Abstract
Targeted expression of a human pituitary tumor derived-fibroblast growth factor receptor-4 (FGFR4) recapitulates pituitary tumorigenesis. We have shown that FGFR4 is a target for Ikaros, a zinc finger-containing transcription factor that localizes to heterochromatin regions and participates in higher order chromatin complexes and control of gene expression. We report here the expression of Ikaros and functional differences between its alternatively spliced variants in human pituitary tumors. Ik1 expression was detected in human pituitary tumors and we also identified a truncated isoform consistent with the non-DNA-binding Ik6 isoform in a subset of adenomas by reverse transcriptase-polymerase chain reaction, sequencing, and Western immunoblotting. Transfection of Ik6 in GH4 pituitary cells resulted in predominantly cytoplasmic expression as compared to Ik1, which resulted in exclusively nuclear expression as determined by immunofluorescence and immunoblotting of fractionated protein. Immunohistochemistry of primary human pituitary adenomas localized Ikaros expression to the nuclear compartment but also in the cytoplasm, the latter consistent with Ik6. Expression of Ikaros and truncated non-DNA-binding isoforms was also suggested by electromobility shift assays using nuclear proteins from primary human pituitary adenomas. Ik6 resulted in reversal of the effects of Ik1 on wild-type 5′ FGFR4 promoter activity, histone acetylation, and regulation of the endogenous gene. We conclude that dominant-negative Ik6 isoforms with their distinct localization and effects on Ik1 action may contribute to the altered expression of FGFR4 and possibly other target genes in human pituitary tumors.
The pathogenetic mechanisms underlying pituitary tumor formation are unknown. 1 Fibroblast growth factors (FGFs) have been implicated. 2 The role of FGF receptors (FGFRs) in pituitary tumorigenesis has been recently highlighted by the identification of a tumor-derived N-terminally truncated isoform of FGFR4 (ptd-FGFR4) that is transforming in vitro and in vivo and causes pituitary tumorigenesis in transgenic mice. 3 ptd-FGFR4 is derived by alternative initiation of the FGFR4 gene upstream of exon 6. The truncated protein lacks a signal peptide, the first immunoglobulin-like domain and part of the second domain; it localizes to the cytoplasm where it is constitutively phosphorylated. In contrast to ptd-FGFR4, wild-type full-length FGFR4 does not result in tumor formation. 3 To examine the mechanism(s) responsible for this altered expression of FGFR4 in pituitary tumors, we examined the promoter of this gene and identified that it is a target for the zinc-finger transcription factor Ikaros (Ik). 4
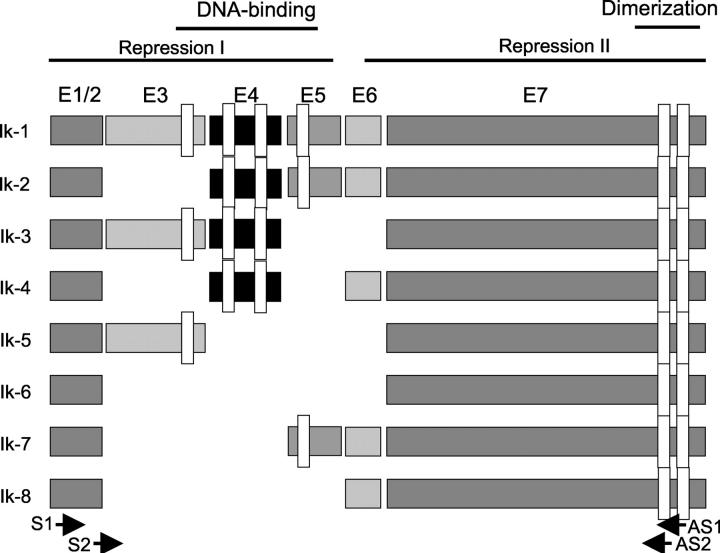
Ikaros is a transcription factor that binds regulatory sequences of genes expressed in lymphoid cells. 5,6 It is the founding member of a family of zinc-finger DNA-binding proteins that are associated with chromatin remodeling. 7 The Ikaros gene contains seven exons that can, by alternative splicing, give rise to at least eight isoforms (Figure 1) ▶ . The isoforms differ in the number of N-terminal zinc finger motifs that bind DNA and contain the nuclear localization signals, resulting in members with and without DNA-binding properties. 5,8,9 All eight Ik isoforms share a common C-terminal domain that contains two zinc finger motifs that are required for hetero- or homodimerization and for interactions with other proteins. 8,10,11 Only isoforms 1 to 3 contain the requisite three or more N-terminal zinc fingers that confer high-affinity binding to an Ik-specific core DNA sequence motif in the promoters of target genes. 8 Ik1 and Ik2 can bind to the same recognition sequences whereas Ik3 and Ik4 interact only with a subset of these motifs. Ik1 and Ik2 proteins can stimulate in vitro transcription as we have demonstrated in the case of the FGFR4 gene. 4
Figure 1.
Schematic representation of the human Ikaros gene and its isoforms. DNA binding, repression/activation domains, and dimerization domains are indicated. The narrow open rectangles represent the specific zinc finger domains. Note that alternative splicing yields mRNA isoforms that encode truncated proteins that differ in the number of N-terminal zinc fingers. The dimerization domain is shared by all eight isoforms. Solid black arrows indicate sites corresponding to nested PCR primers as detailed in Materials and Methods (S1 sense primer, reaction 1; AS1 anti-sense primer, reaction 1; S2 sense primer, reaction 2; AS2 anti-sense primer, reaction 2).
The formation of Ik homo- and heterodimers among the DNA-binding isoforms increases their affinity for DNA, whereas heterodimers between the DNA-binding isoforms and non–DNA-binding isoforms are unable to bind DNA. Thus, Ik proteins with fewer than three N-terminal zinc fingers can negatively interfere with the activity of Ik isoforms that can bind DNA. 8,12 Significantly, the transcription-activating potential of the Ik proteins may correlate with their subcellular localization; 12 the dominant-negative (dn) isoform Ik6 has been shown to have cytoplasmic residence. 13 The various isoforms can act either as activators or repressors, forming an integral component of a functionally diverse chromatin-remodeling network. 7 Thus, the Ikaros system represents an example of a finely tuned system that is the consequence of complex alternative-splicing mechanisms.
