Abstract
Multinucleated foreign body giant cells (FBGCs) form by monocyte-derived macrophage fusion on implanted biomedical devices and are believed to mediate oxidative damage to biomaterial surfaces. Our in vitro system of human macrophage culture and interleukin (IL)-4-induced FBGC formation was developed to study the macrophage fusion mechanism and the physiological significance of FBGCs on implanted biomaterials and at other sites of chronic inflammation. Here, we demonstrate that the antioxidant vitamin E (90% α-tocopherol) moderately induces macrophage fusion and increases IL-4-induced FBGC formation. Moreover, purified α-tocopherol, but not β-, γ-, or δ-tocopherol, most remarkably induces macrophage fusion, leading to cultures of confluent FBGCs below normal plasma concentrations. This is not observed with the similar antioxidants probucol or Trolox, suggesting that the α-tocopherol effects on FBGC formation are independent of its antioxidant activity. Consistent with the reported activation of diacylglycerol kinase by α-tocopherol, the diacylglycerol kinase inhibitor R59022 completely abrogates FBGC formation. R59022 inhibition of IL-4-induced FBGC formation is reversed by α-tocopherol, suggesting that FBGC formation involves diacylglycerol kinase activation. This study suggests a novel role for diacylglycerol kinase in the mechanism of macrophage fusion/FBGC formation at sites of chronic inflammation and reveals that the pleiotropic lipophilic compound, α-tocopherol, is a highly potent macrophage fusion factor.
Studies in our laboratory focus on the inflammatory response to implanted biomaterials and, particularly, on inflammatory cell/biomaterial interactions, including mechanisms of monocyte-derived macrophage adhesion, cytokine secretion, and biomaterial-mediated apoptosis of inflammatory cells. 1-5 A clinically significant consequence of macrophage/biomaterial interactions is adherent macrophage fusion leading to the formation of multinucleated foreign body giant cells (FBGCs) 6 on biomaterial surfaces. 7,8 This phenomenon, a hallmark histiological feature of chronic inflammation, is accompanied by FBGC-mediated biomaterial degradation. 9,10 This is believed to result from the action of reactive oxygen species within an acidified closed compartment between FBGCs and their biomaterial substrate. 11 Long-term biomaterial degradation may lead to biomedical device failure and implant removal. In our in vivo studies with poly(etherurethane) biomaterials designed to minimize material degradation, if vitamin E was incorporated in the polymer, degradation was significantly decreased. 12 These results suggest that the antioxidant capabilities of vitamin E inhibited FBGC-mediated biomaterial degradation, although the effects of vitamin E on macrophages and FBGC formation remain unclear.
Vitamin E was originally described as an essential nutrient for reproduction 13 and is now known to consist of four tocopherols and four tocotrienols, of which α-tocopherol is the most abundant in tissues and possesses the greatest biological activity. 14,15 Although compared to other lipids, vitamin E is a minor component in biological membranes, it is ubiquitous and believed to be important in preserving cell membrane integrity through its antioxidant activity and its association with lipid components that may otherwise destabilize membrane bilayer structure. 15 Therefore, multiple effects of vitamin E are attributed to its free radical scavenging abilities and inhibition of lipid peroxidation, including the inhibition of low-density lipoprotein oxidation leading to cardiovascular disease. 16 In addition, many functions of α-tocopherol have now been described that have been distinguished from or are not clearly associated with antioxidant activity. These include effects on phospholipase A2, cyclooxygenase, and protein kinase C activities, inhibition of expression of adhesion molecules on endothelial cells and monocyte-endothelial cell adhesion, transcriptional regulation of scavenger receptors in macrophages, inhibition of smooth muscle cell proliferation and platelet aggregation, inhibition of the generation of reactive oxygen intermediates from monocytes, and activation of diacylglycerol kinase. 17,18
To correlatively study the mechanisms of macrophage fusion, FBGC formation, and biomaterial degradation under relatively controlled conditions in vitro, we developed a culture system for generating FBGCs from human monocyte-derived macrophages using the lymphokine interleukin (IL)-4 as the fusion-inducing stimulus. 19 IL-4 was selected for our studies because of an early report that IL-4 induced multinucleated giant cell formation from IL-3-treated mouse bone marrow macrophages; although the reported fusion rates were only ∼10%, 20 the giant cells obtained were morphologically similar to those observed on retrieved biomaterials. In contrast, in separate studies with human blood monocytes, it was concluded that IL-4 was not a human macrophage fusion factor, and the earlier findings were attributed to species-specific effects. 21,22 Nevertheless, by using autologous human serum to supplement the culture medium and permitting time for monocyte-to-macrophage development before the application of IL-4, we were subsequently able to demonstrate that IL-4 is indeed a potent human macrophage fusion factor that generates large multinucleated giant cells of the foreign body type. 19 The relevance and participation of IL-4 in FBGC formation on biomaterials in vivo was confirmed by antibody neutralization of IL-4 activity. 23
The in vitro FBGCs obtained with IL-4 from human monocyte-derived macrophages are morphologically indistinguishable from those that form on implanted biomaterials, 19 which may be >1 mm in diameter and occupy up to 25% of implant surface area. 24 These giant cells apparently arise from remarkable degrees of macrophage fusion accompanied by extensive cytoplasmic spreading and often contain >100 randomly arranged nuclei. To date, IL-4 and the related lymphokine IL-13 25 are the only human macrophage fusion factors known to induce these very large FBGCs. Under otherwise identical culture conditions, we found that macrophage fusion induced by interferon-γ plus IL-3 22 leads to multinucleated giant cells of the Langhans type. 19 Other investigators have also used certain lectins to generate Langhans-type giant cells, 26 which exhibit a characteristic circular arrangement of nuclei within a relatively much smaller and generally circular or ovoid cytoplasm as documented more than a century ago. 27 The physiological significance of morphological variants of multinucleated giant cells is unknown.
Initial mechanistic studies exploiting the in vitro IL-4-induction of FBGCs demonstrated a role for IL-4-induced mannose receptor activity 28 in the macrophage fusion mechanism. 29 These results were extended to include similar roles for IL-13. 25 Further, we demonstrated that macrophage fusion leading to FBGC formation is material surface property-dependent, 1,30 is influenced by adsorbed plasma proteins, 31-33 and depends on the participation of β1 and β2 integrins. 2 In this study, we investigate the effects of tocopherols on FBGC formation using exogenous vitamin E, purified tocopherols, other antioxidants, and metabolic inhibitors.
Materials and Methods
Materials
Vitamin E acetate and probucol were from Sigma Chemical Co. (St. Louis, MO); purified tocopherols from Calbiochem (La Jolla, CA); and R59022, R59949, and Trolox from Biomol (Plymouth Meeting, PA). Reagents were dissolved in ethanol or dimethyl sulfoxide as required and added to cells in culture such that these combined solvents were present at ≤0.25% v/v. Recombinant human IL-4 (R&D Systems, Minneapolis, MN) was reconstituted in 0.5% bovine serum albumin (low endotoxin, Sigma) in phosphate-buffered saline (PBS) as recommended by the manufacturer and stored in aliquots at −20°C before use.
