Abstract
Several recent studies have focused on similarities between glomerular podocytes and neurons because the two cells share a specialized cytoskeletal organization and several expression-restricted proteins, such as nephrin and synaptopodin. In neurons, the small guanosine triphosphatase Rab3A and its effector rabphilin-3A form a complex required for the correct docking of synaptic vesicles to their target membrane. Because rabphilin-3A binds in neurons to cytoskeletal proteins also important for podocyte homeostasis, and the complex rabphilin-3A-Rab3A has been demonstrated in neurons and neuroendocrine cells, the aim of our work was to investigate their possible expression and regulation in podocytes. Normal kidneys from mouse, rat, and human were studied by immunohistochemistry, Western blotting, and reverse transcriptase-polymerase chain reaction to evaluate the expression of Rab3A and rabphilin-3A. Double-staining immunohistochemistry and immunogold electron microscopy were then used to precisely localize the two proteins at the cellular and subcellular levels. Rab-3A and rabphilin-3A regulations in disease were then analyzed in growth hormone-transgenic mice, a well established model of focal and segmental glomerulosclerosis, and in human biopsies from proteinuric patients. Our results demonstrated that rabphilin-3A and Rab3A are present in normal mouse, rat, and human kidneys, with an exclusively glomerular expression and a comma-like pattern of positivity along the glomerular capillary wall, suggestive for podocyte staining. Co-localization of both molecules with synaptopodin confirmed their presence in podocytes. By immunogold electron microscopy both proteins were found around vesicles contained in podocyte foot processes. Their expression was increased in growth hormone-transgenic mice compared to their wild-type counterpart, and in a subset of biopsies from proteinuric patients. Our data, demonstrating the presence of two synaptic proteins in podocytes, further supports similarities between cytoskeletal and vesicular organization of podocytes and neurons. The altered expression observed in mouse and human proteinuric diseases suggests a possible role for these molecules in glomerulopathies.
Glomerular podocytes, the last barrier of glomerular filtration, are highly specialized branched cells provided with interdigitating foot processes that externally cover the entire glomerular capillary surface. Recent advances in cell biology and genetics have expanded our knowledge about these cells, especially through the identification of several functionally important specific podocyte proteins. 1 Some of these proteins, such as nephrin, GLEPP-1 (glomerular epithelial protein-1), synaptopodin, and the amino acid transporters CAT3 (cationic amino acid transporter-3) and EAAT2 (excitatory amino acid transporter-2), have been found to be specifically shared by podocytes and neurons, 2-5 two process-bearing cells that, although derived from different embryological layers, have several features in common, 6 such as a high level of differentiation and specialization and a similar cytoskeletal organization. Moreover, they possess cell-type-specific intercellular contacts: slit membranes in podocytes and synapses in neurons.
Rabphilin-3A is a synaptic vesicle protein, first discovered as a binding partner and effector of Rab3A, a member of the Rab family of guanosine triphosphate (GTP) hydrolases (G proteins). 7 Rabphilin-3A binds to Rab3A only in its GTP-bound state, and the complex is required for the correct docking of synaptic vesicles to their target membrane. 8 Binding of rabphilin-3A to Rab3A occurs via the amino (NH2)-terminal half of rabphilin-3A. In absence of Rab3A, rabphilin-3A can bind to the cytoskeletal protein α-actinin increasing its actin filament bundling activity. 9 The carboxy (COOH) terminus of rabphilin-3A contains two C2 (protein kinase C-homology-2) domains (C2A and C2B) that bind to calcium and phospholipids and are homologous to the C2 domains of synaptotagmin. 10 In addition, the C2 domains can bind to another cytoskeletal protein, β-adducin, an actin-binding molecule that acts in the assembly of spectrin-actin complexes at the plasma membrane. 11 These biochemical properties of rabphilin-3A, in particular its ability to bind calcium, phospholipids, and cytoskeletal proteins, and the demonstration of the Rab3A-rabphilin-3A complex in synapses and neuroendocrine cells, prompted our attention in considering these proteins as possible players in podocyte biology.
Here we demonstrate that rabphilin-3A and Rab3A are present in the kidney and specifically localize in podocytes. Moreover, their expression is altered in mouse and human proteinuric diseases, suggesting their possible role in glomerulopathies.
Materials and Methods
Materials
Normal kidney and normal brain tissue were obtained from 3-month-old animals, namely five Sprague-Dawley rats, five 129/SVLMJ mice, four CD-1 mice [the wild-type (WT) littermates of growth hormone (GH)-transgenics; average weight, 43.6 ± 2.0 g; average urine albumin/urine creatinine, 28.0 ± 10.2 μg/mg]. Normal human kidney was from normal areas of 10 human kidneys uninvolved by neoplasia from tumor nephrectomies. Diseased kidney samples were from four 3-month-old GH-transgenic mice (average weight, 70.6 ± 5.2 g; average urine albumin/urine creatinine, 8560 ± 5838 μg/mg) 12 and 15 biopsies of patients with proteinuric diseases (five minimal change disease, five primary membranous nephropathy, five primary focal and segmental glomerulosclerosis).
Tissue samples for light microscopy were fixed in 4% buffered paraformaldehyde and embedded in paraffin. Routine stainings were performed on 2-μm-thick sections according to standard techniques. For immunohistochemistry, unfixed renal tissue was embedded in OCT (optimum cutting temperature cryoembedding matrix) (Miles Scientific, Naperville, IL), snap-frozen in a mixture of isopentane and dry-ice, and stored at −80°C. Subsequently, 5-μm kidney sections and 8-μm brain sections were placed on slides, fixed in cold acetone, and stored at −20°C until immunostained. For immunogold electron microscopy, normal rat tissue was fixed in a mixture of formaldehyde, glutaraldehyde, and phosphate buffer, soaked in glucose and frozen, or alternatively embedded in Lowicril K4M resin (Electron Microscopy Sciences, Società Italiana Chimici, Rome, Italy), as previously described. 13 Glomeruli from the remaining mouse and rat tissue were separated by filtering through 200-, 100-, and 70-μm sieves, whereas human glomeruli were manually microdissected from the normal kidneys. All glomeruli were alternatively stored at −20°C after dipping in RNA-Later (Ambion Inc., Austin, TX) for mRNA extraction, or at −80°C until proteins were extracted.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
RNA was isolated from mouse, rat, and human glomeruli and from rat and mouse brain using the RNAqueous kit (Ambion Inc.). All procedures were performed according to the manufacturer’s protocol: briefly, the extracted glomeruli were homogenized in lysing/binding solution, RNA was isolated from the lysate using a glass fibers filter that can bind specifically RNA and eluted from the filter using an elution buffer. Subsequently RNA was treated with DNase to remove traces of DNA contamination. RNA quality was tested by the Agilent 2001 Bioanalyzer (Agilent Technologies Italia, Cernusco sul Naviglio, Milan, Italy). mRNA was then reverse-transcribed using the Omniscript Reverse Transcriptase kit (Qiagen Inc., Milan, Italy) with Oligo dT Primer p(DT)15 (Roche Diagnostic Corp., Indianapolis, IN).
