Abstract
Assembly of collagen into fibrils is widely studied as a spontaneous and entropy-driven process. To determine whether vascular smooth muscle cells (SMCs) impact the formation of collagen fibrils, we microscopically tracked the conversion of soluble to insoluble collagen in human SMC cultures, using fluorescent type I collagen at concentrations less than that which supported self-assembly. Collagen microaggregates were found to form on the cell surface, initially as punctate collections and then as an increasingly intricate network of fibrils. These fibrils displayed 67-nm periodicity and were found in membrane-delimited cellular invaginations. Fibril assembly was inhibited by an anti-α2β1 integrin antibody and accelerated by an α2β1 integrin antibody that stimulates a high-affinity binding state. Newly assembled collagen fibrils were also found to co-localize with newly assembled fibronectin fibrils. Moreover, inhibition of fibronectin assembly with an anti-α5β1 integrin antibody completely inhibited collagen assembly. Collagen fibril formation was also linked to the cytoskeleton. Fibrils formed on the stretched tails of SMCs, ran parallel to actin microfilament bundles, and formed poorly on SMCs transduced with retrovirus containing cDNA for dominant-negative RhoA and robustly on SMCs expressing constitutively active RhoA. Lysophosphatidic acid, which activates RhoA and stimulates fibronectin assembly, stimulated collagen fibril formation, establishing for the first time that collagen polymerization can be regulated by soluble agonists of cell function. Thus, collagen fibril formation is under close cellular control and is dynamically integrated with fibronectin assembly, opening new possibilities for modifying collagen deposition.
The fundamental importance of type I collagen can be inferred by its presence in almost all human tissues and by the nonviability of embryos deficient in this extracellularmatrix (ECM) protein. 1,2 Type I collagen exerts its roles, as a load-bearing structure and regulator of cell function, only after it has polymerized into fibrils. This assembly process proceeds after proteolytic removal of the globular termini of the secreted procollagen molecules. The resulting fibrils can vary considerably in size and organization. Fibril diameters can range from 20 to 500 nm and the fibrils themselves can organize into diverse patterns including bundles, weaves, and layers. 3,4 This broad diversity in fibril size and topology has important implications for tissue function. For example, optical transparency in the cornea is conferred by collagen that has been assembled into thin fibrils in an orthogonal lattice, whereas the enormous tensile strength of tendon is because of thick collagen fibrils in parallel bundles. The precise manner in which collagen fibrils are assembled and spatially organized is thus critical to organ development and repair.
Current understanding of collagen fibril assembly is based primarily on the long-standing recognition that molecules of type I collagen can self-assemble. It has been appreciated for more than 40 years that solubilized, tissue-extracted collagen will polymerize spontaneously when physiological pH, temperature, and ionic strength are restored. 5-7 Collagen fibrils can also be generated in vitro by subjecting soluble type I procollagen to sequential cleavage of its propeptide termini by procollagen metalloproteinases. This action reduces the solubility of the protein and initiates the entropy-driven self-assembly process. 8,9 Although only the latter approach involves the physiologically relevant step of procollagen cleavage, both in vitro systems yield early collagen fibrils with characteristics similar to those found in developing tissues. 3,10 Moreover, both approaches have been valuable for elucidating conditions for collagen self-assembly and in defining controlling elements for this within the collagen molecule. 7,11
Self-assembly however cannot by itself explain the diverse morphology of collagen fibrils found in tissues, and determinants other than those intrinsic to the collagen molecule are likely required. In this regard, collagen-associating ECM molecules, including decorin, fibromodulin, and lumican, have been found to impact the size and architecture of type I collagen fibrils. 12-14 Recently, the elaboration of collagen structures by mouse embryonic cells has been shown to require the assembly of fibronectin fibrils. 15 Therefore, in the context of a cellular environment, interactions between collagen and noncollagen molecules seem to be important for collagen fibril formation and organization.
It remains difficult however, in a cell-based system, to experimentally separate the process of collagen assembly from the cellular production and secretion of collagen. No study to date has specifically examined the assembly of collagen (ie, the conversion of soluble collagen to an insoluble fibril) in the presence of cells. Likewise, in the presence of cells it is a challenge to distinguish the phenomenon of collagen self-assembly, in which only collagen-collagen interactions are at play, from interactions between collagen and other proteins that might drive assembly. This includes potential interactions with cell-surface ECM receptors and with other ECM fibrils, such as fibronectin, that depend on the cell for polymerization. 16,17
An important context for collagen fibril assembly is the blood vessel wall. The manner in which collagen fibrils are assembled in the vasculature is critical to the mechanical properties of both the normal and diseased artery. The major source of type I collagen in the vasculature is vascular smooth muscle cells (SMCs). SMCs can influence higher levels of fibril organization, as illustrated by their ability to contract a preformed collagen fibril lattice. 18 However, it is not known whether SMCs have control over the process by which a collagen fibril is generated from its soluble precursors.
To determine whether SMCs play a meaningful role in the de novo assembly of type I collagen, we developed a cell culture system in which fluorescence-labeled collagen, in a soluble, unpolymerized state, is applied to SMC cultures. This allowed us to microscopically track the conversion of soluble to insoluble collagen on the cell surface. As such, cell-associated collagen assembly process could be distinguished from collagen production and also be compared with collagen assembly in the absence of the cell. The findings established that SMCs have a pronounced effect on the assembly of collagen fibrils through a mechanism that involves α2β1 integrin and RhoA-mediated signaling. Moreover, using differentially labeled precursors we established that collagen polymerization was intimately related to fibronectin polymerization. Finally, we observed that collagen polymerization could be regulated by exogenous mediators of cell behavior. The results thus identify a central role for SMCs in orchestrating the formation of collagen fibrils, implying that cellular information drives collagen assembly in the vasculature.
Materials and Methods
SMC Culture
Primary cultures of human arterial SMCs were initiated by explant outgrowth from segments of internal thoracic artery retrieved at the time of coronary artery bypass surgery. 19 The identity of vascular SMCs was confirmed morphologically and by immunostaining with a monoclonal antibody to smooth muscle α-actin (1A4; DAKO, Mississauga, Canada). Cells were grown in M199 (Life Technologies, Inc., Gaithersburg, MD), supplemented with 10% fetal bovine serum in the absence of ascorbate. Experiments were performed using SMCs in the third to seventh subculture, cultured on glass coverslips precoated with 100 μg/ml of human fibronectin.
