Abstract
Little information is available regarding whether substance abuse enhances hepatitis C virus (HCV) replication and promotes HCV disease progression. We investigated whether morphine alters HCV mRNA expression in HCV replicon-containing liver cells. Morphine significantly increased HCV mRNA expression, an effect which could be abolished by either of the opioid receptor antagonists, naltrexone or β-funaltrexamine. Investigation of the mechanism responsible for this enhancement of HCV replicon expression demonstrated that morphine activated NF-κB promoter and that caffeic acid phenethyl ester, a specific inhibitor of the activation of NF-κB, blocked morphine-activated HCV RNA expression. In addition, morphine compromised the anti-HCV effect of interferon alpha (IFN-α). Our in vitro data indicate that morphine may play an important role as a positive regulator of HCV replication in human hepatic cells and may compromise IFN-α therapy.
Hepatitis C virus (HCV) is a positive-strand RNA virus of the flavivirus family, which was first molecularly cloned in 1989. 1 HCV has at least six distinct but related genotypes and more than 50 subtypes. Genotype 1 is the most common type found in the United States, while genotypes 2 and 3 are the most prevalent in Europe and Asia. Approximately 4 million people in the United States and 170 million people worldwide have been infected with HCV. 1-4 HCV often escapes clearance by the host’s immune system and leads to the establishment of a persistent infection in approximately 70% of infected individuals. 5,6 A subset of patients with chronic HCV infection develop cirrhosis, liver failure, and hepatocellular carcinoma. 7-9 Treatment of HCV infection with interferon alpha (IFN-α) and ribavirin is associated with a sustained response rate of less than 50%. 6,10,11 The limited therapeutic efficacy of available treatments and the absence of an effective HCV vaccine to prevent HCV infection underscore the importance of extensive studies on the immunopathogenesis of HCV disease.
Injection drug users (IDUs) are the single largest group at risk for HCV infection. 12-14 The rates of HCV infection among past and current IDUs are extremely high, ranging from 70% to over 90% (antibody positive for HCV) in the United States. 15-19 The institution of bloodbank screening measures in developed countries has greatly decreased the risk of transfusion-associated hepatitis; however, new cases continue to occur mainly as a result of injection drug use that frequently includes abuse of opiates. Although it is known that injection drug use contributes significantly to HCV transmission, there is little information available regarding whether drug abuse (in particular opioid abuse) enhances susceptibility to HCV infection in HCV-seronegative individuals or adversely affects HCV disease in HCV-infected IDUs by increasing HCV replication and/or promoting HCV disease progression. Lack of knowledge about the impact of drug abuse on HCV disease is a major barrier to fundamental understanding of HCV-related mobility and mortality among drug abusers and to developing new therapeutic approaches. Thus, it is critical to investigate the impact of drugs of abuse on HCV replication in the target host cells, in particular, liver cells.
Although in vitro HCV replication is extremely robust (10 trillion virion particles per day), 20 growing HCV in in vitro cell culture systems has been found to be very difficult. Although there have been a variety of HCV genome-containing cell culture systems established, the expression HCV RNA in these cultures is low and unstable. 21 Recent genetic manipulations of the RNA of HCV virions have produced high levels of replication in cell lines derived from hepatocytes (Huh7), offering a more feasible means to study viral RNA and protein synthesis. 22,23 The establishment of a subgenomic replicon system is an important advance in the investigation of molecular biology of HCV replication, 21-23 and provides the first effective model cell system for the study of the dynamics of virus replication. 21 The HCV replicon system has been used successfully to examine the anti-HCV effect of IFN-α. 23,24 In this study, we investigated whether morphine, the active metabolite of heroin, affects HCV replicon expression and compromises the anti-HCV effect of IFN-α in HCV replicon-containing hepatic cells.
Materials and Methods
Reagents
The following reagents were used in the reported experiments. Morphine sulfate injectable solution (15 mg/ml) was purchased from Elkins-Sinn, Inc. (Cherry Hill, NJ). Naltrexone, the opiate receptor antagonist, was obtained from Sigma-Aldrich (St. Louis, MO). β-funaltrexamine, a second opiate receptor antagonist, was purchased from Tocris Cookson Inc. (Ballwin, MO). Recombinant human IFN-α and the antibodies against IFN-α and IFN-γ were obtained from R & D Systems Inc. (Minneapolis, MN). Caffeic acid phenethyl ester (CAPE) was purchased from Calbiochem-Novabiochem Corp. (San Diego, CA).
Cell Lines
Huh.8 and Huh7 cells were obtained from Dr. Charles Rice (Washington University School of Medicine and Apath, L.L.C., St. Louis, MO). FCA-1 cells were obtained from Dr. Christoph Seeger (Fox Chase Cancer Center, Philadelphia, PA). Huh7, the parental cell line of Huh.8 and FCA-1, is derived from a human hepatoma. 25 Huh.8 is a cell clone containing a G418-selectable HCV RNA replicon with wild-type HCV nonstructural protein NS5A sequence. 23 The replicon in Huh.8 cells contains both the 5′ NTR and 3′ NTR as well as the open reading frame of the nonstructural proteins NS3–5B. 23 Southern blots are negative for replicon nucleic acid integrated into the host chromosome in Huh.8 cells. 23 FCA-1 cells contain a subgenomic replicon from a known infectious HCV clone 24 with several consensus mutations in NS3 as well as in NS5A (NS3: E177G; NS5A: D1229E, I1299V). 24 Huh.8, FCA-1, and Huh7 cells were maintained in T75 flasks (Corning Incorporated, Corning, NY) containing Dulbecco’s modified Eagle’s medium (Gibco-BRL, Grand Island, NY) supplemented with 10% (v/v) fetal bovine serum (HyClone, Logan, UT), 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mmol/L L-glutamine, and 0.1 mmol/L minimum essential medium non-essential amino acids. The flasks were maintained in a humidified atmosphere of 95% air and 5% CO2 at 37°C. The culture medium for Huh.8 and FCA-1 cells contained G418 (750 μg/ml). Cells were passed every 2 or 3 days and seeded in 24-well plates (10 5 cells/well) or 96-well plates (2 × 104 cells/well). NT2-N cells were derived from NTera2/cl.D1 (NT2) cells, a human teratocarcinoma cell line, and consisted of >95% neuronal cells. 26 The total RNA extracted from NT2-N cells was used as a positive control for μ-opioid receptor mRNA. 27
Morphine Treatment
Huh.8 and FCA-1 cells were plated in 96-well plates and incubated with or without morphine (10−10 ∼ 10−6 mol/L) for up to 96 hours. The selection of morphine concentrations used in the experiments was based on the experience of the investigators and on the published literature. Morphine concentrations ranging from 10−6 to 10−16 mol/L have been used in both in vitro and ex vivo model systems by many investigators. 28-30 This range is consistent with reported therapeutic serum concentrations of morphine and methadone in patients which range from 10−8 to 10−6 mol/L. 28 At concentrations of 10−6 mol/L or lower, there was no cytotoxic effect of morphine on Huh.8, Huh7, and FCA-1 cells, as demonstrated by trypan blue dye staining. This result is in agreement with findings by others which have demonstrated that morphine at concentrations of 10 nmol/L or less did not produce irreversible damage to human hepatocytes. 31 For multiple time-point experiments, cells were re-treated with morphine every 24 hours. In the experiments requiring combination treatment of cells with morphine and an opioid antagonist, naltrexone (10−8 mol/L) or β-funaltrexamine (10−8 mol/L) was added to the culture 30 minutes before the addition of morphine (10−6 mol/L). To determine whether morphine interferes with the anti-HCV effect of IFN-α, Huh.8 and FCA-1 cells were incubated with morphine (10−6 mol/L) and/or IFN-α (100 U/ml). We selected the tested dose of IFN-α to be sufficient to have an anti-HCV effect in the HCV replicon-containing cells. 23,24 In the experiments to investigate whether CAPE, a specific NF-κB inhibitor, suppressed morphine’s effect on HCV RNA expression, Huh.8 cells were incubated with CAPE (25 μg/ml) for 30 minutes before morphine treatment. The selected concentration of CAPE was based on our pilot experiments which showed that CAPE, at concentrations of 35 μg/ml or less, had no cytotoxic effect on Huh.8 cells.
