Abstract
The pathophysiological role of infiltrating macrophages and their subtypes in idiopathic inflammatory myopathies such as dermatomyositis, polymyositis, and inclusion body myositis is not fully clear. Monocytes exhibit various phenotypes with different functional properties such as release of pro- or anti-inflammatory mediators. Expression of myeloid-related proteins MRP8 and MRP14, two calcium-binding S100-proteins, characterizes a proinflammatory subtype of macrophages. We immunohistochemically investigated expression of MRP8 and MRP14 in muscle biopsies of 33 patients with dermatomyositis, polymyositis, and inclusion body myositis. We found a clear association of expression of MRP8 and MRP14 by infiltrating macrophages with degeneration of myofibers. Because MRP8 and MRP14 are secreted by activated macrophages we investigated if these proteins would have direct extracellular effects on myocytes. We found that the purified MRP8/MRP14 complex inhibited proliferation and differentiation of C2C12 myoblasts and that it induced apoptosis via activation of caspase-3 in a time- and dose-dependent manner. These results indicate that in the course of inflammatory myopathies, activated macrophages can promote destruction and impair regeneration of myocytes via secretion of MRP8/MRP14.
Myositis is a term describing muscle inflammation independent of its etiology. The heterogeneous group of acute and chronic idiopathic inflammatory myopathies (IM) is histologically characterized by signs of destruction and partial regeneration of inflamed muscle fibers. 1,2 The three major types of idiopathic inflammatory myopathies are: dermatomyositis (DM), including childhood DM, polymyositis (PM), and inclusion body myositis (IBM). 3 In all three idiopathic IM the mononuclear cell infiltrates consist predominantly of T lymphocytes and monocytes/macrophages. 1 Despite different underlying pathogenesis the molecular mechanisms of monocyte recruitment and cytokine pattern does not seem to be essentially different in these distinct forms of IM. 4-6 The pathophysiological role of these infiltrating monocytes is not completely known. Since monocytes exhibit various phenotypes with different functional properties it is important to look for defined subpopulations in distinct inflammatory conditions. 7 Myeloid-related protein 8 (MRP8; S100A8) and MRP14 (S100A9) are two calcium-binding proteins belonging to the S100 family. The expression of MRP8 and MRP14 is restricted to granulocytes and to early stages of monocytic differentiation. 8,9 These proteins represent about 40% of the total calcium-binding capacity in monocytes, but are not detectable in mature tissue macrophages. 10-13 The expression of MRP8 and MRP14 in vivo correlates with the activity of inflammatory processes in different murine and human diseases. 11,12,14-17 MRP8 and MRP14 form non-covalently associated complexes with each other. There are reports about heterodimers, tetramers, trimers and homodimers but the physiological relevance of these different complex forms is not yet clear. Structural analysis and data obtained by mass spectrometry indicate a MRP8/MRP14 heterodimer as the basal complex at least in the human system which associates to a (MRP8/MRP14)2 heterotetramer in a calcium-dependent manner. 18-21,22 MRP8 and MRP14 have been shown to play a role during calcium-dependent activation of monocytes probably via modulation of cytoskeletal-membrane interactions. 13,23,24 In addition, both proteins are specifically released by monocytes during the course of inflammatory reactions, and serum concentrations of MRP8 and MRP14 have been shown to correlate well with the activity of inflammatory reactions in various human diseases. 11,12,14,16,17,25 In the present study we investigated the expression and local distribution of MRP8 and MRP14 in different inflammatory muscle disorders (DM, PM, and IBM) as well as their effects on muscle cells in vitro. In all three kinds of IM we found a clear correlation between infiltration of MRP8/MRP14-expressing monocytes and the inflammatory destruction of muscle fibers. Using the myoblast cell line C2C12 we found that MRP8/MRP14 exert direct inhibitory effects on proliferation of myoblasts and on differentiation to myotubes in vitro. In addition, MRP8/MRP14 exhibit a time- and concentration-dependent activation of caspase-3, a central mediator of programmed cell death, resulting in apoptosis of myoblasts/myotubes.
Materials and Methods
Patients
Twelve patients with DM, including 4 patients with childhood DM (5 male and 7 female), 12 patients with PM (2 male and 10 female), and 9 patients with IBM (6 male and 3 female) were included in this study. The diagnoses of different IM were based on clinical history and histological examinations of muscle biopsies. The diagnosis of IBM was confirmed by electron microscopy in all cases. Control specimens were obtained from 7 patients with neuromuscular diseases without any signs of inflammation. This group included 3 patients with amyotrophic lateral sclerosis (ALS) and 4 patients with morphologically normal muscle. Muscle biopsies of IM patients were performed before administration of immunosuppressive drugs or other immunomodulators. Approval from the Institutional Research Board was obtained.
Immunohistochemistry
Biopsies were fixed in formalin for routine histopathology and specimens were sectioned at a thickness of 5 μm and mounted on slides coated with silane. Rabbit anti-sera against recombinant MRP8 (a-MRP8) and MRP14 (a-MRP14) were produced as described earlier. 8,9 Monospecificity of antibodies was analyzed by immunoreactivity against recombinant MRP8 and MRP14, Western blot analysis of lysates of monocytes and granulocytes as well as by immunoreactivity against MRP8 and/or MRP14 transfected fibroblastic cell lines as described earlier. 8,9 To detect complex formation of MRP8 and MRP14 in situ monoclonal antibody 27E10 was used, which detects an epitope exclusively formed by MRP8/MRP14 complexes but not by the isolated subunits. 26 In addition, sections were stained with monoclonal antibodies against human leukocyte common antigen (LCA, clones 2B11 and PD7/26; DAKO Diagnostika, Hamburg, Germany), anti-human CD4 antigen (a-CD4; DAKO Diagnostika) and monoclonal antibody KP1 against CD68 antigen (a-CD68), a 110-kd transmembrane glycoprotein highly expressed by human monocytes and tissue macrophages (DAKO Diagnostika). 27,28 A monoclonal antibody against Ki67 (Dianova, Hamburg, Germany), a proliferation marker, was used for investigation of C2C12 cells in culture. Rabbit anti-human CPP32 (cysteine protease protein; Cell Signaling, Beverly, MA) was used to detect active caspase-3, a central mediator of programmed cell death. In double-labeling experiments sections were labeled for CD68 antigen and either MRP8 or MRP14 as described earlier. 16 Specific primary antibodies were detected using appropriate peroxidase-, alkaline phosphatase-, fluorescein isiothiocyanate (FITC)- or Cy3-conjugated second-stage antibodies against mouse or rabbit IgG, respectively (Dianova). Isotype-matched antibodies without relevant specificity were used as negative controls (Dianova). Finally, sections were counterstained with Mayer’s hematoxylin (Merck, Darmstadt, Germany). Slides were not counterstained after double-labeling procedure.