In Vitro Monocyte/Macrophage Culture and FBGC Formation
Human monocytes were isolated and cultured as previously described. 2,34 Briefly, 2 × 10 5 monocytes per well were plated in Smart Plastic (RGD-modified; ICN, Irvine, CA) 96-well culture plates in 0.1 ml of serum-free medium for macrophages (SFM; Life Technologies, Inc., Grand Island, NY) supplemented with 20% autologous serum. After 1.5 hours, nonadherent cells and serum were removed by washing with PBS containing Ca++ and Mg++ (PBS++, Life Technologies, Inc.) at 37°C. The adherent cells were recovered with 0.2 ml of unsupplemented SFM, and incubated for 3 days at 37°C in a humidified atmosphere of 95% air and 5% CO2. On day 3, the medium was replaced with 0.2 ml of unsupplemented SFM without or with vitamin E, purified tocopherols, or other additives as stated in the figure legends. Where indicated, 10 ng/ml of IL-4 was added to induce macrophage fusion. The macrophages were then incubated until day 7. At this time, the plates were washed twice with PBS++ at 37°C, fixed with methanol, and stained with May-Grünwald/Giemsa for evaluation of macrophage/FBGC morphology, fusion index, and percent adhesion. This is an established culture system in which 15 ng/ml of IL-4 typically induces a maximum of 60 to 70% fusion of human monocyte-derived macrophages on RGD-modified culture surfaces within only 7 days; 31 this is likely because of the integrin dependency of macrophage development and FBGC formation. 2 In this study, we used 10 ng/ml of IL-4 to obtain slightly suboptimal macrophage fusion such that increases because of other additives could be observed.
Determination of Percent Adhesion and Percent FBGC Formation
Adhesion was evaluated for each sample by counting the average number of cells in two representative low-power (×20) microscopic fields (∼250 cells). Results with inhibitors or other reagents are expressed as a percentage of adherent cell numbers in control wells with no other additive, ie, percent adhesion. The overall extent of FBGC formation was evaluated by first determining the macrophage fusion index, ie, the fraction of nuclei within multinucleated giant cells (more than two nuclei) in the same fields. This value was multiplied by percent adhesion for each sample to obtain percentage of FBGC formation. Results represent the mean ± SEM for at least three different monocyte donors. Statistical significance was assigned at the 95% confidence level (P < 0.05) using the unpaired Student’s t-test. Photomicrograph images of representative samples were obtained using a Nikon DXM-1200 digital camera (Nikon, Melville, NY) with ACT-1 software in single image acquisition mode.
Results
Vitamin E Induces Macrophage Fusion and Increases IL-4-Induced FBGC Formation
Plasma or serum α-tocopherol levels are used to assess dietary vitamin E status; acceptable concentrations have been defined as >16 μmol/L, with cardiovascular benefits correlated to concentrations of >30 μmol/L. 35 We cultured human monocyte-derived macrophages in serum-free medium in the presence of vitamin E, probucol, or Trolox at concentrations of 10 to 250 μmol/L in the absence or presence of the macrophage fusion-inducing cytokine IL-4. Probucol, like vitamin E, is a potent lipophilic antioxidant. However, it is structurally dissimilar and has been useful to distinguish antioxidant and nonantioxidant effects of vitamin E. 17,18 Trolox is a water-soluble form of vitamin E that retains antioxidant activity, but is unable to penetrate biological membranes. 36 As shown in Table 1 ▶ , vitamin E alone moderately induces FBGC formation to ∼40% above the untreated control macrophages, which exhibit no observable FBGC formation. At the same concentrations, vitamin E increases the levels of IL-4-induced FBGC formation by approximately the same degrees, such that the FBGC population is essentially confluent. However, these effects are not mimicked by probucol or Trolox, which each, conversely, reduce FBGC formation from the controls with no added antioxidants in the presence of IL-4 (Table 1) ▶ and do not alter morphology compared to untreated macrophages (not shown). These data suggest a novel effect of vitamin E on macrophages that is not solely because of its antioxidant properties.
Table 1.
Effects of Vitamin E, Probucol, or Trolox on Percent FBGC Formation
| Concentration (μmol/L) | Vitamin E | Probucol | Trolox | |||
|---|---|---|---|---|---|---|
| No IL-4 | + IL-4 | No IL-4 | + IL-4 | No IL-4 | + IL-4 | |
| 0 | 0 ± 0 | 51 ± 12 | 0 ± 0 | 51 ± 12 | 0 ± 0 | 51 ± 12 |
| 10 | 30 ± 8* | 70 ± 28 | 3 ± 2 | 27 ± 5* | 1 ± 1 | 25 ± 14 |
| 50 | 40 ± 10* | 94 ± 12* | 3 ± 3 | 26 ± 4* | 1 ± 1 | 20 ± 12* |
| 100 | 39 ± 12* | 100 ± 5* | 2 ± 2 | 26 ± 13 | 0 ± 0 | 19 ± 16* |
| 250 | 32 ± 18* | 89 ± 17* | 1 ± 1 | 25 ± 8* | 3 ± 3 | 13 ± 10* |
Day 3 monocyte-derived macrophages were treated without (No) or with (+) 10 ng/ml of IL-4 and different concentrations of the indicated agents, incubated until day 7, fixed with methanol, and stained with May-Grünwald/Giemsa. Percent adhesion was determined by averaging the number of adherent macrophages in two low-power fields (×20) and expressing the result as a percentage of otherwise untreated macrophages. The macrophage fusion index, ie, the fraction of nuclei within multinucleated giant cells (cells with >2 nuclei) was determined for the same microscopic fields. This value was then multiplied by percent adhesion for each sample to obtain percent FBGC formation.
*Significantly different from control values without or with IL-4 and no other additive (P < 0.05).
Purified α-Tocopherol Alone, but Not Other Tocopherols, Promotes FBGC Formation More Potently than Vitamin E and Increases IL-4-Induced FBGC Formation
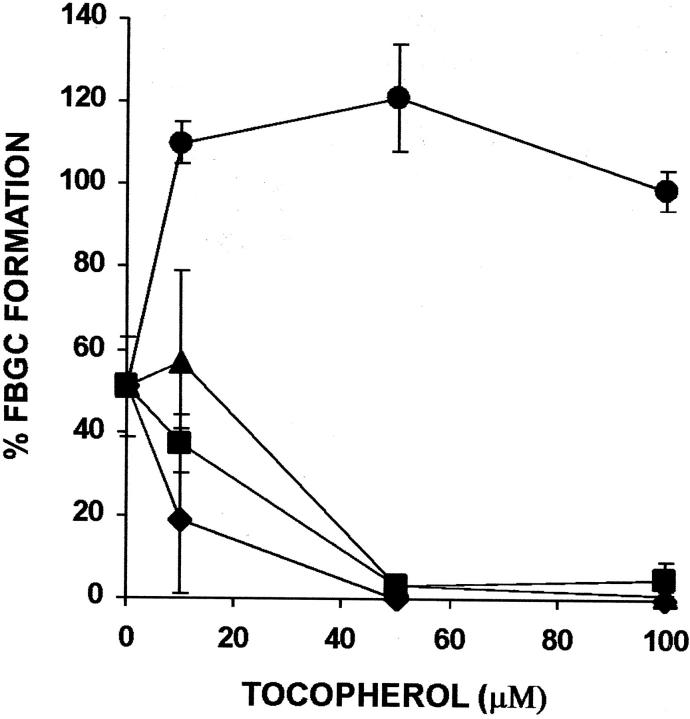
Vitamin E is composed chiefly of α-tocopherol, with β-, γ-, and δ-tocopherols and tocotrienols comprising the remainder. 15 β-tocopherol has similar antioxidant activity, but does not mimic the nonantioxidant effects of α-tocopherol. It is believed to actually inhibit these effects by competition and, like probucol, has been used to distinguish antioxidant and nonantioxidant effects of α-tocopherol. 17 As demonstrated in Figure 1a ▶ , purified α-tocopherol strongly induces FBGC formation in a concentration-dependent manner leading to ∼80% FBGCs at only 10 μmol/L. Therefore, α-tocopherol is considerably more potent than whole vitamin E, and the effective concentration of α-tocopherol in this culture system is below normal plasma levels. However, as shown in Figure 1b ▶ , although this high degree of FBGC formation is maintained by α-tocopherol at concentrations of up to 100 μmol/L, neither the β, γ, or δ isomer induces comparable FBGC formation. In Figure 2, a to c ▶ , representative photomicrographs of macrophages cultured in the absence or presence of vitamin E or α-tocopherol demonstrate no, moderate, or very strong FBGC formation, respectively. In all cases, it can be seen that these multinucleated giant cells appear to be of the foreign body-type, rather than the Langhans-type, morphological variant. These results point to the α-tocopherol component of vitamin E as the macrophage fusion-inducing factor and also suggest that this effect is not because of its antioxidant properties.
