Abstract
In this study, we investigated the role of the hematopoietic cytokine erythropoietin (EPO) during wound healing, the physiological response to tissue injury. We used an in vivo wound-healing assay (fibrin Z-chambers) consisting of fibrin-filled chambers implanted subcutaneously in rats. The fibrin inside the chambers is replaced by granulation tissue consisting of new blood vessels, macrophages and fibroblasts as part of the wound-healing response. Local, exogenous recombinant EPO administration into the fibrin matrix significantly increased granulation tissue formation in a dose-dependent manner. To investigate the physiological role of endogenous EPO during wound healing, we used soluble EPO receptor or anti-EPO monoclonal antibodies to neutralize EPO and observed dose-dependent inhibition of granulation tissue formation, consistent with an important role for EPO in the wound-healing cascade. The ability of recombinant EPO to promote wound healing was associated with a proangiogenic effect during granulation tissue formation. We also found abundant expression of EPO receptor protein in macrophages, cells that play a pivotal role during wound healing. Modulation of wound healing because of administration of recombinant EPO or inhibition of endogenous EPO-EPO receptor correlated with changes in levels of inducible nitric oxide synthase protein in granulation tissue. These data demonstrate a novel function for EPO by providing in vivo evidence for a physiological role during fibrin-induced wound healing.
Erythropoietin (EPO) is a glycoprotein hormone that regulates the production of red blood cells. 1-3 The biological effects of EPO are mediated by its specific interaction with its cell-surface receptor EPOR, a type I cytokine receptor that is expressed in erythroid progenitor cells as well as in several nonhematopoietic cell types. 4 A series of recent studies have provided experimental evidence for diverse nonhematopoietic biological effects of EPO-EPOR signaling. For instance, in the central nervous system, EPO plays an important role in the brain’s response to neuronal injury. 5-9 In other tissues, expression of EPOR in kidney, muscle cells, and intestine is associated with the ability of EPO to induce cellular proliferation. 10-12 Several types of vascular endothelial cells express receptors for EPO 13-15 and previous studies have shown the ability of EPO to stimulate angiogenesis, the generation of new blood vessels from pre-existing vessels. 16 In different experimental systems, recombinant EPO was shown to promote endothelial cell proliferation and migration in rat thoracic aorta 17 and chick chorioallantoic membrane. 18 In the uterus, EPO has been implicated in cyclic endometrial angiogenesis. 19
Wound healing is a complex process that is initiated in response to tissue injury and restores the function and integrity of damaged tissues. Tissue injury is followed by the formation of a fibrin provisional matrix that facilitates the influx of inflammatory and vascular endothelial cells during wound healing. Angiogenesis is an essential component of the physiological wound-healing response that is mediated in large part by cytokines and growth factors. 20,21 In the present study, we hypothesized that EPO may be an important cytokine that is involved in the physiological wound-healing cascade. We investigated the role of EPO during fibrin-induced wound healing in a rodent model consisting of fibrin Z-chambers (F-ZCs), dual porous Plexiglas chambers containing a compound of interest and fibrin matrix, implanted into the subcutaneous tissues of rats and harvested later for analysis of wound-healing response and angiogenesis. 22 We tested the hypothesis that EPO may enhance granulation tissue formation and found that local recombinant EPO administration accelerated fibrin-induced wound healing. We investigated the role for endogenous EPO during wound healing by using soluble EPOR (sER) and anti-EPO monoclonal antibodies (mAbs) to scavenge EPO and observed delayed wound healing associated with EPO-EPOR inhibition. Furthermore, we found EPOR expression in macrophages, cells that are critical mediators of wound-healing response. Modulation of wound healing because of recombinant EPO administration or endogenous EPO-EPOR inhibition correlated with changes in levels of inducible nitric oxide synthase (iNOS) protein in granulation tissue. We also show that stimulation of wound healing after local recombinant EPO administration correlates with increased microvessel density (MVD) in granulation tissue suggesting that the prohealing effect of EPO may be associated, at least in part, with its ability to stimulate blood vessel growth in vivo.
Materials and Methods
Reagents
Recombinant human EPO (hEPO) containing 2.5 mg/ml of human albumin (Procrit) was purchased from Ortho-Biotech (Bridgewater, NJ). Recombinant murine EPO (mEPO) was provided by Amgen (Thousand Oaks, CA). Recombinant basic fibroblast growth factor (bFGF), neutralizing anti-EPO mAb 287, a second monoclonal anti-EPO mAb 2871, and recombinant soluble EPO receptor (sER) consisting of the extracellular domain of EPOR, capable of binding EPO and acting as a potent antagonist were from R&D Systems, Minneapolis, MN. sER, anti-EPO mAb 287, and mAb 2871 were capable of inhibiting murine EPO-mediated proliferation of hematopoietic cells expressing EPOR in vitro, with mAb 2871 being the least potent of all three (unpublished data). Anti-EPO mAbs and sER were reconstituted in phosphate-buffered saline (PBS) and mEPO was diluted in PBS. Anti-EPOR polyclonal antibodies (C-20 and M-20) and anti-actin (I-19) were from Santa Cruz Biotechnology, Santa Cruz, CA, antibody against tissue transglutaminase was from Neomarkers (Fremont, CA; catalog no. MS-279), monoclonal ED-1 antibody (Serotec, Oxford, UK) was used to stain macrophages and iNOS antibody was from Transduction Laboratories, Lexington, KY. Fibrinogen (catalog no. 341578) and thrombin (catalog no. 605160) were from Calbiochem, La Jolla, CA. Human albumin was from Sigma Chemical Co., St. Louis, MO (catalog no. A3782). Dulbecco’s modified Eagle’s medium was used to reconstitute fibrinogen.