RNA Extraction
Total RNA (1 μg) was extracted from Huh.8, FCA-1, Huh7, and NT2-N cells using Tri-Reagent (Molecular Research Center, Cincinnati, OH). In brief, total RNA was extracted by a single-step guanidium thiocyanate-phenol-chloroform extraction. After centrifugation at 13,000 × g for 15 minutes, the RNA-containing aqueous phase was precipitated in isopropanol. RNA precipitates were then washed once in 75% ethanol and re-suspended in 30 μl of RNase-free water.
RT-PCR for μ-Opioid Receptor mRNA
Total RNA (1 μg), extracted from Huh.8, FCA-1, Huh7, or NT2-N cells, was subjected to reverse transcription using the Reverse Transcription System (Promega, Madison, MI) with primer (antisense, see below) specific for μ-opioid receptor for 1 hour at 42°C. The reaction was terminated by incubating the reaction mixture at 99°C for 5 minutes. One-tenth of the resulting cDNA was used as a template for PCR amplification. The PCR amplification was performed for 40 cycles with AmpliTaq Gold (PerkinElmer, Norwalk, CT) in a GeneAmp PCR System 2400 (PerkinElmer). The PCR reaction mixture contained 0.2 mmol/L of dNTPs, 20 pM each of two primers and 1.5 U of AmpliTaq Gold in 1X reaction buffer. The specific μ-opioid receptor primer pair is 5′-GGTACTGGGAAAACCTGCTGAAGATCTGTG-3′ (sense) and 5′-GGTCTC-TAGTGTTCTGACGAATTCGAGTGG-3′ (antisense) and the anticipated PCR product is 441 bp. 32 The primers were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). The PCR program consisted of heat activation of AmpliTaq Gold for 9 minutes at 95°C followed by 40 cycles of 95°C for 45 seconds, 48°C for 45 seconds, and 72°C for 90 seconds, and further elongation at 72°C for 7 minutes. One-fifth (10 μl) of each PCR-amplified cDNA product was electrophoresed on 2% ethidium-bromide-stained agarose gels.
Sequencing Analysis
Sequence analysis is an essential step for independent verification of the identity of PCR-amplified products and confirmation of lack of cross-contamination in PCR assays. Thus, we analyzed the specificity of the PCR-amplified μ-opioid receptor products by sequence analysis. PCR amplified μ-opioid receptor products visualized in 2% agarose gels by ethidium bromide staining were isolated and purified using the Wizard PCR Preps DNA Purification System (Promega). Direct sequencing analysis then was performed by the Nucleic Acid Protein Core Facility at The Children’s Hospital of Philadelphia. GenBank was used for the nucleotide sequence homology search.
Real-Time RT-PCR for HCV RNA Quantification
We used a real-time reverse transcription-polymerase chain reaction (RT-PCR) assay, newly developed in our laboratory, for the quantification of HCV RNA. 33 Cell lysates (4 μl) were added directly to the RT mixture that contained 50 mmol/L KCl, 10 mmol/L Tris-HCI (pH 9.0), 4 mmol/L MgCl2, 0.1% Triton X-100, 20 U of RNasin ribonuclease inhibitor (Promega), 2 pM of an HCV-specific antisense primer (AS311, see below for sequences), 200 μmol/L dNTPs, and 7.5 U of avian myeloblastosis virus reverse transcriptase (Promega) in a final volume of 20 μl. The resultant cDNA was amplified by PCR for 40 cycles. The ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA) was used for real-time analysis. We designed the primer pair (S130/AS311), which is specific for the recognition of the highly conservative 5′-noncoding region (5′-NCR) in HCV genome. 33 These sequences were: 5′-CGGGAGAGCCATAGTGGTCTGCG-3′ (S130) and 5′-CTCGCAAGCACCCTATCAGGCAGTA-3′ (AS311). The probe (molecular beacon, MB) sequence was selected within the primer pair, S130 and AS311, which was designed to be perfectly complementary to the target sequence in 5′-NCR of HCV genome. The following is the sequence of the MB: 5′-FAM-GCGAGCCACCGGAATTGCCAGGACGACC-GCTCGCDABCYL-3′. The stem sequence (underlined) of the MB does not complement the sequences within the loop region. The length of the MB was designed to withstand a slightly higher annealing temperature compared with that of the primers. The MB was labeled at the 5′ end with 6-FAM and the quencher 4-(4′-dimethylaminophenylaso) benzoic acid (DABCYL) at the 3′ end. Both primers and MB were suspended in TE buffer, and stored at −30°C. Thermal cycling conditions were designed as follows: initial denaturation at 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds, and 60°C 60 seconds. Fluorescent measurements were recorded during each annealing step. At the end of each PCR run, data were automatically analyzed by the system and amplification plots were generated. For each PCR reaction, 2 μl of cDNA template was added to 48 μl of PCR Master mixture (5 μl of 1X PCR buffer II, 5 mmol/L MgCl2, 300 nmol/L dNTP, 400 nmol/L of each primer, 1.5 U of AmpliTaq Gold DNA polymerase, 400 nmol/L of molecular beacons, and 24.7 μl of water). The PCR buffer contained 5-carboxy-X-rhodamine (5-ROX) (500 nmol/L) as the reference dye for normalization of the reactions. Any possible fluctuations in ROX signal are used to correct the sample signal. All amplification reactions were performed in triplicate. A standard curve was generated with 10-fold dilutions of HCV 5′-NCR RNA control that had been pre-quantified by a spectrophotometer (Eppendorf Scientific Inc., Westbury, NY). GAPDH RNA expression is used to normalize the RNA concentration in each sample tested.
