Abstract
Although a number of autoimmune diseases are known to develop in postmenopausal women, the mechanisms by which estrogen deficiency influences autoimmune lesions remain unclear. We speculate that antiestrogenic actions might be a potent factor in the formation of pathogenic autoantigens. Previously, we have identified 120-kd α-fodrin as an important autoantigen in Sjögren’s syndrome (SS). When healthy C57BL/6 (B6) mice were treated with an ovariectomy (Ovx), we found a significant increase in TUNEL+-apoptotic epithelial cells in the salivary gland cells associated with α-fodrin cleavage during 2 and 3 weeks after Ovx. By contrast, no apoptotic cells were found in estrogen receptor-α knockout mice. In in vitro studies using primary cultured mouse salivary gland cells and human salivary gland cells, we found a cleavage product of 120-kd α-fodrin in cells that had undergone tamoxifen (Tam)-induced apoptosis through caspase activation, especially caspase-1. Adoptive transfer of α-fodrin-reactive T cells into Ovx-B6 and -SCID mice resulted in the development of autoimmune exocrinopathy quite similar to SS. These results suggest that estrogen deficiency exerts a crucial influence on autoantigen cleavage, and may cause, in part, autoimmune exocrinopathy in postmenopausal women.
Loss of ovarian function following menopause results in functional failures of the immune system, bone metabolism, and endocrine system. Estrogenic action has been suggested to be responsible for the strong female preponderance of many autoimmune diseases, including systemic lupus erythematosus (SLE), scleroderma, rheumatoid arthritis (RA), and Sjögren’s syndrome (SS). 1-4 Sex hormones influence both humoral and cell-mediated immune responses in a number of experimental models. 5-9 Previous reports indicate that the increase in autoantibody production as a result of estrogen deficiency is mediated by cytokines such as interleukin-6 (IL-6), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α), and that estrogen plays an important role in the regulation of B-lymphocyte development in mouse bone marrow. 10-12
Recently, we have demonstrated that the dysfunction of regulatory T cells as a result of estrogen deficiency may play a crucial role on acceleration of organ-specific autoimmune lesions, and that estrogenic action influences target epithelial cells through Fas-mediated apoptosis in a murine model for SS. 13 Although autoimmune diseases are triggered by various environmental factors, such as hormonal changes, microbial infections, stress, and aging, 14,15 much less is known about the role of estrogen deficiency on the formation of autoantigen. We hypothesize that estrogen deficiency may influence the formation of pathogenic autoantigen in target organs through a T-cell-independent pathway.
Previously, we have identified 120-kd α-fodrin as an important autoantigen in both NFS/sld murine SS model and in patients, 16 but the mechanisms of α-fodrin cleavage in the salivary gland cells remain unclear. Our recent study has been strongly suggestive of essential roles of caspase cascade for α-fodrin cleavage leading to tissue destruction in primary SS. 17 α-fodrin is a ubiquitous, heterodimeric calmodulin-binding protein that is cleaved by calcium-activated protease (calpain) in apoptotic cells and caspase through Fas-mediated apoptosis in Jurkat cells. 18-20 The fodrin α-subunit of various cells has also been shown to be cleaved in association with apoptosis. 21-23 Several reports have demonstrated that estrogen may play an inhibitory role on apoptosis in endothelial cells, breast cancer cells, cardiac myocytes, prostate cells, and neuronal cells. 24-27 Moreover, it has been noted that some enzymatic activities are elevated in postmenopausal women compared with normal healthy women. 28,29
The aim of this study was to analyze the effect of estrogen deficiency on the formation of pathogenic autoantigen. Moreover, caspase activity in mouse salivary gland cells stimulated by an antiestrogenic action has been analyzed, indicating that estrogen deficiencies may play a pivotal role in autoantigen cleavage initially triggered in the salivary and lacrimal gland.
Materials and Methods
Mice and Treatments
Female C57BL/6 (B6) mice (H-2b) were purchased from Japan SLC (Shizuoka, Japan), and maintained in a specific pathogen-free (SPF) mouse colony and given food and water ad libitum. Estrogen receptor-α knockout mice (ERαKO) on a B6 strain background were purchased from Taconic (Germantown, NY). Female SCID C.B-17-scid/scid mice (H-2d), purchased from Japan SLC (Shizuoka), were used to confirm cell transfer experiment. Normal female B6 (H-2b), and BALB/c mice (H-2d), purchased from Japan SLC (Shizuoka), were used to obtain antigen-stimulated T cells. Mice were ovariectomized (Ovx) at 4 weeks of age and compared with sham-operated (Sham, in both strain) mice. At 1 to 6 weeks after Ovx, all organs were removed from the mice and analyzed.
Histological Analysis
All organs were removed from mice, fixed with 4.0% phosphate-buffered formaldehyde (pH 7.2), and prepared for histological examination. The sections were stained with hematoxylin and eosin. The disease incidence was determined using the histological score of inflammatory lesions by White and Casarret, 30 estimated by three independent, well-trained pathologist in a blinded manner.
In Situ End-Labeling of Fragmented DNA (TUNEL)
Apoptotic cells were detected in sections using the in situ TUNEL Kit (Wako Pure Chemical Industries, Ltd., Osaka, Japan). Briefly, sections were incubated with proteinase K (20 μg/ml) for 10 minutes, and then presoaked in TdT buffer (0.5 μmol/L cacodylate, 1 μmol/L CoCl, 0.5 μmol/L dithiothreitol, 0.05% bovine serum albumin, 0.15 mol/L NaCl) for 10 minutes. Sections were incubated for 2 hours at 37°C in 25 ml of TdT solution, containing 1X terminal transferase buffer, 0.5 nmol of biotin-dUTP, and 10 U of TdT (Wako Pure Chemical). After the TdT reaction, sections were soaked in TdT blocking buffer (300 nmol/L NaCl, 30 mmol/L tri-sodium citrate-2-hydrate), incubated with HRP-conjugated streptavidin for 30 minutes at room temperature, and developed for 10 minutes in phosphate-buffered citrate (pH 5.8) containing 0.6 mg/ml DAB. Nuclei were counterstained with hematoxylin.
Western Blot Analysis
Westrn blot analysis with anti-human α-fodrin (Affiniti, Mamhead, UK) was performed. Briefly, the cells were incubated in 20 mmol/L Tris-HCl (pH 8.0), 20 mmol/L NaCl, 0.5% Triton X-100, 5 mmol/L ethylenediaminetetraacetate (EDTA), and 3 mmol/L MgCl2 lysis buffer. After centrifugation for 20 minutes at 12,000 × g at 4°C, supernatant was extracted and used for sample. Ten micrograms of each sample per well was used for sodium dodecyl sulfide-polyacrylamide gel electrophoresis. Protein binding was visualized with enhanced chemiluminescence Western blotting reagent (Amersham Biosciences, Arlington Heights, IL). Control for protein loading was provided by anti-human α-tubulin, or GAPDH monoclonal antibody (Sigma Chemical Co., St. Louis, MO). To detect caspase-1 in cultured human cells and mouse tissues, Western blot analysis was performed by the indicated methods using anti-human caspase-1 (Sigma Chemical Co.), and anti-human active form caspase-1 (p20 subunit; Upstate Biotech, Charlottesville, VA) polyclonal antibody. An anti-human caspase-1 polyclonal antibody is known to cross-react with mouse lysate.
