Abstract
Mastocytosis is a rare disease characterized by accumulation of mast cells in tissues. To investigate whether an altered regulation of mast cell apoptosis might be involved in the pathogenesis of mastocytosis, expression of the apoptosis-preventing molecules bcl-2 and bcl-xL was studied by immunohistochemistry in skin and bone marrow lesions of mastocytosis patients. In addition, reverse transcription-polymerase chain reaction was used to investigate levels of bcl-2 and bcl-xL mRNA in cutaneous mastocytosis lesions. Since activating mutations of c-kit are known to be associated with some forms of mastocytosis, human mast cell cultures were also stimulated via c-kit and the expression of bcl-2 and bcl-xL was assessed by immunoblotting. In patients with mastocytosis, the expression of bcl-2 protein but not bcl-xL in cutaneous mast cells was significantly enhanced, compared to healthy controls. Evaluating different subgroups of adult and pediatric mastocytosis patients, all groups were found to express significantly increased levels of bcl-2 protein, and none of the patient groups was found to overexpress bcl-xL, with the exception of solitary mastocytomas that showed a tendency for up-regulated bcl-xL protein. Furthermore, the expression of bcl-2 mRNA was significantly enhanced in cutaneous lesions of adult and pediatric patients, while bcl-xL mRNA levels were only slightly increased in pediatric, but not in adult patients with mastocytosis. In contrast to the skin lesions, bone marrow infiltrates of patients with systemic mastocytosis showed only low or absent immunoreactivity for bcl-2, but marked expression of bcl-xL. In vitro, stimulation of two different mast cell culture systems by activation of c-kit resulted in up-regulation of bcl-2 and also in an increase of bcl-xL, although less pronounced. Thus, overexpression of bcl-2 and bcl-xL leading to prolonged survival of mast cells may contribute to the pathogenesis of mastocytosis. Our findings may help to develop new strategies for the treatment of this disease.
Mastocytosis represents a heterogeneous group of diseases characterized by an increase of mast cells in different organs. 1,2 Cutaneous mastocytosis is limited to the skin, preferentially develops in infancy, often shows spontaneous regression after several years, and can be categorized into a maculopapular (traditionally named urticaria pigmentosa), nodular (also named mastocytoma and presents as solitary or multiple lesions), diffuse, and telangiectatic form. 3-5 Systemic mastocytosis is defined by infiltrates of mast cells in the bone marrow or other extracutaneous organs, presents with or without skin lesions, usually affects adults, and shows an indolent or aggressive clinical course. 3 Recent studies have demonstrated that activating mutations of c-kit, the receptor of the mast cell growth factor stem cell factor (SCF), play a key role in the pathogenesis of sporadic adult onset mastocytosis. 6 However, these mutations alone do not explain the heterogeneity of adult disease and are usually not present in pediatric and familial mastocytosis. 1,7-10 To investigate additional mechanisms by which mast cell numbers are regulated in mastocytosis, we hypothesized that an altered control of mast cell apoptosis might also be involved in the pathogenesis of the disease.
Members of the bcl-2 family of proteins play important roles in regulating cell survival and apoptosis. 11 The bcl-2 family includes pro-apoptotic members and anti-apoptotic proteins such as bcl-2 and bcl-xL that inhibit apoptosis by blocking the release of cytochrome C. 12 Bcl-2 and bcl-xL have both been shown to prevent apoptosis in response to a wide variety of stimuli, including growth factor deprivation, γ-irradiation, and activation of death receptors. 11,13 On the other hand, the two survival proteins are expressed in a reciprocal pattern during cellular development, suggesting also unique functions for bcl-2 and bcl-xL. 14-16 Overexpression of bcl-2 and bcl-xL causes aberrant accumulation of cells and thus leads to neoplasia. 17,18 In fact, an increased expression of bcl-2 and bcl-xL has been found in a variety of different cancers. 19-23 In many neoplastic cells, high expression of bcl-2 and bcl-xL also correlates with resistance to conventional chemotherapy. 19,20,24
In vitro studies have demonstrated that survival of murine and human mast cells also depends on bcl-2 and bcl-xL. 25-32 Bcl-2-deficient mice show reduced numbers of mast cells in the stomach mucosa, although numbers of cutaneous mast cells are comparable to wild-type controls. 33 Recently, bone marrow mast cells of patients with systemic mastocytosis have been reported to strongly express bcl-xL. 34 In contrast, expression of bcl-2 was absent or low, with the exception of two patients with mast cell leukemia that exhibited higher levels of bcl-2 in their bone marrow. 34,35
In the present study, we show for the first time that bcl-2 expression is strongly enhanced in cutaneous mast cells of patients with different forms of mastocytosis, whereas expression of bcl-xL is mainly unaltered or only slightly increased in lesions of short duration. In the same patients, bone marrow infiltrates fail to express bcl-2, but strongly express bcl-xL. These results further support the concept that alterations in the control of apoptosis may contribute to accumulation of mast cells in mastocytosis and also suggest that survival of cutaneous mast cells in this disease may be differentially regulated compared to bone marrow mast cells.
Materials and Methods
Patients and Tissue Samples
Cutaneous biopsies were obtained from a total of 39 patients with mastocytosis for diagnostic purposes. The diagnosis of mastocytosis with cutaneous involvement was made on the basis of clinical appearance and an increase of mast cells on histology, according to published criteria. 3,4 Paraffin-embedded sections of 32 mastocytosis patients (12 adult and 20 pediatric patients) were analyzed by immunohistochemistry and compared with 7 healthy controls. Out of the 32 patients, 12 adults and 6 children had maculopapular cutaneous mastocytosis (urticaria pigmentosa), 7 children had solitary mastocytomas, and 7 children had multiple mastocytomas. 4,5 Lesional mast cell counts of skin biopsies were 6.58 ± 1.75 mast cells/mm2 (mean ± SEM) in 12 adult mastocytosis patients and 39.68 ± 5.31 mast cells/mm2 in 20 pediatric patients (29.23 ± 6.42 mast cells/mm2 in 6 maculopapular mastocytosis, 38.81 ± 5.90 mast cells/mm2 in 7 solitary mastocytoma, and 49.50 ± 11.73 mast cells/mm2 in 7 multiple mastocytoma patients), compared with 1.01 ± 0.07 mast cells/mm2 in 7 healthy controls. In addition, small snap-frozen pieces of 9 mastocytosis biopsies (2 biopsies that had also been used for immunohistochemical staining and 7 additional biopsies of different patients with mastocytosis) from 3 adult patients with maculopapular mastocytosis and 6 pediatric patients with maculopapular mastocytosis or mastocytomas as well as frozen tissue of 6 healthy controls were used for RNA isolation.