Collagen Assembly by SMCs
Solubilized collagen was obtained either from bovine skin, after pepsin digestion and solubilization in HCl (Vitrogen; Cohesion Technologies, Palo Alto, CA), or from rat tail, solubilized in acetic acid. 20 The collagen preparations were dialyzed against borate-buffered saline (170 mmol/L boric acid, 170 mmol/L sodium tetraborate, 75 mmol/L NaCl, pH 9.3) at 4°C overnight and then labeled with either fluorescein isothiocyanate (FITC) or Texas Red (Molecular Probes, Eugene, OR) by transferring the dialysate to a solution of borate-buffered saline containing 30 mg/ml of the fluorochrome and mixing in the dark at 4°C for 6 hours. Acidic pH was restored and unbound FITC or Texas Red was removed by dialysis against 0.1% acetic acid at 4°C for 4 days. The concentration of labeled collagen was measured spectrophotometrically by measuring absorption at 280 nm and either 493 nm or 595 nm wavelengths.
To assay collagen assembly, labeled soluble collagen (500 μg/ml) was mixed with ice-cold M199 with 10% fetal bovine serum to the final designated concentration (0.2 to 10 μg/ml). This was added to SMC cultures that were maintained on ice for 2 minutes, at room temperature for 2 minutes, and then at 37°C for designated intervals. Cultures were then washed in phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde. Cells were then stained with Hoechst 33258 (Molecular Probes), mounted in glycerol/PBS (9:1), and collagen fibril formation was evaluated by fluorescence microscopy (Axiovert S100; Zeiss). To assess for collagen self-assembly under these conditions, the identical procedure was performed without cells. The effect of collagen-binding integrins on SMC-directed assembly was assessed using an α1β1 integrin-specific blocking antibody (5E8D9; Upstate Biotechnology Inc., Lake Placid, NY), an α2β1 integrin-specific blocking antibody (BHA2.1), 21 and an α2β1 integrin-specific stimulating antibody (JBS2), 22 each added 30 minutes before addition of fluorescent collagen.
Electron Microscopy
Solubilized collagen was added to SMCs cultured on plastic coverslips (Therminox; Nalge-Nunc) in ascorbate-free medium. At designated times the cultures were fixed with 2% phosphate-buffered glutaraldehyde, postfixed in 1% osmium tetroxide, and then embedded in Polybed/araldite epoxy. Coverslips with cells and collagen were cross-sectioned followed by sequential staining with 2% uranyl acetate in 70% ethanol and Reynolds aqueous lead citrate. Specimens were viewed with a transmission electron microscope (Phillips 410) at 60 kV.
Fibronectin Assembly by SMCs
Fibronectin assembly by human SMCs was evaluated as we have described previously, 23 using fluorescently labeled, plasma-derived human fibronectin. Fibronectin protomers were labeled with Oregon Green conjugated to an amine-reactive succinimidyl ester (Molecular Probes). Labeled soluble fibronectin (20 μg/ml) was added to SMCs cultured in M199, supplemented with fibronectin-free fetal bovine serum, together with Texas Red-labeled soluble collagen. In some experiments, labeled collagen was added to cultures after they had been incubated with labeled fibronectin protomers for 1 hour and then extensively washed.
Infection of Human SMCs with Retrovirus Containing Mutant RhoA
cDNA fragments encoding dominant-negative (T19N RhoA) or constitutively active (Q63L RhoA) RhoA, kindly provided by Dr. Gary Bokoch (Scripps Research Institute, La Jolla, CA), 24 were excised from pRK5 Myc and cloned into the retroviral gene delivery vector pLNCX (BD Biosciences Clontech, Palo Alto, CA). Retroviral delivery constructs were introduced into the Phoenix-amphotropic retrovirus packaging/producing cell line (American Type Culture Collection, Rockville, MD) 25 by CaCl2-mediated transfection. The virus-containing culture supernatant was harvested 48 to 72 hours later and, after centrifugation and filtration (0.45-μm pore filters), was added to proliferating human SMCs for 48 hours. To obtain stably expressing cells, transductants were selected in M199 containing 500 μg/ml of G418. Cells were maintained in G418-containing medium for two passages before use in the fibril assembly assay.
Staining for Actin Filaments
Paraformaldehyde-fixed SMCs were permeabilized in cold acetone for 15 seconds, nonspecific binding sites were blocked with 5% normal goat serum, and cells were incubated with Texas Red-labeled phalloidin (1:100 dilution, Molecular Probes) for 1 hour.
Results
SMC-Associated Collagen Fibril Assembly Is Distinct from Cell-Free Fibril Assembly
To evaluate the assembly of collagen in SMC cultures, independent of collagen production, we labeled tissue-extracted, soluble collagen with FITC or Texas Red and microscopically tracked fibril formation after subjecting the collagen solution to physiological temperature and pH. This was undertaken in both cellular and noncellular environments. Nonfibrillar precipitation of the collagen was minimized by adding the labeled precursors to precooled culture medium that was then gradually warmed to 37°C. Two different collagen preparations were used, pepsin-digested bovine skin collagen solubilized in HCl (Vitrogen collagen) and acetic acid-solubilized rat tail collagen. The two preparations were studied recognizing that the method of collagen preparation might influence its subsequent polymerization.
We first established the threshold concentration below which collagen self-assembly was not apparent, by adding precursor to culture media in the absence of cells. This threshold proved to be 2 μg/ml for Vitrogen collagen and 5 μg/ml for rat tail collagen. At higher concentrations, fibrils 6 to 12 μm in length and similar to self-assembled fibrils previously described 7,8 settled onto the fibronectin-coated coverslips (Figure 1a) ▶ . At concentrations below these thresholds, fibrils were not detected on the substrate or suspended in the media (Figure 1b) ▶ . However, when a subthreshold concentration of collagen was added to media bathing a monolayer of human SMCs, an insoluble collagen network of formed on the cells. As illustrated in Figure 1c ▶ , within 1 hour of adding Vitrogen collagen, there was a finely punctate distribution of fluorescent collagen on the cell surface (Figure 1c) ▶ . Fibril-like structures were generally not evident at this time but in the ensuing 2 hours, short fibrils formed on the apical surface of cells (Figure 1d) ▶ . By 24 hours, an intricate pericellular network developed (Figure 1 ▶ ; c to e). This network was seen with precursor concentrations as low as 0.2 μg/ml. The network that formed using rat tail collagen was similar to that using Vitrogen collagen, with somewhat less cell-to-cell bridging of fibrils (Figure 1f) ▶ . In neither case were fibrils found in regions devoid of cells. Fibril formation was not influenced by the substrate on which SMCs were grown, with no discernable difference in fibrils seen for SMCs on fibronectin, vitronectin, or rat tail collagen (data not shown).
Figure 1.
