Abstract
The overexpression of transforming growth factor (TGF)-β and Smad-mediated intracellular TGF-β signaling in the kidney underlies the development of renal scarring from pathological matrix accumulation. However, changes in the Smad proteins during the progression of kidney disease are unclear. In this study, we investigated the regulation of Smad proteins in the glomeruli of rats with anti-thymocyte serum nephritis. We found that Smad2 protein decreased markedly in nephritic glomeruli, whereas no significant changes were observed in the levels of Smad3 and Smad4 proteins. In contrast, the level of Smad2 mRNA in nephritic glomeruli did not differ significantly from that in control glomeruli. Based on recent reports of the ubiquitin-mediated degradation of Smad2, we investigated the degradation and ubiquitination activity directed against Smad2 in glomerular extracts. Both the degradation and ubiquitination of Smad2 were markedly increased in glomerular extracts from rats with nephritis. We also found that Smurf2, a ubiquitin ligase for Smad2, was increased in the nephritic glomerular extracts. These data suggest that the decrease in Smad2 resulted from enhanced ubiquitin-dependent degradation of Smad2 mediated by Smurf2, and is involved in the regulation of Smad2-mediated TGF-β signaling in nephritic glomeruli.
Transforming growth factor (TGF)-β is a multifunctional signaling protein that regulates the cell cycle, apoptosis, differentiation, and extracellular matrix production. 1 TGF-β plays a significant role in the progression of kidney fibrosis in clinical and experimental kidney diseases. 2-9 TGF-β signals through two distinct serine/threonine kinase TGF-β receptors (TβR): type I (TβRI) and type II (TβRII). On TGF-β binding to TβRII, TβRI is recruited and activated by the constitutively active and autophosphorylated TβRII. Activated TβRI then directly signals to downstream intracellular substrates, the Smads. Smads 1, 2, 3, 5, and 8 make up the receptor-regulated Smad (R-Smad) subfamily, and all contain a conserved carboxyl terminal SSXS motif; Smad4 is a collaborating Smad (or common Smad, Co-Smad); and Smads 6 and 7 form the inhibitory Smad (I-Smad) subfamily. R-Smads 1, 5, and 8 appear to specifically mediate signaling downstream from bone morphogenic protein and its receptors, whereas R-Smads 2 and 3 function in the TGF-β and activin signaling pathways. Activated R-Smads form heteromeric R-Smad Co-Smad complexes and translocate to the nucleus to regulate the transcription of target genes with co-activators or co-repressors. 10-14
Protein ubiquitination and subsequent proteasomal degradation is a common regulatory mechanism. 15 Several studies have demonstrated that Smads undergo ubiquitin-proteasome-mediated degradation. 16-19 Protein ubiquitination is carried out by a sequence of three enzymes, the E1 ubiquitin-activating enzymes, the E2 ubiquitin-conjugating enzymes, and the E3 ubiquitin ligases. Recently, a novel class of E3-type HECT-domain ubiquitin ligases, designated Smurfs (Smad-ubiquitination regulatory factors), has been shown to interact with Smads and is implicated in their ubiquitination. Smurf1 regulates the amount of Smad1 in the cytoplasm of unstimulated cells. 20 Smurf2 targets Smad1, Smad2, 17,18 and the transcriptional co-repressor SnoN, 21 but not Smad3, 18 for ubiquitination and degradation. Smurf2 has also been found to bind to Smad7 and induce ubiquitin-dependent degradation of the active TGF-β receptor complex and Smad7. 22 Smad3 ubiquitination, mediated by the SCF/Roc1 E3 ligase complex, has also been reported. 19
The renal TGF-β system has been widely studied, and several recent reports demonstrated the involvement of a Smad-mediated TGF-β signaling pathway in the progression of kidney disease 23-26 ; however, the regulation of TGF-β-Smad signaling has not yet been elucidated in kidney disease. In the present study, we investigated the glomerular expression of Smads 2, 3, and 4 in anti-thymocyte serum (ATS) nephritis, in which the glomeruli show a transient increase in TGF-β1 expression. We also examined the ubiquitination and degradation of Smad2, as well as the glomerular expression of Smurf2, because significant decreases in Smad2 protein were noted in ATS nephritic glomeruli.