PCR was performed using TaqPCR Core Kit (Qiagen) in combination with the primers listed in Tables 1 and 2 ▶ (tested for specificity with the Nucleotide Blast from the NCBI homepage http://www.ncbi.nlm.nih.gov/BLAST/). In each species, rabphilin-3A sequence amplification was obtained by two primer pairs selected from both the NH2 and C2 domain sequences. Given the homology among Rab3 isoforms (A, B, C, D), Rab3A primers were designed from nonoverlapping fragments and PCR was conducted in stringent conditions. For both rabphilin-3A and Rab3A, to confirm the amplification of the desired sequence, nested-PCR followed the first round of amplification.
Table 1.
Rabphilin-3A Primer Sequences and Predicted Product Size
| Molecule | ID number | Primer sequence | Sequence position | Product size |
|---|---|---|---|---|
| Mouse rabphilin-3A (external, NH2) | D29965 | GTG AAC CGA TGG ATG TAC CC | 241–260 | 402 |
| GAT CTT GCA GAG CCA TAC CG | 623–642 | |||
| Mouse rabphilin-3A (internal, NH2) | GAC AGG CAG AGG AAG CAG GAA GA | 337–359 | 299 | |
| CAG AGC CAT ACC GGA TGC GGA | 615–635 | |||
| Mouse rabphilin-3A (external, C2) | GAT GAA GAG AGC AGG GAC CA | 1749–1768 | 504 | |
| TTC TCG TTC TGC AGT TGG TG | 2233–2252 | |||
| Mouse rabphilin-3A (internal, C2) | GTC AAG CTC TGG CTG AAA CC | 1954–1973 | 195 | |
| GCA GCC TCC GAT GTA ATC AT | 2129–2148 | |||
| Rat rabphilin-3A (external, NH2) | U12571 | GAC ACT GTG GTG AAC CGT TG | 289–308 | 503 |
| TTT ATA GGC ATG GGC TGT GG | 772–791 | |||
| Rat rabphilin-3A (internal, NH2) | GAC AGG CAG AGG AAG CAG GAA GA | 394–416 | 377 | |
| AGG ACC TGT TTT GGG AAA CC | 751–770 | |||
| Rat rabphilin-3A (external, C2) | TTT CCA GAG CAG AGC ACA GA | 1213–1232 | 506 | |
| CCA AAC TTG TCC TCG TCA CA | 1699–1718 | |||
| Rat rabphilin-3A (internal, C2) | TGA CCA AGA CAA CAG CAA CC | 1458–1477 | 222 | |
| TTC CTC TGC ATG TCC TCC TC | 1660–1679 | |||
| Human rabphilin-3A (external, NH2) | NM_014954 | GAG GAA GCA GGA AGA GCT GA | 461–480 | 377 |
| TAG GCT GTG GGA GGA CCT GT | 818–837 | |||
| Human rabphilin-3A (internal, NH2) | TGA GGA GAA AGA AAT CAT CA | 485–504 | 297 | |
| TTC CAC ACC TCC CTC TG | 765–781 | |||
| Human rabphilin-3A (external, C2) | CTC CGT CTG TGA TGA GGA CA | 1769–1788 | 504 | |
| TTG GAC TTG CCG ATG TCA TA | 2253–2272 | |||
| Human rabphilin-3A (internal, C2) | AGC CCA ACC AGA GGA AGA AT | 1843–1862 | 205 | |
| CGT ATG ATG CCC ACA ATG AG | 2028–2047 |
PCR amplifications of the extracted mRNAs without RT revealed no PCR product, excluding amplification of genomic DNA, and were taken as negative controls. Amplification products obtained from rat and mouse normal brain cDNAs and amplification of the housekeeping genes, GAPDH and β-actin, were run together and taken as positive controls.
Western Blot
Glomerular proteins from rat and mouse glomeruli were extracted by lysis with a detergent-based buffer. Total glomerular proteins (100 μg) were run on a 12% sodium dodecyl sulfate-polyacrylamide electrophoresis gel and transferred to a nitrocellulose membrane (HybondECL, Amersham Biosciences) by electroblotting. Membranes were stained with Ponceau Red to control for adequate transfer and equally loaded amounts. After blocking, each membrane was incubated with the following primary antibodies: polyclonal rabbit anti-rabphilin-3A (BD Transduction Laboratories, Milan, Italy) and polyclonal rabbit anti-Rab3A (Synaptic Systems, Goettingen, Germany) followed by the proper secondary antibody (horseradish peroxidase-linked sheep anti-rabbit) (Amersham Biosciences). Positive reaction products were identified by enhanced chemiluminescence (ECL, Amersham) and autoradiography. Positive controls were performed using a brain protein extract provided together with the polyclonal anti-rabphilin antibody from BD Transduction Laboratories. Negative controls were performed by loading buffer instead of proteins on the sodium dodecyl sulfate-polyacrylamide electrophoresis gel or by substituting buffer or proper control immunoglobulins (ie, immunoglobulins of the same class and at the same concentration of the primary antibody) (Zymed) for the primary antibody. Positive and negative controls were run concurrently.
Immunohistochemistry
Immunohistochemistry was performed on 5-μm-thick acetone-fixed kidney sections, on 8-μm-thick brain sections, and on 1-μm-thick semithin sections. Briefly, rat and human material were sequentially hydrated, incubated with the primary monoclonal mouse anti-rabphilin-3A (BD Transduction Laboratories) and monoclonal mouse anti-Rab3A (Synaptic Systems), then by the secondary fluorescein isothiocyanate-labeled or cyanine3 (Cy3)-labeled goat anti-mouse secondary antibody (Zymed, Histoline, Milan, Italy).