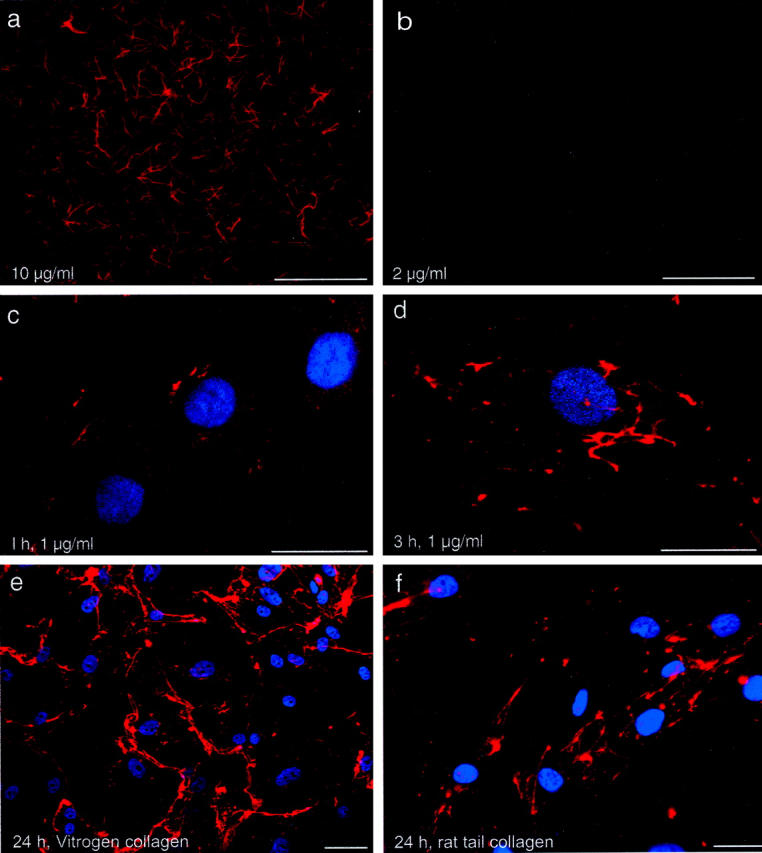
SMCs stimulate collagen fibril assembly. In vitro collagen assembly was performed by incubating Texas Red-labeled, acid-solubilized collagen at 37°C in culture medium (M199 plus 10% fetal bovine serum, pH 7.4). Fibrils that formed and attached to the substrate were fixed in paraformaldehyde and imaged microscopically. a: Spontaneously assembled fibrils that formed and adhered to a fibronectin-coated coverslip after incubating 10 μg/ml of Texas Red-Vitrogen collagen in cell-free culture media for 3 hours at 37°C. b: Fibrils are not evident when 2 μg/ml of Texas Red-collagen is incubated with culture medium, indicating this concentration to be below the critical threshold for self-assembly under these conditions. c and d: Confocal microscopic images acquired 1 hour (c) and 3 hours (d) after addition of 1 μg/ml of Texas Red-labeled Vitrogen collagen to human SMCs. Punctate accumulations of fluorescence are evident initially, followed by small fibrils on the surface of SMCs. e: Lower magnification image (nonconfocal) 24 hours after addition of soluble collagen to SMCs, showing extensive fibril formation. f: Cell-associated fibrils also formed after the addition of 1 μg/ml of Texas Red-labeled rat-tail collagen to SMC cultures. Nuclei were counterstained with Hoechst 33258. Scale bars, 50 μm.
Solubilized Collagen Polymerizes into Native Fibrils on the SMC Surface
When collagen precipitates from solution it can potentially assume nonfibrillar forms that are not relevant to in vivo circumstances, or abnormal fibril variants that are only rarely observed in vivo. 26 To elucidate the ultrastructure of collagen polymerizing on the SMCs, cultures were studied by transmission electron microscopy. SMCs were cultured in ascorbate-free medium to minimize endogenous collagen production. Under these conditions, and in the absence of exogenous collagen precursor, no fibrils were evident by electron microscopy 36 hours after cell plating. Likewise, when soluble collagen was added in the absence of SMCs, only scattered amorphous aggregates were found on the substrate. However, addition of soluble collagen to SMC cultures yielded cross-banded fibrils. The banding displayed 67-nm periodicity, confirming the structures to be native collagen fibrils (Figure 2a) ▶ . It was also noteworthy that assembled collagen fibrils were found in association with distinct morphological conformations of the cell surface. Some fibrils were found within plasma membrane-delimited spaces. These spaces were much larger than uptake vesicles, with a greater degree of clarity. They were thus consistent with recesses and invaginations of the cell surface. Newly assembled fibrils were also found adjacent to discrete cell surface depressions and at sites of plasma membrane apposition between cells (Figure 2b) ▶ . These observations suggest that participation of the cell surface is a feature of collagen assembly.
Figure 2.
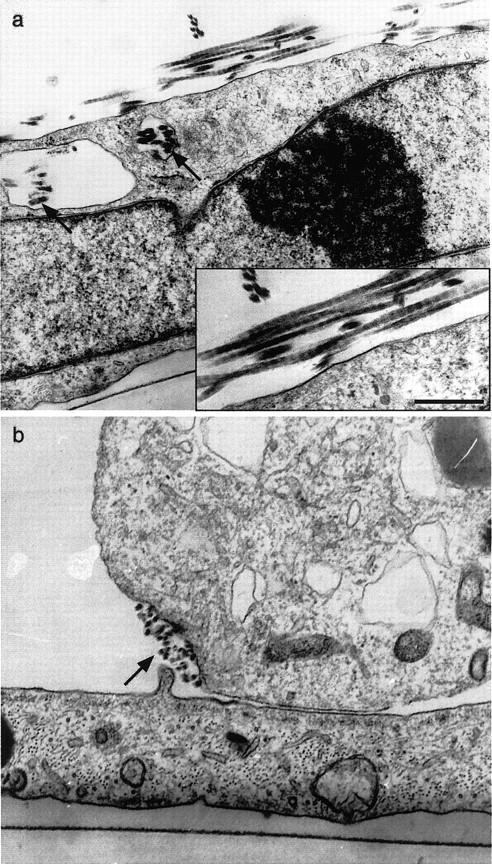
Native collagen fibrils assemble in human SMC cultures. Electron micrographs of human SMCs, cultured in ascorbate-deficient media, 24 hours after the addition of soluble, bovine skin collagen. Cross-striated collagen fibrils are apparent near the cell surface (a and at higher magnification in inset). Fibrils can also be seen in membrane-delimited cellular invaginations (arrows). b: Collection of fibrils seen in cross-section within a cell-surface depression at a zone of apposition with an adjacent cell. Scale bar, 500 nm (a).
α2β1 Integrin Is Involved in SMC-Associated Collagen Assembly
We considered that the basis of cell-associated collagen polymerization could be the provision of a local microenvironment favorable to self-assembly or a more direct interaction that involved surface receptors. SMCs express α1β1 and α2β2 integrin-type collagen receptors. 27 Therefore, to test if SMC-associated collagen assembly was mediated by either of these collagen receptors, fluorescent collagen monomers were added to SMC cultures in the presence of specific anti-integrin antibodies. As illustrated in Figure 3 ▶ , the extensive collagen fibril network that formed by adding Vitrogen to SMCs for 48 hours was reduced by BHA2.1, an anti-α2β1 integrin antibody. Similar inhibition was observed for rat tail collagen (data not shown). Fibril formation was however unaffected by a function-blocking antibody against α1β1 integrin. We reasoned that if α2β1 integrin mediates collagen fibril assembly, stimulating its function should enhance the process. This in fact was observed. Cultures incubated with JBS2, an α2β1 integrin-activating antibody that stimulates a high-affinity binding state and augments attachment to collagen, 22 showed greater fibril assembly. This accentuation was most apparent during early assembly (eg, 6 hours) (Figure 3, d and e) ▶ .