Out of the12 adult patients analyzed by immunohistochemistry, 5 patients with systemic mastocytosis 3 associated with maculopapular cutaneous mastocytosis were chosen who had both a skin and bone marrow biopsy within the same year and their paraffin-embedded bone marrow biopsies were also investigated by immunohistochemistry. All patients displayed focal dense infiltrates of mast cells in the bone marrow. In 4 of these patients, tryptase levels were available and showed comparable systemic involvement (range of tryptase levels, 60.7 to 72.3 μg/L; mean, 67.3 μg/L; n = 4; measured by UniCAP Tryptase Fluoroenzyme Immunoassay, Pharmacia Diagnostics, Uppsala, Sweden).
Most of the adult patients analyzed in this study were tested in the past for the presence of a codon 816 c-kit mutation and were all found to express this mutation. 7 Written, informed consent was obtained from all patients and controls before biopsies were performed. The study was approved by the ethical committee of the Humboldt University, Berlin, Germany.
Immunohistochemistry
For analysis of mast cells in cutaneous biopsies, all sections were first stained with toluidine blue at pH 0.5 for 24 hours, and costaining of mast cells with antibodies against bcl-2 and bcl-xL was then assessed in serial sections, as previously described. 36,37 Before exposure to antibodies, antigen unmasking was performed by incubating the skin sections in a commercial antigen retrieval buffer (pH 9.9) (Target Retrieval Solution; DakoCytomation, Hamburg, Germany) for 30 minutes at 95°C. Immunoreactivity for bcl-2 and bcl-xL was then investigated using a mouse anti-human bcl-2 mAb (clone 100/D5; dilution 1:100; Zymed Laboratories, South San Francisco, CA), a mouse anti-human bcl-xL mAb (clone 7B2.5; dilution 1:500; DPC Biermann, Bad Nauheim, Germany), and the alkaline phosphatase anti-alkaline phosphatase (APAAP) technique. 37 Optimal dilutions of the antibodies were tested with appropriate positive and negative control specimens such as tonsils or tumors. Evaluation of skin sections was performed by recording the distribution of positive cells and by counting the numbers of toluidine blue-positive and immunoreactive mast cells in at least five microscopic fields at high-power magnification (×400). Data are expressed as percentage (mean ± SEM) of bcl-2- or bcl-xL-positive mast cells.
To compare the expression of bcl-2 and bcl-xL in skin and bone marrow sections and to exclude differences due to methodology, 5 adult patients with systemic mastocytosis who had both a skin and bone marrow biopsy within the same year were chosen and their skin sections, which had previously been stained by the APAAP technique, as well as their bone marrow sections, were also pretreated by incubation in 10 mmol/L citrate buffer at pH 6.0 in a microwave oven at 750 W three times for 4 minutes each and stained using a mouse anti-human bcl-2 mAb (clone 124; dilution 1:40; DakoCytomation), a mouse anti-human bcl-xL mAb (clone H-5; dilution 1:100; Santa Cruz Biotechnology, Santa Cruz, CA), and a commercial immunohistochemistry kit (EnVision AP; DakoCytomation). Again, optimal dilutions of the antibodies were tested with appropriate positive and negative controls. In the cutaneous sections, the APAAP and the EnVision AP technique showed the same results. For detection of mast cell infiltrates in the bone marrow sections, staining with chloroacetate esterase was performed on serial sections and compared to staining reactions obtained with the antibodies against bcl-2 and bcl-xL. In these 5 patients, the intensity of the EnVision AP-stained cutaneous and bone marrow sections was evaluated semiquantitatively and graded as 0 (bone marrow: reactivity in <5% of dense focal infiltrates containing mast cells; skin: reactivity in <5% of mast cells), 1 (bone marrow: reactivity in 5% to 35% of infiltrates; skin: reactivity in 5% to 35% of mast cells), 2 (bone marrow: reactivity in 35% to 65% of infiltrates; skin: reactivity in 35% to 65% of mast cells), or 3 (bone marrow: reactivity in >65% of infiltrates; skin: reactivity in >65% of mast cells).
RT-PCR
Total RNA was isolated from frozen skin biopsies using the RNeasy Kit (Qiagen, Hilden, Germany) and treated with RNase-free DNase I (Roche Molecular Biochemicals, Mannheim, Germany). After isopropanol precipitation, 1 μg of total RNA was reverse-transcribed with M-MLV Reverse Transcriptase (Life Technologies, Karlsruhe, Germany) using random hexamers. Polymerase chain reaction (PCR) was performed using the following primers for bcl-2: 5′- CAGCTGCACCTGACGCCCTT-3′ and reverse 5′- CCCAGCCTCCGTTATCCTGGA-3′, the following primers for bcl-xL: 5′- AGGCAGGCGACGAGTTTGAACTG-3′ and reverse 5′-CAGGAACCAGCGGTTGAAGCGT-3′, the following primers for tryptase: 5′- GGAGCTGGAGGAGCCCGTGA-3′ and reverse 5′- ACCTGGGTAGGAAGCAGTGGTG-3′, and the following primers for GAPDH: 5′-GCTGTAGCCAAATTCGTTGTC-3′ and reverse 5′- GATGACATCAGAAGGTGGTG-3′. In all experiments, PCR conditions were standardized using a master mixture containing Taq polymerase, MgCl2, and 2 μl cDNA/50 μl PCR volume. PCR products were purified using the QIAquick Nucleotide Removal Kit (Qiagen) and spectrofluorometrically quantified using the double-stranded DNA-binding dye PicoGreen (Molecular Probes, Leiden, Netherlands). Results are given in ng bcl-2, bcl-xL or tryptase mRNA/ng GAPDH mRNA.
Cells
The human basophilic cell line KU-812 (kindly provided by the Institute for Allergy Research, Borstel, Germany), 38 known to express non-mutated c-kit, 7 was maintained in RPMI 1640 supplemented with 20% fetal calf serum, 2 mmol/L L-glutamine, antibiotics, and 0.04% 2-mercaptoethanol. Primary cord blood-derived mast cells were obtained from mononuclear cells of heparinized umbilical cord blood after incubation with a selective medium containing 100 ng/ml SCF (Peprotech, Rocky Hill, NJ) for 8 to 12 weeks, as described. 39 Mast cell development was confirmed by toluidine blue staining and fluorescence-activated cell sorter analysis of tryptase and KIT expression.