Primary Culture of Mouse Salivary Gland (MSG) Cells
Mouse salivary gland (MSG) epithelial cells were prepared as previously described. 13,17 Briefly, mouse salivary glands were minced into 1-mm2 pieces, washed with Hank’s balanced salt solution (HBSS) without Ca2+ and Mg2+, and placed in a 60-mm dish containing HBSS with 0.76 μg/ml EDTA, 4.9 μg/ml L-ascorbic acid, and 4.9 μg/ml reduced glutathione. Fragments were washed with Dulbecco’s modified Eagle’s medium/soybean trypsin inhibitor, and placed in a mixture of collagenase (750 U/ml of type I) and hyarulonidase (500 U/ml of type IV) dissolved in DMEM/F12 containing 10% fetal bovine serum (FBS). The digest suspension was passed through a 100-μm nylon mesh filter. Adherant cells after culture in DMEM containing 10% FBS for 24 hours at 37°C were isolated as salivary gland epithelial cells. Apoptotic cells were detected by flow cytometry with an EPICS flow cytometer (Beckman Coulter, Miami, FL) using the Annexin V-FITC Apoptosis Detection kit (Genzyme, Cambridge, MA).
Cell Culture
MSG cells, human salivary gland adenocarcinoma cell line (HSG), 31 breast cancer cell line (MCF-7), colon cancer cells (HT29, Colo201), and Jurkat cells were cultured in the DMEM and RPMI-1640 including 10% FBS at 37°C. When the cells were treated with tamoxifen (Tam) (ICN Biochemicals, Costa Mesa, CA) and 17β-estradiol (Wako Pure Chemical), they were cultured in the medium without FBS. Apoptotic cells were detected by flow cytometry with an EPICS flow cytometer (Beckman Coulter) using the Annexin V-FITC Apoptosis Detection kit (Genzyme).
Cell Transfection
We used polymerase chain reaction (PCR) techniques to generate derivatives of a human caspase-1 promoter-luciferase construct. The following forward and reverse oligomers were used as primers to create a promoter of the human caspase-1 gene: 5′CACTGCAGATTGAGAAACTCTTCACTG3′, 5′GATCTAGAGGCTTTTCTCTCCTCCCT3′. The PCR-amplified promoter fragments, including Pst-1/Xba-1 site, were cloned into the multiple cloning site of the pGL3-basic vector (Promega, Charbonnieres, France), upstream of the luciferase gene. The caspase-1 promoter-luciferase gene were transfected into HSG cells using LipofectAMIN (Promega). The vector pGL3-basic (lacking a promoter) and the vector (Promega) pGL3-control served as negative and positive controls, respectively. Briefly, the transfection medium, containing 10 μg of plasmid DNA and 60 μl of Lipofectin reagent in 2 ml of serum-free DMEM was incubated for 20 minutes at room temperature and then diluted with serum-free DMEM to a final volume of 5 ml and added to HSG cells, plated the day before. The transfection process occurred at 37°C for 5 hours, then 5 ml of DMEM containing 20% fetal calf serum (FCS) was added to the cells.
Luciferase Assay
The transfected cells were incubated for 24 hours and stimulated during the last 2 hours with Tam (1 × 10−7M). Pretreatment with 17β-estradiol (1 × 10−9M) was performed during last 12 hours. After rinsing with phosphate-buffered saline, cells were lysed with reporter lysis buffer (Promega), and cell extracts were used for luciferase assay with the Promega kit in a luminometer (Promega). To control transfection efficiency, pSVβ-galactosidase plasmid (Promega) was cotransfected with the luciferase reporter constructs in a 1:4 ratio. The results showed that the difference in the relative efficiency of transfection between constructs was negligible.
Caspase Activities
Caspase activities in Tam-induced apoptosis in HSG cell extracts and mouse various tissues were assayed using Caspase-Family Colorimetric Substrate Set (BioVision Inc., Palo Alto, CA). Briefly, tissue or cell lysates were incubated with pNA-conjugated substrates (200 mmol/L, caspase-1, -2, -3, -5, -6, -8, and -9 substrate: Ac-YVAD-pNA, Ac-VDVAD-pNA, Ac-DEVD-pNA, Ac-WEHD-pNA, Ac-VEID-pNA, Ac-IETD-pNA, and Ac-LEHD-pNA) at 37°C for 2 hours. Absorbance of each sample was read at 405 nm in a microtiter plate reader using a 96-well plate. Fold-increase in caspase activity was determined by comparing these results with the level of the uninduced control. The caspase inhibitors z-VAD-fmk (1 μmol/L), Ac-DEVD-CHO (1 μmol/L), and Ac-WEHD-CHO (0.5 μmol/L) (Sigma Chemical Co.) were added to the Tam-stimulated HSG cells. After the incubation for 48 hours, apoptotic cells were detected using Annexin-V flow cytometric analysis.
Adoptive Transfer
To obtain α-fodrin-reactive T cells, B6 mice were injected subcutaneously with 20 μg recombinant α-fodrin protein (JS-1) and Freund’s complete adjuvant (ICN Biochemicals) at 4 weeks of age, and i.p. injections of 20 μg JS-1 and Freund’s incomplete adjuvant (ICN Biochemicals) were performed at 6 weeks of age. OVA (10 μg/head)-reactive T cells were obtained as the same manner for control experiments. After 2 weeks later (8 weeks of age), mice were sacrificed and the splenic T cells were obtained as donor cells. As recipients, female B6 mice were ovariectomized (Ovx, n = 7) or sham-operated (Sham, n = 5) at 4 weeks of age. After 2 weeks (6 weeks of age), Ovx- and Sham-mice were transferred i.p. with 5 × 106 α-fodrin-reactive T cells. The transferred mice were analyzed at 4 and 8 weeks after the cell transfer. To confirm cell transfer experiments, Ovx- and Sham-SCID mice (n = 7 and n = 5) were transferred with α-fodrin-reactive T cells obtained from BALB/c mice in the same manner.
Proliferation Assay
Splenic T cells were performed on proliferation assay against various antigens including recombinant α-fodrin protein (JS-1). Single cell suspensions were cultured in 96-well flat bottom microtiter plate (5 × 105 cells/well) in RPMI-1640 containing 10% FCS, penicillin/streptomycin, and β-mercaptoethanol. Cells were cultured with 10 μg/ml JS-1, and 2.0 μg/ml Con A (EY Laboratories, San Mateo, CA). The control antigens used were OVA (5 μg/ml), lysozyme (5 μg/ml), and albumin (5 μg/ml) (Sigma, St. Louis, MO).3[H]thymidine incorporation during the last 20 hours of the culture was evaluated using an automatoid β-liquid scintillation counter.