Figure 3.
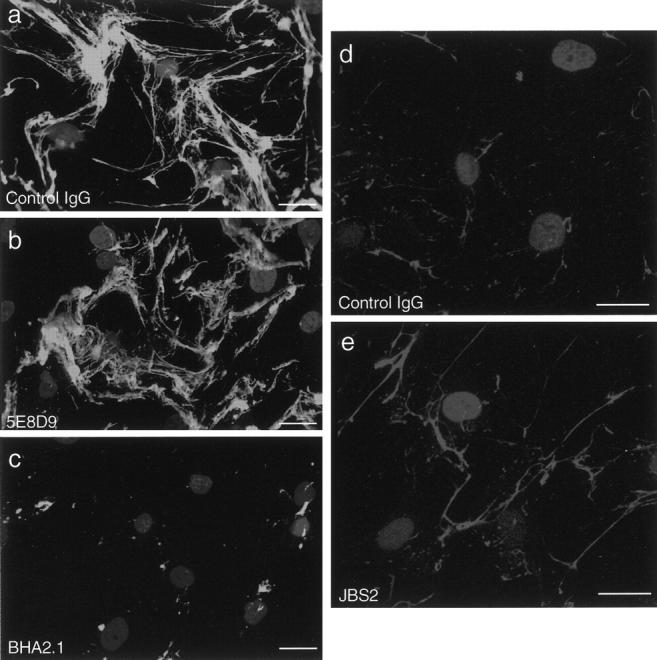
SMC-associated collagen assembly is mediated by α2β1 integrin. Human SMCs were incubated for 48 hours with solubilized FITC-labeled collagen (2 μg/ml) and either control IgG (a), an anti-α1β1 integrin antibody (5E8D9, 20 μg/ml) (b), or an anti-α2β1 integrin antibody (BHA2.1, 20 μg/ml) (c). Antibodies were added 30 minutes before addition of FITC-collagen monomers. Fibril assembly was also evaluated in cultures preincubated for 30 minutes with anti-α2β1 integrin-stimulating antibody (JBS2, 1:50) and then incubated with Texas Red-labeled collagen (1 μg/ml) for 6 hours. Compared to control conditions (d), the fibers in JBS2-treated cultures were more abundant and straighter (e). Washed cultures were fixed in paraformaldehyde and nuclei were stained with Hoechst 33258. Scale bars, 30 μm.
SMC-Mediated Collagen Assembly Is Integrated with Fibronectin Assembly
Another binding partner for collagen is fibronectin, and it has been recognized for several years that collagen fibrils can co-localize with fibronectin fibrils. 28 It is also known that fibronectin polymerization is dependent on interaction with cell-surface receptors. 29 Therefore, to determine whether there was a relationship between the assembly of collagen on SMCs and assembly of fibronectin, we co-incubated SMC cultures with Texas Red-labeled soluble collagen and Oregon Green-labeled soluble fibronectin. As shown in Figure 4A ▶ , newly assembled collagen fibrils co-localized with newly assembled fibronectin fibrils. To ensure that this finding was not because of association of collagen and fibronectin precursors in solution, before contacting the cell surface, we performed experiments in which fibronectin was incubated with the cells for 1 hour, the cultures were extensively washed, and then labeled collagen was added for another 12 hours. Under these circumstances, fibril co-localization was consistently evident, although the fluorescent collagen fibrils tended to extend beyond the fluorescent regions of the fibronectin fibrils, in keeping with the brief incubation period of the latter. We also found that the early fibronectin formations were always in the form of linear fibrils, whereas the earliest collagen formations could be found as fine punctate accumulations (Figure 1c) ▶ .
Figure 4.
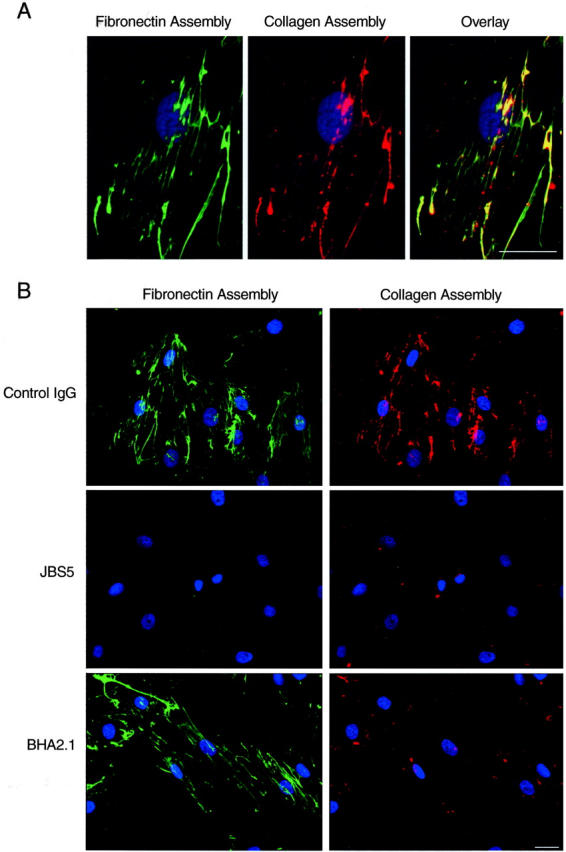
SMC-directed collagen assembly is integrated with α5β1 integrin-mediated fibronectin assembly. A: Human SMCs co-incubated for 16 hours with Oregon Green-labeled fibronectin and Texas Red-labeled bovine skin collagen showing co-localization of assembling fibrils. B: SMCs co-incubated with Oregon Green-labeled fibronectin and Texas Red-labeled collagen together with control IgG, anti-α5β1 integrin antibody (JBS5, 20 μg/ml), or anti-α2β1 integrin antibody (BHA2.1, 20 μg/ml). Nuclei were stained with Hoechst 33258. Scale bar, 40 μm.
We have previously shown that the assembly of fibronectin by human SMCs is dependent on α5β1 integrin. 23 Given the close physical association of actively assembling collagen and fibronectin fibrils, we sought to determine whether disrupting the α5β1 integrin-fibronectin interaction influenced collagen assembly. For this, we incubated SMCs with JBS5, an anti-α5β1 integrin antibody, together with fluorescent fibronectin and fluorescent collagen precursors. As shown in Figure 4b ▶ , JBS5 completed inhibited fibronectin assembly. Importantly, it also fully inhibited collagen fibril assembly. In some cells, a fine punctate collagen fluorescence could be seen, but fibril formation was not. We next determined if fibronectin assembly was influenced by α2β1 integrin, by incubating cultures with BHA2.1. Under these conditions, collagen fibril formation was reduced but fibronectin assembly was unaffected (Figure 4B) ▶ .