Purification of MRP8 and MRP14 from Human Neutrophils
Complexes of MRP8 and MRP14 were isolated from human granulocytes as described in detail previously. 29 Briefly, granulocytes were lysed in homogenization buffer (20 mmol/L Tris, 1 mmol/L EGTA, 1 mmol/L ethylenediaminetetraacetate (EDTA), 1 mmol/L dithiothreitol (DTT), 1% NP40 pH8.5) supplemented with a protease-inhibitor cocktail (Roche Diagnostics, Mannheim, Germany) using a Branson sonifier (model 250; Branson Ultrasonics, Danbury, CT). After ultracentrifugation (100,000 × g for 150 minutes at 4°C using Centrikon T-1065; Kontron Instruments, Munich, Germany), proteins were precipitated by 70% ammonium sulfate. After centrifugation the supernatant was dialyzed and proteins were separated using anion exchange chromatography (MonoQ, Amersham Biosciences, Inc., Piscataway, NJ). At this stage, MRP8/MRP14 complexes appeared to be essentially pure (>98%). The identity of MRP8 and MRP14 was ascertained by amino acid sequencing and mass spectrometry (UV-MALDI-MS). 22,29 Proteins were dialyzed against Dulbecco’s modified Eagle’s medium (DMEM).
Cell Culture
Murine C2C12 myoblasts were cultured at 37°C in a humidified atmosphere of 5% CO2 in DMEM with high glucose (concentration of CaCl2 200 mg/L, no zinc supplementation), supplemented with 15% fetal bovine serum (FBS; Greiner Bio-One, Frickenhausen, Germany), 100 U/ml penicillin G, and 100 μg/ml streptomycin sulfate. Cells were grown at subconfluency of 80% to 90% and were passaged every 3 days. To induce differentiation of myoblasts into myotubes, confluent cells were incubated for an additional 3 days in the presence of 5% FBS. To examine the effects of MRP8/MRP14 complexes on C2C12 myoblasts, 5 × 104 cells were plated in 8-well Lab-Tek chambers (Lab-Tek Chamber Slide System; Nalge Nunc International Corp., Naperville, IL) with 300 μl medium supplemented with 5% or 15% FBS as indicated. 30,31 The cultures were incubated with different concentrations of MRP8/MRP14 (range from 25 to 1600 μg/ml) for 16 to 72 hours. After indicated time periods cells were fixed and stained with hematoxylin and eosin (H&E) to evaluate differentiation of myoblasts to myotubes by morphological criteria. In parallel, cells were fixed in glutaraldehyde for ultrastructural evaluation of apoptosis by transmission electron microscopy (TEM). In addition, activity of lactate dehydrogenase (LDH) was measured in supernatants as a marker of cell death.
Electron Microscopy
For ultrastructural analysis murine C2C12 myoblasts were incubated with 800 μg/ml MRP8/MRP14 or medium as control for 72 hours. After this time cells were processed using a standard protocol. 32 Briefly, cultured cells were washed in PBS and centrifuged for 5 minutes at 200 × g and fixed in 2% glutaraldehyde, in 0.1 mol/L sodium cacodylate buffer (pH 7.4) for 1 hour at room temperature. Cells were washed twice in Tris-buffer alone, postfixed for 1 hour at room temperature in 2% OsO4 in PBS, dehydrated in ethanol and embedded in Epon. Thin sections were contrasted with uranylacetate and lead citrate and examined on a Philips electron microscope for morphological signs of apoptosis.
Detection of Apoptosis by DNA Fragmentation
The leakage of fragmented DNA from apoptotic nuclei was measured according to the method of Nicoletti et al. 33 C2C12 myoblasts were treated for the indicated time with different concentrations of MRP8/MRP14. Cells were harvested, washed, centrifuged, and the cell pellet was resuspended in hypotonic lysis buffer (1% sodium citrate, 0,1% Triton X-100, 50 μg/ml propidium iodide) and subsequently analyzed by flow cytometry with the FL-2/FSC profile. Nuclei to the left of the 2 N peak containing hypodiploid DNA were considered as apoptotic. Flow cytometry analyses were performed on a FACScalibur (BD Biosciences Clontech, Heidelberg, Germany) using CellQuest analysis software.
Fluorimetric Assay of Caspase Activity
Cytosolic cell extracts were prepared by lysing 5 × 104 C2C12 cells in 150 μl buffer containing 0.5% NP-40, 20 mmol/L HEPES (pH 7.4), 84 mmol/L KCl, 10 mmol/L MgCl2, 0.2 mmol/L EDTA, 0.2 mmol/L EGTA, 1 mmol/L DTT, 5 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, and 1 mmol/L phenylmethylsulfonyl fluoride. Caspase activities were determined by incubation of 50 μl of cell lysates with 50 μmol/L of the fluorogenic substrate DEVD-AMC (N-acetyl-Asp-Glu-Val-Asp-aminomethyl-coumarin; Bachem, Heidelberg, Germany) in 200 μl buffer containing 50 mmol/L HEPES pH 7.3, 100 mmol/L NaCl, 10% sucrose, 0.1% CHAPS and 10 mmol/L DTT. The release of aminomethylcoumarin was measured by fluorometry using an excitation wavelength of 360 nm and an emission wavelength of 475 nm.
Western Blotting
For detection of myogenin as a differentiation marker 1 × 105 C2C12 myoblasts were plated in 8-well Lab-Tek chambers with 300 μl medium and cultured in growth medium for 1 day, and in differentiation medium with 5% FBS for 3 more days. MRP8/MRP14 was added to the culture medium (range from 50 to 800 μg/ml). Subsequently, cells were harvested, washed and lysed in 70 μl sodium dodecyl sulfate (SDS) buffer. The protein concentration was determined by Bradford test and equal amounts of protein (15 μg) were loaded on a 12.5% SDS-polyacrylamide gel electrophoresis, and blotted onto a nitrocellulose membrane. Filters were equilibrated in TBST for 10 minutes, blocked with TBST plus 4% nonfat dry milk powder, and myogenin was detected with a specific antibody (clone F5D; BD PharMingen, San Diego, CA). Rabbit anti-mouse IgG peroxidase reaction was used for immunostaining.
Statistical Analysis
Mann-Whitney U-test (for values without normal distribution) was performed to determine significant differences of MRP8 and MRP14 expression between distinct categories of IM and to describe significant results in the cell culture experiments. P values greater than 0.05 were considered to be not significant.
Results
Phenotyping of Leukocytes in Control Biopsies without Signs of IM
Three patients with ALS and 4 muscle biopsies without any pathological feature served as controls. In all sections, labeling for MRP8 and MRP14 revealed only a few single positive mononuclear cells. Staining for CD68 showed some positive tissue macrophages, which were negative for MRP8 or MRP14. LCA+ and CD4+ cells were rarely (< 1%) observed in sections of these patients.
Expression of MRP8 and MRP14 in IM
In all kinds of IM infiltrating monocytes presented a significant expression of MRP8 and MRP14 as well as the leukocyte markers LCA, CD4, and CD68 in the endo- and/or perimysium.