Animal Studies and F-ZCs
The Duke Institutional Animal Care and Use Committee approved all animal protocols. Female Fischer 344 rats of an average weight of >150 g were selected for these studies. The animals were kept in temperature-controlled rooms (24°C) on a 12-hour light-dark cycle with access to rodent chow and bottled tap water ad libitum. The F-ZC is a fibrin gel-based in vivo assay in which fibrinogen, thrombin, and the compound of interest are added to a dual porous chamber through a side port (Figure 1A) ▶ and the chambers are then surgically implanted (four chambers per animal) in the subcutaneous tissues at the dorsum of rats as described. 22-25 As a positive control, we performed an experiment to test the effect of bFGF, a proangiogenic growth factor that is known to promote wound healing. 26 Two rats were used for surgical implantation of eight chambers containing bFGF (final concentration of 1 μg/ml) and two control rats were implanted with eight chambers containing vehicle (PBS). At day 6, the F-ZCs were removed and the contents of four randomly assigned chambers in each group (control and bFGF) were fixed in 10% formalin for paraffin embedding for assessment of wound healing and the remaining four chamber contents were snap-frozen for protein analyses. To investigate the effect of EPO on wound healing, we tested increasing doses of recombinant EPO (human or murine) or EPO-EPOR inhibitors in F-ZCs, harvested either at day 6 or day 9. Negative controls included vehicle (PBS or PBS with 2.5 mg/ml of human albumin). For each treatment group and dose, three animals were used leading to a total of 12 chambers in each experiment. For every treatment group, seven or eight randomly chosen chambers were used for formalin fixation and paraffin embedding. The remaining four or five chambers were frozen in liquid nitrogen for protein analyses. The effect of murine EPO was tested in two experiments and the data from two experiments pooled, leading to a total of 15 chambers (of 24 implanted) in each dose group analyzed for wound healing at day 6. A second negative control group consisted of heat-inactivated murine EPO and all 12 implanted chambers were assessed for wound-healing response. For each recombinant human EPO treatment group, 8 of the 12 implanted chambers were randomly assigned for formalin fixation for measurement of wound-healing response at day 6 and the remaining four chambers were snap-frozen for protein analyses. To investigate the role of endogenous EPO, we administered sER or anti-EPO mAbs into F-ZCs that were harvested at day 9. For each inhibitor treatment group, 12 chambers were implanted and in the control group 16 chambers (4 rats) were implanted. In every dose group, five random chambers were snap-frozen for protein analyses and the remaining chambers (7 in each treatment group and 11 in control group) were processed for analysis of wound healing at day 9. In all experiments, every implanted chamber that was randomly assigned for immunohistochemistry was analyzed for wound healing and every granulation tissue measurement was included in the data analysis except one chamber in the low-dose sER group (1 μg/ml) was damaged during paraffin embedding, precluding measurement of wound healing. To assess wound-healing response, maximum thickness of granulation tissue inside each F-ZC was measured from hematoxylin and eosin (H&E)-stained tissue sections. 22 To assess angiogenic response, MVD was estimated using immunohistochemistry as described below. All measurements of granulation tissue thickness and MVD were performed independently by two pathologists (ZAH and KA) in a blinded manner.
Figure 1.
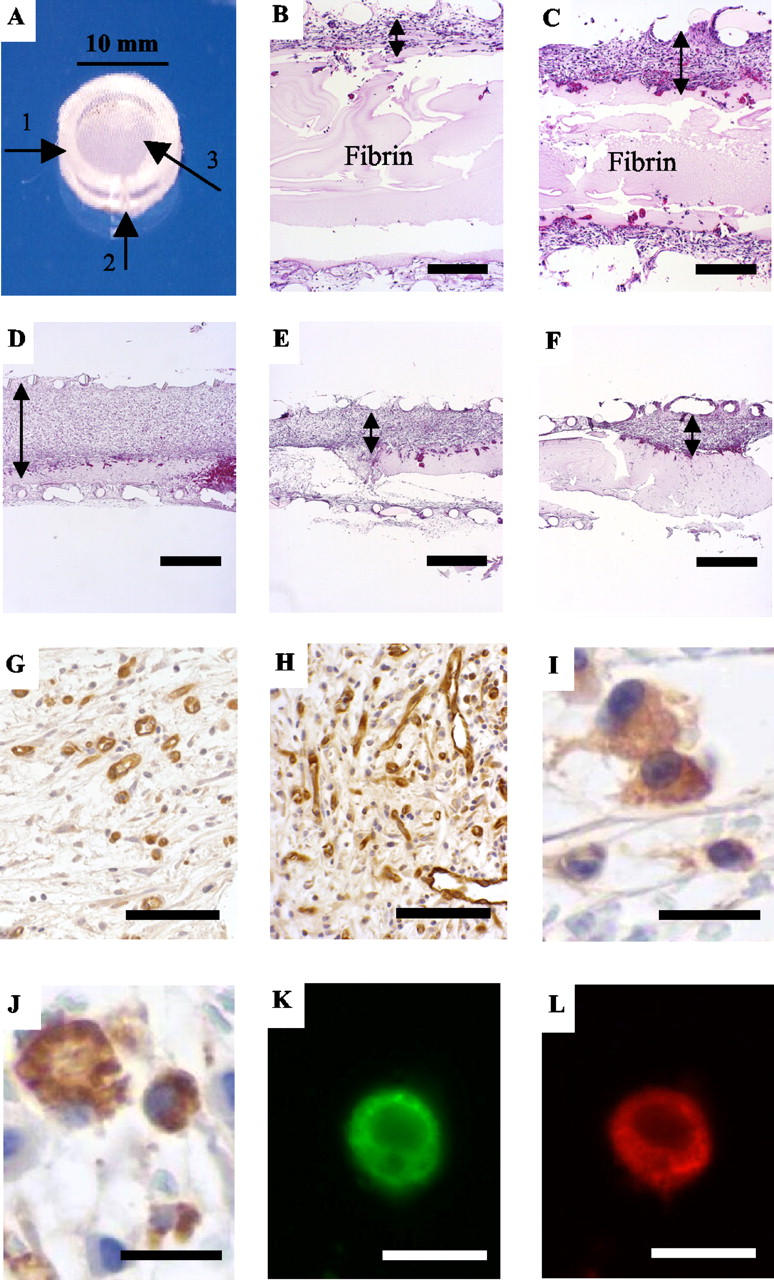
Effect of recombinant EPO, soluble EPOR, and anti-EPO antibodies on granulation tissue formation and angiogenesis in F-ZCs and EPOR expression in wound macrophages. A: Z-chamber. Arrows indicate the Plexiglas ring (1), side port through which fibrinogen and thrombin solution are added (2), and nylon mesh of 180-μm pore size glued to both sides of the ring (3). B and C: Representative H&E-stained tissue sections from F-ZCs illustrating the effect of EPO on granulation tissue thickness at day 6 after treatment with vehicle (B) or recombinant EPO (C). Thickness of granulation tissue developed inside the F-ZC was used as a measure of the healing response. Lines with arrowheads indicate the maximum granulation tissue thickness inside F-ZC. The fibrin in the chamber is indicated. The granulation tissue in EPO-treated F-ZCs (C) is distinctly increased compared to control (B). Black bars, 200 μm. D, E, and F: Representative H&E-stained tissue sections illustrating the effect of sER (E) and neutralizing anti-EPO antibody mAb 287 (F) on granulation tissue thickness in F-ZCs at day 9 compared to control (D). Lines with arrowheads indicate maximum granulation tissue thickness that is decreased in F-ZCs treated with EPO-EPOR inhibitors (E and F) compared to control (D). Black bars, 500 μm. G and H: Representative tissue sections stained with blood vessel marker demonstrating increased MVD in granulation tissue on day 6 in F-ZCs containing EPO (H) compared to control (G). Black bars, 100 μm. I and J: Expression of EPOR (I) and macrophage marker ED-1 (J) in wound macrophages by immunohistochemistry of formalin-fixed, paraffin-embedded granulation tissue sections. Black bars, 15 μm. K and L: EPOR expression in wound macrophages identified by double labeling with both anti-EPOR (green) and anti-ED-1 (red) and visualized by fluorescence microscopy. White bars, 15 μm.
