Abstract
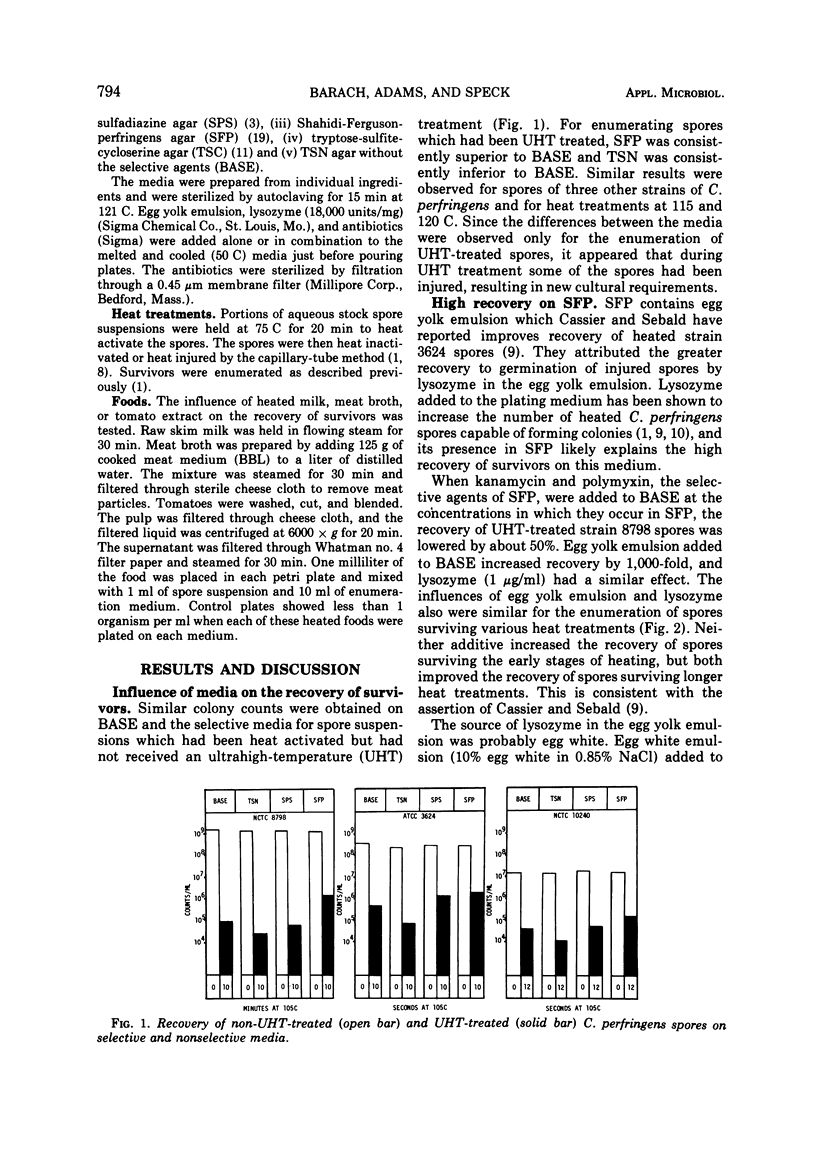
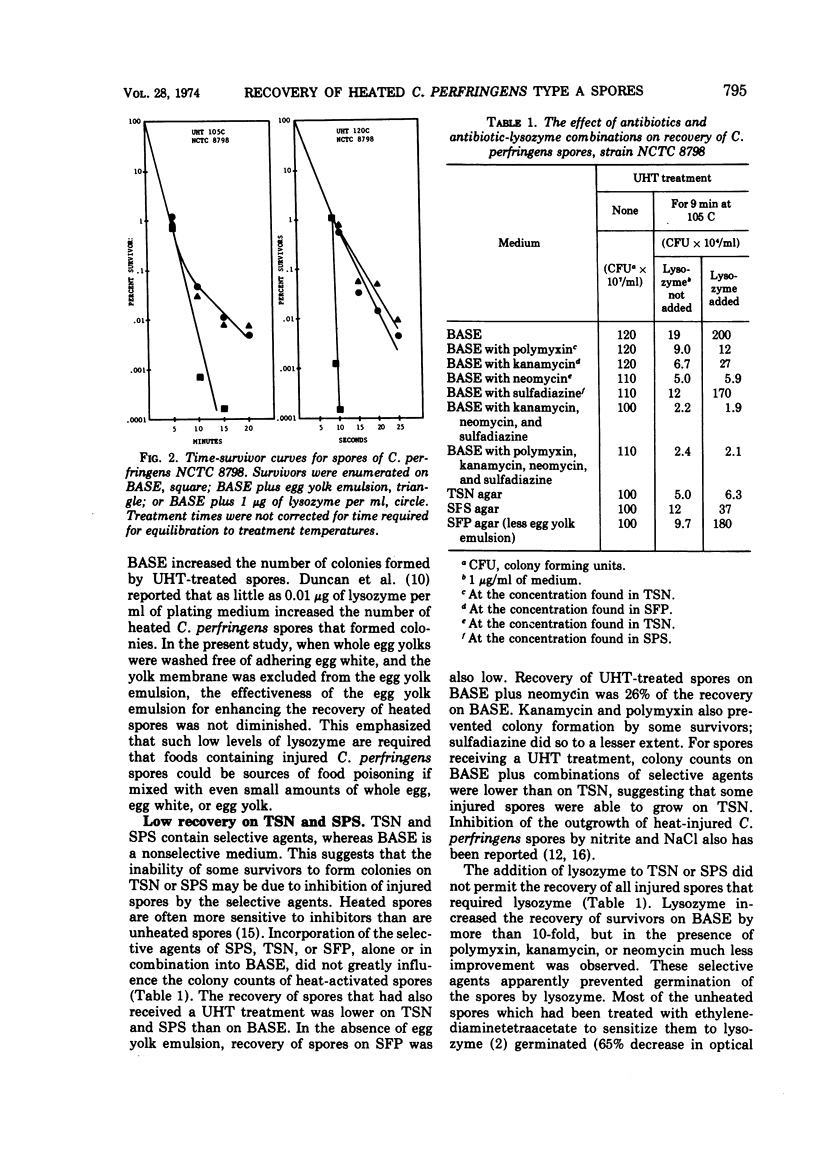
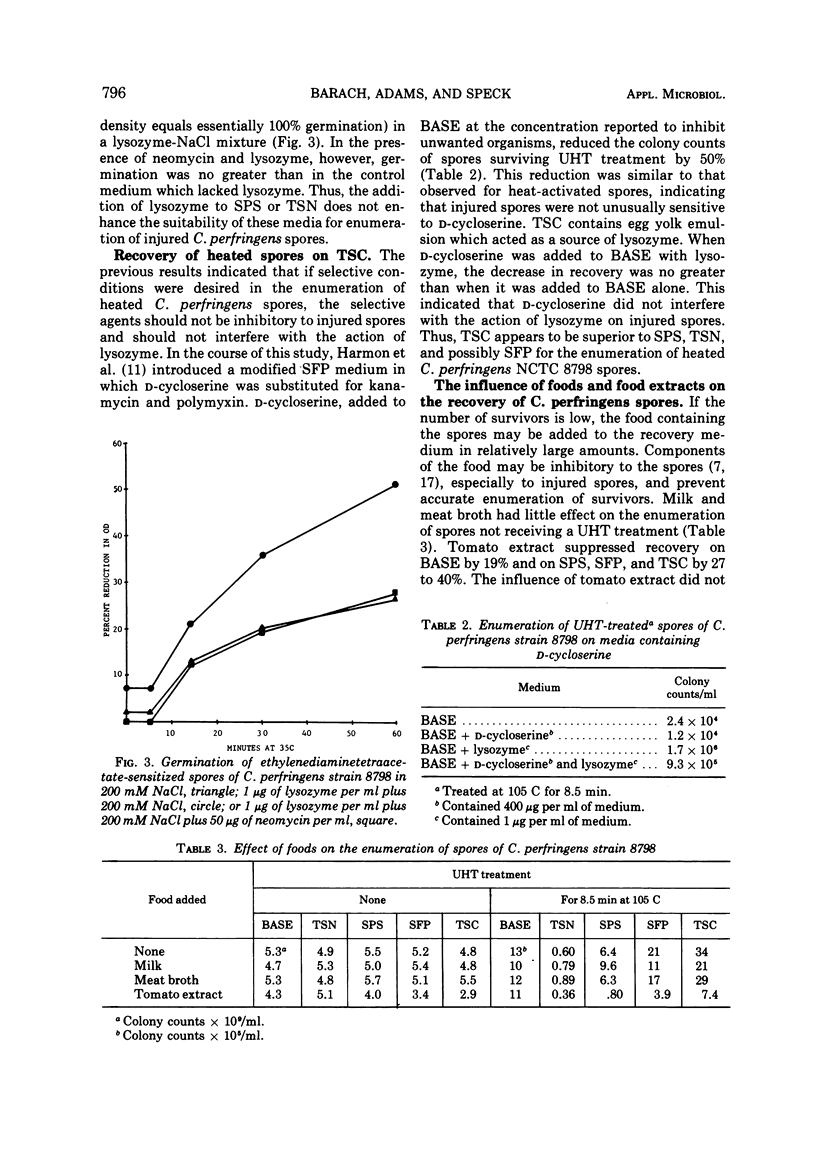
The enumeration of Clostridium perfringens spores on sulfite-polymyxin-sulfadiazine agar (SPS), tryptone-sulfite-neomycin agar (TSN), Shahidi-Ferguson-perfringens agar (SFP), tryptone-sulfite-cycloserine agar (TSC), and TSN lacking antibiotics (BASE) was studied. The spores were heated at 105 to 120 C by the capillary-tube method. The media were about equally efficient for the enumeration of heat-activated spores. Efficiency of the media for the recovery of spores surviving heat treatments at ultrahigh temperatures varied as follows: TSC ≥ SFP > BASE > SPS > TSN. Greater recovery when survivors were enumerated on TSC or SFP was attributed to germination of injured spores by the lysozyme present in the egg yolk emulsion used in these media. Low recovery of survivors on TSN and SPS was due to both the absence of lysozyme and inhibition of injured spores by the selective agents of these media. Recovery of heated spores was reduced greatly by polymyxin, neomycin, and kanamycin, and slightly by sulfadiazine and D-cycloserine. The addition of lysozyme to SPS or TSN did not improve the percentage of heat-injured spores recovered because the selective agents of these media interfered with the action of lysozyme. The suitability of the selective media for the enumeration of survivors was greatly affected by the presence of certain foods.