Western Blot Analysis
KU-812 cells and cord blood-derived mast cells were cultured with or without 100 ng/ml SCF (Peprotech) for 48 hours, lysed, and concentration of total protein was measured using a Bradford assay (Bio-Rad Laboratories, Hercules, CA). Fifty μg of each protein sample was electrophoresed on sodium dodecyl sulfate gels containing 15% polyacrylamide and transferred to nitrocellulose filters. Equal loading and transfer of proteins was ensured by staining with Ponceau S. Bcl-2 protein was detected using a mouse anti-human bcl-2 mAb (clone 100; dilution 1:200; Santa Cruz Biotechnology) and bcl-xL was detected using a mouse anti-human bcl-xL mAb (clone H-5; dilution 1:200; Santa Cruz Biotechnology), followed by a horseradish peroxidase-conjugated rabbit anti-mouse antibody (DakoCytomation). Immunoblots were visualized with an ECL chemiluminescence detection system (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK). Optical densities of immunoreactive bands were monitored using Quantity One software (Bio-Rad Laboratories).
Statistical Analysis
Statistical differences were evaluated using Student’s t-test for unpaired values. P values were determined by a two-sided calculation, and a P value of less than 0.05 was considered significant.
Results
Expression of Bcl-2 Protein Is Enhanced in Cutaneous Mastocytosis Lesions
To study the expression of apoptosis-preventing molecules in cutaneous biopsies of mastocytosis patients, paraffin-embedded sections of 32 patients with different forms of mastocytosis and 7 healthy controls were analyzed by immunohistochemistry using antibodies against bcl-2 and bcl-xL (Table 1 ▶ ; Figure 1, A and B ▶ ). In patients with mastocytosis, the expression of bcl-2 protein in mast cells was significantly enhanced compared to controls (Table 1 ▶ ; Figure 1A ▶ ), suggesting that prolonged survival of mast cells may, at least in some patients, be associated with increased mast cell numbers. In contrast, mast cell immunoreactivity for bcl-xL was comparable between mastocytosis and control skin (Table 1 ▶ ; Figure 1B ▶ ), although a high variation of bcl-xL expression was observed with up-regulation in about half of all patients. Evaluating different subgroups of patients (Table 1) ▶ , all groups showed an increased reactivity for bcl-2 in lesional mast cells, with most pronounced levels in maculopapular mastocytosis of adult onset, and none of the patient groups showed a significantly altered expression of bcl-xL, even though a tendency for enhanced levels of bcl-xL was noted in solitary mastocytomas. These lesions had also the lowest duration, namely a mean duration of 2.83 months, compared to 25 months in multiple mastocytomas, 10.5 months in maculopapular mastocytosis of childhood onset, and 11.18 years in maculopapular mastocytosis of adult onset.
Table 1.
Percentage of Cutaneous Mast Cells Reactive for Bcl-2 and Bcl-xL in Patients with Different Forms of Mastocytosis and Healthy Controls
| Patient groups | Bcl-2 | Bcl-xL | ||||
|---|---|---|---|---|---|---|
| Mean ± SEM (%) | n | P value | Mean ± SEM (%) | n | P value | |
| All patients with mastocytosis | 44.8 ± 4.64 | 32 | 0.000 | 18.99 ± 4.41 | 30 | 0.729 |
| All mastocytosis patients with adult onset | 53.58 ± 8.18 | 12 | 0.000 | 15.30 ± 8.12 | 12 | 0.993 |
| Maculopapular mastocytosis, adult onset | 53.58 ± 8.18 | 12 | 0.000 | 15.30 ± 8.12 | 12 | 0.993 |
| All mastocytosis patients with childhood onset | 39.59 ± 5.23 | 20 | 0.000 | 21.44 ± 4.88 | 18 | 0.570 |
| Maculopapular mastocytosis, childhood onset | 28.22 ± 6.27 | 6 | 0.045 | 17.50 ± 10.26 | 6 | 0.887 |
| Solitary mastocytomas, childhood onset | 47.14 ± 8.93 | 7 | 0.007 | 27.17 ± 6.48 | 6 | 0.326 |
| Multiple mastocytomas, childhood onset | 41.79 ± 9.49 | 7 | 0.019 | 19.67 ± 7.66 | 6 | 0.735 |
| All patients with maculopapular mastocytosis | 45.13 ± 6.48 | 18 | 0.000 | 16.03 ± 6.41 | 18 | 0.956 |
| All patients with mastocytomas | 44.46 ± 6.55 | 14 | 0.000 | 23.42 ± 5.13 | 12 | 0.460 |
| Healthy controls | 9.43 ± 3.65 | 7 | 15.41 ± 8.30 | 7 | ||
Figure 1.
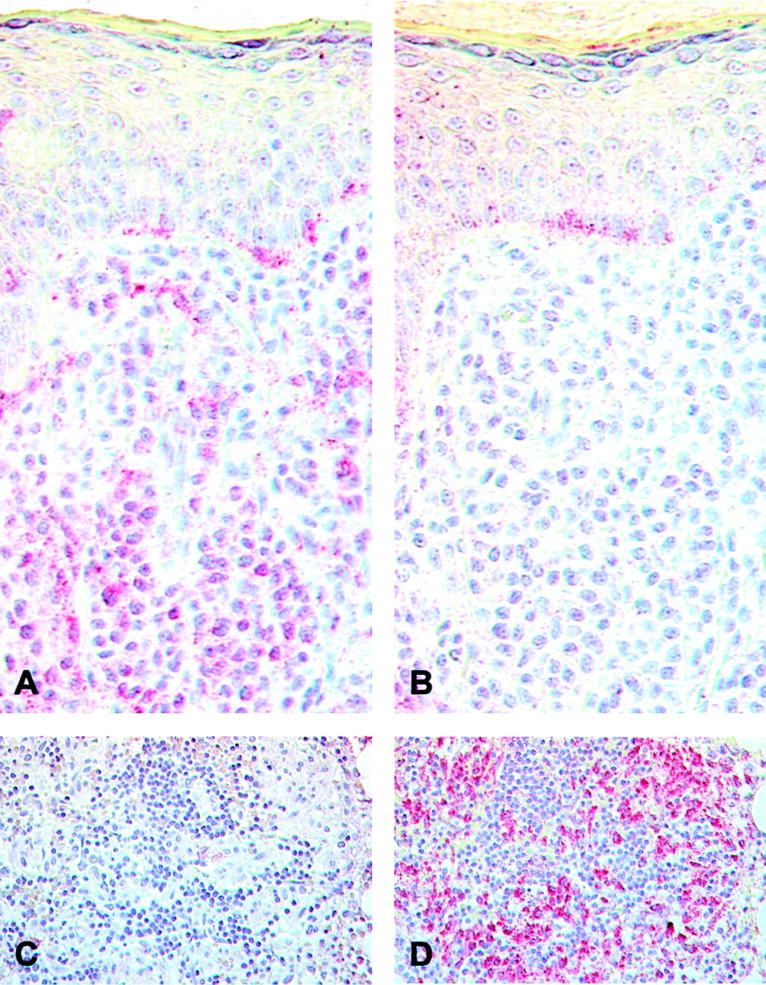
Expression of bcl-2 (A and C) and bcl-xL protein (B and D) in cutaneous (A and B) and bone marrow lesions (C and D) of mastocytosis. Immunohistochemical staining of paraffin-embedded sections of skin and bone marrow biopsies of patients with mastocytosis was performed using antibodies against bcl-2 and bcl-xL and the EnVision AP kit (DakoCytomation). Note bcl-2 staining of practically all dermal mast cells crowding the dermis, and of some basal epidermal cells in a solitary mastocytoma (A). In contrast, only rare dermal mast cells and some basal keratinocytes of the same mastocytoma react with bcl-xL (B). Bone marrow infiltrates of patients with systemic mastocytosis, on the other hand, fail to express bcl-2 (C), but show pronounced expression of bcl-xL (D). Original magnification: ×400 (A and B); ×250 (C and D).
