Abstract
Accumulation of T cells and macrophages in atherosclerotic plaques and the formation of antibodies directed against plaque proteins suggests that adaptive immunity contributes to the development of atherosclerosis. The contribution of Th1 and Th2 helper cell subsets to atherogenesis was studied in a murine model by interbreeding apolipoprotein E-deficient (apoE−/−) mice with mice deficient in key cytokines that drive either Th1 responses [interleukin (IL)-12] or Th2 responses (IL-4). Compared to apoE−/− mice, apoE−/−/IL-12−/− mice had a 52% reduction in plaque area in the aortic root at 30 weeks of age (P < 0.001). ApoE−/−/IL-4−/− mice had a 27% reduction in plaque area compared to apoE−/− mice (P < 0.05) at 30 weeks of age, but their plaques were significantly larger than in apoE−/−/IL-12−/− mice at this stage (P < 0.05). By 45 weeks of age, there were no significant differences in lesion sizes in the aortic root between the strains, however apoE−/−/IL-4−/− mice showed a 58% and 64% decrease in disease in their aortic arch compared to apoE−/− (P < 0.05) and apoE−/−/IL-12−/− (P < 0.05) mice, respectively, and a 78% decrease in thoracic lesions compared to apoE−/−/IL-12−/− (P < 0.05). This suggests that both Th1 and Th2 cytokines play roles throughout the development of atherosclerosis in various vascular sites in apoE−/− mice.
Atherosclerosis has many of the hallmarks of a chronic immune inflammatory disease, although its pathogenesis is not fully understood. The role of resident smooth muscle cells, endothelial cells, and infiltrating cells such as macrophages in the development of atherosclerotic lesions has been extensively studied, but less so the role of infiltrating T lymphocytes and adaptive immunity.
CD4+ and CD8+ T cells are present throughout the development of lesions in humans. 1-3 The co-localization of T cells and macrophages within lesions, 4 the expression of MHC class II molecules 4,5 and interleukin (IL)-2 receptor 4 and presence of CD40/CD40 ligand 5 is consistent with the involvement of adaptive cell-mediated immunity in atherogenesis. The observation that a significant proportion of T cells express activation markers and have heterogeneous T-cell receptor gene rearrangement patterns, 6 suggests that T cells may be stimulated by a variety of local antigens such as oxidized low density lipoprotein (oxLDL), 7,8 heat shock proteins, 9,10 Chlamydia pneumoniae, 11 or other microbes 12 or are recruited by mechanisms independent of their antigen specificity.
The contribution of adaptive immunity to the progression of atherosclerosis has been investigated using apolipoprotein E-deficient (apoE−/−) mice. These hypercholesterolemic mice spontaneously develop atherosclerosis with similar pathology to human disease. 13,14 Interbreeding of these mice with immunodeficient recombination activating gene (RAG)-deficient mice or severe combined immunodeficient mice (SCID) has helped elucidate the role of adaptive immunity in the pathogenesis of atherosclerosis. When RAG-2−/− mice, which lack functional T and B cells, are combined with apoE−/− mice and fed a standard chow diet, a significant decrease in lesion size is observed. 15 Similarly, RAG-1-deficient apoE−/− mice showed a twofold decrease in aortic lesion size 16 and apoE−/−/SCID/SCID mice showed a 73% decrease in fatty streak development. 17 Transfer of CD4+ T cells from apoE−/− mice to apoE−/−/SCID/SCID mice was associated with infiltration of these T cells into developing lesions and increased lesion size, suggesting acquisition of adaptive immunity accelerates atherosclerosis. 17
Feeding immunodeficient mice with a moderate (Western diet) or a high-fat diet results in serum cholesterol levels many fold higher than in human disease and obscures the effects of adaptive immunity on the development of atherosclerosis. 16,18 These effects may be partially explained by the ability of high cholesterol diets to modulate the Th1/Th2 immune response in apoE−/− mice. 19 Immunosuppression with cyclosporin A in C57BL/6 mice fed a high cholesterol diet caused an increase in early plaque development, although cyclosporin-induced endothelial cell injury may contribute to this response. 20
Plaque T cells are primarily of the T-helper 1 (Th1) subtype, secreting cytokines such as interferon (IFN)-γ, IL-2, and tumor necrosis factor-α and -β, 21-23 which are involved in macrophage activation and inflammation. 12 IL-12, produced by many cell types including plaque macrophages, is important for Th1 differentiation 21 and stimulates proliferation and differentiation of natural killer and T cells. 24 IL-12 has also been shown to affect humoral responses by switching the immunoglobulin isotype to IgG2a in mice. 25 IL-12 is expressed in the aortas of young apoE−/− mice and administration of IL-12 increases plasma levels of IgG2a and IgM anti-oxLDL antibodies in the blood and accelerates development of atherosclerotic lesions in apoE−/− mice. 26
Th2 type cytokines, including IL-4, IL-5, and IL-10 are also expressed in human plaques but are somewhat less abundant than Th1 type cytokines. 21,22 IL-4 is the major cytokine directing Th2 differentiation of Th0 cells. 27 It is expressed by T cells in atheroma of severely hypercholesterolemic apoE−/− mice, 20 although T-cell clones generated from human atheromatous plaques seldom express a Th2 profile of cytokines (high IL-4 and low IFN-γ). 28 IL-4 is produced by macrophages, mast cells, and others 29,30 and promotes synthesis of IgE 31 and allergic responses. It has the ability to inhibit Th1 responses, reduce macrophage activation, and IFN-γ production and reduce procoagulant activity expression by endothelial cells. 29,30 IL-4 also up-regulates expression of the oxLDL-binding scavenger receptor 32 and inhibits inducible nitric oxide synthase and cyclooxygenase 2. 33,34
The contribution of Th1 and Th2 helper cell responses to the development of atherosclerosis remains unclear. This question was addressed in the current study by examining the development of atherosclerosis in apoE−/− mice deficient in key cytokines responsible for directing either Th1 (IL-12) or Th2 (IL-4) responses.
Materials and Methods
Mice
ApoE−/− mice 35 were obtained from Animal Resources Center, Canning Vale, Australia. IL-4−/− mice 36 and IL-12−/− (Jackson Laboratories, Bar Harbor, ME) mice 37 were all bred on a C57BL/6 background. The apoE−/−/IL-4−/− and apoE−/−/IL-12−/− mice were generated by crossbreeding of apoE−/− and either IL-4−/− or IL-12−/− mice. Mice with homozygous deficiency of both apoE and either IL-4 or IL-12 were selected in the F2 offspring by genotyping using polymerase chain reaction-based protocols. These F2 mice were used as breeders to generate double-deficient stock for experimental studies.
