Abstract
Since ovarian carcinoma cells detach from the primary lesion and metastasize via peritoneal dissemination, we hypothesized that these cells are exposed to hypoxia, which may affect cell attachment and invasiveness. To address this hypothesis, we first examined in vivo the immunohistochemical expression of hypoxia-inducible factor-1α (HIF-1α) and its topological correlation with E-cadherin expression in ovarian carcinomas. We then examined in vitro the effect of hypoxia on the mRNA and protein expressions of E-cadherin using two ovarian cancer cell lines, SKOV3 and OVCAR3, and normal ovarian surface epithelial (OSE) cells. In addition, hypoxia-induced change in the expression of SNAIL, a transcriptional factor repressing E-cadherin expression, was also analyzed. Finally, we examined the facilitation of invasiveness of ovarian cancer cells under hypoxia using Matrigel invasion assay. Immunohistochemically, nuclear localization of HIF-1α was observed in 32 of the 76 (42%) carcinomas studied, and showed a topological correlation with loss of E-cadherin expression. Northern blotting, real-time PCR and Western blotting demonstrated that E-cadherin expression was remarkably decreased under hypoxia in both SKOV3 and OVCAR3 cells, but not in normal OSE cells. mRNA expression of SNAIL was increased under hypoxia in both ovarian cancer cell lines. Invasion assay revealed that hypoxia increases the invasiveness of ovarian cancer cells. Accordingly, the present study demonstrated that hypoxia induces down-regulation of E-cadherin in ovarian carcinoma cells, via up-regulation of the transcriptional repressor SNAIL. These findings suggest that hypoxia plays an important role in the change in intercellular attachment, which may be involved in the initiation of tumor progression of ovarian cancer cells.
Epithelial ovarian carcinoma is the leading cause of death from female genital malignancies, and more than half of patients are diagnosed at the advanced stage of the disease. 1 The prognosis of patients with advanced ovarian cancer is most likely related to the degree of peritoneal dissemination. Although the process of cancer metastasis appears to be regulated by a variety of gene products, 2 little is known about the molecular aspects of peritoneal dissemination of ovarian carcinoma cells. One of the important events in the first step of metastasis is the reduction of cellular adhesion between the tumor cells, facilitating invasion into the surrounding tissues and vascular channels. 3 E-cadherin and the associated catenin complex have been recognized as performing key roles in cell adhesion. Reduced expression of E-cadherin has been reported in various human cancers, being associated with tumor progression. 4-6
The search for factors affecting the progression and behavior of tumors has revealed the importance of the microenvironment surrounding the tumor cells. Peritoneal dissemination is a metastatic process in which the cancer cells detach from the primary tumor, attach to the peritoneum, and re-grow at this site. It is therefore hypothesized that ovarian cancer cells leaving the primary tumor experience lower oxygen levels. 7 Hypoxia is known to induce hypoxia-inducible factor-1α (HIF-1α), which binds to the hypoxia-response elements of various target genes and activates the transcription of these genes controlling glucose transport, angiogenesis, erythropoiesis, and vasomotor regulation, and thus may increase the survival of tumor cells. 8-10 With the progressive and rapid growth of malignant tumors, cancer cells in the ischemic condition are expected to transform to the “metastatic” phenotype through a reduction in intercellular adhesion as well as an increase in cell motility and invasiveness.
However, little is known about the hypoxic status in vivo and its functional relationship with intercellular adhesion in malignant tumors. Therefore, we first immunohistochemically examined the expression and localization of HIF-1α with relevance to those of E-cadherin in epithelial ovarian carcinomas. Based on the topological correlation between the two molecules, we hypothesized that hypoxia may affect the cell attachment and invasiveness of ovarian cancer cells. Our preliminary screening using microarray analysis showed possible hypoxia-induced changes in the expression of adhesion molecules including E-cadherin in ovarian cancer cells. 11 In this study, therefore, we examined the effect of hypoxia on the change in expression of E-cadherin at the mRNA and protein levels in ovarian cancer cell lines, as well as in the normal counterpart, ovarian surface epithelial (OSE) cells. Hypoxia-induced change in the expression of SNAIL, a transcriptional factor repressing E-cadherin expression, was also analyzed. Finally, the hypoxic effect on cell invasiveness was examined by in vitro invasion assay.
Materials and Methods
Tissue Samples
Seventy-six cases of primary epithelial ovarian carcinoma were selected from the pathology file of Shinshu University Hospital. The tissues were obtained from patients who underwent laparotomy at our department. None had received preoperative chemotherapy or radiotherapy. These specimens were fixed in 10% phosphate-buffered formalin and embedded in paraffin. Serial sections of 3-μm thickness were made for hematoxylin and eosin staining and for immunohistochemistry. Histologically, 27 of the 76 were serous, 7 were mucinous, 17 were endometrioid, and 25 were clear cell carcinomas. According to the classification of the International Federation of Gynecology and Obstetrics (FIGO), 37 were classified as stage I, 10 were stage II, 24 were stage III, and 5 were stage IV. Each tissue was used with the approval of the Ethical Committee of Shinshu University, after obtaining written informed consent from the patients.
Immunohistochemical Analyses
Immunostaining of E-Cadherin
Immunohistochemical staining was performed on paraffin-embedded sections by the streptavidin-biotin-peroxidase complex method using a Histofine SAB-PO kit (Nichirei, Tokyo, Japan). The primary antibodies were anti-E-cadherin monoclonal antibodies (number 20820, clone 36) (Transduction Laboratories, Lexington, KY) and were used at a dilution of 1:250. After deparaffinization and rehydration, the sections were boiled in 0.01 mol/L citrate buffer (pH 6.0) for 15 minutes in a microwave oven. They were then treated with 0.3% hydrogen peroxide and incubated with 10% normal rabbit serum to block non-specific binding. The sections were incubated with a primary antibody at 4°C overnight. After washing in phosphate-buffered saline (PBS), they were incubated with biotinylated goat anti-mouse IgG, followed by treatment with peroxidase-conjugated streptavidin and stained with diaminobenzidine with 0.15% hydrogen peroxide. Counterstaining was performed with hematoxylin. Immunoreactivity was semiquantitatively estimated: negative (-) corresponded to 0% positive cells, weakly positive (+) to 1% to 50% positive cells, and strongly positive (++) to >50% positive cells.
Immunostaining of HIF-1α
Serial, formalin-fixed and paraffin-embedded sections were used for HIF-1α immunostaining using the Catalyzed Signal Amplification System (DAKO, Carpinteria, CA), as described previously. 10 In brief, after deparaffinization and rehydration, the sections were treated with target retrieval solution (DAKO) at 95°C for 45 minutes. Primary antibody, mouse anti-HIF-1α monoclonal antibody (Novus Biologicals, Littleton, CO), was used at a dilution of 1:1000. They were counterstained with hematoxylin. Specific immunoreactivity for HIF-1α was observed in the cytoplasm and/or in the nuclei of the tumor cells. Cytoplasmic immunostaining was semiquantitatively estimated: negative (-) corresponded to 0% positive cells, weakly positive (+) to 1% to 50% positive cells, and strongly positive (++) to >50% positive cells. Since nuclear immunostaining was observed sporadically in the tumor cells, the cases were classified as positive (presence of tumor cells with nuclear staining) or negative (absence of tumor cells with nuclear staining).