Results
Apoptosis Induced in Salivary Gland Cells by Ovariectomy (Ovx)
To examine the in vivo effects of estrogen deficiency in normal B6 and ERαKO mice, Ovx was performed at the age of 4 weeks. A radioimmunoassay confirmed that β-estradiol was not detectable in the sera of Ovx-mice (Ovx-B6 and Ovx-ERαKO, not detected; Sham-B6, 27.4 ± 2.8 pg/ml; Sham-ERαKO, 26.8 ± 3.6 pg/ml). At 1 to 6 weeks after Ovx, an in situ apoptosis detection assay was performed using all organs. Although we found a significant increase in TUNEL+ apoptotic cells in the salivary gland sections of B6 mice, but not ERαKO mice, at 2 or 3 weeks after Ovx (Figure 1) ▶ , no significant apoptosis was observed in any other organs of Ovx and Sham mice. To define TUNEL labeling due to the effect of Ovx in vivo, we examined Annexin V-flow cytometric analysis using primary cultured MSG cells, indicating that a small proportion of apoptotic cells (12.5%) in Ovx mice was found, but not in Sham mice (3.1%) (Figure 1A) ▶ . No significant difference in number of TUNEL+ apoptotic cells of Ovx and Sham mice was observed at 0, 1, 4, 5, and 6 weeks after Ovx (Figure 1B) ▶ . Thus, apoptotic changes in the salivary glands of normal mice were observed transiently at 2 or 3 weeks after Ovx, supposing that antiestrogenic action to the epithelial tissues seems to be transient in vivo.
Figure 1.
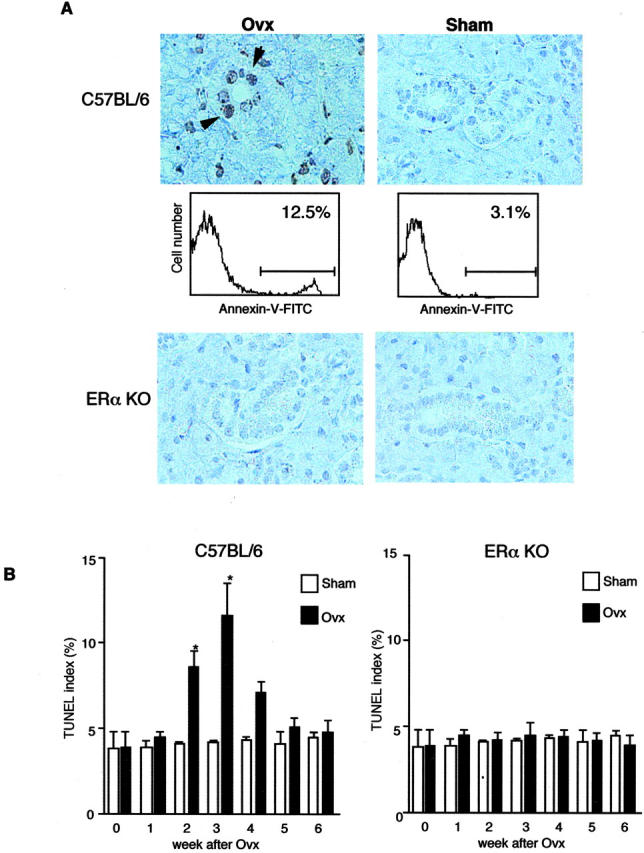
A: Detection of TUNEL+-apoptotic cells in the salivary gland sections from Ovx- and Sham-B6 mice, but not from ERαKO mice, at 3 weeks after Ovx (arrows). Annexin V-flow cytometric analysis using MSG cells demonstrated 12.5% positive cells detected in Ovx-B6 mice, but 3.1% positive in Sham-B6 mice. Data are representative in triplicate. B: A significant increase of apoptotic epithelial cells was observed in the salivary gland tissues from Ovx-B6 mice, not from ERαKO mice, restricted at 2 and 3 weeks after Ovx. The percentage of epithelial cells staining positively with TUNEL was enumerated using a 10- × 20-grid net micrometer disk covering an objective of area 0.16 mm2. Data were analyzed in 10 fields per section and expressed as mean percent ± SD in five mice examined per group. (*P < 0.05; Student’s t-test.)
Effect of Estrogen Deficiency on α-Fodrin Proteolysis
We next investigated whether estrogen deficiency is involved in the formation of pathogenic autoantigens in salivary glands. To analyze α-fodrin proteolysis in the salivary glands from Ovx-B6 mice, Western blot analysis was performed using tissue samples. Although an intense band expressing 120-kd α-fodrin was found in the salivary gland samples from Ovx-B6 mice, no cleavage products of α-fodrin were observed in other organs from Ovx mice or any of the samples from Sham mice, while expression of α-tubulin as a control was present (Figure 2) ▶ . Thus, estrogen deficiency induces in vivo proteolysis of α-fodrin in association with apoptosis in the normal salivary gland cells.
Figure 2.
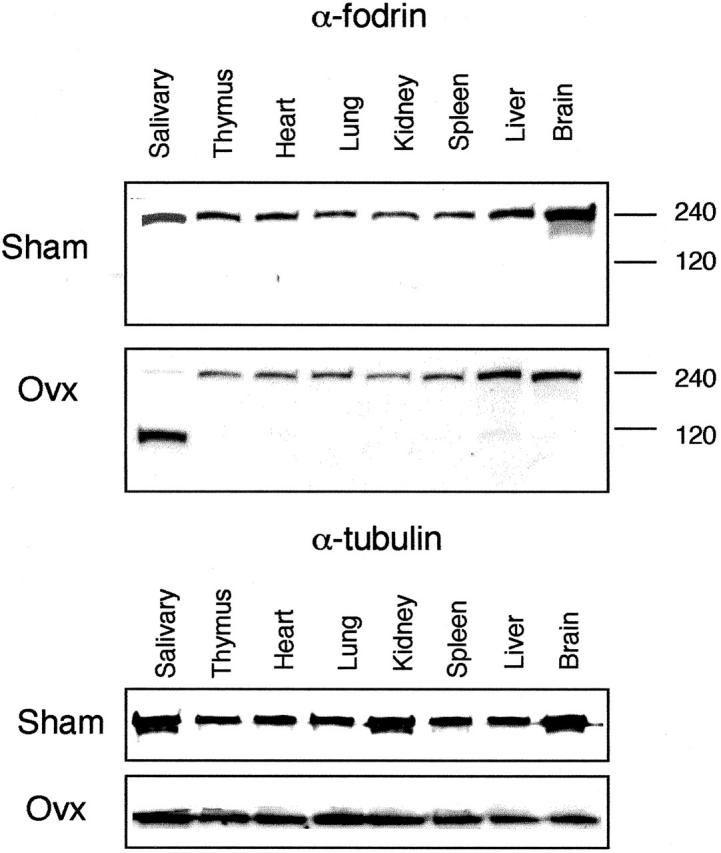
Detection of α-fodrin cleavage in various tissues from Ovx- and Sham-B6 mice on Western blot analysis showing distinct band of 120 kd in the salivary gland tissue alone from Ovx-B6 mice. α-tubulin protein as internal control was present. Data are representative of five mice in each group.