To avoid nonspecific staining, a direct immunofluorescence was performed in mouse specimens after directly conjugating the primary monoclonal antibodies with fluorescent markers (Alexa Fluor 488 and 568) using the Zenon One labeling kit (Molecular Probes Europe BV, Leiden, The Netherlands) according to the manufacturer’s instructions. Similarly, for double staining, a direct immunofluorescence was conducted using alternatively in sequence two of the following labeled monoclonals: mouse anti-rabphilin-3A, mouse anti-Rab3A, and the podocyte-specific mouse anti-synaptopodin (Progen Biotechnik GmbH, Heidelberg, Germany). Nuclear counterstaining was performed by 4′,6-diamidino-2-phenyindole (Sigma Chimica, Milan, Italy). Analysis of double-staining and co-localization studies were conducted on digitized images by Q-Fluor software (Leica Italia, Milan, Italy).
Specificity of antibody labeling was demonstrated by the lack of staining after applying as primary antibody a monoclonal mouse anti-synaptotagmin (BD Transduction Laboratories), chosen because of the high homology of the C2 domains of synaptotagmin and rabphilin-3A, or after substituting proper control immunoglobulins (Zymed) for the primary antibody. Positive controls were performed by applying anti-rabphilin-3A and Rab-3A on brain sections. To test the immunoreactivity of the tissue, a monoclonal mouse anti-vimentin antibody (Zymed), that stains glomeruli and vessels, was also applied on kidney sections. Positive and negative controls were run concurrently. Slides were mounted with Vectashield aqueous mounting medium (Vector Laboratories, DBA Italia SRL, Milan, Italy).
A semiquantitative evaluation (0 = negative, 1 = mild positivity, 2 = intense positivity) of rabphilin-3A and Rab3A staining was performed in mouse WT and GH-transgenic animals by scoring 30 glomeruli per section. The same semiquantitative score was applied to evaluate Rab3A positivity in all human renal tissues on an average of 9.3 ± 1.7 glomeruli/biopsy.
Immunogold Electron Microscopy
An indirect immunogold labeling procedure was performed on ultrathin sections, as previously described. 13 Briefly, after blocking, the material was incubated with the primary monoclonal mouse anti-rabphilin-3A, and monoclonal mouse anti-Rab3A, then by the secondary 10-nm gold-conjugated goat anti-mouse secondary antibody (Aurion, DBA, Milan, Italy). Specificity of antibody labeling was demonstrated by the lack of staining after applying as primary antibody a monoclonal mouse anti-synaptotagmin, or after substituting proper controlimmunoglobulins (Zymed) for the primary antibody. As positive controls, anti-rabphilin-3A and anti-Rab3A were applied on brain tissues. Furthermore, to test the reactivity of kidney tissue, a monoclonal mouse anti-synaptopodyn antibody (Zymed), that stains glomerular podocytes, was also applied. Positive and negative controls were run together.
Results
By RT-PCR and nested-PCR, we demonstrated mRNA of both rabphilin-3A and Rab3A in normal mouse, rat, and human glomeruli (Figure 1) ▶ . The glomerular presence of both proteins was then detected by immunoblot (Figure 2) ▶ .
Figure 1.
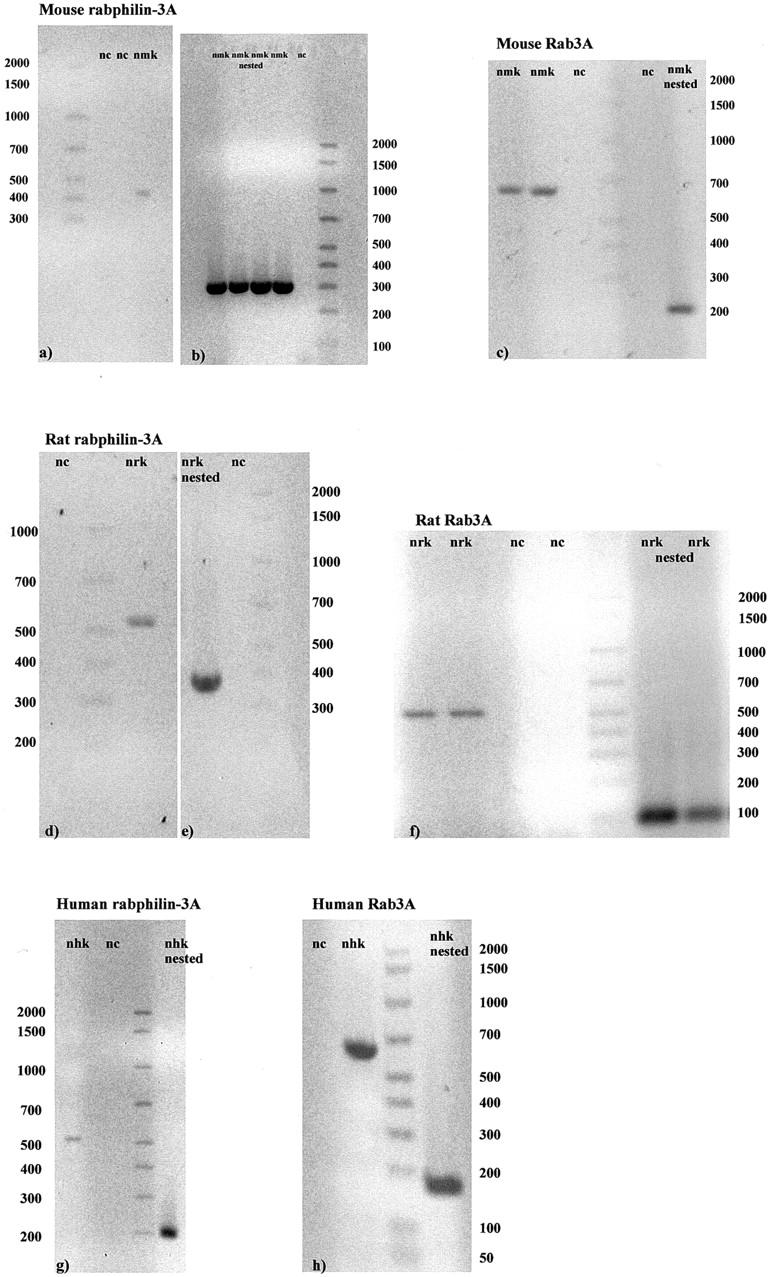
Examples of RT-PCR and nested PCR are shown for normal mouse (a–c), rat (d–f), and human (g, h) kidney glomeruli. All bands are of the expected size (see Tables 1 and 2 ▶ ). Rabphilin-3A amplification products have been obtained by NH2 primers in mouse (a, b) and rat (d, e), and by C2 primers in human (g). nc, negative control; nmk, normal mouse kidney; nrk, normal rat kidney; nhk, normal human kidney.
Figure 2.