Cell Culture
Ovarian Cancer Cells
The ovarian cancer cell lines OVCAR3 and SKOV3 were purchased from the ATCC (Rockville, MD). SKOV3 was cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA) with 5% fetal calf serum (FCS) (Biological Industries, Grand Island, NY) and 1% antibiotic-antimycotic solution (Invitrogen). OVCAR3 were cultured in RPMI-1640 (Sigma, St. Louis, MO) with 5% FCS and 1% antibiotic-antimycotic solution. Incubation was carried out at 37°C under 5% CO2 in air.
Normal Ovarian Surface Epithelial (OSE) Cells
Ovarian surface epithelium was obtained from five women who were treated surgically for benign gynecological disease, after obtaining written informed consent from each patient. At the operation, OSE cells were collected by scraping the surface of the ovaries with a surgical blade, and were immediately cultured on collagen type I-coated plastic dishes (Iwaki Glass, Chiba, Japan) in a 1:1 mixture of Medium 199 (Invitrogen) and MCDB 105 (Sigma) containing 15% FCS (Biological Industries). 10 The purity of cultured OSE cells was confirmed by positive immunostaining for cytokeratin and negative immunostaining for factor VIII. 10
For the reverse transcription-polymerase chain reaction (RT-PCR) and Western blotting, the cultured cells were transferred to a 75-cm 2 flask (5 × 10 5 cells/flask) and incubated at 37°C under 20% O2 as control, or at 37°C under 5% O2 as hypoxia for 72 hours. 10,11
For in vitro invasion assay, it was difficult to perform the experiments using normal OSE cells. Instead, we used SV40 large T-immortalized OSE cells (IOSE 398), kindly provided by Dr. Nelly Auersperg at The University of British Columbia, Vancouver, Canada.
Total RNA Extraction
Total RNA was extracted by the acid guanidinium-phenol-chloroform method as described previously. 12,13 One microgram of total RNA was treated with 1 U/10 μl DNase I (Life Technologies, Gaithersburg, MD).
RT-PCR
RT was performed using an RNA PCR Kit (Takara Shuzo, Otsu, Japan), the 1 μg RNA sample being added to 20 μl of a reaction mixture consisting of 10 mmol/L Tris-HCl (pH 8.3), 50 mmol/L KCl, 5 mmol/L MgCl2, 1 mmol/L dNTP mixture, 1 U/μl of RNase inhibitor, 0.25 U/μl of avian myeloblastosis virus-derived reverse transcriptase, and 0.125 μmol/L of oligo d(T)-adaptor primer. Using a thermal cycler (Gene Amp PCR System 2400-R; PerkinElmer, Norwalk, CT), the reaction mixture was incubated at 42°C for 30 minutes, heated at 99°C for 5 minutes, and then cooled down to 5°C for 5 minutes.
One microliter of the RT products, containing 50 ng reverse-transcribed total RNA, was amplified by adding 20 μl of a PCR reaction mixture containing 10 mmol/L Tris-HCl (pH 8.3), 50 mmol/L KCl, 2.5 U/100 μl of TaKaRa TaqDNA polymerase, with 0.2 μmol/L of a set of 20- to 21-mer oligonucleotide primers either for E-cadherin, HIF-1α, or SNAIL and for glyceraldehyde-3-phosphate dehydrogenase (G3PDH) cDNA. Primers were synthesized to encompass a specific segment of the cDNA sequence of E-cadherin 14 (sense, 5′-TCCATTTCTTGGTCTACGCC′ and antisense, 5′-CACCTTCAGCCAACCTGTTT-3′), HIF-1α 15 (sense, 5′-CCCTGCAGTAGGTTTCTGCT-3′ and antisense, 5′-CTCAAAGTCGGACAGCCTCA-3′),SNAIL 16 (sense, 5′-AATCGGAAGCCTAACTACAAG-3′ and antisense, 5′-AGGAAGAGACTGAAGTAGAG-3′), or of G3PDH (sense, 5′-ACGACCACTTTGTCAAGCTC-3′ and antisense 5′-TCACA GTTGCCATGTAGACC-3′, spanning between exons 7 and 8). The corresponding cDNA fragments were denatured at 94°C for 30 seconds, annealed at 58°C for 1 minute, and extended at 72°C for 1 minute. After 35 cycles of amplification, the PCR products were analyzed on a 2% agarose gel, and the bands were visualized using ethidium bromide during exposure to an UV transilluminator. The density of the bands on the gel was quantified by densitometric analysis using a Quantity One Scan System (ATTO, Tokyo, Japan). Gene expression was presented by the relative yield of PCR product from the target sequence to that from the G3PDH gene. Mean values from three independent experiments were taken as results.
Real-Time PCR
To confirm the results of RT-PCR, mRNA expression was also analyzed using real-time PCR (Roche LightCycler; Roche Diagnostics, Mannheim, Germany). A master-mix of the following reaction components was prepared to the indicated end-concentration: 6.4 μl water, 1.2 μl MgCl2 (4 mmol/L), 0.2 μl forward primer (0.4 mol/L), 0.2 μl reverse primer (0.4 mol/L) and 1.0 μl LightCycler Fast Start DNA Master SYBR Green I (Roche Diagnostics). Nine microliters of master-mix was added to glass capillaries and 1 μl volume, containing 50 ng reverse-transcribed total RNA, and was added as a PCR template. The capillaries were closed, centrifuged, and placed into the rotor. To improve SYBR Green I quantification, the temperature of the fluorescence measurement point was set at 72°C. At the completion of cycling, melting curve analysis was carried out to establish the specificity of the amplicons produced. The level of expression of each mRNA and their estimated crossing points in each sample were determined relative to the standard preparation using the Light Cycler computer software. A ratio of specific mRNA/G3PDH amplification was then calculated to correct for any differences in efficiency. The relative abundance of the mRNAs, expressed as fold changes, was extrapolated from crossing point data. A difference of one PCR cycle in crossing-point number represents a twofold change in mRNA expression.
Northern Blotting
Fifteeen micrograms of total RNA were electrophoresed on a denaturing 1% agarose gel containing formaldehyde and transferred to Hybond-N membranes (Amersham Biosciences Inc., Buckinghamshire, UK). The membranes were prehybridized at 42°C for 6 hours in 50% formamide, 5X saline-sodium phosphate-EDTA, 5X Denhardt’s solution, 1% sodium dodecyl sulfate (SDS), and 100 μg/ml denatured herring sperm DNA. For probes, genomic DNA obtained from OSE was used as a template, [γ32P]deoxy-CTP (Amersham Biosciences Inc.) was labeled by PCR using templates and primers specific for E-cadherin, and G3PDH was used as an internal control. After prehybridization, the filters were hybridized with probe at 42°C for 12 hours. The hybridized filters were washed in 1X SSC with 0.1% SDS at 55°C for 20 minutes. The bands were analyzed using a MacBas system (Fuji Photo Film, Tokyo, Japan) and were then quantitated by densitometric analysis using a Quantity One Scan System.