Materials and Methods
Experimental Animals and Design
All studies were conducted in accordance with the guidelines for the care and use of laboratory animals of Hamamatsu University School of Medicine. ATS was raised in Suffolk sheep by subcutaneous immunization with Wistar-rat thymocytes conjugated with complete Freund’s adjuvant. Sera were heat-inactivated (56°C for 30 minutes) and adsorbed three times with Wistar-rat erythrocytes. Male Wistar rats, weighing 150 g, were injected intravenously with 0.5 ml ATS.
Rats were maintained in metabolic cages, and 24 hours of urine output was collected on days 0, 3, 7, 14, and 28. Urinary protein was measured with a pyrogallol red-molybdate protein assay kit (Wako, Osaka, Japan). Rats were killed on days 0, 3, 4, 5, 6, 7, 14, and 28, and the kidneys were immediately perfused in situ with ice-cold 0.1 mol/L phosphate-buffered saline (PBS) (pH 7.4), and removed. Parts of the renal cortices were fixed with 4% paraformaldehyde in PBS for 4 hours, rinsed with PBS, dehydrated in graded ethanol, and embedded in paraffin. Kidney sections (3-μm-thick) were stained with periodic acid-Schiff stain and analyzed under a light microscope. Glomeruli were isolated from the remaining cortices using a graded sieving technique 27 under sterile conditions, and were saved for glomerular culture, protein extraction, and total RNA extraction.
Measurement of TGF-β1 in Cultured Glomeruli
Isolated glomeruli, 10,000/well, were cultured in 1 ml RPMI medium containing penicillin and streptomycin. After 24 hours incubation, the medium was collected, centrifuged for 10 minutes at 9000 × g, and the resulting supernatants were used for the measurement of TGF-β1 with the TGF-β1 Emax Immunoassay System (Promega, Madison, WI) according to the manufacturer’s instructions.
Western Blot Analysis
Isolated glomeruli (approximately 20,000) were homogenized in 300 μl RIPA buffer (25 mmol/L Tris-HCl [pH 7.4], 150 mmol/L NaCl, 0.1% sodium dodecyl sulfate [SDS], 0.5% Triton X-100, 0.5% sodium deoxycholate) containing protease inhibitors (150 μg/ml phenylmethylsulfonyl fluoride [PMSF], 5 μg/ml aprotinin, 5 μg/ml pepstatin, 5 μg/ml leupeptin) at 4°C. After incubation for 12 hours, the lysates were centrifuged at 4°C for 10 minutes at 9000 × g The protein concentrations of the lysates were measured with a pyrogallol red-molybdate protein assay kit (Wako). Soluble lysates were boiled for 7 minutes with 2X SDS sample buffer (100 mmol/L Tris-HCl [pH 6.8], 4% SDS, 0.2% bromophenol blue, 20% glycerol). Soluble lysates were loaded and separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) using 4.2% acrylamide for the stacking gel and 8% acrylamide for the resolving gel. Protein was transferred to polyvinylidene difluoride membranes and probed with antibodies against human Smad2 (rabbit polyclonal antibody; Zymed Laboratories Inc., San Francisco, CA), human phospho-Smad2 (rabbit polyclonal antibody; Upstate Biotechnology, Waltham, MA), human Smad3 (rabbit polyclonal antibody; Santa Cruz Biotechnology, Santa Cruz, CA), human Smad4 (mouse monoclonal antibody; Santa Cruz Biotechnology), human Smurf2 (goat polyclonal antibody; Santa Cruz Biotechnology), and β-actin (mouse monoclonal antibody; Sigma Chemical Co., St. Louis, MO). The primary antibodies that bound to the target proteins were detected using horseradish peroxidase-conjugated anti-rabbit IgG, anti-mouse IgG (Promega), or anti-goat IgG antibodies (Cortex Biochem, San Leandro, CA), as appropriate. The antibodies were visualized with Western Lightning (PerkinElmer Life Sciences Inc., Boston, MA). Glomerular extracts were obtained from three rats per group per time point of observation, and were mixed together and analyzed by Western blotting. To confirm that the bands detected by the anti-human Smurf2 antibody in the rat glomerular samples were Smurf2 protein, excess Smurf2 blocking peptide (Santa Cruz Biotechnology) was pre-incubated with the antibody before probing. Human Smurf2 protein was extracted from HEK293 cells that were transfected with the plasmid vector pcDNA3 containing flag-tagged full-length human Smurf2 cDNA (kindly provided by Dr. Kohei Miyazono of the University of Tokyo with the permission of Dr. Xin-Hua Feng of Baylor College of Medicine) using FuGENE 6 Transfection Reagent (Roche Molecular Biochemicals, Indianapolis, IN) and used as a positive control.