Gene-targeting experiments have established that Ikaros factors are essential for normal lymphoid development. 14 Mice homozygous for a null mutation in Ik1 lack B- and T-lymphocyte differentiation, 15 and those homozygous for a dn form of Ik lack all lymphocytes. 16 Heterozygous Ik-deficient mice exhibit hyperactive T-cell receptor-mediated proliferative responses and eventually develop leukemias and lymphomas. 11,17 Animals heterozygous for the dn forms of Ikaros (isoforms that lack the DNA-binding domain) develop T-cell lymphoproliferative disorders similar to human T-lymphoblastic leukemia or lymphoma, presumably by inactivation of the normal Ik allele. 11 Thus, Ik appears to be an essential molecular switch in early cell differentiation and lineage commitment and altered Ik expression may play an important role in tumorigenesis.
In this study we sought to determine whether the Ikaros gene is expressed in human pituitary tumors, whether it undergoes alternative splicing, and whether the generated Ikaros isoforms can have distinct effects on the regulation of its putative target FGFR4 in the pituitary.
Materials and Methods
Cell Culture and Tissue Specimens
The rat pituitary tumor-derived GH4C1 cell line was grown in Dulbecco’s modified Eagle’s medium (Life Technologies, Inc., Rockville, MD) medium with high glucose supplemented with 15% horse and fetal bovine serum (Sigma, Oakville, Ontario, Canada), 2 mmol/L glutamine, 100 IU/ml penicillin, and 100 μg/ml of streptomycin (37°C, 95% humidity, 5% CO2 atmosphere incubation). Fifty primary human pituitary samples were obtained at the time of trans-sphenoidal pituitary surgery as previously described. 18 All patients provided informed consent.
RNA Extraction and Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analysis
RNA was extracted from cells and human pituitary tissue using Trizol (Life Technologies, Inc., Rockville, MD). Reverse transcription was performed according to the manufacturer’s protocol (Perkin Elmer Cetus Corp., Norwalk, CT). The integrity of RNA quality was examined by means of PCR amplification of the housekeeping gene PGK-1 using the following primers: forward 5′-GCTGACAAGTTTGATGAGAAT-3′ and reverse 5′-AGGACTTTACCTTCCAGGAGC-3′. Primary and nested PCR for Ik amplification was performed throughout 35 cycles under the following conditions: (94°C 5 minutes, 94°C 50 seconds, 57°C 50 seconds, and 72°C 1 minute 50 seconds, and finally 72°C for 7 minutes) using the following primers: forward (P1) 5′-ATGGATGCTGACGAGGGTCAAGAC-3′ and reverse (P1As) 5′-TTAGCTCATGTGGAAGCGGTGCTC-3′ and nested forward (P2), 5′-CTCATCAGGGAAGGAAAGCC-3′ and reverse (P2As), 5′-GGTGTACATGACGTGATCCAGG-3′. The P1 and P2 are located, respectively, at +169 and +201 from the 5′-end start site of the Ik1 cDNA and +1728 and +1603 for P1As and P2As, respectively. Purified RT-PCR products were cloned using the TA method (Invitrogen, San Diego, CA). Cloned PCR products were purified using the Qiagen Plasmid Mini-Prep isolation kit (Qiagen, Mississauga, Ontario, Canada) and subjected to nucleotide sequencing using T7 and M13 reverse-sequencing primers (Amersham Pharmacia, Piscataway, NJ). Resulting sequences were compared with the GenBank database published human and mouse Ik cDNA sequences (accession codes U40462 and NM009578, respectively).
Western Blotting Analysis
Total protein was extracted from total cell lysates and quantified. Cell fractionation was performed by the hypotonic/Nonidet P-40 lysis method. Briefly, cells were washed in Tris-buffered saline, swollen in homogenization buffer [10 mmol/L HEPES, pH 7.9, 10 mmol/L KCl, 0.1 mmol/L ethylenediaminetetraacetic acid (EDTA), 0.1 mmol/L ethylene glycolbis, 1 mmol/L dithiothreitol, and 0.5 mmol/L phenylmethyl sulfonyl fluoride (PMSF)] and vortexed in homogenization buffer containing 0.6% Nonidet P-40. Supernatant containing cytoplasm and plasma membranes were removed and the pellet containing the nuclear fraction was suspended in a resuspension buffer: 250 mmol/L Tris, pH 7.8, 60 mmol/L KCl, 1 mmol/L dithiothreitol, and 1 mmol/L PMSF.
Protein concentrations were determined using the Bio-Rad method. Forty μg of whole lysates were separated on 10% sodium dodecyl sulfate denaturing polyacrylamide gels, transferred onto nylon membrane (Millipore, Bedford, MA) at 100 V for 1 hour at room temperature. Blots were incubated with a mouse monoclonal antibody (4E9; kindly provided by K. Georgopoulos, Harvard University, Boston, MA) that recognizes the C-terminal fragments of Ikaros proteins 8 at 1:2000 dilution in phosphate-buffered saline (PBS)-5% nonfat milk with 0.1% Tween at 4°C overnight, followed by washing with PBS-Tween-20 four times at 10 minutes each at room temperature and incubated with secondary antibody of peroxidase-conjugated goat anti-mouse IgG (1:2000) for 1 hour at room temperature with agitation. Proteins were detected using a chemiluminescence method (ECL; Amersham Bai d’Urfé, Quebec, Canada).
Immunocytochemical Localization of Ikaros in the Pituitary
Immunolocalization of Ik was performed with the 4E9 mouse monoclonal antibody as described above that recognizes the C-terminal tail of Ik. Pituitary tissues were fixed in formalin and embedded in paraffin; after microwave antigen retrieval, immunolocalization was performed with the primary antibody at a dilution of 1:400 and detected with the streptavidin-biotin-peroxidase complex technique and 3,3′-diaminobenzidine. Co-localization with pituitary hormones was performed with double staining as previously described. 19 For subcellular localization after transfection, cells were grown on glass coverslips, fixed in 1% formalin in PBS, the primary antiserum was localized with fluorescein-tagged secondary antibody and visualized with a Bio-Rad MRC 600 confocal microscope. The specificity of reactions was verified by preabsorbing the primary antibody with purified Ik1 when localizing transfected Ik1 and with Ik6 when localizing transfected Ik6.