Western Blotting
Cells were lysed in a lysis buffer: 50 mmol/L Tris-HCl (pH 8.0), 0.25 mol/L NaCl, 0.5% NP-40, 1 mmol/L PMSF (Sigma), 1 μg/ml aprotinin (Roche Diagnostics), 1 μg/ml leupeptin (Roche Diagnostics), and 20 μg/ml TPCK (Roche Diagnostics). The lysates were centrifuged at 13,000 × g for 20 minutes at 4°C and the supernatants were stored at −80°C. Extracts equivalent to 30 μg of total protein were separated by SDS-polyacrylamide gel electrophoresis (8% acrylamide) and transferred onto nitrocellulose membranes (Hybond TM-C super; Amersham Biosciences Inc.). The membranes were blocked in TBST (0.2 mol/L NaCl, 10 mmol/L Tris, pH 7.4, 0.2% Tween-20) containing 5% nonfat dry milk and 0.02% NaN3 for 1 hour, then incubated with mouse monoclonal antibodies against E-cadherin, HIF-1α and β-actin (Santa Cruz, St Louis, MO) in TBST containing 5% nonfat dry milk. The membranes were then incubated with sheep anti-mouse Ig (Amersham Biosciences Inc.) in TBST containing 2% nonfat dry milk. Bound antibodies were detected with an enhanced chemiluminescence system (Amersham Biosciences Inc.).
In Vitro Invasion Assay
Cell invasion through reconstituted basement membrane Matrigel was assayed by a method reported previously. 17 Briefly, polycarbonate membranes (8.0-μm pore size) of the upper compartment of transwell culture chambers were coated with 5% Matrigel (BD Biosciences, Bedford, MA). Subconfluent cells were starved for 24 hours and harvested with 0.05% trypsin containing 0.02% EDTA, washed twice with PBS, and resuspended at 1 × 10 6 cells/ml in serum-free medium with 0.1% bovine serum albumin (BSA). The cell suspension (500 μl) was placed in the upper compartment, and the lower compartment was immediately filled with 500 μl of serum-free medium containing 0.1% BSA. After 20 hours of incubation, at 37°C under 20% O2 as control, or at 37°C under 5% O2 as hypoxia, the cells on the upper surface of the filter were removed carefully with a cotton swab, the membranes were stained with Diff-Quik solution (Kokusai-Shiyaku, Kobe, Japan) and the cells that had migrated through the membrane to the lower surface were counted in five different fields under a light microscope at ×100 magnification. Each experiment was performed in triplicate wells and repeated three times.
Statistical Analyses
The Kruskal-Wallis test, Scheffé test, and Mann-Whitney U test were used to compare the immunoreactivity of E-cadherin and HIF-1α according to the FIGO stage and histological type. The Mann-Whitney U-test was used to assess the differences in the invasion assay. Differences were considered to be significant at P < 0.05. These analyses were made using the StatView system (Abacus, Berkeley, CA).
Results
Loss of E-Cadherin Expression is Associated with HIF-1α Expression
Expression of E-Cadherin
The immunohistochemical expression of E-cadherin was observed along the cytoplasmic membrane (Figure 1) ▶ , and strongly positive in 56 (74%), weakly positive in 17 (22%), and negative in 3 (4%) of the 76 ovarian carcinomas (Table 1) ▶ . According to the FIGO stage classification, strong expression of E-cadherin was found in 32 of the 37 stage I (86%), 7 of the 10 stage II (70%), 17 of the 24 stage III (71%), and none of the 5 stage IV (0%) tumors. Strong immunostaining of E-cadherin was significantly higher in stage I/II (83%) than in stage III/IV (59%) (P < 0.05). Regarding the histological type, the expression of E-cadherin was strongly positive in 17 of the 27 serous (63%), 7 of the 7 mucinous (100%), 12 of the 17 endometrioid (71%), and 20 of the 25 clear cell (80%) carcinomas. There was no significant difference according to the histological type.
Figure 1.
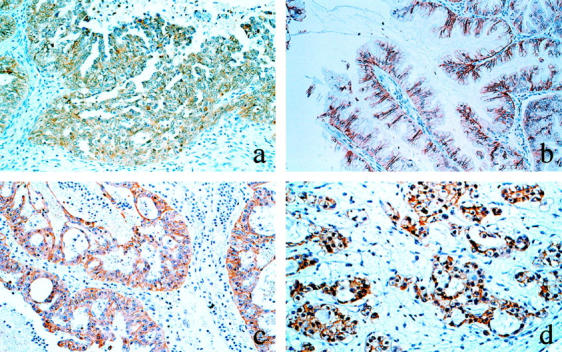
Immunohistochemical staining of E-cadherin in ovarian carcinomas. a: Serous carcinoma. b: Mucinous carcinoma. c: Endometrioid carcinoma. d: Clear cell carcinoma. Magnification, ×100
Table 1.
Immunohistochemical Expression of E-Cadherin in Ovarian Carcinomas According to FIGO Stage and Histological Type
| Total | E-cadherin expression | % | |||||
|---|---|---|---|---|---|---|---|
| − | + | ++ | ≧++ | ||||
| 76 | 2 | 18 | 56 | 56/76 | 74 | ||
| FIGO stage | |||||||
| I | 37 | 0 | 5 | 32 | 32/37 | 86 | I+II |
| II | 10 | 0 | 3 | 7 | 7/10 | 70 | 39/47 (83%)* |
| III | 24 | 0 | 7 | 17 | 17/24 | 71 | III+IV |
| IV | 5 | 2 | 3 | 0 | 0/5 | 0 | 17/29 (59%)* |
| Histology | |||||||
| Serous | 27 | 2 | 8 | 17 | 17/27 | 63 | |
| Mucinous | 7 | 0 | 0 | 7 | 7/7 | 100 | |
| Endometrioid | 17 | 0 | 5 | 12 | 12/17 | 71 | |
| Clear cell | 25 | 0 | 5 | 20 | 20/25 | 80 | |
*, P < 0.05.
−, 0% positive cells; +, 1–50% positive cells; ++, >50% positive cells.
Expression of HIF-1α
The immunohistochemical expression of HIF-1α in the nuclei was observed in 32 of the 76 (42%) ovarian carcinomas (Table 2) ▶ . According to the FIGO stage, the nuclear expression of HIF-1α was found in 17 of the 37 stage I (46%), 4 of the 10 stage II (40%), 9 of the 24 stage III (38%), and 2 of the 5 stage IV (40%) tumors. With regard to the histological type, it was observed in 11 of the 27 serous (41%), 2 of the 7 mucinous (29%), 6 of the 17 endometrioid (22%), and 13 of the 25 clear cell carcinomas (52%) (Table 2) ▶ . There were no significant differences in the nuclear expression of HIF-1α according to the FIGO stage or histological type. Irrespective of the stage and histology, however, cancer cells with nuclear HIF-1α immunoreactivity were observed frequently in the tip of the papillary projection of the tumor (Figure 2a) ▶ or in the vicinity of the necrotic area (Figure 2b) ▶ .
Table 2.