In keratinocytes, hair follicles, sweat glands, sebaceous glands, and endothelial cells, bcl-2 protein was variably expressed, ranging from staining throughout the epidermis to restriction of reactivity to basal keratinocytes (Figure 1A) ▶ or lack of epithelial expression at all. There was no significant difference of epithelial bcl-2 staining between mastocytosis and control skin, and a relationship between epidermal and mast cell reactivity could not be established either. For bcl-xL, variable immunoreactivity was also observed in epithelial cells, especially in basal keratinocytes (Figure 1B) ▶ .
Enhanced Expression of Bcl-2 mRNA in Cutaneous Mastocytosis Lesions
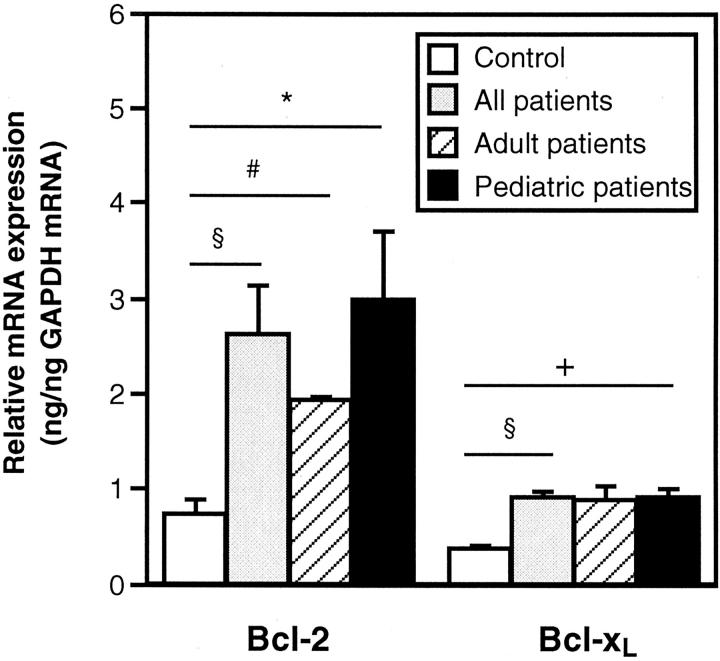
To quantify the expression of bcl-2 and bcl-xL mRNA in mastocytosis skin, RT-PCR was performed using frozen cutaneous biopsies of 9 patients with mastocytosis (3 adult and 6 pediatric patients) and 6 healthy controls (Figure 2) ▶ . Tryptase mRNA, which is predominantly expressed in mast cells, was also measured and served as positive control (data not shown). In accordance with the immunohistochemistry data, expression of bcl-2 mRNA was significantly enhanced in mastocytosis patients (Figure 2) ▶ . Analyzing adult and pediatric patients separately, both patient groups showed an increased expression of bcl-2. Mean bcl-2 levels in children exceeded that in adults, although there was no statistically significant difference between the two patient groups. In addition, a small but significant increase of bcl-xL mRNA was seen in all mastocytosis patients as well as in the pediatric subgroup, but not in the adult group, confirming the observed tendency for enhanced bcl-xL protein expression in children with solitary mastocytomas. As anticipated, patients were also found to express increased levels of tryptase mRNA, compared to controls (2.97 ± 0.31 ng/ng GAPDH mRNA (mean ± SEM) in 9 mastocytosis patients versus 1.03 ± 0.21 ng/ng GAPDH mRNA in 6 healthy controls, P < 0.000).
Figure 2.
Cutaneous expression of bcl-2 mRNA is significantly enhanced in mastocytosis. Analyzing adult and pediatric patients separately, both patient groups show a significant increase of bcl-2 mRNA. In addition, significantly enhanced levels of bcl-xL mRNA are observed in all patients as well as in the pediatric subgroup. Total RNA of frozen cutaneous biopsies of 9 patients with mastocytosis (3 adult and 6 pediatric patients) and 6 healthy controls was isolated and expression of bcl-2 and bcl-xL mRNA analyzed by RT-PCR. Results are presented as relative expression of bcl-2 and bcl-xL mRNA, compared to mRNA levels of the housekeeping gene GAPDH, and expressed as means ± SEM. * indicates P < 0.05; §, P < 0.01; +, P < 0.005 and #, P < 0.001 compared with controls.
Expression of Bcl-xL, but not Bcl-2 Protein, is Enhanced in Bone Marrow Infiltrates of Patients with Systemic Mastocytosis
In contrast to the observed overexpression of bcl-2 in cutaneous mastocytosis lesions, a recent study has reported that mast cell infiltrates in bone marrow of patients with systemic mastocytosis are associated with enhanced levels of bcl-xL, but usually fail to express bcl-2. 34 To make sure that the contrasting expression of bcl-2 and bcl-xL in skin and bone marrow lesions is not related to differences in immunohistochemical methods or patient material, bone marrow biopsies from 5 adult patients with systemic mastocytosis associated with cutaneous lesions and cutaneous biopsies of the same 5 patients were analyzed for expression of bcl-2 and bcl-xL by immunohistochemistry using exactly the same method. In all 5 patients, an increased expression of bcl-2 protein in cutaneous mast cells had previously been observed using the APAAP technique (Table 1) ▶ . In bone marrow infiltrates of these 5 patients, only low or absent expression of bcl-2 (Table 2 ▶ ; Figure 1C ▶ ), but marked expression of bcl-xL (Table 2 ▶ ; Figure 1D ▶ ) was found. Using the same staining procedures, cutaneous mastocytosis sections again showed marked immunoreactivity for bcl-2, but not for bcl-xL (Table 2) ▶ , thus confirming that patients with systemic mastocytosis show enhanced expression of bcl-2 in cutaneous mast cells and at the same time overexpression of bcl-xL in bone marrow lesions.