Plasmids
The human FGFR4 gene wild-type reporter construct P(−115/+99)-Luc and the same with mutation of the Ik-binding site FGFR4 (mIk)-Luc were described previously. 4 The convention for sequence coordinates with +1 as the first base of the coding sequence in exon 1 was adopted. The Ik1 and Ik6 cDNA encoding expression vectors were generously provided by Dr. K. Georgopoulos. 20
Transfection and Luciferase Assays
All plasmid reporters were prepared by column chromatography (QiaGen, Missisauga, Ontario, Canada) for sequencing and transfections. Cells were transfected by the lipofectamine method according to the manufacturer’s protocol (Gibco, Burlington, Ontario, Canada). Cells were plated into six-well cluster dishes (7 × 105 cells per well), transfected the following day with 3 μl or 5 μl/well of lipofectamine and 2 μg of DNA per well as indicated. The total amount of transfected DNA was kept constant by adding empty vector. Transfection efficiency was monitored by simultaneous co-transfection with a β-galactosidase control expression plasmid CMV-βgal (20 ng/well). Histone deacetylase inhibition was performed using trichostatin-A (Sigma, Oakville, Ontario, Canada) at a concentration of 100 ng/ml for 12 hours. Forty-eight hours after all transfections, cells were lysed in buffer containing 25 mmol/L glycylglycine, 15 mmol/L MgSO4, 4 mmol/L EGTA, 1% Triton X, and 1 mmol/L dithiothreitol. Luciferase activity was measured for 20 seconds in a luminometer. β-galactosidase activity was measured to normalize for variations in transfection efficiency. Promoter activity of each construct was expressed as firefly luciferase/β-galactosidase activity. Each experiment was independently performed on three separate occasions with triplicate wells in each experiment. Stable transfections of GH4 cells with Ik6 were performed as previously described. 18
Preparation of Nuclear Extracts
Nuclear extracts were prepared by washing cells in 1× PBS and lysis in 100 μl of buffer containing (10 mmol/L HEPES, pH 7.9, 1 mmol/L dithiothreitol, 1 mmol/L EDTA, 60 mmol/L KCl, 0.5% Nonidet P-40, 1 mmol/L PMSF) 5 minutes on ice. The pellet was resuspended into 100 μl of the nuclear resuspension buffer (0.25 mmol/L Tris-HCl, pH 7.8, 60 mmol/L KCl, 1 mmol/L dithiothreitol, 1.5 mmol/L PMSF) and lysed with three cycles of freezing and thawing to 37°C. After centrifugation at 13,000 rpm for 10 minutes at 4°C, the clear supernatant was collected for further analysis.
Electrophoretic Mobility Shift Assays (EMSAs)
Oligonucleotides were end-labeled with [α-32P]dCTP using the Klenow fragment of DNA polymerase. Five to 10 μg of nuclear protein extracts and 2 ng of labeled oligonucleotides were allowed to bind for 30 minutes at room temperature in a final volume of 20 μl of binding buffer [20 mmol/L HEPES (pH 7.9), 50 mmol/L KCl, 1 mmol/L EDTA, 1 mmol/L dithiothreitol, 0.5 mmol/L MgCl2, 2% glycerol, and 1 μg poly(dI-dC) (Pharmacia, Piscataway, NJ)]. Protein-DNA complexes were resolved in 4% polyacrylamide gels containing 0.5× Tris borate-EDTA. Double-stranded oligonucleotides were used as probes and for competition as follows: wild-type Ik: 5′-AAGAAGCGGGAGTGACAGG-3′ and its complementary strand; Pit-1: 5′TGTCTTCCTGAATATGAATAAGAAATA-3′ and its complementary strand (synthesized by Sigma, Oakville, Ontario, Canada). The complementary strands were annealed in an annealing buffer consisting of 10 mmol/L Tris-Cl, pH 8.0, 50 mmol/L NaCl, 1 mmol/L EDTA.
Gel shift probes were radiolabeled using Klenow fragment I (Boehringer, Ontario, Canada), and purified with a G50 spin column. For EMSA, 100 cpm of P32-labeled probe was incubated with 5 μg of nuclear extracts at room temperature for 30 minutes in a binding reaction consisting of 20 mmol/L HEPES (pH 7.9), 50 mmol/L KCl, 1 mmol/L EDTA, 1 mmol/L dithiothreitol, 0.5 mmol/L MgCl2, 2% glycerol, and 1 μg poly(dI-dC) (Pharmacia) in a final volume of 20 μl. For competition assay, 10 or 100 molar excess of unlabeled fragment was added as competitor DNA or antibody to the relevant transcription factor was added 30 minutes before addition of radiolabeled probe. Samples were electrophoresed on 4% polyacrylamide nondenatured gels containing 0.5% Tris-borate buffer and 2% glycerol. Gels were dried under vacuum and autoradiographed.
Chromatin Immunoprecipitation (ChIP) Assay
GH4 cells were co-transfected with FGFR4-Luc reporter containing the 5′ promoter (−115/+99) with either the Ik1 or Ik6 expression vector or their empty control vector. The chromatin immunoprecipitation assay was performed in accordance with the manufacturer’s recommendations (UBI, Lake Placid, NY). Histone was cross-linked to DNA by the addition of formaldehyde. One quarter of the total lysate was used for monitoring total DNA input by PCR. The rest of the lysate was cleared with a salmon sperm DNA/protein G-agarose slurry. Half of the cleared lysate was incubated with anti-acetylated-histone 3 (AcH3) antibody overnight at 4°C and the other non-AcH3 antibody immunoprecipitated protein was used as a negative control both of which were also examined by immunoblotting with anti-AcH3 antibody. For PCR analysis, the eluted immunocomplexes were digested with proteinase K, and DNA was purified by phenol extraction and PCR amplification. This was performed under the following conditions: (95°C for 4 minutes followed by 35 cycles of 95°C for 45 seconds, 58°C for 45 seconds, and 72°C for 50 seconds, and finally 72°C for 7 minutes) using with the following primers: forward 5′-GTGGAAGGAGGGGCGGGC-3′ and reverse primers 5′-GAGGAGGCGGCGGAGTGAGG-3′ corresponding to nucleotides 1040 to 1058 and 1217 to 1236 of the FGFR4 promoter sequence respectively (GenBank accession no. Y13901) yielding a 196-bp product. Similarly, the following primers: forward 5′ GCAATGGCACACATTGCAG-3′ and reverse primers 5′-AGTCCTAAGAGAACCACTGC-3′ were used to generate a 364-bp fragment from the 5′ (−422) prolactin promoter.