Immunohistochemistry and Protein Analyses
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded tissues removed from F-ZCs. Primary antibody against tissue transglutaminase (TG100, 1:10, endothelial cell marker) was used using procedures described previously. 22,27-29 Controls for immunohistochemistry were treated with mouse or rabbit IgG instead of primary antibody and were negative in any reactivity. MVD was estimated as described by Weidner and colleagues. 30 Briefly, three hot spots or areas with the highest visible blood vessel density (marked by the vessel marker) per section were selected and the number of blood vessels having a visible lumen were counted at high-power field (×400) by two pathologists (ZAH and KA) blinded to the samples. 22 A total of 9 to 18 fields in three to six randomly chosen sections were analyzed for each group (control or treatment). The data were pooled for the controls or treated tissues and mean values were calculated for each group. Macrophage influx into granulation tissue was assessed on images captured from ED-1-immunostained F-ZC sections. Images of three ×400 fields from each section showing highest density of macrophages were analyzed. Macrophages were counted using Zeiss interactive digital imaging software KS 300. A total of four randomly chosen sections were analyzed for each group (control or treatment). To determine iNOS protein levels in granulation tissue, Western blots were performed using tissues obtained from F-ZCs. The granulation tissues from four or five chambers from each treatment or control group were pooled and tissues were homogenized in 1 ml of ice-cold lysis buffer containing 20 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 10% glycerol, 5 mmol/L ethylenediaminetetraacetic acid, 10 mmol/L NaF, 1% Triton X-100, supplemented with proteolytic inhibitor cocktail (Roche, Indianapolis, IN) followed by sonification and centrifugation at 13,000 × g for 20 minutes at 4°C. The concentration of the soluble proteins was determined using the Bio-Rad protein assay, proteins (100 μg/lane) were mixed with 2× Laemmli buffer, heated to 95°C for 5 minutes and separated by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by electrophoretic transfer to polyvinylidene difluoride (Immobilon-P) membranes (Millipore, Bedford, MA). The membranes were blocked with TBST [10 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 0.05% (v/v) Tween 20] with 5% nonfat dry milk for 1 hour at room temperature. Immunoblots were incubated with primary antibodies and the appropriate secondary antibodies according to the manufacturers’ instructions and the immune complexes were detected using SuperSignal Chemiluminescent Substrate (Pierce, Rockford, IL) and autoradiography. The relative amount of iNOS protein in each lane was determined with a densitometer.
EPOR Expression in Wound Macrophages and Immunofluorescence Studies
Immunostaining was performed on sections (5 μm) of formalin-fixed, paraffin-embedded granulation tissues from F-ZCs. EPOR expression was analyzed using polyclonal rabbit anti-mouse antibody against EPOR (M-20) and EPOR immunoreactivity was confirmed with a second EPOR antibody (C-20) that has been validated previously. 7,31-33 Both antibodies were incubated overnight at 4°C at a dilution of 1:50 and visualized with horseradish peroxidase/diaminobenzidine and counterstained with hematoxylin. Monoclonal ED-1 antibody (1:50) was used to label wound macrophages. Controls were treated with rabbit or mouse IgG instead of primary antibody and were negative in any reactivity. For immunofluorescence studies, double labeling of wound macrophages was performed with anti-EPOR (M-20) and the macrophage marker ED-1. Fluorescein isothiocyanate-conjugated donkey anti-rabbit secondary antibody (1:200; Jackson Laboratories, Bar Harbor, ME) was used to visualize EPOR and rhodamine red-conjugated donkey anti-mouse antibody (1:200, Jackson Laboratories) was used to visualize ED-1. Fluorescence microscopy was performed under ×630 magnification using Zeiss Axioskop 2 and digital images were captured using KS300 imaging software.
Statistical Analyses
GraphPad InStat version 3.00 for Windows (San Diego, CA) software was used for all analyses. The data are expressed as mean ± SE. Every granulation tissue and MVD measurement was included in the statistical analyses. For comparison of two groups, Student’s t-tests or nonparametric Mann-Whitney tests were performed as indicated. When more than two groups were compared, analysis of variance was performed followed by Bonferroni multiple comparisons post hoc test. P < 0.05 was considered statistically significant.
Results
Recombinant EPO Promotes Wound Healing
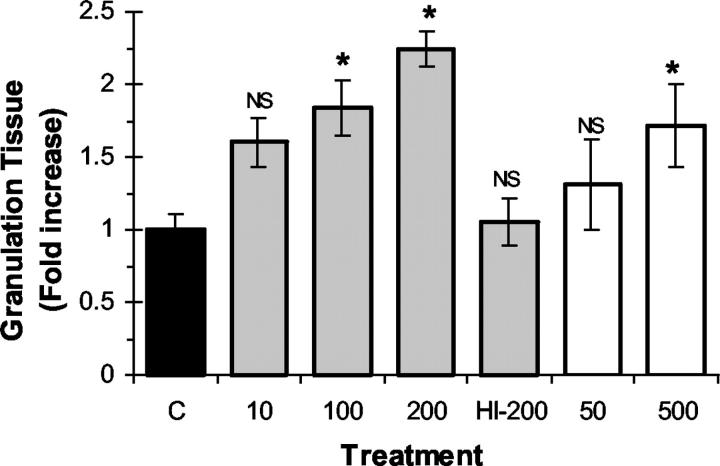
We first investigated the effect of local recombinant EPO administration on wound-healing response in F-ZCs. In this model, the fibrin inside the Z-chamber initiates a wound-healing response that is associated with stimulation of angiogenesis and the production of granulation tissue that can be quantitated. 22-24 To test the hypothesis that EPO may enhance wound-healing response, recombinant EPO was administered at incremental concentrations into F-ZCs that were surgically implanted in the subcutaneous tissues of rats. As negative controls, vehicle-only or heat-inactivated EPO were placed into F-ZCs. To assess wound-healing response, we measured the thickness of granulation tissue that developed in the chambers in the presence or absence of EPO. In a separate experiment, we tested the effect of bFGF, a proangiogenic growth factor that is known to promote wound healing 26 and found that local bFGF administration (1 μg/ml) into F-ZCs resulted in an ∼3.5-fold increase in granulation tissue thickness from 159.5 ± 9.8 μm in controls to 567 ± 43.1 μm in bFGF-treated chambers at day 6 (P < 0.05, Mann-Whitney U = 16.00, n = 4 in each group). Figure 1 ▶ illustrates representative photomicrographs of the effect of EPO on granulation tissue thickness in F-ZC at day 6 of wound healing (compare B and C). In the presence of EPO, granulation tissue thickness increased at all concentrations tested in a dose-dependent manner. The summary of measurements performed on paraffin-embedded F-ZCs is shown in Table 1 ▶ and quantitative representation of the effect of EPO on granulation tissue formation is illustrated in Figure 2 ▶ . In negative control F-ZCs containing vehicle only, the mean granulation tissue thickness was 150.6 ± 16.8 μm. Local administration of high-dose mEPO (200 U/ml) was associated with a 2.2-fold increase in granulation tissue thickness to 338 ± 17.2 μm (P < 0.001). Human recombinant EPO also promoted fibrin-induced wound healing associated with a dose-dependent, significant increase in granulation tissue thickness to 285 ± 42.8 μm compared to controls (P < 0.05). As a negative control using heat-inactivated EPO, the granulation tissue thickness was 158.4 ± 23.8 μm, not different from controls with vehicle only (Table 1 ▶ and Figure 2 ▶ ). Taken together, these experimental data demonstrate for the first time the ability of local, one time EPO administration to stimulate fibrin-induced wound-healing response.