All animals used in the study were maintained under specific pathogen-free conditions and fed a normal chow diet. Surgical procedures were performed under methoxyflurane anesthesia (Medical Developments Australia, Melbourne, Australia). After 6, 15, 30, or 45 weeks, blood was collected into sodium citrate anti-coagulant via tail bleed. Cells were used in flow cytometric analysis of circulating leukocyte subsets and plasma was used for cholesterol assay and determination of immunoglobulin titers against oxLDL. Aortas were perfused with phosphate-buffered saline (PBS) (pH 7.4), followed by PLP fixative (0.1 mol/L phosphate buffer, 0.2 mol/L lysine, 8% paraformaldehyde, pH 7.4). Full-length aortas were dissected and removed. After fixation for 4 hours in PLP, tissues were processed through 7% sucrose, embedded in OCT (optimal cutting temperature) compound (Sakura Finetechnical Co., Tokyo, Japan), frozen in liquid nitrogen, and stored at −70°C until sectioning.
Flow Cytometric Analysis of Lymphocyte Subsets
After tail bleeding, 200 μl of mouse blood was incubated with 1 μl each of anti-mouse CD4-R-PE, CD8a-APC, and CD45R/B220-FITC (BD PharMingen, San Diego, CA) for 30 minutes and analyzed through a Cytomation Mo-Flo (Ft. Collins, CO) fluorescence-activated cell sorter. CD4, CD8, and B220 subsets were determined as a proportion of total lymphocyte counts.
Lesion Quantitation
In aortic root analysis, 10-μm serial sections of PLP-fixed tissue were cut from the proximal aorta. Every second section, beginning at the point that the aortic valves were no longer visible, but valve cusps were still prominent, was stained with 0.5% w/v oil-red-O (Sigma Chemical Co., St. Louis, MO) and counterstained with hematoxylin. Digital photos were taken of five sections of aorta, 40 μm apart, and the average area of oil-red-O-stained material across the five sections was determined using Sigma-Scan software (SPSS Science, IL). Arch, thoracic, and abdominal analyses 38 were performed by dissecting full-length aortas, exposing their luminal surface to oil-red-O and measuring the stained area via Stereo Investigator software (MicroBrightField, Colchester, VT). The extent of the lesions was expressed as a percentage of the total luminal surface area in these regions to adjust for the variable size of the aortic lumens available for assessment.
Cholesterol Measurement
Total cholesterol was measured in mouse plasma by a standard commercial cholesterol esterase enzymatic assay using a Dimension RxL Chemistry Analyser (DADE Behring Diagnostics, Sydney, Australia) with reagents and calibrators supplied by Dade Behring.
Immunohistochemistry
Ten-μm sections of aorta were stained with a panel of monoclonal rat anti-mouse antibodies directed against Mac1-positive macrophages (M1/70) and CD3 (KT3)-, CD4 (GK1.5)-, and CD8 (53-6.72)-positive cells. Bound antibody was visualized via incubation with biotinylated rabbit anti-rat Ig (DAKO, Carpinteria, CA), followed by streptavidin-horseradish peroxidase (Silenus Labs, Victoria, Australia) and 3,3′ diaminobenzidine (Sigma) as the chromogenic substrate. Sections were counterstained with hematoxylin. Macrophage staining area was measured using SigmaScan software as above and CD3-, CD4-, and CD8-positive cells were counted in a blinded protocol. A minimum of three sections were evaluated for each antibody per animal.
Measurement of OxLDL Antibody
Human LDL (Calbiochem-Novabiochem Corp, Darmstadt, Germany) was diluted in sterile PBS to a concentration of 1 mg/ml and oxidized via incubation with 200 μmol/L of CuSO4 overnight at 37°C. Oxidation was terminated by the addition of 0.3 mmol/L of Na2EDTA. The degree of oxidation was assessed as previously described. 39 Nunc Maxisorb (Nalge Nunc International, Denmark) 96-well plates were coated overnight at 4°C with 50 μl of 10 μg/ml native or 10 μg/ml oxLDL in PBS. Plates were blocked with 1% bovine serum albumin/PBS and 50 μl of mouse plasma at a dilution of 1:80 was added for 2 hours at room temperature, followed by anti-mouse IgG-horseradish peroxidase (1:2000; Amersham Pharmacia Biotech, UK) and addition of the substrate TMB (3,3′-5,5′ tetramethylbenzidine liquid substrate; Sigma). Plates were read at 450 nm. Specific anti-oxLDL antibody was determined by subtracting the OD of the wells containing native LDL from those coated with oxLDL.
Plasma IgG isotypes of oxLDL antibodies were determined by coating plates with native and oxLDL as above and incubating with a 1:10 dilution of mouse plasma overnight at 4°C. Plates were then incubated with anti-mouse IgG3-horseradish peroxidase (1:2000; Southern Biotech, AL) or biotinylated anti-mouse IgG1 or IgG2a (1:1000; BD PharMingen) for 2 hours at room temperature followed by streptavidin-horseradish peroxidase (1:1000) and TMB. Specific anti-oxLDL antibodies were defined as above.
Statistical Analyses
Statistical analyses were performed using Student’s t-test or analysis of variance followed by the Newman-Kuels multiple comparison test using GraphPad Prism software (GraphPad Software Inc., CA)
Results
Generation of Compound Knockout Mice
ApoE−/−/IL-4−/− and apoE−/−/IL-12−/− mice were fertile and showed no developmental abnormalities. The weights of the single and compound knockouts were not different at each of the time points examined (data not shown). ApoE−/−/IL-4−/− mice had higher numbers of circulating CD4+ and CD8+ cells, and fewer B220+ cells than the other strains. This effect appeared to be maintained at each age point and the aggregated results demonstrated statistical significance (Table 1) ▶ .
Table 1.
Blood Lymphocyte Subset Analysis in C57BL/6 and Atherosclerosis-Prone Mice
| C57BL/6 | apoE−/− | apoE−/−/IL-4−/− | apoE−/−/IL-12−/− | |
|---|---|---|---|---|
| CD4 | 14.4 ± 0.5* | 10.8 ± 0.7†§ | 16.2 ± 0.7 | 12.3 ± 0.6†¶ |
| CD8 | 9.4 ± 0.3* | 8.2 ± 0.4† | 10.5 ± 0.4 | 8.7 ± 0.3‡ |
| B220 | 66.0 ± 0.9† | 64.3 ± 1.2† | 56.9 ± 1.2 | 64.5 ± 1.2† |
| n = 50 | n = 53 | n = 47 | n = 53 |
Values are percent of total lymphocyte counts determined by flow cytometry. Data mean ± SEM of pooled data from all age groups.
* P < 0.05 versus apoE−/−/IL-4−/−;
†P < 0.001 versus apoE−/−/IL-4−/−;
‡P < 0.01 versus apoE−/−/IL-4−/−;
§P < 0.001 versus C57BL/6;
¶P < 0.05 versus C57BL/6.