mRNA Analysis
Total RNA was extracted from isolated glomeruli that had been stored in RNAlater (Ambion Inc., Austin, TX) at −20°C, by guanidinium isothiocyanate extraction and column purification using an RNeasy kit (Qiagen, Tokyo, Japan). Reverse transcription (RT) of the RNA was performed using a first-strand cDNA synthesis kit (Roche Molecular Biochemicals) and 1 μg total RNA. After the completion of cDNA synthesis, the enzyme was heat-inactivated for 5 minutes at 95°C. The polymerase chain reaction (PCR) with QuantiTect SYBR Green PCR Master Mix (Qiagen) was used to analyze Smad2 mRNA. All PCR primers were designed using the online software Primer 3 (http://www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi). Primers for rat Smad2 were 5′-CCAGGTCTCTTGATGGTCGT-3′ and 5′-GGCGGCAGTTCTGTTAGAAT-3′, giving an amplified RT-PCR product of 253 bp. Primers for 18S ribosomal RNA, used as an internal control, were 5′-CCCTTCCGTCAATTCCTTTA-3′ and 5′-GGAGGTTCGAAGACGATCAG-3′, giving an amplified product of 173 bp. Cycling conditions were: 15 minutes preincubation at 95°C, 15 seconds denaturation at 95°C, 20 seconds annealing at 60°C, and 10 seconds extension at 72°C, using a LightCycler (Roche Diagnostics, Minato-ku, Tokyo). The fluorescent product was detected at the end of each cycle. Product specificity was confirmed in initial experiments by agarose gel electrophoresis and routinely by melting-curve analysis. Real-time PCR data were analyzed according to the manufacturer’s instructions.
Preparation of Recombinant Smad2 and Smad3
Plasmid vector pGEX-4T-1 containing the glutathione-S-transferase (GST) gene and full-length human Smad2 or Smad3 cDNAs were kindly provided by Dr. Hidetoshi Hayashi of Nagoya City University. Recombinant GST-Smad2 and GST-Smad3 were expressed in Escherichia coli strain BL21 transformed with the recombinant plasmid. GST fusion proteins were purified according to the manufacturer’s instructions (Amersham Biosciences Inc., Piscataway, NJ).
Degradation Assays for Smad2
To investigate the degradation of glomerular Smad2, we used an in vitro degradation assay for endogenous Smad2 to evaluate the glomerular extracts collected on day 7 from rats with ATS nephritis, and those from control animals.
A portion of the isolated glomeruli was lysed in a buffer containing 50 mmol/L Tris-HCl (pH 7.5), 300 mmol/L NaCl, 0.5% Triton X-100, 150 μg/ml PMSF, 5 μg/ml aprotinin, 5 μg/ml pepstatin, and 5 μg/ml leupeptin. The lysates were homogenized by pipetting 50 times, incubated for 30 minutes at 4°C, and centrifuged for 10 minutes at 9000 × g. The resulting supernatants (glomerular extracts) were used in the degradation assay. Glomerular extracts, 5.5 μl in 7.5 μl ubiquitination mixture (50 mmol/L Tris-HCl [pH 8.3], 5 mmol/L MgCl2, 2 mmol/L dithiothreitol [DTT], 5 mmol/L adenosine-5′-triphosphate, and 2 mg/ml ubiquitin) were incubated for 0, 1, 2, 4, or 6 hours at 37°C. After incubation, each sample was boiled for 7 minutes with 2X SDS sample buffer and subjected to SDS-PAGE on an 8% gel, followed by immunoblot analysis with anti-Smad2 antibody. The degradation assay was also performed with 1X proteasome inhibitor mix (0.25 mmol/L MG132, 0.25 mmol/L MG115).