An Intact Actin Cytoskeleton Is Required for SMC-Mediated Collagen Assembly
We observed that newly assembled collagen fibrils tended to orient along the long axis of the cell. As well, at lower cell densities human SMCs display a more irregular shape with thin projections, and in these circumstances collagen fibrils were found running along the length of these projections (Figure 5a) ▶ . The relationship between collagen assembly and cell shape was further evaluated by staining the actin cytoskeleton with Texas Red phalloidin, 6 hours after incubating cells with FITC-labeled collagen monomers. This revealed newly assembled collagen fibrils that generally co-aligned with the underlying actin microfilament bundles (Figure 5b) ▶ . When actin microfilament bundles were disrupted by incubating SMCs with cytochalasin D (10 μmol/L), almost no collagen fibril assembly could be detected on the cells (Figure 5, c and d) ▶ .
Figure 5.
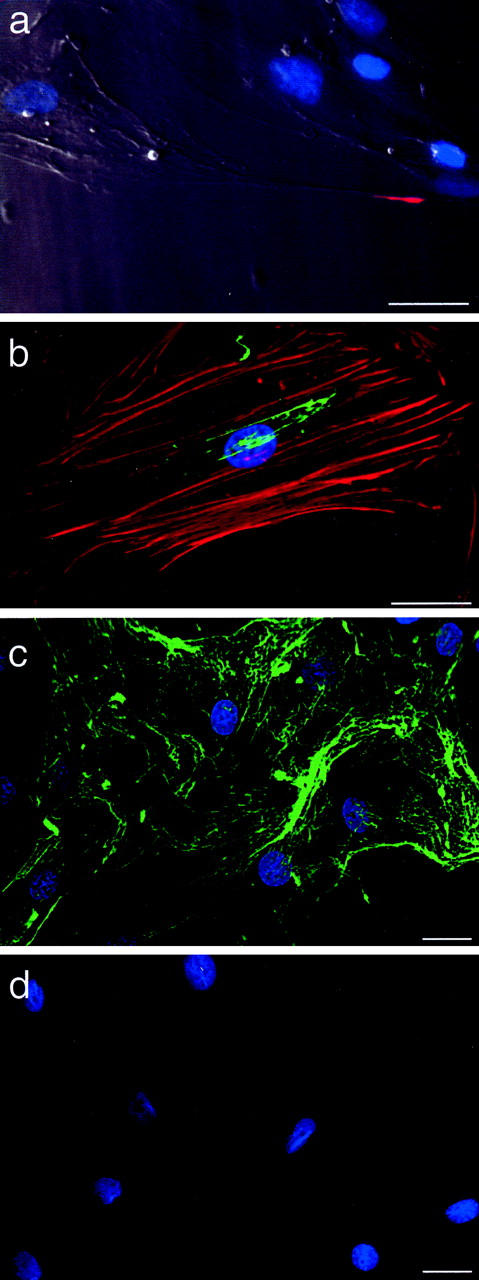
SMC-directed collagen assembly is dependent on the actin cytoskeleton. a: Collagen fibrils formed over the stretched tail of human SMCs (arrow) 6 hours after addition of soluble, Texas Red-labeled rat tail collagen (1 μg/ml) to cultures (overlay of phase contrast with fluorescence image). b: Six hours after addition of soluble, FITC-labeled bovine skin collagen (2 μg/ml) to cultures, early fibrils can be seen oriented parallel to the actin microfilament bundles, the latter stained using Texas Red-labeled phalloidin. c and d: Collagen assembly was evaluated in SMC cultures incubated for 24 hours with FITC-collagen and either DMSO (c) or cytochalasin D (10 μmol/L) (d), both added 1 hour before addition of solubilized collagen. Nuclei were counterstained with Hoechst 33258. Scale bars, 30 μm.
RhoA Mediates SMC-Directed Collagen Assembly
The relationship between the actin cytoskeleton and collagen assembly raised the possibility that signaling cascades impinging on actin assembly might influence collagen polymerization. The small GTPase RhoA is central to actin microfilament bundle assembly in several cells, apparent by injecting or overexpressing mutant forms. 30 Human muscle cells are substantially resistant to DNA transfection. Therefore, to perturb RhoA function we infected human SMCs with retrovirus containing the coding sequence for dominant-negative RhoA (T19N RhoA), constitutively active RhoA (N63L RhoA), or vector alone (pLNCX). Stably expressing SMCs were then selected with G418 and the ability of the cells to assemble a collagen fibril matrix throughout 24 hours was assessed. As shown in Figure 6 ▶ , collagen fibril assembly was substantially reduced in SMCs expressing dominant-negative RhoA (Figure 6, a and b) ▶ . In contrast, a dense mat-like pattern of fibrils formed throughout the same 24-hour period by cells expressing constitutively active RhoA (Figure 6c) ▶ . Actin microfilament bundles, detected by staining with Texas Red-labeled phalloidin, were scant in SMCs expressing T19N RhoA and well-developed in SMCs expressing N63L RhoA (data not shown).
Figure 6.
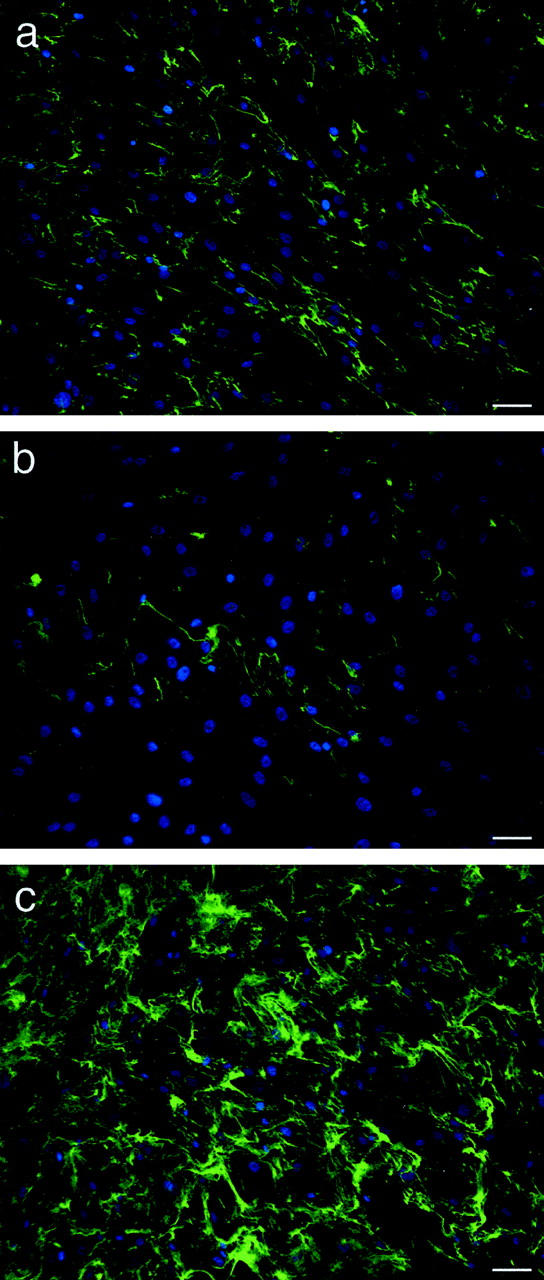
SMC-directed collagen assembly is mediated by RhoA. Human arterial SMCs were infected with retrovirus containing empty vector (pLNCX, a), cDNA for dominant-negative RhoA (pLNCX-T19N RhoA, b), or cDNA for constitutively active RhoA (pLNCX-N63L RhoA, c). Transductants were selected by G418 treatment and then incubated with FITC-labeled collagen (2 μg/ml) for 24 hours. Collagen fibrils are reduced on SMCs expressing dominant-negative RhoA and extensive on SMCs expressing constitutively active RhoA. Nuclei were counterstained with Hoechst 33258. Scale bars, 50 μm.
