows a phenotypic spectrum, ranging from a smooth-muscle tumor to an IPT. Thus, the observed phenotypic heterogeneity most likely reflects divergence during neoplastic progression, with the subsequent expansion of morphologically and molecularly distinct but cytogenetically related clones. The reason for this diversity remains obscure. One may hypothesize, however, that secondary genomic rearrangements induced by virus integration and subsequent relocation could impose somatic changes of cellular genes encoding functions responsible for tumor’s gene expression profile.
Notably, the EBV-ALK interaction in EBV-associated smooth-muscle tumors or IPTs has never been investigated. Whether these tumors might be induced by direct ALK-EBV fusion warrants further studies.
Acknowledgments
We thank Dr. Eric Schoenmakers and Eric Huys for help in providing EBV DNA probe, and Belinda Carleer and Lut Mekers for excellent technical assistance.
Footnotes
Address reprint requests to Maria Debiec-Rychter, M.D., Ph.D., Center for Human Genetics, Katholieke Universiteit Leuven, Herestraat 49, B-3000 Leuven, Belgium. E-mail: Maria.Debiec-Rychter@med.kuleuven.ac.be.
This text presents research results of the Belgian program on Interuniversity Poles of Attraction initiated by the Belgian Sate, Prime Minister’s Office, Science Policy Programming. Its authors assume the scientific responsibility. The authors would like to acknowledge the COST support through the COST ACTION B19 “Molecular cytogenetics of solid tumors” in carrying out this work.
References
- 1.Lee ES, Locker J, Nalesnik M, Reyes J, Jaffe R, Alashari M, Nour B, Tzakis A, Dickman PS: The association of Epstein-Barr virus with smooth-muscle tumors occurring after organ transplantation. N Engl J Med 1995, 332:19-25 [DOI] [PubMed] [Google Scholar]
- 2.Davidoff AM, Hebra A, Clark BJ, 3rd, Tomaszewski JE, Montone KT, Ruchelli E, Lan HT: Epstein-Barr virus-associated hepatic smooth-muscle neoplasm in a cardiac transplant recipient. Transplantation 1996, 61:515-517 [DOI] [PubMed] [Google Scholar]
- 3.Wu TT, Swerdlow SH, Locker J, Bahler D, Randhawa P, Yunis EJ, Dickman PS, Nalesnik MA: Recurrent Epstein-Barr virus-associated lesions in organ transplant recipients. Hum Pathol 1996, 27:157-164 [DOI] [PubMed] [Google Scholar]
- 4.Rogatsch H, Bonatti H, Menet A, Larcher C, Feichtinger H, Dirnhofer S: Epstein-Barr virus-associated multicentric leiomyosarcoma in an adult patient after heart transplantation: case report and review of the literature. Am J Surg Pathol 2000, 24:614-6215 [DOI] [PubMed] [Google Scholar]
- 5.Cheuk W, Li PC, Chan JK: Epstein-Barr virus-associated smooth-muscle tumour: adistinctive mesenchymal tumour of immunocompromised individuals. Pathology 2002, 34:245-249 [DOI] [PubMed] [Google Scholar]
- 6.Arber DA, Kamel OW, van de Rijn M, Davis RE, Medeiros LJ, Jaffe ES, Weiss LM: Frequent presence of the Epstein-Barr virus in inflammatory pseudotumor. Hum Pathol 1995, 26:1093-1098 [DOI] [PubMed] [Google Scholar]
- 7.Cheuk W, Chan JK, Shek TW, Chang JH, Tsou MH, Yuen NW, Ng WF, Chan AC, Prat J: Inflammatory pseudotumor-like follicular dendritic cell tumor: a distinctive low-grade malignant intra-abdominal neoplasm with consistent Epstein-Barr virus association. Am J Surg Pathol 2001, 25:721-7318 [DOI] [PubMed] [Google Scholar]
- 8.Griffin BE: Epstein-Barr virus (EBV) and human disease: facts, opinions and problems. Mutation Res 2000, 462:395-409 [DOI] [PubMed] [Google Scholar]