Immunoblot Assay
Total cell lysates were prepared from Huh.8 cells (105 cells/well in a 24-well plate) treated with or without morphine (10−6 mol/L) using a lysis buffer (Promega). The protein concentration was determined by detergent-compatible (DC) protein assay kit (Bio-Rad, Hercules, CA). The immunoblot analysis of HCV NS5 protein was performed using a Bio-Dot SF apparatus as described by the manufacturer (Bio-Rad). Briefly, total protein (0.5 μg) extracted from Huh.8 cells and treated with or without morphine (10−6 mol/L) was applied onto a nitrocellulose (NC) membrane. After being blocked with PBS containing 5% nonfat dry milk for 1 hour at room temperature, the membrane was incubated with a mouse monoclonal anti-HCV NS5 antibody (a gift from Dr. Bill Sun of Thomas Jefferson University, Philadelphia, PA) at 4°C overnight. After being washed three times with PBS, the NC membrane was incubated with horseradish peroxidase-conjugated goat anti-mouse IgG for 1 hour. Bound antibody was visualized by developing the membrane in SuperSignal West Pico Chemiluminescent Substrate Kit (PIERCE, Rockford, IL). The results were recorded on film (Eastman Kodak, Rochester, NY).
NF-κB Promoter Activation Assay
We used the plasmid (pNF-κB-Luc) containing NF-κB promoter linked with a luciferase gene that was developed by Dr. Petrak (Laboratory of Immune Cell Biology, National Cancer Institute, National Institutes of Health, Bethesda, MD). 34 Two copies of the mouse κ light chain enhancer 35 were cloned into pBLCAT3 vector, 36 and then the construct was modified by replacing the CAT reporter with the luciferase gene obtained from pGEM-Luc. 34 Plasmid DNA was prepared by Miniprep techniques, according to the manufacturer’s instruction (Wizard Plus Minipreps, Promega) and used in the transfection experiments. For each transfection experiment, the Huh.8 and Huh7 cells were seeded in a 6-well tissue culture plate at the density of 3 × 105 cells/well 1 day before the transfection. The cells were transfected with the pNF-κB-Luc using FuGENE 6 Transfection Reagent (Roche Molecular Bilchemicals, Indianapolis, IN) with a ratio of FuGENE 6: plasmid 6:1 (μl:μg). Six hours after the transient transfection, the cells were incubated with or without morphine (10−6 mol/L) for 24 hours. At the termination of the experiments, cells were harvested and washed twice with PBS by centrifugation at 3,300 × g for 3 minutes at room temperature. The cell pellets were lysed with 0.25 ml of 1X Reporter Lysis Buffer (Promega) and a cycle of freezing and thawing in dry ice. Cell-free lysates were obtained by centrifugation at 10,000 × g for 30 seconds at room temperature. The effects of morphine on the activation of NF-κB promoter in these transiently transfected cells were determined by NF-κB promoter-driven luciferase activity. Luciferase activity in cell lysate (50 μl/sample) was quantified using a Luciferase Assay System (Promega) and a Luminometer. The results were presented as relative light units.
Statistical Analysis
All variables were tested in triplicate and experiments were repeated at least three times. Triplicate wells had variability of less than 15%. One-way analysis of variance was used to test for differences in means and a posthoc t-test was used for comparisons. Differences were considered significant if P < 0.05.
Results
Huh.8, FCA-1, and Huh7 Cells Express μ-Opioid Receptor
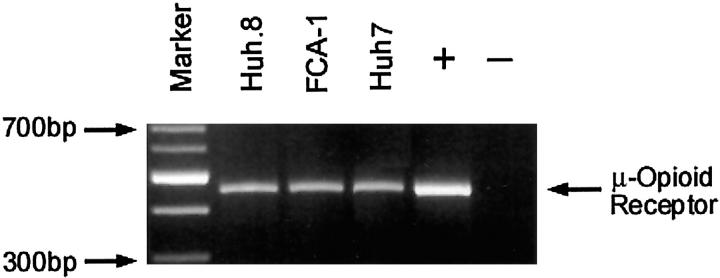
The biological functions of morphine are mediated by μ-opioid receptors. Thus, it is important to determine whether Huh.8, FCA-1, and Huh7 cells express μ-opioid receptors. Since the liver produces endogenous opiates and expresses their receptors, 37,38 we examined whether μ-opioid receptors also are present in Huh.8, FCA-1, and Huh7 cells. We performed a conventional RT-PCR assay using a primer pair specific for μ-opioid receptor detection in Huh.8, FCA-1, and Huh7 cells. Ethidium bromide staining of RT-PCR-amplified products from Huh.8, FCA-1, and Huh7 cells showed a visible 441-bp band that was identical to the band amplified from RNA isolated from NT2-N cells, a human neuronal cell line (Figure 1) ▶ .
Figure 1.
μ-Opioid receptor expression in Huh.8, FCA-1, and Huh7 cells. RT-PCR analysis of μ-opioid receptor gene expression. Total RNA was extracted from Huh.8, FCA-1, and Huh7 cells and subjected to RT-PCR using specific primers for μ-opioid receptor. Sizes are estimated from DNA ladder (100-bp fragments) co-electrophoresed as markers. Lane 1: Markers; lane 2: Huh.8 cells; lane 3: FCA-1 cells; lane 4: Huh7 cells; lane 5: NT2-N cells as a positive control (+); lane 6: negative control (-, the same cells as used in the positive control but processed without reverse transcriptase).