Quantification of Atherosclerotic Lesions
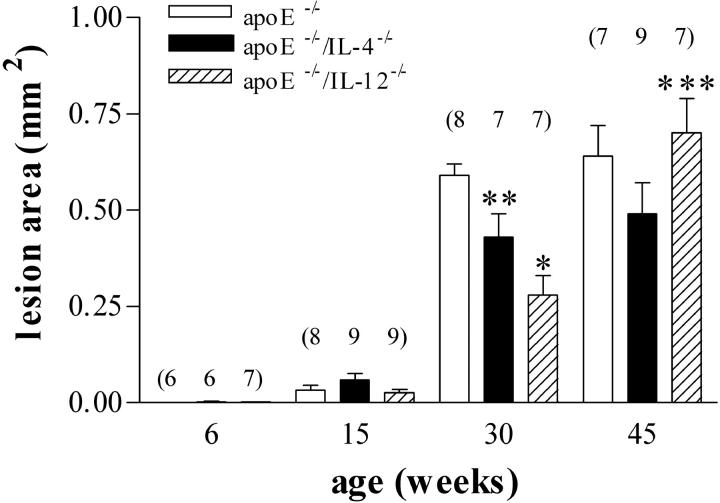
The extent of lesion formation in the aortic root was assessed on tissue stained with oil-red-O at 6, 15, 30, and 45 weeks of age. Digital image analysis was used to quantify lesion area. Previous studies have suggested that IFN-γ deficiency may affect atherogenesis in a gender-specific manner. 40 However, two studies show conflicting results regarding the effects of IFN-γ deficiency on atherogenesis in female mice. 40,41 We found no significant effects of gender on development of atherosclerosis (Table 2) ▶ . Because there were no differences in disease between males and females, lesion area results (Figures 1 and 2) ▶ are representative of pooled data from mice of both sexes. In the aortic root (Figure 1) ▶ , no measurable disease was apparent at 6 weeks of age. At 15 weeks, small but measurable amounts of fatty streak deposits were observed within the aortic root of the atherosclerotic-prone mice. At 30 weeks of age, large fibro-fatty lesions with a significant cellular infiltrate were apparent in all atherosclerotic-prone mice. The mean lesion size in apoE−/−/IL-4−/− mice (0.42 ± 0.06 mm2, P < 0.05) and apoE−/−/IL-12−/− (0.28 ± 0.05 mm2, P < 0.001) mice was significantly reduced by 27% and 52%, respectively, in comparison to apoE−/− mice (0.58 ± 0.02 mm2). IL-12 deficiency afforded significantly greater protection than IL-4 deficiency (P < 0.05).
Table 2.
Effect of Gender on Extent of Atherosclerosis
| 30 Weeks | 45 Weeks | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| apoE−/− | 0.62 ± 0.04 | 0.56 ± 0.02 | 0.53 ± 0.07 | 0.67 ± 0.13 |
| apoE−/−/IL-4−/− | 0.41 ± 0.07 | 0.43 ± 0.11 | 0.48 ± 0.08 | 0.54 ± 0.13 |
| apoE−/−/IL-12−/− | 0.32 ± 0.07 | 0.23 ± 0.05 | 0.62 ± 0.08 | 0.75 ± 0.10 |
Values are lesion area (mm2) ± SEM in the aortic root. There were no significant differences in lesion area between genders in any strain at either time point. n = 3 to 5 in each group.
Figure 1.
Effect of IL-12 and IL-4 deficiency on plaque area in the aortic root of apoE−/− mice. Small quantifiable lesions were detectable in all three groups at 15 weeks. Maximal increase in the size of lesions occurred between 15 and 30 weeks. Lesions in IL-4-deficient apoE−/− mice were significantly smaller than in apoE−/− at this time. Lesions in IL-12-deficient apoE−/− mice were significantly smaller than both IL-4-deficient apoE−/− mice and apoE−/− mice. Lesion area in IL-12-deficient apoE−/− mice increased significantly between 30 and 45 weeks. Numbers of mice in each group are shown in parentheses. *, P < 0.001 versus apoE−/−; **, P < 0.05 versus apoE−/−/IL-12−/− and apoE−/−; ***, P = 0.0002 versus 30-week apoE−/−/IL-12−/−.
The extent of plaque formation in apoE−/− and apoE−/−/IL-4−/− mice did not increase significantly after 30 weeks, but a significant (142%) increase occurred between these time points in apoE−/−/IL-12−/− (0.28 ± 0.05 mm2 versus 0.68 ± 0.06 mm2, P = 0.0002). At 45 weeks of age, the mean lesion sizes were not significantly different between the single and compound knockout mice.
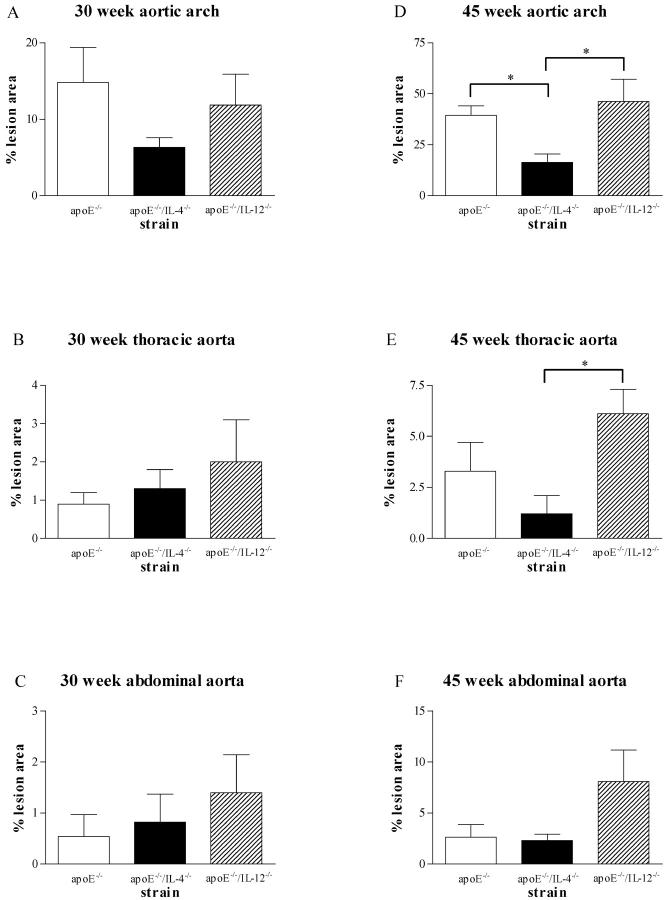
Measurement of disease in other areas of the aorta (Figure 2) ▶ showed that at 30 weeks of age, apoE−/−/IL-4−/− mice had a 57% reduction in disease in the aortic arch compared with apoE−/− mice (6.3 ± 1.3% versus 14.8 ± 4.6% intimal area) and apoE−/−/IL-12−/− also had less disease than apoE−/− (11.8 ± 4.1%) but neither difference reached statistical significance (Figure 2A) ▶ . There was minimal atherosclerosis present in the thoracic and abdominal regions at 30 weeks and no statistical differences were apparent between any of the lesion-prone mice (Figure 2, B and C) ▶ .