Tamoxifen (Tam)-Induced Apoptosis and α-Fodrin Cleavage in Salivary Gland Cells
It has been reported that the antiestrogen tamoxifen (Tam) induces cell death in the human breast cancer cell line MCF-7. 32 To examine whether Tam induces apoptosis in the mouse and human salivary gland (MSG and HSG) cells, the cells were treated with 1 × 10−10 to 1 × 10−6 (M) Tam for 48 hours. We found a time- and concentration-dependent increase in number of apoptotic MSG and HSG cells until 48 hours (data not shown). In contrast to the colon cancer cells (HT-29 and Colo201) or Jurkat cells, apoptosis was induced in MSG, HSG, and MCF7 cells treated with Tam (Figure 3A) ▶ . Of importance is that the 240-kd α-fodrin in Tam-induced apoptotic MSG and HSG cells was cleaved into 120-kd fragment in a time-dependent manner on Western blotting (Figure 3B) ▶ . In Tam-induced apoptotic MCF7 cells, negligible levels of cleaved products of α-fodrin were found on Western blotting. We next examined whether estrogen could inhibit Tam-induced apoptosis of MSG and HSG cells. As shown in Figure 3C ▶ , Tam-induced apoptosis of MSG and HSG cells was significantly reduced by the pretreatment with estrogen. These data indicate that apoptosis of mouse and human salivary gland cells followed by α-fodrin cleavage into 120-kd fragment could be induced by an antiestrogenic action.
Figure 3.
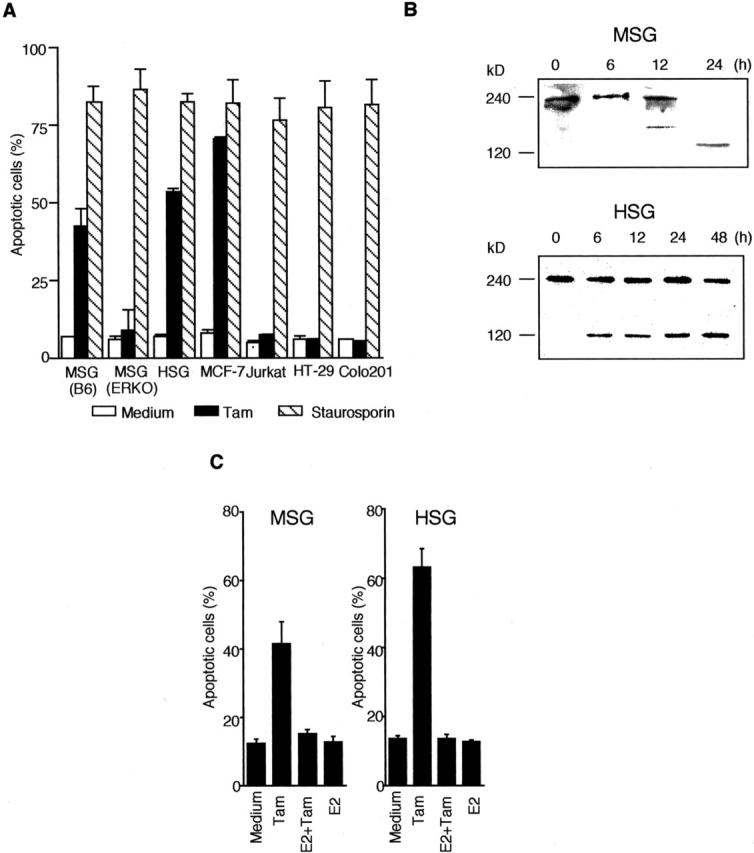
A: Effect of Tam (1 × 10−7 M) on apoptosis in various mouse and human cells (MSG, HSG, MCF-7, Jurkat, HT-29, and Colo201). Staurosporin (1 μmol/L) was used as common apoptotic reagent. B: α-fodrin cleavage into 120 kd in MSG and HSG cells induced by Tam. Western blot analysis with mouse monoclonal Ab to α-fodrin was performed in MSG and HSG cells stimulated with Tam (1 × 10−7 M) for 24 or 48 hours. The data are representative of three individual experiments. C: Tam-induced apoptosis of MSG and HSG cells was significantly reduced by the pretreatment with estrogen. Apoptotic cells were detected by flow cytometer using propidium lodide staining and Annexin V-FITC. The data are the mean ± SD from three individual experiments.
Participation of Caspases in Estrogen-Deficient Salivary Gland Cells
We next investigated whether apoptotic proteases are involved in the α-fodrin cleavage during Tam-induced apoptosis in MSG and HSG cells. A significant increase in caspase-1 activity was detected with relatively elevated caspase-3 and -8 activity on Tam-induced apoptotic HSG cells (Figure 4A) ▶ . In addition, Tam-induced apoptosis in HSG cells was inhibited considerably by treatment with caspase inhibitors zVAD, DEVD, and WEHD (Figure 4B) ▶ . We next examined the level of the caspase activities (caspase-1, -2, -3, -5, -6, -8, and -9) in various organs from Ovx- and non-Ovx-B6 mice. Figure 4C ▶ shows that a significantly elevated caspase-1 activity in the salivary gland tissues is observed in Ovx-B6 mice, with slight elevation of caspase-3 and 8 activity. No differences in caspase activities were observed in other organs. These results suggest that estrogen deficiency stimulates caspase activity, especially caspase-1, in the salivary gland tissues in vivo. We then examined whether Tam could influence the promoter activity of caspase-1 in HSG cells. After transfection with the plasmid containing the caspase-1 promoter-ligated upstream of the luciferase gene, HSG cells were stimulated with Tam, and then luciferase assay was performed. Figure 5A ▶ shows an increased promoter activity of caspase-1 after stimulation with Tam in HSG cells, but not in MCF-7, Jurkat, HT-29, and colo201 cells. The increase in caspase-1 promoter activity observed in HSG cells was significantly reduced by the addition of estrogen. We observed a time-dependent expression of caspase-1 (p20) activities until 48 hours on Western blot analysis (Figure 5B) ▶ . Figure 5C ▶ shows that a distinct expression of caspase-1 (p20) in the salivary gland tissues is observed in Ovx-B6 mice. These results suggest that the salivary gland cell apoptosis could be induced by caspase activation, especially caspase-1, in estrogen deficient state.
Figure 4.