Lysophosphatidic Acid (LPA) Stimulates SMC-Directed Collagen Assembly
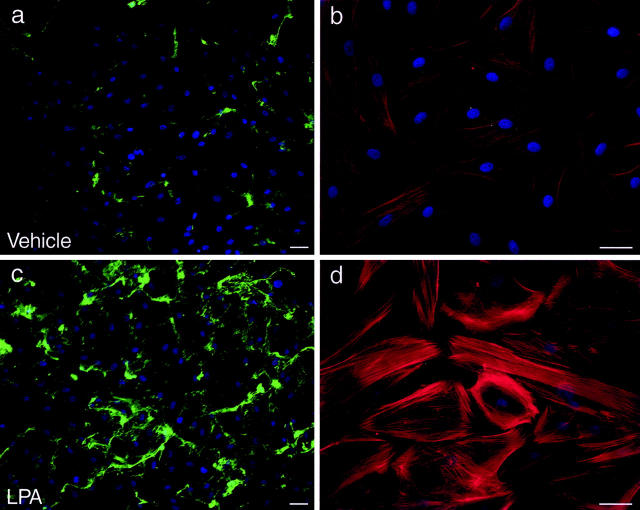
The observation that SMC-directed collagen fibril was affected by perturbations in cell signaling raised the possibility that extracellular factors that can activate RhoA might be capable of controlling the assembly process. To test this, human SMCs were incubated with 20 μmol/L of LPA, which can stimulate RhoA activation and actin stress fiber assembly, 31 in the presence of FITC-labeled solubilized collagen. As illustrated in Figure 7 ▶ , assembled collagen fibrils were much more abundant in SMCs stimulated with LPA than in vehicle-treated cultures, changes that paralleled the increase in actin stress fibers. These data provide the first demonstration that soluble agonists in the extracellular milieu can acutely modulate the assembly of type I collagen fibrils.
Figure 7.
LPA stimulates SMC-directed collagen assembly. SMCs were incubated for 12 hours with 2 μg/ml of FITC-labeled soluble collagen and either vehicle (a, b) or LPA (20 μmol/L) (c, d), added 30 minutes before addition of collagen. Cultures were fixed in 4% paraformaldehyde and nuclei stained with Hoechst 33258. Cultures were also stained with Texas Red-labeled phalloidin to show actin microfilament bundles (b and d, control and LPA-treated, respectively). The Texas Red fluorescence images were acquired with identical exposure times. Scale bars, 50 μm.
Discussion
The assembly of collagen into fibrils is vital for tissue development, repair, and function. It is well established that collagen can self-polymerize and, given this, details of the collagen assembly process have been studied extensively in cell-free systems. However, such an approach does not account for potentially important contributions of the collagen-producing cell itself. By introducing labeled, soluble collagen to human vascular SMC cultures, we have studied the assembly of type I collagen fibrils in a cellular environment. In doing so, we identified a dominant and regulatable role for SMCs in collagen fibril polymerization. SMCs orchestrated the formation of a robust fibril network at concentrations of precursor well below that required for microscopically detectable collagen self-assembly. The assembly process involved the collagen-binding α2β1 integrin and was also intimately associated with fibronectin polymerization, a process dependent on cellular interaction. Thus, notwithstanding the ability of collagen to self-assemble, the construction of collagen fibrils at the surface of SMCs is under substantial cellular control.
The model used to evaluate the role of SMCs in collagen assembly, by necessity, exposed solubilized collagen to precipitation conditions, with the potential to form biologically irrelevant collagen aggregates. However, the assembled fibrils displayed the hallmark 67-nm periodicity of native collagen fibrils, supporting the relevance of the in vitro process. It is also noteworthy that similar patterns of fibril formation occurred with two collagen preparations that differ in species, method of extraction, and likely glycosylation status and presence of nonmonomeric forms. 32 Thus, SMC-associated polymerization is not a phenomenon that proceeds with only a specific collagen preparation, but appears to be a robust response to cell-collagen interactions.
It is also notable that assembled collagen fibrils were found within spaces formed by invaginations of the plasma membrane. Previous ultrastructural studies of chick embryo tendons have documented that initial events in collagen fibrillogenesis occur within cellular recesses. 33 It was postulated that the contents of these spaces, including fibril-modifying molecules, are closely regulated by the cell to facilitate fibril growth. Whether this mechanism operates in the culture system used here is unknown. Nevertheless, the presence of newly formed collagen fibrils within plasma membrane-delimited cytoplasmic folds and depressions supports the validity of the culture model used and highlights the importance of the cell surface in the collagen polymerization process.
Type I collagen first accumulated on the cell surface as punctate collections. These aggregations were less apparent when α2β1 integrin was blocked, and fibril formation proceeded less efficiently. Conversely, activation of α2β1 integrin by a stimulating antibody enhanced collagen assembly. Previous studies have established that engagement of α2β1 integrin by a preformed collagen fibril matrix has an impact on the collagen environment, by inducing collagenase expression as well as mediating collagen contraction. 34,35 The current findings indicate that α2β1 integrin also participates in the SMC-mediated transition of soluble to insoluble collagen. Recently, the presence of collagen fibers in mutant mouse embryonic fibroblast cell lines was found to be greater when these cells were transfected with α2β1 integrin. 36 Collagen fibril assembly was not directly assessed in that study and other factors such as collagen turnover could contribute to the results. Nevertheless, the findings are consistent with our observation that α2β1 integrin contributes to collagen polymerization.