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANGELOTTI R., HALL H. E., FOTER M. J., LEWIS K. H. Quantitation of Clostridium perfringens in foods. Appl Microbiol. 1962 May;10:193–199. doi: 10.1128/am.10.3.193-199.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D. M. Inactivation of Clostridium perfringens type A spores at ultrahigh temperatures. Appl Microbiol. 1973 Sep;26(3):282–287. doi: 10.1128/am.26.3.282-287.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D. M. Sensitization by ethylenediaminetetraacetate of Clostridium perfringens type A spores to germination by lysozyme. J Bacteriol. 1973 Oct;116(1):500–502. doi: 10.1128/jb.116.1.500-502.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busta F. F., Adams D. M. Identification of a germination system involved in the heat injury of Bacillus subtilis spores. Appl Microbiol. 1972 Sep;24(3):412–417. doi: 10.1128/am.24.3.412-417.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busta F. F. Milk component(s) inhibitory to Bacillus stearothermophilus. J Dairy Sci. 1966 Jul;49(7):751–756. doi: 10.3168/jds.S0022-0302(66)87940-7. [DOI] [PubMed] [Google Scholar]
- Cassier M., Sebald M. Germination lysozyme-dépendante des spores de Clostridium perfringens ATCC 3624 après traitement thermique. Ann Inst Pasteur (Paris) 1969 Sep;117(3):312–324. [PubMed] [Google Scholar]
