Abstract
There is evidence that angiotensin II, vascular endothelial growth factor (VEGF), angiopoietins, and their cognate receptors participate in retinal angiogenesis. We investigated whether angiotensin type 2-receptor blockade (AT2-RB) reduces retinal angiogenesis and alters the expression of VEGF/VEGF-R2 and angiopoietin-Tie2. Retinopathy of prematurity (ROP) was induced in Sprague Dawley (SD) rats by exposure to 80% oxygen from postnatal (P) days 0 to 11, followed by 7 days in room air. ROP shams were in room air from P0–18. A group of ROP rats received the AT2-RB, PD123319, by mini-osmotic pump (5 mg/kg/day) from P11–18 (angiogenesis period). Evaluation of the retinal status of the AT2 receptor indicated that this receptor, as assessed by real-time PCR, immunohistochemistry, and in vitro autoradiography, was present in the retina, was more abundant than the AT1 receptor in the neonatal retina, and was increased in the ROP model. AT2-RB reduced retinal angiogenesis. VEGF and VEGF-R2 mRNA were increased in ROP and localized to blood vessels, ganglion cells, and the inner nuclear layer, and were decreased by PD123319. Angiopoietin2 and Tie2, but not angiopoietin1 mRNA were increased with ROP, and angiopoietin2 was reduced with PD123319. This study has identified a potential retinoprotective role for AT2-RB possibly mediated via interactions with VEGF- and angiopoietin-dependent pathways.
Retinopathy of prematurity (ROP) and proliferative diabetic retinopathy (PDR) are characterized by extensive angiogenesis, a process which threatens vision and can ultimately lead to blindness. 1-3 There is accumulating evidence that angiotensin II (ANG II), the effector peptide of the renin-angiotensin system (RAS), is a key pathophysiological factor in a number of retinal vascular disorders including ROP and PDR. 4-7 In addition to its well characterized hemodynamic actions, ANG II is also a growth factor, stimulating endothelial and smooth muscle cell proliferation, 8-10 pericyte migration, 11,12 and smooth muscle cell hypertrophy. 13 The actions of ANG II are largely mediated by two receptors; angiotensin type 1 (AT1) and angiotensin type 2 (AT2). 14 Most of the blood pressure and cell growth effects of ANG II are viewed to be mediated by the AT1 receptor subtype. 14 In contrast, the function of the AT2 receptor subtype is less well defined, with reports of both anti-growth and pro-growth effects. 9,14-19 Furthermore, the AT2 receptor is highly expressed in developing organs and is postulated to be involved in tissue growth and cell differentiation. 20,21
Recent studies indicate that vascular endothelial growth factor (VEGF) may be the major mediator of the angiogenic effects of ANG II. 9,11 VEGF is a ligand for the receptor tyrosine kinase (RTK) receptors, Flt-1 (VEGF-R1) and Flk-1 (VEGF-R2), and is a potent permeability and angiogenic factor. 22-24 With respect to the retina, recent studies show that blockade of the RAS attenuates angiogenesis and the rise in VEGF and VEGF-R2 expression in vitro, 11 in ROP, 7 and in streptozotocin diabetic rats. 25 Apart from the VEGF RTK family, only one other family of RTKs, comprising Tie1 and Tie2, is largely endothelial cell specific. The roles of Tie1 and Tie2 have been demonstrated in studies that have involved deletion of these receptors. Specifically, Tie2 knockout mice die early in development because of immature blood vessels and lack of vessel development, 26 and following deletion of Tie1 in mice, death occurs in utero with defects linked to hemodynamic and transcapillary fluid exchange. 26 Ligands for the Tie2 receptor include angiopoietin 1 (Ang1) and angiopoietin 2 (Ang2). 27,28 Ang1 phosphorylates Tie2 in cultured endothelial cells, whereas Ang2 is a naturally occurring antagonist of Ang1, competing with Ang1 for binding to Tie2. 27 Recent studies indicate that Ang1 and Ang2 facilitate VEGF-induced angiogenesis, with Ang1 promoting vascular network maturation and Ang2 initiating angiogenesis. 29
There are limited studies which have examined the relationship between the RAS, VEGF/VEGF-R2, and angiopoietin-Tie2 systems in blood vessel growth. 30,31 Furthermore, to our knowledge the interaction between the AT2 receptor and VEGF/VEGF-R2 and angiopoietin-Tie2 in an in vivo model of retinal angiogenesis is unknown. In the present study we aimed firstly to determine whether AT2 receptor blockade (AT2-RB) would attenuate retinal angiogenesis in a rat model of ROP, and secondly whether this was associated with alterations in VEGF/VEGF-R2 and angiopoietin-Tie2 gene expression.
Materials and Methods
Protocol 1: Autoradiographic Localization of AT1 and AT2 Receptors in the Developing and Adult Rat Retina
Experimental procedures were consistent with the guidelines of the Australian National Health and Medical Research Council Code of Practice for the Care and Use of Animals for Scientific Purposes. Sprague Dawley (SD) rats at postnatal days 1, 7, 14, 21, and 90 were anesthetized with pentobarbital sodium (Nembutal, 60 mg/kg/body weight, i.p.; Boehringer, Ingelheim, Germany) and their eyes were enucleated and embedded in cryomatrix (Sakura Finetechnical Co., Ltd., Tokyo, Japan), and then immersed in iso-pentane and snap-frozen in liquid nitrogen. Twenty-μm sections were placed on glass slides and the sections dehydrated at 4°C overnight and stored at −80°C. ANG II receptor binding was determined by in vitro autoradiography using the radioligand [125I]-[Sar1,Ile8]-ANG II, as previously described. 32 Briefly, sections were incubated with [125I]-[Sar1,Ile8]-ANG II alone for determination of total binding, in the presence of both the AT1 receptor blocker valsartan (10−5 mol/L, Novartis Pharma AG, Basel, Switzerland) and the AT2-RB, PD123319 (10−5 mol/L, Parke-Davis, Ann Arbor, MI, USA) for non-specific binding, PD123319 (10−5 mol/L) for AT1 receptor binding and valsartan for AT2 receptor binding.
After incubation with [125I]-[Sar1,Ile8]-ANG II, the sections were washed four times for 1 minute each in ice-cold 0.1 mol/L sodium phosphate buffer to remove non-specifically bound radioligand. The sections were then dried under a stream of cold air, loaded into X-ray film cassettes together with a set of radioactive standards and apposed to X-ray film (Kodak BioMax film, Kodak, Rochester, NY, USA) for 2 weeks at room temperature and were subsequently developed. The optical densities in each section were quantified using a computerized image analysis system (AIS, Analytical Imaging Research, Ontario, Canada). Radioactive standards were fitted to calibration curves and the disintegration per minute of I125 per millimeter2 of tissue (dpm/mm2) was estimated. Total binding to AT1 and AT2 receptors was calculated by subtraction of non-specific binding. 32
Protocol 2: Retinopathy of Prematurity (ROP) Study
SD rats were randomly divided into shams, ROP, or ROP rats treated with the AT2-RB, PD123319 (Sigma Chemical Co., St. Louis, MO, USA, 5 mg/kg/day). As previously described, 7 sham rats were newborn pups housed in room air for 18 days and administered vehicle from postnatal days 11 to 18. ROP rats were newborn pups housed in sealed chambers containing 80 ± 2% O2 and 2% CO2 from postnatal days 0 to 11 (hyperoxic period). Pups were then placed in room air for 7 days (retinal angiogenesis period) and received vehicle. Each day in the chamber, pups were removed and placed in room air for 3 hours. Treated ROP rats received PD123319 during the retinal angiogenesis period, postnatal days 11 to 18. Mini-osmotic pumps (Alzet model 2004, Alza Corporation, Palo Alto, CA, USA) were used to deliver vehicle or the AT2-RB, and were surgically inserted into the abdominal cavity of 11-day-old pups during brief anesthesia with enflurane (Ethrane, Abbott Laboratories, WA, Australia). The insertion wound was then closed with a small stitch. The mini-osmotic pump delivers agents at a dose of 0.25 μl/hr (Alzet model 2004, Alza). The chosen dose of PD123319 was based on previous autoradiographic studies by our group that have shown PD123319 to block the AT2 receptor in blood vessels and kidney. 16,32 At postnatal day 18, animals were anesthetized with pentobarbital sodium and eyes collected. Six to eight rats per group were studied.