Results
Human Pituitary Adenomas Express Ik mRNA and Its Alternatively Spliced Isoforms
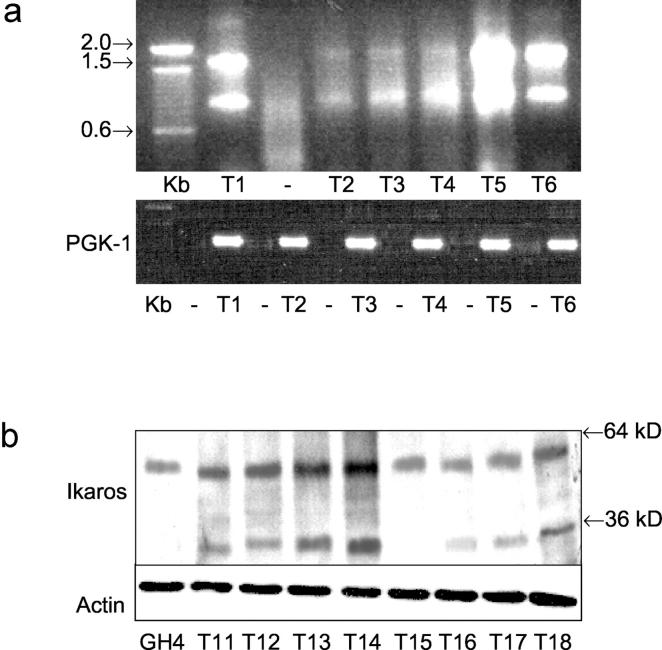
Figure 1 ▶ depicts the organization of the human Ik gene and its known alternatively spliced products. Ik1 is the largest of all Ik isoforms encoding four Cys-2 to His-2 zinc fingers at its N-terminus (F1, F2, F3, and F4) and two at its C-terminus (F5 and F6). Ik2 shares two of the four N-terminal zinc fingers (F2 and F3) whereas Ik3 shares three of the N-terminal zinc fingers (F1, F2, F3). Using a nested RT-PCR approach with the depicted primers, 18 of 30 human pituitary adenomas composed of each of the hormone-producing types revealed two products measuring 0.9 and 1.5 kb (Figure 2a) ▶ . Sequencing of the 1.5-kb PCR products confirmed Ikaros sequence and the smaller 0.9-kb bands corresponded to Ik transcripts from which the N-terminal zinc finger-encoding exons were spliced out. The latter would be expected to yield the Ik6 isoform lacking DNA-binding potential.
Figure 2.
Detection of Ikaros isoforms in human pituitary tumors. a: RT-PCR detection of Ik isoforms. RNA from primary human pituitary adenomas was reverse-transcribed and amplified using nested PCR primers as shown in Figure 1 ▶ . The expected product sizes are approximately as follows: Ik1 to Ik3, 1.3 to 1.5 kb; Ik4 to Ik8, 0.9 kb. Pituitary tumors (T1 to T6) express Ik1 to Ik3 and also yield a band of 0.9 kb; sample T1 submitted for PCR without reverse transcription (−) yields no product. The identity of all PCR products was confirmed by DNA sequencing; the 0.9-kb bands were verified by sequencing to be Ik6. The products of the RT reactions amplified with PGK-1 primers yield the expected 338-bp product, confirming intact quality of RNA (bottom). b: Ik isoform expression in human pituitary tumors by Western blotting. Lysates from GH4 cells and primary human pituitary tumors (T) were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with an Ik antibody that recognizes the C-tail that is common to all Ik isoforms. Note the expression of Ik1 at ∼57 kd and a smaller protein consistent with the size of Ik6 (∼36 kd) identified in several pituitary tumors. Immunoblotting with an anti-actin antibody confirms similar protein loading (bottom).
Ik1 and Ik6 Are Localized to Distinct Cellular Compartments in the Pituitary
We have previously shown that the Ik gene is expressed in primary mouse pituitary and in rat GH4 pituitary cells. 4 To investigate the translation of the Ik gene and to localize the products of its alternatively spliced isoforms in the human pituitary, primary human pituitary adenomas were examined by Western blotting and immunocytochemistry. Human pituitary adenomas provide small samples and only 18 could be examined by Western blotting; 15 of these expressed a 57-kd protein corresponding to Ik1 (Figure 2b) ▶ . As predicted by the RT-PCR findings, 12 of these tumors also expressed immunoreactive proteins of ∼36 kd in size (Figure 2b) ▶ , corresponding to the dominant-negative (dn) isoforms Ik4 to Ik8. Densitometric analysis of Western blots showed Ik6 to Ik1 expression ranging from 0.26 to 0.72 in these 12 pituitary tumors.
The subcellular localization of Ik isoforms in the pituitary was examined by confocal microscopy. GH4 cells transfected with Ik1 cDNA exhibited a pattern of nuclear positivity that is characteristic of classical transcription factors (Figure 3a ▶ , left). In contrast, cells transfected with Ik6 yielded predominantly cytoplasmic staining (Figure 3a ▶ , right). These findings were further supported by immunoblotting of fractionated proteins where Ik6-transfected cells exhibited strong reactivity in cytoplasmic fractions in contrast to the exclusive nuclear reactivity in Ik1-transfected cells (Figure 3b) ▶ .
Figure 3.
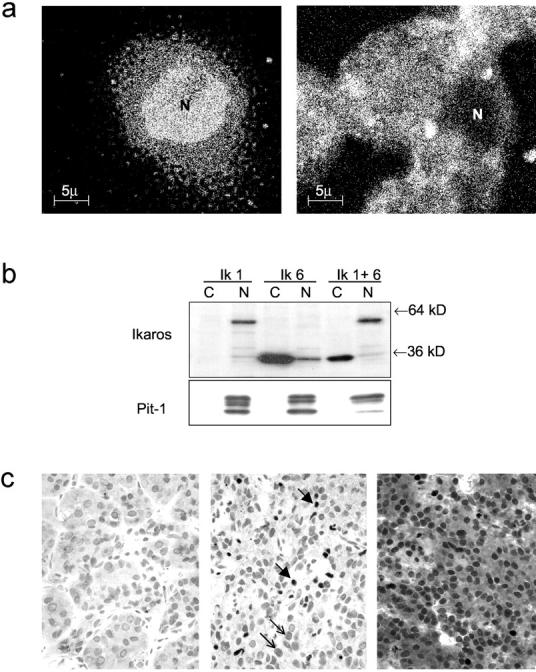
Localization of Ik1 and Ik6 in pituitary cells. a: Immunofluorescence detection of Ik isoforms. GH4 pituitary cells were transiently transfected with Ik1 or Ik6. Note the predominantly nuclear (N) staining in Ik1-transfected cells (left) in contrast to the cytoplasmic fluorescence detected in Ik6-transfected cells (right). b: Western blotting of fractionated protein lysates from Ik-1-transfected GH4 cells detects a 57-kd protein predominantly in nuclear fractions (N); Ik-6-transfected cells exhibit a 36-kd protein predominantly in cytoplasmic fractions (C) with some component in nuclear fractions (N). Cells transfected with both Ik1 and Ik6 (Ik 1 + 6) show nuclear Ik1 and predominantly cytoplasmic Ik6 reactivity. Pit-1 reactivity was used as a positive control to verify appropriate fractionation of protein. c: Immunocytochemical localization of Ik in the human pituitary. The nontumorous adenohypophysis (left) is negative. A pituitary adenoma that expressed Ik1 (middle) exhibits focal strong nuclear staining (thick arrows). An adenoma that expressed Ik1 and Ik6 (right) exhibits strong nuclear and cytoplasmic staining.