Figure 1.
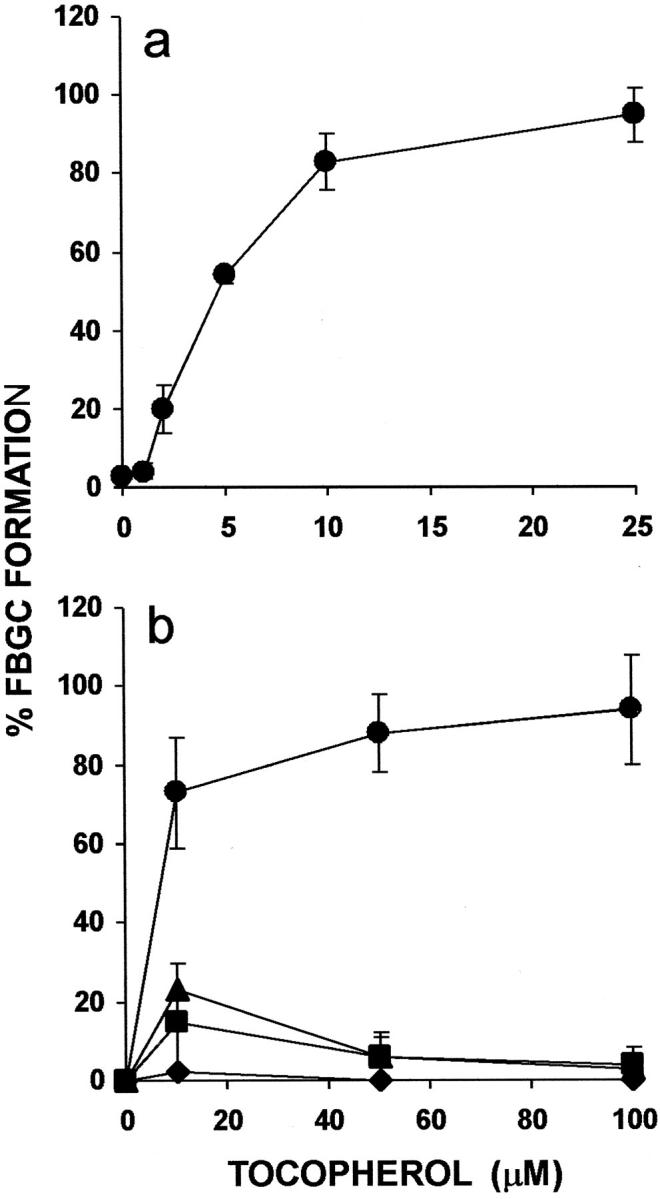
Effects of purified tocopherols on FBGC formation. a: Dose dependency of α-tocopherol. b: Comparison of tocopherols. The indicated concentrations of purified α-tocopherol (circles), β-tocopherol (squares), γ-tocopherol (triangles), or δ-tocopherol (diamonds) were added to day 3 macrophages, and the cultures were fixed and stained with May-Grünwald/Giemsa on day 7. Results are expressed as percent FBGC formation ± SEM, n = 3 monocyte donors that are different for a and b.
Figure 2.
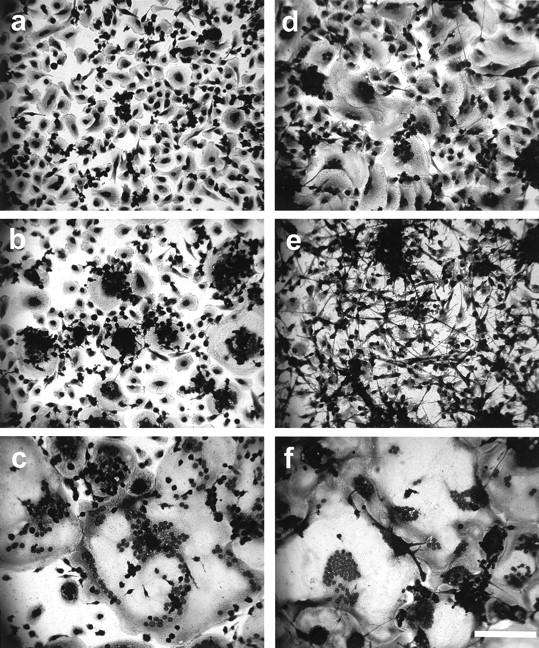
Vitamin E- or α-tocopherol-induced FBGC formation, and reversal of R59022 inhibition of IL-4-induced FBGC formation by α-tocopherol. Day 3 macrophages were otherwise untreated (a) or treated with 50 μmol/L vitamin E (b), 50 μmol/L α-tocopherol (c), 10 ng/ml IL-4 (d), 10 ng/ml IL-4 plus 10 μmol/L R59022 (e), or 10 ng/ml IL-4 plus 10 μmol/L R59022 plus 100 μmol/L α-tocopherol (f), and fixed and stained with May-Grünwald/Giemsa on day 7. Scale bar, 100 μm.
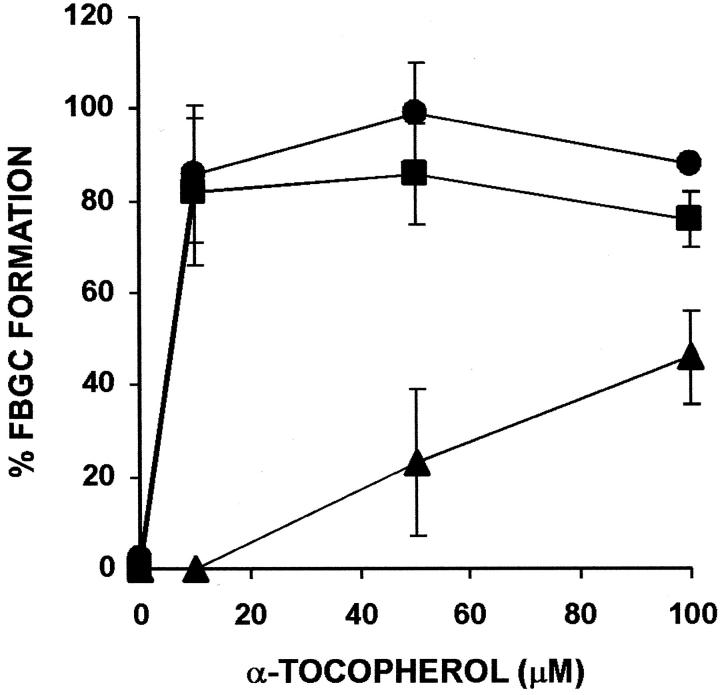
We further explored the effects of purified tocopherols on macrophage fusion by asking how they would influence percentage of FBGC formation induced by IL-4. As indicated in Figure 3 ▶ , α-tocopherol strongly increases IL-4-induced FBGC formation (50%) essentially to confluence (≥100%). Again, neither β-, γ-, or δ-tocopherol performs similarly at these concentrations, each actually decreasing IL-4-induced FBGC formation relative to the IL-4-only control.