Dermatomyositis
In 12 biopsies of patients with DM about 17% of all infiltrating cells were positive for MRP8, 12% were positive for MRP14, and 16% were positive for CD68. Immunohistochemical experiments revealed the predominance of two different monocytic phenotypes in the infiltrate of DM. Serial sections of the same biopsy showed parallel expression of CD68 as well as MRP8 and MRP14 around necrotic fibers (Figure 1, A and B) ▶ which was confirmed by double-labeling experiments (Figure 1, C and D) ▶ and confocal microscopy (Figure 1, G and H) ▶ . In contrast, CD68+ cells around blood vessels or the perimysium (Figure 1E) ▶ showed almost no immunoreactivity for MRP8 or MRP14 (Figure 1F) ▶ in the absence of myotube necrosis. Infiltrates consisted of nearly 10% CD4+ T cells; about 45% were positive for LCA.
Figure 1.
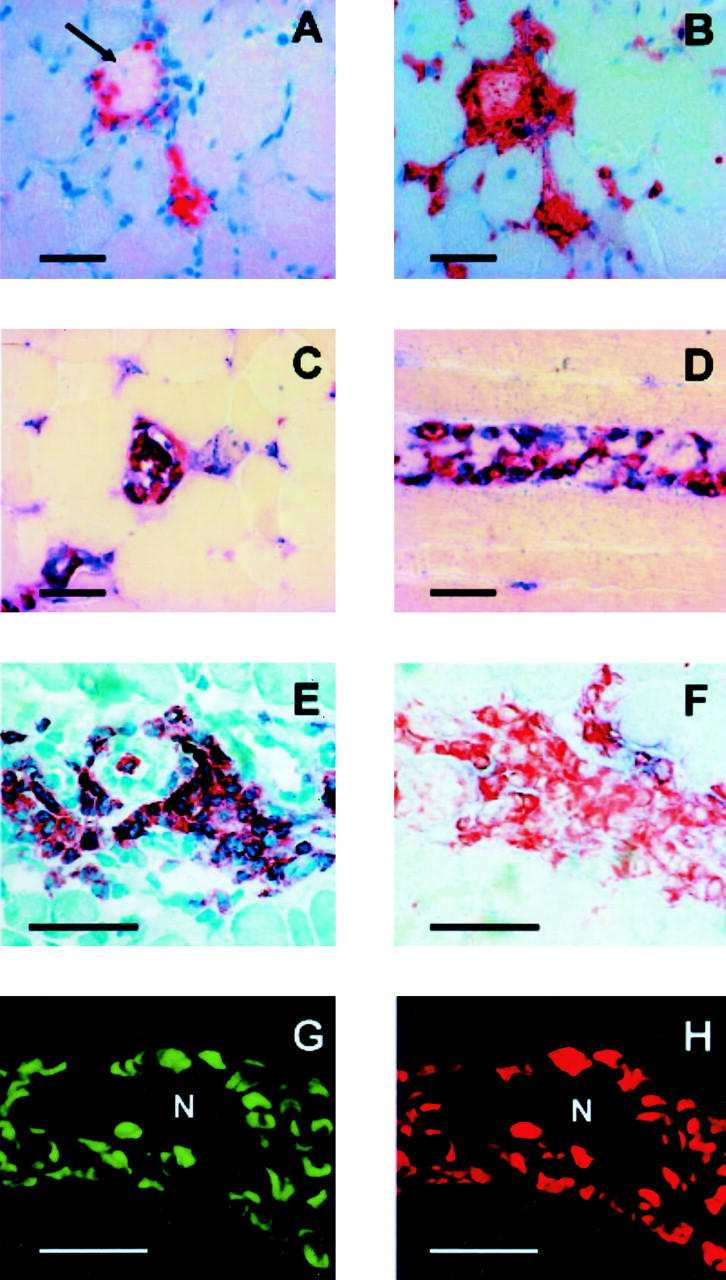
MRP14-expressing CD68+ cells are associated with necrosis of muscle fibers (see Immunohistochemistry). 5-μm sections of DM were stained by immunohistochemistry (A to F) or immunofluorescence (G and H). A: Immunoperoxidase staining of DM with an antibody against CD68 on a cross-section of a necrotic muscle fiber (arrow). B: Serial section of the same biopsy showed parallel expression of MRP8 around the necrotic fiber as well as extracellular secreted MRP8 within the muscle destruction. Sections were counterstained with H&E. C and D: Double-labeling experiments with a-CD68 (blue, alkaline phosphatase) and a-MRP8 (C, red, peroxidase) or a-MRP14 (D, red, peroxidase) of a cross-section (C) and a longitudinal section (D) of a necrotic muscle fiber revealed a clear expression of both proteins in CD68+ macrophages (no counterstaining). E: A high number of CD68+ cells is also detectable in the perimysium of DM in the absence of muscle fiber necrosis (H&E staining). F: Serial section of the same biopsy was labeled in a double-labeling experiment with a-CD68 (red, peroxidase) and a-MRP14 (blue, alkaline phosphatase, no counterstaining). Only a minority of CD68+ cells shows a low expression of MRP14 in the absence of necrotic muscle fibers. G and H: Immunofluorescence staining for CD68 (G, FITC) and MRP14 (H, Cy3) in a necrotic muscle fiber (N) in DM analyzed by confocal laser microscopy confirmed high expression of MRP14 by almost all CD68+ macrophages. Bar, 50 μm.
Polymyositis
Immunohistochemical stainings in 12 patients with PM partly resembled that of DM. The percentages of positive cells in inflammatory infiltrates are 16% for CD68, 21% for CD4, and 50% for LCA. These results do not differ significantly from that obtained in DM. However, the percentage of MRP8-expressing cells (8%) was significantly lower than in DM (P value 0,04), their percentage of infiltrating cells expressing MRP14 was 14%. Double-labeling experiments with a-MRP8 or a-MRP14 and a-CD68 showed a similar prevalence of two different cellular phenotypes in the infiltrate as described for DM above. Expression of MRP8 and MRP14 by CD68+ cells was pronounced in and around necrotic muscle fibers whereas monocytes negative for MRP8 and MRP14 were found primarily in the perimysium (not shown).
Inclusion Body Myositis
In 9 patients the diagnosis of IBM was verified by electron microscopy, which demonstrated typical cytoplasmatic and intranuclear tubulofilaments. The immunohistochemical features were similar to the findings in DM or PM. Double-labeling experiments with a-MRP8 or a-MRP14 and a-CD68 showed a high association of MRP8 and MRP14 expression in monocytes associated with muscle fiber necrosis (about 90% of CD68+ cells express MRP8/MRP14; not shown).
Immunoreactivity of monoclonal antibody 27E10 parallels stainings for MRP8 and MRP14, indicating that complexes of MRP8/MRP14 are the predominant form expressed by monocytes in IM. This finding was constant in all three inflammatory muscle disorders.