These data allowed us to examine the expression of Ikaros isoforms in a series of primary human pituitary tumors by immunohistochemistry. Ik staining was undetectable in nontumorous pituitary adjacent to adenomas (Figure 3c ▶ , left). Pituitary adenomas exhibited focal nuclear staining for Ik (Figure 3c ▶ , middle). In 28 of 50 adenomas examined, there was also diffuse cytoplasmic staining for Ik (Figure 3c ▶ , right), consistent with Ik6 expression. Absorption of the primary antibody with purified Ik6 protein resulted in complete loss of staining (data not shown). There was no correlation between Ik reactivity and tumor type.
Detection of Ik in Human Pituitary Adenomas by EMSA
The DNA-binding potential of Ik generated in pituitary tumors was examined by gel EMSA using an Ikaros oligonucleotide probe that contains the Ik-binding site. We have previously shown that this Ik oligonucleotide probe contains the conserved Ik-binding motif (-GGGA-) and forms specific complexes with nuclear proteins from pituitary GH4 cells. 4 Proteins from primary pituitary adenomas resulted in distinct complexes that were specifically competed by excess unlabeled Ik oligonucleotide but not by the same oligonucleotide with mutation of the Ik-binding site (mIk) (Figure 4) ▶ . Those complexes that were specifically competed with the excess Ik oligonucleotide were also supershifted by Ik antibody (Figure 4) ▶ . The integrity of nuclear protein from these adenomas was confirmed by formation of specific complexes with an oligonucleotide probe containing the pituitary-specific Pit-1-binding site.
Figure 4.
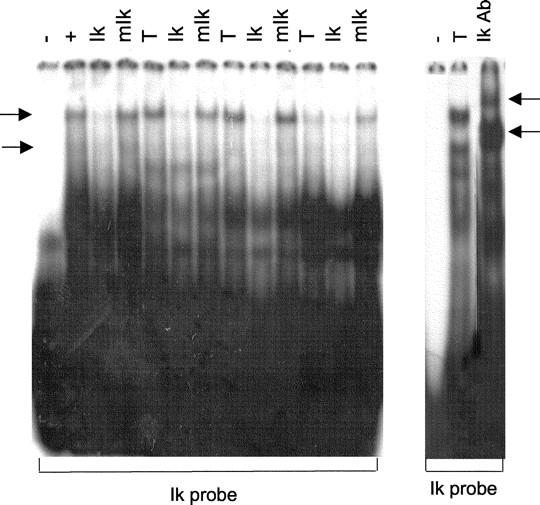
Examination of DNA-binding activity of Ik proteins in human pituitary tumors by EMSA. Nuclear proteins from primary human pituitary tumor samples (T) were allowed to compete with a 32P-ATP-labeled oligonucleotide probe containing the Ik-binding site. Note formation of complexes indicated by side arrows by GH4 cell nuclear extracts (+). Pituitary tumors (T) formed complexes with the Ik probe that were competed by 100 molar excess of unlabeled Ik oligonucleotide (Ik) but not by the same oligonucleotide with mutation of the Ik-binding site (mIk). Those complexes that were specifically competed by the wild-type Ik oligonucleotide were supershifted by Ik antibody (right arrows).
Correlation of Ikaros and FGFR4 Expression in Human Pituitary Tumors
Among the 50 tumors examined, 28 had cytoplasmic positivity for Ik; 18 of these were proven to express Ik6 by RT-PCR and 12 by Western blotting. All 28 of these tumors expressed ptd-FGFR4 as shown by RT-PCR and immunohistochemistry with diffuse cytoplasmic staining. No tumors exhibited membrane staining for FGFR4 and none expressed intact FGFR4 by RT-PCR. 3
Ik1 and Ik6 Differentially Regulate FGFR4 in the Pituitary
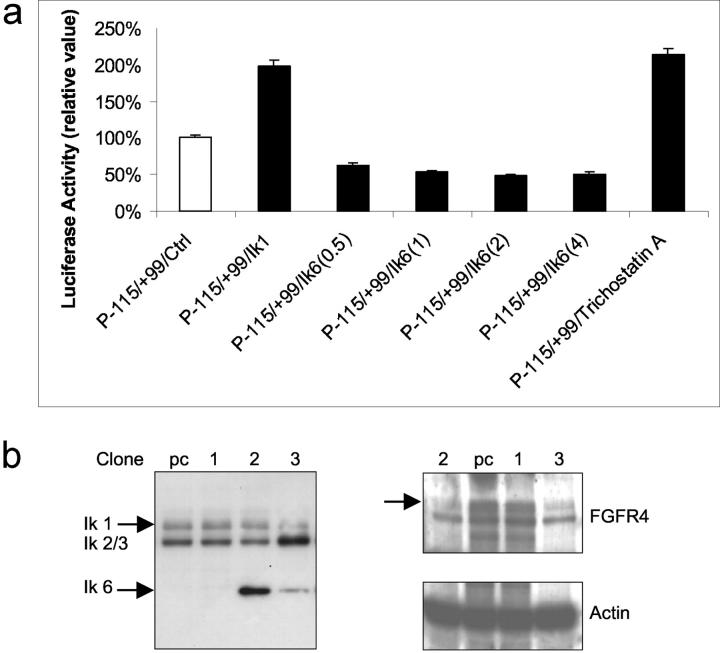
To compare the functional contribution of Ik1 and Ik6 in regulating pituitary FGFR4, we examined the wild-type 5′ P(−115/+99)-Luc FGFR4 or the same promoter with mutated Ik-binding site (mIk P(−115/+99)-Luc FGFR4) and the effects of co-transfection with Ik1 or Ik6. Figure 5a ▶ demonstrates the stimulating effect of Ik1 transfection on the wild-type FGFR4 promoter in GH4 pituitary cells. In contrast, transfection of Ik6 in doses ranging from 0.5 to 4 μg resulted in significant inhibition (ranging from 35 to 52%) of the 5′ FGFR4 promoter. Mutation of the Ik-binding site diminished basal promoter activity by ∼30% and completely eliminated the response to Ik1 transfection (not shown). Neither Ik1 nor Ik6 transfection altered pituitary prolactin (PRL) (−422-Luc) promoter activity under the same conditions (data not shown).