Table 1.
Summary of Measurements Performed on Paraffin-Embedded F-ZCs in Response to Recombinant EPO Administration
| Treatment | GT (μm) | Difference (GT) | MVD | Difference (MVD) |
|---|---|---|---|---|
| Control | 150.6 ± 16.8 | N/A, n = 15 | 12.94 ± 0.39 | N/A, n = 18 |
| mEPO (10) | 240.8 ± 25.6 | 60%, NS, n = 15 | 25.83 ± 0.8 | 93%, P < 0.001, n = 18 |
| mEPO (100) | 277.4 ± 28.6 | 84%, P < 0.01, n = 15 | 24.05 ± 1.1 | 86%, P < 0.001, n = 18 |
| mEPO (200) | 338 ± 17.2 | 124%, P < 0.001, n = 15 | 25.17 ± 1.19 | 95%, P < 0.001, n = 18 |
| mEPO-HI (200) | 158.4 ± 23.8 | 5%, NS, n = 12 | 14.06 ± 1.72 | 8%, NS, n = 18 |
| hEPO (50) | 197.6 ± 46.2 | 31%, NS, n = 8 | 20.67 ± 1.53 | 60%, P < 0.001, n = 9 |
| hEPO (500) | 285 ± 42.8 | 89%, P < 0.05, n = 8 | 26.22 ± 1.52 | 102%, P < 0.001, n = 9 |
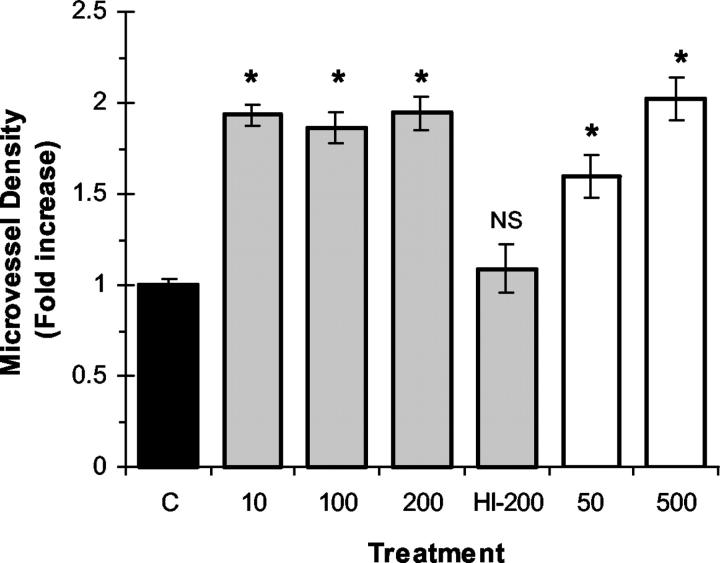
The concentration of recombinant EPO in each treatment group is indicated in brackets (U/ml). Data represents mean ± SE values. The difference from controls of each granulation tissue thickness (GT) and microvessel density (MVD) measurement is indicated as percentage of increase from control. Statistical analysis (ANOVA) was performed as described in Materials and Methods and P < 0.05 is considered statistically significant. The number of F-ZCs analyzed for granulation tissue thickness (GT) and total number of hot-spots counted for estimation of MVD are indicated in each group (n). mEPO; mouse EPO; hEPO; human EPO; N/A, not applicable; NS; not significant; mEPO-HI; heat-inactivated EPO-negative control.
Figure 2.
Effect of recombinant EPO on granulation tissue formation during wound healing. F-ZCs were harvested at day 6 after implantation and granulation tissue formation was measured as described in Materials and Methods. The granulation tissue thickness in each treatment group was expressed as fold change from control (y axis). mEPO (gray bars) was administered into F-ZCs at the indicated concentrations ranging from 10 to 200 U/ml and hEPO (white bars) was administered at concentrations of 50 and 500 U/ml. The negative control chambers (C) contained vehicle (black bar) or 200 U/ml of heat-inactivated mEPO (HI-200). The mean values (±SE) are shown. *, P < 0.05 (analysis of variance). NS, not significant. Table 1 ▶ illustrates the measurement data, number of measurements in each group, and the P values.