MRP8/MRP14 Complex Inhibits Proliferation of C2C12 Myoblasts in Vitro
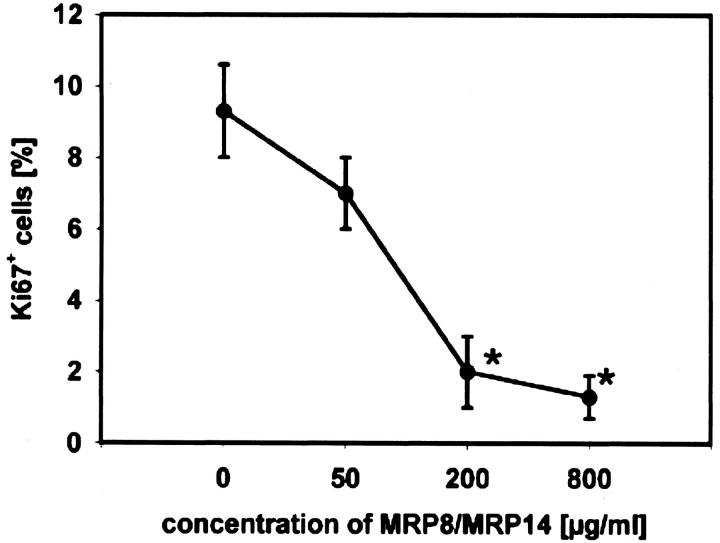
The complex of both proteins represents the predominant extracellular form. 12,34 Since presence of MRP8/MRP14 complexes was such a characteristic feature in all three types of IM and since it was associated with muscle necrosis, we wondered whether MRP8/MRP14 would have a direct effect on striated muscle cells. To investigate direct effects of MRP8/MRP14 complexes on proliferation of C2C12 myoblasts in vitro, cells were cultured for 24 hours with varying concentrations of MRP8/MRP14 and then stained with anti-Ki67 (Figure 2) ▶ . We found a significant and dose-dependent inhibition of proliferation by MRP8/MRP14 treatment compared to untreated controls. This inhibitory effect on proliferation was constant over at least 3 days (data not shown).
Figure 2.
MRP8/MRP14 inhibit proliferation of C2C12 myoblasts. Proliferation of C2C12 myoblast was analyzed using monoclonal antibody Ki67. Cells were cultured with different concentrations of MRP8/MRP14 or medium as control for 24 hours. At least 500 cells were counted and the ratio of Ki67+ cells to the total number was calculated. The results are shown as percentages of positive cells. Asterisks indicate statistically significant differences compared to untreated controls (P ≤ 0.002).
MRP8/MRP14 Complex Inhibits Differentiation of C2C12 Myoblasts
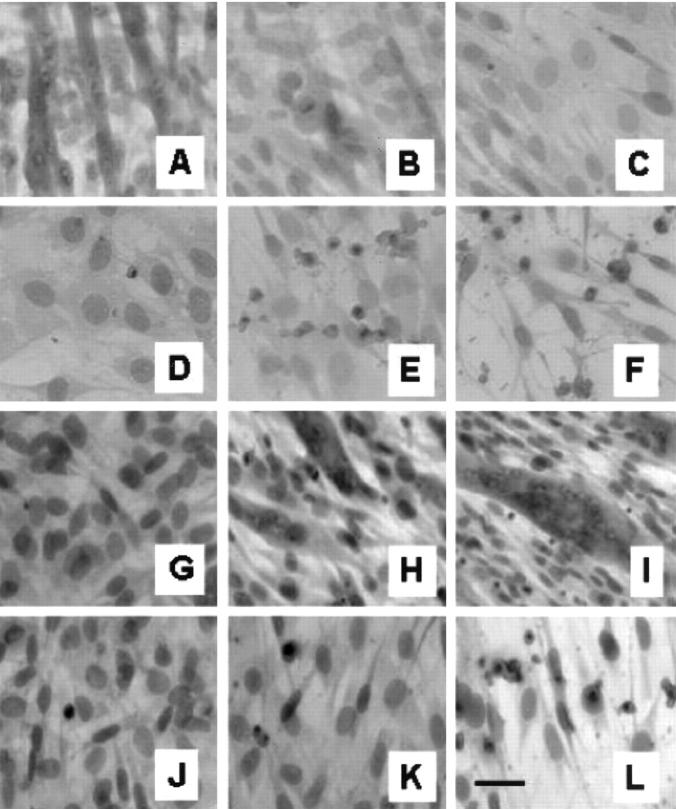
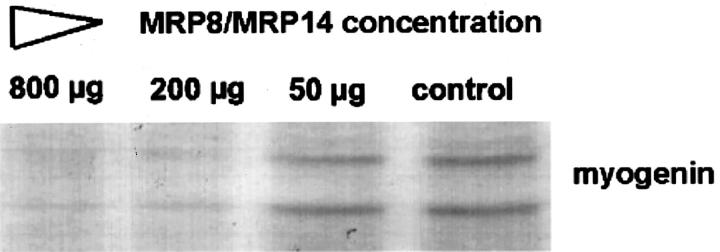
To investigate the effects of MRP8/MRP14 on differentiation, C2C12 myoblasts were cultured for 72 hours in differentiation medium (containing 5% FBS) in the presence of varying doses of MRP8/MRP14. During this period of time, control cells show a clear differentiation of myoblasts to myocytes indicated by a pronounced formation of myotubes. This process of differentiation was almost completely inhibited in a time- and dose-dependent manner by addition of MRP8/MRP14 (Figure 3) ▶ . In parallel, expression of myogenin was analyzed by Western blot as a marker of differentiation. 35 While 72 hours after induction of differentiation untreated C2C12 myoblast showed a significant increase of myogenin expression, MRP8/MRP14 treatment resulted in marked inhibition of myogenin expression (Figure 4) ▶ .
Figure 3.
MRP8/MRP14 block differentiation of C2C12 myoblasts to myotubes. A to F: To analyze the effect of MRP8/MRP14 on proliferation and differentiation, C2C12 myoblast were cultured in medium, as described in Materials and Methods, with indicated concentrations of MRP8/MRP14 for 72 hours. After this time cells were fixed and stained with H&E. There was a significant and dose-dependent inhibition of the formation of myotubes after treatment with MRP8/MRP14 compared to untreated control cells. At high concentrations MRP8/MRP14 induce morphological features of apoptosis. A: Control. B: 100 μg/ml MRP8/MRP14. C: 200 μg/ml. D: 400 μg/ml. E: 800 μg/ml. F: 1600 μg/ml. G to L: Cells were cultured in medium (G to I) or 400 μg/ml MRP8/MRP14 (J to L). Cells were fixed and stained with H&E after 0 hours (G and J), 24 hours (H and K), or 72 hours (J and L). Bar, 10 μm.
Figure 4.
MRP8/MRP14 inhibit expression of myogenin. C2C12 cells were cultured in the presence of different concentrations of MRP8/MRP14 in differentiation medium for 72 hours. Immunoblotting shows the two protein bands known to be recognized by a monoclonal antibody against myogenin (clone F5D). The different lanes represent myogenin expression of C2C12 cells after 72 hours treatment with decreasing concentrations (800, 200, 50, 0 μg/ml) of MRP8/MRP14. There was an impressive inhibition of myogenin expression by MRP8/MRP14 treatment.