In Vitro Assays of Ubiquitination Activity Directed against Smad2
To determine whether glomerular levels of Smad2 are regulated by ubiquitin-dependent degradation, we performed an in vitro assay of ubiquitination activity directed against exogenous recombinant GST-Smad2 in the glomerular extracts from rats with ATS nephritis on days 3, 4, 5, 6, and 7, and in those from control animals (day 0).
Glomerular extracts were centrifuged for 8 hours at 100,000 × g, and the resulting supernatants (S100) were used in the ubiquitination assay, as described previously. 29 In brief, 2 μl GST-Smad2 or Smad3 fusion protein solution was incubated with 5.5 μl glomerular extracts in 7.5 μl ubiquitination mixture (50 mmol/L Tris-HCl [pH 8.3], 5 mmol/L MgCl2, 2 mmol/L DTT, 10 mmol/L phosphocreatinine, 0.2 units/ml phosphocreatinine kinase, 5 mmol/L adenosine-5′-triphosphate, 50 μg/ml ubiquitin aldehyde, 1X protease inhibitor mix, 1X proteasome inhibitor mix, 2 mg/ml ubiquitin, 60 nmol/L E1 [BostonBiochem, Cambridge, MA], 870 nmol/L UbcH5b [BostonBiochem]). After incubation for 30 minutes at 30°C, the substrates were subjected to SDS-PAGE on a 6% gel, followed by immunoblot analysis with anti-Smad2 or anti-Smad3 antibody. Portions of the substrates were also immunoprecipitated with anti-Smad2 antibody and the resulting precipitates were subjected to SDS-PAGE on a 6% gel, followed by immunoblot analysis with anti-ubiquitin (Medical and Biological Laboratories Co. Ltd., Nagoya, Japan) or anti-Smad2 antibody.
Statistical Analysis
Data are presented as means ± SE. Groups were compared using unpaired Student’s t-test. P < 0.05 was considered significant.
Results
Urinary Protein Excretion and Histological Kidney Lesions
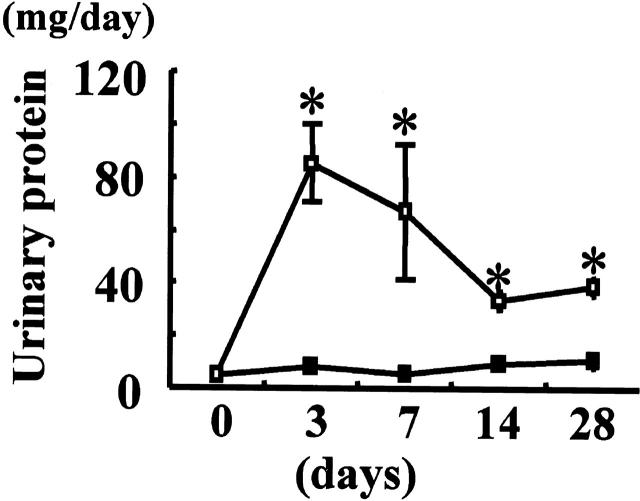
Urinary protein excretion increased immediately after ATS injection, peaked on day 3, and then decreased gradually to the excretion levels seen in the control animals by day 28 (Figure 1) ▶ . Increases in mesangial matrix accumulation and mesangial cell proliferation were noted in the glomeruli of rats injected with ATS, which peaked on day 7, ameliorated gradually thereafter (Figure 2) ▶ , and returned to almost normal levels by 8 weeks. These findings are compatible with our previous report. 28
Figure 1.
Urinary protein excretion in rats with ATS nephritis. Urinary protein excretion was measured over 24 hours in rats injected with anti-thymocyte serum (ATS) on day 0 (open squares, days 3 and 7, n = 6; day 14, n = 5; day 28, n = 3) and control animals (closed squares, days 0, 3, 7, 14, and 28, n = 3). Values are given as mean ± SE. *, P < 0.05 versus control rats.
Figure 2.