The assembly of collagen by SMCs was not however exclusively dependent on collagen-integrin interactions. Rather, the process was also intimately associated with fibronectin assembly. Assembly of both fibronectin and collagen proceeded at the same locations on the cell surface with close co-localization. Furthermore, when fibronectin assembly was inhibited by blocking α5β1 integrin, collagen assembly was also abrogated. Indeed the extent of collagen assembly inhibition under these circumstances was more striking than when α2β1 integrin was blocked. Using fibronectin-null mouse embryo cells, it was recently shown that retention of thrombospondin-1 and type I collagen in fibrillar structures depended on an intact fibronectin fibril matrix. 15 Our findings indicate that, in human vascular SMCs, it is the collagen polymerization process itself that is impacted by fibronectin. Thus, the polymerization of collagen proceeds in a manner that is spatially and functionally integrated with polymerization of fibronectin. Interestingly, α2β1 integrin blockade impeded collagen assembly but not fibronectin assembly. As well, α5β1 integrin blockade did not appear to eliminate the fine punctate collections of collagen on the cell surface. Although further studies are necessary to elucidate the precise events, we speculate that α2β1 integrin may serve to nucleate collagen fibril precursors on the cell surface, which facilitates subsequent interactions with assembling fibronectin polymers.
Early collagen fibrils were prominent along the stretched tails of SMCs and were oriented in the same direction as actin microfilament bundles. In addition, collagen assembly did not proceed after disruption of contractile forces by cytochalasin D. Moreover, fibril assembly was substantially influenced by the function of RhoA, a GTPase critical to actin microfilament bundle assembly. Assembly was impaired on cells expressing dominant-negative RhoA and accentuated on cells expressing constitutively active RhoA. Collectively, these findings establish that actin assembly is a critical regulator of collagen assembly. Presumably, forces generated from the actin-based cytoskeleton are transmitted to the collagen molecules in a manner that facilitates their polymerization and/or draws early collagen fibrils into preferred alignment for growth and packing. The transfer of force from actin to collagen could be via α2β1 integrin, or via an α5β1 integrin-fibronectin-collagen linkage. The latter process is consistent with the finding that fibronectin polymerization requires cell-generated mechanical forces that are regulated by RhoA. 37 This further supports a paradigm wherein polymerization of collagen and fibronectin may be integrated, as a supramolecular assembly process.
The lipid mediator LPA, an upstream, environmental signal for actin assembly, was also found to stimulate collagen assembly. LPA has been shown to activate RhoA and also stimulate fibronectin assembly. 38 The current findings therefore further support the involvement of RhoA, the cytoskeleton, and fibronectin in SMC-mediated collagen assembly. Moreover, the effect of LPA on collagen assembly illustrates that collagen fibril assembly can be modulated by soluble agonists of cell function.
In the vasculature, excess or abnormal collagen fibril deposition may contribute to hypertension and to aberrant remodeling after angioplasty. 39-41 The recent observation that collagen self-assembly involves inhibitable binding between the telopeptide region and sites in the triple helix suggests that blocking self-assembly is a potential therapeutic approach. 11 The current findings however open new possibilities to preventing collagen fibril accumulation such as targeting specific binding sites or the cytoskeletal events that influence fibril assembly. Exploiting the cellular interactions that direct fibril formation might also provide a basis for enhancing collagen assembly, recognizing that there are circumstanceswhereby accelerating collagen fibril assembly could be therapeutic. An important example of this is the formation of a mechanically sound fibrous cap on the surface of atherosclerotic plaques. This process, which is mediated by vascular SMCs, is critical to preventing heart attacks in predisposed individuals. 42
In summary, vascular SMCs play a substantial role in orchestrating the assembly of type I collagen fibrils. This assembly process is accomplished through a dynamic relationship between nascent collagen, α2β1 integrin, polymerizing cell-bound fibronectin, and the cytoskeleton. This cell-based level of control likely integrates with previously recognized determinants of collagen assembly—those intrinsic to the collagen molecule and those related to collagen-associated ECM such as decorin—to ensure that the collagen fibril needs of developing and repairing blood vessels are met. Because SMC-associated fibril assembly is regulatable, the findings raise new possibilities for the therapeutic control of collagen deposition.
Acknowledgments
We thank Garry Nolan (Stanford University) for the Phoenix cell line and Gary Bokoch (Scripps Research Institute) for the mutant RhoA constructs. We also thank Subrata Chakrabarti and Robert Hammond (London Health Sciences Centre) for assistance with electron microscopy.
Footnotes
Address reprint requests to J. Geoffrey Pickering, M.D., Ph.D., London Health Science Centre, 339 Windermere Rd., London, Ontario, Canada N6A 5A5. E-mail: gpickering@robarts.ca.
Supported by the Canadian Institutes of Health Research (grant MT11715), the Heart and Stroke Foundation of Canada (grant T4458), a Premiers Research Excellence Award, and a Heart and Stroke Foundation of Ontario Career Investigator Award (to J. G. P.).
References
- 1.Jaenisch R, Harbers K, Schnieke A, Lohler J, Chumakov I, Jahner D, Grotkopp D, Hoffmann E: Germline integration of Moloney murine leukemia virus at the Mov13 locus leads to recessive lethal mutation and early embryonic death. Cell 1983, 32:209-216 [DOI] [PubMed] [Google Scholar]
- 2.Breindl M, Harbers K, Jaenisch R: Retrovirus-induced lethal mutation in collagen I gene of mice is associated with an altered chromatin structure. Cell 1984, 38:9-16 [DOI] [PubMed] [Google Scholar]
- 3.Kadler KE, Holmes DF, Trotter JA, Chapman JA: Collagen fibril formation. Biochem J 1996, 316:1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trelstad RL, Silver RH: Matrix assembly. HED eds. Cell Biology of the Extracellular Matrix. 1981:pp 179-216 Plenum Publishing Corp. New York
- 5.Gross J, Kirk D: Heat precipitation of collagen from neutral salt solutions: some rate regulating factors. J Biol Chem 1958, 233:355-360 [PubMed] [Google Scholar]
- 6.Wood GC: The precipitation of collagen fibers from solution. Int Rev Connect Tissue Res 1964, 2:1-31 [DOI] [PubMed] [Google Scholar]
- 7.Williams BR, Gelman RA, Poppke DC, Piez KA: Collagen fibril formation. Optimal in vitro conditions and preliminary kinetic results. J Biol Chem 1978, 253:6578-6585 [PubMed] [Google Scholar]
- 8.Miyahara M, Njieha FK, Prockop DJ: Formation of collagen fibrils in vitro by cleavage of procollagen with procollagen proteinases. J Biol Chem 1982, 257:8442-8448 [PubMed] [Google Scholar]