MRP8/MRP14 Complex Induces Apoptosis in C2C12 Cells
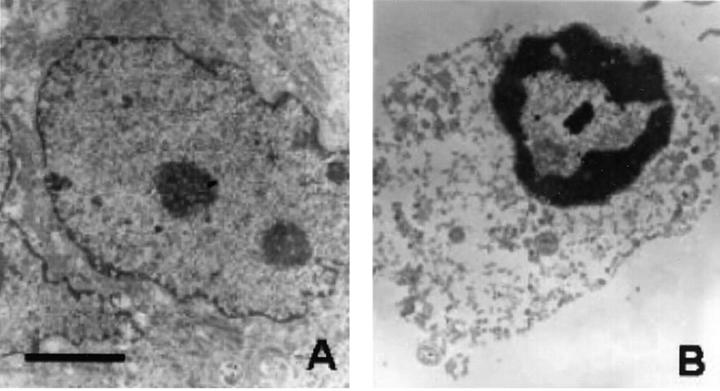
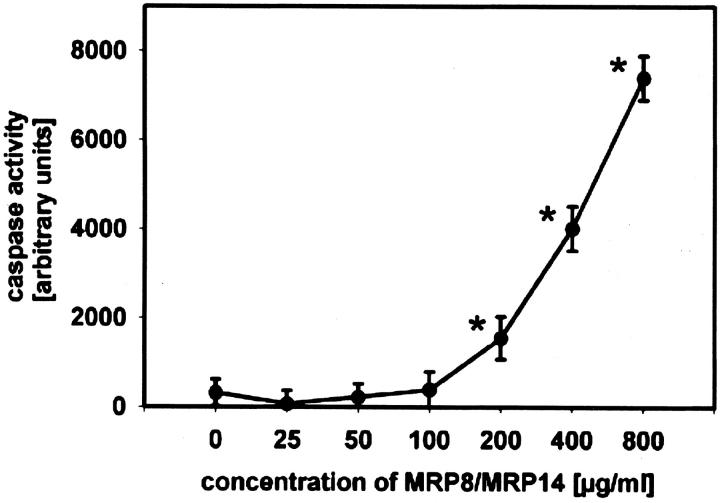
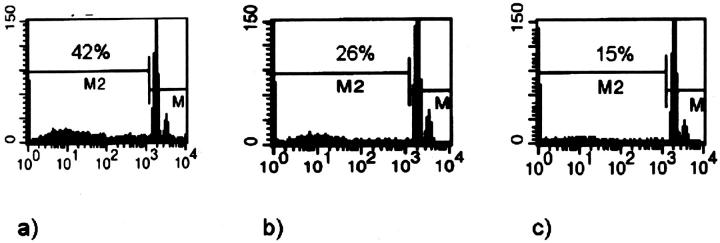
Since expression of MRP8/MRP14 was locally associated with degeneration of muscle fibers we investigated effects of MRP8/MRP14 on viability of muscle cells. After treatment of C2C12 myoblasts with MRP8/MRP14 for 72 hours many cells displayed changes in their morphology in a dose-dependent manner, eg, a decrease in cell volume and chromatin condensation by conventional microscopy, indicating induction of apoptosis. To confirm this finding we performed ultrastructural analysis by TEM. In contrast to controls, MRP8/MRP14-treated C2C12 cells show typical nuclear features of apoptosis, such as condensation of the chromatin along the inner surface of the nuclear envelope and nuclear shrinkage (Figure 5) ▶ . There was no clumping of nuclear chromatin as observed in necrotic nuclei. To further prove these morphological and ultrastructural signs of apoptosis by an independent method we determined activity of caspase-3 in lysates of C2C12 cells as an early central mediator of programmed cell death at certain time periods during MRP8/MRP14 treatment. There was a clear dose- and time-dependent induction of caspase-3 activity after MRP8/MRP14 treatment with a maximum at 16 hours preceding morphological changes described above (Figure 6) ▶ . In addition, subsequent leakage of fragmented DNA from apoptotic nuclei as a later event of apoptosis was observed after incubation with MRP8/MRP14 for 72 hours and detected by the method of Nicoletti et al (Figure 7) ▶ . 33,36
Figure 5.
MRP8/MRP14 induce morphological features of apoptosis in myoblasts. Ultrastructural changes of C2C12 myoblasts induced by treatment with MRP8/MRP14 are consistent with apoptosis. Presented here are control C2C12 myoblast grown under normal culture conditions (A) and grown under addition of 800 μg/ml MRP8/MRP14 for 48 hours (B). After preparation for ultrastructural analysis control C2C12 myoblast present a nucleus with well-defined margins, regular distribution of chromatin and a nucleolus. Myoblasts treated with MRP8/MRP14 show typical nuclear features of apoptosis such as condensation of the chromatin along the inner surface of the nuclear envelope and nuclear shrinkage. There was no clumping of nuclear chromatin as observed in necrotic nuclei (original magnification, ×4600; bar, 2 μm).
Figure 6.
MRP8/MRP14 activate caspase-3 in C2C12 cells. C2C12 myoblasts were incubated with different concentrations of MRP8/MRP14 or medium as control and prepared as described in Material and Methods. After 16 hours the cell lysates were incubated with the fluorogenic caspase substrate DEVD-AMC and measured in a spectrofluorometer. Caspase activity is given in arbitrary units. Asterisks indicate statistically significant differences compared to untreated controls (P ≤ 0.002).
Figure 7.
MRP8/MRP14 induce DNA fragmentation in myoblasts/myocytes. The leakage of fragmented DNA from apoptotic nuclei was measured by the method of Nicoletti et al (see Material and Methods). 33 The figure presents histograms of flow cytometry after staining with propidium iodide. C2C12 myoblasts were cultured in the presence of different concentrations of MRP8/MRP14 for 72 hours (A, 800 μg; B, 400 μg; C, 200 μg). There is a high percentage of fragmented DNA after treatment with 800 μg/ml MRP8/MRP14. The result for 200 μg/ml MRP8/MRP14 was similar to untreated controls.
Discussion
Characterization of cellular events in IM has revealed different aspects of possible pathomechanisms involved in DM, PM, and IBM. 1,37-39 In DM, immune complex and complement-mediated alterations of endothelial cells have been considered to be the primary event. In PM and IBM, destruction of muscle fibers was observed to be associated with invasion of oligoclonal, autoaggressive CD45RO- and CD8-positive T cells and macrophages. 4,40 Deposition of immune complexes at the vascular endothelium as well as cytokines secreted by autoaggressive T cells are known to result in activation of the macrophage system. Macrophages represent a substantial percentage of leukocytes in the endo- and perimysial inflammatory infiltrates of all three disorders. 1 Their presence as well as distinct proinflammatory effector mechanisms of these cells, eg, expression of cytokines or adhesion receptors, has been shown to be a common feature in DM, PM, and IBM. 4-6 However, the exact sequence of events and ensuing muscle-destroying mechanisms are not yet clear. It has to be considered that monocytes and macrophages do not represent a homogeneous cell population, but that different subpopulations may exhibit antagonistic inflammatory properties. Depending on their stage of differentiation and activation monocytes/macrophages secrete pro- or anti-inflammatory mediators and may be involved in propagation as well as in suppression of inflammatory reactions. 7 Pathogenetical concepts involving macrophages focus on their possible interactions with T cells and on their generally damaging potential by release of degrading enzymes and of chemokines. Other or more distinct effector mechanisms have not been demonstrated yet.