Effect of Morphine on HCV Replicon Expression
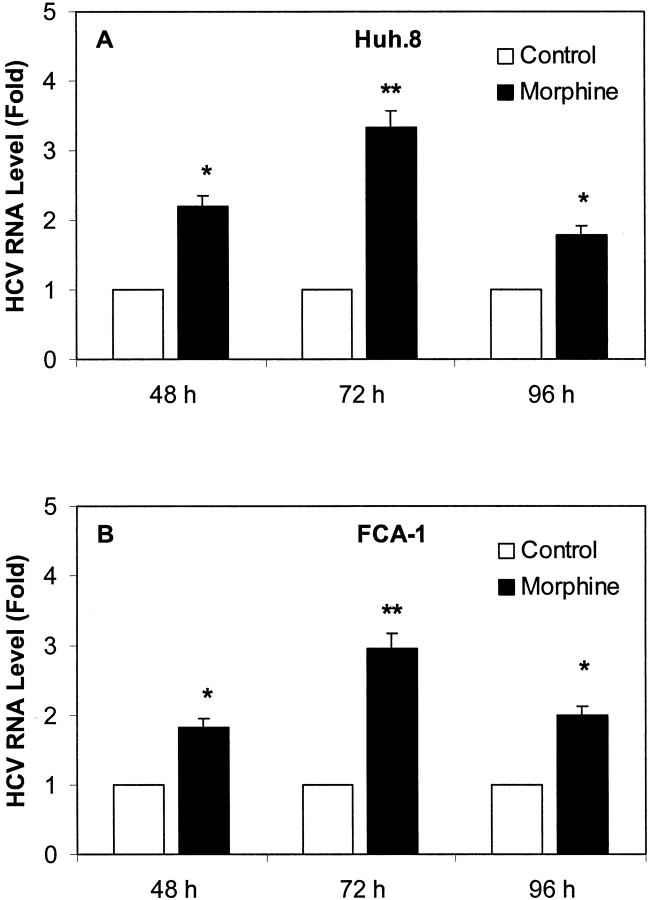
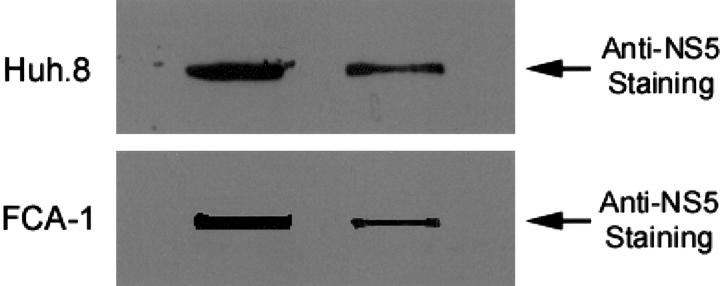
To evaluate the effect of morphine on HCV RNA expression in Huh.8 and FCA-1 cells, we incubated these cells with or without morphine at different concentrations (10−10 to 10−6 mol/L). Cell lysates then were subjected to real-time RT-PCR for the analysis of HCV RNA levels at 72 hours after morphine treatment. The addition of morphine to Huh.8 and FCA-1 cells enhanced HCV RNA expression in a concentration-dependent fashion (Figure 2) ▶ . To determine whether the effect of morphine on HCV RNA expression was time-dependent, we incubated Huh.8 and FCA-1 cells with morphine (10−6 mol/L) and collected cells lysates for HCV RNA quantification at 3 different time points (48 hours, 72 hours, and 96 hours) post morphine treatment. The maximum HCV RNA expression in morphine-treated Huh.8 cells was observed at 72 hours post treatment (Figure 3) ▶ . Because NS5 protein plays a critical role in HCV replication, we also examined whether morphine altered HCV NS5 protein expression in Huh.8 and FCA-1 cells using an immunoblot assay. Compared with untreated Huh.8 and FCA-1 cells, morphine (10−6 mol/L)-treated cells expressed higher levels of NS5 protein, as demonstrated by the enhanced intensity of the protein band (Figure 4) ▶ .
Figure 2.
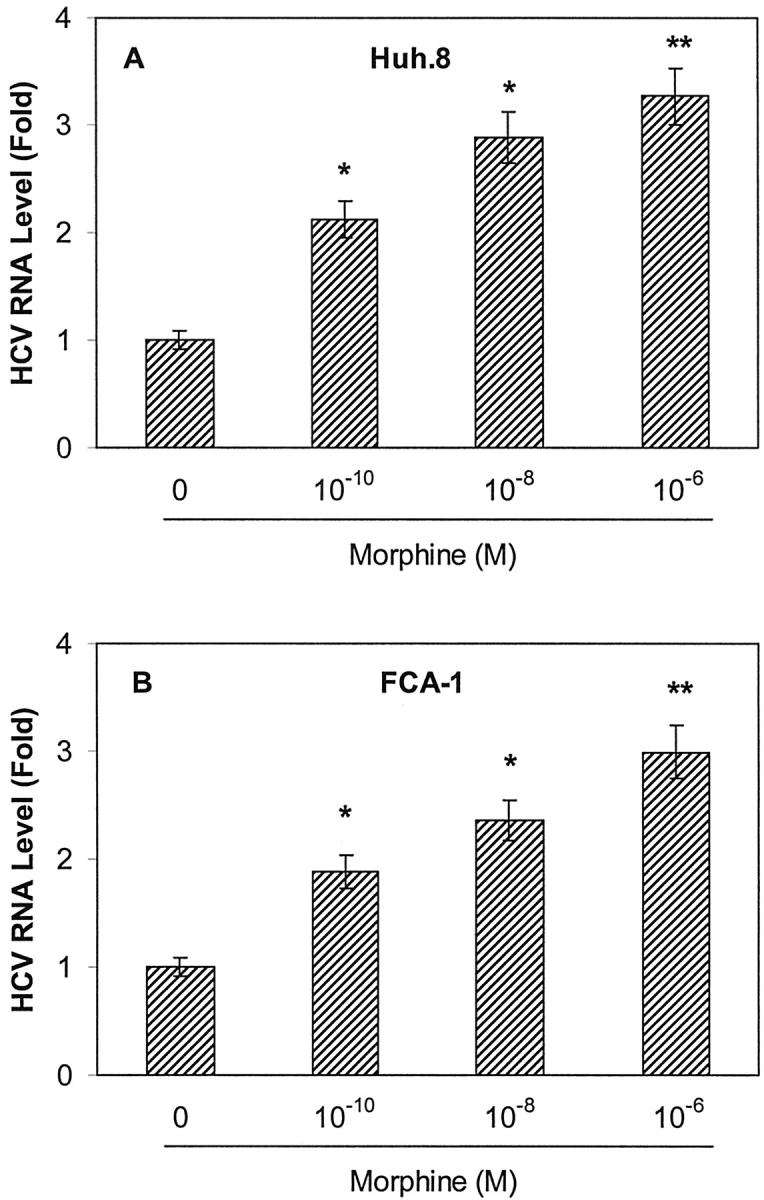
Effect of morphine on HCV RNA expression in Huh.8 (A) and FCA-1 (B) cells. Huh.8 and FCA-1 cells (2 × 104 cells/well) in a 96-well plate were incubated with or without morphine at indicated concentrations for 72 hours and cell lysates were then subjected to real-time RT-PCR for HCV and GAPDH RNA quantification. The data are expressed as HCV RNA levels relative (Fold) to untreated control, which is defined as 1.0. The results shown are the mean ± SD of triplicate cultures, representative of five independent experiments (*, P < 0.05; **, P < 0.01).
Figure 3.
Effect of morphine on HCV RNA expression in Huh.8 (A) and FCA-1 (B) cells at different time points. Huh.8 cells and FCA-1 (2 × 104 cells/well) in a 96-well plate were incubated with or without morphine at 10−6 mol/L. Cell lysates were then subjected to real-time RT-PCR for HCV and GAPDH RNA quantification at the indicated time points post-treatment. The data are expressed as HCV RNA levels relative (Fold) to untreated control, which is defined as 1.0. The results shown are the mean ± SD of triplicate cultures, representative of five independent experiments (*, P < 0.05; **, P < 0.01).
Figure 4.
Effect of morphine on HCV NS5 protein expression in Huh.8 (A) and FCA-1 (B) cells. Huh.8 and FCA-1 cells (105 cells/well) plated in a 24-well plate were incubated with or without (Control) morphine at 10−6 mol/L for 72 hours. Cell lysates were quantified by DC protein assay kit. Equal amounts (0.5 μg) of protein extracted from treated and untreated Huh.8 and FCA-1 cells were applied onto a NC membrane for immunoblot assay. The results were recorded on the film (2 minutes exposure). One representative result from three experiments is shown.