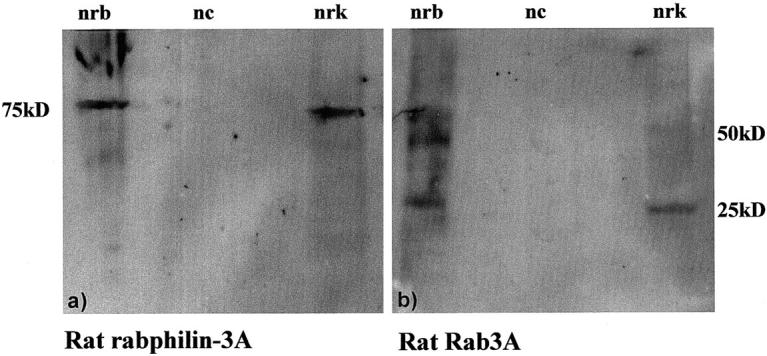
Western blot results obtained using a protein extract from normal rat brain (nrb) and normal rat kidney glomeruli (nrk). A band of ∼75 kd is present in a, corresponding to the molecular weight of rabphilin-3A (78 kd). In b, a band of 25 kd has the expected molecular weight of Rab3A, and other two bands, of ∼50 and 75 kd, more evident in the protein extract from brain, are likely dimer and trimer forms of the same molecule. nc, negative control.
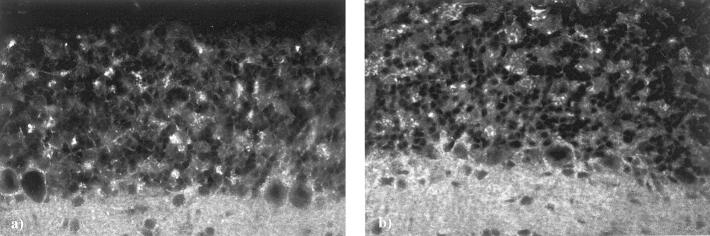
Single stain immunofluorescence of normal rat and mouse kidney tissue demonstrated a specific glomerular positivity for rabphilin-3A and Rab3A, with a comma-like pattern of positivity along the glomerular capillary wall (Figure 3) ▶ . The interstitium was completely negative. The anti-synaptotagmin antibody, selected because of the high homology of the C2 domains of synaptotagmin and rabphilin-3A, always gave negative results (Figure 4) ▶ . Positive controls in brain sections demonstrated the highest immunoreactivity of both anti-rabphilin-3A and anti-Rab3A in the cerebellum (Figure 5) ▶ .
Figure 3.
Normal mouse (a, c) and normal rat (b, d) kidney tissue. The immunostaining obtained by antibodies against rabphilin-3A (a, b) and Rab3A (c, d) is present only at glomerular level, with a comma-like pattern of positivity along the glomerular capillary wall. The interstitium is completely negative. Original magnifications, × 200 [direct (a, c) and indirect (b, d) immunofluorescence].
Figure 4.
Normal mouse (a) and normal rat (b) kidney tissue. Applying the anti-synaptotagmin antibody the tissue remains completely negative both at glomerular and interstitial level. Original magnifications, ×200 [direct (a) and indirect (b) immunofluorescence].
Figure 5.
Normal rat brain. Immunopositive small dots of both rabphilin-3A (a) and Rab3A (b) are spread in the cerebellum, and show the same pattern of positivity, especially evident in the granular layer. Purkinje and granular cell bodies are negative. Original magnifications, ×200 (indirect immunofluorescence).
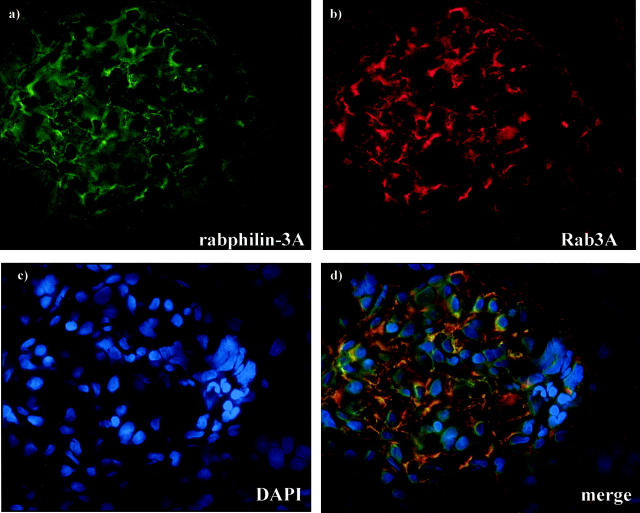
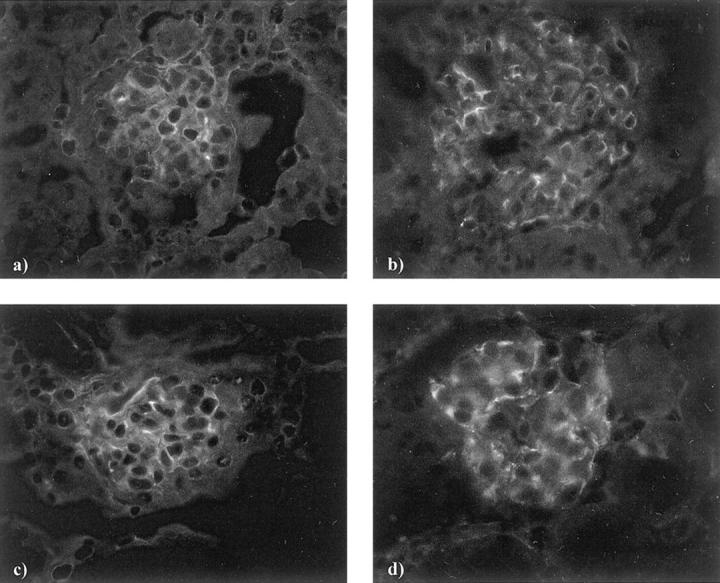
Double staining in normal rat and mouse kidneys with anti-rabphilin-3A and anti-Rab3A showed that the two proteins co-localize (Figure 6) ▶ , and alternatively coupling rabphilin-3A or Rab3A with the anti-synaptopodin antibody, we observed a precise co-localization as well, demonstrating the exclusive podocyte location of both synaptic molecules (Figure 7) ▶ . The specific podocyte staining was also evident when immunohistochemistry was performed on semithin sections (Figure 8) ▶ .
Figure 6.