Histology of the ROP Study
One eye from each animal was removed and fixed for 3 hours in Bouin’s fixative. Eyes were processed in graded alcohols, embedded in paraffin wax and then serially sectioned at 3 μm, 90° to the optic nerve. Every 10th section was deparaffinized and stained with Mayer’s hematoxylin (5 minutes) and eosin (5 minutes) (Amber Scientific Laboratories, Belmont, Australia). 7
Quantitation of Blood Vessel Profiles in the Inner Retina of ROP Rats
Three paraffin sections were randomly chosen from one eye from each animal and stained with hematoxylin and eosin. Using an established technique, 7 blood vessel profiles (BVPs) were counted in the inner retina and included vessels adherent to the inner limiting membrane (ILM). The inner retina comprised the ILM, ganglion cell layer (GCL), and inner plexiform layer (IPL). A BVP is defined as an endothelial cell or a blood vessel with a lumen. Briefly, the entire inner retina was sampled using a non-biased stereological test frame. The method involves projecting images of retina onto a computer screen via a close-circuit camera. A stereological test grid measuring 150 × 150 mm and divided into 16 unit areas is then superimposed onto each image and an automated stage used to advance across the entire retina by means of an unbiased counting frame. Approximately 500 unit areas are counted per retina. The results are expressed as BVPs per high-powered field of inner retina which represents BVPs/stereological test grid. The method is performed by two investigators masked to the experimental groups.
Reverse Transcription-Polymerase Chain Reaction
Following anesthesia, one eye was removed and the retina dissected and snap-frozen in liquid nitrogen. Three micrograms of total RNA was extracted from each retina using the Qiagen Rneasy Mini kit (Qiagen, Valencia, CA, USA) and then synthesized to cDNA using the Superscript First-Strand Synthesis system for RT-PCR (Gibco BRL, Grand Island, NY, USA). VEGF, VEGF-R2, Ang1, Ang2, Tie2, renin, AT1, and AT2 gene expression were analyzed by real-time quantitative RT-PCR performed with the TaqMan system based on real-time detection of accumulated fluorescence (ABI Prism 7700, Perkin-Elmer Inc, Foster City, CA, USA), as previously described. 33 Primers and Taqman probes for VEGF, VEGF-R2, Ang1, Ang2, Tie2, and the endogenous reference 18S rRNA were constructed with the help of Primer Express (ABI Prism 7700, Perkin-Elmer). For amplification of the VEGF cDNA, the forward primer was 5′GCGGGCTGCTGCAATG-3′ and the reverse primer was 5′TGCAACGCGAGTCTGTGTTT-3′. The probe specific for VEGF was FAM-5′−TGCCCACGTCGGAGAGCAACGT-3′-TAMRA;FAM = 6-carboxyfluorescein, TAMRA (quencher) = 6-carboxy-tetramethylrhodamine. For the VEGF-R2 cDNA, the forward primer was 5′ CCACTTCTGTCTTGCCACACA 3′ and the reverse primer was 5′ CCAACCAATTAAGACCTTCTG 3′. The probe specific to VEGF-R2 was FAM-5′−CCCTCCCCAGTGCTCAGTATTTTAGCTTTG-3′-TAMRA. The forward primer for Ang1 was 5′ AGATACAACAGAATGCGGTTCAAA 3′ and the reverse primer was 5′ TGAGACAAGAGGCTGGTTCCTAT 3′. The specific probe for Ang1 was FAM-5′−CCACACGGCCACCATGCTGG-3′-TAMRA. The forward primer for Ang2 cDNA was 5′ GCTGGGCAACGAGTTTGTCT 3′ and the reverse primer was 5′ CAGTCCTTCAGCTGGATCTTCA 3′. The probe for Ang2 was FAM-5′−CTGACCAGTGGGCATCGCTACGTG-3′-TAMRA. The forward primer for the Tie2 receptor was 5′ CTGAGAACAACATAGGATCAAGCAA 3′ and the reverse primer was 5′ TTTCCCCCTCCAAGGTCTTT 3′. The probe for the Tie2 receptor was FAM-5′−CCCAAGAAATTAGGACACTTCCAGCCCC-3′-TAMRA. The forward renin primer was 5′ AACATTACCAGGGCAACTTTCACT 3′ and the reverse 5′ ACCCCCTTCATGGTGATCTG 3. The specific probe for renin was FAM-5′-TGAGCATCAGCAAGGCCGGCT-3′-TAMRA. The probes and primers for both the AT1 and AT2 receptors are as previously described. 33 The amplification was performed with the following time course: 50°C, 2 minutes and 10 minutes at 95°C; and 40 cycles of 94°C, 20 seconds, 60°C, 1 minute. Each sample was tested in triplicate. Results were expressed as relative to control retinas, which were arbitrarily assigned a value of 1.
In Situ Hybridization for VEGF and VEGF-R2 in ROP Rats
Riboprobes were synthesized from cDNAs encoding mouse VEGF and VEGF-R2 (Dr. S. Stacker, Ludwig Institute, Parkville, Australia). 25 Three-μm paraffin sections of eye were incubated with antisense and sense VEGF and VEGF-R2 probes and gene expression quanitifed in the inner retina (ILM, GCL, and IPL) as previously described. 25
AT1 and AT2 Receptor Immunohistochemistry in ROP Rats
Three-μm paraffin sections of eye were incubated with either a polyclonal antibody to the AT1 (sc 1173, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or AT2 receptor (sc 9040). Positive controls were sections of rat kidney and adrenal, while negative controls were sections of eye incubated with 0.1mol/L phosphate-buffered saline instead of the primary antibody. The avidin-biotin peroxidase technique was performed and localization of the AT1 and AT2 receptors was revealed with diaminobenzoic acid (Sigma). Sections were counterstained with Mayer’s hematoxylin.
Statistics
Data were analyzed using Statview for Windows (version 5.0.1, SAS Institute Inc., Cary, NC, USA). A two-way AVOVA with Fisher’s post-hoc comparison was applied, with P < 0.05 considered statistically significant.