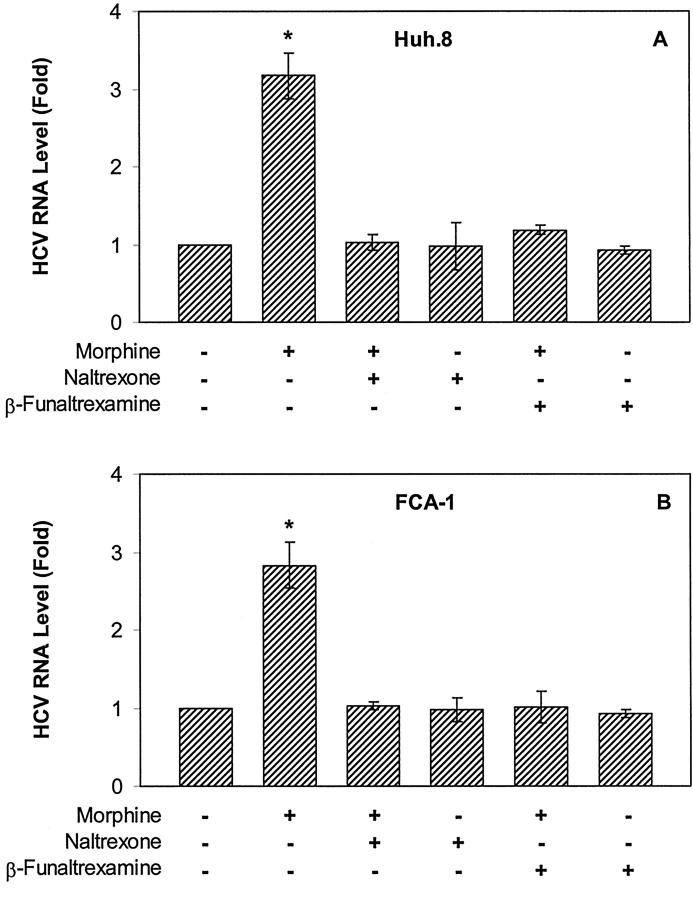
Opioid Receptor Antagonists Abrogate Morphine’s Effect on HCV Replicon Expression
Since we were able to demonstrate that μ-opioid receptor is present in Huh.8 and FCA-1 cells (Figure 1) ▶ , we hypothesized that the effect of morphine on HCV RNA expression in these cells is mediated through the μ-opioid receptor. To test this hypothesis, we first pre-treated the Huh.8 and FCA-1 cells with naltrexone, the pan-opioid receptor antagonist, for 30 minutes before the addition of morphine. Naltrexone completely abrogated the enhancing effect of morphine on HCV RNA expression, while naltrexone alone had little effect on HCV RNA expression (Figure 5) ▶ . Since morphine has a high affinity for the μ-opioid receptor, we examined whether the μ-opioid receptor was responsible for this morphine-mediated action. Thus, we tested the effect of β-funaltrexamine, a specific μ-opioid receptor antagonist, on HCV RNA expression. β-funaltrexamine completely blocked the morphine-mediated up-regulation of HCV RNA expression in Huh.8 and FCA-1 cells, while the addition of β-funaltrexamine alone into Huh.8 and FCA-1 cell cultures did not affect HCV RNA expression (Figure 5) ▶ .
Figure 5.
Antagonizing effect of naltrexone or β-funaltrexamine on morphine-induced HCV RNA expression in Huh.8 (A) and FCA-1 (B) cells. Huh.8 cells (2 × 104 cells/well) plated in a 96-well plate were incubated with naltrexone (10−8 mol/L) or β-funaltrexamine (10−8 mol/L) for 30 minutes before the addition of morphine (10−6 mol/L). Cell lysates then were subjected to real-time RT-PCR for HCV and GAPDH RNA quantification 72 hours after morphine treatment. The data are expressed as HCV RNA levels relative (Fold) to untreated control, which is defined as 1.0. The results shown are the mean ± SD of triplicate cultures, representative of five independent experiments (*, P < 0.01).
Morphine Compromises the Anti-HCV Effect of IFN-α
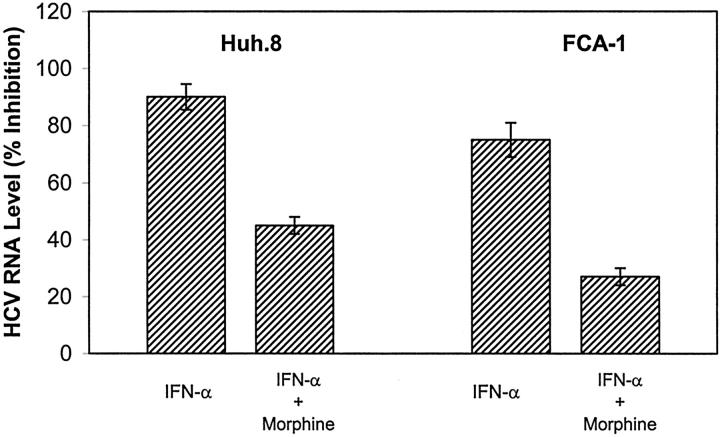
Anti-HCV therapy with IFN-α is ineffective in about 70% of treated patients. 39-41 The mechanism responsible for the failure of IFN-α to inhibit HCV replication is largely unknown. Since IDUs are the primary group at risk for HCV infection, 12-14 it is important to understand whether abuse of injection drugs such as opioids plays a role in resistance to IFN-α therapy. Thus, we examined whether morphine had a negative impact on the anti-HCV effect of IFN-α in Huh.8 and FCA-1 cells. As expected, IFN-α, when added to Huh.8 and FCA-1 cell cultures, significantly inhibited HCV RNA expression in both Huh.8 and FCA-1 cells (Figure 6) ▶ . The inhibitory effect of IFN-α on HCV RNA expression in these cells, however, was significantly compromised by morphine (Figure 6) ▶ .
Figure 6.
Effect of morphine on the anti-HCV activity of IFN-α in Huh.8 and FCA-1 cells. Huh.8 and FCA-1 cells (2 × 104 cells/well) in a 96-well plate were incubated with or without morphine (10−6 mol/L) and/or IFN-α (100 U/ml) for 72 hours. Cell lysates then were subjected to real-time RT-PCR for HCV and GAPDH RNA quantification. The data are expressed as HCV RNA levels (% inhibition) relative to untreated control that is defined as 0. The results shown are the mean ± SD of triplicate cultures, representative of five independent experiments.