Cytoplasmic and Nuclear Expression of HIF-1α in Ovarian Carcinomas According to FIGO Stage and Histological Type
| Total | Cytoplasmic expression | Nuclear expression | |||||||
|---|---|---|---|---|---|---|---|---|---|
| − | + | ++ | ≧+ | % | − | + | % | ||
| 76 | 14 | 40 | 22 | 62/76 | 82 | 44 | 32 | 42 | |
| FIGO stage | |||||||||
| I | 37 | 7 | 16 | 14 | 30/37 | 81 | 20 | 17 | 46 |
| II | 10 | 3 | 5 | 2 | 7/10 | 70 | 6 | 4 | 40 |
| III | 24 | 3 | 17 | 4 | 21/24 | 88 | 15 | 9 | 38 |
| IV | 5 | 1 | 2 | 2 | 4/5 | 80 | 3 | 2 | 40 |
| Histology | |||||||||
| Serous | 27 | 5 | 14 | 8 | 22/27 | 82 | 16 | 11 | 41 |
| Mucinous | 7 | 3 | 3 | 1 | 4/7 | 57 | 5 | 2 | 29 |
| Endometrioid | 17 | 2 | 9 | 6 | 15/17 | 88 | 11 | 6 | 35 |
| Clear cell | 25 | 4 | 14 | 7 | 21/25 | 84 | 12 | 13 | 52 |
Cytoplasmic immunostaining was estimated as follows: −, 0% positive cells; +, 1–50% positive cells; ++, >50% positive cells.
Nuclear immunostaining was classified as follows: −, absence of tumor cells with nuclear staining; +, presence of tumor cells with nuclear staining.
Figure 2.
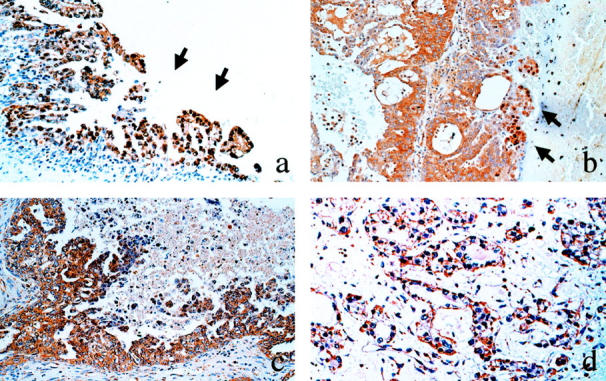
Immunohistochemical staining of HIF-1α in ovarian carcinoma tissues. Nuclear localization of HIF-1α is sporadically observed in the tumor cells in the tip of the papillary projection (a) or in the vicinity of necrosis (b). Cytoplasmic localization of HIF-1α is more frequently observed (c and d). a: Serous carcinoma. b: Endometrioid carcinoma. c: Serous carcinoma. d: Clear cell carcinoma. Magnification, ×100
The immunohistochemical expression of HIF-1α in the cytoplasm was strongly positive in 22 (29%) (Figure 2, c and d) ▶ , weakly positive in 40 (53%), and negative in the remaining 14 (18%) of the 76 ovarian carcinomas. There were no significant differences in the cytoplasmic HIF-1α expression according to the FIGO stage or histological type (Table 2) ▶ .
Topological Correlation between E-Cadherin and HIF-1α
Closer observation of the immunoreactivity for E-cadherin and HIF-1α using the serial sections disclosed that the tumor cells with nuclear HIF-1α expression were associated with reduced expression of E-cadherin compared with the surrounding tumor cells that were negative for HIF-1α (Figure 3) ▶ . Such reduced expression of E-cadherin along with nuclear HIF-1α expression was observed in 21 of the 32 cases (66%), especially in the cells at the tip of the papillary projection of the tumor (Figure 3, a and b) ▶ or in the vicinity of the necrotic area (Figure 3, c and d) ▶ . In the remaining 11 cases, E-cadherin expression was preserved, although the tumor cells expressed HIF-1α in the nuclei.
Figure 3.
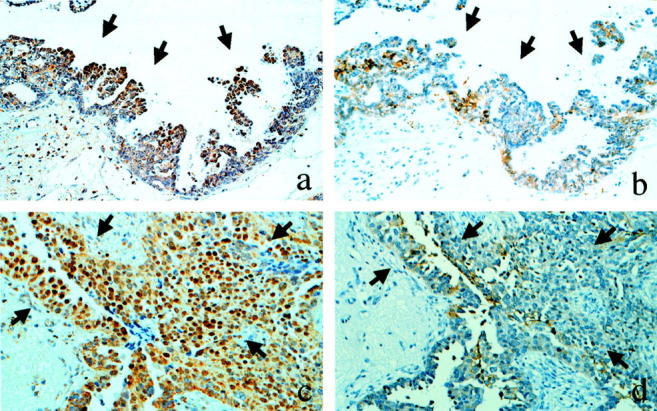
Topological correlation between HIF-1α (a and c) and E-cadherin (b and d). In the serial sections for immunolocalization of HIF-1α and E-cadherin, the tumor cells with nuclear expression of HIF-1α (a and c; arrows) are associated with reduced or loss of expression of E-cadherin (b and d; arrows), compared with the surrounding tumor cells negative for HIF-1α. Magnification, ×100
With regard to the expression of HIF-1α in the cytoplasm, there was no apparent topological correlation with reduced expression of E-cadherin. However, the 32 cases with nuclear HIF-1α expression were either strongly positive (14 cases) or weakly positive (18 cases) for cytoplasmic HIF-1α expression. Reduced expression of E-cadherin was observed in 9 of the 14 cases with strong expression, and in 12 of the 18 cases with weak expression for HIF-1α in the cytoplasm.
Hypoxia Attenuates E-Cadherin Expression in Ovarian Cancer Cells
mRNA Expression of E-Cadherin
To clarify whether hypoxic conditions decrease E-cadherin mRNA expression, we performed Northern blotting. Specific bands for E-cadherin and G3PDH mRNA were detected (Figure 4A) ▶ . In the 2 ovarian cancer cell lines, the band intensities for E-cadherin were reduced under hypoxia compared with those under normoxia by 19% in SKOV3 and by 36% in OVCAR3. In normal OSE cells, however, the expression of E-cadherin was very low and showed no difference between the cells cultured in hypoxia and normoxia (Figure 4, A and B) ▶ .
Figure 4.
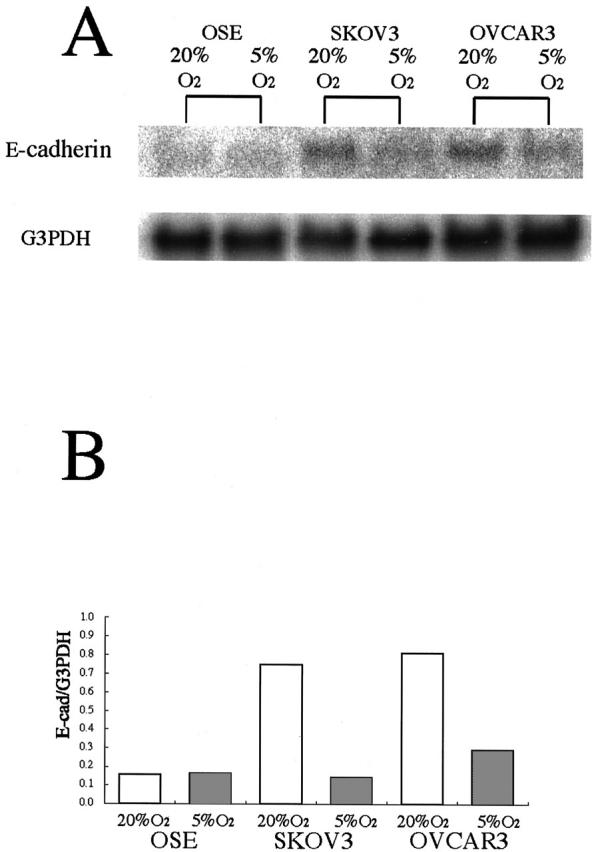
Northern blot analysis showing the effect of hypoxia on E-cadherin mRNA in OSE cells and in two ovarian cancer cell lines. A: The specific bands for E-cadherin mRNAs are decreased under hypoxia in SKOV3 and OVCAR3, but not in OSE. B: The density of the bands was quantitated by densitometric analysis. Data are presented after normalization with the G3PDH bands.