Figure 5.
Ik1 and Ik6 differentially regulate 5′ FGFR4 promoter activity. a: GH4 cells were transiently co-transfected with the minimal FGFR4 P(−115/+99) and expression vectors encoding either Ik1 or Ik6 as indicated. Note similar activation of wild-type FGFR4 promoter by Ik1 or by the deacetylase inhibitor trichostatin-A. In contrast, transfection of increasing amounts of Ik6 (in μg of DNA/well) results in inhibition of 5′FGFR4 promoter activity. Disruption of the Ik-binding site in FGFR4 (mIk) completely abrogated the effect of Ik1 (not shown). All transfections included corresponding empty control vectors along with 20 ng of pCMVβgal to normalize for transfection efficiency. Data are presented as the mean luciferase activity adjusted for β-gal activity (±SD) and compared with control wells of three separate experiments, each performed in triplicate (P < 0.005). b: Ik6 attenuates endogenous FGFR expression in pituitary cells. GH4 cells were stably transfected with Ik6 and clones of varying degrees of Ik6 protein expression selected for FGFR4 analysis by Western blotting. Note the reduced expression of endogenous FGFR4 reactivity (110/90-kd doublet) in clones expressing moderate to high amounts of Ik6 compared to a negative clone or empty vector-transfected control cells.
To further examine the influence of Ik6 on the endogenous gene, FGFR4 protein expression was examined in Ik6 stably transfected GH4 cells. Clones of varying degrees of Ik6 expression were selected and subjected to Western immunoblotting for endogenous FGFR4. Figure 5b ▶ demonstrates reduction of FGFR4 immunoreactivity even in clones where transfected Ik6 is expressed at lower levels than endogenous Ik1–3 and in the range identified in primary pituitary tumors. These data provide further evidence of the dominant-negative properties of Ik6 on Ik1–3 action in GH4 cells and indicate that FGFR4 represents a target of Ik action in the pituitary.
Ik1 and Ik6 Differentially Deacetylate Histones on the FGFR4 Promoter in Pituitary Cells
As Ik1 has been shown to potentially recruit components of the histone deacetylase complex (HDAC) to some promoters in a gene and cell-specific manner, we first sought to determine whether inhibition of deacetylase activity would alter FGFR4 activity in GH4 pituitary cells. Using trichostatin-A as an HDAC inhibitor we noted a similar degree of stimulation of FGFR4 promoter activity to that achieved by Ik1 transfection (Figure 5a) ▶ in these cells.
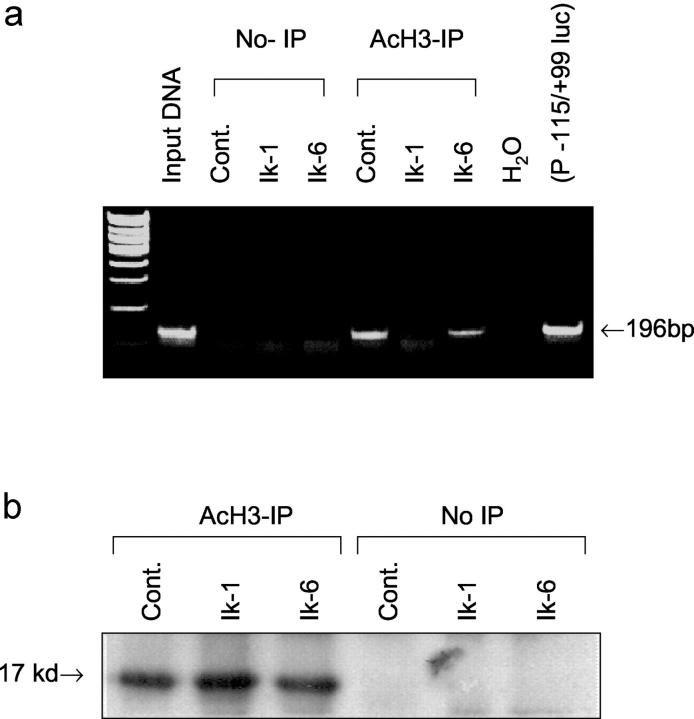
As Ik proteins have been shown to associate with the transcriptional co-repressors mSin3 and Mi-2 that are part of the HDAC, and the potential for Ik isoforms may lead to cell type-dependent Ik repression, we examined whether Ikaros can regulate histone deacetylation on the FGFR4 promoter. A chromatin immunoprecipitation (ChIP) assay was used to assess the acetylation status of histones on the 5′ FGFR4 promoter. GH4 pituitary cells were co-transfected with the 5′ FGFR4 (−115/+99) promoter along with Ik1, Ik6, or their control empty vector. Formaldehyde cross-linked chromatin isolated from these transfected cells was immunoprecipitated with a specific antibody to acetylated histone H3 (AcH3) and subjected to PCR amplification using primers for the FGFR4 promoter. If the FGFR4 promoter is associated with acetylated histones, PCR analysis of the immunoprecipitates would be expected to yield a visualized product. GH4 cells that constitutively express full-length FGFR4 4 demonstrated histone acetylation in empty vector-control transfected cells (Figure 6a) ▶ . In contrast, Ik1-transfected cells failed to yield a product, providing evidence for the ability of Ik1 to recruit an HDAC complex to the FGFR4 5′ promoter. In sharp contrast to Ik1, Ik6-transfected cells yielded PCR products from AcH3 antibody immunoprecipitates. Immunoprecipitation followed by immunoblotting with the AcH3 antibody showed no difference in global histone acetylation in response to Ik1 or Ik6 transfection (Figure 6b) ▶ . These findings, together with lack of transcriptional effect by Ik1 or Ik6 on the PRL promoter (see above) are consistent with a gene-specific role for Ikaros in the pituitary.
Figure 6.
Ik1 and Ik6 display distinct effects on chromatin histone deacetylation on the FGFR4 promoter. Chromatin immunoprecipitation (ChIP) was performed as described in Materials and Methods. a: DNA from lysate before immunoprecipitation (input DNA) or from plasmid FGFR4 (P−115/+99-Luc) DNA were amplified as positive controls. GH4 cells were co-transfected with 5′ FGFR4 promoter in combination with expression vectors encoding either Ik1, Ik6, or empty vector control. Cross-linked chromatin was immunoprecipitated with antibody to acetyl histone 3 (AcH3) and subjected to PCR analysis. Note the effect of Ik1 on histone deacetylation resulting in absence of PCR product from AcH3 antibody immunoprecipitates. Omission of AcH3 antibody immunoprecipitation (non-IP) results in absence of PCR products from all corresponding transfections. b: Corresponding immunoblotting with AcH3 antibody of immunoprecipitates reveals no effect or difference between Ik1 and Ik6 on global histone acetylation in pituitary cells.