Results
Autoradiographic Binding for AT1 and AT2 Receptors in the Developing and Adult Rat Retina
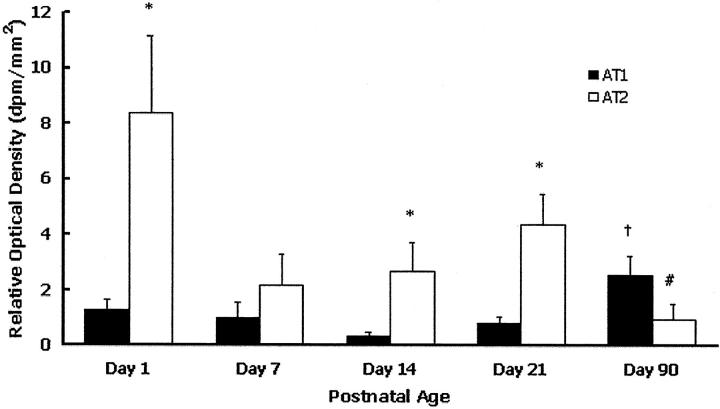
In the retina of SD rats, binding for both AT1 and AT2 receptors was present from postnatal days 1 to 90 (Figure 1) ▶ . AT2 receptor binding sites were more abundant than AT1 at postnatal days 1, 14 and 21 (Figure 1) ▶ . In adult rats, at postnatal day 90, AT2 and AT1 receptors were in similar amounts due to the reduced abundance of AT2 receptors and increased abundance of AT1 receptors compared to the developing retina (Figure 1) ▶ .
Figure 1.
Autoradiographic analysis of AT1 and AT2 receptor binding sites in the retina of neonatal and adult Sprague Dawley rats. Values are mean ± SEM. N = 6 rats per group. dpm/mm2, represents the disintegration per minute of I125 per millimeter2 of tissue. *, P < 0.05 compared to AT1 receptor binding at a particular time point; †, P < 0.05 compared to AT1 receptor binding at postnatal day 1; #, P < 0.05 compared to AT2 receptor binding at postnatal days 1, 14, and 21.
Quantitation of Blood Vessel Profiles in the Inner Retina of ROP Rats
In sham rats, the vasculature of the inner retina appeared normal (Figure 2,A and D) ▶ . In ROP rats, clusters of blood vessels were adherent to the ILM and numerous blood vessels were observed in the inner retina (Figure 2, B and D) ▶ . In ROP rats treated with PD123319, fewer blood vessels were attached to the ILM and observed in the inner retina (Figure 2, C and D) ▶ .
Figure 2.
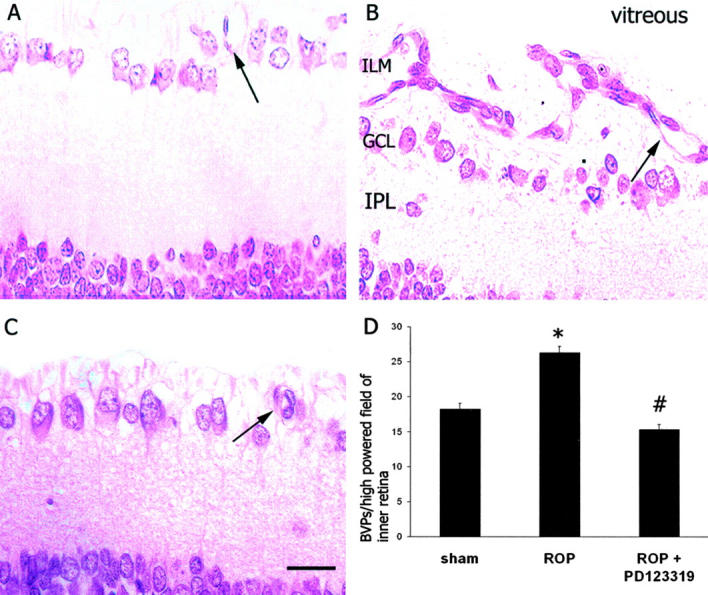
A–C: Three-μm paraffin sections of inner retina from Sprague Dawley rats following retinopathy of prematurity (ROP) and AT2 receptor blockade. Stain, hematoxylin and eosin. ILM, inner limiting membrane; GCL, ganglion cell layer; IPL, inner plexiform layer. Magnification, ×400. Bar, 30 μm. A: ROP sham. B: ROP. C: ROP + PD123319. Arrows denote blood vessels in the inner retina. D: Quantitation of blood vessel profiles (BVPs) per high-powered field of inner retina (BVPs/stereological test grid). Values are mean ± SEM. N = 6 to 8 rats per group. *, P < 0.001 compared to sham; #, P < 0.01 compared to ROP.
Gene Expression for VEGF, VEGF-R2, Ang1, Ang2, and Tie2 in ROP Rats
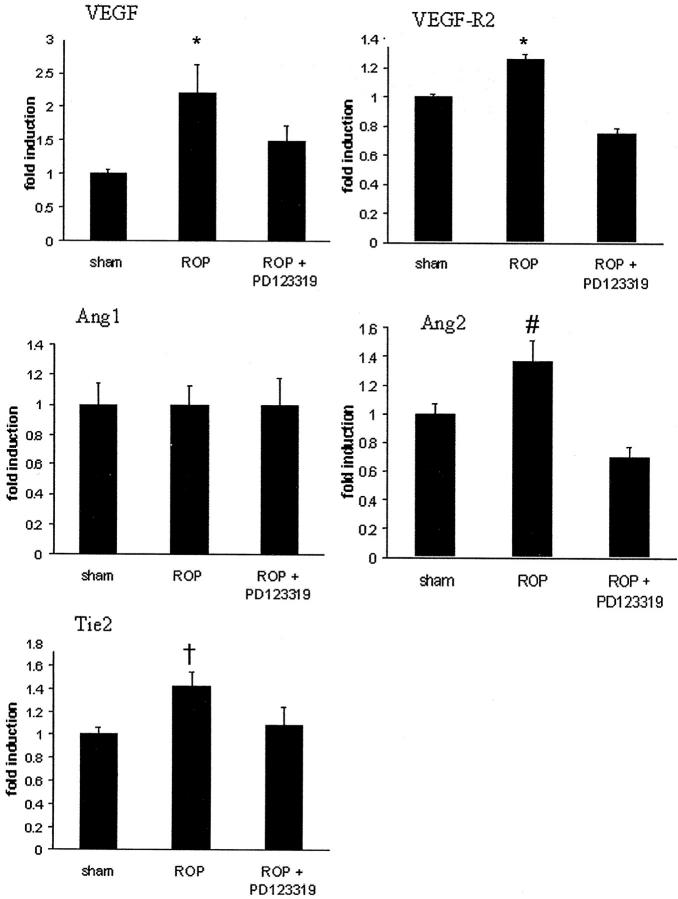
Real-time PCR revealed that VEGF, VEGF-R2, Ang1, Ang2, and Tie2 mRNA were present in sham retina (Figure 3) ▶ . ROP was associated with an increase in VEGF, VEGF-R2, Ang2, and Tie2 mRNA, whereas Ang1 was unchanged compared to sham rats. PD123319 treatment to ROP rats attenuated the rise in VEGF, VEGF-R2, and Ang2 but had no effect on Tie2 mRNA. In ROP rats, PD123319 did not alter Ang1 gene expression (Figure 3) ▶ .
Figure 3.
Real-time PCR showing gene expression for VEGF, VEGF-R2, Ang1, Ang2, and Tie2 in retina from Sprague Dawley rats following retinopathy of prematurity (ROP) and AT2 receptor blockade. Values are mean ± SEM. N = 6 to 8 rats per group. *, P < 0.01 compared to sham and ROP + PD123319; #, P < 0.05 compared to sham and ROP+PD123319; †, P < 0.05 compared to sham.