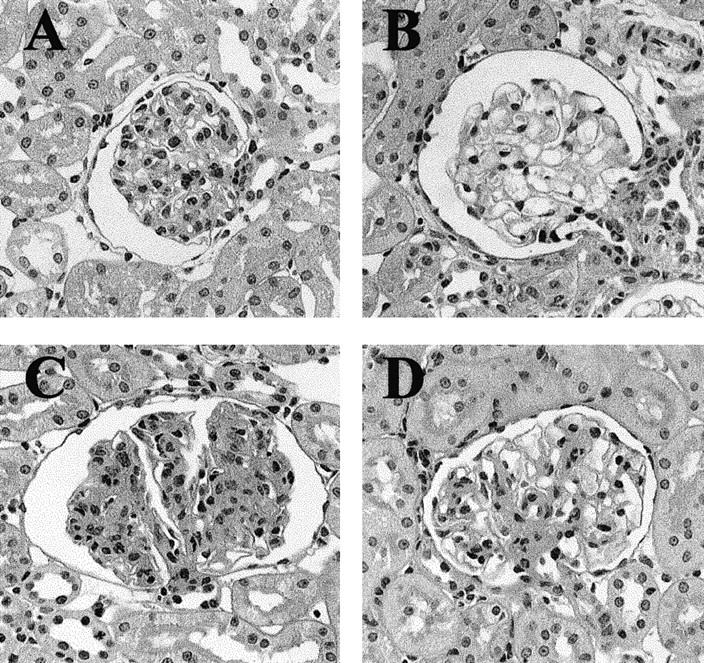
Light micrographs of rat kidneys. A: Control tissue. Kidney sections from animals (B) 3 days, (C) 7 days, and (D) 28 days after injection with ATS. Magnification, ×400; periodic acid-Schiff stain.
Glomerular TGF-β1
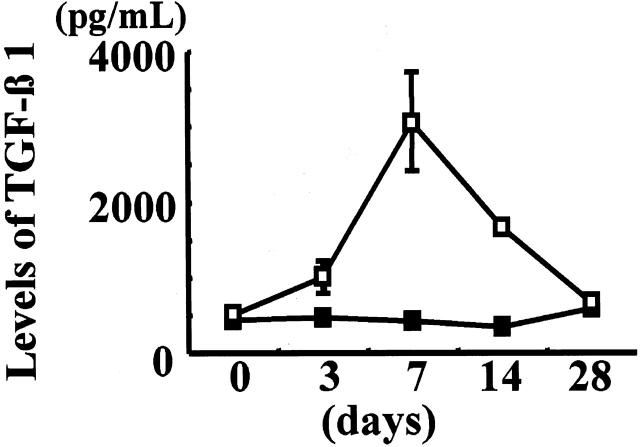
The levels of TGF-β1 in the medium of cultured glomeruli increased after ATS injection, peaked on day 7, and declined thereafter (Figure 3) ▶ . The peak value for glomerular TGF-β1 in rats with ATS nephritis was approximately six times higher than that observed in control animals (day 0).
Figure 3.
Secretion levels of TGF-β1 from ATS nephritic glomeruli. TGF-β1 in the culture medium of glomeruli isolated from rats injected with ATS and from control animals (day 0) were measured by ELISA. Glomeruli isolated from three rats were combined in each group and used for the measurement of TGF-β1. Values are given as mean ± SE (open squares, ATS nephritic glomeruli, six rats each on days 0, 3, and 7, and three rats each on days 14 and 28; closed squares, control glomeruli, three rats each on days 0, 3, 7, 14, and 28).
Levels of Smads 2, 3, and 4 in Isolated Glomeruli
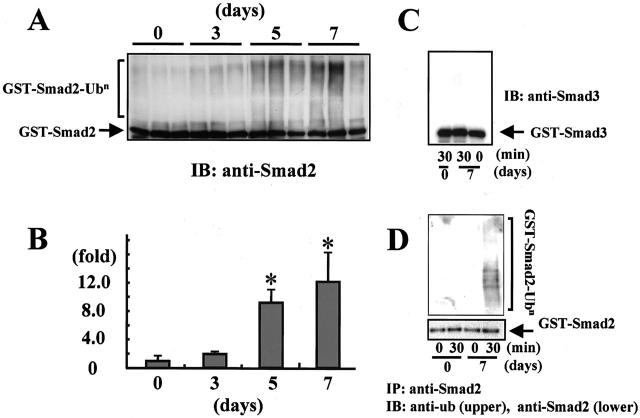
We investigated the intracellular signaling molecules Smads 2, 3, and 4 in the glomeruli isolated from rats with ATS nephritis. Western blot analysis showed a marked decrease in the levels of Smad2 in glomeruli isolated from rats with ATS nephritis on days 3 to 7 relative to the levels in control glomeruli (day 0) (Figure 4A) ▶ . In contrast to the decreased Smad2 in ATS nephritic glomeruli, no marked differences were noted in the levels of Smad3 or Smad4 between control glomeruli and ATS nephritic glomeruli.