- Duncan C. L., Labbe R. G., Reich R. R. Germination of heat- and alkali-altered spores of Clostridium perfringens type A by lysozyme and an initiation protein. J Bacteriol. 1972 Feb;109(2):550–559. doi: 10.1128/jb.109.2.550-559.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon S. M., Kautter D. A., Peeler J. T. Improved medium for enumeration of Clostridium perfringens. Appl Microbiol. 1971 Oct;22(4):688–692. doi: 10.1128/am.22.4.688-692.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe R. G., Duncan C. L. Growth from spores of Clostridium perfringens in the presence of sodium nitrite. Appl Microbiol. 1970 Feb;19(2):353–359. doi: 10.1128/am.19.2.353-359.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSHALL R. S., STEENBERGEN J. F., MCCLUNG L. S. RAPID TECHNIQUE FOR THE ENUMERATION OF CLOSTRIDIUM PERFINGENS. Appl Microbiol. 1965 Jul;13:559–563. doi: 10.1128/am.13.4.559-563.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T. A. Heat and radiation resistance and activation of spores of Clostridium welchii. J Appl Bacteriol. 1968 Mar;31(1):133–144. doi: 10.1111/j.1365-2672.1968.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Rosen B., Christiansen L. N., Busta F. F. Inhibition of Bacillus megaterium by a trimethylamine oxide-associated browning reaction product. Appl Microbiol. 1970 Jul;20(1):113–116. doi: 10.1128/am.20.1.113-116.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi S. A., Ferguson A. R. New quantitative, qualitative, and confirmatory media for rapid analysis of food for Clostridium perfringens. Appl Microbiol. 1971 Mar;21(3):500–506. doi: 10.1128/am.21.3.500-506.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