Figure 2.
Effect of IL-12 and IL-4 deficiency on plaque area in the aortic arch and thoracic and abdominal aorta of apoE−/− mice. At 30 weeks (A–C) there were no significant differences by analysis of variance in disease levels between the three strains in any of the vascular sites. At 45 weeks, apoE−/−/IL-4−/− mice had significantly less disease in the arch than both apoE−/− and apoE−/−/IL-12−/− (D; *, P < 0.05) and significantly less than apoE−/−/IL-12−/− in the thoracic aorta (E; *, P < 0.05) (n = 4 to 7 in each group).
By 45 weeks in the aortic arch, apoE−/−/IL-4−/− mice had significantly less disease than both apoE−/− and apoE−/−/IL-12−/− mice (Figure 2D ▶ ; 16.4 ± 4.1% versus 39.3 ± 4.8%, P < 0.05; and 46.2 ± 11.0%, P < 0.05, respectively). ApoE−/−/IL-4−/− mice also had a 60% reduction in disease in their thoracic aorta compared with apoE−/− and a 78% reduction compared with apoE−/−/IL-12−/− with the latter reaching statistical significance (Figure 2E ▶ ; 1.3 ± 0.9% versus 3.3 ± 1.4% and 6.1 ± 1.2%, P < 0.05, respectively). ApoE−/−/IL-12−/− mice showed a more than threefold increase in abdominal lesions at 45 weeks compared to both apoE−/− and apoE−/−/IL-4−/− mice (Figure 2F ▶ ; 8.1 ± 3.1% versus 2.6 ± 1.3% and 2.3 ± 0.7%, respectively).
Plasma Cholesterol
Plasma from four animals of each strain and age group was assayed for total cholesterol. There was no significant effect of age on plasma cholesterol levels in any strain (data not shown). Pooled data across all age groups demonstrated no significant difference in plasma cholesterol levels between atherosclerosis prone mice. Each of the atherosclerosis-prone strains had significantly higher levels than normal C57BL/6 mice (P < 0.001) (Table 3) ▶ .
Table 3.
Plasma Cholesterol Levels
| C57BL/6 | apoE−/− | apoE−/−/IL-4−/− | apoE−/−/IL-12−/− |
|---|---|---|---|
| 2.07 ± 0.07 | 6.51 ± 0.46* | 7.46 ± 0.52* | 6.76 ± 0.59* |
| n = 16 | n = 16 | n = 16 | n = 16 |
Values are total cholesterol (mmol/L) and represent mean ± SEM of pooled data from all age groups.
*P < 0.001 versus C57BL/6.
Immunohistology
The extent of infiltration of macrophages was determined by area stained with anti-mouse Mac1, because it was not possible to count individual foam cells in plaques. Plaque macrophage infiltration showed similar trends to total lesion size at both 30 and 45 weeks (Figure 3A) ▶ . Lesions in apoE−/−/IL-12−/− mice had significantly less macrophage infiltration at 30 weeks than lesions in apoE−/−/IL-4−/− mice (0.08 ± 0.01 mm2 versus 0.14 ± 0.02 mm2, P = 0.026) and a 49% reduction compared to apoE−/− mice (0.16 ± 0.06 mm2), which did not reach statistical significance (P = 0.117).
Figure 3.
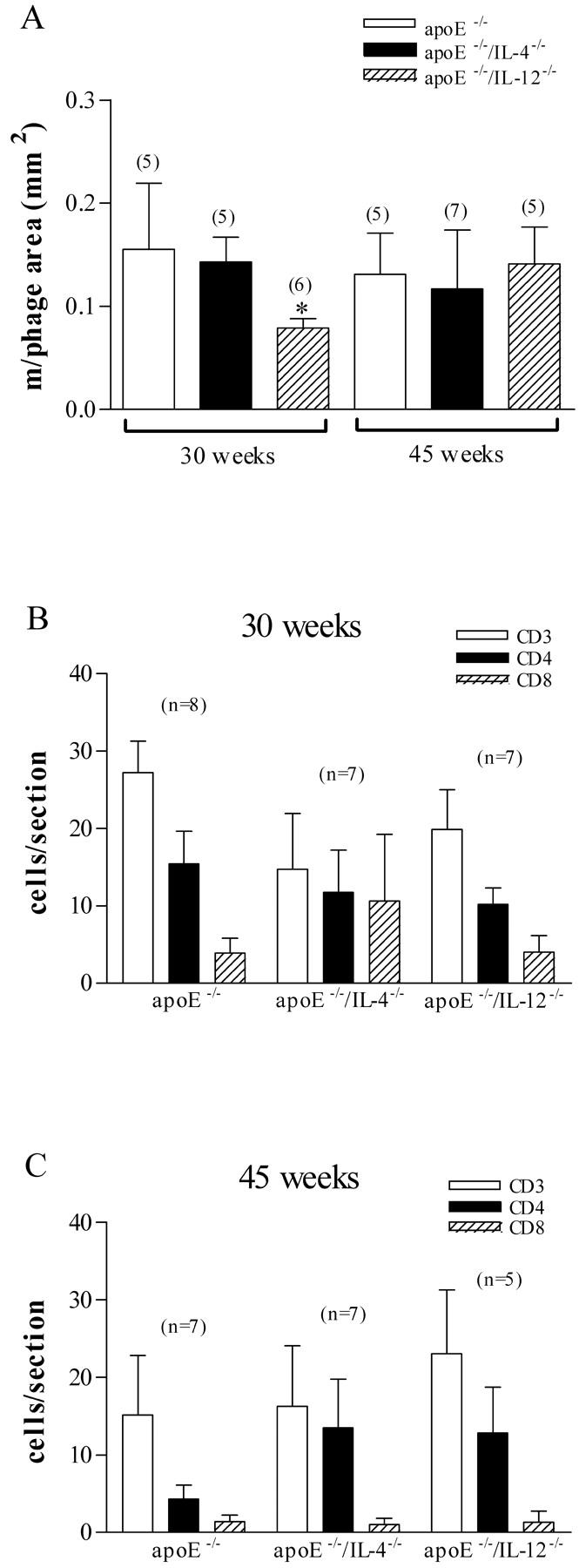
Effect of IL-12 and IL-4 deficiency on mononuclear cell accumulation in lesions in apoE−/− mice. Macrophage accumulation (A) was significantly less in IL-12-deficient apoE−/− mice at 30 weeks (*, P = 0.026 versus apoE−/−/IL-4−/−). There were no significant differences in CD3, CD4, or CD8 T-cell accumulation (B and C) between any of the strains at either time point. Numbers of mice in each group are shown in parentheses.