Morphine Activates NF-κB
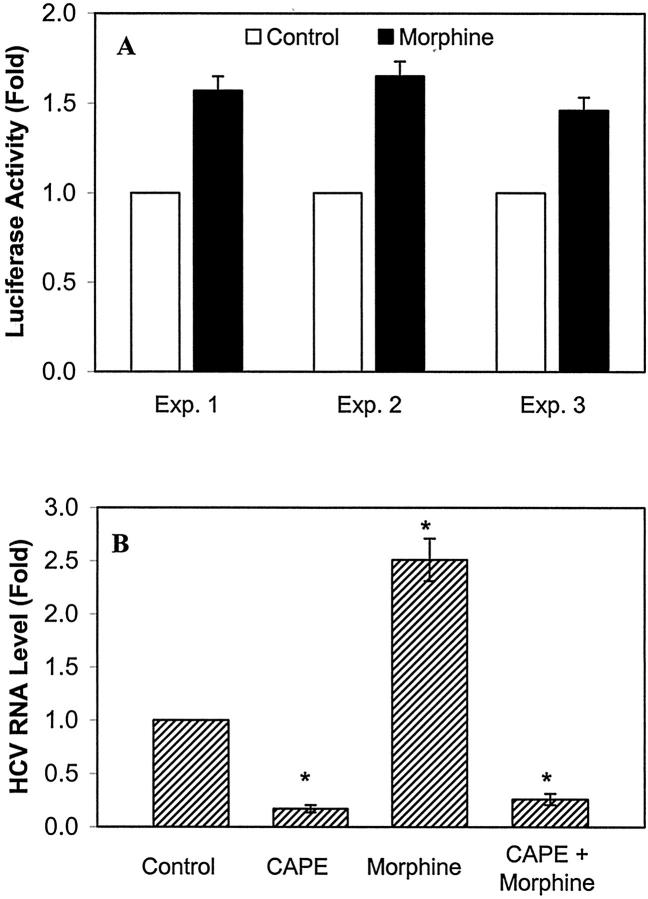
To investigate the possible mechanism(s) responsible for morphine-mediated up-regulation of HCV RNA expression, we examined whether morphine activates NF-κB, an important nuclear transcription factor that controls viral replication and cytokine production. 42-44 Huh7 cells (the parental cell line for Huh.8 and FCA-1) were transfected with the plasmid (pNF-κB-Luc) that contains NF-κB promoter linked to a luciferase gene. The transfected Huh7 cells then were incubated with or without morphine. As shown in the Figure 7A ▶ , morphine enhanced the NF-κB promoter-directed luciferase activity in Huh7 cells. To determine further whether NF-κB was directly involved in morphine-induced HCV RNA expression, we examined whether CAPE, a specific NF-κB inhibitor, blocked morphine’s action on Huh.8 cells. CAPE not only inhibited HCV RNA expression in Huh.8 cells but also abolished the enhancing effect of morphine on HCV RNA expression (Figure 7B) ▶ .
Figure 7.
The role of NF-κB in morphine-mediated HCV RNA expression. A: Effect of morphine on NF-κB promoter in pNF-κB-Luc-transfected Huh7 cells. Huh7 cells in 6-well plate were transfected with pNF-κB-Luc for 24 hours and then incubated with or without morphine (10−6 mol/L) for 24 hours. NF-κB promoter-directed luciferase activity quantitated from the cell-free lysates were normalized using the total protein of the cell-free lysates. The data are means of triplicate cultures ± SD, representative of three independent experiments. B: Effect of CAPE on morphine-enhanced HCV RNA expression in Huh.8 cells. Huh.8 cells (2 × 104 cells/well) in a 96-well plate were incubated with or without CAPE (25 μg/ml) and/or morphine (10−6 mol/L). CAPE was added to the cell cultures 30 minutes before the addition of morphine. Cell lysates were then subjected to real-time RT-PCR for HCV and GAPDH RNA quantification 72 hours after morphine treatment. The data are expressed as HCV RNA levels relative (Fold) to untreated control, which is defined as 1. The results shown are the mean ± SD of triplicate cultures, representative of five independent experiments (*, P < 0.01).
Discussion
In this study, we examined the overall hypothesis that opioids play a role as a cofactor in promoting HCV replication in liver cells. We also investigated whether morphine has a negative effect on the therapeutic, anti-HCV effect of IFN-α, the only cytokine approved for HCV therapy. We used recently developed HCV replicon-containing cell lines for this study. Huh.8, derived from a human hepatoma cell line (Huh7), is a cell clone containing a G418 selectable HCV RNA replicon with wild-type HCV nonstructural protein NS5A sequence. 23 We also used the FCA-1 line 24 that also was generated from Huh7 cells. FCA-1 cells containing a subgenomic replicon derived from a known infectious HCV clone also were used. 24 Since FCA-1 cells were established for testing the mechanism(s) of the IFN-α response against HCV, 24 we investigated whether morphine has the ability to compromise the anti-HCV activity of IFN-α in these cells. It should be noted that, although these cell clones mimic only some aspects of HCV replication, they offer an important means to study viral RNA and protein synthesis. 21-23 The HCV replicon system has been used successfully to examine the anti-HCV effect of IFN-α, 23,24 although the mechanism responsible for therapeutic action of IFN-α in this system is still obscure. 24
Since opioids exert their biological functions through the activation of opioid receptors, it is important to determine whether liver cells express the μ-opioid receptor. In this study, we demonstrated that both Huh.8, FCA-1, and Huh7 cells expressed μ-opioid receptor mRNA (Figure 1) ▶ . Our finding is in agreement with other reports showing that the liver produces endogenous opiates and expresses μ− and δ-opioid receptors. 37,38 Because of low level expression of membrane opioid receptor on non-neuronal cells and the lack of commercially available antibody that is appropriate for detecting surface μ-opioid receptor by immunofluorescence or Western blot assays, we were unable to determine whether Huh.8 and Huh7 cells express μ-opioid receptor at the protein level. To determine whether opioids such as morphine act through a biological receptor-mediated mechanism to promote HCV replication, we examined whether naltrexone, a pan-opioid receptor antagonist, blocked the promoting effect of morphine on HCV RNA expression in Huh.8, and FCA-1 cells (Figure 5) ▶ . Since morphine has a high affinity and selectivity for the μ-opioid receptor, 45-47 we further investigated whether μ-opioid receptor mediates morphine’s action. We examined whether β-funaltrexamine, a specific μ-opioid receptor antagonist, blocks morphine-mediated HCV RNA up-regulation. Our data showed that β-funaltrexamine abrogated the enhancing effect of morphine on HCV RNA expression in both Huh. 8 and FCA-1 cells (Figure 5) ▶ . These data strongly support the notion that the μ-opioid receptor mediates morphine-induced HCV RNA expression in HCV replicon-containing cells.
We further investigated the possible mechanism(s) responsible for morphine-mediated up-regulation of HCV RNA expression. Since NF-κB is a critical nuclear transcription factor involved in the activation of viral replication and cytokine production, 42-44 we investigated whether morphine, through the activation of NF-κB, enhances HCV expression in Huh.8 cells. We first examined whether morphine activates NF-κB promoter. Because Huh.8 cells contain a HCV replicon that may affect NF-κB promoter activity, we used the parental cell line (Huh7) of Huh.8 to define the role of morphine in the activation of NF-κB promoter. We demonstrated that morphine enhanced NF-κB promoter-directed luciferase activity in Huh7 cells (Figure 7A) ▶ . We further confirmed that NF-κB is directly involved in morphine-mediated up-regulation of HCV RNA expression by demonstrating that CAPE, a specific inhibitor for NF-κB, 48 could block the enhancing effect of morphine on HCV RNA expression. We found that CAPE not only inhibited HCV RNA expression in Huh.8 cells but also abolished the enhancing effect of morphine on HCV RNA expression (Figure 7B) ▶ . Our findings indicate that NF-κB activation may be critical in regulation of HCV RNA expression in Huh.8 cells and may be one of the mechanisms responsible for morphine’s action. Further investigation into mutual interactions between NF-κB expression and HCV replication will provide insight into factors that control HCV gene expression and NF-κB activation.