In Situ Hybridization for VEGF and VEGF-R2 in ROP Rats
In sham rats, VEGF and VEGF-R2 mRNA were detected in the GCL, weakly on blood vessels and in the inner nuclear layer and retinal pigment epithelium (Figure 4,A and a ▶ and Figure 5 ▶ ). In ROP rats, VEGF and VEGF-R2 mRNA were increased compared to sham rats in the GCL, blood vessels, INL, and RPE (Figure 4, B and b ▶ and Figure 5 ▶ ). In ROP rats treated with PD123319 (Figure 4, C and c ▶ and Figure 5 ▶ ) both VEGF and VEGF-R2 gene expression in the inner retina were reduced to the level of sham rats. In all animals, hybridization signal for VEGF and VEGF-R2 was not observed in the outer nuclear layer.
Figure 4.
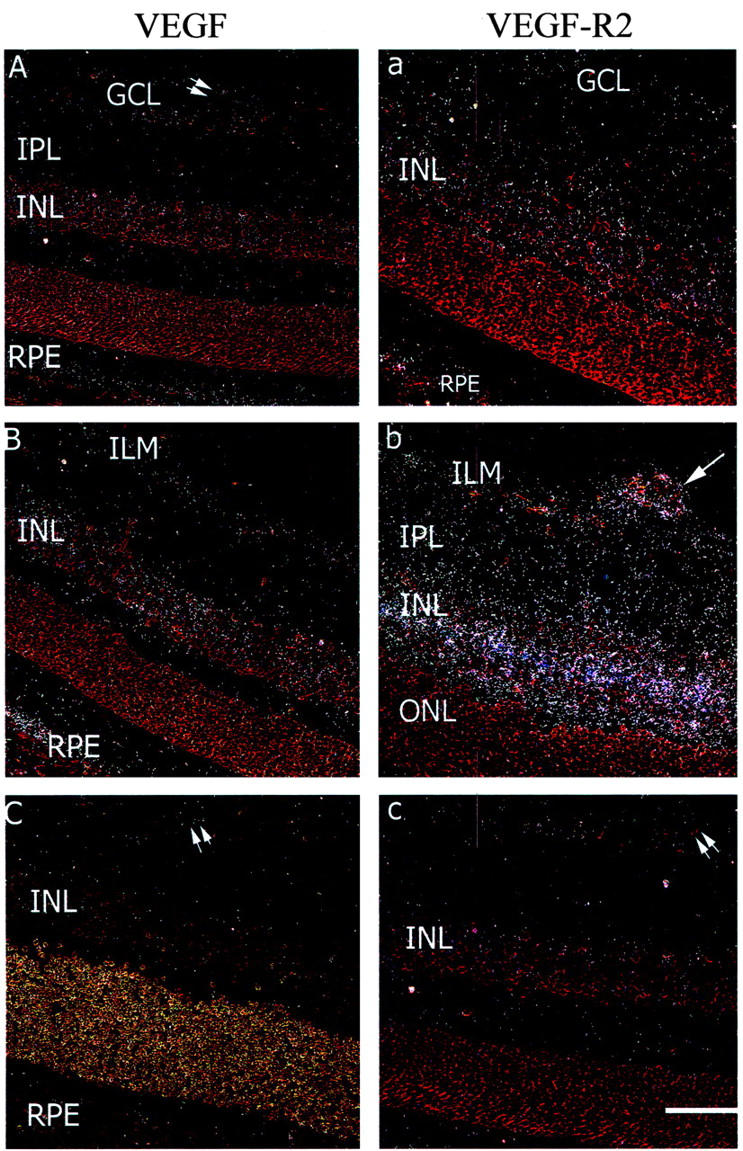
Dark field sections showing VEGF (A–C) and VEGF-R2 (a–c) gene expression in retina from 18-day-old Sprague Dawley rats following retinopathy of prematurity (ROP) and AT2 receptor blockade. Stain, hematoxylin and eosin. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium. Magnification, ×180. Bar, 55 μm. Weak hybridization signal is observed in the GCL, blood vessels, IPL, and INL of sham animals (A and a). Hybridization signal for both VEGF and VEGFR-2 is increased in the GCL, blood vessels, IPL, and INL with ROP (B and b) and reduced in ROP rats with the AT2-RB, PD123319 (C and c). In all sections, specific hybridization signal is not observed in the ONL. Arrow denotes hybridization signal on blood vessels. Double arrows denote blood vessels with minimal or no hybridization signal.
Figure 5.
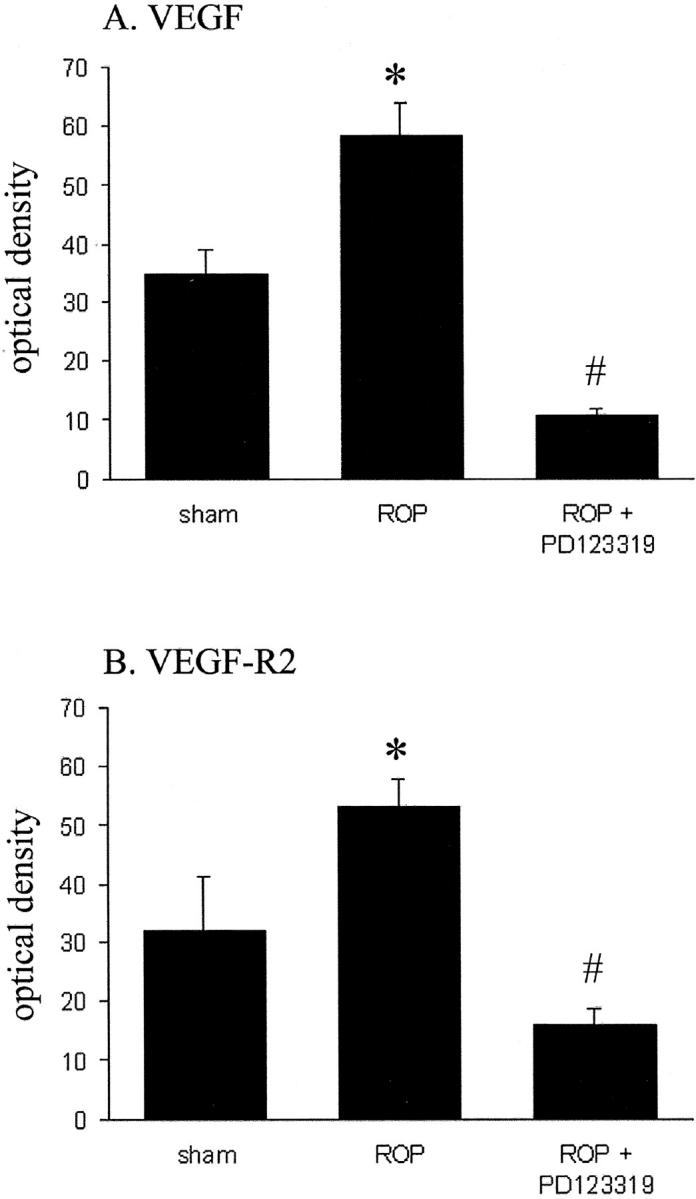
Optical density of VEGF (A) and VEGF-R2 (B) mRNA in Sprague Dawley rats following retinopathy of prematurity (ROP) and AT2 receptor blockade. Values are mean ± SEM. N = 6 to 8 rats per group. *, P < 0.05 compared to all groups, #, P < 0.05 compared to sham.