CD3+, CD4+, and CD8+ cells were counted in 30- and 45-week lesions (Figure 3, B and C) ▶ . CD4+ cells were consistently more abundant than CD8+ cells in each strain and at each age group. The apparent lower numbers of infiltrating CD3+ and CD4+ cells in apoE−/−/IL-12−/− and apoE−/−/IL-4−/− mice at 30 weeks were not statistically different from apoE−/− (Figure 3B) ▶ . At 45 weeks (Figure 3C) ▶ , the number of infiltrating CD4+ cells in apoE−/− mice was significantly reduced compared with 30 weeks (15.4 ± 4.2 cells/cross-section versus 4.3 ± 1.8 cells/cross-section, P = 0.037). There were no differences in T-cell numbers between 30 and 45 weeks in the apoE−/−/IL-12−/− or apoE−/−/IL-4−/− mice.
Development of Antibody Responses to OxLDL
C57BL/6 mice showed very low levels of circulating IgG directed against oxLDL, which did not increase with age. Atherosclerotic-prone groups of mice had significantly higher titers of anti-oxLDL IgG that increased with age (Figure 4A) ▶ . There were no significant differences in anti-oxLDL titers between any of the atherosclerosis prone groups at comparable time points.
Figure 4.
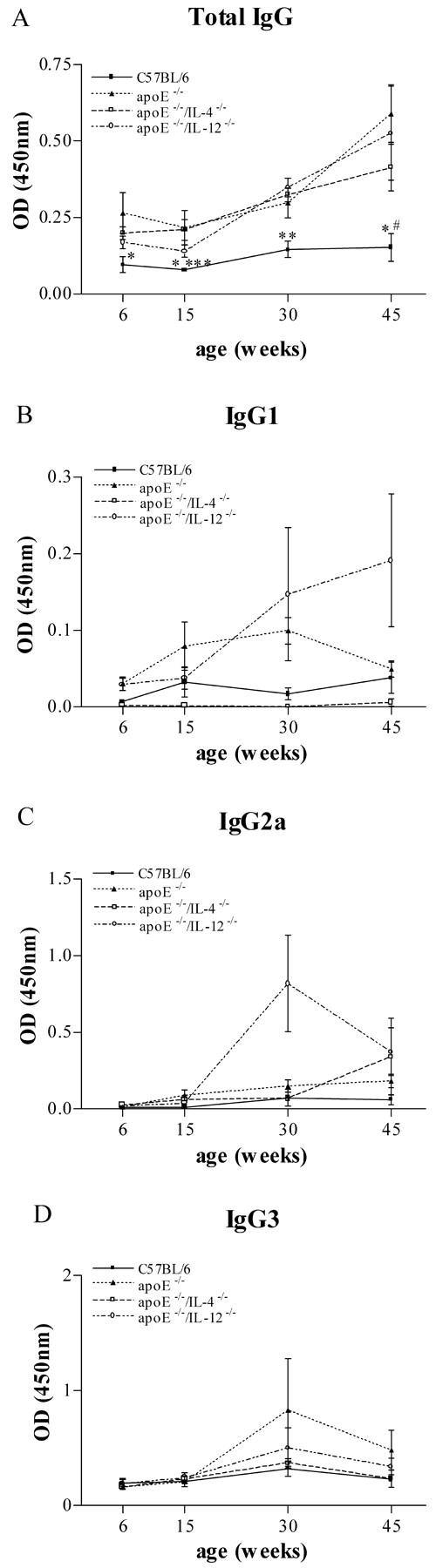
Total plasma IgG antibodies to oxLDL (A) in C57BL/6 mice, apoE−/−, IL-4-deficient apoE−/−, and IL-12-deficient apoE−/−. Plasma from six animals was evaluated in each group at each time point. *, P < 0.05 versus apoE−/−; **, P < 0.01 versus apoE−/−, apoE−/−/IL-4−/−, and apoE−/−/IL-12−/−; ***, P < 0.05 versus apoE−/−/IL-4−/−; #, P < 0.05 versus apoE−/−/IL-12−/−. Plasma anti-oxLDL IgG isotypes (B–D) in C57BL/6 mice, apoE−/−, IL-4-deficient apoE−/−, and IL-12-deficient apoE−/−. Plasma from four to eight animals was evaluated in each group at each time point.
Anti-oxLDL IgG subclasses were measured to determine whether significant Th1 or Th2 bias of the humoral immune response to this putative atherogenic antigen could be demonstrated in the absence of IL-12 or IL-4. As shown (Figure 4; B, C, and D) ▶ , apoE−/−/IL-4−/− mice produced extremely low levels of IgG1 antibodies compared to apoE−/− and apoE−/−/IL-12−/− mice, consistent with diminished Th2 responses. These IgG1 levels were below the background level in nonatherosclerotic C57BL/6 mice. ApoE−/−/IL-12−/− mice had the highest levels of IgG1 at 30 and 45 weeks, consistent with augmented Th2 responses in the absence of IL-12, although these results were not statistically significant. Both apoE−/−/IL-12−/− and apoE−/−/IL-4−/− had diminished IgG3 responses at 30 and 45 weeks of age compared with apoE−/− but these were not statistically significant.
Discussion
Evidence from human and experimental studies suggests involvement of Th1 adaptive immune responses in atherogenesis. Th1 cytokines such as IFN-γ and IL-12, are present in atherosclerotic lesions in both humans and in animal models 21,22,26 whereas Th2 cytokines such as IL-4 and IL-5 are found in the minority of human lesions. 21,22 IL-10, which inhibits Th1 responses, is not found in lesions in apoE−/− mice until 6 months of age. Th2-biased immune responses to modified LDL are only apparent in apoE-deficient mice after feeding a high-fat diet to induce severe hypercholesterolemia. 20 T cells cloned from human aortic plaques more frequently show a Th1 than Th2 cytokine profile, although the vast majority of T cell clones appear to be Th0. 28 Although these data suggest a dominant role for Th1 responses in disease pathogenesis, the role of Th1 and Th2 cytokines in atherosclerosis has not been directly compared in vivo.