- 9.Kutok JL, Pinkus GS, Dorfman DM, Flechter CDM: Inflammatory pseudotumor of lymph node and spleen: an entity biologically distinct from inflammatory myofibroblastic tumor. Hum Pathol 2001, 32:1382-1387 [DOI] [PubMed] [Google Scholar]
- 10.Knecht H, Berger C, Rothenberger S, Odermatt BF, Brousset P: The role of Epstein-Barr virus in neoplastic transformation. Oncology 2001, 60:289-302 [DOI] [PubMed] [Google Scholar]
- 11.Cahir McFarland ED, Izumi KM, Mosialos G: Epstein-Barr virus transformation: involvement of latent membrane protein1-mediated activation of NF-κβ. Oncogene 1999, 18:6959-6964 [DOI] [PubMed] [Google Scholar]
- 12.Morris SW, Naeve C, Mathew P, James PL, Kirstein MN, Cui X, Witte DP: ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin’s lymphoma encodes a neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK). Oncogene 1997, 14:2175-2188 [DOI] [PubMed] [Google Scholar]
- 13.Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, Look AT: Fusion of a kinase gene ALK, to nucleolar protein gene NPM, in non-Hodgkin’s lymphoma. Science 1994, 263:1281-1284 [DOI] [PubMed] [Google Scholar]
- 14.Griffin CA, Hawkins AL, Dvorak C, Henkle C, Ellingham T, Perlman EJ: Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res 1999, 59:2776-2780 [PubMed] [Google Scholar]
- 15.Lawrence B, Perez-Atayde A, Hibbard MK, Rubin BP, Dal Cin P, Pinkus JL, Pinkus GS, Xiao S, Yi ES, Fletcher CDM, Fletcher JA: TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol 2000, 157:377-384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bridge JA, Kanamori M, Ma Z, Pickering D, Hill DA, Lydiatt W, Lui MY, Colleoni GW, Antonescu CR, Ladanyi M, Morris SW: Fusion of the ALK gene to the clathrin heavy chain gene, CLTC, in inflammatory myofibroblastic tumor. Am J Pathol 2001, 159:411-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cools J, Wlodarska I, Somers R, Mentens N, Pedeutour F, Maes B, De Wolf-Peeters C, Pauwels P, Hagemeijer A, Marynen P: Identification of novel fusion partners of ALK, the anaplastic lymphoma kinases, in anaplastic large-cell lymphoma and inflammatory myofibroblastic tumor. Genes Chromosomes Cancer 2002, 34:354-362 [DOI] [PubMed] [Google Scholar]
- 18.Coffin CM, Patel A, Perkins S, Elenitoba-Johnson KSJ, Perlman E, Griffin CA: ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol 2001, 14:569-576 [DOI] [PubMed] [Google Scholar]
- 19.Neuhauser TS, Derringer GA, Thompson LD: Splenic inflammatory myofibroblastic tumor (inflammatory pseudotumor): a clinicopathologic and immunophenotypic study of 12 cases. Arch Pathol Lab Med 2001, 125:379-385 [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Roman JJ, Ocejo-Vinyals G, Sanchez-Velasco P, Nieto EH, Leyva-Cobian F, Val-Bernal JF: Presence of human herpesvirus-8 DNA sequences and overexpression of human IL-6 and cyclin D1 in inflammatory myofibroblastic tumor (inflammatory pseudotumor). Lab Invest 2000, 80:1121-1126 [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Roman JJ, Sanchez-Velasco P, Ocejo-Vinyals G, Hernandez-Nieto E, Val-Bernal JF: Human herpesvirus-8 genes are expressed in pulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). Am J Surg Pathol 2001, 25:624-629 [DOI] [PubMed] [Google Scholar]
- 22.: ISCN (1995): Mitelman F eds. International System for Human Genetic Nomenclature. 1995. S. Karger Basel
- 23.Mathew P, Sanger WG, Weisenburger DD, Valentine M, Valentine V, Pickering D, Higgins C, Hess M, Cui X, Srivastava DK, Morris SW: Detection of the t(2;5)(p23;q35) and NPM-ALK fusion in non-Hodgkin’s lymphoma by two-color fluorescence in situ hybridization. Blood 1997, 89:1678-1685 [PubMed] [Google Scholar]