Discussion
We have previously demonstrated that FGFR4 promoter regulation involves a multiprotein complex that includes ubiquitous factors such as Sp1 and tissue-specific factors. 4 Using deletional mapping, we defined a 214-bp (−115/+99) functional promoter in the pituitary that included a fragment containing important binding sites for the hematopoietic zinc finger-containing transcription factor Ikaros (Ik) flanked by binding sites for Sp1- and Ets-type factors. Specific DNA binding by Sp1-, Ets-, and Ik-like factors were identified by oligonucleotide competition and transcriptional function was demonstrated by co-transfection of Sp1, Ets, or Ik with disruption of the Ik-binding site. Specific Ik expression in rodent pituitary cells was identified by immunocytochemistry and confirmed in GH4 cells by Western blotting. 4 We show here for the first time the expression and subcellular localization of Ikaros and the Ik6 isoform in the human pituitary by multiple techniques. Our data indicate that expression of Ik6 and compartmentalization may be of critical functional importance in regulating FGFR4 promoter activity.
Expression of the non-DNA-binding isoform of Ik, Ik6, has been identified in approximately a third of cases of human T-cell acute leukemias. In our study, we detected the expression of Ik6 in human pituitary tumors. Expression of non-DNA-binding Ik isoforms such as Ik6 could result in dysregulated expression of target genes including FGFR4 that are essential for normal development and maturation. Our findings in the pituitary suggest that in contrast to Ik1, Ik6 interrupts activation of the 5′ FGFR4 promoter, consistent with a dominant-negative role for Ik6 in pituitary tumors. These data are also consistent with the notion that only Ik isoforms with DNA-binding domains when bound in cis-to Ik-binding sites are able to activate gene transcription. 8,12,21 Thus, the balance between DNA and non-DNA-binding Ik isoforms may contribute to a cell- and gene-specific response of either transcriptional activation or repression. Whether Ik6 has independent functions in addition to its ability to form inactive heterodimers with other Ikaros members remains to be shown. For example, another truncated Ik isoform (Ik7) appears to have the capacity to regulate the adhesion molecule L-selectin and migration in three-dimensional collagen gels in a cell-specific manner independent of Ik1. 22 Our finding of Ik6 in the cytoplasm further supports the potential for Ik1-independent function(s) for truncated Ik isoform action in pituitary tumors.
Protein acetylation plays a crucial role in regulating transcriptional activity. Acetylation complexes (such as CBP/p300) or deacetylation complexes (including HDAC) are usually recruited to DNA-bound transcription factors in response to signaling pathways. Histone hyperacetylation by histone acetyl-transferases (HATs) is generally associated with transcriptional activation, presumably by remodeling nucleosomal structure into an open conformation that is more accessible to transcription complexes. Conversely, HDAC recruitment is generally associated with transcriptional repression reversing the chromatin remodeling process. This gene repression can be cell-type- and promoter-specific. In the case of Ik it has been shown to be mediated through at least two repression domains that interact with the HDAC complexes containing mSin3 23 and Mi-2 proteins. 24 The earlier findings of histone under-acetylation in the vicinity of Ik recruitment sites 23 supported the significance of HDAC in mediating Ik action. Our findings of the ability of Ik1 to recruit an HDAC complex to the 5′ FGFR4 promoter supports this mechanism. However, we also demonstrate that the HDAC inhibitor trichostatin can activate this promoter, which is typical of the effect of histone acetylation on gene activation. Other groups have similarly noted such discordance between Ik-mediated gene activation and heterochromatin complex formation. 25 These apparently paradoxical findings can be reconciled by a model in which Ik1 is in direct association with its target genes in a predominantly restrictive chromatin environment. As previously proposed, 26 the heterochromatin compartment houses genes that are more tightly regulated, such as during the cell cycle or development. We propose that FGFR4 belongs to this category of tightly regulated genes that are typically repressed in many adult tissues but can be reactivated under unique conditions, presumably when specific activators and Ik are both present in the pericentromeric heterochromatin compartment. In this model, Ik functions as a potentiator by remodeling a densely packaged chromatin environment, facilitating activator access under unique conditions.
FGFR4 has been implicated as a modulator of erythroid cell 27 and pituitary cell proliferation. 3 We propose that FGFR4 represents an important target for Ik action in human pituitary tumors. Our data indicate that altered Ik splicing and isoform generation may contribute to an Ik isoform environment that can target FGFR4 promoter activity. This Ik1-mediated complex likely involves HDAC as well as non-HDAC components. The net influence of these Ik isoform/HDAC/non-HDAC complex interactions may ultimately disfavor utilization of the wild-type promoter over downstream cryptic promoters that may interact with other factors, such as AP-2α, 28 resulting in the genesis of tumorigenic truncated receptor isoforms such as ptd-FGFR4.
Acknowledgments
We thank Dr. K. Georgopoulos for the contribution of Ikaros antibody and expression vectors and Mr. Kelvin So for technical assistance.
Footnotes
Address reprint requests to Dr. Sylvia L. Asa, University Health Network, 610 University Ave., 4–302, Toronto, Ontario, Canada M5G 2M9. E-mail: sylvia.asa@uhn.on.ca.
Supported by the Canadian Institutes of Health Research (grant MT-14404 to S. E. and S. L. A.) and by the Toronto Medical Laboratories.