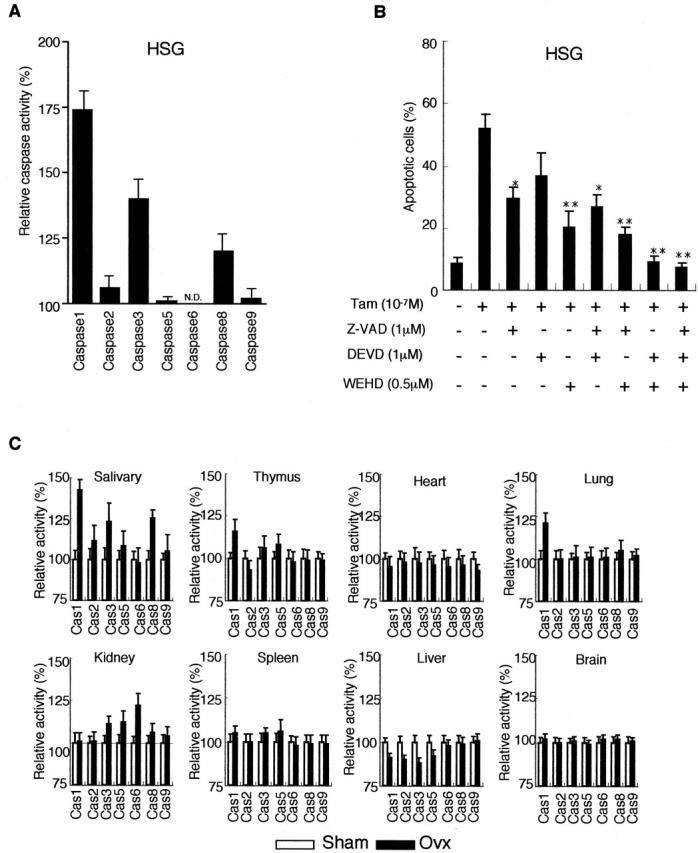
A: A significant increase in caspase-1 activity with relatively elevated caspase-3, and caspase-8 activity on Tam-induced apoptosis in HSG cells. B: Tam-induced apoptosis in HSG cells was inhibited considerably by the treatment with caspase inhibitors zVAD, DEVD, and WEHD. C: A significantly elevated caspase-1 activity in the salivary gland tissues is observed in Ovx-B6 mice with slight elevation of caspase-3, and caspase-8 activity. No significant differences in caspase activities were observed in other organs.
Figure 5.
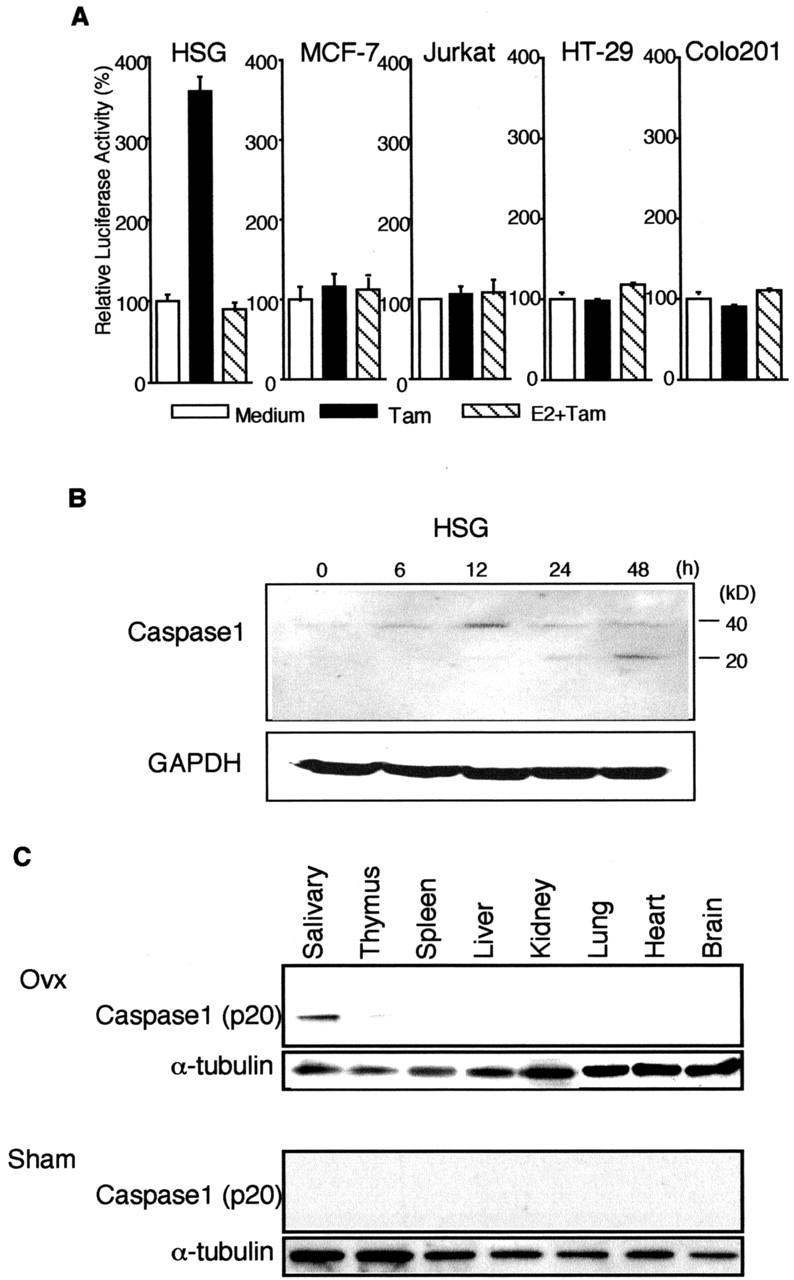
A: Luciferase assay of caspase-1 promoter activities in HSG cells stimulated with Tam. HSG, MCF-7, Jurkat, HT-29, and colo201 cells, after transfection with caspase-1 promoter Luc construct, were stimulated for 2 hours without or with 1 × 10−7 M Tam. Estrogen (17β-estradiol, 1 × 10−8 M) was added to the cells 12 hours before Tam stimulation. The results are the mean values of three independent experiments run in triplicate. B: Western blot analysis of caspase-1 in apoptotic HSG cells stimulated with Tam, showing an increase in procaspase-1 (40 kd) at 12 hours, and a time-dependent increase in caspase-1 active form (p20). Cytosolic extracts were prepared from HSG cells which were treated with Tam (1 × 10−7 M) for various times. C: Detection of caspase-1 active form (p20) in the salivary gland tissue, but not in various organs from Ovx- and Sham-B6 mice on Western blot analysis. α-tubulin proteins were used as internal control. Data were representative in triplicate.
Adoptive Transfer of α-Fodrin-Reactive T Cells into Ovx-B6 and -SCID Mice
We examined the adoptive transfer experiments using α-fodrin-reactive T cells into Ovx-B6 mice as shown in protocol (Figure 6A) ▶ . Before the cell transfer, we confirmed the proliferative T-cell response against JS-1, but not against lysozyme, albumin, and ovalbumin, of JS-1-immunized mice (Figure 6B) ▶ . Consequently, inflammatory lesions developed exclusively in the salivary and lacrimal gland at 4 and 8 weeks after the transfer with 5 × 106 α-fodrin-reactive T cells, not with 5 × 106 OVA-reactive T cells, while no inflammatory lesions in any other organs were detectable (Table 1 ▶ and Figure 6C ▶ ). When 5 × 106 α-fodrin-reactive CD4+ or CD8+ fractionated T cells were transferred into Ovx-B6 and Ovx-SCID mice, no inflammatory lesions were observed in any organs (data not shown). Proliferative T-cell response against recombinant α-fodrin (JS-1) was clearly observed in spleen cells from transferred Ovx-B6 mice (Figure 6D) ▶ . In Ovx-SCID mice, inflammatory lesions were also induced in the salivary and lacrimal gland at 8 weeks after the transfer with 5 × 10 6 α-fodrin-reactive T cells, while no inflammatory lesions in any other organs were detectable (Table 1) ▶ . Significant proliferative T-cell response against recombinant α-fodrin (JS-1) was observed in spleen cells from transferred Ovx-SCID mice (Figure 6E) ▶ .