In the present study we investigated the expression of MRP8 and MRP14 in the infiltrate of different types of IM. Both proteins are expressed in early differentiation stages of monocytes. 8,9,41 Expression of MRP8 and MRP14 in vivo correlates well with disease activity in different models of inflammation in mice as well as in various kinds of human inflammatory diseases. 11,12,14,16,17,25 Analyzing expression of MRP8 and MRP14 in IM we found two different phenotypes of infiltrating monocytes/macrophages with a close association of highly MRP8/MRP14-expressing monocytes with destructed muscle fibers in DM, PM, and IBM. Since both proteins are secreted by monocytes at local sites of inflammation via a so-called alternative pathway of secretion 16,34 we looked for direct effects of extracellular MRP8/MRP14 on myocytes. We observed a direct inhibitory action of MRP8/MRP14 complexes on proliferation of C2C12 myoblasts as well as on differentiation of this myoblastic cell line to myocytes in vitro. Inhibition of differentiation by MRP8/MRP14 was reflected by a significant reduction of myotube formation and confirmed by an inhibitory effect on the expression of myocytic differentiation markers such as myogenin. 35,42 In addition, proliferation of C2C12 cells was blocked in the presence of MRP8/MRP14. At higher concentrations MRP8/MRP14 induced characteristic morphological and ultrastructural changes of apoptosis in C2C12 cells as shown by light and electron microscopy. This finding was confirmed by two independent assays, eg, detection of DNA fragmentation and determination of caspase-3 activity. Induction of apoptosis has been described for rat MRP8/MRP14 in murine fibroblasts and carcinoma cells. This effect depends on de novo protein synthesis and is attenuated by the presence of different divalent cations as zinc or copper. However, the underlying molecular mechanism and intracellular signal pathways inducing apoptosis in different cells are not yet clear. 43-45 A potential candidate to be involved in this process is the scavenger receptor CD36 which is supposed to be involved in apoptosis as well as in interaction with MRP8/MRP14. 46,47
Besides signs of destruction, there are also clear signs of muscle regeneration during IM reflected, eg, by expression of neural cell adhesion molecule in myocytes. They could be considered an important compensatory mechanism against the predominating destructive processes which is induced as a constitutive part within the inflammatory response. 48 Impairment of this response would thus increase the overall destructive outcome. Our data clearly indicate that MRP8/MRP14 inhibit mechanisms of regeneration by inhibition of myoblast proliferation and differentiation to myotubes. Although it is not possible to determine the exact concentrations of MRP8 and MRP14 at the local sites of destruction of myofibers in IM, MRP8/MRP14 concentrations used in our in vitro assays are not higher than those demonstrated in various inflammatory exudates in vivo such as wound secretions and synovial fluid of rheumatoid arthritis. 16,49 The detection of such large amounts of MRP8/MRP14 in certain inflammatory conditions thus indicates that our in vitro data bear relevance in vivo.
In addition, our findings clearly demonstrate that MRP8/MRP14 induce apoptosis of myoblasts in vitro. However, our data cannot reflect to what extent this mechanism is involved in the pathogenesis of DM, PM, and IBM in vivo. Concentrations of MRP8/MRP14 necessary to induce apoptosis in vitro are much higher compared to those sufficient to inhibit proliferation and differentiation of myocytes. Furthermore, there is a controversial debate whether or not apoptosis is involved in IM. While Fas/FasL expression and morphological signs of muscle apoptosis were found in at least PM and DM by Sugiura et al 39 other independent investigators detected only minor DNA fragmentation, only a few cells with characteristic apoptotic features and no signs of marked caspase activation in situ in IM. In addition, several anti-apoptotic molecules are up-regulated in myocytes during IM. 50-56
In spite of different etiology possibly involved in induction of DM, PM, IBM, their pathophysiological pathways converge on severely disturbing the physiological balance between destructive and regenerative processes and shifting it toward degeneration of muscle fibers. The complete array of molecular effector mechanisms resulting in this destructive process is currently not clear. All three forms of IM present with pathomechanisms resulting in activation of the macrophage system, eg, synthesis of IL-1, tumor necrosis factor, or MCP-1. 1,4,5 The obvious association of MRP8/MRP14 expression with myofiber destruction in vivo and the clear interference of MRP8/MRP14 with myocyte regeneration in vitro points to a novel common role of macrophages in the destructive processes of different IM. Identification of proinflammatory stimuli leading to recruitment of MRP8/MRP14-expressing cells and further analysis of the molecular mechanisms underlying release and extracellular functions of these calcium-binding proteins may thus offer novel molecular targets for future therapeutic strategies to inhibit destructive effects of monocytes in IM.
Acknowledgments
We thank Eva Nattkemper, Astrid Knischewski, Sandra Oberfeld and Karin Fischer for technical assistance.
Footnotes
Address reprint requests to Cord Sunderkötter, M.D., Department of Dermatology and Allergology, University of Ulm, Maienweg 12, D-89081 Ulm, Germany. E-mail: cord.sunderk@medizin.uni-ulm.de.
Supported by grants from the Deutsche Forschungsgemeinschaft (Ro-1190/2–2 and SFB 293) and Interdizisplinäres Zentrum für Klinische Forschung (C16, D15).