Soluble EPOR and Anti-EPO Antibodies Delay Wound-Healing Response
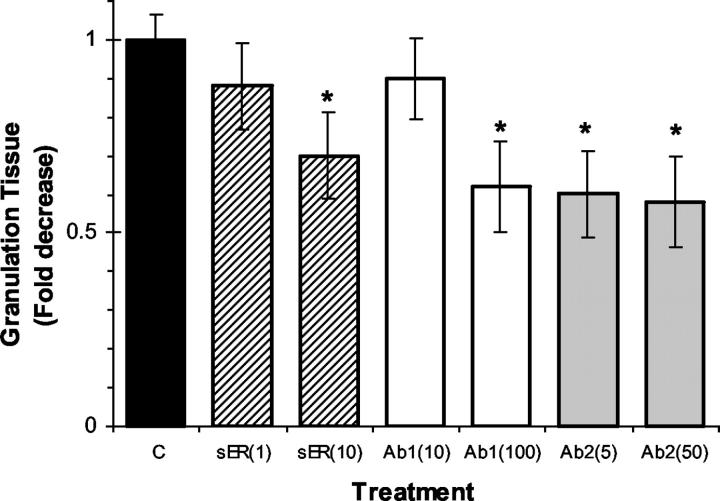
We then investigated the effect of EPO antagonists on wound-healing response. We hypothesized that if endogenous EPO is involved in the wound-healing cascade, administration of compounds into F-ZCs that scavenge endogenous EPO may be associated with partial inhibition of wound-healing response. To examine the role of endogenous EPO, we administered sER or one of two different anti-EPO mAbs into F-ZCs. The wound-healing response was assessed by measuring granulation tissue thickness in the chambers at day 9 after implantation of the chambers in rats. We selected day 9 as F-ZC harvest time because in previous experiments we had observed that the negative controls approach their peak granulation tissue formation at day 9, enabling us to demonstrate any delay in wound healing in response to EPO-EPOR inhibition. Two different concentrations were tested for each compound in F-ZCs. The summary of measurements performed on paraffin-embedded F-ZCs are presented in Table 2 ▶ and quantitative representation of the effects on granulation tissue formation is shown in Figure 3 ▶ . As indicated in Table 2 ▶ , sER administration was associated with a dose-dependent, significant, 30% decrease in granulation tissue thickness from 585.4 ± 37.2 μm in controls to 411.4 ± 65 μm (P < 0.05; compare Figure 1, D and E ▶ ). Local administration of mAbs against EPO was also associated with decreased granulation tissue thickness (Table 2) ▶ . Representative photomicrographs are shown in Figure 1 ▶ (compare D and F). After the administration of mAb 2871, we observed a concentration-dependent 37% decrease in granulation tissue thickness to 368.4 ± 68 μm (P < 0.01) and in the presence of the second antibody mAb 287, granulation tissue thickness decreased significantly to 342.8 ± 68.8 μm, a reduction of 41% compared to controls (P < 0.01). These data indicate that local administration of sER or anti-EPO antibodies results in partial inhibition of wound-healing response at day 9. The significant inhibitory effects on granulation tissue formation were observed after only a one time administration of the compounds. These findings demonstrate an important role for endogenous EPO in the physiological wound-healing cascade.
Table 2.
Summary of Measurements Performed on Paraffin-Embedded F-ZCs in Response to Soluble EPOR (sER) and Monoclonal Anti-EPO Antibody Administration
| Treatment | GT (μm) | Difference |
|---|---|---|
| Control | 585.4 ± 37.2 | N/A, n = 11 |
| sER (1) | 520 ± 66 | 11%, NS, n = 6 |
| sER (10) | 411.4 ± 65 | 30%, P < 0.05, n = 7 |
| mAb 2871 (10) | 528.4 ± 61.4 | 10%, NS, n = 7 |
| mAb 2871 (100) | 368.4 ± 68 | 37%, P < 0.01, n = 7 |
| mAb 287 (5) | 351.4 ± 64.4 | 40%, P < 0.01, n = 7 |
| mAb 287 (50) | 342.8 ± 68.8 | 41%, P < 0.01, n = 7 |
The final concentration of compound in each treatment group is indicated in brackets (μg/ml). Granulation tissue (GT) measurement data is presented with mean ± SE values. The difference from control of each GT measurement is indicated as percentage of decrease from control. Statistical analysis (ANOVA) was performed as described in Materials and Methods and P < 0.05 is considered statistically significant. The numbers of F-ZCs analyzed for granulation tissue thickness are indicated in each group (n). N/A, not applicable; NS, not significant.
Figure 3.
Effect of soluble EPOR and anti-EPO antibodies on granulation tissue formation during wound healing. Soluble EPOR (sER, patterned bars), monoclonal anti-EPO antibodies mAb 2871 (Ab1, white bars), and mAb 287 (Ab2, gray bars) were administered once, locally into F-ZCs at final concentrations indicated in brackets (μg/ml). Control chambers (C) contained vehicle alone (black bar). F-ZCs were harvested at day 9 and granulation tissue thickness in each chamber was measured and expressed as fold decrease from control (y axis). Mean values (±SE) are shown. *, P < 0.05 (analysis of variance). Table 2 ▶ illustrates the measurement data, number of measurements in each group, and P values.
Prohealing Effect of EPO Is Associated with Increased Angiogenesis
We investigated whether the EPO-mediated increase in wound-healing response may be associated with enhanced angiogenesis as measured by MVD in granulation tissue. Tissue sections from granulation tissue that developed within F-ZCs were evaluated for blood vessels by immunohistochemistry to estimate MVD as described in Materials and Methods. In a separate experiment as a positive control, administration of the proangiogenic growth factor bFGF (1 μg/ml) into F-ZCs resulted in an increase in MVD from 13.83 ± 1.3 in untreated controls to 26.16 ± 1.4 in bFGF-treated chambers at day 6 (P < 0.0001 by t-test, n = 12 in each group). Representative photomicrographs illustrating the effect of EPO on MVD are presented in Figure 1 ▶ (compare G and H), summary of MVD measurements are presented in Table 1 ▶ and quantitative representation of MVD data are illustrated in Figure 4 ▶ . Local administration of EPO in F-ZCs resulted in significantly increased MVD in granulation tissue compared to controls at day 6 (P < 0.001). The MVD in granulation tissue of negative controls containing vehicle was 12.94 ± 0.39 (Table 1) ▶ . In response to local EPO administration, we observed an increase in MVD to 25.17 ± 1.19 with mouse EPO and to 26.22 ± 1.52 with human EPO (P < 0.001). As a negative control using heat-inactivated EPO, the MVD was 14.06 ± 1.72, not significantly different from controls without added EPO (Table 1 ▶ and Figure 4 ▶ ). Taken together, these experimental results indicate that the ability of EPO to promote wound healing is associated with a significant increase of MVD in granulation tissue.
Figure 4.
Effect of recombinant EPO on angiogenesis during wound healing. mEPO (gray bars) was administered locally into F-ZCs at the indicated concentrations ranging from 10 to 200 U/ml and hEPO (white bars) was administered at 50 and 500 U/ml. The negative control chambers (C) contained vehicle (black bar) or 200 U/ml of heat-inactivated mEPO (HI-200). The F-ZCs were harvested at day 6 after implantation, MVD in granulation tissues was determined as described in Materials and Methods and expressed as fold increase from controls (y axis). Data represents mean values (±SE). *, P < 0.05 (analysis of variance). NS, not significant. Table 1 ▶ illustrates the MVD data, number of measurements in each group, and P values.