- 9.Kadler KE, Hojima Y, Prockop DJ: Assembly of collagen fibrils de novo by cleavage of the type I pC-collagen with procollagen C-proteinase. Assay of critical concentration demonstrates that collagen self-assembly is a classical example of an entropy-driven process. J Biol Chem 1987, 262:15696-15701 [PubMed] [Google Scholar]
- 10.Holmes DF, Lowe MP, Chapman JA: Vertebrate (chick) collagen fibrils formed in vivo can exhibit a reversal in molecular polarity. J Mol Biol 1994, 235:80-83 [DOI] [PubMed] [Google Scholar]
- 11.Prockop DJ, Fertala A: Inhibition of the self-assembly of collagen I into fibrils with synthetic peptides. Demonstration that assembly is driven by specific binding sites on the monomers. J Biol Chem 1998, 273:15598-15604 [DOI] [PubMed] [Google Scholar]
- 12.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV: Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol 1997, 136:729-743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H: Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol 1998, 141:1277-1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svensson L, Aszodi A, Reinholt FP, Fassler R, Heinegard D, Oldberg A: Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem 1999, 274:9636-9647 [DOI] [PubMed] [Google Scholar]
- 15.Sottile J, Hocking DC: Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell 2002, 13:3546-3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu C, Keivens VM, O’Toole TE, McDonald J, Ginsberg MH: Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell 1995, 83:715-724 [DOI] [PubMed] [Google Scholar]
- 17.Schwarzbauer JE, Sechler JL: Fibronectin fibrillogenesis: a paradigm for extracellular matrix assembly. Curr Opin Cell Biol 1999, 11:622-627 [DOI] [PubMed] [Google Scholar]
- 18.Lee RT, Berditchevski F, Cheng GC, Hemler ME: Integrin-mediated collagen matrix reorganization by cultured human vascular smooth muscle cells. Circ Res 1995, 76:209-214 [DOI] [PubMed] [Google Scholar]
- 19.Pickering JG, Bacha P, Weir L, Jekanowski J, Nichols JC, Isner JM: Prevention of smooth muscle cell outgrowth from human atherosclerotic plaque by a recombinant fusion protein specific for the epidermal growth factor receptor. J Clin Invest 1993, 91:724-729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell E, Ivarsson B, Merrill C: Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci USA 1979, 76:1274-1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hangan D, Uniyal S, Morris V, MacDonald I, von Ballestrem C, Chau T, Schmidt E, Chambers A, Groom A, Chan BMC: Integrin VLA-2 (α2β1) function in post-extravasation movement of human rhabdomyosarcoma cells in the liver. Cancer Res 1996, 56:3142-3149 [PubMed] [Google Scholar]
- 22.Ho WC, Heinemann C, Hangan D, Uniyal S, Morris VL, Chan BM: Modulation of in vivo migratory function of alpha 2 beta 1 integrin in mouse liver. Mol Biol Cell 1997, 8:1863-1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pickering JG, Chow LH, Li S, Rogers KA, Rocnik EF, Zhong R, Chan BM: α5β1 integrin expression and luminal edge fibronectin matrix assembly by smooth muscle cells after arterial injury. Am J Pathol 2000, 156:453-465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S, Han J, Sells MA, Chernoff J, Knaus UG, Ulevitch RJ, Bokoch GM: Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J Biol Chem 1995, 270:23934-23936 [DOI] [PubMed] [Google Scholar]
- 25.Pear WS, Nolan GP, Scott ML, Baltimore D: Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA 1993, 90:8392-8396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paige MF, Rainey JK, Goh MC: Fibrous long spacing collagen ultrastructure elucidated by atomic force microscopy. Biophys J 1998, 74:3211-3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pickering JG, Uniyal S, Ford C, Chau T, Laurin MA, Chow LH, Ellis CG, Fish J, Chan BMC: Fibroblast growth factor-2 potentiates vascular smooth muscle cell migration to platelet-derived growth factor: upregulation of α2β1 integrin and disassembly of actin filaments. Circ Res 1997, 80:627-637 [DOI] [PubMed] [Google Scholar]
- 28.Furcht LT, Smith D, Wendelschafer-Crabb G, Mosher DF, Foidart JM: Fibronectin presence in native collagen fibrils of human fibroblasts: immunoperoxidase and immunoferritin localization. J Histochem Cytochem 1980, 28:1319-1333 [DOI] [PubMed] [Google Scholar]
- 29.Wu C, Bauer JS, Juliano RL, McDonald JA: The alpha 5 beta 1 integrin fibronectin receptor, but not the alpha 5 cytoplasmic domain, functions in an early and essential step in fibronectin matrix assembly. J Biol Chem 1993, 268:21883-21888 [PubMed] [Google Scholar]
- 30.Hall A: Rho GTPases and the actin cytoskeleton. Science 1998, 279:509-514 [DOI] [PubMed] [Google Scholar]
- 31.Ridley AJ, Hall A: The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992, 70:389-399 [DOI] [PubMed] [Google Scholar]
- 32.Vogel W, Gisg GD, Alves F, Pawson T: The discoiding domain receptor tyrosine kinases are activated by collagen. Mol Cell 1997, 1:13-23 [DOI] [PubMed] [Google Scholar]
- 33.Birk DE, Trelstad RL: Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. J Cell Biol 1986, 103:231-240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langholz O, Rockel D, Mauch C, Kozlowska E, Bank I, Krieg T: Collagen and collagenase gene expression in three-dimensional collagen lattices are differentially regulated by α1β1 and α2β1 integrins. J Cell Biol 1995, 131:1903-1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein CE, Dressel D, Steinmayer T, Mauch C, Eckes B, Krieg T, Bankert RB, Weber L: Integrin α2β1 is upregulated in fibroblasts and highly aggressive melanoma cells in three-dimensional collagen lattices and mediates the reorganization of collagen I fibrils. J Cell Biol 1991, 115:1427-1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Velling T, Risteli J, Krister W, Mosher DF, Johansson S: Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins a11β1 and a2β1. J Biol Chem 2002, 277:37377-37381 [DOI] [PubMed] [Google Scholar]
- 37.Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K: Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol 1998, 141:539-551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Q, Magnusson MK, Mosher DF: Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Mol Biol Cell 1997, 8:1415-1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strauss BH, Chisholm RJ, Keeley FW, Gotlieb AI, Logan RA, Armstrong PW: Extracellular matrix remodeling after balloon angioplasty injury in a rabbit model of restenosis. Circ Res 1994, 75:650-658 [DOI] [PubMed] [Google Scholar]
- 40.Pickering JG, Ford CM, Chow LH: Evidence for rapid accumulation and persistently disordered architecture of fibrillar collagen in human coronary restenosis lesions. Am J Cardiol 1996, 78:633-637 [DOI] [PubMed] [Google Scholar]
- 41.Geary RL, Nikkari ST, Wagner WD, Williams JK, Adams MR, Dean RH: Wound healing: a paradigm for lumen narrowing after arterial reconstruction. J Vasc Surg 1998, 27:96-106 [DOI] [PubMed] [Google Scholar]
- 42.Libby P: Molecular basis of acute coronary syndromes. Circulation 1995, 91:2844-2850 [DOI] [PubMed] [Google Scholar]