References
- 1.Mantegazza R, Bernasconi P, Confalonieri P, Cornelio F: Inflammatory myopathies and systemic disorders: a review of immunopathogenetic mechanisms and clinical features. J Neurol 1997, 244:277-287 [DOI] [PubMed] [Google Scholar]
- 2.Kalovidouris AE: Mechanisms of inflammation and histopathology in inflammatory myopathy. Rheum Dis Clin North Am 1994, 20:881-897 [PubMed] [Google Scholar]
- 3.Amato AA, Barohn RJ: Idiopathic inflammatory myopathies. Neurol Clin 1997, 15:615-648 [DOI] [PubMed] [Google Scholar]
- 4.Lundberg IE: The role of cytokines, chemokines, and adhesion molecules in the pathogenesis of idiopathic inflammatory myopathies. Curr Rheumatol Rep 2000, 2:216-224 [DOI] [PubMed] [Google Scholar]
- 5.Bartoli C, Civatte M, Pellissier JF, Figarella-Branger D: CCR2A and CCR2B, the two isoforms of the monocyte chemoattractant protein-1 receptor are up-regulated and expressed by different cell subsets in idiopathic inflammatory myopathies. Acta Neuropathol (Berl) 2001, 102:385-392 [DOI] [PubMed] [Google Scholar]
- 6.Drosos AA, Dalakas MC: Identification of macrophages in the muscle biopsy preparations: a comparative study using specific monoclonal antimacrophage antibodies and acid phosphatase reaction. Muscle Nerve 1995, 18:242-244 [DOI] [PubMed] [Google Scholar]
- 7.Mosser DM: The many faces of macrophage activation. J Leukoc Biol 2003, 73:209-212 [DOI] [PubMed] [Google Scholar]
- 8.Roth J, Goebeler M, van den Bos C, Sorg C: Expression of calcium-binding proteins MRP8 and MRP14 is associated with distinct monocytic differentiation pathways in HL-60 cells. Biochem Biophys Res Commun 1993, 191:565-570 [DOI] [PubMed] [Google Scholar]
- 9.Odink K, Cerletti N, Bruggen J, Clerc RG, Tarcsay L, Zwadlo G, Gerhards G, Schlegel R, Sorg C: Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature 1987, 330:80-82 [DOI] [PubMed] [Google Scholar]
- 10.Kerkhoff C, Klempt M, Sorg C: Novel insights into structure and function of MRP8 (S100A8) and MRP14 (S100A9). Biochim Biophys Acta 1998, 1448:200-211 [DOI] [PubMed] [Google Scholar]
- 11.Roth J, Sunderkotter C, Goebeler M, Gutwald J, Sorg C: Expression of the calcium-binding proteins MRP8 and MRP14 by early infiltrating cells in experimental contact dermatitis. Int Arch Allergy Immunol 1992, 98:140-145 [DOI] [PubMed] [Google Scholar]
- 12.Roth J, Teigelkamp S, Wilke M, Grun L, Tummler B, Sorg C: Complex pattern of the myelo-monocytic differentiation antigens MRP8 and MRP14 during chronic airway inflammation. Immunobiology 1992, 186:304-314 [DOI] [PubMed] [Google Scholar]
- 13.van den Bos C, Roth J, Koch HG, Hartmann M, Sorg C: Phosphorylation of MRP14, an S100 protein expressed during monocytic differentiation, modulates Ca(2+)-dependent translocation from cytoplasm to membranes and cytoskeleton. J Immunol 1996, 156:1247-1254 [PubMed] [Google Scholar]
- 14.Goebeler M, Roth J, Burwinkel F, Vollmer E, Boecker W, Sorg C: Expression and complex formation of S100-like proteins MRP8 and MRP14 by macrophages during renal allograft rejection. Transplantation 1994, 58:355-361 [PubMed] [Google Scholar]
- 15.Sunderkötter C, Kunz M, Steinbrink K, Meinardus H-G, Goebeler M, Bildau H, Sorg C: Resistance of mice to experimental leishmaniasis is associated with more rapid appearance of mature macrophages in vitro and in vivo. J Immunol 1993, 151:4891-4901 [PubMed] [Google Scholar]
- 16.Frosch M, Strey A, Vogl T, Wulffraat NM, Kuis W, Sunderkotter C, Harms E, Sorg C, Roth J: Myeloid-related proteins 8 and 14 are specifically secreted during interaction of phagocytes and activated endothelium and are useful markers for monitoring disease activity in pauciarticular-onset juvenile rheumatoid arthritis. Arthritis Rheum 2000, 43:628-637 [DOI] [PubMed] [Google Scholar]
- 17.Roth J, Goebeler M, Sorg C: S100A8 and S100A9 in inflammatory diseases. Lancet 2001, 357:1041. [DOI] [PubMed] [Google Scholar]
- 18.Berntzen HB, Fagerhol MK: L1, a major granulocyte protein; isolation of high quantities of its subunits. Scand J Clin Lab Invest 1990, 50:769-774 [DOI] [PubMed] [Google Scholar]
- 19.Propper C, Huang X, Roth J, Sorg C, Nacken W: Analysis of the MRP8-MRP14 protein-protein interaction by the two-hybrid system suggests a prominent role of the C-terminal domain of S100 proteins in dimer formation. J Biol Chem 1999, 274:183-188 [DOI] [PubMed] [Google Scholar]
- 20.Hunter MJ, Chazin WJ: High-level expression and dimer characterization of the S100 EF-hand proteins, migration inhibitory factor-related proteins 8 and 14. J Biol Chem 1998, 273:12427-12435 [DOI] [PubMed] [Google Scholar]
- 21.Teigelkamp S, Bhardwaj RS, Roth J, Meinardus-Hager G, Karas M, Sorg C: Calcium-dependent complex assembly of the myeloic differentiation proteins MRP-8 and MRP-14. J Biol Chem 1991, 266:13462-13467 [PubMed] [Google Scholar]
- 22.Vogl T, Roth J, Sorg C, Hillenkamp F, Strupat K: Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 detected by ultraviolet matrix-assisted laser desorption/ionization mass spectrometry. J Am Soc Mass Spectrom 1999, 10:1124-1130 [DOI] [PubMed] [Google Scholar]
- 23.Roth J, Burwinkel F, van den Bos C, Goebeler M, Vollmer E, Sorg C: MRP8 and MRP14, S-100-like proteins associated with myeloid differentiation, are translocated to plasma membrane and intermediate filaments in a calcium-dependent manner. Blood 1993, 82:1875-1883 [PubMed] [Google Scholar]
- 24.Vogl T, Propper C, Hartmann M, Strey A, Strupat K, van den Bos C, Sorg C, Roth J: S100A12 is expressed exclusively by granulocytes and acts independently from MRP8 and MRP14. J Biol Chem 1999, 274:25291-25296 [DOI] [PubMed] [Google Scholar]
- 25.Roth J, Vogl T, Sorg C, Sunderkotter C: Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol 2003, 24:155-158 [DOI] [PubMed] [Google Scholar]
- 26.Bhardwaj RS, Zotz C, Zwadlo-Klarwasser G, Roth J, Goebeler M, Mahnke K, Falk M, Meinardus-Hager G, Sorg C: The calcium-binding proteins MRP8 and MRP14 form a membrane-associated heterodimer in a subset of monocytes/macrophages present in acute but absent in chronic inflammatory lesions. Eur J Immunol 1992, 22:1891-1897 [DOI] [PubMed] [Google Scholar]
- 27.Holness CL, Simmons DL: Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood 1993, 81:1607-1613 [PubMed] [Google Scholar]