EPOR Is Expressed in Wound Macrophages
We investigated the expression of EPOR protein in granulation tissue that developed during wound healing by immunohistochemistry using anti-EPOR polyclonal antibodies as described in Materials and Methods. We found strong EPOR immunoreactivity not only in vascular endothelial cells, but interestingly in macrophages in granulation tissue (Figure 1I) ▶ , cells that play a pivotal role during wound healing, confirmed by immunostaining for macrophage marker ED-1 (Figure 1J) ▶ . Expression of EPOR in wound macrophages was further validated by performing double immunofluorescence for macrophage marker and EPOR on the same granulation tissue section. Figure 1, K and L ▶ , illustrates representative images of a wound macrophage exhibiting both EPOR (Figure 1K) ▶ and ED-1 expression (Figure 1L) ▶ . Wound macrophage EPOR expression is a unique finding that has not been reported previously.
Recombinant EPO Administration and Endogenous EPO-EPOR Inhibition Modulate iNOS Expression during Wound Healing
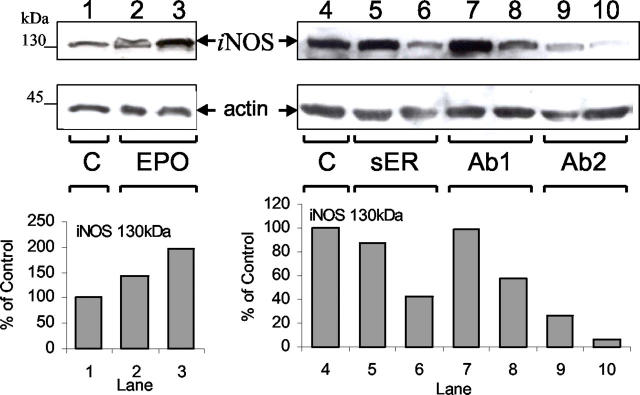
The inducible isoform of nitric oxide synthase, iNOS, plays an important role during wound healing 34 and we investigated whether the modulation of wound healing in response to recombinant EPO administration or endogenous EPO-EPOR inhibition was associated with changes in iNOS protein levels in granulation tissue. Granulation tissues from EPO-treated, EPO-EPOR antagonist-treated, and untreated control F-ZCs were solubilized and analyzed by immunoblotting for iNOS expression. Figure 5 ▶ illustrates the results of representative Western blot assays for iNOS expression in granulation tissue. The enhanced wound healing because of recombinant EPO administration on day 6 was associated with a concentration-dependent increase in iNOS protein (Figure 5 ▶ , lanes 1 to 3). Densitometric analysis revealed an increase of 43% with low-dose EPO (Figure 5 ▶ , lane 1) and 97% with high-dose EPO (Figure 5 ▶ , lane 3) compared to untreated control (Figure 5 ▶ , lane 1). Conversely, the significant decrease in granulation tissue formation in response to EPO-EPOR inhibition on day 9 using sER or anti-EPO monoclonal antibodies was associated with a concentration-dependent reduction in iNOS levels as illustrated in Figure 5 ▶ (lanes 4 to 10). The primary source of iNOS in granulation tissue are macrophages, 35 and a possible mechanism for changes in iNOS levels in granulation tissue may involve an increase or decrease in macrophage influx associated with EPO administration or EPO-EPOR inhibition, respectively. To determine whether the observed modulation of iNOS levels were a function of the number of macrophages in the granulation tissue, we quantitated macrophage influx using ED-1 immunostaining of granulation tissue sections from EPO treatment, EPO-EPOR inhibition and control groups. The mean (± SE) macrophage numbers in granulation tissues at day 6 were similar in EPO-treated (500 U/ml) samples (97 ± 6/high-power field, n = 12) and untreated negative controls (99 ± 7/high-power field, n = 12). We also found that the reduction in iNOS levels associated with administration of EPO-EPOR inhibitors and decreased granulation tissue formation was not because of decreased macrophage influx. In granulation tissues at day 9, macrophage influx per high-power field in untreated controls was 92 ± 5.6, not significantly different from macrophage influx after administration of high concentrations of sER (93 ± 5.9), mAb 2871 (87 ± 3.8), and mAb 287 (90 ± 5.8) (n = 12 in each group). These findings indicate that modulation of iNOS associated with administration of recombinant EPO or inhibition of EPO-EPOR during wound healing is not because of changes in macrophage influx into granulation tissue, the primary source of iNOS.
Figure 5.
Immunoblot for iNOS expression in granulation tissue during wound healing. Granulation tissues from F-ZCs were harvested at day 6 after administration of EPO (left, lanes 1 to 3) or day 9 after soluble EPOR (sER), mAb 2871 (Ab1), or mAb 287 (Ab2) administration (right, lanes 4 to 10). Western blotting was performed as described in Materials and Methods. Lane 1, control day 6 (C); lane 2, EPO 50 U/ml; lane 3, EPO 500 U/ml; lane 4, control day 9 (C); lane 5, sER 1 μg/ml; lane 6, sER 10 μg/ml; lane 7, Ab1 10 μg/ml; lane 8, Ab1 100 μg/ml; lane 9, Ab2 5 μg/ml; and lane 10, Ab2 50 μg/ml. Comparable loading and integrity of proteins in each lane were confirmed by hybridization of an immunoblot to anti-actin antibody (bottom blots). Arrows indicate iNOS and actin immunoreactivity. Densitometric analysis for the 130-kd iNOS bands for this representative experiment are illustrated in the graphs below the blots.