This issue was addressed in the apoE-deficient mouse model of atherosclerosis by crossbreeding with IL-4- and IL-12-deficient mice to generate combined knockouts with a deficiency of either Th1 (IL-12-deficient) or Th2 (IL-4-deficient) immune responses. IL-4 is the major cytokine that directs differentiation of Th0 cells to Th2 cells. IL-4−/− mice exhibit deficiencies in Th2 responses with reduced production of IL-5, IL-9, and IL-10 36 and decreased secretion of IgG1 and IgE. 42 IL-12 is the cytokine responsible for development of Th1 cells. IL-12−/− mice exhibit impaired IFN-γ production, increased IL-4 production, and reduction in delayed-type hypersensitivity response indicating impaired Th1 responses. 37 Combined apoE−/−/IL-12−/− and apoE−/−/IL-4−/− mice exhibited normal fertility, development, and growth. Plasma cholesterol levels were equivalent in all these atherosclerosis-prone strains with levels in the range of 6.5 to 7.5 mmol/L. Previous studies on IL-4−/− mice, 38,43 and others investigating administration of IL-12 26 or IL-18 44 to apoE−/− mice, also report no effects of these cytokines on plasma cholesterol levels. ApoE−/−/IL-4−/− mice had minor differences in their circulating lymphocyte subsets compared to apoE−/−/IL-12−/− and apoE−/− mice with a modest (8%) decrease in B cells, which may reflect absence of IgG1-secreting clones. This abnormality was not reported in the original description of IL-4-deficient mice. 36
In the aortic root, apoE−/− mice showed most rapid development of lesions between 15 and 30 weeks of age. ApoE−/−/IL-12−/− mice showed significantly reduced disease development during this interval with a 52% reduction in the extent of atherosclerosis at 30 weeks of age. Between 30 and 45 weeks of age, the size of lesions did not increase significantly in apoE−/− mice, but in apoE−/−/IL-12−/− mice, lesions increased significantly to reach a similar size to those in apoE−/− mice. Reduced plaque size in apoE−/−/IL-12−/− mice was accompanied by reduced macrophage infiltration at 30 weeks but the small decrease in intra-plaque T cells was not significantly different from apoE−/− mice. Plaque macrophages and T cells, like plaque area, were equivalent in all groups at 45 weeks. These results suggest that Th1 responses accelerate development of atherosclerosis in the aortic root during the phase of rapid development but do not affect the eventual extent of advanced disease at this site.
At 30 weeks, IL-12 deficiency resulted in a modest (20%) decrease in lesion area in the aortic arch. Minimal atherosclerosis was present in the thoracic and abdominal aorta and IL-12 deficiency had no significant impact on lesion area at these sites.
At 45 weeks, IL-12 deficiency had no effect on atherogenesis in the aortic root or arch but was associated with an 84% increase in lesion area in the thoracic aorta and a threefold increase in abdominal lesion area. Although neither of these increases reached statistical significance, they suggest that late in disease progression, IL-12 may have site-specific effects, similar to previously published effects of IL-4 deficiency late in the progression of atherosclerosis. 38
The extent of protection afforded by IL-12 deficiency in the aortic root was similar to that reported in combined IFN-γ receptor-deficient/apoE-deficient mice (which also have impaired Th1 responses) fed a high-fat (Western) diet for 12 weeks. 41 Despite modestly higher plasma cholesterol levels, IFN-γ receptor−/−/apoE−/− mice showed a 59% reduction in plaque size compared to apoE-deficient controls. The progression of disease at later time points was not evaluated and it is unknown whether these mice also show a late catch-up in disease. A twofold increase in lesion size, in the ascending aorta and arch, in apoE−/− mice receiving recurrent pharmacological doses of IL-12 26 or IL-18 44 provides further evidence for a proatherogenic role for Th1.
In the aortic root, apoE−/−/IL-4−/− mice also showed retarded development of disease between 15 and 30 weeks of age with a 27% reduction in plaque area compared to apoE−/− mice at 30 weeks. The extent of macrophage and T-cell infiltration was not decreased at this time. By 45 weeks of age, plaque area in apoE−/−/IL-4−/− was equivalent to apoE−/−. This is consistent with the observation that fatty streak formation is significantly reduced in the aortic sinus in atherosclerotic IL-4-deficient mice immunized with heat shock protein 65 or Mycobacterium tuberculosis. 43 Other studies have found that fatty streak formation in the aortic root was not influenced by the absence of IL-4. 45 However, the lesions in this study were not as advanced as in our present study and these animals were fed a high-fat diet, which is known to modulate the immune process in atherosclerosis 19 and obscure the effects of adaptive immunity on lesion development. 16,18
In the aortic arch, apoE−/−/IL-4−/− mice had reduced atherosclerosis at both 30 and, significantly, at 45 weeks compared with apoE−/− mice. King and colleagues 38 also found that IL-4 deficiency resulted in a similar reduction in lesion size in the arch and thoracic aorta but did not find a difference in extent of disease in the aortic root. ApoE−/−/IL-4−/− mice also had reduced lesion area in comparison to apoE−/−/IL-12−/− mice at 45 weeks in the arch, thoracic, and abdominal aorta and less in the aortic root suggesting that Th1 and Th2 cytokines are having opposing effects on atherogenesis at this time point.
Given that animals lacking IL-4 would be expected to show a bias toward a Th1/proinflammatory state and therefore, possibly exhibit a greater level of disease, mechanisms for IL-4 outside the Th1/Th2 paradigm may play an important role in the pathogenesis of atherosclerosis. IL-4 has been shown to influence mononuclear cell recruitment by stimulating an increase in expression of macrophage chemoattractant protein-1 46 and influence cell adhesion by causing an increase in expression of VCAM-1 by endothelial 47 and vascular smooth muscle 48 cells. IL-4 also causes an increase in production of 15-lipoxygenase, an enzyme implicated in oxidizing LDL to its atherogenic form 49 and it is known to increase scavenger receptor expression by macrophages, 50 which may increase their uptake of modified lipid and accelerate early lesion development. Proinflammatory effects of IL-4 have been demonstrated in autoimmune uveoretinitis 51 and adjuvant-induced arthritis, 52 suggesting additional roles for this cytokine that may be independent of its effects on Th1/Th2 immune bias.
The atherosclerotic-prone mice had increased serum levels of anti-oxLDL antibodies compared with C57BL/6 mice fed the identical diet. The serum total IgG levels increased progressively with time in the atherosclerosis-prone mice but did not change in C57BL/6 mice. Measurements of IgG isotypes provide an assessment of the Th1 and Th2 bias of the anti-oxLDL Ig responses. In mice, IgG1 production is indicative of a Th2 response and this is characteristically low in IL-4-deficient mice. In apoE−/−/IL-4−/− mice, IgG1 was undetectable throughout development of disease (below levels in C57BL/6 mice) indicating deficient Th2 responses as expected. IgG1 levels were highest in apoE−/−/IL-12−/− mice, consistent with a bias toward a Th2 response. The anti-oxLDL IgG2a titers in apoE−/−/IL-12−/− mice showed considerable fluctuation during the development of disease, with unexpectedly high levels at 30 weeks that were not significantly different from apoE−/− mice. The reduced IgG3 levels in the apoE−/−/IL-12−/− mice, together with elevated IgG1 levels, is consistent with a Th1-deficient, Th2-biased response to oxLDL.