Protein Expression of E-Cadherin and HIF-1α
Specific bands for E-cadherin and HIF-1α under normoxia were detected by Western blotting in both SKOV3 and OVCAR3, but not in OSE cells (Figure 5A) ▶ . β-actin was expressed at 42 kd in all of the samples. In the ovarian cancer cell lines, the expression of E-cadherin was decreased under hypoxia by 21% in SKOV3 and by 56% in OVCAR3 (Figure 5, A and B) ▶ . On the other hand, the expression of 120 kd HIF-1α was increased under hypoxia by 190% in SKOV3 and by 228% in OVCAR3 (Figure 5A) ▶ . Apparently, normal OSE cells did not express E-cadherin or HIF-1α, and hypoxia did not affect the expression of either E-cadherin or HIF-1α.
Figure 5.
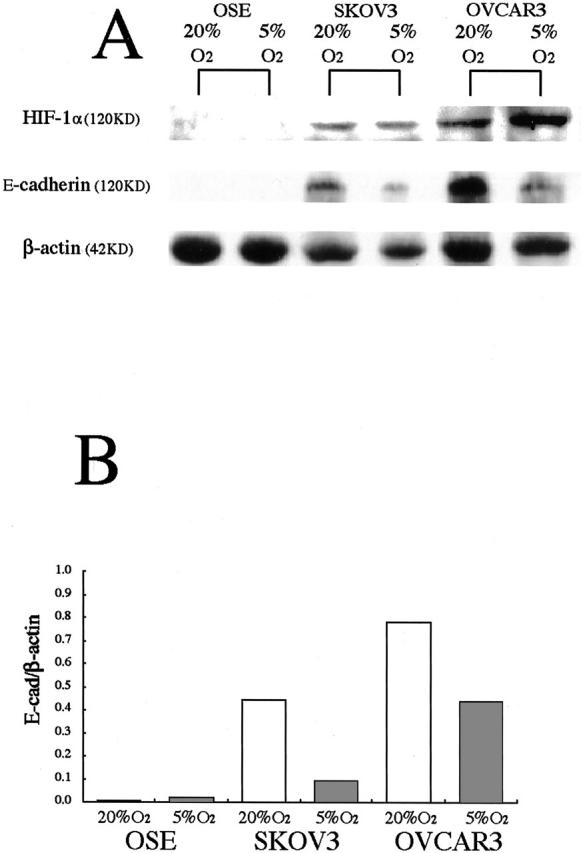
Western blot analysis showing the effect of hypoxia on HIF-1α and E-cadherin proteins in OSE cells and in two ovarian cancer cell lines. A: Specific bands for HIF-1α and E-cadherin were detected, and the band density of HIF-1α is increased in two ovarian cancer cells, but not in OSE cells under hypoxia. E-cadherin expression is remarkably decreased in two ovarian cancer cells, but not in OSE cells under hypoxia. B: The density of the E-cadherin bands was quantified by densitometric analysis. Data are presented after normalization with the β-actin bands.
mRNA Expression of SNAIL
To address the mechanism involved in hypoxia-induced change in the E-cadherin expression, we examined the mRNA expression of SNAIL, a transcriptional repressor of E-cadherin, using RT-PCR. A specific band for SNAIL was detected in cultured cells in both 20% O2 and 5% O2 (Figure 6) ▶ . In both ovarian cancer cell lines, the band density of SNAIL was increased under hypoxia by 178% in SKOV3 and 277% in OVCAR3, compared with under normoxia (Figure 6, A and B) ▶ . Along with the increase in SNAIL mRNA, the band density of E-cadherin was decreased under hypoxia (Figure 6, A and C) ▶ . We confirmed the above results using real-time RT-PCR (Table 3) ▶ . mRNA expression of SNAIL was increased under hypoxia by 172% in SKOV3 and 250% in OVCAR3. Simultaneously, mRNA expression of E-cadherin was decreased by 40% in SKOV3 and 48% in OVCAR3, compared with under normoxia. In contrast, the expression of SNAIL and E-cadherin was not changed in OSE cells.
Figure 6.
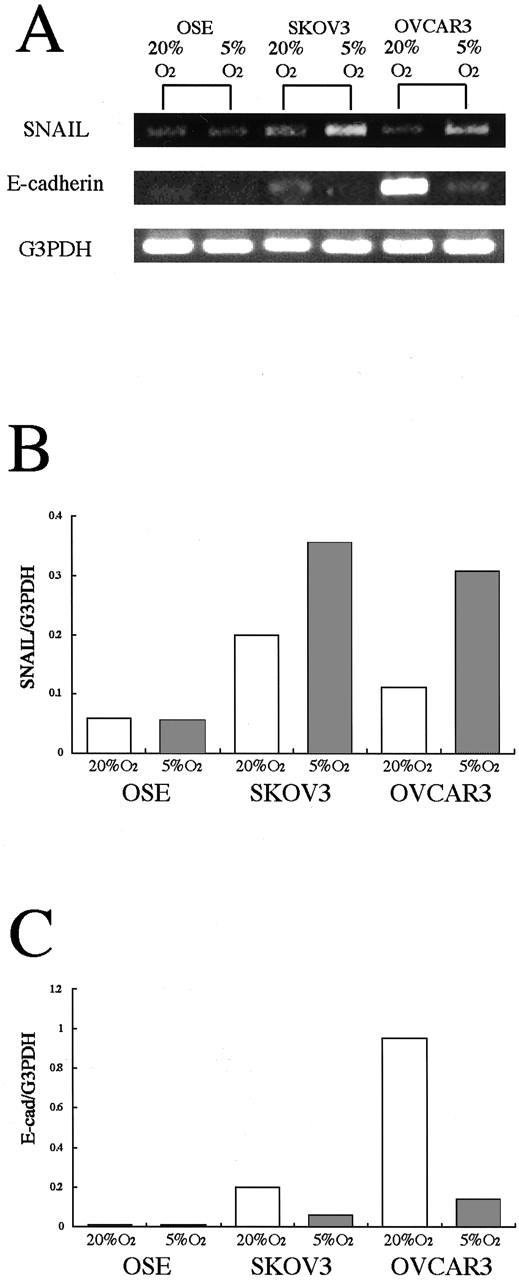
RT-PCR analysis showing the effect of hypoxia on the expression of SNAIL and E-cadherin mRNA in OSE cells and in two ovarian cancer cell lines. A: A specific band for SNAIL mRNA is detected in normoxia and is increased under hypoxia in two cultured ovarian cancer cells, but not in OSE cells. Along with SNAIL up-regulation, the expression of E-cadherin mRNA is decreased in two ovarian cancer cells, but not in OSE cells. B: The density of the bands for SNAIL was quantitated by densitometric analysis. C: The density of the bands for E-cadherin was quantitated by densitometric analysis. Data are expressed after normalization with the G3PDH bands.