Table 2.
Intensity of Immunoreactivity for Bcl-2 and Bcl-xL in Bone Marrow Infiltrates Compared to Cutaneous Lesions in Five Adult Patients with Systemic Mastocytosis
| Patient number | Bone marrow | Skin | ||
|---|---|---|---|---|
| Bcl-2 (Score) | Bcl-xL (Score) | Bcl-2 (Score) | Bcl-xL (Score) | |
| 1 | 0–1 | 3 | 2 | 0 |
| 2 | 0 | 2–3 | 2 | 0 |
| 3 | 0 | 2 | 1 | 0 |
| 4 | 0 | 2 | 2 | 0 |
| 5 | 0 | 1 | 2 | 0 |
Activation of c-kit Is Associated with Enhanced Expression of Bcl-2 Protein in Vitro
Somatic mutations causing constitutive activation and phosphorylation of KIT are known to be associated with all sporadic adult and with rare atypical pediatric mastocytosis. 7,8 To assess whether activation of c-kit may be linked to an altered expression of bcl-2 and bcl-xL, the basophilic precursor cell line KU-812 expressing non-mutated c-kit was stimulated with the KIT ligand SCF for 48 hours, and the expression of bcl-2 and bcl-xL protein was analyzed by Western blotting (Figure 3A) ▶ . The results indicated that c-kit activation leads to a marked up-regulation of bcl-2. In comparison, the expression of bcl-xL was mainly unaltered or only slightly increased after treatment with SCF (data not shown). In accordance, primary cord blood-derived mast cells cultured in SCF showed strongly enhanced levels of bcl-2 (Figure 3B) ▶ and only slightly up-regulated bcl-xL levels (data not shown), compared to cells deprived of SCF for 48 hours, confirming our previous study using human mast cells cultured from peripheral blood. 30
Figure 3.
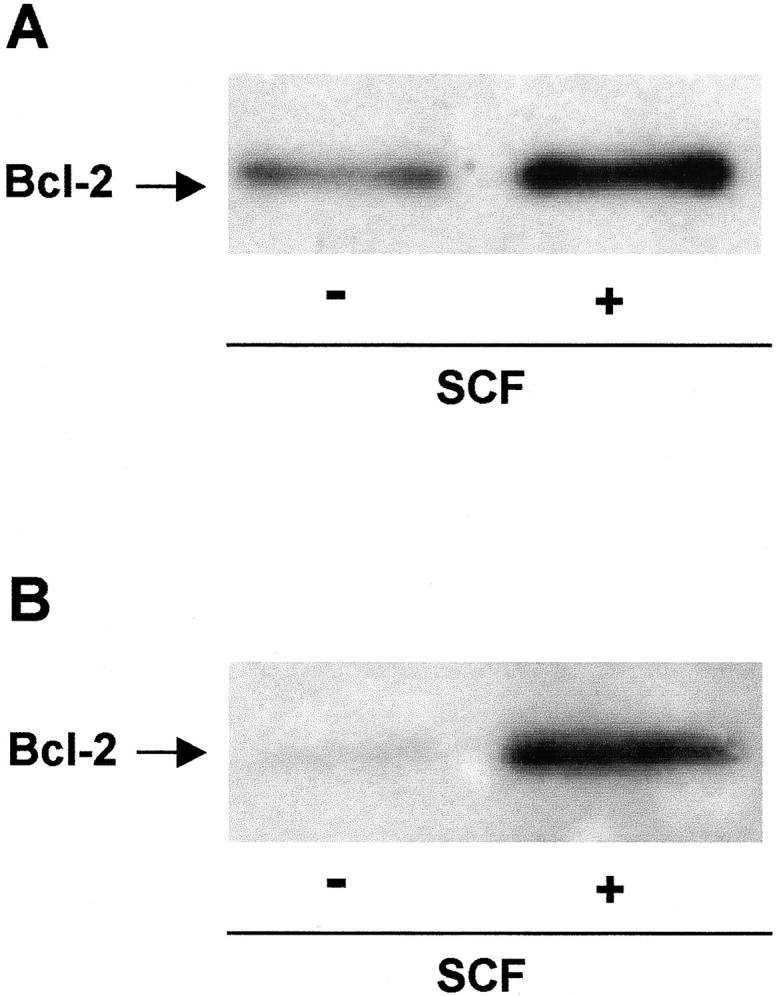
C-kit activation leads to up-regulation of bcl-2 expression in vitro. The basophilic cell line kU-812 (A) and primary cord-blood-derived mast cells (B) were each cultured in the absence or presence of 100 ng/ml SCF for 48 hours and the expression of bcl-2 was analyzed by Western blotting. Data are representative of three to four independent experiments with similar results.
Discussion
In this study, we provide evidence that the anti-apoptotic molecule bcl-2 is overexpressed in mast cells of cutaneous mastocytosis lesions. Expression of the related apoptosis-preventing molecule bcl-xL in cutaneous mast cells was, in contrast, largely comparable to healthy controls. In comparison, some of the same patients with systemic mastocytosis failed to express bcl-2 but overexpressed bcl-xL in their bone marrow lesions. Furthermore, up-regulation of bcl-2 was found in human mast cell cultures after stimulation of c-kit.
Overexpression of bcl-2 and bcl-xL has been linked to a variety of different cancers, including many hematopoietic malignancies. 19-23 Although the mechanism by which bcl-2 family proteins promote neoplasia is not fully understood, it is well accepted that enhanced expression of bcl-2 and bcl-xL allows cells to live longer and thus to accumulate genetic alterations. 11,22 Two previous studies have addressed the role of bcl-2 proteins in mastocytosis. 34,35 Using immunohistochemistry, Jordan et al 34 have demonstrated that mast cells in focal bone marrow infiltrates of adult patients with systemic mastocytosis show strong immunoreactivity for bcl-xL, but usually fail to express bcl-2. Out of 22 patients investigated, only one patient with mast cell leukemia was found to express bcl-2 in a small fraction of bone marrow mast cells. In accordance, Cervero et al 35 have reported on one patient with mast cell leukemia and overexpression of bcl-2, as assessed by quantitative flow cytometry, in bone marrow mast cells as well as peripheral blood mast cells that were present in this case. Eight other patients with indolent systemic mastocytosis expressed normal levels of bcl-2 in bone marrow mast cells. 35 The present study shows, on the other hand, overexpression of bcl-2 and largely unaltered expression of bcl-xL in cutaneous mast cells in different forms of mastocytosis (Table 1 ▶ ; Figure 1, A and B ▶ ; Figure 2 ▶ ). In addition, overexpression of bcl-xL compared to low or absent expression of bcl-2 in bone marrow lesions of patients with systemic mastocytosis was confirmed (Table 2 ▶ ; Figure 1, C and D ▶ ). These findings demonstrate for the first time that dysregulation of apoptosis leading to prolonged survival of mast cells is operative not only in bone marrow mast cells, but also in cutaneous mast cells. Since mastocytosis is thought to represent a clonal disease, 40,41 it can also be assumed that the up-regulation of bcl-2 in cutaneous mast cells as well as the overexpression of bcl-xL in bone marrow mast cells are not primary pathological defects, but rather secondary events following other survival-promoting signals present in mastocytosis mast cells.