Figure 4.
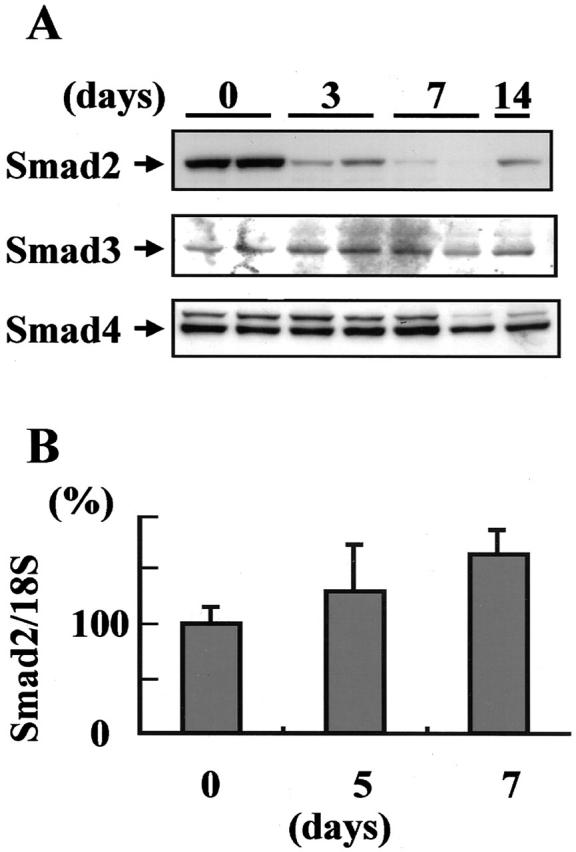
Down-regulation of Smad2 protein in ATS nephritic glomeruli. A: Western blot analysis of Smad2, Smad3, and Smad4 proteins was performed using glomeruli isolated from rats with ATS nephritis on days 3, 7, and 14, and from control animals (day 0). Glomeruli isolated from three rats were combined in each group and subjected to Western blot analysis. B: Total RNA prepared from isolated glomeruli was subjected to real-time RT-PCR for Smad2 mRNA and 18S ribosomal RNA (internal control). The mRNA levels of Smad2/18S rRNA in the glomeruli isolated from rats with ATS nephritis on day 5 (n = 4) and day 7 (n = 6), and from control animals (day 0) (n = 4) are indicated graphically.
To clarify whether the reduction in Smad2 was the result of Smad2 mRNA down-regulation, we evaluated the mRNA expression of Smad2 in isolated glomeruli by real-time RT-PCR. No significant differences in Smad2 mRNA expression were noted between control glomeruli (day 0) and ATS nephritic glomeruli on days 5 and 7 (Figure 4B) ▶ . Based on these data, we speculated that decreases in the levels of Smad2 might be caused by protein degradation in ATS nephritic glomeruli.
Degradation of Endogenous Smad2
To investigate whether the degradation of Smad2 was elevated in ATS nephritic glomeruli, we subjected glomerular extracts to an in vitro degradation assay. Degradation of endogenous Smad2 progressed in the glomerular extracts collected from rats with ATS nephritis on day 7 (Figure 5, A and B) ▶ , when the glomerular levels of Smad2 protein were markedly decreased, as indicated in Figure 4A ▶ . In contrast, no significant reduction in Smad2 was noted in the glomerular extracts collected from control animals (day 0). Degradation of endogenous Smad2 was prevented by the addition of proteasome inhibitors (Figure 5C) ▶ . These data indicate that the proteasome-mediated degradation of Smad2 was enhanced in ATS nephritic glomeruli.