Normal rat kidney. The double staining has been performed by fluorescein isothiocyanate-labeled anti-rabphilin-3A (a) and Cy3-labeled anti-Rab-3A (b) antibodies. They both show a scattered comma-like pattern of glomerular positivity. Nuclear counterstain has been obtained by 4′,6-diamidino-2-phenyindole (c). Merging the images (d), yellow dots show the co-localization of the two molecules. Original magnifications, ×250 (direct immunofluorescence).
Figure 7.
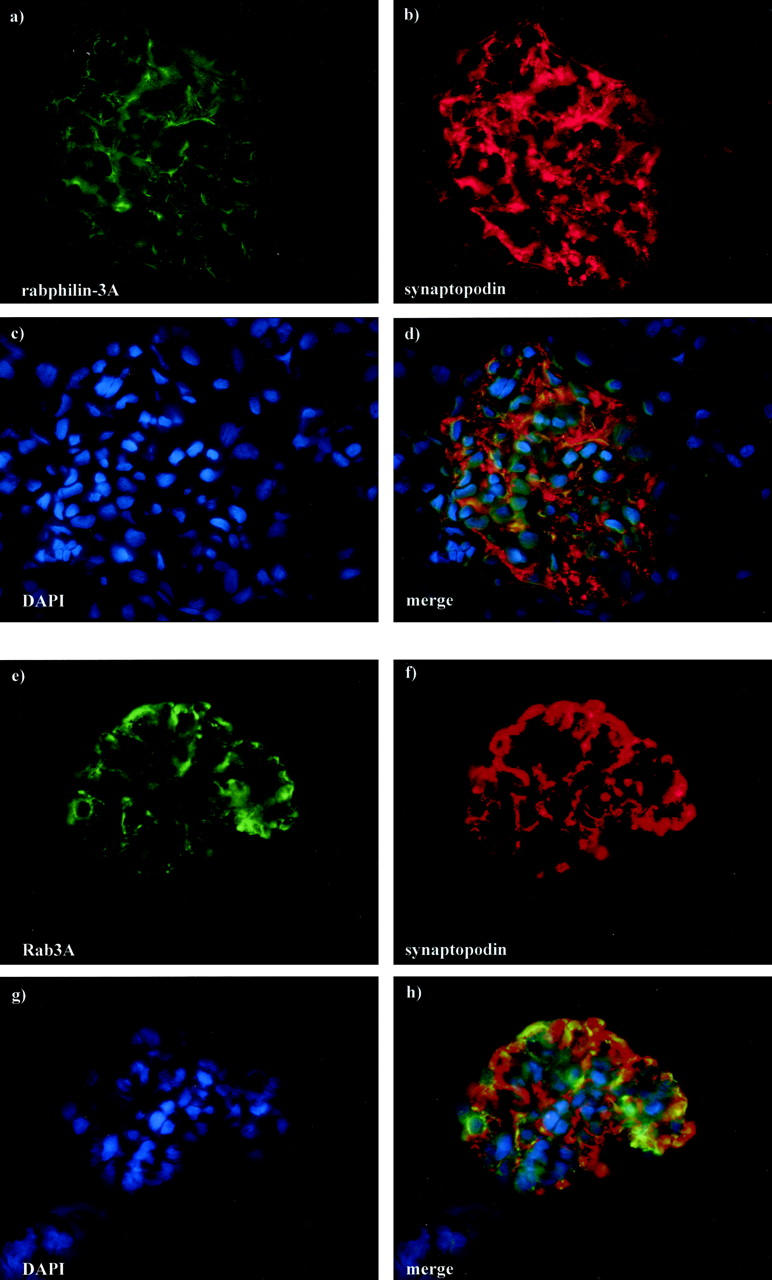
Normal rat kidney. The double staining has been performed by fluorescein isothiocyanate-labeled anti-rabphilin-3A (a) or fluorescein isothiocyanate-labeled anti-Rab3A (e) and Cy3-labeled anti-synaptopodin (b, f) antibodies. Compared to the comma-like glomerular positivity in a and e, the anti-synaptopodin antibody (b, f) globally stains the glomerular podocytes. Nuclear counterstain has been obtained by 4′,6-diamidino-2-phenyindole (c, g). Co-localization is evident in yellow (d, h). Original magnifications, ×250 (direct immunofluorescence)
Figure 8.
Normal rat kidney. Immunostaining on semithin (1 μm) sections clearly shows the podocyte-specific labeling given by anti-rabphilin-3A (a) and anti-Rab3A (b) antibodies, with evident white dots on the external side of the glomerular capillary wall. Original magnifications, ×500 (indirect immunofluorescence).
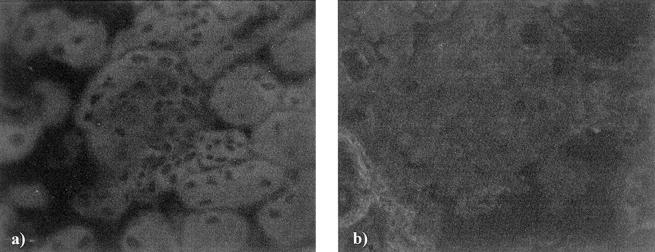
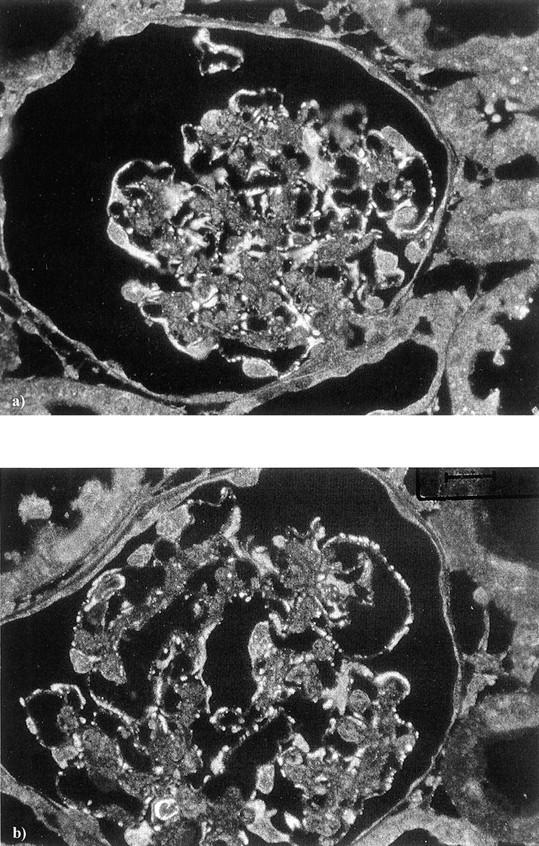
By immunogold electron microscopy both rabphilin-3A and Rab3A were clearly detected around vesicles contained in approximately one of five podocyte foot processes (Figure 9) ▶ . As expected, GH transgenic mice showed enlarged glomeruli with areas of segmental sclerosis (Figure 10, a and b) ▶ . Apart from sclerotic areas, almost all glomeruli were characterized by an enhanced staining for both synaptic proteins, compared to their WT counterparts (Figure 10; c to f) ▶ .