Figure 3.
Effects of purified tocopherols on IL-4-induced FBGC formation. The indicated concentrations of purified α-tocopherol (circles), β-tocopherol (squares), γ-tocopherol (triangles), or δ-tocopherol (diamonds) were added to day 3 macrophages together with 10 ng/ml of IL-4, and the cultures were fixed and stained with May-Grünwald/Giemsa on day 7. Results are expressed as percent FBGC formation ± SEM, n = 3 monocyte donors.
The Effects of Vitamin E or α-Tocopherol on Macrophage/FBGC Adhesion
Macrophage development and adhesion are required for IL-4-induced FBGC formation in vitro. 19 If adhesion is sufficiently restricted, either pharmacologically or by culture material surface modifications, FBGC formation cannot occur. 1-3,30 Therefore, we asked whether the effects of vitamin E and α-tocopherol on FBGC formation could be related to effects on macrophage adhesion. Compared to probucol and Trolox, vitamin E and α-tocopherol each appear to increase the adhesion of otherwise untreated macrophages slightly (Table 2) ▶ , although these increases are not statistically significant. In IL-4-treated macrophages, α-tocopherol has a more pronounced effect, significantly increasing adhesion in the 10 to 100 μmol/L range (Table 3) ▶ . In contrast, other tocopherols, and particularly δ-tocopherol, decrease percent adhesion without or with IL-4 at the same concentrations at which α-tocopherol promotes macrophage fusion. Thus, the negative effects of these isomers on FBGC formation may result from inhibition of adhesion, and their effects on macrophage fusion cannot be clearly evaluated. Although both probucol and Trolox decrease percentage of FBGC formation induced by IL-4 (Table 1) ▶ , neither of these reagents decreases the percentage of macrophage adhesion without or with IL-4 (Tables 2 and 3) ▶ .
Table 2.
Effects of Vitamin E, Tocopherols, Probucol, or Trolox on Percent Adhesion of Otherwise Untreated Macrophages
| Concentration (μmol/L) | Vitamin E | Tocopherols | Probucol | Trolox | |||
|---|---|---|---|---|---|---|---|
| α | β | γ | δ | ||||
| 10 | 114 ± 5* | 123 ± 30 | 44 ± 14* | 57 ± 5* | 8 ± 8* | 93 ± 11 | 99 ± 4 |
| 50 | 115 ± 15 | 118 ± 12 | 31 ± 10* | 22 ± 21* | 0 ± 0* | 94 ± 14 | 103 ± 6 |
| 100 | 120 ± 6* | 134 ± 24 | 28 ± 17* | 15 ± 15* | 1 ± 1* | 101 ± 8 | 110 ± 3 |
| 250 | 105 ± 19 | 117 ± 20 | 61 ± 17* | 17 ± 13* | 0 ± 0* | 97 ± 9 | 116 ± 13 |
Day 3 monocyte-derived macrophages were treated with different concentrations of the indicated agents, incubated until day 7, fixed with methanol, and stained with May-Grünwald/Giemsa. The number of adherent macrophages in two low-power fields (×20) was averaged and expressed as a percentage of otherwise untreated macrophages.
*Significantly different from the control value with no other additive (P < 0.05).
Table 3.
Effects of Vitamin E, Tocopherols, Probucol, or Trolox on Percent Adhesion of IL-4-Treated Macrophages/FBGC
| Concentration (μmol/L) | Vitamin E | Tocopherols | Probucol | Trolox | |||
|---|---|---|---|---|---|---|---|
| α | β | γ | δ | ||||
| 10 | 119 ± 35 | 141 ± 17* | 48 ± 10* | 115 ± 1 | 81 ± 30 | 107 ± 9 | 114 ± 8 |
| 50 | 126 ± 33 | 149 ± 13* | 18 ± 6* | 25 ± 9* | 0 ± 0* | 98 ± 8 | 105 ± 1 |
| 100 | 130 ± 25 | 131 ± 15* | 27 ± 8* | 16 ± 9* | 0 ± 0* | 105 ± 10 | 118 ± 13 |
| 250 | 122 ± 39 | 112 ± 20 | 18 ± 18* | 14 ± 9* | 0 ± 0* | 99 ± 10 | 126 ± 5* |
Day 3 monocyte-derived macrophages were treated with 10 ng/ml of IL-4 and different concentrations of the indicated agents, incubated until day 7, fixed with methanol, and stained with May-Grünwald/Giemsa. The number of adherent macrophages in two low-power fields (×20) was averaged and expressed as a percentage of otherwise untreated macrophages.
*Significantly different from the control value with IL-4 with no other additive (P < 0.05).
The Diacylglycerol Kinase Inhibitor, R59022, Prevents FBGC Formation, an Effect that Is Reversed by α-Tocopherol
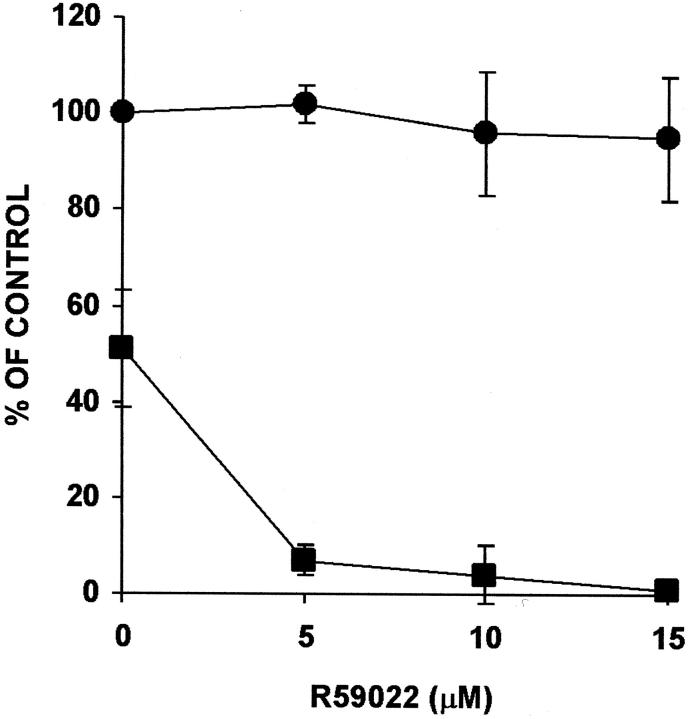
Among the reported effects of α-tocopherol is the activation of diacylglycerol kinase. 18,37 To investigate the mechanism by which α-tocopherol increases FBGC formation, we tested the effects of R59022 and R59949, which are inhibitors of diacylglycerol kinase activity, 38,39 on α-tocopherol-induced FBGC formation. These results are presented in Figure 4 ▶ , which demonstrates that 15 μmol/L of R59022 completely abrogates the high degree of FBGC formation induced by 10 μmol/L of α-tocopherol, but increasing concentrations of α-tocopherol are able to reverse the inhibition. Essentially identical results are observed with R59949 (data not shown). In addition, and as shown in Figure 5 ▶ , although percent adhesion is not decreased, R59022 completely prevents IL-4-induced FBGC formation and severely restricts cytoplasmic spreading (Figure 2 ▶ , compare d and e).