Figure 5.
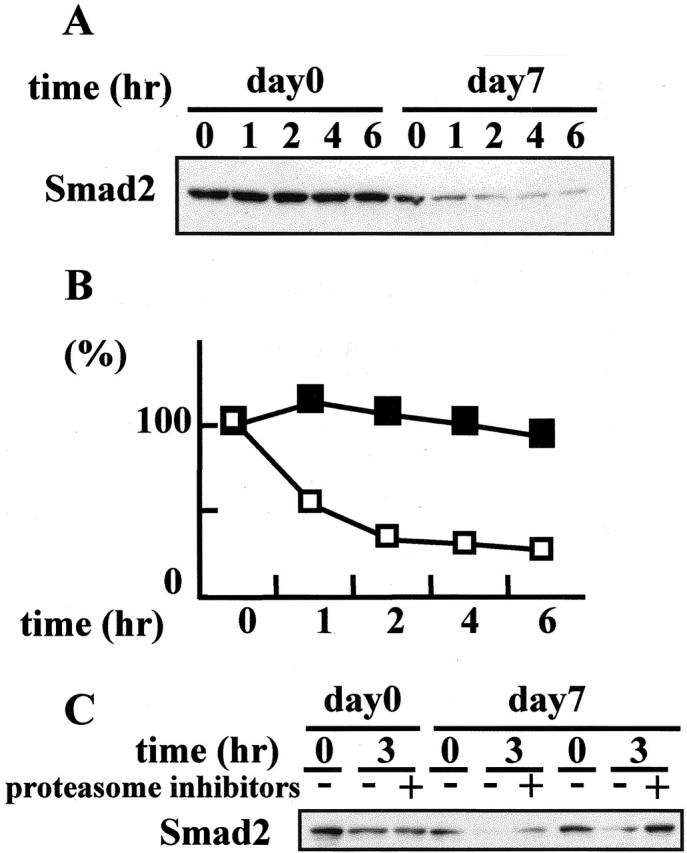
Enhanced degradation of Smad2 in ATS nephritic glomeruli. A: To evaluate the degradation activity directed against endogenous Smad2 protein in ATS nephritis, glomerular extracts collected on day 7 from rats with ATS nephritis and from control animals (day 0) were subjected to an in vitro degradation assay. The glomerular extracts were mixed with the ubiquitination mixture, incubated for 0 to 6 hours at 37°C, and the reaction mixtures were subjected to immunoblot analysis to detect the degradation of endogenous Smad2 protein using anti-Smad2 antibody. B: The Smad2 protein signals in A were quantified by densitometry. The densitometric ratios were calculated relative to the densitometric values at zero time in each group (open squares, ATS nephritic glomeruli; closed squares, control glomeruli). C: In vitro degradation assay of endogenous Smad2 with or without proteasome inhibitors.
Ubiquitination Activity Directed against Exogenous Recombinant GST-Smad2 in Isolated Glomeruli
Recently, the degradation of Smad2 through the ubiquitin-proteasome pathway has been demonstrated in cultured cells. To clarify whether the increased degradation of Smad2 in nephritic glomeruli involves the ubiquitin system, we investigated the in vitro ubiquitination activity directed against Smad2 in glomerular extracts using recombinant GST-Smad2 protein as the substrate. As shown in Figure 6A ▶ , significant ladders of polyubiquitinated Smad2 were observed when recombinant GST-Smad2 proteins were incubated with glomerular extracts collected from rats with ATS nephritis. Densitometric analysis demonstrated that the increases in polyubiquitinated Smad2 after incubation with the glomerular extracts obtained from ATS nephritic rats at days 5 to 7 were 9- to 12-fold higher than the increases observed after incubation with glomerular extracts from control animals (day 0) (Figure 6B) ▶ . In contrast, no significant ladders of polyubiquitinated GST-Smad3 protein were observed when recombinant GST-Smad3 protein was incubated for 30 minutes with the glomerular extracts obtained from control rats (day 0) or from ATS nephritic rats at day 7 (Figure 6C) ▶ . Ladders of polyubiquitinated GST-Smad2 were also detected in the anti-Smad2-immunoprecipitated substrates of glomerular extracts from rats with ATS nephritis (Figure 6D) ▶ .