Figure 9.
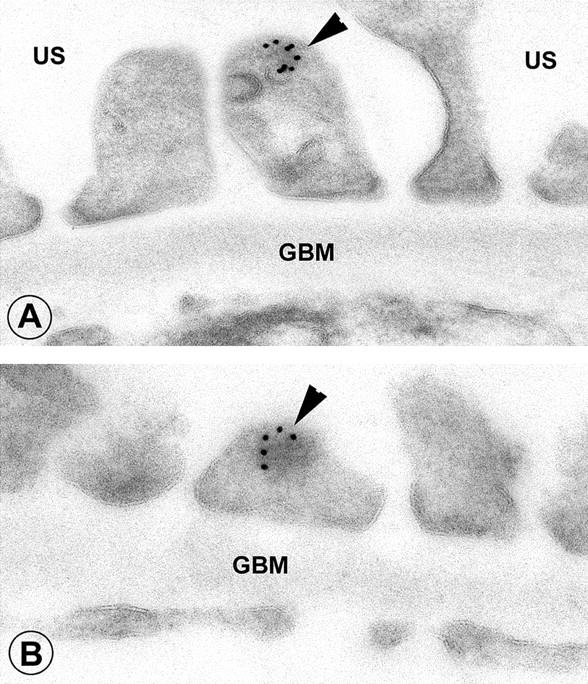
Normal rat kidney. A: Staining obtained by the anti-rabphilin-3A antibody. B: An example of anti-Rab3A is given. In both cases, the immunogold particles are roundly distributed along vesicles contained in podocyte foot processes (arrowheads). US, urinary space; GBM, glomerular basement membrane. Original magnifications, ×36,000 (immunogold electron microscopy, 10-nm gold particles).
Figure 10.
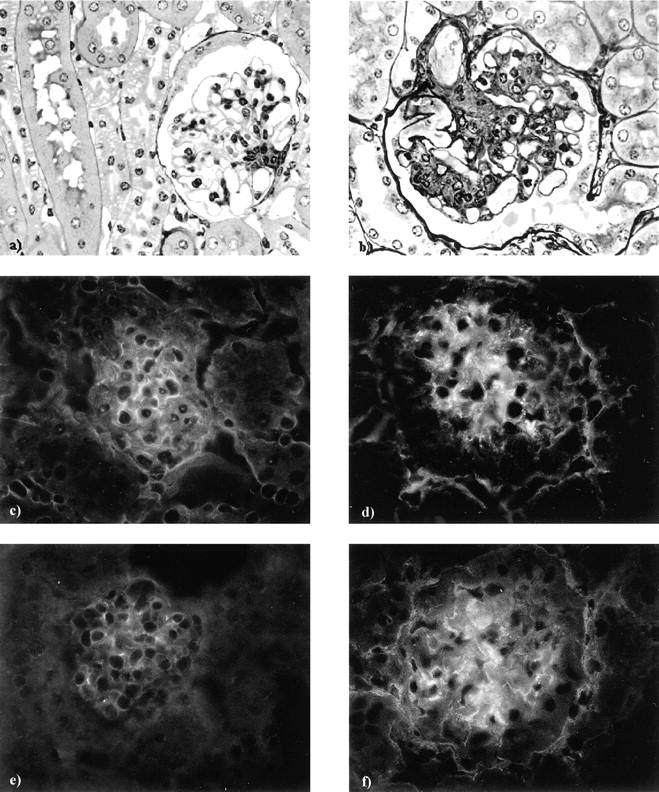
By light microscopy, a glomerulus taken from a GH-transgenic mouse (b) shows glomerular hypertrophy, mesangial expansion, and segmental hyalinosis, all these alterations being completely absent from a glomerulus taken from the WT littermate control (a). By immunofluorescence, glomeruli from GH-transgenic animals (d, f) show an increased staining for both rabphilin-3A (d) and Rab3A (f), compared to the staining of their WT littermates (c, e). Original magnifications, ×200 [glycolmethacrylate sections, methenamine silver/periodic acid-Schiff (a, b), and direct immunofluorescence (c–f)].
The primary antibody against rabphilin-3A was not suitable for human tissues and we obtained negative results. As a consequence, in human specimens we studied only the expression of Rab3A. In normal kidneys Rab3A was expressed specifically in glomeruli with a comma-like labeling along the glomerular capillary wall (Figure 11, a and b) ▶ . Compared to normal tissues, a subset of biopsies from human proteinuric diseases, independent of diagnosis, showed an enhanced Rab3A expression in all glomeruli (Figure 11, c and d) ▶ . By chi-square analysis, we found that the enhanced Rab3A expression was inversely associated with the amount of urinary proteins (P = 0.012).
Figure 11.
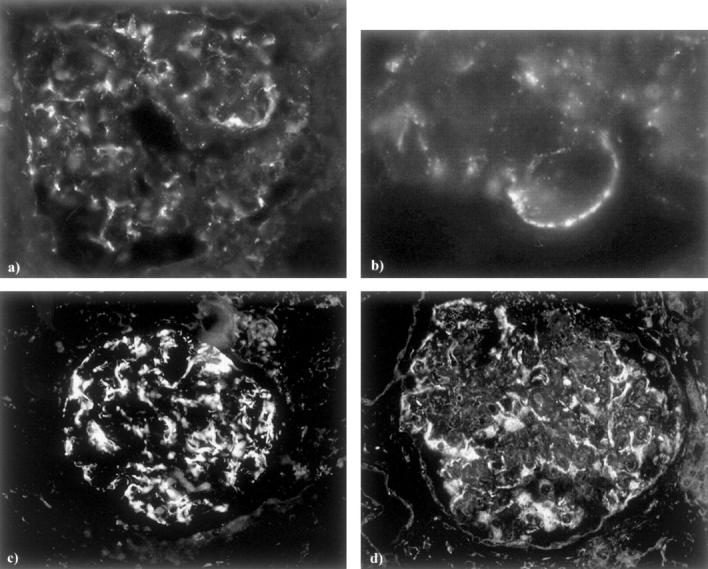
Rab3A staining in normal human kidney (a, b) shows a glomerular comma-like pattern. At higher magnification (b), white dots along the glomerular capillary wall are evident. The increased Rab3A staining is evident in glomeruli from a biopsy of minimal change disease (c) and one of membranous nephropathy (d). Original magnifications, ×200 [indirect immunofluorescence, (a, c, d); ×500 (b)].