Figure 4.
Effect of R59022 on α-tocopherol-induced FBGC formation. The indicated concentrations of purified α-tocopherol were added to day 3 macrophages in the absence (circles) or the presence of 5 μmol/L (squares) or 15 μmol/L (triangles) of R59022, and the cultures were fixed and stained with May-Grünwald/Giemsa on day 7. Results are expressed as percent FBGC formation ± SEM, n = 3 monocyte donors.
Figure 5.
Effect of R59022 on macrophage/FBGC adhesion and FBGC formation in IL-4-treated cultures. The indicated concentrations of R59022 were added to day 3 macrophages, and the cultures were fixed and stained with May-Grünwald/Giemsa on day 7. Results are expressed as percent adhesion (circles) or percent FBGC formation (squares) ± SEM, n = 3 monocyte donors.
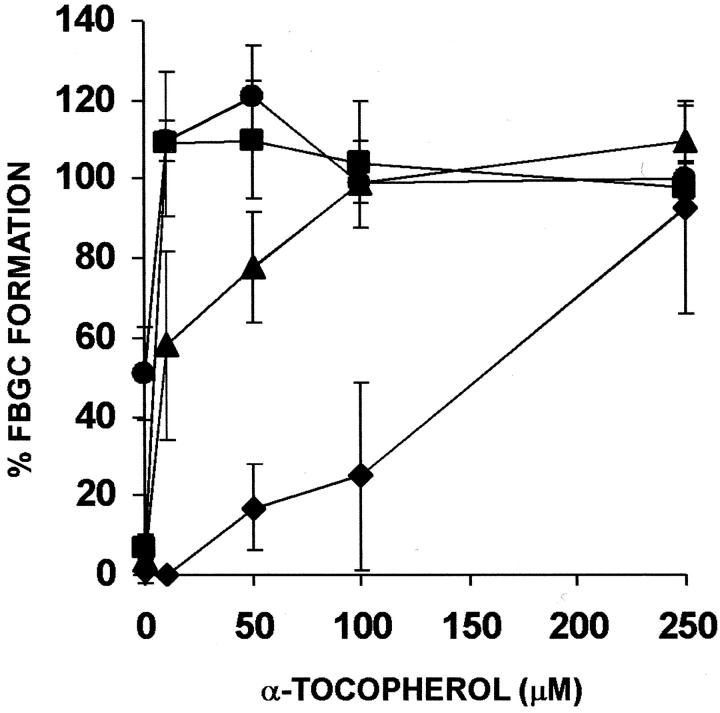
We then asked whether α-tocopherol would reverse R59022-mediated inhibition of IL-4-induced FBGC formation. The results are shown morphologically in Figure 2f ▶ and depicted in Figure 6 ▶ , which demonstrates that, at inhibitory concentrations of R59022 (10 or 15 μmol/L), increasing concentrations of α-tocopherol overcome the inhibition of IL-4-induced FBGC formation. These combined results suggest that both the α-tocopherol-mediated induction of macrophage fusion and enhancement of IL-4-induced FBGC formation are dependent on diacylglycerol kinase activity.
Figure 6.
Effect of R59022 on IL-4-induced FBGC formation, and reversal of R59022 inhibition of IL-4-induced FBGC formation by α-tocopherol. The indicated concentrations of purified α-tocopherol were added to day 3 macrophages together with 10 ng/ml of IL-4 in the absence (circles) or the presence of 5 μmol/L (squares), 10 μmol/L (triangles), or 15 μmol/L (diamonds) of R59022, and the cultures were fixed and stained with May-Grünwald/Giemsa on day 7. Results are expressed as percent FBGC formation ± SEM, n = 3 monocyte donors.
Phosphatidate phosphohydrolase catalyzes the dephosphorylation of phosphatidic acid, which is the product of diacylglycerol kinase activity on diacylglycerol. 38,40 The phosphatidate phosphohydrolase inhibitor propranolol would thus be expected to increase the pool of phosphatidic acid by inhibiting its conversion to diacylglycerol. In further experiments, we observed increases in IL-4-induced macrophage fusion to 89 ± 10% and 119 ± 3% with 5 or 10 μmol/L of propranolol, respectively. The resulting FBGCs are morphologically similar to those obtained with α-tocopherol (not shown).
Discussion
We report that α-tocopherol, the major component of vitamin E, induces extensive human monocyte-derived macrophage fusion leading to FBGC formation in vitro. This novel phenomenon is apparently independent of antioxidant activity per se and occurs with high potency below normal plasma α-tocopherol concentrations. In addition, we find that α-tocopherol increases the degree of macrophage fusion obtainable with IL-4 in vitro. Finally, we report that both α-tocopherol- and IL-4-mediated macrophage fusion are abrogated by R59022, suggesting that FBGC formation induced by either of these fusion factors proceeds along a common pathway involving activation of a diacylglycerol kinase. Thus, in addition to IL-4 and IL-13, and as well as the multiple other functions currently ascribed to this lipophilic vitamin, α-tocopherol may play a role in the formation of multinucleated FBGCs at sites of chronic inflammation in vivo.
The participation of diacylglycerol kinase in the mechanism of macrophage fusion would extend an early observation that phorbol myristate acetate, a synthetic diacylglycerol analog and activator of protein kinase C, induces macrophage fusion. 41 Although the resultant multinucleated cells were not typical foreign body- or Langhans-type, this was the first evidence that diacylglycerol metabolism plays a role in macrophage fusion. More recently, we observed that inhibition of phosphatidylinositol-specific phospholipase C or protein kinase C activity has substantial effects on IL-4-induced FBGC formation, 42 indicating that the phospholipase C-mediated generation of diacylglycerol is a critical step in this process. Additionally, the present results suggest that the metabolism of diacylglycerol by diacylglycerol kinase is an event without which macrophage adhesion can be maintained, but macrophage fusion cannot occur.
Although macrophage fusion can be separately evaluated and distinguished from adhesion in vitro, adhesion with cytoplasmic spreading is prerequisite for macrophage fusion. IL-4-induced FBGC formation does not occur with insufficient densities of adherent macrophages. 43 The α-tocopherol-mediated increases in adhesion could contribute somewhat to α-tocopherol enhancement of IL-4-induced FBGC formation. However, although α-tocopherol clearly promotes extensive cytoplasmic spreading, it very strongly induces FBGC formation in the absence of IL-4 without statistically significant increases in macrophage adhesion. Furthermore, R59022 does not prevent macrophage adhesion at concentrations that prevent IL-4-induced FBGC formation, which α-tocopherol is able to restore. These combined results suggest that α-tocopherol activates diacylglycerol kinase, and that the functional outcome of diacylglycerol kinase activity leading to macrophage fusion may include cytoplasmic spreading.