Gene Expression for RAS Components in ROP Rats
Real-time PCR revealed that renin and AT1 and AT2 receptor mRNA were detected in sham retina and increased with ROP (Figure 6) ▶ . In ROP rats treated with the AT2 receptor blocker, PD123319, renin gene expression and AT1 and AT2 receptor mRNA were unchanged compared to ROP rats (Figure 6) ▶ .
Figure 6.
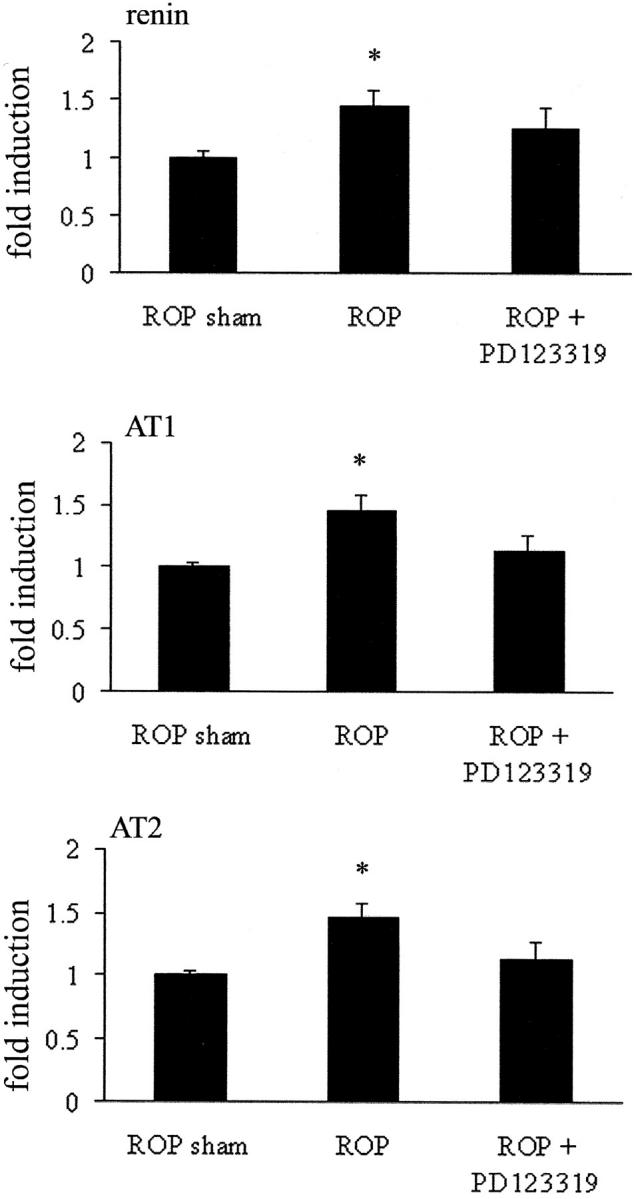
Real-time PCR for renin and AT1 and AT2 receptors in retina from 18-day-old Sprague Dawley rats following retinopathy of prematurity (ROP) and AT2 receptor blockade. Values are mean ± SEM. N = 6 to 8 rats per group. *, P < 0.05 compared to sham.
Immunohistochemistry for AT1 and AT2 Receptors in ROP Rats
In shams, ROP and ROP + PD123319, immunolabeling for both the AT1 and AT2 receptors was observed in blood vessels, ILM, and the cytoplasm of cells in the inner nuclear layer (INL)(Figure 7) ▶ . Sections incubated with phosphate-buffered saline instead of the primary antibodies showed no specific immunolabeling (Figure 7) ▶ . There were no apparent changes in the intensity or distribution of immunolabeling for the AT1 and AT2 receptors among the three groups.
Figure 7.
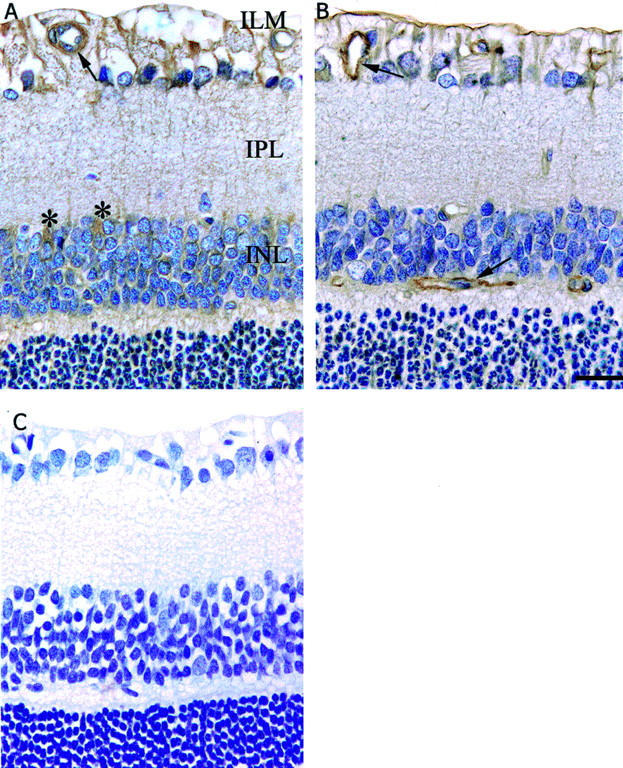
Immunolabeling for AT1 and AT2 receptors in the retina of Sprague Dawley rats following retinopathy of prematurity and treated with AT2 receptor blockade. Counterstain, hematoxylin. ILM, inner limiting membrane; IPL, inner plexiform layer; INL, inner nuclear layer. Magnification, ×320. Bar, 25 μm. A: AT1 receptor. B: AT2 receptor. C: Negative control. Arrows denote labeling on blood vessels. Asterisks denotes labeling in the cytoplasm of cells in the INL.
Discussion
This study demonstrates that retinal angiogenesis in a rat model of ROP is associated with up-regulation of the RAS, namely AT1 and AT2 receptors, and renin gene expression which is accompanied by an increase in two pathways that are involved in endothelial cell growth, VEGF and angiopoietin. The novelty of the study is that AT2-RB reduces retinal angiogenesis with a concomitant reduction in the expression of VEGF, VEGF-R2, and Ang2. Furthermore, our findings are consistent with an angiogenic role for angiopoietin2 (Ang2) in the in vivo environment of high VEGF in the hypoxic retina. Overall, these findings demonstrate an important interaction between the RAS and these angiogenic cytokines and their receptors in mediating retinal angiogenesis.
Angiotensin II (ANG II) has pro-angiogenic effects in various situations such as sponge implant models of angiogenesis, 34 chick chorioallantoic membrane, 35 rat cremaster muscle, 19 rat carotid artery, 36 and cornea. 37 There is evidence that the AT1 receptor mediates the proliferative effects of ANG II 7,19,38 however, the role of the AT2 receptor in these processes is less well understood with reports of pro-, anti-, and no effects on cell growth and angiogenesis. 9,14-20,39,40 In the present study, we found gene expression for the AT2 receptor to be up-regulated in ROP and AT2-RB to attenuate retinal angiogenesis, indicating that the AT2 receptor promotes retinal endothelial cell growth. AT1 receptor mRNA is also increased in ROP and this finding, together with our previous report that AT1-RB reduces retinal angiogenesis in ROP, 7 is consistent with AT1 and AT2 receptors having similar pro-angiogenic roles in ROP. A trophic action for both the AT1 and AT2 receptors in the retina is consistent with reports that AT1-RB and AT2-RB are anti-proliferative in proximal tubules, 16 the mesenteric arterial tree, 32 and mouse spleen lymphocytes. 41 There is also evidence that the AT2 receptor promotes cell growth and inflammation in vascular smooth muscle cells, rat embryo, and cultured cells. 15,17,42 Previous studies by our laboratory have reported combined AT1-RB and AT2-RB to be more effective than monotherapy in attenuating cell proliferation and tissue injury in an experimental model of renal disease. 33 In view of the present findings, this approach is worth considering in terms of potential therapeutic strategies for the treatment of retinal angiogenesis in ROP and proliferative diabetic retinopathy. However, there is evidence that the AT2 receptor constrains the pro-growth effects of the AT1 receptor in various tissues 14,18,19,43 and therefore it is possible that the influence of AT2 receptors on cell growth is tissue and injury specific.