Figure 6.
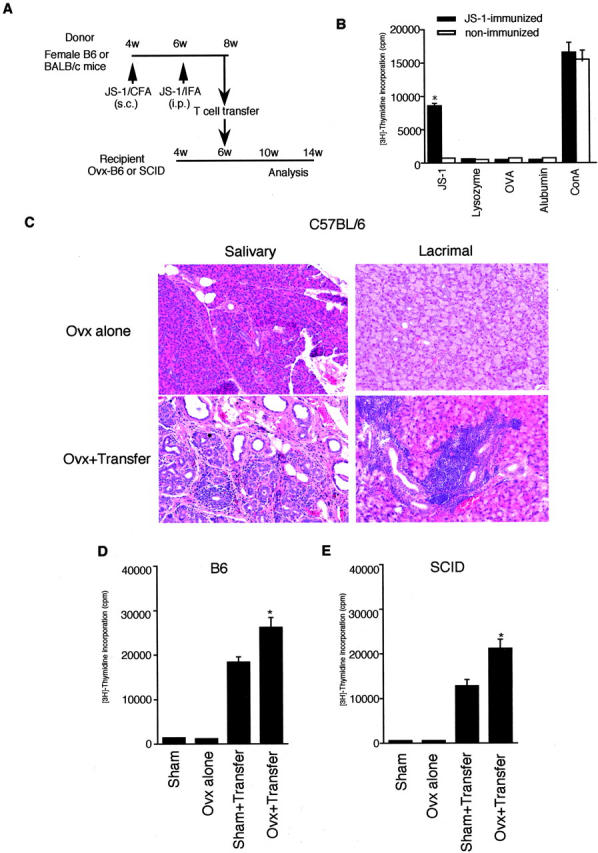
A: Experimental protocol of adoptive transfer using B6 and SCID mice as indicated. B: Significant proliferative response of the splenic T cells against recombinant α-fodrin (JS-1, 10 μg/ml) were confirmed before the transfer (*P < 0.01, Student’s t-test). Antigen-stimulated blastogenesis was measured in spleen T cells from immunized and non-immunized mice. No proliferative responses were found to lysozyme (5 μg/ml), albumin (5 μg/ml), and OVA (5 μg/ml). Data are expressed as counts per minute ± SD in triplicate. C: Representative histological features of the salivary and lacrimal glands in Ovx+transfer, and Ovx-alone mice (hematoxylin and eosin, magnification, ×120). D: Significant proliferative response of the splenic T cells against recombinant α-fodrin (JS-1, 10 μg/ml) in Ovx+transfer B6 mice was observed (*P < 0.01, Student’s t-test). E: Significant proliferative response of the splenic T cells against recombinant α-fodrin (JS-1, 10 μg/ml) in Ovx+transfer SCID mice was observed (*P < 0.05, Student’s t-test). Data are expressed as counts per minute ± SD in triplicate.
Table 1.
Frequency of Inflammatory Lesions in the Salivary and Lacrimal Glands in Transferred C57BL/6 and SCID Mice Treated with Ovx
| Treatment | No. of mice | No. of mice with lesions* | ||
|---|---|---|---|---|
| Submandibular | Parotid | Lacrimal | ||
| 4 weeks after transfer (C57BL/6) | ||||
| Ovx alone† | 5 | 0/5 | 0/5 | 0/5 |
| Sham | 5 | 0/5 | 0/5 | 0/5 |
| Ovx+transfer‡ | 7 | 5/7 | 7/7 | 6/7 |
| Sham+transfer | 7 | 0/7 | 0/7 | 0/7 |
| Ovx+transfer (OVA)§ | 5 | 0/5 | 0/5 | 0/5 |
| 8 weeks after transfer (C57BL/6) | ||||
| Ovx alone† | 5 | 0/5 | 0/5 | 0/5 |
| Sham | 5 | 0/5 | 0/5 | 0/5 |
| Ovx+transfer§ | 7 | 6/7 | 7/7 | 7/7 |
| Sham+transfer | 7 | 0/7 | 0/7 | 0/7 |
| Ovx+transfer (OVA)§ | 5 | 0/5 | 0/5 | 0/5 |
| 8 weeks after transfer (SCID) | ||||
| Ovx alone† | 5 | 0/5 | 0/5 | 0/5 |
| Sham | 5 | 0/5 | 0/5 | 0/5 |
| Ovx+transfer‡ | 7 | 5/7 | 6/7 | 6/7 |
| Sham+transfer | 7 | 0/7 | 0/7 | 0/7 |
*Histological evaluation for frequency of inflammatory lesions in the salivary and lacrimal glands was done according to the method proposed by White and Casarett. 30
†Female C57BL/6 (B6) and SCID mice were ovariectomized (Ovx) at 4 weeks of age.
‡Ovx-B6 and SCID mice were transferred intraperitoneally with 5 × 106 α-fodrin-reactive T cells at 6 weeks of age.
§Ovx-B6 mice were transferred with 5 × 106 OVA-reactive T cells. α-fodrin-reactive T cells were obtained from B6 or BALB/c mice as described in detail in the text.
Discussion
The mechanisms responsible for the development of autoimmune diseases during the postmenopausal stage are still unclear. Previous reports concerning gender differences in autoimmunity have suggested that estrogen influences the cytokine production of effector cells and autoantibody production. 33,34 The distinct immune environments in males and females underlie many of the gender-related differences in autoimmunity. These environments are established by the cytokines that are released by immune cells, particularly T helper (Th) lymphocytes. Sex hormones, pituitary hormones including prolactin, and growth hormones, as well as liver-derived insulin-like growth factor-1 affect autoimmune diseases by modulating cytokine productions. 35,36 Women have higher levels of these hormones than men. Estrogen withdrawal after menopause leads to an increase in the production of cytokines, such as granulocyte-macrophage colony-stimulating factor, IL-1, IL-6, and TNF-α. 37 Although many studies have described the effects of estrogen on cytokine productions in effector cells, much less is known about the effect of estrogen deficiency in target organs of postmenopausal women. Thus, it is required to determine how estrogen deficiency influences the expression of autoantigens in target cells before the infiltration of lymphocytes into the salivary glands.