In summary, these studies show IL-12-dependent Th1 responses appear to have a dominant role in the root early in disease development and IL-12 deficiency was associated with reduced plaque size and macrophage accumulation at 30 weeks at this site. By 45 weeks however, IL-12 deficiency was associated with an increase in atherosclerosis at all vascular sites. IL-4-dependent Th2 responses play a lesser role in early lesion development in the aortic root. However, IL-4 appears to play a significant and opposing role to IL-12 later in disease development, especially in the aortic arch and thoracic aorta. This data suggests IL-12 and IL-4 both play a role in immune responses during the early phase of rapid development of atherosclerosis in the aortic root in mice and have an ongoing role in other vascular sites.
Acknowledgments
We thank Alice Wright and Paul Hutchinson for their technical assistance.
Footnotes
Address reprint requests to Piers Davenport, Monash University Department of Medicine, Level 5 Block E, Monash Medical Centre, 246 Clayton Rd., Clayton, Victoria, Australia 3168. E-mail: piers.davenport@med.monash.edu.au.
Supported by the National Health and Medical Research Council of Australia.
P. G. T. is a Principal Research Fellow of National Health and Medical Research Council of Australia.
References
- 1.Xu QB, Oberhuber G, Gruschwitz M, Wick G: Immunology of atherosclerosis: cellular composition and major histocompatibility complex class II antigen expression in aortic intima, fatty streaks, and atherosclerotic plaques in young and aged human specimens. Clin Immunol Immunopathol 1990, 56:344-359 [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK, Jonasson L, Lojsthed B, Stemme S, Kocher O, Gabbiani G: Localization of T lymphocytes and macrophages in fibrous and complicated human atherosclerotic plaques. Atherosclerosis 1988, 72:135-141 [DOI] [PubMed] [Google Scholar]
- 3.Emeson EE, Robertson AL, Jr: T lymphocytes in aortic and coronary intimas. Their potential role in atherogenesis. Am J Pathol 1988, 130:369-376 [PMC free article] [PubMed] [Google Scholar]
- 4.Van Der Wal AC, Das PK, Bentz Van De Berg D, Van Der Loos CM, Becker AE: Atherosclerotic lesions in humans: in situ immunophenotypic analysis suggesting an immune mediated response. Lab Invest 1989, 61:166-170 [PubMed] [Google Scholar]
- 5.Jonasson L, Holm J, Skalli O, Gabbiani G, Hansson GK: Expression of class II transplantation antigen on vascular smooth muscle cells in human atherosclerosis. J Clin Invest 1985, 76:125-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stemme S, Rymo L, Hansson GK: Polyclonal origin of T lymphocytes in human atherosclerotic plaques. Lab Invest 1991, 65:654-660 [PubMed] [Google Scholar]
- 7.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK: Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis 1986, 6:131-138 [DOI] [PubMed] [Google Scholar]
- 8.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK: T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci USA 1995, 92:3893-3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Q, Willeit J, Marosi M, Kleindienst R, Oberhollenzer F, Kiechl S, Stulnig T, Luef G, Wick G: Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis. Lancet 1993, 341:255-259 [DOI] [PubMed] [Google Scholar]
- 10.Xu Q, Kleindienst R, Waitz W, Dietrich H, Wick G: Increased expression of heat shock protein 65 coincides with a population of infiltrating T lymphocytes in atherosclerotic lesions of rabbits specifically responding to heat shock protein 65. J Clin Invest 1993, 91:2693-2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saikku P, Leinonen M, Mattila K, Ekman MR, Nieminen MS, Makela PH, Huttunen JK, Valtonen V: Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet 1988, 2:983-986 [DOI] [PubMed] [Google Scholar]
- 12.Hansson GK: Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol 2001, 21:1876-1890 [DOI] [PubMed] [Google Scholar]
- 13.Zhang SH, Reddick RL, Piedrahita JA, Maeda N: Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 1992, 258:468-471 [DOI] [PubMed] [Google Scholar]
- 14.Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL: Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 1992, 71:343-353 [DOI] [PubMed] [Google Scholar]
- 15.Reardon CA, Blachowicz L, White T, Cabana V, Wang Y, Lukens J, Bluestone J, Getz GS: Effect of immune deficiency on lipoproteins and atherosclerosis in male apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 2001, 21:1011-1016 [DOI] [PubMed] [Google Scholar]
- 16.Dansky HM, Charlton SA, Harper MM, Smith JD: T and B lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci USA 1997, 94:4642-4646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X, Nicoletti A, Elhage R, Hansson GK: Transfer of CD4+ T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation 2000, 102:2919-2922 [DOI] [PubMed] [Google Scholar]
- 18.Daugherty A, Pure E, Delfel-Butteiger D, Chen S, Leferovich J, Roselaar SE, Rader DJ: The effects of total lymphocyte deficiency on the extent of atherosclerosis in apolipoprotein E−/− mice. J Clin Invest 1997, 100:1575-1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou X, Paulsson G, Stemme ST, Hansson GK: Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J Clin Invest 1998, 101:1717-1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emeson EE, Shen ML: Accelerated atherosclerosis in hyperlipidemic C57BL/6 mice treated with cyclosporin A. Am J Pathol 1993, 142:1906-1915 [PMC free article] [PubMed] [Google Scholar]
- 21.Uyemura K, Demer LL, Castle SC, Jullien D, Berliner JA, Gately MK, Warrier RR, Pham N, Fogelman AM, Modlin RL: Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Invest 1996, 97:2130-2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frostegard J, Ulfgren A-K, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK: Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 1999, 145:33-43 [DOI] [PubMed] [Google Scholar]
- 23.Schreyer SA, Vick CM, LeBoeuf RC: Loss of lymphotoxin-α but not tumor necrosis factor-α reduces atherosclerosis in mice. J Biol Chem 2002, 277:12364-12368 [DOI] [PubMed] [Google Scholar]