Our previous study in a rat model of ROP 7 and a report by Lonchampt et al 40 in mice with ROP, have shown that administration of AT1-RB into either the peritoneum or subcutaneously, reduced retinal neovascularization. In the present study, AT2-RB also attenuated retinal neovascularization in rats with ROP, however this was not observed in the study by Lonchampt and colleagues. 40 One explanation for the differences between the two studies may relate to the mode of administration of the AT2-RB, PD123319. We administered PD123319 by mini-osmotic pump to ensure continuous infusion of the AT2-RB. Our group has previously reported that PD123319 requires continuous administration to effectively block the AT2 receptor in blood vessels and kidney using in vitro and in vivo autoradiography techniques. 16,32 By contrast, the daily administration of a single subcutaneous dose of PD123319 by Lonchampt and colleagues 40 which failed to confer retinal protection was not clearly demonstrated by that group to block the AT2 receptor in vivo. However, it must be acknowledged that the abundance of AT1 and AT2 receptors in the mouse and rat retina may also account for the discrepancies between the studies. We found AT2 receptor binding to be generally higher than AT1 during postnatal development of the rat retina, and both receptors to be in similar amounts in adult rats. To our knowledge, the abundance of AT1 and AT2 receptors in retina has not previously been evaluated, although there are reports that both receptors are expressed in the developing and adult rat retina. 44,45 The abundance of AT2 receptors in the rat retina may explain our findings of an anti-angiogenic response with PD123319 in ROP, and are in accordance with the view that the AT2 receptor is highly expressed in fetal tissues to influence cell differentiation and growth. 21,46
The cellular distribution of the AT2 receptor in the retina is also important when evaluating the growth effects of this receptor. A previous in vitro study has reported PD123319 to not attenuate ANG II-stimulated retinal pericyte migration. 12 In the present study, we found in both sham and untreated ROP rats, that both AT1 and AT2 receptors were localized not only to blood vessels but also to the ILM and nuclei in the INL, which presumably represent macroglial Muller cells. Importantly, Muller cells are associated with retinal blood vessels 47 and are sites of VEGF and VEGF-R2 expression. 7,25,48 It is therefore possible that ANG II via the AT2 receptor may influence retinal angiogenesis via a paracrine effect on VEGF in certain cells that support the retinal microvasculature.
It is clear that ANG II up-regulates VEGF gene expression in a variety of cell types including retinal endothelial cells. 11,49-51 In terms of angiogenesis, studies using blockade of the RAS in cancer and experimental models of angiogenesis have highlighted this interaction. 9,52 Similar findings have been reported in ocular tissues including bovine retinal endothelial cells (BREC) 11 and streptozotocin diabetes. 25 Of interest is our finding in ROP 7 where we reported that ACE inhibition but not AT1-RB attenuated the rise in VEGF and VEGF-R2 gene expression. These findings together with those of the present study, which clearly demonstrate a reduction in VEGF and VEGF-R2 mRNA with AT2-RB, highlight for the first time a previously unrecognized interaction between the AT2 receptor and the VEGF/VEGF-R2 system in the ischemic-induced angiogenesis of ROP.
In vitro studies indicate that in cooperaton with VEGF, Ang1 can potentiate vascular maturation, whereas Ang2 initiates extensive angiogenesis. 29 In agreement is a recent in vivo study using the pupillary membrane, a transient ocular microvessel network, which shows that following VEGF inhibition, Ang2 promotes endothelial cell death and blood vessel regression. 53 This concept can be extended to the retina, as Ang2-deficient mice have abnormal vascular development, and following ROP exhibit no retinal neovascularization. 54 Furthermore, inhibition of the Tie2 receptor with adenovirus-mediated gene delivery also attenuates angiogenesis in mouse models of ROP and choroidal neovascularization. 55 The findings of the present study support an angiogenic role for Ang2 and Tie2 as both were increased with ROP in the setting of elevated VEGF, whereas Ang1 mRNA remained unchanged. In ROP, the stimulus for up-regulation of VEGF is tissue hypoxia, 7,25,48 which may also be the case for Ang2 and Tie2 as initially observed in rat glioma cells and BRECs. 56,57 In contrast to Ang2, Ang1’s role in retinal angiogenesis may be protective. A recent study has indicated that intravitreal administration of Ang1 to newly diabetic rats normalizes retinal VEGF and intercellular adhesion molecule-1 mRNA and protein levels, and reduces leukocyte adhesion and breakdown of the blood retinal barrier. 58
There is evidence of a potential interaction between the RAS and VEGF/VEGF-R2 and angiopoietin-Tie2. In BRECs and a rat model of choroidal neovascularization, the pro-angiogenic effects of ANG II involve up-regulation of Ang2 but not Ang1 in a dose- and time-dependent manner. 30 Although retinal ANG II was not measured in the present study, the rise in Ang2 mRNA with ROP was accompanied by an increase in renin mRNA, and we have previously reported activation of prorenin in ROP rats. 7 Since renin is a major rate-limiting step in the RAS, this would be expected to lead to an increase in the local production of ANG II. These findings together with a reduction in Ang2 mRNA following AT2-RB indicate an interaction between the retinal RAS and Ang2-Tie2 systems in promoting retinal angiogenesis. Previous studies in BRECs 30 and cultured cardiac cells 31 are in agreement with an interaction between the RAS and angiopoietins, with AT1-RB reducing Ang2 mRNA. Whether ANG II modulates the gene expression of Tie2 in retinal cells is not known, however in cultured cardiac endothelial cells, ANG II was reported to have no effect on Tie2 mRNA. 31 Similarly, in the present study, AT2-RB did not influence the gene expression of Tie2.
In context of previous studies linking components of the RAS to retinal disease, the present study extends our understanding of this issue by demonstrating not only the presence of the AT2 receptor in the retina which appears to be up-regulated in the ROP model but also demonstrates that blockade of this receptor subtype is anti-angiogenic. The findings linking retinal vascular protection to changes in VEGF and angiopoietin-dependent pathways highlights the potential impact of the interaction between the RAS and angiogenic cytokines and their receptors in retinal angiogenesis.
Acknowledgments
We thank the National Health and Medical Research Council of Australia and Juvenile Diabetes Research Foundation International.
Footnotes
Address reprint requests to Dr. Jennifer Wilkinson-Berka, Department of Physiology, The University of Melbourne, Parkville, Victoria, Australia, 3010. E-mail: j.berka@physiology.unimelb.edu.au.
Supported by National Health and Medical Research Council of Australia and Juvenile Diabetes Foundation International.