- 28.Pulford KA, Rigney EM, Micklem KJ, Jones M, Stross WP, Gatter KC, Mason DY: KP1: a new monoclonal antibody that detects a monocyte/macrophage associated antigen in routinely processed tissue sections. J Clin Pathol 1989, 42:414-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Bos C, Rammes A, Vogl T, Boynton R, Zaia J, Sorg C, Roth J: Copurification of P6, MRP8, and MRP14 from human granulocytes and separation of individual proteins. Protein Expr Purif 1998, 13:313-318 [DOI] [PubMed] [Google Scholar]
- 30.Lawson MA, Purslow PP: Differentiation of myoblasts in serum-free media: effects of modified media are cell line-specific. Cells Tissues Organs 2000, 167:130-137 [DOI] [PubMed] [Google Scholar]
- 31.Nagao M, Kaziro Y, Itoh H: Thrombin-induced inhibition of myoblast differentiation is mediated by Gβγ. FEBS Lett 2000, 472:297-301 [DOI] [PubMed] [Google Scholar]
- 32.Sunderkotter C, Wittkowski W, Hoffmann K: Fenestrated capillaries in the ventral sebaceous gland of the Djungarian hamster, Phodopus sungorus. Acta Anat 1990, 139:45-48 [PubMed] [Google Scholar]
- 33.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C: A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 1991, 139:271-279 [DOI] [PubMed] [Google Scholar]
- 34.Rammes A, Roth J, Goebeler M, Klempt M, Hartmann M, Sorg C: Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J Biol Chem 1997, 272:9496-9502 [DOI] [PubMed] [Google Scholar]
- 35.Layne MD, Farmer SR: Tumor necrosis factor-α and basic fibroblast growth factor differentially inhibit the insulin-like growth factor-I induced expression of myogenin in C2C12 myoblasts. Exp Cell Res 1999, 249:177-187 [DOI] [PubMed] [Google Scholar]
- 36.Los M, Wesselborg S, Schulze-Osthoff K: The role of caspases in development, immunity, and apoptotic signal transduction: lessons from knockout mice. Immunity 1999, 10:629-639 [DOI] [PubMed] [Google Scholar]
- 37.De Bleecker JL, Meire VI, Declercq W, Van Aken EH: Immunolocalization of tumor necrosis factor-α and its receptors in inflammatory myopathies. Neuromuscul Disord 1999, 9:239-246 [DOI] [PubMed] [Google Scholar]
- 38.Mozaffar T, Pestronk A: Myopathy with anti-Jo-1 antibodies: pathology in perimysium and neighbouring muscle fibres. J Neurol Neurosurg Psychiatry 2000, 68:472-478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugiura T, Murakawa Y, Nagai A, Kondo M, Kobayashi S: Fas and Fas ligand interaction induces apoptosis in inflammatory myopathies: cD4+ T cells cause muscle cell injury directly in polymyositis. Arthritis Rheum 1999, 42:291-298 [DOI] [PubMed] [Google Scholar]
- 40.Hohlfeld R, Engel AG, Goebels N, Behrens L: Cellular immune mechanisms in inflammatory myopathies. Curr Opin Rheumatol 1997, 9:520-526 [DOI] [PubMed] [Google Scholar]
- 41.Roth J, Goebeler M, Wrocklage V, van den Bos C, Sorg C: Expression of the calcium-binding proteins MRP8 and MRP14 in monocytes is regulated by a calcium-induced suppressor mechanism. Biochem J 1994, 301:655-660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bardouille C, Lehmann J, Heimann P, Jockusch H: Growth and differentiation of permanent and secondary mouse myogenic cell lines on microcarriers. Appl Microbiol Biotechnol 2001, 55:556-562 [DOI] [PubMed] [Google Scholar]
- 43.Mikami M, Yamazaki M, Yui S: Kinetical analysis of tumor cell death-inducing mechanism by polymorphonuclear leukocyte-derived calprotectin: involvement of protein synthesis and generation of reactive oxygen species in target cells. Microbiol Immunol 1998, 42:211-221 [DOI] [PubMed] [Google Scholar]
- 44.Yui S, Mikami M, Tsurumaki K, Yamazaki M: Growth-inhibitory and apoptosis-inducing activities of calprotectin derived from inflammatory exudate cells on normal fibroblasts: regulation by metal ions. J Leukoc Biol 1997, 61:50-57 [PubMed] [Google Scholar]
- 45.Murao S, Collart F, Huberman E: A protein complex expressed during terminal differentiation of monomyelocytic cells is an inhibitor of cell growth. Cell Growth Differ 1990, 1:447-454 [PubMed] [Google Scholar]
- 46.Febbraio M, Hajjar DP, Silverstein RL: CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest 2001, 108:785-791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerkhoff C, Sorg C, Tandon NN, Nacken W: Interaction of S100A8/S100A9-arachidonic acid complexes with the scavenger receptor CD36 may facilitate fatty acid uptake by endothelial cells. Biochemistry 2001, 40:241-248 [DOI] [PubMed] [Google Scholar]
- 48.Illa I, Leon-Monzon M, Dalakas MC: Regenerating and denervated human muscle fibers and satellite cells express neural cell adhesion molecule recognized by monoclonal antibodies to natural killer cells. Ann Neurol 1992, 31:46-52 [DOI] [PubMed] [Google Scholar]
- 49.Thorey IS, Roth J, Regenbogen J, Halle JP, Bittner M, Vogl T, Kaesler S, Bugnon P, Reitmaier B, Durka S, Graf A, Wockner M, Rieger N, Konstantinow A, Wolf E, Goppelt A, Werner S: The Ca2+-binding proteins S100A8 and S100A9 are encoded by novel injury-regulated genes. J Biol Chem 2001, 276:35818-35825 [DOI] [PubMed] [Google Scholar]
- 50.Schneider C, Dalakas MC, Toyka KV, Said G, Hartung HP, Gold R: T-cell apoptosis in inflammatory neuromuscular disorders associated with human immunodeficiency virus infection. Arch Neurol 1999, 56:79-83 [DOI] [PubMed] [Google Scholar]
- 51.Li M, Dalakas MC: Expression of human IAP-like protein in skeletal muscle: a possible explanation for the rare incidence of muscle fiber apoptosis in T-cell mediated inflammatory myopathies. J Neuroimmunol 2000, 106:1-5 [DOI] [PubMed] [Google Scholar]
- 52.Falcini F, Calzolari A, Generini S, Pignone A, Simonini G, Zulian F, Matucci-Cerinic M: Bcl-2, p53 and c-myc expression in juvenile dermatomyositis. Clin Exp Rheumatol 2000, 18:643-646 [PubMed] [Google Scholar]
- 53.Behrens L, Bender A, Johnson MA, Hohlfeld R: Cytotoxic mechanisms in inflammatory myopathies. Co-expression of Fas and protective Bcl-2 in muscle fibres and inflammatory cells. Brain 1997, 120:929-938 [DOI] [PubMed] [Google Scholar]
- 54.Nagaraju K, Casciola-Rosen L, Rosen A, Thompson C, Loeffler L, Parker T, Danning C, Rochon PJ, Gillespie J, Plotz P: The inhibition of apoptosis in myositis and in normal muscle cells. J Immunol 2000, 164:5459-5465 [DOI] [PubMed] [Google Scholar]
- 55.Migheli A, Mongini T, Doriguzzi C, Chiado-Piat L, Piva R, Ugo I, Palmucci L: Muscle apoptosis in humans occurs in normal and denervated muscle, but not in myotonic dystrophy, dystrophinopathies or inflammatory disease. Neurogenetics 1997, 1:81-87 [DOI] [PubMed] [Google Scholar]
- 56.Vattemi G, Tonin P, Filosto M, Spagnolo M, Rizzuto N, Tomelleri G: T-cell anti-apoptotic mechanisms in inflammatory myopathies. J Neuroimmunol 2000, 111:146-151 [DOI] [PubMed] [Google Scholar]