Adult patients with sporadic mastocytosis are known to carry activating mutations of c-kit. 6-8 To investigate whether activation of mast cells via c-kit induces enhanced expression of bcl-2 and bcl-xL, immunoblotting experiments with mast cell cultures were performed (Figure 3) ▶ . Using two different mast cell systems, we found that stimulation of c-kit in vitro leads to marked overexpression of bcl-2 and, less pronounced, also to enhanced levels of bcl-xL. This, in part, is in agreement with our previous study on human mast cells cultured from peripheral blood CD34+ progenitor cells that exhibited a decreased expression of bcl-2 as well as bcl-xL after deprivation of SCF. 30 In accordance with our present data, a study was published while this paper was under revision demonstrating up-regulation of bcl-2 in human lung mast cells as well as in the human mast cell line HMC-1 in response to treatment with SCF by RT-PCR and immunocytochemistry. 32 In addition, another group has also observed up-regulation of bcl-2 mRNA, but not bcl-xL mRNA, in rat peritoneal mast cells following incubation with SCF. 26
Our in vitro findings point to the possibility that activating c-kit mutations present in adult sporadic mastocytosis may be associated with bcl-2 overexpression in cutaneous mast cells. However, a similarly enhanced expression of bcl-2 was also observed in pediatric patients who fail to carry these activating mutations. 7,8 It can, therefore, be speculated that other defects, which finally lead to the same apoptosis-inhibiting mechanism, are operative in pediatric mastocytosis. Since mastocytosis is known to resolve spontaneously in about half of all pediatric patients, especially in patients with mastocytomas 42 who were shown here to also express significantly enhanced bcl-2 levels, it can also be assumed that overexpression of bcl-2 in cutaneous mast cells represents, at least in some patients, a temporary rather than a stable defect. In contrast to bcl-2 upregulation, however, overexpression of bcl-xL in bone marrow lesions has so far only been demonstrated in adult patients usually associated with c-kit mutations and, thus, it cannot be excluded that bcl-xL up-regulation is more directly linked to c-kit mutations.
In lymphocytes, bcl-2 and bcl-xL have been shown to be expressed in reciprocal patterns during development and maturation. 14,15 While bcl-2 plays a critical role in controlling long-term survival and maintenance of resting cells, bcl-xL appears to mainly regulate survival during early development and after activation of T- and B-cells. 11,15 Our data demonstrating preferential expression of bcl-2 in the more mature cutaneous mast cells, in contrast to the expression of bcl-xL in immature bone marrow mast cells, 34 suggest that bcl-2 and bcl-xL are also differentially regulated during the life-span of a mast cell. Supporting this concept, a tendency for enhanced bcl-xL protein levels and significantly increased bcl-xL mRNA levels were observed in pediatric patients with mastocytosis lesions of short duration (Table 1 ▶ ; Figure 2 ▶ ). In addition, two studies have shown that activation of FcεRI in murine mast cells is associated with increased expression of bcl-xL, but not bcl-2, paralleling the findings in lymphocytes. 29,31 However, systematic studies on the precise roles of bcl-2 and bcl-xL in mast cells are still missing.
Recent studies have shown that several transcription factors are able to modulate KIT-associated bcl-2 and bcl-xL expression, ie, experiments with transfected cell lines have demonstrated that the c-kit mutation in codon 816 typical for mastocytosis leads to activation of STAT3 (signal transducer and activator of transcription 3) and STAT1 via the phosphatidylinositol-3-kinase/Akt pathway followed by up-regulation of the STAT3 downstream target bcl-xL. 43 In addition, the hematopoietic transcription factor GATA1 has been shown to take part in controlling SCF-mediated expression of bcl-2 and bcl-xL in erythroid progenitor cells. 44 GATA1 has also been found to play an essential role during mast cell differentiation in vivo. 45 It can therefore be speculated that certain transcription factors such as STAT3 and GATA1 may be involved in modulating the expression of bcl-2 and bcl-xL in mastocytosis. Studies are underway to explore the role of these transcription factors in different forms of mastocytosis.
The present study, demonstrating overexpression of the anti-apoptotic molecule bcl-2 in mast cells of cutaneous mastocytosis lesions compared to overexpression of the related molecule bcl-xL in bone marrow infiltrates of systemic mastocytosis, may help to explain the pathogenesis of the disease in patients with and without c-kit mutations. In addition, the present data suggest that bcl-2 and bcl-xL may regulate different functions during the development of mast cells. Finally, our results may serve to develop new strategies for the treatment of mastocytosis, in analogy to in vitro and in vivo studies in various other neoplastic diseases expressing bcl-2 where bcl-2 antisense oligonucleotides were effective in either directly reducing tumor growth or in enhancing their sensitivity to chemotherapy, radiation or also to additional antisense oligonucleotides. 46-51 Whether bcl-2 antisense oligonucleotides are also able to induce apoptosis of mast cells or to chemosensitize mast cells, remains to be studied in future experiments.
Acknowledgments
We thank C. Berns, from the Department of Dermatology, University of Cologne; C. Wesendahl and R. Nordheim, from the Department of Dermatology, Charité, Humboldt University; and S. Landsberg, from the Institute of Pathology, University of Cologne, for excellent technical assistance.
Footnotes
Address reprint requests to Karin Hartmann, M.D., Department of Dermatology, University of Cologne, Joseph-Stelzmann-Str. 9, 50931 Cologne, Germany. E-mail: karin.hartmann@uni-koeln.de.
Supported by grant 99.049.1 from the Wilhelm Sander Foundation to K.H. and B.M.H.