References
- 1.Klein R, Klein B, Moss S: Epidemiology of proliferative diabetic retinopathy. Diabetes Care 1992, 15:1875-1891 [DOI] [PubMed] [Google Scholar]
- 2.Fryczkowski A, Hodes B, Walker J: Diabetic choroidal and iris vasculature scanning electron microscopy findings. Int Ophthalmol Clin 1989, 13:269-279 [DOI] [PubMed] [Google Scholar]
- 3.Phelps DL: Retinopathy of prematurity. Pediatr Rev 1995, 16:50-56 [DOI] [PubMed] [Google Scholar]
- 4.Schalekamp MADH: Renin-angiotensin system components and endothelial proteins as markers of diabetic microvascular disease. Clin Invest 1993, 71:S3-S6 [DOI] [PubMed] [Google Scholar]
- 5.Danser AHJ, Van den Dorpel MA, Deinum J, Derkx FHM, Franken AAM, Peperkamp E, de Jong PTVM, Schalekamp MADH: Renin, prorenin, and immunoreactive renin in vitreous fluid from eyes with and without diabetic retinopathy. J Clin Endocrinol Metab 1989, 68:160-167 [DOI] [PubMed] [Google Scholar]
- 6.Chaturvedi M, Sjolie AK, Stephenson JM, Abrahamian H, Keipes M: Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. Lancet 1998, 351:28-31 [DOI] [PubMed] [Google Scholar]
- 7.Moravski CJ, Kelly DJ, Cooper ME, Gilbert RE, Bertram J, Shahinfar S, Skinner SL, Wilkinson-Berka JL: Retinal neovascularization is prevented by blockade of the renin-angiotensin system. Hypertens 2000, 36:1099-1104 [DOI] [PubMed] [Google Scholar]
- 8.Le Noble FA, Schreurs NH, van Straaten HW, Slaaf D, Smits JF, Rogg H, Struijker-Boudier HA: Evidence for a novel angiotensin II receptor involved in angiogenesis in chick embryo chorioallantoic membrane. Am J Physiol 1993, 264:460-465 [DOI] [PubMed] [Google Scholar]
- 9.Tamarat R, Silvestre JS, Durie M, Levy BI: Angiotensin II angiogenic effect in vivo involves vascular endothelial growth factor- and inflammation-related pathways. Lab Invest 2002, 82:747-756 [DOI] [PubMed] [Google Scholar]
- 10.Itoh H, Mukoyama M, Pratt RE, Gibbons GH, Dzau VJ: Multiple autocrine growth factors modulate vascular smooth muscle cell growth in response to angiotensin II. J Clin Invest 1993, 91:2268-2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otani A, Takagi H, Suzuma K, Honda Y: Angiotensin II potentiates endothelial growth factor-induced angiogenic activity in retinal microcapillary endothelial cells. Circ Res 1998, 82:619-628 [DOI] [PubMed] [Google Scholar]
- 12.Nadal JA, Scicli GM, Carbini LA, Scicli AG: Angiotensin II stimulates migration of retinal microvascular pericytes: involvement of TGF-β and PDGF-BB. Am J Physiol 2002, 282:H739-H748 [DOI] [PubMed] [Google Scholar]
- 13.Perio C, Rodriguezlopez AM, Angulo J, Regadera J, Marin J, Sanchezferrer CF, Lopeznovoa JM: Endogenous angiotensin II and cell hypertrophy in vascular smooth muscle cultures from hypertensive Ren-2 transgenic rats. Cell Physiol Biochem 1998, 8:106-116 [DOI] [PubMed] [Google Scholar]
- 14.Chung O, Kuhl H, Stoll M, Unger T: Physiological and pharmacological implications of AT1 versus AT2 receptors. Kidney Int 1998, 54:S95-S99 [DOI] [PubMed] [Google Scholar]
- 15.Tebbs C, Pratten MK, Broughton Pipkin F: Angiotensin II is a growth factor in the peri-implantation rat embryo. J Anat 1999, 195:75-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao Z, Kelly DJ, Cox A, Casley D, Forbes JM, Martinello P, Dean R, Gilbert RE, Cooper ME: Angiotensin type 2 receptor is expressed in the adult rat kidney and promotes cellular proliferation and apoptosis. Kidney Int 2000, 58:2437-2451 [DOI] [PubMed] [Google Scholar]
- 17.Levy BI, Benessiano J, Henrion D, Caputo L, Heymes C, Duriez M, Poitevin P, Samuel JL: Chronic blockade of AT2-subtype receptors prevents the effect of angiotensin II on the rat vascular structure. J Clin Invest 1996, 98:418-425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakajima M, Hutchinson HG, Fujinaga M, Hayashida W, Morishita R, Zhang L, Horiuchi M, Pratt RE, Dzau VJ: The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1 receptor: gain-of-function study using gene transfer. Proc Natl Acad Sci USA 1995, 92:10663-10667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munzenmaier DH, Greene AS: Opposing actions of angiotensin II on microvascular growth and arterial blood pressure. Hypertens 1996, 27:760-765 [DOI] [PubMed] [Google Scholar]
- 20.Akishita M, Ito M, Lehtonen JYA, Daviet L, Dzau VJ, Horiuchi M: Expression of the AT2 receptor developmentally programs extracellular signal-regulated kinase activity and influences fetal vascular growth. J Clin Invest 1999, 103:63-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grady EF, Sechi LA, Griffin CA, Schambelan M, Kalinyak JE: Expression of AT2 receptors in the developing rat fetus. J Clin Invest 1991, 88:921-933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrara N: Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol 2001, 280:C1358-1366 [DOI] [PubMed] [Google Scholar]
- 23.De Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT: The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 1992, 255:989-991 [DOI] [PubMed] [Google Scholar]
- 24.Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ullrich A: High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 1993, 72:835-846 [DOI] [PubMed] [Google Scholar]
- 25.Gilbert R, Vranes D, Berka JL, Cox A, Kelly DJ, Stacker S, Cooper ME: Vascular endothelial growth factor and its receptors in control and diabetic rat eyes. Lab Invest 1998, 78:1017-1027 [PubMed] [Google Scholar]
- 26.Sato TN, Tozawa Y, Deutsch U, Wolburgbuchholz K, Fujiwara Y, Gendronmaguire M, Gridley T, Wolburg H, Risau W, Qin Y: Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 1995, 376:70-74 [DOI] [PubMed] [Google Scholar]
- 27.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand S, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD: Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997, 277:55-60 [DOI] [PubMed] [Google Scholar]
- 28.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD: Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 1996, 87:1161-1169 [DOI] [PubMed] [Google Scholar]
- 29.Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, Yancopoulos GD, Isner JM: Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res 1998, 83:233-240 [DOI] [PubMed] [Google Scholar]
- 30.Otani A, Takagi H, Oh H, Koyama S, Honda Y: Angiotensin II induces expression of the Tie2 receptor ligand, angiopoietin-2, in bovine retinal endothelial cells. Diabetes 2001, 50:867-875 [DOI] [PubMed] [Google Scholar]