The nonantioxidant α-tocopherol-mediated inhibition of smooth muscle cell proliferation is believed to be brought about by dephosphorylation of protein kinase C. 18,44 However, the α-tocopherol-mediated activation of diacylglycerol kinase 37 results in phosphorylation of diacylglycerol and the generation of phosphatidic acid. 38,45 Phosphatidic acid is well recognized as an important intra- and extracellular messenger with multiple effects, 40 including the activation of neutrophil cytosolic phospholipase A2 46 and actin polymerization. 47 Phosphatidic acid and its lysophosphatidic acid product, resulting from phospholipase A2 activity, have both been implicated in monocyte chemotaxis and migration. 48 Lysophosphatidic acid has been further correlated to cell survival, 49 cytoskeletal alterations, 50 and migration and matrix assembly. 51,52 The present results may be related to a report of neutrophil granule-plasma membrane fusion in which phosphatidic acid is implicated as a fusogenic phospholipid. 53 Inasmuch as both α-tocopherol- and IL-4-induced FBGC formation are attenuated by R59022, the possibility is raised that the phosphorylation of diacylglycerol to phosphatidic acid and/or lysophosphatidic acid is part of common pathway initiated by these macrophage fusion factors. In FBGC formation, this pathway may lead to macrophage-macrophage interactions and/or the extensive cytoskeletal rearrangements and cytoplasmic spreading that are characteristic of these large multinucleated cells. Our combined results, including the enhancement of IL-4-induced FBGC formation by the phosphatidate phosphohydrolase inhibitor propranolol, are consistent with this interpretation.
Other mechanisms by which macrophage fusion could be promoted by α-tocopherol involve structural stabilization of the plasma membrane during FBGC formation. Vitamin E is well known as a membrane stabilizer and is believed to perform this function by associating with lysophospholipids and mimicking the missing lipid tail structure. 15 In addition, α-tocopherol is reported to stabilize lysosomal membranes by protection from phospholipase A2-mediated damage. 54 Thus, although the complete inhibition of FBGC formation by R59022 is completely restored by α-tocopherol, the influences of other mechanisms cannot be ruled out, including effects on macrophage adhesion as discussed above.
The mechanism for the inhibition of IL-4-induced FBGC formation by probucol or Trolox is shown to be independent of effects on adhesion and is otherwise not clear. There may be as yet undescribed effects of probucol or Trolox on macrophages or on components in the culture medium that play a role, perhaps in modifying the expression of receptors required for macrophage fusion. 28 For example, probucol, but not vitamin E, is reported to increase the cholesterol ester content of macrophages, 55 with possible alterations in macrophage phenotype. The inhibition of FBGC formation by β-, γ-, or δ-tocopherols may be because of their competition with α-tocopherol for cellular binding sites, as is believed to occur with β-tocopherol. 17 This may also account for the discrepancy in strength of FBGC induction between purified α-tocopherol and vitamin E, which contains other tocopherols and tocotrienols.
The present discovery that α-tocopherol induces very large FBGC formation extends the brief list of known human macrophage fusion factors and enlarges the basis from which to investigate the phenotype of these giant multinucleated cells. Except for the demonstrated deleterious effects of FBGC formation on biomaterials, 9,10 the physiological significance of this hallmark histological feature of chronic inflammation has not been elucidated. Biomaterial degradation is believed to result from frustrated phagocytosis 56 by FBGC and oxidative activities at the FBGC/biomaterial interface. This is consistent with an apparent antioxidant mechanism for vitamin E-mediated inhibition of poly(etherurethane) degradation in vivo. 12
In consideration of the present findings, however, it is notable that α-tocopherol also exerts certain nonantioxidant effects on monocytes/macrophages in common with IL-4 and IL-13, such as inhibition of monocyte adhesion to endothelium and respiratory burst activity. 17,57,58 IL-4 and IL-13 are well known to have negative regulatory effects on many so-called proinflammatory functions of these cells 59 leading to a state of alternative macrophage activation characterized by mannose receptor expression 28 and with proposed roles in wound healing, angiogenesis, and tissue remodeling. 60-64 Related to this, a recently recognized nonantioxidant effect of α-tocopherol is to increase the expression of connective tissue growth factor, 65 which is believed to participate in connective tissue fibrosis at sites of chronic tissue injury and wound healing. 66 Inasmuch as a frequent consequence of biomedical device implantation is fibrous encapsulation of the implant, 7 biomaterial-associated FBGCs may produce connective tissue growth factor and/or other fibrogenic cytokines, thereby contributing to this clinical complication.
A report demonstrating the polarized distribution of a lysosomal antigen and a Na+K+-ATPase in FBGCs 67 implies that the multinucleated giant cell is a differentiated cell type with specialized functional capabilities. The evidence to date and the theory of frustrated phagocytosis are consistent with macrophage/FBGC capabilities that are deleterious to biomaterial surfaces in the initial stages of FBGC formation. In later phases, however, it is possible that the high degree of multinucleation achieved by continued macrophage fusion, and exhibited by very large biomaterial adherent FBGCs in vivo and by IL-4-, IL-13-, or α-tocopherol-induced FBGCs in vitro, reflects a more highly differentiated multinucleated cell phenotype. In these FBGCs, activities that were initially deleterious to polymer surfaces may have been depleted or down-modulated and replaced by wound-healing, angiogenic, or tissue-remodeling capabilities consistent with a state of alternative activation. Accordingly, the presence of vitamin E in poly(etherurethane) biomaterial may have accelerated the acquisition of this alternative FBGC phenotype and thereby decreased biomaterial degradation in vivo, 12 not solely through antioxidant mechanisms, but also in a manner independent of its antioxidant effects. Our in vitro system of FBGC induction with the lymphokines IL-4 or IL-13, and now with the lipophilic vitamin α-tocopherol, provides a means for further addressing these and other unknowns regarding the formation and physiological significance of multinucleated giant cells at sites of inflammation.
Footnotes
Address reprint requests to Dr. James M. Anderson, Institute of Pathology, Case Western Reserve University, 2085 Adelbert Rd., Cleveland, OH 44106. E-mail: akm2@po.cwru.edu.
Supported by the National Heart, Lung, and Blood Institute, Devices and Technology Branch (grant HL55714).