- 24.Trinchieri G, Wysocka M, D’Andrea A, Rengaraju M, Aste-Amezaga M, Kubin M, Valiante NM, Chehimi J: Natural killer cell stimulatory factor (NKSF) or interleukin-12 is a key regulator of immune response and inflammation. Prog Growth Factor Res 1992, 4:355-368 [DOI] [PubMed] [Google Scholar]
- 25.Morris SC, Madden KB, Adamovicz JJ, Gause WC, Hubbard BR, Gately MK, Finkelman FD: Effects of IL-12 on in vivo cytokine gene expression and Ig isotype selection. J Immunol 1994, 152:1047-1056 [PubMed] [Google Scholar]
- 26.Lee T-S, Yen H-C, Pan C-C, Chau L-Y: The role of interleukin 12 in the development of atherosclerosis in apoE-deficient mice. Arterioscler Thromb Vasc Biol 1999, 19:734-742 [DOI] [PubMed] [Google Scholar]
- 27.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL: Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 1986, 136:2348-2357 [PubMed] [Google Scholar]
- 28.de Boer OJ, van der Wal AC, Verhagen CE, Becker AE: Cytokine secretion profiles of cloned T cells from human aortic atherosclerotic plaques. J Pathol 1999, 188:174-179 [DOI] [PubMed] [Google Scholar]
- 29.Seder RA, Paul WE: Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol 1994, 12:635-673 [DOI] [PubMed] [Google Scholar]
- 30.Paul WE, Seder RA: Lymphocyte responses and cytokines. Cell 1994, 76:241-251 [DOI] [PubMed] [Google Scholar]
- 31.Coffman RL, Ohara J, Bond MW, Carty J, Zlotnik A, Paul WE: B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol 1986, 136:4538-4541 [PubMed] [Google Scholar]
- 32.Yesner LM, Huh HY, Pearce SF, Silverstein RL: Regulation of monocyte CD36 and thrombospondin-1 expression by soluble mediators. Arterioscler Thromb Vasc Biol 1996, 16:1019-1025 [DOI] [PubMed] [Google Scholar]
- 33.Bogdan C, Vodovotz Y, Paik J, Xie QW, Nathan C: Mechanism of suppression of nitric oxide synthase expression by interleukin-4 in primary mouse macrophages. J Leukoc Biol 1994, 55:227-233 [DOI] [PubMed] [Google Scholar]
- 34.Mehindate K, al-Daccak R, Aoudjit F, Damdoumi F, Fortier M, Borgeat P, Mourad W: Interleukin-4, transforming growth factor beta 1, and dexamethasone inhibit superantigen-induced prostaglandin E2-dependent collagenase gene expression through their action on cyclooxygenase-2 and cytosolic phospholipase A2. Lab Invest 1996, 75:529-538 [PubMed] [Google Scholar]
- 35.Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N: Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci USA 1992, 89:4471-4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G: Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature 1993, 362:245-248 [DOI] [PubMed] [Google Scholar]
- 37.Magram J, Connaughton SE, Warrier RR, Carvajal DM, Wu CY, Ferrante J, Stewart C, Sarmiento U, Faherty DA, Gately MK: IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity 1996, 4:471-481 [DOI] [PubMed] [Google Scholar]
- 38.King VL, Szilvassy SJ, Daugherty A: Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler Thromb Vasc Biol 2002, 22:456-461 [DOI] [PubMed] [Google Scholar]
- 39.Hoff HF, Whitaker TE, O’Neil J: Oxidation of low density lipoprotein leads to particle aggregation and altered macrophage recognition. J Biol Chem 1992, 267:602-609 [PubMed] [Google Scholar]
- 40.Whitman SC, Ravisankar P, Daugherty A: IFN-gamma deficiency exerts gender-specific effects on atherogenesis in apolipoprotein E−/− mice. J Interferon Cytokine Res 2002, 22:661-670 [DOI] [PubMed] [Google Scholar]
- 41.Gupta S, Pablo AM, Jiang X-C, Wang N, Tall AR, Schindler C: IFN-gamma potentiates atherosclerosis in apoE knock-out mice. J Clin Invest 1997, 99:2752-2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuehn R, Rajewsky K, Mueller W: Generation and analysis of inteleukin-4 deficient mice. Science 1991, 254:707-710 [DOI] [PubMed] [Google Scholar]
- 43.George J, Shoenfeld Y, Gilburd B, Afek A, Shaish A, Harats D: Requisite role for interleukin-4 in the acceleration of fatty streaks induced by heat shock protein 65 or Mycobacterium tuberculosis. Circ Res 2000, 86:1203-1210 [DOI] [PubMed] [Google Scholar]
- 44.Whitman SC, Ravisankar P, Daugherty A: Interleukin-18 enhances atherosclerosis in apolipoprotein E (−/−) mice through release of interferon-gamma. Circ Res 2002, 90:E34-E38 [DOI] [PubMed] [Google Scholar]
- 45.George J, Mulkins M, Shaish A, Casey S, Schatzman R, Sigal E, Harats D: Interleukin (IL)-4 deficiency does not influence fatty streak formation in C57BL/6 mice. Atherosclerosis 2000, 153:403-411 [DOI] [PubMed] [Google Scholar]
- 46.Winsor GL, Waterhouse CC, MacLellan RL, Stadnyk AW: Interleukin-4 and IFN-gamma differentially stimulate macrophage chemoattractant protein-1 (MCP-1) and eotaxin production by intestinal epithelial cells. J Interferon Cytokine Res 2000, 20:299-308 [DOI] [PubMed] [Google Scholar]
- 47.Iademarco MF, Barks JL, Dean DC: Regulation of vascular cell adhesion molecule-1 expression by IL-4 and TNF-alpha in cultured endothelial cells. J Clin Invest 1995, 95:264-271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barks JL, McQuillan JJ, Iademarco MF: TNF-alpha and IL-4 synergistically increase vascular cell adhesion molecule-1 expression in cultured vascular smooth muscle cells. J Immunol 1997, 159:4532-4538 [PubMed] [Google Scholar]
- 49.Conrad DJ, Kuhn H, Mulkins M, Highland E, Sigal E: Specific inflammatory cytokines regulate the expression of human monocyte 15-lipoxygenase. Proc Natl Acad Sci USA 1992, 89:217-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, Witztum JL, Funk CD, Conrad D, Glass CK: Interleukin-4-dependent production of PPAR-γ ligands in macrophages by 12/15-lipoxygenase. Nature 1999, 400:378-382 [DOI] [PubMed] [Google Scholar]
- 51.Ramanathan S, de Kozak Y, Saoudi A, Goureau O, Van der Meide PH, Druet P, Bellon B: Recombinant IL-4 aggravates experimental autoimmune uveoretinitis in rats. J Immunol 1996, 157:2209-2215 [PubMed] [Google Scholar]
- 52.Jacobs MJ, van den Hoek AE, van Lent PL, van de Loo FA, van de Putte LB, van den Berg WB: Role of IL-2 and IL-4 in exacerbations of murine antigen-induced arthritis. Immunology 1994, 83:390-396 [PMC free article] [PubMed] [Google Scholar]