Discussion
In the present work we demonstrate that two synaptic proteins, rabphilin-3A and Rab3A, are found in glomeruli and specifically localize in podocytes. Rab3A belongs to Rab proteins, the largest family of small GTPases, whose ability is to function as molecular switches that oscillate between guanosine triphosphate (GTP)- and guanosine diphosphate (GDP)-bound conformations. They interact with their effectors only in the activated GTP-bound state and constitute an important regulatory system that provides compartmental specificity to eukaryotic cells. 14 It is well established that Rab proteins are involved in the tethering/docking of vesicles to their target membrane, leading to membrane SNARE (soluble N-ethylmaleimide-sensitive factor-attachment protein receptors)-mediated fusion. It seems that all processes of vesicle trafficking can be supervised and directed by Rab members and their effectors, that are mostly ubiquitous, but highly vesicle-specific. 15
Among these proteins, Rab3A and its effector rabphilin-3A seem to possess not only vesicle specificity, but also cell specificity, because they have been mainly found in the nervous system, where they are required for correct docking of synaptic vesicles to the target membrane. Also in our hands, confirming previous results, 16 both proteins have shown a dot-like pattern of immunoreactivity especially evident in the granular layer of cerebellum, an area particularly rich in synapses. In neuroendocrine and endocrine cells Rab3A and rabphilin-3A are involved in processes of highly regulated exocytosis, 17,18 although their precise role still needs to be completely clarified. The extensive conservation of both proteins from Caenorhabditis elegans to vertebrates 19 implies that they play a critical role and is consistent with our finding of a similar expression in mouse, rat, and human.
When Rab3A is GTP-bound and recruits its specific effector rabphilin-3A, it is the effector that regulates the vesicle motility along cytoskeletal elements and mediates the docking to the acceptor membrane. 20 Podocytes possess a well-developed cytoskeleton: F-actin localizes to the submembranous region and maintains the infrastructure of the membrane domains and their complex digitated shape by continuous remodeling. 21 Not surprisingly, most of the newly discovered podocyte-associated proteins, such as nephrin and CD2-associated protein (CD2AP), have been found strictly connected to the actin cytoskeleton. 1 Rabphilin-3A interacts at least with two cytoskeletal molecules, α-actinin and β-adducin, 9,11 both important, at least to our knowledge, for podocyte homeostasis.
α-actinin (the isoform 4) is highly expressed in podocytes and has recently gained attention because its mutations have been found to associate with familial forms of focal and segmental glomerulosclerosis, 22 demonstrating its essential role in the maintenance of podocyte integrity. Although the exact function of α-actinin in the podocyte is not known at present, the molecule has been recently proposed to participate in processes of exocytosis 23 and endocytosis. 24 As far as rabphilin-3A, it has been shown that binding to α-actinin is able to increase the actin bundling activity of α-actinin. 9 Because the binding is inhibited by the GTP-bound form of Rab3A, the authors have hypothesized that, through switching the GTPase on and off and alternatively binding of rabphilin to Rab3A and α-actinin, these molecules behave like a timer that prepares vesicles for docking/fusion events by cytoskeletal reorganization. Further studies are needed to understand whether these processes are operative also in podocytes and whether they account for at least some of the cytoskeletal remodeling observed in normal glomeruli and altered in disease.
Adducin is an ubiquitously expressed calmodulin-binding protein, first purified from human erythrocyte cytoskeleton and from brain membranes. 25 It localizes at spectrin-actin junctions in erythrocyte membrane skeletons and co-localizes with spectrin at sites of cell-cell contact in epithelial cells and in dendritic spines. Adducin is believed to promote association of spectrin with actin and its function is to cap the fast-growing ends of actin filaments. It exists in three isoforms, α, β, and γ: although α- and γ-adducin are ubiquitous, β-adducin is more restricted and tissue-specific and has been found especially in the brain. 25 We have found β-adducin expressed in normal podocytes and are currently studying a rat strain with a β-adducin point mutation that is highly proteinuric (unpublished results), but the significance of β-adducin-rabphilin-3A interaction remains to be fully analyzed even in the brain.
We know that Rab proteins confer specificity to vesicles. 20 It is noteworthy that, among more than 60 Rab proteins discovered in mammals, most of them ubiquitous, podocytes possess Rab3A and its specific effector rabphilin-3A, which are expressed only in cells capable of highly regulated exocytosis. It has been well known that podocytes are involved in many glomerular functions and, apart from the maintenance of the filtration barrier, they are responsible for the turnover of glomerular basement membrane components and for the ability to produce a variety of cytokines and growth factors, 26 but these activities still need to be fully analyzed. Our immunogold results seem to confirm the precise location of rabphilin-3A and Rab3A around foot process vesicles. As a consequence, we believe that our results should present a starting point for better understanding podocyte exocytotic functions. Furthermore, in murine and human proteinuric conditions the expression of these molecules can increase, supporting the idea that the Rab3A-rabphilin-3A complex can play a role not only in normal but also in damaged podocytes.
Table 2.
Rab3A Primer Sequences and Predicted Product Size
| Molecule | ID number | Primer sequence | Sequence position | Product size |
|---|---|---|---|---|
| Mouse Rab3A (external) | NM_009001 | GAC TCT CGC TAT GGG CAG AA | 16–35 | 642 |
| GGC ACA ATC CTG ATG AGG TG | 638–657 | |||
| Mouse Rab3A (internal) | ATC AAA ACT TAC TCG TGG GAC AAT | 358–381 | 221 | |
| GTA TCC AGG GAC TCT GAC ATC TTC | 555–578 | |||
| Rat Rab3A (external) | NM_013018 | TCG ATA TGG GCA GAA GGA GT | 101–120 | 533 |
| CAC AGA TCA CGT CCA CCA GA | 614–633 | |||
| Rat Rab3A (internal) | ACT TCA AGG TCA AAA CCA TCT ACC | 253–276 | 104 | |
| GTA GTA GGC TGT GGT GAT GGT TC | 334–356 | |||
| Human Rab3A (external) | XM_054457 | CGC TAT GGG CAG AAG GAG T | 49–67 | 642 |
| TCA GCA GGC GCA GTC CTG G | 672–690 | |||
| Human Rab3A (internal) | ATC TAT CGC AAC GAC AAG AGG AT | 217–239 | 192 | |
| ATT GTC CCA TGA GTA GGT CTT GAT | 385–408 |
Footnotes
Address reprint requests to Maria Pia Rastaldi, M.D., Renal Immunopathology Laboratory, Associazione Nuova Nefrologia, c/o San Carlo Borromeo Hospital, Via Pio II, 3, 20153, Milan, Italy. E-mail: mp.rastaldi@oscb.sined.net.