Table 3.
mRNA Expression of SNAIL and E-Cadherin Using Real-Time RT-PCR
| 20% O2 | 5% O2 | Ratio (5% O2/20% O2) | |
|---|---|---|---|
| SNAIL/G3PDH | |||
| OSE | 2.14 ± 0.51 × 10−4 | 2.33 ± 0.65 × 10−4 | 1.09 ± 0.09 |
| SKOV3 | 1.79 ± 0.52 × 10−4 | 3.15 ± 1.49 × 10−4 | 1.72 ± 0.40 |
| OVCAR3 | 1.30 ± 0.11 × 10−4 | 3.23 ± 0.97 × 10−4 | 2.50 ± 0.77 |
| E-Cadherin/G3PDH | |||
| OSE* | 0.69 × 10−3 | 0.72 × 10−3 | 1.04 |
| SKOV3 | 2.55 ± 0.69 × 10−3 | 1.03 ± 0.42 × 10−3 | 0.40 ± 0.11 |
| OVCAR3 | 4.37 ± 2.03 × 10−3 | 1.81 ± 0.59 × 10−3 | 0.48 ± 0.30 |
*E-cadherin expression was detected in OSE cells from only one of three cases examined.
Hypoxia Facilitates Invasiveness of Ovarian Cancer Cells
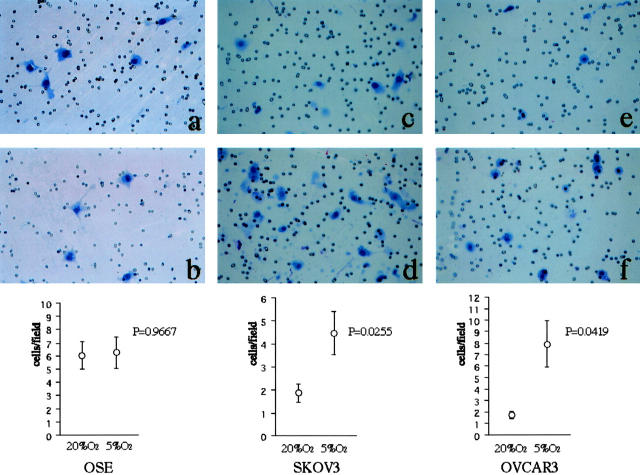
Finally, we examined whether hypoxia stimulates the invasiveness of ovarian cancer cells along with the suppression of E-cadherin expression, using Matrigel invasion assay. The cells were incubated for 20 hours in either normoxia or hypoxia, and the number of cells per field that passed through the Matrigel-coated membranes was counted. We tested the effect of hypoxia in immortalized OSE cells, and found that hypoxia did not affect their behavior (Figure 7, a and b) ▶ . In contrast, the invasive capacity of the ovarian cancer cells was significantly facilitated under hypoxia compared with that under normoxia in both SKOV3 (P = 0.0255) (Figure 7, c and d) ▶ and OVCAR3 (P = 0.0419) cells (Figure 7, e and f) ▶ .
Figure 7.
In vitro invasion assay showing the effect of hypoxia on the invasiveness of ovarian cancer cells. a: Photographs showing the cells that have passed through the Matrigel-coated membranes; OSE, normoxia. b: OSE, hypoxia. c: SKOV3, normoxia. d: SKOV3, hypoxia. e: OVCAR3, normoxia. f: OVCAR3, hypoxia. Original magnification, ×200. The number of both SKOV3 and OVCAR3 cells invading the Matrigel is significantly increased under hypoxic condition compared with under normoxia. Hypoxic effect was not seen in OSE cells.
Discussion
The present study showed that the expression of E-cadherin in ovarian carcinomas was reduced in 26% of the cases examined. Epithelial ovarian tumors including benign, borderline, and frankly invasive tumors are known to be characterized by the expression of E-cadherin protein, whereas normal OSE cells lack its expression. 18 Down-regulation of E-cadherin was reported in a substantial percentage of carcinomas or borderline tumors, when compared with benign epithelium. 19 Reduced expressions of E-cadherin and catenins have been observed in various human malignancies, often being associated with reduced differentiation and often correlating with the presence of lymph node or blood bone metastasis, 20,21 suggesting an essential role in the capability to invade locally and spread to distant organs. Our study showed that E-cadherin expression is more frequently reduced in advancing stages of the disease; strong immunostaining was seen in 83% of stage I/II cases versus 59% of stage III/IV cases. Thus, reduced expression of E-cadherin may also be important in the tumor progression of ovarian carcinomas.
In our study, the expression of HIF-1α in the nuclei and cytoplasm was observed in 42% and 82% of ovarian carcinomas cases, respectively. It has recently been reported that increased levels of HIF-1α are found in human cancers and are associated with tumor progression and angiogenesis. 22-24 In ovarian cancer patients, strong expression of HIF-1α protein was significantly correlated with short survival. 25 In addition, our study showed that the nuclear HIF-1α expression was topologically correlated with loss of E-cadherin expression. HIF-1α is known to be translocated into the nucleus under hypoxia, and is involved in gene transcription. 8,9 In fact, in ovarian carcinomas, nuclear expression of HIF-1α was frequently observed in tumor cells at the tip of the papillary proliferation or in the vicinity of necrosis. Such localization of HIF-1α seems to be consistent with reports showing that the tumor cells at these sites are under hypoxia. 26 The expression and localization of HIF-1α was reported to be well correlated with tissue hypoxia. 27 We previously reported that ovarian cancer cells at the tip of the papillary projection apart from blood vessels exhibit stronger expression of VEGF, 10 which is well known to be up-regulated by hypoxia. 28,29 Therefore, our in vivo observation of an inverse correlation between HIF-1α and E-cadherin suggests that nuclear localization of HIF-1α along with down-regulation of E-cadherin might have occurred under hypoxia in ovarian carcinomas.