In this study, we have demonstrated a significant apoptosis associated with α-fodrin cleavage in the salivary gland cells of estrogen deficient healthy B6 mice. Moreover, inflammatory lesions developed exclusively in the salivary and lacrimal gland after the adoptive transfer with α-fodrin-reactive T cells in both Ovx-B6 and Ovx-SCID mice. These data indicate that α-fodrin cleavage triggered by estrogen deficiency plays a role in the development of autoimmune exocrinopathy in the salivary and lacrimal gland. By contrast, apoptotic cells in the salivary glands were not found in ERαKO mice. In in vitro studies using primary cultured MSG and HSG, we found a cleavage product of 120-kd α-fodrin in cells that had undergone Tam-induced apoptosis, not in other type of cells including MCF-7. Since pretreatment with estrogen inhibits the Tam-induced apoptosis of MSG and HSG cells, estrogen may play a crucial role in the apoptosis-related signal pathway. When we analyzed whether cystein proteases are involved in Tam-induced apoptosis of HSG cells, we observed a time-dependent increase in the active forms of caspase-1. In addition, we found that the promoter activity of caspase-1 was significantly increased when HSG cells transfected with the promoter-caspase-1 gene were stimulated with Tam. Indeed, active forms of caspase-1 was detected only in the salivary gland tissues from Ovx-B6 mice. It has been reported that estrogen inhibits the apoptosis of human myocytes and endothelial cells by reducing the activity of caspase-1-like protease. 25,26 On the other hand, estrogen has been shown to inhibit bone resorption by directly inducing apoptosis of bone-resorbing osteoclasts. 38 Our results suggest that antiestrogenic actions might induce the salivary gland apoptosis through a caspase-1-mediated pathway. It is assumed that estrogen acts as a negative regulator of caspase-1 activity in the salivary gland cells.
When MSG and HSG cells were induced to undergo apoptosis using Tam, the 240-kd α-fodrin was cleaved into a single detectable fragment of 120 kd. Among the substrates cleaved during apoptosis are nuclear autoantigen targets for systemic autoimmune diseases such as PARP, U1–70-kd, the nuclear lamin, and DNA-dependent kinase. 39-43 In organ-specific autoimmune diseases, no evidence in in vivo cleavage of self proteins during apoptosis has been demonstrated. Our data suggest that antiestrogenic actions have a potent effect on the proteolysis of α-fodrin autoantigen in the salivary gland through up-regulation of caspase-1 activity. These results strongly suggest that α-fodrin fragments induced by Ovx may play an important role on the development of autoimmune lesions as a pathogenic autoantigen. Molecular mechanisms responsible for tissue-specific apoptosis induced by estrogen deficiency are further investigated.
In conclusion, we have demonstrated that antiestrogenic actions, including estrogen deficiency or Tam stimulation, may have a crucial influence on apoptosis and α-fodrin proteolysis through an increased caspase activity in the salivary gland cells, suggesting a novel mechanism for the development of organ-specific autoimmunity in postmenopausal women.
Footnotes
Address reprint requests to Yoshio Hayashi, Department of Pathology, Tokushima University School of Dentistry, 3 Kuramotocho, Tokushima 770, Japan. E-mail: hayashi@dent.tokushima-u.ac.jp.
This work was supported in part by Grants-in-Aid for Scientific Research (numbers 12307040 and 12557022) from the Ministry of Education, Science and Culture of Japan.
References
- 1.Lahita RG: The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol 1999, 11:352-356 [DOI] [PubMed] [Google Scholar]
- 2.Bateman A, Singh A, Kral T, Solomon S: The immune-hypothalamic-pituitary-adrenal axis. Endocrinol Rev 1989, 10:92-112 [DOI] [PubMed] [Google Scholar]
- 3.Grossman C: Possible underlying mechanisms of sexual dimorphism in the immune response, fact and hypothesis. J Steroid Biochem 1989, 34:241-251 [DOI] [PubMed] [Google Scholar]
- 4.Mooradian A, Morley J, Korenman S: Biological actions of androgens. Endocrinol Rev 1987, 8:1-28 [DOI] [PubMed] [Google Scholar]
- 5.Keisler LW, Vom-Saal FS, Keisler DH, Walker SE: Hormonal manipulation of the prenatal environment alters reproductive morphology and increases longevity in autoimmune NZB/W mice. Biol Reprod 1991, 44:707-716 [DOI] [PubMed] [Google Scholar]
- 6.Carlsten H, Nilsson N, Jonsson R, Backman K, Holmdahl R, Tarkowski A: Estrogen accelerates immune complex glomerulonephritis but ameliorates T cell-mediated vasculitis and sialadenitis in autoimmune MRL lpr/lpr mice. Cell Immunol 1992, 144:190-202 [DOI] [PubMed] [Google Scholar]
- 7.Jansson L, Olsson T, Holmdahl R: Estrogen induces a potent suppression of experimental autoimmune encephalomyelitis and collagen-induced arthritis in mice. J Neuroimmunol 1994, 53:203-207 [DOI] [PubMed] [Google Scholar]
- 8.Hawkins T, Gala R, Dunbar J: The effect of neonatal sex hormone manipulation on the incidence of diabetes in nonobese diabetic mice. Proc Soc Exp Biol Med 1993, 202:201-205 [DOI] [PubMed] [Google Scholar]
- 9.Jansson L, Mattsson A, Mattsson R, Holmdah IR: Estrogen-induced suppression of collagen arthritis: V: physiological level of estrogen in DBA/1 mice is therapeutic on established arthritis, suppresses anti-type II collagen T-cell dependent immunity and stimulates polyclonal B-cell activity. J Autoimmun 1990, 3:257-270 [DOI] [PubMed] [Google Scholar]
- 10.Ishimi Y, Miyaura C, Ohmura M, Onoe Y, Sato T, Uchiyama Y, Ito M, Wang X, Suda T, Ikegami S: Selective effects of genistein, a soybean isoflavone, on B-lymphopoiesis and bone loss caused by estrogen deficiency. Endocrinol 1999, 140:1893-1900 [DOI] [PubMed] [Google Scholar]
- 11.Masuzawa T, Miyaura C, Onoe Y, Kusano K, Ohta H, Nozawa S, Suda T: Estrogen deficiency stimulates B lymphopoiesis in mouse bone marrow. J Clin Invest 1994, 94:1090-1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poli V, Balena R, Fattori E, Markatos A, Yamamoto M, Tanaka H, Ciliberto G, Rodan GA, Costantini F: Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J 1994, 13:1189-1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishimaru N, Saegusa K, Yanagi K, Haneji N, Saito I, Hayashi Y: Estrogen deficiency accelerates autoimmune exocrinopathy in murine Sjogren’s syndrome through Fas-mediated apoptosis. Am J Pathol 1999, 155:173-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayes M: Epidemiologic studies of environmental agents and systemic autoimmune diseases. Environ Health Perspect 1999, 107:743-748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Z, Granucci F, Yeh L, Schaffer P, Cantor H: Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science 1998, 279:1344-1347 [DOI] [PubMed] [Google Scholar]