Supported by the European Union grant QLG1-2000-00619.
References
- 1.Kerjaschki D: Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest 2001, 108:1583-1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Putaala H, Soininen R, Kilpeläinen P, Wartiovaara J, Tryggvason K: The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet 2001, 10:1-8 [DOI] [PubMed] [Google Scholar]
- 3.Thomas PE, Wharram BL, Goyal M, Wiggins JE, Holzman LB, Wiggins RC: GLEPP1, a renal glomerular epithelial cell (podocyte) membrane protein-tyrosine phosphatase. Identification, molecular cloning, and characterization in rabbit. J Biol Chem 1994, 269:19953-19962 [PubMed] [Google Scholar]
- 4.Mundel P, Heid HW, Mundel TM, Kruger M, Reiser J, Kriz W: Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol 1997, 139:193-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gloy J, Reitinger S, Fischer KG, Schriber R, Boucherot A, Kunzelmann K, Mundel P, Pavenstädt H: Amino acid transport in podocytes. Am J Physiol 2000, 278:F999-F1005 [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi N, Mundel P: A role of microtubules during the formation of cell processes in neuronal and non-neuronal cells. Cell Tissue Res 1998, 291:163-174 [DOI] [PubMed] [Google Scholar]
- 7.Shirataki H, Kaibuchi K, Sakoda T, Kishida S, Yamaguchi T, Wada K, Miyazaki M, Takai Y: Rabphilin-3A, a putative target protein for smg p25A/rab3A p25 small GTP-binding protein related to synaptotagmin. Mol Cell Biol 1993, 13:2061-2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns ME, Sasaki T, Takai Y, Augustine GJ: Rabphilin-3A: a multifunctional regulator of synaptic vesicle traffic. J Gen Physiol 1998, 111:243-255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato M, Sasaki T, Ohya T, Nakanishi H, Nishioka H, Imamura M, Takai Y: Physical and functional interaction of rabphilin-3A with α-actinin. J Biol Chem 1996, 271:31775-31778 [DOI] [PubMed] [Google Scholar]
- 10.Südhof TC: The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature 1995, 375:645-653 [DOI] [PubMed] [Google Scholar]
- 11.Miyazaki M, Shirataki H, Kohno H, Kaibuchi K, Tsugita A, Takai Y: Identification as β-adducin of a protein interacting with rabphilin-3A in the presence of calcium and phosphatidylserine. Biochem Biophys Res Commun 1994, 205:460-466 [DOI] [PubMed] [Google Scholar]
- 12.Wanke R, Wolf E, Hermanns W, Folger S, Buchmuller T, Brem G: The GH-transgenic mouse as an experimental model for growth research: clinical and pathological studies. Horm Res 1992, 37(Suppl 3):74-87 [DOI] [PubMed] [Google Scholar]
- 13.Regele HM, Fillipovic E, Langer B, Poczewki H, Kraxberger I, Bittner RE, Kerjaschki D: Glomerular expression of dystroglycans is reduced in minimal change nephrosis but not in focal segmental glomerulosclerosis. J Am Soc Nephrol 2000, 11:403-412 [DOI] [PubMed] [Google Scholar]
- 14.Ostermeier C, Brunger AT: Structural basis of Rab effector specificity: crystal structure of the small G protein Rab3A complexed with the effector domain of rabphilin-3A. Cell 1999, 96:363-374 [DOI] [PubMed] [Google Scholar]
- 15.Zerial M, McBride H: Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2001, 2:107-119 [DOI] [PubMed] [Google Scholar]
- 16.Mizoguchi A, Yano Y, Hamaguchi H, Yanagida H, Chizuka I, Zahraoui A, Shirataki H, Sasaki T, Takay Y: Localization of rabphilin-3A on the synaptic vesicle. Biochem Biophys Res Comm 1994, 202:1235-1243 [DOI] [PubMed] [Google Scholar]
- 17.Fischer von Mollard G, Mignery GA, Baumert M, Perin MS, Hanson TJ, Burger PM, Jahn R, Südhof TC: Rab3A is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc Natl Acad Sci USA 1990, 87:1988-1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holz RW, Brondyk WH, Senter RA, Kuizon L, Macara IG: Evidence for the involvement of Rab3A in Ca(2+)-dependent exocytosis from adrenal chromaffin cells. J Biol Chem 1994, 269:10229-10234 [PubMed] [Google Scholar]
- 19.Staunton J, Ganetzky B, Nonet ML: Rabphilin potentiates soluble N-ethylmaleimide sensitive factor attachment protein receptor function independently of rab3. J Neurosci 2001, 21:9255-9264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzales L, Scheller RH: Regulation of membrane trafficking: structural insights from a Rab/effector complex. Cell 1999, 96:755-758 [DOI] [PubMed] [Google Scholar]
- 21.Simons M, Saffrich R, Reiser J, Mundel P: Directed membrane transport is involved in process formation in cultured podocytes. J Am Soc Nephrol 1999, 10:1633-1639 [DOI] [PubMed] [Google Scholar]
- 22.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguez-Perez JC, Allen PG, Beggs AH, Pollak MR: Mutations in ACTN4, encoding α-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 2000, 24:251-256 [DOI] [PubMed] [Google Scholar]
- 23.Dubernard V, Arbeille BB, Lemesle MB, Legrand C: Evidence for an alpha-granular pool of the cytoskeletal protein alpha-actinin in human platelets that redistributes with the adhesive glycoprotein thrombospondin-1 during the exocytotic process. Arterioscler Thromb Vasc Biol 1997, 17:2293-2305 [DOI] [PubMed] [Google Scholar]
- 24.Araki N, Hatae T, Yamada T, Hirohashi S: Actinin-4 is preferentially involved in circular ruffling and macropinocytosis in mouse macrophages: analysis by fluorescence ratio imaging. J Cell Sci 2000, 113:3329-3340 [DOI] [PubMed] [Google Scholar]
- 25.Matsuoka Y, Li X, Bennett V: Adducin: structure, function and regulation. Cell Mol Life Sci 2000, 57:884-895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adler S: Glomerular epithelial cells. Neilson EG Couser WG eds. Immunologic Renal Diseases. 1997:pp 655-667 Lippincott-Raven Philadelphia