Based on the above findings, we hypothesized that hypoxia may directly or indirectly down-regulate the expression of E-cadherin, resulting in a decrease in cell attachment and facilitation of invasiveness. To address this hypothesis, we performed in vitro experiments using ovarian cancer cell lines, SKOV3 and OVCAR3, cultured under normoxic/hypoxic conditions. In our preliminary experiments by microarray analysis using Human Cancer CHIP version 2.1 (Takara Shuzo, Otsu, Japan), in which 425 human cancer-related genes and 11 control housekeeping genes could be screened, hypoxic condition decreased the expression of E-cadherin and β-catenin but did not change the expression of other adhesion molecules such as integrins. 11 Therefore, we then analyzed the hypoxia-induced change in the expression of E-cadherin at the mRNA and protein levels using Northern blotting, real-time RT-PCR, and Western blotting. The results indicated that hypoxic condition reduces the expression of E-cadherin mRNA by 19% in SKOV3 and by 36% in OVCAR3 cells. In addition, the percent decrease in E-cadherin protein in SKOV3 and OVCAR3 cells was 21% and 56%, respectively. Interestingly, such hypoxia-induced change in the E-cadherin expression was not observed in normal OSE cells. It is known that OSE cells have a unique character, not only of the epithelial but also of the mesenchymal nature. 30 E-cadherin expression in OSE cells has been reported to be present at mRNA level but not at protein level, 18 and forced E-cadherin expression resulted in the epithelial differentiation and neoplastic progression. 18 Further studies are needed to explore why normal OSE cells exhibit no or very little expression of E-cadherin. Accordingly, our in vivo and in vitro observations indicate that hypoxia down-regulates the expression of E-cadherin in ovarian cancer cells. To our knowledge, this is the first report to demonstrate the functional relationship between hypoxia and E-cadherin in malignant tumor cells, although this possibility was suggested by Beavon in 1999. 31
There are multiple mechanisms involved in inactivating the E-cadherin-mediated cell adhesion system in cancer, 6 such as gene mutations, promoter hypermethylations, posttranslational truncation, 32 and the recently reported transcriptional repressions. 33,34 In ovarian carcinomas, E-cadherin mutations appear to be infrequent. 35 Methylation rates of E-cadherin were reportedly higher in ovarian cancer than normal tissue, but it has not been correlated with protein expression. 33 Our study focused on the expression of the transcriptional repressor SNAIL, which has been considered important in epithelial-mesenchymal transition not only in embryonic development but also in tumor progression through the direct repression of E-cadherin. 34 An inverse correlation between SNAIL and E-cadherin has been reported in cell lines derived from breast cancer, 34 hepatocellular carcinoma, 36 and in oral squamous cell carcinoma, 37 and melanomas. 38 Our study showed that hypoxia induces the expression of SNAIL mRNA by 172% in SKOV3 and 250% in OVCAR3 cells. Along with up-regulation of SNAIL, the expression of E-cadherin mRNA was decreased under hypoxia in both ovarian cancer cell lines studied. Taken together, it is likely that hypoxia-induced attenuation of the expression of E-cadherin is mediated by up-regulation of SNAIL in ovarian cancer cells. However, it has been reported that the expression of E-cadherin is regulated by a complex interplay of multiple regulatory regions dispersed throughout large parts of the E-cadherin promoter. 39 The structure and function of E-cadherin is sensitive to posttranslational modification. 6 It should also be noted that, in our series of ovarian carcinoma, E-cadherin expression was preserved in 11 of the 32 cases with nuclear HIF-1α expression. These findings suggest the presence of other pathways for the regulation of E-cadherin expression in ovarian carcinoma cells.
Finally, our in vitro invasion assay showed that the invasiveness of both ovarian cancer cells is enhanced under the same hypoxic condition as adopted in the attenuation of E-cadherin expression. These findings suggest that hypoxia-induced changes in the expression of E-cadherin are actually involved in tumor progression. Loss of E-cadherin expression is regarded as a central event in tumor metastasis, as reduction of cell adhesion between tumor cells facilitates their ability to invade. 40 It is likely that malignant tumor cells exhibit uncontrolled growth, eventually outstripping their blood supply, and are exposed to sublethal ischemia, which itself may have the effect of reducing cell adhesion and stimulating invasiveness and angiogenesis. 31 In head and neck cancers, cervical carcinomas and soft tissue sarcomas, it has been reported that the presence of hypoxia may be associated with increased invasiveness and a propensity to metastasis. 41-43 Ovarian cancer cells, especially of serous papillary histology, exhibit papillary growth, and these tumor cells tend to detach from each other, resulting in frequent peritoneal dissemination. 44 We observed a loss of E-cadherin expression along with the induction of HIF-1α in these ovarian cancer cells. These data suggest that hypoxia contributes to tumor progression and to the transformation into “metastatic phenotype” by inactivating E-cadherin via SNAIL in ovarian carcinomas. Therefore, such hypoxia-HIF-1α and hypoxia-SNAIL-cadherin systems may be possible candidates for molecular targeting in the future treatment of malignant ovarian tumors. 45 Further researches on the molecular aspects involved in peritoneal dissemination are needed to develop a novel molecular target therapies for ovarian carcinomas.
Acknowledgments
We thank Dr. Nelly Auersperg for access to the Canadian Ovarian Tissue Bank.
Footnotes
Address reprint requests to Akiko Horiuchi, M.D., Department of Obstetrics and Gynecology, Shinshu University School of Medicine, 3–1-1 Asahi, Matsumoto 390-8621, Japan. E-mail: aki9hori@hsp.md.shinshu-u.ac.jp.
Supported by Grant-in-Aid for Scientific Research to I.K. (number 13470349) from the Ministry of Education, Science, and Culture, Japan.
References
- 1.Murdoch WJ: Ovarian surface epithelium, ovulation and carcinogenesis. Biol Rev Camb Philos Soc 1996, 71:529-543 [DOI] [PubMed] [Google Scholar]
- 2.Gunthert U, Birchmeier W, Schlag PM: Attempts to understand metastasis formation: II. regulatory factors: introduction. Curr Top Microbiol Immunol 1996, 213:V-VII [PubMed] [Google Scholar]
- 3.Bernstein LR, Liotta LA: Molecular mediators of interactions with extracellular matrix components in metastasis and angiogenesis. Curr Opin Oncol 1994, 6:106-113 [DOI] [PubMed] [Google Scholar]
- 4.Shiozaki H, Oka H, Inoue M, Tamura S, Monden M: E-cadherin mediated adhesion system in cancer cells. Cancer 1996, 77:1605-1613 [DOI] [PubMed] [Google Scholar]
- 5.Bukholm IK, Nesland JM, Karesen R, Jacobsen U, Borresen-Dale AL: E-cadherin and α-, β-, and γ-catenin protein expression in relation to metastasis in human breast carcinoma. J Pathol 1998, 185:262-266 [DOI] [PubMed] [Google Scholar]
- 6.Van Aken E, De Wever O, Correia da Rocha AS, Mareel M: Defective E-cadherin/catenin complexes in human cancer. Virchows Arch 2001, 439:725-751 [DOI] [PubMed] [Google Scholar]
- 7.Mutch DG, Williams S: Biology of epithelial ovarian cancer. Clin Obstet Gynecol 1994, 37:406-422 [DOI] [PubMed] [Google Scholar]
- 8.Semenza GL: Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol 1999, 15:551-578 [DOI] [PubMed] [Google Scholar]
- 9.Ratcliffe PJ, O’Rourke JF, Maxwell PH, Pugh CW: Oxygen sensing, hypoxia-inducible factor-1 and the regulation of mammalian gene expression. J Exp Biol 1998, 201:1153-1162 [DOI] [PubMed] [Google Scholar]