References
- 1.Metcalfe DD, Akin C: Mastocytosis: molecular mechanisms and clinical disease heterogeneity. Leuk Res 2001, 25:577-582 [DOI] [PubMed] [Google Scholar]
- 2.Hartmann K, Henz BM: Mastocytosis: recent advances in defining the disease. Br J Dermatol 2001, 144:682-695 [DOI] [PubMed] [Google Scholar]
- 3.Valent P, Horny HP, Escribano L, Longley BJ, Li CY, Schwartz LB, Marone G, Nunez R, Akin C, Sotlar K, Sperr WR, Wolff K, Brunning RD, Parwaresch RM, Austen KF, Lennert K, Metcalfe DD, Vardimann JW, Bennett JM: Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res 2001, 25:603-625 [DOI] [PubMed] [Google Scholar]
- 4.Wolff K, Komar M, Petzelbauer P: Clinical and histopathological aspects of cutaneous mastocytosis. Leuk Res 2001, 25:519-528 [DOI] [PubMed] [Google Scholar]
- 5.Hartmann K, Henz BM: Classification of cutaneous mastocytosis: a modified consensus proposal. Leuk Res 2002, 26:483-484 [DOI] [PubMed] [Google Scholar]
- 6.Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y, Metcalfe DD: Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematological disorder. Proc Natl Acad Sci USA 1995, 92:10560-10564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buettner C, Henz BM, Welker R, Sepp NT, Grabbe J: Identification of activating c-kit mutations in adult-, but not in childhood-onset indolent mastocytosis: a possible explanation for divergent clinical behaviour. J Invest Dermatol 1998, 111:1227-1231 [DOI] [PubMed] [Google Scholar]
- 8.Longley BJ, Metcalfe DD, Tharp M, Wang X, Tyrrell L, Lu S, Heitjan D, Ma Y: Activating and dominant inactivating c-KIT catalytic domain mutations in distinct clinical forms of human mastocytosis. Proc Natl Acad Sci USA 1999, 96:1609-1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosbotham JL, Malik NM, Syrris P, Jeffery S, Bedlow A, Gharraie S, Murday VA, Holden CA, Carter ND: Lack of c-kit mutation in familial urticaria pigmentosa. Br J Dermatol 1999, 140:849-852 [DOI] [PubMed] [Google Scholar]
- 10.Sato-Matsumura KC, Matsumura T, Koizumi H, Sato H, Nagashima K, Ohkawara A: Analysis of c-kit exon 11 and exon 17 of urticaria pigmentosa that occurred in monozygotic twin sisters. Br J Dermatol 1999, 140:1130-1132 [DOI] [PubMed] [Google Scholar]
- 11.Yang E, Korsmeyer SJ: Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood 1996, 88:386-401 [PubMed] [Google Scholar]
- 12.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD: The release of cytochrome c from mitochondria: a primary site for bcl-2 regulation of apoptosis. Science 1997, 275:1132-1136 [DOI] [PubMed] [Google Scholar]
- 13.Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB: Bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 1993, 74:597-608 [DOI] [PubMed] [Google Scholar]
- 14.Gottschalk AR, Boise LH, Thompson CB, Quintans J: Identification of immunosuppressant-induced apoptosis in a murine B-cell line and its prevention by bcl-x but not bcl-2. Proc Natl Acad Sci USA 1994, 91:7350-7354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behrens TW, Mueller DL: Bcl-x and the regulation of survival in the immune system. Immunol Res 1997, 16:149-160 [DOI] [PubMed] [Google Scholar]
- 16.Grad JM, Zeng XR, Boise LH: Regulation of bcl-xL: a little bit of this and a little bit of STAT. Curr Opin Oncol 2000, 12:543-549 [DOI] [PubMed] [Google Scholar]
- 17.Korsmeyer SJ: Bcl-2: a repressor of lymphocyte death. Immunol Today 1992, 13:285-288 [DOI] [PubMed] [Google Scholar]
- 18.Xerri L, Hassoun J, Devilard E, Birnbaum D, Birg F: Bcl-x and the apoptotic machinery of lymphoma cells. Leuk Lymphoma 1998, 28:451-458 [DOI] [PubMed] [Google Scholar]
- 19.Findley HW, Gu L, Yeager AM, Zhou M: Expression and regulation of bcl-2, bcl-xl, and Bax correlate with p53 status and sensitivity to apoptosis in childhood acute lymphoblastic leukemia. Blood 1997, 8:2986-2993 [PubMed] [Google Scholar]
- 20.Kitada S, Andersen J, Akar S, Zapata JM, Takayama S, Krajewski S, Wang HG, Zhang X, Bullrich F, Croce CM, Rai K, Hines J, Reed JC: Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with in vitro and in vivo chemoresponses. Blood 1998, 9:3379-3389 [PubMed] [Google Scholar]
- 21.Salomons GS, Smets LA, Verwijs-Janssen M, Hart AAM, Haarman EG, Kaspers GJL, Van Wering ER, Van der Does-Van den Berg A, Kamps WA: Bcl-2 family members in childhood acute lymphoblastic leukemia: relationships with features at presentation, in vitro and in vivo drug response and long-term clinical outcome. Leukemia 1999, 13:1574-1580 [DOI] [PubMed] [Google Scholar]
- 22.Baliga BC, Kumar S: Role of bcl-2 family of proteins in malignancy. Hematol Oncol 2002, 20:63-74 [DOI] [PubMed] [Google Scholar]
- 23.Kitada S, Pedersen IM, Schimmer AD, Reed JC: Dysregulation of apoptosis genes in hematopoietic malignancies. Oncogene 2002, 13:3459-3474 [DOI] [PubMed] [Google Scholar]
- 24.Tu Y, Renner S, Xu F, Fleishman A, Taylor J, Weisz J, Vescio R, Rettig M, Berenson J, Krajewski S, Reed JC, Lichtenstein A: Bcl-x expression in multiple myeloma: possible indicator of chemoresistance. Cancer Res 1998, 58:256-262 [PubMed] [Google Scholar]
- 25.Yee NS, Paek I, Besmer P: Role of kit-ligand in proliferation and suppression of apoptosis in mast cells: basis for radiosensitivity of white spotting and steel mutant mice. J Exp Med 1994, 179:1777-1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bullock ED, Johnson EM: Nerve growth factor induces the expression of certain cytokine genes and bcl-2 in mast cells: potential role in survival promotion. J Biol Chem 1994, 269:2695-2702 [DOI] [PubMed] [Google Scholar]