- 16.Haneji N, Nakamura T, Takio K, Yanagi K, Higashiyama H, Saito I, Noji S, Sugino H, Hayashi Y: Identification of α-fodrin as a candidate autoantigen in primary Sjogren’s syndrome. Science 1997, 276:604-607 [DOI] [PubMed] [Google Scholar]
- 17.Saegusa K, Ishimaru N, Yanagi K, Mishima K, Arakaki R, Suda T, Saito I, Hayashi Y: Prevention and induction of autoimmune exocrinopathy is dependent on pathogenic autoantigen cleavage in murine Sjögren’s syndrome. J Immunol 2002, 169:1050-1057 [DOI] [PubMed] [Google Scholar]
- 18.Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J: Human ICE/CED-3 protease nomenclature (letter). Cell 1996, 87:171. [DOI] [PubMed] [Google Scholar]
- 19.Martin SJ, O’Brien GA, Nishioka WK, McGahon AJ, Mahboubi A, Saido TC, Green DR: Proteolysis of fodrin (non-erythroid spectrin) during apoptosis. J Biol Chem 1995, 270:6425-6428 [DOI] [PubMed] [Google Scholar]
- 20.Martin SJ, Finucane DM, Amarante-Mendes GP, O’Brien GA, Green DR: Phosphatidylserine externalization during CD95-induced apoptosis of cells and cytoplasts requires ICE/CED-3 protease activity. J Biol Chem 1996, 271:28753-28756 [DOI] [PubMed] [Google Scholar]
- 21.Janicke RU, Sprengart ML, Wati MR, Porter AG: Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem 1998, 273:9357-9360 [DOI] [PubMed] [Google Scholar]
- 22.Cryns VL, Bergeron L, Zhu H, Li H, Yuan J: Specific cleavage of alpha-fodrin during Fas- and tumor necrosis factor-induced apoptosis is mediated by an interleukin-1β-converting enzyme/Ced-3 protease distinct from the poly(ADP-ribose) polymerase protease. J Biol Chem 1996, 271:31277-31282 [DOI] [PubMed] [Google Scholar]
- 23.Wang KK, Posmantur R, Nath R, McGinnis K, Whitton M, Talanian RV, Glantz SB, Morrow JS: Simultaneous degradation of αII- and βII-spectrin by caspase-3 (CPP32) in apoptotic cells. J Biol Chem 1998, 273:22490-22497 [DOI] [PubMed] [Google Scholar]
- 24.Szende B, Romics I, Vass L: Apoptosis in prostate cancer after hormonal treatment (letter). Lancet 1993, 342:1422. [DOI] [PubMed] [Google Scholar]
- 25.Spyridopoulos I, Sullivan A, Kearney M, Isner J, Losordo D: Estrogen-receptor-mediated inhibition of human endothelial cell apoptosis: estradiol as a survival factor. Circulation 1997, 95:1505-1514 [DOI] [PubMed] [Google Scholar]
- 26.Pelzer T, Schumann M, Neumann M, DeJager T, Stimpel M, Serfling E, Neyses L: 17β-estradiol prevents programmed cell death in cardiac myocytes. Biochem Biophys Res Commun 2000, 268:192-200 [DOI] [PubMed] [Google Scholar]
- 27.Pike CJ: Estrogen modulates neuronal Bcl-xL expression and β-amyloid-induced apoptosis: relevance to Alzheimer’s disease. J Neurochem 1999, 72:1552-1563 [DOI] [PubMed] [Google Scholar]
- 28.Acarturk F, Robinson JR: Vaginal permeability and enzymatic activity studies in normal and ovariectomized rabbits. Pharm Res 1996, 13:779-783 [DOI] [PubMed] [Google Scholar]
- 29.Oner P, Bekpinar S, Cinar F, Argun A: Relationship of some endogenous sex steroid hormones to leukocyte arylsulphatase A activities in pre- and postmenopausal healthy women. Horm Metab Res 1994, 26:301-304 [DOI] [PubMed] [Google Scholar]
- 30.White SC, Casarett GW: Induction of experimental autoallergic sialadenitis. J Immunol 1974, 112:178-185 [PubMed] [Google Scholar]
- 31.Shirasuna K, Sato M, Miyazaki T: A neoplastic epithelial duct cell line established form an irradiated human salivary gland. Cancer 1981, 48:745-752 [DOI] [PubMed] [Google Scholar]
- 32.Kyprianou N, English HF, Davidson NE, Isaacs JT: Programmed cell death during regression of the MCF-7 human breast cancer following estrogen ablation. Cancer Res 1991, 51:162-166 [PubMed] [Google Scholar]
- 33.Ansar-Ahmed S, Penhale WJ, Talal N: Sex hormones, immune responses, and autoimmune diseases. Mechanisms of sex hormone action. Am J Pathol 1985, 121:531-551 [PMC free article] [PubMed] [Google Scholar]
- 34.Ansar-Ahmed S, Dauphinee M, Montoya A, Talal N: Estrogen induces normal murine CD5+ B cells to produce autoantibodies. J Immunol 1989, 142:2647-2653 [PubMed] [Google Scholar]
- 35.Luca IC: Prolactin secretion and the immune system. Rev Med Chir Soc Med Nat Iasi 1997, 101:56-59 [PubMed] [Google Scholar]
- 36.Whitacre CC, Reingold SC, O’Looney PA: A gender gap in autoimmunity. Science 1999, 283:1277-1278 [DOI] [PubMed] [Google Scholar]
- 37.Kimble RB, Srivastava S, Ross FP, Matayoshi A, Pacifici R: Estrogen deficiency increases the ability of stromal cells to support murine osteoclastogenesis via an interleukin-1 and tumor necrosis factor-mediated stimulation of macrophage colony-stimulating factor production. J Biol Chem 1996, 271:28890-28897 [DOI] [PubMed] [Google Scholar]
- 38.Kameda T, Mano H, Yuasa T, Mori Y, Miyazawa K, Shiokawa M, Nakamaru Y, Hiroi E, Hiura K, Kameda A, Yang N, Hakeda Y, Kumegawa M: Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J Exp Med 1997, 186:489-495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casciola-Rosen L, Nicholson DW, Chong T, Rowan KR, Thornberry NA, Miller DK, Rosen A: Apopain/CPP32 cleaves proteins that are essential for cellular repair: a fundamental principle of apoptotic death. J Exp Med 1996, 183:1957-19648642305 [Google Scholar]
- 40.Oberhammer FA, Hochegger K, Froschl G, Tiefenbacher R, Pavelka M: Chromatin condensation during apoptosis is accompanied by degradation of lamin A+B, without enhanced activation of cdc2 kinase. J Cell Biol 1994, 126:827-837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casciola-Rosen LA, Anhalt GA, Rosen A: DNA-dependent protein kinase is one of a subset of autoantigens specifically cleaved early during apoptosis. J Exp Med 1995, 182:1625-1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tu S, Cerione RA: Cdc42 is a substrate for caspases and influences Fas-induced apoptosis. J Biol Chem 2001, 276:19656-19633 [DOI] [PubMed] [Google Scholar]
- 43.Slee EA, Adrain C, Martin SJ: Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem 2001, 276:7320-7326 [DOI] [PubMed] [Google Scholar]