Discussion
The present study demonstrates a novel nonhematopoietic function for EPO as a cytokine with an important role during wound healing. The diminished healing response after administration of EPO antagonists provides evidence for the involvement of an endogenous EPO-EPOR system in the wound-healing cascade. Our studies further show that local EPO administration significantly accelerates fibrin-induced wound-healing response. The ability of recombinant EPO to promote wound healing correlates with increased angiogenesis in granulation tissue suggesting that the prohealing effect of EPO is associated, at least in part, with its stimulatory effect on the proliferation and migration of endothelial cells during wound healing. It is important to note that the enhancement in wound healing and angiogenesis observed in our experiments was achieved with only a one time administration of EPO locally in the fibrin matrix of subcutaneous chambers. Whether systemic, intravascular EPO administration would augment wound healing and exhibit proangiogenic effects in distant peripheral tissues remains to be determined. This question is relevant because localized angiogenic responses have been reported to vary depending on the route of administration of angiogenic molecules. 16 For instance, although bFGF was reported to stimulate angiogenic responses when administered locally, systemic intravascular infusion did not promote endothelial mitogenesis and neovascularization. 36,37
Wound-healing response consists of two major components: angiogenesis mediated by endothelial cells and pericytes; and matrix formation orchestrated by fibroblasts and smooth muscle cells with macrophages, the major cell type in granulation tissue, playing a critical role in both regulation of angiogenesis and matrix formation. 20,28,38 In the present study, we show for the first time that macrophages express EPOR protein. Macrophages are responsible for production of cytokines and growth factors essential to the healing cascade and they are also the predominant cell type expressing iNOS in healing wounds. 35 The importance of iNOS expression during wound healing is highlighted by the phenotype of iNOS knockout mice that exhibit impaired wound-healing response associated with reduced epithelialization and wound contraction. 39 The delayed wound repair in iNOS-deficient mice was reversed after topical adenovirus-mediated iNOS gene transfer. 40 Furthermore, direct transfection of iNOS gene in cutaneous wounds enhanced wound strength, collagen synthesis, epithelialization, and wound contraction. 41 In our studies, we showed that the diminished wound-healing response with EPO-EPOR inhibition paralleled reduction in iNOS levels in granulation tissue and conversely, enhanced wound healing after recombinant EPO administration was associated with increased iNOS expression. Because macrophages are the main source of iNOS expression during wound healing, 35 we determined whether administration of recombinant EPO or inhibition of EPO-EPOR affected the influx of macrophages into the granulation tissue. We found that changes in iNOS levels could not be explained on the basis of differences in macrophage numbers in granulation tissue suggesting that mechanisms other than macrophage influx are involved in modulation of iNOS expression associated with EPO administration or EPO-EPOR inhibition during wound healing.
Recombinant human EPO has been widely used in clinical practice for the treatment or prevention of anemia associated with renal failure, cancer, chemo-radiotherapy, human immunodeficiency virus infection, and surgery. Our studies indicate a novel function for EPO by providing in vivo evidence for a physiological role as a proangiogenic cytokine that modulates wound-healing response. Given the excellent clinical safety profile of recombinant EPO and its ability to accelerate wound healing, EPO may be investigated in new clinical applications that are comparable, for instance, to the use of recombinant bFGF to facilitate wound healing 42,43 and in stimulation of therapeutic angiogenesis. 44 Furthermore, exploration of the functional significance of EPOR expression and signaling in macrophages and in disorders associated with unregulated, pathological angiogenesis such as that observed in cancer 33 and diabetic retinopathy 45 may provide insight into specific signaling pathways that mediate the diverse hematopoietic and nonhematopoietic effects of EPO.
Acknowledgments
We thank Dr. Charles S. Greenberg for helpful discussions and his support and encouragement during the initial stages of this study and Amgen for generously providing recombinant murine EPO.
Footnotes
Address reprint requests to Murat O. Arcasoy, M.D., F.A.C.P., Duke University School of Medicine, Department of Medicine, DUMC Box 3912, Durham, NC 27710. E-mail: arcas001@mc.duke.edu.
Supported in part by the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases grant DK-02566 to M. O. A.).
References
- 1.Krantz SB: Erythropoietin. Blood 1991, 77:419-434 [PubMed] [Google Scholar]
- 2.Wu H, Liu X, Jaenisch R, Lodish HF: Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell 1995, 83:59-67 [DOI] [PubMed] [Google Scholar]
- 3.Lin CS, Lim SK, D’Agati V, Costantini F: Differential effects of an erythropoietin receptor gene disruption on primitive and definitive erythropoiesis. Genes Dev 1996, 10:154-164 [DOI] [PubMed] [Google Scholar]
- 4.D’Andrea AD, Lodish HF, Wong GG: Expression cloning of the murine erythropoietin receptor. Cell 1989, 57:277-285 [DOI] [PubMed] [Google Scholar]
- 5.Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R: Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience 1997, 76:105-116 [DOI] [PubMed] [Google Scholar]
- 6.Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R: In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci USA 1998, 95:4635-4640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A: Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA 2000, 97:10526-10531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siren AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P: Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA 2001, 98:4044-4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Digicaylioglu M, Lipton SA: Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature 2001, 412:641-647 [DOI] [PubMed] [Google Scholar]
- 10.Westenfelder C, Biddle DL, Baranowski RL: Human, rat, and mouse kidney cells express functional erythropoietin receptors. Kidney Int 1999, 55:808-820 [DOI] [PubMed] [Google Scholar]
- 11.Ogilvie M, Yu X, Nicolas-Metral V, Pulido SM, Liu C, Ruegg UT, Noguchi CT: Erythropoietin stimulates proliferation and interferes with differentiation of myoblasts. J Biol Chem 2000, 275:39754-39761 [DOI] [PubMed] [Google Scholar]