- 24.Dierlamm J, Wlodarska I, Michaux L, La Starza R, Zeller W, Mecucci C, Van den Berghe H: Successful use of the same slide for constitutive fluorescence in situ hybridization experiments. Genes Chrom Cancer 1996, 16:261-264 [DOI] [PubMed] [Google Scholar]
- 25.Debiec-Rychter M, Van Valckenborgh I, Van den Broeck C, Hagemeijer A, Van de Ven WJM, Kas K, Van Damme B, Voz ML: Histologic localization of PLAG1 (pleomorphic adenoma gene 1) in pleomorphic adenoma of the salivary gland: cytogenetic evidence of common origin of phenotypically diverse cells. Lab Invest 2001, 81:1289-1297 [DOI] [PubMed] [Google Scholar]
- 26.Cessna MH, Zhou H, Sanger WG, Perkins SL, Tripp S, Pickering D, Daines C, Coffin CM: Expression of ALK1 and p80 in inflammatory myofibroblastic tumor and its mesenchymal mimics: a study of 135 cases. Mod Pathol 2002, 15:931-938 [DOI] [PubMed] [Google Scholar]
- 27.Lyavieris P, Fabre M, Waguet J, Bernard O: Inflammatory pseudotumor after liver transplantation. J Pediatr Gastroenterol Nutr 2000, 31:309-312 [DOI] [PubMed] [Google Scholar]
- 28.Tai YS, Lin PW, Chen SG, Chang KC: Inflammatory pseudotumor of the liver in a patient with immunodeficiency virus infection. Hepatogastroenterology 1998, 45:1760-1763 [PubMed] [Google Scholar]
- 29.Chan KW, Chan KL, Lam KY: Inflammatory pseudotumor of epididymis and Epstein-Barr virus: a study of two cases. Pathology 1997, 29:100-101 [DOI] [PubMed] [Google Scholar]
- 30.Arber DA, Weiss LM, Chang KL: Detection of Epstein-Barr virus in inflammatory pseudotumor. Semin Diagn Pathol 1998, 15:155-160 [PubMed] [Google Scholar]
- 31.Dal Cin P, Sciot R, Fletcher CDM, Samson I, De Vos R, Mandahl N, Willen H, Larson O, Van den Berghe H: Inflammatory leiomyosarcoma may be characterized by specific near-haploid chromosome changes. J Pathol 1998, 185:112-115 [DOI] [PubMed] [Google Scholar]
- 32.Wolf J, Jox A, Skarbek H, Pukrop T, Bartnitzke S, Pawlita M, Diehl V, Bullerdiek J: Selective loss of integrated Epstein-Barr virus genomes after long-term cultivation of Burkitt’s lymphoma x B-lymphoblastoid cell hybrids due to chromatin instability at the integration site. Virology 1995, 212:179-185 [DOI] [PubMed] [Google Scholar]
- 33.Lin CT, Chan WY, Chen W, Huang HM, Wu HC, Hsu MM, Chuang SM, Wang CC: Characterization of seven newly established nosopharyngeal carcinoma cell lines. Lab Invest 1993, 68:716-727 [PubMed] [Google Scholar]
- 34.Srinivas SK, Sample JT, Sixbey Jw: Spontaneous loss of viral episomes accompanying Epstein-Barr virus reactivation in a Burkitt’s lymphoma cell line. J Infect Dis 1998, 177:1705-1709 [DOI] [PubMed] [Google Scholar]
- 35.Ohshima K, Suzumiya J, Kanda M, Kato A, Kikuchi M: Integrated and episomal forms of Epstein-Barr virus (EBV) in EBV-associated disease. Cancer Lett 1998, 122:43-50 [DOI] [PubMed] [Google Scholar]
- 36.Maes B, Vanhentenrijk V, Wlodarska I, Cools J, Peeters B, Marynen P, De Wolf-Peeters C: The NPM-ALK and the ATIC-ALK fusion genes can be detected in non-neoplastic cells. Am J Pathol 2001, 158:2185-2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kutok JL, Aster JC: Molecular biology of anaplastic lymphoma kinase-positive anaplastic large-cell lymphoma. J Clin Oncology 2002, 20:3691-3702 [DOI] [PubMed] [Google Scholar]
- 38.Agarwal S, Ramanathan U, Naresh KN: Epstein-Barr virus association and ALK gene expression in anaplastic large-cell lymphoma. Hum Pathol 2002, 33:146-152 [DOI] [PubMed] [Google Scholar]