- 10.Horiuchi A, Imai T, Shimizu M, Oka K, Wang C, Nikaido T, Konishi I: Hypoxia-induced changes in the expression of HIF-1α, VEGF and cell cycle-related molecules in ovarian cancer cells. Anticancer Res 2002, 22:2697-2702 [PubMed] [Google Scholar]
- 11.Imai T, Horiuchi A, Wang C, Nikaido T, Konishi I: Hypoxia-induced changes in the expression of cell adhesion molecules in ovarian cancer cells. Mok JE Quinn MA Namkoong SE Kim YT eds. 9th Biennial Meeting of the International Gynecologic Cancer Society. 2002:pp 99-101 Monduzzi Editore Bologna
- 12.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 13.Horiuchi A, Nikaido T, Ito K, Zhai Y, Orii A, Taniguchi S, Toki T, Fujii S: Reduced expression of calponin h1 in leiomyosarcoma of the uterus. Lab Invest 1998, 78:839-846 [PubMed] [Google Scholar]
- 14.Mialhe A, Levacher G, Champelovier P, Martel V, Serres M, Knudsen K, Seigneurin D: Expression of E-, P-, N-cadherins and catenins in human bladder carcinoma cell lines. J Urol 2000, 164:826-835 [DOI] [PubMed] [Google Scholar]
- 15.Wang GL, Jiang BH, Rue EA, Semenza GL: Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 1995, 92:5510-5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng CW, Wu PE, Yu JC, Huang CS, Yue CT, Wu CW, Shen CY: Mechanisms of inactivation of E-cadherin in breast carcinoma: modification of the two-hit hypothesis of tumor suppressor gene. Oncogene 2001, 20:3814-3823 [DOI] [PubMed] [Google Scholar]
- 17.Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN: A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res 1987, 47:3239-3245 [PubMed] [Google Scholar]
- 18.Auersperg N, Pan J, Grove BD, Peterson T, Fisher J, Maines-Bandiera S, Somasiri A, Roskelley CD: E-cadherin induces mesenchymal-to-epithelial transition in human ovarian surface epithelium. Proc Natl Acad Sci USA 1999, 96:6249-6254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darai E, Scoazec JY, Walker-Combrouze F, Mlika-Cabanne N, Feldmann G, Madelenat P, Potet F: Expression of cadherins in benign, borderline, and malignant ovarian epithelial tumors: a clinicopathologic study of 60 cases. Hum Pathol 1997, 28:922-928 [DOI] [PubMed] [Google Scholar]
- 20.Tomlinson JS, Alpaugh ML, Barsky SH: An intact overexpressed E-cadherin/α, β-catenin axis characterizes the lymphovascular emboli of inflammatory breast carcinoma. Cancer Res 2001, 61:5231-5241 [PubMed] [Google Scholar]
- 21.Cai J, Ikeguchi M, Tsujitani S, Maeta M, Liu J, Kaibara N: Significant correlation between micrometastasis in the lymph nodes and reduced expression of E-cadherin in early gastric cancer. Gastric Cancer 2001, 4:66-74 [DOI] [PubMed] [Google Scholar]
- 22.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW: Overexpression of hypoxia-inducible factor 1α in common human cancers and their metastases. Cancer Res 1999, 59:5830-5835 [PubMed] [Google Scholar]
- 23.Semenza GL: HIF-1 and human disease: one highly involved factor. Genes Dev 2000, 14:1983-1991 [PubMed] [Google Scholar]
- 24.Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL, Bedi A: Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1α. Genes Dev 2000, 14:34-44 [PMC free article] [PubMed] [Google Scholar]
- 25.Birner P, Schindl M, Obermair A, Breitenecker G, Oberhuber G: Expression of hypoxia-inducible factor 1α in epithelial ovarian tumors: its impact on prognosis and on response to chemotherapy. Clin Cancer Res 2001, 7:1661-1668 [PubMed] [Google Scholar]
- 26.Nakayama K, Kanzaki A, Hata K, Katabuchi H, Okamura H, Miyazaki K, Fukumoto M, Takebayashi Y: Hypoxia-inducible factor 1α (HIF-1α) gene expression in human ovarian carcinoma. Cancer Lett 2002, 176:215-223 [DOI] [PubMed] [Google Scholar]
- 27.Vukovic V, Haugland HK, Nicklee T, Morrison AJ, Hedley DW: Hypoxia-inducible factor-1α is an intrinsic marker for hypoxia in cervical cancer xenografts. Cancer Res 2001, 61:7394-7398 [PubMed] [Google Scholar]
- 28.Yamamoto S, Konishi I, Mandai M, Kuroda H, Komatsu T, Nanbu K, Sakahara H, Mori T: Expression of vascular endothelial growth factor (VEGF) in epithelial ovarian neoplasms: correlation with clinicopathology and patient survival, and analysis of serum VEGF levels. Br J Cancer 1997, 76:1221-1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shweiki D, Itin A, Soffer D, Keshet E: Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992, 35:843-845 [DOI] [PubMed] [Google Scholar]
- 30.Wong AS, Maines-Bandiera SL, Rosen B, Wheelock MJ, Johnson KR, Leung PC, Roskelley CD, Auersperg N: Constitutive and conditional cadherin expression in cultured human ovarian surface epithelium: influence of family history of ovarian cancer. Int J Cancer 1999, 81:180-188 [DOI] [PubMed] [Google Scholar]
- 31.Beavon IR: Regulation of E-cadherin: does hypoxia initiate the metastatic cascade? Mol Pathol 1999, 52:179-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rashid MG, Sanda MG, Vallorosi CJ, Rios-Doria J, Rubin MA, Day ML: Posttranslational truncation and inactivation of human E-cadherin distinguishes prostate cancer from matched normal prostate. Cancer Res 2001, 61:489-492 [PubMed] [Google Scholar]
- 33.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A: The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2000, 2:84-89 [DOI] [PubMed] [Google Scholar]
- 34.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA: The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2000, 2:76-83 [DOI] [PubMed] [Google Scholar]
- 35.Risinger JI, Berchuck A, Kohler MF, Boyd J: Mutations of the E-cadherin gene in human gynecologic cancers. Nat Genet 1994, 7:98-102 [DOI] [PubMed] [Google Scholar]
- 36.Jiao W, Miyazaki K, Kitajima Y: Inverse correlation between E-cadherin and Snail expression in hepatocellular carcinoma cell lines in vitro and in vivo. Br J Cancer 2002, 86:98-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yokoyama K, Kamata N, Hayashi E, Hoteiya T, Ueda N, Fujimoto R, Nagayama M: Reverse correlation of E-cadherin and snail expression in oral squamous cell carcinoma cells in vitro. Oral Oncol 2001, 37:65-71 [DOI] [PubMed] [Google Scholar]
- 38.Poser I, Dominguez D, de Herreros AG, Varnai A, Buettner R, Bosserhoff AK: Loss of E-cadherin expression in melanoma cells involves up-regulation of the transcriptional repressor Snail. J Biol Chem 2001, 276:24661-24666 [DOI] [PubMed] [Google Scholar]
- 39.Stemmler MP, Hecht A, Kinzel B, Kemler R: Analysis of regulatory elements of E-cadherin with reporter gene constructs in transgenic mouse embryos. Dev Dyn 2003, 227:238-245 [DOI] [PubMed] [Google Scholar]
- 40.Beavon IR: The E-cadherin-catenin complex in tumour metastasis: structure, function and regulation. Eur J Cancer 2000, 36:1607-1620 [DOI] [PubMed] [Google Scholar]
- 41.Sundfor K, Lyng H, Rofstad EK: Tumour hypoxia and vascular density as predictors of metastasis in squamous cell carcinoma of the uterine cervix. Br J Cancer 1998, 78:822-827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW: Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 1997, 38:285-289 [DOI] [PubMed] [Google Scholar]
- 43.Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Dodge RK, Charles HC, Samulski TV, Prosnitz LR, Dewhirst MW: Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas. Cancer Res 1996, 56:5347-5350 [PubMed] [Google Scholar]
- 44.Partridge EE, Phillips JL, Menck HR: The National Cancer Data Base report on ovarian cancer treatment in United States hospitals. Cancer 1996, 78:2236-2246 [DOI] [PubMed] [Google Scholar]
- 45.Ratcliffe PJ, Pugh CW, Maxwell PH: Targeting tumors through the HIF system. Nat Med 2000, 6:1315-1316 [DOI] [PubMed] [Google Scholar]