- 12.Juul SE, Ledbetter DJ, Joyce AE, Dame C, Christensen RD, Zhao Y, DeMarco V: Erythropoietin acts as a trophic factor in neonatal rat intestine. Gut 2001, 49:182-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anagnostou A, Lee ES, Kessimian N, Levinson R, Steiner M: Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proc Natl Acad Sci USA 1990, 87:5978-5982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anagnostou A, Liu Z, Steiner M, Chin K, Lee ES, Kessimian N, Noguchi CT: Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci USA 1994, 91:3974-3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaji R, Okada T, Moriya M, Naito M, Tsuruo T, Miyatake K, Nakano Y: Brain capillary endothelial cells express two forms of erythropoietin receptor mRNA. Eur J Biochem 1996, 239:494-500 [DOI] [PubMed] [Google Scholar]
- 16.Folkman J, Shing Y: Angiogenesis. J Biol Chem 1992, 267:10931-10934 [PubMed] [Google Scholar]
- 17.Carlini RG, Reyes AA, Rothstein M: Recombinant human erythropoietin stimulates angiogenesis in vitro. Kidney Int 1995, 47:740-745 [DOI] [PubMed] [Google Scholar]
- 18.Ribatti D, Presta M, Vacca A, Ria R, Giuliani R, Dell’Era P, Nico B, Roncali L, Dammacco F: Human erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood 1999, 93:2627-2636 [PubMed] [Google Scholar]
- 19.Yasuda Y, Masuda S, Chikuma M, Inoue K, Nagao M, Sasaki R: Estrogen-dependent production of erythropoietin in uterus and its implication in uterine angiogenesis. J Biol Chem 1998, 273:25381-25387 [DOI] [PubMed] [Google Scholar]
- 20.Martin P: Wound healing—aiming for perfect skin regeneration. Science 1997, 276:75-81 [DOI] [PubMed] [Google Scholar]
- 21.Haroon ZA, Peters KG, Greenberg CS, Dewhirst MW: Angiogenesis and oxygen transport in solid tumors. Teicher BA eds. Antiangiogenic Agents in Cancer Therapy. 1999:pp 3-21 Humana Press Inc. Totowa
- 22.Haroon ZA, Amin K, Saito W, Wilson W, Greenberg CS, Dewhirst MW: SU5416 delays wound healing through inhibition of TGF-beta 1 activation. Cancer Biol Ther 2002, 1:121-126 [DOI] [PubMed] [Google Scholar]
- 23.Dvorak HF, Harvey VS, Estrella P, Brown LF, McDonagh J, Dvorak AM: Fibrin containing gels induce angiogenesis. Implications for tumor stroma generation and wound healing. Lab Invest 1987, 57:673-686 [PubMed] [Google Scholar]
- 24.Brown LF, Dvorak AM, Dvorak HF: Leaky vessels, fibrin deposition, and fibrosis: a sequence of events common to solid tumors and to many other types of disease. Am Rev Respir Dis 1989, 140:1104-1107 [DOI] [PubMed] [Google Scholar]
- 25.Rohr S, Toti F, Brisson C, Albert A, Freund M, Meyer C, Cazenave JP: Quantitative image analysis of angiogenesis in rats implanted with a fibrin gel chamber. Nouv Rev Fr Hematol 1992, 34:287-294 [PubMed] [Google Scholar]
- 26.Broadley KN, Aquino AM, Woodward SC, Buckley-Sturrock A, Sato Y, Rifkin DB, Davidson JM: Monospecific antibodies implicate basic fibroblast growth factor in normal wound repair. Lab Invest 1989, 61:571-575 [PubMed] [Google Scholar]
- 27.Haroon ZA, Hettasch JM, Lai TS, Dewhirst MW, Greenberg CS: Tissue transglutaminase is expressed, active, and directly involved in rat dermal wound healing and angiogenesis. EMBO J 1999, 13:1787-1795 [DOI] [PubMed] [Google Scholar]
- 28.Haroon ZA, Raleigh JA, Greenberg CS, Dewhirst MW: Early wound healing exhibits cytokine surge without evidence of hypoxia. Ann Surg 2000, 231:137-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haroon ZA, Wannenburg T, Gupta M, Greenberg CS, Wallin R, Sane DC: Localization of tissue transglutaminase in human carotid and coronary artery atherosclerosis: implications for plaque stability and progression. Lab Invest 2001, 81:83-93 [DOI] [PubMed] [Google Scholar]
- 30.Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G: Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst 1992, 84:1875-1887 [DOI] [PubMed] [Google Scholar]
- 31.Juul SE, Yachnis AT, Christensen RD: Tissue distribution of erythropoietin and erythropoietin receptor in the developing human fetus. Early Hum Dev 1998, 52:235-249 [DOI] [PubMed] [Google Scholar]
- 32.Fairchild Benyo D, Conrad KP: Expression of the erythropoietin receptor by trophoblast cells in the human placenta. Biol Reprod 1999, 60:861-870 [DOI] [PubMed] [Google Scholar]
- 33.Arcasoy MO, Amin K, Karayal AF, Chou SC, Raleigh JA, Varia MA, Haroon ZA: Functional significance of erythropoietin receptor expression in breast cancer. Lab Invest 2002, 82:911-918 [DOI] [PubMed] [Google Scholar]
- 34.Witte MB, Barbul A: Role of nitric oxide in wound repair. Am J Surg 2002, 183:406-412 [DOI] [PubMed] [Google Scholar]
- 35.Reichner JS, Meszaros AJ, Louis CA, Henry WL, Jr, Mastrofrancesco B, Martin BA, Albina JE: Molecular and metabolic evidence for the restricted expression of inducible nitric oxide synthase in healing wounds. Am J Pathol 1999, 154:1097-1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whalen GF, Shing Y, Folkman J: The fate of intravenously administered bFGF and the effect of heparin. Growth Factors 1989, 1:157-164 [DOI] [PubMed] [Google Scholar]
- 37.Folkman J: Therapeutic angiogenesis in ischemic limbs. Circulation 1998, 97:1108-1110 [DOI] [PubMed] [Google Scholar]
- 38.Lingen MW: Role of leukocytes and endothelial cells in the development of angiogenesis in inflammation and wound healing. Arch Pathol Lab Med 2001, 125:67-71 [DOI] [PubMed] [Google Scholar]
- 39.Shi HP, Efron DT, Most D, Tantry US, Barbul A: Supplemental dietary arginine enhances wound healing in normal but not inducible nitric oxide synthase knockout mice. Surgery 2000, 128:374-378 [DOI] [PubMed] [Google Scholar]
- 40.Yamasaki K, Edington HD, McClosky C, Tzeng E, Lizonova A, Kovesdi I, Steed DL, Billiar TR: Reversal of impaired wound repair in iNOS-deficient mice by topical adenoviral-mediated iNOS gene transfer. J Clin Invest 1998, 101:967-971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thornton FJ, Schaffer MR, Witte MB, Moldawer LL, MacKay SL, Abouhamze A, Tannahill CL, Barbul A: Enhanced collagen accumulation following direct transfection of the inducible nitric oxide synthase gene in cutaneous wounds. Biochem Biophys Res Commun 1998, 246:654-659 [DOI] [PubMed] [Google Scholar]
- 42.Robson MC, Phillips LG, Lawrence WT, Bishop JB, Youngerman JS, Hayward PG, Broemeling LD, Heggers JP: The safety and effect of topically applied recombinant basic fibroblast growth factor on the healing of chronic pressure sores. Ann Surg 1992, 216:401-406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu X, Shen Z, Chen Y, Xie J, Guo Z, Zhang M, Sheng Z: Randomised placebo-controlled trial of use of topical recombinant bovine basic fibroblast growth factor for second-degree burns. Lancet 1998, 352:1661-1664 [DOI] [PubMed] [Google Scholar]
- 44.Lederman RJ, Mendelsohn FO, Anderson RD, Saucedo JF, Tenaglia AN, Hermiller JB, Hillegass WB, Rocha-Singh K, Moon TE, Whitehouse MJ, Annex BH: Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet 2002, 359:2053-2058 [DOI] [PubMed] [Google Scholar]
- 45.Junk AK, Mammis A, Savitz SI, Singh M, Roth S, Malhotra S, Rosenbaum PS, Cerami A, Brines M, Rosenbaum DM: Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc Natl Acad Sci USA 2002, 99:10659-10664 [DOI] [PMC free article] [PubMed] [Google Scholar]