The HCV subgenomic replicon-containing cell line is an excellent in vitro model 24 to investigate whether opioids such as morphine interfere with IFN-α treatment. Clinical trials indicate a therapeutic benefit of IFN-α treatment in chronic HCV infection. 49,50 In fact, IFN-α, with or without ribavirin, is the only compound approved for the treatment of chronic HCV infection. IFN-α has been shown to inhibit HCV RNA expression in Huh.8 cells as demonstrated by a reduction in HCV RNA expression over time. 23 IFN-α also reduced HCV replicon expression in FCA-1 cells. 24 Our data (Figure 6) ▶ showing that IFN-α significantly inhibited HCV RNA expression in both Huh.8 and FCA-1 cells confirm these observations. The currently available combination therapy with IFN-α and ribavirin is effective in less than 50% of treated subjects. 6,10,11 The mechanism responsible for resistance against IFN-α therapy is not understood. Thus, it is important to identify factor(s) responsible for the failure of IFN-α treatment in IDUs, the subgroup of the population that comprise the single largest risk group for HCV infection. 12-14 We hypothesized that morphine may have a negative impact on the anti-HCV effect of IFN-α. Our data, showing that morphine compromised the anti-HCV effect of IFN-α in both Huh.8 and FCA-1 cells (Figure 6) ▶ , supported this hypothesis. These in vitro observations suggest that abuse of drugs such as heroin, or therapeutic use of morphine, may undermine the anti-HCV effect of IFN-α-based therapy in vivo. Further epidemiological studies are necessary to confirm the in vivo impact of opioid abuse on the development of chronic HCV infection and resistance to IFN-α therapy.
Acknowledgments
We thank Dr. Charles Rice for generously providing us with the Huh.8 and Huh7 cell lines. We also are very grateful to Dr. Christoph Seeger for providing the FCA-1 cells for this study.
Footnotes
Address reprint requests to Dr. Wen-Zhe Ho, Division of Immunologic and Infectious Diseases, The Children’s Hospital of Philadelphia, University of Pennsylvania School of Medicine, 34th Street & Civic Center Boulevard, Philadelphia, PA 19104. E-mail: ho@email.chop.edu.
Supported by National Institutes of Health grants DA-12815 and DA-16022 (to W. Z. H.) and MH-49981 and AA-13547 (to S. D. D.).
References
- 1.Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M: Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 1989, 244:359-362 [DOI] [PubMed] [Google Scholar]
- 2.Kuo G, Choo QL, Alter HJ, Gitnick GL, Redeker AG, Purcell RH, Miyamura T, Dienstag JL, Alter MJ, Stevens CE: An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science 1989, 244:362-364 [DOI] [PubMed] [Google Scholar]
- 3.Strader DB, Seeff LB: The natural history of chronic hepatitis C infection. Eur J Gastroenterol Hepatol 1996, 8:324-328 [DOI] [PubMed] [Google Scholar]
- 4.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, Kaslow RA, Margolis HS: The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med 1999, 341:556-562 [DOI] [PubMed] [Google Scholar]
- 5.Alter MJ: Epidemiology of hepatitis C in the West. Semin Liver Dis 1995, 15:5-14 [DOI] [PubMed] [Google Scholar]
- 6.Alter HJ, Seeff LB: Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis 2000, 20:17-35 [DOI] [PubMed] [Google Scholar]
- 7.Alter MJ, Margolis HS, Krawczynski K, Judson FN, Mares A, Alexander WJ, Hu PY, Miller JK, Gerber MA, Sampliner RE: The natural history of community-acquired hepatitis C in the United States: the Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. N Engl J Med 1992, 327:1899-1905 [DOI] [PubMed] [Google Scholar]
- 8.Tong MJ, el-Farra NS, Reikes AR, Co RL: Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med 1995, 332:1463-1466 [DOI] [PubMed] [Google Scholar]
- 9.Kiyosawa K, Sodeyama T, Tanaka E, Gibo Y, Yoshizawa K, Nakano Y, Furuta S, Akahane Y, Nishioka K, Purcell RH: Interrelationship of blood transfusion, non-A, non-B hepatitis and hepatocellular carcinoma: analysis by detection of antibody to hepatitis C virus. Hepatology 1990, 12:671-675 [DOI] [PubMed] [Google Scholar]
- 10.Davis GL, Esteban-Mur R, Rustgi V, Hoefs J, Gordon SC, Trepo C, Shiffman ML, Zeuzem S, Craxi A, Ling MH, Albrecht J: Interferon α-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. International Hepatitis Interventional Therapy Group. N Engl J Med 1998, 339:1493-1499 [DOI] [PubMed] [Google Scholar]
- 11.McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling MH, Cort S, Albrecht JK: Interferon α-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med 1998, 339:1485-1492 [DOI] [PubMed] [Google Scholar]
- 12.Alter MJ: Epidemiology of hepatitis C. Hepatology 1997, 26:62S-65S [DOI] [PubMed] [Google Scholar]
- 13.Alter MJ: Hepatitis C virus infection in the United States. J Hepatol 1999, 31:88-91 [DOI] [PubMed] [Google Scholar]
- 14.Thomas DL, Cannon RO, Shapiro CN, Hook EW, 3rd, Alter MJ, Quinn TC: Hepatitis C, hepatitis B, and human immunodeficiency virus infections among non-intravenous drug-using patients attending clinics for sexually transmitted diseases. J Infect Dis 1994, 169:990-995 [DOI] [PubMed] [Google Scholar]
- 15.Williams I: Epidemiology of hepatitis C in the United States. Am J Med 1999, 107:2S-9S [DOI] [PubMed] [Google Scholar]
- 16.McCarthy JJ, Flynn N: Hepatitis C in methadone maintenance patients: prevalence and public policy implications. J Addict Dis 2001, 20:19-31 [DOI] [PubMed] [Google Scholar]
- 17.Edlin BR, Seal KH, Lorvick J, Kral AH, Ciccarone DH, Moore LD, Lo B: Is it justifiable to withhold treatment for hepatitis C from illicit-drug users? N Engl J Med 2001, 345:211-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang CJ, Lin CH, Lee CT, Chang SJ, Ko YC, Liu HW: Hepatitis C virus infection among short-term intravenous drug users in southern Taiwan. Eur J Epidemiol 1999, 15:597-601 [DOI] [PubMed] [Google Scholar]
- 19.Oliveira ML, Bastos FI, Telles PR, Yoshida CF, Schatzmayr HG, Paetzold U, Pauli G, Schreier E: Prevalence and risk factors for HBV, HCV and HDV infections among injecting drug users from Rio de Janeiro, Brazil. Braz J Med Biol Res 1999, 32:1107-1114 [DOI] [PubMed] [Google Scholar]
- 20.Neumann AU, Lam NP, Dahari H, Gretch DR, Wiley TE, Layden TJ, Perelson AS: Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-α therapy. Science 1998, 282:103-107 [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg S: Recent advances in the molecular biology of hepatitis C virus. J Mol Biol 2001, 313:451-464 [DOI] [PubMed] [Google Scholar]