- 27.Mekori YA, Oh CK, Dastych J, Goff JP, Adachi S, Bianchine PJ, Worobec A, Semere T, Pierce JH, Metcalfe DD: Characterization of a mast cell line that lacks the extracellular domain of membrane c-kit. Immunology 1997, 90:518-525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartmann K, Metcalfe DD: Regulation and dysregulation of mast cell survival and apoptosis. Marone G Lichtenstein LM Galli SJ eds. Mast Cells and Basophils. 2000:51-60 Academic Press New York
- 29.Kalesnikoff J, Huber M, Lam V, Damen JE, Zhang J, Siraganian RP, Krystal G: Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity 2001, 14:801-811 [DOI] [PubMed] [Google Scholar]
- 30.Mekori YA, Gilfillan AM, Akin C, Hartmann K, Metcalfe DD: Human mast cell apoptosis is regulated through bcl-2 and bcl-xL. J Clin Immunol 2001, 21:171-174 [DOI] [PubMed] [Google Scholar]
- 31.Xiang Z, Ahmed AA, Moeller C, Nakayama K, Hatakeyama S, Nilsson G: Essential role of the prosurvival bcl-2 homologue A1 in mast cell survival after allergic activation. J Exp Med 2001, 194:1561-1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baghestanian M, Jordan JH, Kiener HP, Bevec D, Agis H, Fritsch G, Mueller MR, Bankl HC, Schernthaner GH, Lechner K, Valent P: Activation of human mast cells through stem cell factor receptor (KIT) is associated with expression of bcl-2. Int Arch Allergy Immunol 2002, 129:228-236 [DOI] [PubMed] [Google Scholar]
- 33.Maurer M, Tsai M, Metz M, Fish S, Korsmeyer SJ, Galli SJ: A role for Bax in the regulation of apoptosis in mouse mast cells. J Invest Dermatol 2000, 114:1205-1206 [DOI] [PubMed] [Google Scholar]
- 34.Jordan JH, Walchshofer S, Jurecka W, Mosberger I, Sperr WR, Wolff K, Chott A, Buehring HJ, Lechner K, Horny HP, Valent P: Immunohistochemical properties of bone marrow mast cells in systemic mastocytosis: evidence for expression of CD2, CD117/Kit, and bcl-xL. Hum Pathol 2001, 32:545-552 [DOI] [PubMed] [Google Scholar]
- 35.Cervero C, Escribano L, San Miguel JF, Diaz-Agustin B, Bravo P, Villarrubia J, Garcia-Sanz R, Velasco JL, Herrera P, Vargas M, Gonzalez M, Navarro JL, Orfao A: Expression of bcl-2 by human bone marrow mast cells and its overexpression in mast cell leukemia. Am J Hematol 1999, 60:191-195 [DOI] [PubMed] [Google Scholar]
- 36.Haas N, Toppe E, Henz BM: Microscopic morphology of different types of urticaria. Arch Dermatol 1998, 134:41-46 [DOI] [PubMed] [Google Scholar]
- 37.Hermes B, Prochazka AK, Haas N, Jurgovsky K, Sticherling M, Henz BM: Up-regulation of TNF-α and IL-3 expression in lesional and uninvolved skin in different types of urticaria. J Allergy Clin Immunol 1991, 103:307-314 [DOI] [PubMed] [Google Scholar]
- 38.Kishi K: A new leukemia cell line with Philadelphia chromosome characterized as basophil precursors. Leuk Res 1985, 9:381-390 [DOI] [PubMed] [Google Scholar]
- 39.Hartmann K, Henz BM, Krueger-Krasagakes S, Koehl J, Burger R, Guhl S, Haase I, Lippert U, Zuberbier T: C3a and C5a stimulate chemotaxis of human mast cells. Blood 1997, 89:2863-2870 [PubMed] [Google Scholar]
- 40.Longley BJ, Tyrrell L, Lu SZ, Ma YS, Langley K, Ding TG, Duffy T, Jacobs P, Tang LH, Modlin I: Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet 1996, 12:312-314 [DOI] [PubMed] [Google Scholar]
- 41.Valent P, Escribano L, Parwaresch RM, Schemmel V, Schwartz LB, Sotlar K, Sperr WR, Horny HP: Recent advances in mastocytosis research. Summary of the Vienna Mastocytosis Meeting 1998. Int Arch Allergy Immunol 1999, 120:1-7 [DOI] [PubMed] [Google Scholar]
- 42.Hartmann K, Metcalfe DD: Pediatric mastocytosis. Metcalfe DD Soter NA eds. Mast Cell Disorders. 2000:625-640 Saunders Philadelphia
- 43.Ning ZQ, Li J, Arceci RJ: Signal transducer and activator of transcription 3 activation is required for Asp816 mutant c-kit-mediated cytokine-independent survival and proliferation in human leukemia cells. Blood 2001, 97:3559-3567 [DOI] [PubMed] [Google Scholar]
- 44.Kapur R, Zhang L: A novel mechanism of cooperation between c-Kit and erythropoietin receptor. J Biol Chem 2001, 276:1099-1106 [DOI] [PubMed] [Google Scholar]
- 45.Migliaccio AR, Rana RA, Sanchez M, Lorenzini R, Centurione L, Bianchi L, Vannucchi AM, Migliaccio G, Orkin SH: GATA-1 as a regulator of mast cell differentiation revealed by the phenotype of GATA-1 low mouse mutant. J Exp Med 2003, 197:281-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jansen B, Schlagbauer-Wadl H, Brown BD, Bryan RN, Van Elsas A, Mueller M, Wolff K, Eichler HG, Pehamberger H: Bcl-2 antisense therapy chemosensitizes human melanoma in SCID mice. Nat Med 1998, 4:232-234 [DOI] [PubMed] [Google Scholar]
- 47.Schlagbauer-Wadl H, Klosner G, Heere-Ress E, Waltering S, Moll I, Wolff K, Pehamberger H, Jansen B: Bcl-2 antisense oligonucloetides (G3139) inhibit merkel cell carcinoma growth in SCID mice. J Invest Dermatol 2000, 114:725-730 [DOI] [PubMed] [Google Scholar]
- 48.Flaherty KT, Stevenson JP, O’Dwyer PJ: Antisense therapeutics: lessons from early clinical trials. Curr Opin Oncol 2001, 13:499-505 [DOI] [PubMed] [Google Scholar]
- 49.Jahrsdorfer B, Jox R, Muhlenhoff L, Tschoep K, Krug A, Rothenfusser S, Meinhardt G, Emmerich B, Endres S, Hartmann G: Modulation of malignant B-cell activation and apoptosis by bcl-2 antisense ODN and immunostimulatory CpG ODN. J Leukoc Biol 2002, 72:83-92 [PubMed] [Google Scholar]
- 50.Gleave M, Miyake H, Zangemeister-Wittke U, Jansen B: Antisense therapy: current status in prostate cancer and other malignancies. Cancer Metastasis Rev 2002, 21:79-92 [DOI] [PubMed] [Google Scholar]
- 51.Reed JC: Apoptosis-based therapies. Nat Rev Drug Discov 2002, 1:111-121 [DOI] [PubMed] [Google Scholar]