References
- 1.Asa SL, Ezzat S: The pathogenesis of pituitary tumours Nat Rev Cancer 2002, 2:836-849 [DOI] [PubMed] [Google Scholar]
- 2.Ezzat S, Smyth HS, Ramyar L, Asa SL: Heterogeneous in vivo and in vitro expression of basic fibroblast growth factor by human pituitary adenomas J Clin Endocrinol Metab 1995, 80:878-884 [DOI] [PubMed] [Google Scholar]
- 3.Ezzat S, Zheng L, Zhu XF, Wu GE, Asa SL: Targeted expression of a human pituitary tumor-derived isoform of FGF receptor-4 recapitulates pituitary tumorigenesis J Clin Invest 2002, 109:69-78 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Yu S, Asa SL, Ezzat S: Fibroblast growth factor receptor 4 is a target for the zinc-finger transcription factor Ikaros in the pituitary Mol Endocrinol 2002, 16:1069-1078 [DOI] [PubMed] [Google Scholar]
- 5.Molnar A, Wu P, Largespada DA, Vortkamp A, Scherer S, Copeland NG, Jenkins NA, Bruns G, Georgopoulos K: The Ikaros gene encodes a family of lymphocyte-restricted zinc finger DNA binding proteins, highly conserved in human and mouse J Immunol 1996, 156:585-592 [PubMed] [Google Scholar]
- 6.Georgopoulos K, Winandy S, Avitahl N: The role of the Ikaros gene in lymphocyte development and homeostasis Annu Rev Immunol 1997, 15:155-176 [DOI] [PubMed] [Google Scholar]
- 7.Georgopoulos K: Haematopoietic cell-fate decisions, chromatin regulation and Ikaros Nat Rev Immunol 2002, 2:162-174 [DOI] [PubMed] [Google Scholar]
- 8.Sun L, Liu A, Georgopoulos K: Zinc finger-mediated protein interactions modulate Ikaros activity, a molecular control of lymphocyte development EMBO J 1996, 15:5358-5369 [PMC free article] [PubMed] [Google Scholar]
- 9.Hahm K, Ernst P, Lo K, Kim GS, Turck C, Smale ST: The lymphoid transcription factor LyF-1 is encoded by specific, alternatively spliced mRNAs derived from the Ikaros gene Mol Cell Biol 1994, 14:7111-7123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hahm K, Cobb BS, McCarty AS, Brown KE, Klug CA, Lee R, Akashi K, Weissman IL, Fisher AG, Smale ST: Helios, a T cell-restricted Ikaros family member that quantitatively associates with Ikaros at centromeric heterochromatin Genes Dev 1998, 12:782-796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winandy S, Wu P, Georgopoulos K: A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma Cell 1995, 83:289-299 [DOI] [PubMed] [Google Scholar]
- 12.Molnar A, Georgopoulos K: The Ikaros gene encodes a family of functionally diverse zinc finger DNA-binding proteins Mol Cell Biol 1994, 14:8292-8303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun L, Heerema N, Crotty L, Wu X, Navara C, Vassilev A, Sensel M, Reaman GH, Uckun FM: Expression of dominant-negative and mutant isoforms of the antileukemic transcription factor Ikaros in infant acute lymphoblastic leukemia Proc Natl Acad Sci USA 1999, 96:680-685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortes M, Wong E, Koipally J, Georgopoulos K: Control of lymphocyte development by the Ikaros gene family Curr Opin Immunol 1999, 11:167-171 [DOI] [PubMed] [Google Scholar]
- 15.Winandy S, Wu L, Wang JH, Georgopoulos K: Pre-T cell receptor (TCR) and TCR-controlled checkpoints in T cell differentiation are set by Ikaros J Exp Med 1999, 190:1039-1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang JH, Nichogiannopoulou A, Wu L, Sun L, Sharpe AH, Bigby M, Georgopoulos K: Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation Immunity 1996, 5:537-549 [DOI] [PubMed] [Google Scholar]
- 17.Avitahl N, Winandy S, Friedrich C, Jones B, Ge Y, Georgopoulos K: Ikaros sets thresholds for T cell activation and regulates chromosome propagation Immunity 1999, 10:333-343 [DOI] [PubMed] [Google Scholar]
- 18.Asa SL, Ramyar L, Murphy PR, Li AW, Ezzat S: The endogenous fibroblast growth factor-2 antisense gene product regulates pituitary cell growth and hormone production Mol Endocrinol 2001, 15:589-599 [DOI] [PubMed] [Google Scholar]
- 19.Asa SL, Coschigano KT, Bellush L, Kopchick JJ, Ezzat S: Evidence for growth hormone (GH) autoregulation in pituitary somatotrophs in GH antagonist-transgenic mice and GH receptor-deficient mice Am J Pathol 2000, 156:1009-1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koipally J, Kim J, Jones B, Jackson A, Avitahl N, Winandy S, Trevisan M, Nichogiannopoulou A, Kelley C, Georgopoulos K: Ikaros chromatin remodeling complexes in the control of differentiation of the hemo-lymphoid system Cold Spring Harbor Symp Quant Biol 1999, 64:79-86 [DOI] [PubMed] [Google Scholar]
- 21.Wargnier A, Lafaurie C, Legros-Maida S, Bourge JF, Sigaux F, Sasportes M, Paul P: Down-regulation of human granzyme B expression by glucocorticoids. Dexamethasone inhibits binding to the Ikaros and AP-1 regulatory elements of the granzyme B promoter J Biol Chem 1998, 273:35326-35331 [DOI] [PubMed] [Google Scholar]
- 22.Christopherson I, Piechoki M, Liu G, Ratner S, Galy A: Regulation of L-selectin expression by a dominant negative Ikaros protein J Leukoc Biol 2001, 69:675-683 [PubMed] [Google Scholar]
- 23.Koipally J, Renold A, Kim J, Georgopoulos K: Repression by Ikaros and Aiolos is mediated through histone deacetylase complexes EMBO J 1999, 18:3090-3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J, Sif S, Jones B, Jackson A, Koipally J, Heller E, Winandy S, Viel A, Sawyer A, Ikeda T, Kingston R, Georgopoulos K: Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes Immunity 1999, 10:345-355 [DOI] [PubMed] [Google Scholar]
- 25.Sabbattini P, Lundgren M, Georgiou A, Chow C, Warnes G, Dillon N: Binding of Ikaros to the lambda5 promoter silences transcription through a mechanism that does not require heterochromatin formation EMBO J 2001, 20:2812-2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koipally J, Heller EJ, Seavitt JR, Georgopoulos K: Unconventional potentiation of gene expression by Ikaros J Biol Chem 2002, 277:13007-13015 [DOI] [PubMed] [Google Scholar]
- 27.Koritschoner NP, Bartunek P, Knespel S, Blendinger G, Zenke M: The fibroblast growth factor receptor FGFR-4 acts as a ligand dependent modulator of erythroid cell proliferation Oncogene 1999, 18:5904-5914 [DOI] [PubMed] [Google Scholar]
- 28.Yu SJ, Asa SL, Weigel RJ, Ezzat S: Pituitary tumor AP-2alpha recognizes a cryptic promoter in intron 4 of fibroblast growth factor receptor 4 J Biol Chem 2003, 278:19597-19602 [DOI] [PubMed] [Google Scholar]