- 22.Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R: Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 1999, 285:110-113 [DOI] [PubMed] [Google Scholar]
- 23.Blight KJ, Kolykhalov AA, Rice CM: Efficient initiation of HCV RNA replication in cell culture. Science 2000, 290:1972-1974 [DOI] [PubMed] [Google Scholar]
- 24.Guo JT, Bichko VV, Seeger C: Effect of α interferon on the hepatitis C virus replicon. J Virol 2001, 75:8516-8523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J: Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res 1982, 42:3858-3863 [PubMed] [Google Scholar]
- 26.Pleasure SJ, Page C, Lee VM: Pure, postmitotic, polarized human neurons derived from NTera 2 cells provide a system for expressing exogenous proteins in terminally differentiated neurons. J Neurosci 1992, 12:1802-1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beczkowska IW, Gracy KN, Pickel VM, Inturrisi CE: Inducible expression of N-methyl-D-aspartate receptor, and δ and μ opioid receptor messenger RNAs and protein in the NT2-N human cell line. Neuroscience 1997, 79:855-862 [DOI] [PubMed] [Google Scholar]
- 28.Peterson PK, Gekker G, Brummitt C, Pentel P, Bullock M, Simpson M, Hitt J, Sharp B: Suppression of human peripheral blood mononuclear cell function by methadone and morphine. J Infect Dis 1989, 159:480-487 [DOI] [PubMed] [Google Scholar]
- 29.Nair MPN, Schwartz SA, Polasani R, Hou J, Sweet A, Chadha KC: Immunoregulatory effects of morphine on human lymphocytes. Clin Diagn Lab Immunol 1997, 4:127-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahajan SD, Schwartz SA, Shanahan TC, Chawda RP, Nair MP: Morphine regulates gene expression of α- and β-chemokines and their receptors on astroglial cells via the opioid mu receptor. J Immunol 2002, 169:3589-3599 [DOI] [PubMed] [Google Scholar]
- 31.Gomez-Lechon MJ, Ponsoda X, Jover R, Fabra R, Trullenque R, Castell JV: Hepatotoxicity of the opioids morphine, heroin, meperidine, and methadone to cultured human hepatocytes. Mol Toxicol 1987, 1:453-463 [PubMed] [Google Scholar]
- 32.Chuang TK, Killam KF, Jr, Chuang LF, Kung HF, Sheng WS, Chao CC, Yu L, Chuang RY: μ opioid receptor gene expression in immune cells. Biochem Biophys Res Commun 1995, 216:922-930 [DOI] [PubMed] [Google Scholar]
- 33.Yang JH, Lai JP, Douglas SD, Metzger D, Zhu XH, Ho WZ: Real-time RT-PCR for quantitation of hepatitis C virus RNA. J Virol Methods 2002, 102:119-128 [DOI] [PubMed] [Google Scholar]
- 34.Petrak D, Memon SA, Birrer MJ, Ashwell JD, Zacharchuk CM: Dominant negative mutant of c-Jun inhibits NF-AT transcriptional activity and prevents IL-2 gene transcription. J Immunol 1994, 153:2046-2051 [PubMed] [Google Scholar]
- 35.Pierce JW, Lenardo M, Baltimore D: Oligonucleotide that binds nuclear factor NF-κ B acts as a lymphoid-specific and inducible enhancer element. Proc Natl Acad Sci USA 1988, 85:1482-1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luckow B, Schutz G: CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res 1987, 15:5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khawaja XZ, Green IC, Thorpe JR, Titheradge MA: The occurrence and receptor specificity of endogenous opioid peptides within the pancreas and liver of the rat. Comparison with brain. Biochem J 1990, 267:233-240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wittert G, Hope P, Pyle D: Tissue distribution of opioid receptor gene expression in the rat. Biochem Biophys Res Commun 1996, 218:877-881 [DOI] [PubMed] [Google Scholar]
- 39.Di Bisceglie AM, Martin P, Kassianides C, Lisker-Melman M, Murray L, Waggoner J, Goodman Z, Banks SM, Hoofnagle JH: Recombinant interferon α therapy for chronic hepatitis C: a randomized, double-blind, placebo-controlled trial. N Engl J Med 1989, 321:1506-1510 [DOI] [PubMed] [Google Scholar]
- 40.Davis GL, Balart LA, Schiff ER, Lindsay K, Bodenheimer HC, Jr, Perrillo RP, Carey W, Jacobson IM, Payne J, Dienstag JL: Treatment of chronic hepatitis C with recombinant interferon α: a multicenter randomized, controlled trial. Hepatitis Interventional Therapy Group. N Engl J Med 1989, 321:1501-1506 [DOI] [PubMed] [Google Scholar]
- 41.Poynard T, Bedossa P, Chevallier M, Mathurin P, Lemonnier C, Trepo C, Couzigou P, Payen JL, Sajus M, Costa JM: A comparison of three interferon α-2b regimens for the long-term treatment of chronic non-A, non-B hepatitis. Multicenter Study Group. N Engl J Med 1995, 332:1457-1462 [DOI] [PubMed] [Google Scholar]
- 42.Powell JD, Bednarik DP, Folks TM, Jehuda-Cohen T, Villinger F, Sell KW, Ansari AA: Inhibition of cellular activation of retroviral replication by CD8+ T cells derived from non-human primates. Clin Exp Immunol 1993, 91:473-481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swingler S, Morris A, Easton A: Tumour necrosis factor α and interleukin-1 β induce specific subunits of NFKB to bind the HIV-1 enhancer: characterisation of transcription factors controlling human immunodeficiency virus type 1 gene expression in neural cells. Biochem Biophys Res Commun 1994, 203:623-630 [DOI] [PubMed] [Google Scholar]
- 44.Ghosh S, Karin M: Missing pieces in the NF-κB puzzle. Cell 2002, 109:S81-S96 [DOI] [PubMed] [Google Scholar]
- 45.Herz A: Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997, 129:99-111 [DOI] [PubMed] [Google Scholar]
- 46.Peterson PK, Molitor TW, Chao CC: The opioid-cytokine connection. J Neuroimmunol 1998, 83:63-69 [DOI] [PubMed] [Google Scholar]
- 47.Peterson PK, Gekker G, Hu S, Lokensgard J, Portoghese PS, Chao CC: Endomorphin-1 potentiates HIV-1 expression in human brain cell cultures: implication of an atypical μ-opioid receptor. Neuropharmacology 1999, 38:273-278 [DOI] [PubMed] [Google Scholar]
- 48.Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB: Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κ B. Proc Natl Acad Sci USA 1996, 93:9090-9095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corrao G, Arico S: Independent and combined action of hepatitis C virus infection and alcohol consumption on the risk of symptomatic liver cirrhosis. Hepatology 1998, 27:914-919 [DOI] [PubMed] [Google Scholar]
- 50.Wiley TE, McCarthy M, Breidi L, Layden TJ: Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology 1998, 28:805-809 [DOI] [PubMed] [Google Scholar]