References
- 1.Jenney CR, Anderson JM: Alkylsilane-modified surfaces: inhibition of human macrophage adhesion and foreign body giant cell formation. J Biomed Mater Res 1999, 46:11-21 [DOI] [PubMed] [Google Scholar]
- 2.McNally AK, Anderson JM: Beta1 and beta2 integrins mediate adhesion during macrophage fusion and multinucleated foreign body giant cell formation. Am J Pathol 2002, 160:621-630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodbeck WG, Nakayama Y, Matsuda T, Colton E, Ziats NP, Anderson JM: Biomaterial surface chemistry dictates adherent monocyte/macrophage cytokine expression in vitro. Cytokine 2002, 18:311-319 [DOI] [PubMed] [Google Scholar]
- 4.Brodbeck WG, Patel J, Voskerician G, Christenson E, Shive MS, Nakayama N, Matsuda T, Ziats NP, Anderson JM: Biomaterial adherent macrophage apoptosis is increased by hydrophilic and anionic substrates in vivo. Proc Natl Acad Sci USA 2002, 99:10287-10292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodbeck WG, Shive MS, Colton E, Nakayama Y, Matsuda T, Anderson JM: Influence of biomaterial surface chemistry on apoptosis of adherent cells. J Biomed Mater Res 2001, 55:661-668 [DOI] [PubMed] [Google Scholar]
- 6.Murch AR, Grounds MD, Marshall CA, Papadimitriou JM: Direct evidence that inflammatory multinucleate cells form by fusion. J Pathol 1982, 137:177-180 [DOI] [PubMed] [Google Scholar]
- 7.Anderson JM: Inflammatory response to implants. Am Soc Artif Intern Organs 1988, 11:101-107 [DOI] [PubMed] [Google Scholar]
- 8.Anderson JM: Multinucleated giant cells. Curr Opin Hematol 2000, 7:40-47 [DOI] [PubMed] [Google Scholar]
- 9.Zhao Q, Topham NS, Anderson JM, Hiltner A, Lodoen G, Payet R: Foreign body giant cells and polyurethane biostability: in vivo correlation of cell adhesion and surface cracking. J Biomed Mater Res 1991, 25:177-183 [DOI] [PubMed] [Google Scholar]
- 10.Wiggins MJ, Wilkoff B, Anderson JM, Hiltner A: Biodegradation of polyether polyurethane inner insulation in bipolar pacemaker leads. J Biomed Mater Res 2001, 58:302-307 [DOI] [PubMed] [Google Scholar]
- 11.Heiple JM, Wright SD, Allen NS, Silverstein SC: Macrophages form circular zones of close apposition to IgG-coated surfaces. Cell Motil Cytoskel 1990, 15:260-270 [DOI] [PubMed] [Google Scholar]
- 12.Schubert MA, Wiggins MJ, DeFife KM, Hiltner A, Anderson JM: Vitamin E as an antioxidant for poly(etherurethane urea): in vivo studies. J Biomed Mater Res 1996, 32:493-504 [DOI] [PubMed] [Google Scholar]
- 13.Evans HM, Bishop KS: On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 1922, 56:650-651 [DOI] [PubMed] [Google Scholar]
- 14.Brigelius-Flohe R, Traber MG: Vitamin E: function and metabolism. EMBO J 1999, 13:1145-1155 [PubMed] [Google Scholar]
- 15.Wang X, Quinn PJ: The location and function of vitamin E in membranes. Mol Membr Biol 2000, 17:143-156 [DOI] [PubMed] [Google Scholar]
- 16.Esterbauer H, Dieber Rotheneder M, Striegl G, Waeg G: Role of vitamin E in preventing the oxidation of low density lipoprotein. Am J Clin Nutr 1991, 53:314S-321S [DOI] [PubMed] [Google Scholar]
- 17.Azzi A, Stocker A: Vitamin E: non-antioxidant roles. Prog Lipid Res 2000, 39:231-255 [DOI] [PubMed] [Google Scholar]
- 18.Azzi A, Breyer I, Feher M, Pastori M, Ricchiarelli R, Spycher S, Staffieri M, Stocker A, Zimmer S, Zing J: Specific cellular responses to α-tocopherol. J Nutr 2000, 130:1649-1652 [DOI] [PubMed] [Google Scholar]
- 19.McNally AK, Anderson JM: Interleukin-4 induces foreign body giant cells from human monocytes/macrophages. Differential lymphokine regulation of macrophage fusion leads to morphological variants of multinucleated giant cells. Am J Pathol 1995, 147:1487-1499 [PMC free article] [PubMed] [Google Scholar]
- 20.McInnis A, Rennick DM: Interleukin-4 induces cultured monocytes/macrophages to form giant multinucleated cells. J Exp Med 1988, 167:598-611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Most J, Neumayer HP, Dierich MP: Cytokine-induced generation of multinucleated giant cells in vitro requires interferon-γ and expression of LFA-1. Eur J Immunol 1990, 20:1661-1667 [DOI] [PubMed] [Google Scholar]
- 22.Enelow RI, Sullivan GW, Holliday TC, Mandell GL: Induction of multinucleated giant cell formation from in vitro culture of human monocytes with interleukin-3 and interferon-γ: comparison with other stimulating factors. Am J Respir Cell Mol Biol 1992, 6:57-62 [DOI] [PubMed] [Google Scholar]
- 23.Kao WJ, McNally AK, Hiltner A, Anderson JM: Role for interleukin-4 in foreign body giant cell formation on a poly(etherurethane urea) in vivo. J Biomed Mater Res 1995, 29:1267-1275 [DOI] [PubMed] [Google Scholar]
- 24.Zhao QH, Anderson JM, Hiltner A, Lodoen GA, Payet CR: Theoretical analysis on cell size distribution and kinetics of foreign body giant cell formation in vivo on polyurethane elastomers. J Biomed Mater Res 1992, 26:1019-1038 [DOI] [PubMed] [Google Scholar]
- 25.DeFife KM, Jenney CR, McNally AK, Colton E, Anderson JM: Interleukin-13 induces monocyte/macrophage fusion and macrophage mannose receptor expression. J Immunol 1997, 158:3385-3390 [PubMed] [Google Scholar]
- 26.Takashima T, Ohnishi K, Tsuyuguchi I, Kishimoto S: Differential regulation of formation of multinucleated giant cells from concanavalin A-stimulated human blood monocytes by IFN-γ and IL-4. J Immunol 1993, 150:3002-3010 [PubMed] [Google Scholar]
- 27.Langhans T: Uber riezenzellen mit wandstandigen kernen in tuberkeln und die fibrose form des tuberkels. Arch Pathol Anat 1868, 42:382-404 [Google Scholar]
- 28.Stein M, Keshav S, Harris N, Gordon S: Interleukin-4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med 1992, 176:287-292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNally AK, Anderson JM: Interleukin-4-induced macrophage fusion is prevented by inhibitors of mannose receptor activity. Am J Pathol 1996, 149:975-985 [PMC free article] [PubMed] [Google Scholar]
- 30.McNally AK, Anderson JM: The lymphokine interleukin-4 induces foreign body giant cell formation from human macrophages in a material surface property-dependent manner in vitro. Fifth World Biomaterials Congress 1996, May 29-June 2, Toronto, Canada
- 31.Anderson JM, DeFife K, McNally A, Collier T, Jenney C: Monocyte, macrophage and foreign body giant cell interactions with molecularly engineered surfaces. J Mater Sci Mater Med 1999, 10:579-588 [DOI] [PubMed] [Google Scholar]
- 32.Jenney CR, Anderson JM: Adsorbed serum proteins responsible for surface dependent human macrophage behavior. J Biomed Mater Res 2000, 49:435-447 [DOI] [PubMed] [Google Scholar]
- 33.Jenney CR, Anderson JM: Adsorbed IgG: a potent adhesive substrate for human macrophages. J Biomed Mater Res 2000, 50:281-290 [DOI] [PubMed] [Google Scholar]
- 34.McNally AK, Anderson JM: Complement C3 participation in monocyte adhesion to different surfaces. Proc Natl Acad Sci USA 1994, 91:10119-10123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrisey PA, Sheehy PJ: Optimal nutrition: vitamin E. Proc Nutr Soc 1999, 58:459-468 [DOI] [PubMed] [Google Scholar]
- 36.Chan SS, Monteiro HP, Schindler F, Stern A, Junqueia VB: Alpha-tocopherol modulates tyrosine phosphorylation in human neutrophils by inhibition of protein kinase C activity and activation of tyrosine phosphatases. Free Radic Res 2001, 35:843-856 [DOI] [PubMed] [Google Scholar]