- 31.Fujiyama S, Matsubara H, Nozawa Y, Maruyama K, Mori Y, Tsutsumi Y, Masaki H, Uchiyama Y, Koyama Y, Nose A, Iba O, Tateishi E, Ogata N, Jyo N, Higashiyama S, Iwasaka T: Angiotensin AT1 and AT2 receptors differentially regulate angiopoietin-2 and vascular endothelial growth factor expression and angiogenesis by modulating heparin binding-epidermal growth factor (EGF)-mediated EGF receptor transactivation. Circ Res 2001, 88:22-29 [DOI] [PubMed] [Google Scholar]
- 32.Cao Z, Dean R, Wu L, Casley D, Cooper ME: Role of angiotensin receptor subtypes in mesenteric vascular proliferation and hypertrophy. Hypertens 1999, 34:408-414 [DOI] [PubMed] [Google Scholar]
- 33.Cao Z, Bonnet F, Candido R, Nesteroff SP, Burns WC, Kawaci H, Shimizu F, Carey RM, de Gasparo M, Cooper ME: Angiotensin type 2 receptor antagonism confers renal protection in a rat model of progressive renal injury. J Am Soc Nephrol 2002, 13:1773-1787 [DOI] [PubMed] [Google Scholar]
- 34.Hu DE, Hiley CR, Fan TP: Comparative studies of the angiogenic activity of vasoactive intestinal peptide, endothelins-1 and –3, and angiotensin II in a rat sponge model. Br J Pharmacol 1996, 117:545-551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LeNoble FAC, Hekking JWM, Van Straaten HWM, Slaaf DW, Struykey Boudier HAJ: Angiotensin II stimulates angiogenesis in the chorio-allantoic membrane of the chick embryo. Eur J Pharmacol 1991, 195:305-306 [DOI] [PubMed] [Google Scholar]
- 36.Scheidegger KJ, Wood JM: Local application of angiotensin II to the rat carotid artery induces adventitial thickening. J Vasc Res 1997, 34:436-446 [DOI] [PubMed] [Google Scholar]
- 37.Fernandez L, Twickler J, Mead A: Neovascularization produced by angiotensin II. J Lab Clin Med 1985, 105:141-145 [PubMed] [Google Scholar]
- 38.Emanueli C, Salis MB, Stacca T, Pinna A, Gaspa L, Maddeddu P: Angiotensin AT(1) receptor signalling modulates reparative angiogenesis induced by limb ischaemia. Br J Pharmacol 2002, 135:87-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabri A, Levy BI, Poetevin P, Caputo L, Faggin E, Marotte F, Rappaprot L, Samuel JL: Differential roles of AT1 and AT2 receptor subtypes in vascular trophic and phenotypic changes in response to stimulation with angiotensin II. Arterioscler Thromb Vasc Biol 1997, 17:257-264 [DOI] [PubMed] [Google Scholar]
- 40.Lonchampt M, Pennel L, Duhault J: Hyperoxia/normoxia-driven retinal angiogenesis in mice: a role for angiotensin II. Invest Ophthalmol Vis Sci 2001, 42:429-432 [PubMed] [Google Scholar]
- 41.Kunert-Radek J, Stepien H, Komorowski J, Pawlikowski M: Stimulatory effect of angiotensin II on the proliferation of mouse spleen lymphocytes in vitro is mediated via both types of angiotensin II receptors. Biochem Biophys Res Commun 1994, 198:1034-1039 [DOI] [PubMed] [Google Scholar]
- 42.Wolf G, Wenzel U, Burns KD, Harris RC, Stahl RAK, Thaiss F: Angiotensin II activates nuclear transcription factor-kB through AT1 and AT2 receptors. Kidney Int 2002, 61:1886-1995 [DOI] [PubMed] [Google Scholar]
- 43.Stoll M, Steckelings UM, Paul M, Bottari SP, Metzger R, Unger T: The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J Clin Invest 1995, 95:651-657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wheeler-Schilling TH, Kohler K, Sautter M, Guenther E: Angiotensin II receptor subtype gene expression and cellular localization in the retina and non-neuronal ocular tissues of the rat. Eur J Neurosci 1999, 11:3387-3394 [DOI] [PubMed] [Google Scholar]
- 45.Murata M, Nakagawa M, Takahashi S: Expression and localization of angiotensin II type 1 receptor mRNA in rat ocular tissues. Ophthalmologica 1997, 211:384-386 [DOI] [PubMed] [Google Scholar]
- 46.Daud AI, Bumpus FM, Husain A: Angiotensin II: does it have a direct obligatte role in ovulation? Science 1989, 245:870-871 [DOI] [PubMed] [Google Scholar]
- 47.Nork T, Wallow I, Sramek S, Anderson G: Muller cells involvement in proliferative diabetic retinopathy. Arch Ophthalmol 1987, 105:1424-1429 [DOI] [PubMed] [Google Scholar]
- 48.Stone J, Itin A, Alon T, Peer J, Gnessin H, Chan-Ling T, Keshet E: Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci 1995, 15:4738-4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gruden G, Thomas S, Burt D, Zhou W, Chusney G, Gnudi L, Viberti G: Interaction of angiotensin II and mechanical stretch on vascular endothelial growth factor production by human mesangial cells. J Am Soc Nephrol 1999, 10:730-737 [DOI] [PubMed] [Google Scholar]
- 50.Sasaki K, Murohara T, Ikeda H, Sugaya T, Shimada T, Shintani S, Imaizumi T: Evidence for the importance of angiotensin II type 1 receptor in ischemia-induced angiogenesis. J Clin Invest 2002, 109:603-611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amaral S, Roman R, Greene A: Renin gene transfer restores angiogenesis and vascular endothelial growth factor expression in Dahl’s rats. Hypertens 2001, 37:386-390 [DOI] [PubMed] [Google Scholar]
- 52.Yoshiji H, Kuriyama S, Kawata M, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Fukui H: The angiotensin-I-converting enzyme inhibitor perindopril suppresses tumor growth and angiogenesis: possible role of the vascular endothelial growth factor. Clin Cancer Res 2001, 7:1073-1078 [PubMed] [Google Scholar]
- 53.Lobov IB, Brooks PC, Lang RA: Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci USA 2002, 99:11205-11210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hackett SF, Ozaki H, Strauss RW, Wahlin K, Suri C, Maisonpierre P, Yancopoulos G, Campochiaro PA: Angiopoietin 2 expression in the retina: up-regulation during physiologic and pathologic neovascularization. J Cell Physiol 2000, 184:275-284 [DOI] [PubMed] [Google Scholar]
- 55.Hangai M, Moon YS, Kitaya N, Chan CK, Wu DY, Peters KG, Ryan SJ, Hinton DR: Systemically expressed soluble Tie2 inhibits intraocular neovascularization. Hum Gene Ther 2001, 12:1311-1321 [DOI] [PubMed] [Google Scholar]
- 56.Enholm B, Paavonen K, Ristimaki A, Kumar V, Gunji Y, Klefstrom J, Kivinen L, Laiho M, Olofsson B, Joukov V, Eriksson U, Alitalo K: Comparison of VEGF, VEGF-B, VEGF-C, and Ang-1 mRNA regulation by serum, growth factors, oncoproteins, and hypoxia. Oncogene 1997, 14:2475-2483 [DOI] [PubMed] [Google Scholar]
- 57.Oh H, Takagi H, Suzuma K, Otani A, Matsumura M, Honda Y: Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J Biol Chem 1999, 274:15732-15739 [DOI] [PubMed] [Google Scholar]
- 58.Joussen AM, Poulaki V, Tsujikawa A, Qin W, Qaum T, Xu Q, Moromizato Y, Bursell SE, Wiegand SJ, Rudge J, Ioffe E, Yancopoulos GD, Adamis AP: Suppression of diabetic retinopathy with angiopoietin-1. Am J Pathol 2002, 160:1683-1693 [DOI] [PMC free article] [PubMed] [Google Scholar]