- 37.Lee IK, Koya D, Ishi H, Kanoh H, King GL: d-α-Tocopherol prevents the hyperglycemia-induced activation of diacylglycerol (DAG)-protein kinase C (PKC) pathway in vascular smooth muscle cell by an increase of DAG kinase activity. Diabetes Res Clin Pract 2001, 45:183-190 [DOI] [PubMed] [Google Scholar]
- 38.Blitterswijk WJ, Houssa B: Properties and functions of diacylglycerol kinases. Cell Signalling 2000, 12:595-605 [DOI] [PubMed] [Google Scholar]
- 39.Jiang Y, Sakane F, Kanoh H, Walsh JP: Selectivity of the diacylglycerol kinase inhibitor 3-{2-(4-[bis-(4-fluorophenyl)methylene]-1-piperidinyl)ethyl}-2,3-dihydro-2-thioxo-4(1H)quinazolinone (R59949) among diacylglycerol kinase subtypes. Biochem Pharmacol 2000, 59:763-772 [DOI] [PubMed] [Google Scholar]
- 40.English D, Cui Y, Siddiqui RA: Messenger functions of phosphatidic acid. Chem Phys Lipids 1996, 80:117-132 [DOI] [PubMed] [Google Scholar]
- 41.Hassan NF, Kamani N, Meszaros MM, Douglas SD: Induction of multinucleated giant cell formation from human blood monocytes by phorbol myristate acetate in in vitro culture. J Immunol 1989, 143:2179-2184 [PubMed] [Google Scholar]
- 42.McNally AK, Anderson JM: Foreign body giant cell formation in vitro: the effects of inhibitors of phospholipid and arachidonic acid metabolism. Sixth World Biomaterials Congress 2000, May 15–20, Kamuela, Hawaii
- 43.McNally AK: Mechanisms of monocyte/macrophage adhesion and macrophage fusion on different surfaces. PhD Thesis 1994. Case Western Reserve University Cleveland, Ohio
- 44.Tasinato A, Boscoboinik D, Bartoli GM, Maroni P, Azzi A: d-α-Tocopherol inhibition of vascular smooth muscle cell proliferation occurs at physiological concentrations, correlates with protein kinase C inhibition, and is independent of its antioxidant properties. Proc Natl Acad Sci USA 1995, 92:12190-12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Topham MK, Prescott SM: Mammalian diacylglycerol kinases, a family of lipid kinases with signaling functions. J Biol Chem 1999, 274:11447-11450 [DOI] [PubMed] [Google Scholar]
- 46.Bauldry SA, Wooten RE: Induction of cytosolic phospholipase A2 activity by phosphatidic acid and diacylglycerides in permeabilized human neutrophils: interrelationship between phospholipases D and A2. Biochem J 1997, 322:353-363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ha KS, Exton JH: Activation of actin polymerization by phosphatidic acid derived from phosphatidylcholine in HC9 fibroblasts. J Cell Biol 1993, 123:1789-1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou D, Luini W, Bernasconi S, Diomede L, Salmona M, Mantovani A, Sozzani S: Phosphatidic acid and lysophosphatidic acid induce haptotactic migration of human monocytes. J Immunol 1995, 270:25549-25556 [DOI] [PubMed] [Google Scholar]
- 49.Koh JS, Lieberthal W, Heydrick S, Levine JS: Lysophosphatidic acid is a major serum noncytokine survival factor for murine macrophage which acts via the phosphatidylinositol 3-kinase signaling pathway. J Clin Invest 1998, 102:716-727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Q, Checovich WJ, Peters DM, Albrecht RM, Mosher DF: Modulation of cell surface fibronectin assembly sites by lysophosphatidic acid. J Cell Biol 1994, 127:1447-1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakai T, Manuel de la Pena J, Mosher DF: Synergism among lysophosphatidic acid, β1A integrins, and epidermal growth factor or platelet-derived growth factor in mediation of cell migration. J Biol Chem 1999, 274:15480-15486 [DOI] [PubMed] [Google Scholar]
- 52.Olorundare OE, Peyruchaud O, Albrecht RM, Mosher DF: Assembly of a fibronectin matrix by adherent platelets stimulated by lysophosphatidic acid and other agonists. Hemost Thromb Vasc Biol 2001, 98:117-124 [DOI] [PubMed] [Google Scholar]
- 53.Harsh DM, Blackwood RA: Phospholipase A2-mediated fusion of neutrophil-derived membranes is augmented by phosphatidic acid. Biochem Biophys Res Comm 2001, 282:480-486 [DOI] [PubMed] [Google Scholar]
- 54.Mukherjee AK, Ghosal SK, Maity CR: Lysosomal membrane stabilization by α-tocopherol against the damaging action of Vipera russelli venom phospholipase A2. Cell Mol Life Sci 1997, 53:152-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trach CC, Wulfroth PM, Severs NJ, Robenek H: Influence of native and modified lipoproteins on migration of mouse peritoneal macrophages and the effect of the antioxidants vitamin E and probucol. Eur J Cell Biol 1996, 71:199-205 [PubMed] [Google Scholar]
- 56.Henson PM, Henson JE, Fittschen C, Kimani G, Bratton DL, Riches DWH: Phagocytic cells: degranulation and secretion. Gallin JI Goldstein IM Snyderman R eds. Inflammation: Basic Principles and Clinical Correlates. 1988:pp 363-390 Raven New York
- 57.Islam KN, Devaraj S, Jialal I: Alpha-tocopherol enrichment of monocytes decreases agonist-induced adhesion to human endothelial cells. Circulation 1988, 98:2255-2261 [DOI] [PubMed] [Google Scholar]
- 58.Devaraj S, Li D, Jialal I: The effects of alpha-tocopherol supplementation on monocyte function. Decreased lipid oxidation, interleukin-1 beta secretion, and monocyte adhesion to endothelium. J Clin Invest 1996, 98:756-763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malefyt RW: Role of interleukin-10, interleukin-4, and interleukin-13 in resolving inflammatory responses. Gallin JI Snyderman R eds. Inflammation: Basic Principles and Clinical Correlates. 1999:pp 837-849 Lippincott Williams & Wilkins Philadelphia
- 60.Kodelja V, Muller C, Tenorio C, Schebesch C, Orfanos CE, Goerdt S: Differences in angiogenic potential of classically vs. alternatively activated macrophages. Immunobiology 1997, 197:478-483 [DOI] [PubMed] [Google Scholar]
- 61.Kodelja V, Muller C, Politz O, Hakij N, Orfanos CE, Goerdt S: Alternative macrophage activation-associated CC-chemokine-1, a novel structural homologue of macrophage inflammatory protein-1α with a Th2-associated expression pattern. J Immunol 1998, 160:1411-1418 [PubMed] [Google Scholar]
- 62.Goerdt S, Politz O, Schledzewski K, Birk R, Gratchev A, Guillot P, Hakiy N, Klemke CD, Dippel E, Kodelja V, Orfanos CE: Alternative versus classical activation of macrophages. Pathobiology 1999, 67:222-226 [DOI] [PubMed] [Google Scholar]
- 63.Gratchev A, Guillot P, Haliy N, Politz O, Orfanos CE, Scheledzewski K, Goerdt S: Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein βIG-H3. Scan J Immunol 2001, 53:386-392 [DOI] [PubMed] [Google Scholar]
- 64.Mosser DM: The many faces of macrophage activation. J Leukoc Biol 2003, 73:209-212 [DOI] [PubMed] [Google Scholar]
- 65.Azzi A, Ricciarelli R, Zingg J-M: Non-antioxidant molecular functions of α-tocopherol (vitamin E). FEBS Lett 2002, 519:8-10 [DOI] [PubMed] [Google Scholar]
- 66.Leask A, Holmes A, Abraham DJ: Connective tissue growth factor: a new and important player in the pathogenesis of fibrosis. Curr Rheumatol Rep 2002, 4:136-142 [DOI] [PubMed] [Google Scholar]
- 67.Vignery A, Niven-Fairchild T, Ingbar DH, Caplan M: Polarized distribution of Na+K+-ATPase in giant cells elicited in vivo and in vitro. J Histochem Cytochem 1989, 37:1265-1271 [DOI] [PubMed] [Google Scholar]