Figure 6.
Increased ubiquitination of Smad2 protein in ATS nephritic glomeruli. A: Ubiquitination of Smad2 in ATS nephritic glomeruli was measured by an in vitro ubiquitination assay. The glomerular extracts isolated on day 3, 5, and 7 from rats with ATS nephritis and from control animals (day 0) were incubated with recombinant GST-Smad2 protein for 30 minutes at 30°C. The reaction mixtures were subjected to immunoblot analysis using anti-Smad2 antibody. B: The densitometric ratios were calculated relative to the control densitometric values in A (n = 3). Values are given as mean ± SE. *, P < 0.05 versus control rats. C: Ubiquitination activity directed against exogenous recombinant GST-Smad3 proteins present in the glomerular extracts isolated from rats with ATS nephritis on day 7 and from control animals (day 0) was measured by an in vitro ubiquitination assay of Smad3. D: The reaction mixtures from the in vitro ubiquitination assays of Smad2 were immunoprecipitated by anti-Smad2 antibody. The precipitated proteins were subjected to immunoblot analysis using anti-ubiquitin antibody (upper panel). A ubiquitination ladder was detected in reactants for 30 minutes of day 7 extract. The anti-Smad2-immunoprecipitated proteins in the reaction mixtures were also immunoblotted using anti-Smad2 antibody (lower panel), to demonstrate that equal amounts of recombinant GST-Smad2 protein were subjected to the ubiquitination activity assay in each sample.
Levels of Smad2, Phospho-Smad2, and Smurf2, and the Ubiquitination Activity Directed against GST-Smad2 in Isolated Glomeruli
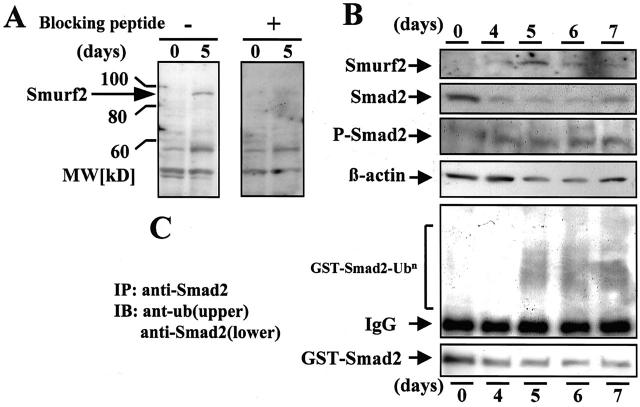
Smurf2 is a member of the HECT family of E3 ubiquitin ligases, and interacts with Smad2. We used Western blot analyses of Smad2, phospho-Smad2, and Smurf2 in the glomerular extracts of rats with ATS nephritis to investigate the relationship between the levels of Smad2 and Smurf2 in nephritic glomeruli. To confirm the specificity of the anti-Smurf2 antibody used in the present study, blocking peptide and positive control samples were used. Pretreatment of the anti-human-Smurf2 antibody with Smurf2 blocking peptides completely erased the Smurf2-specific band at 90 kd in the glomerular extracts collected from ATS nephritic rats on day 5 (Figure 7A) ▶ , and from the protein extracts from HEK293 cells transfected with human Smurf2 cDNA which were used as positive control samples (not shown). Marked decreases in Smad2 and slight increases in phospho-Smad2 were noted on days 5, 6, and 7. In contrast, the glomerular levels of Smurf2 increased in almost inverse proportion to those of Smad2 (Figure 7B) ▶ . Smurf2 protein was not detectable at day 0, became detectable at day 4, peaked at day 5, and began decreasing gradually thereafter.
Figure 7.
Increase in Smurf2 expression in ATS nephritic glomeruli. A: To evaluate the accessibility of anti-Smurf2 antibody to rat Smurf2 protein in Western blot analysis, anti-human-Smurf2 antibody (E-16; Santa Cruz Biotechnology) was pretreated with or without excess Smurf2 blocking peptide. The resultant antibodies were used for Western blotting to detect rat Smurf2 in the glomerular samples collected on day 5 from rats with ATS nephritis and from control animals (day 0). A 90-kd band (arrow) corresponding to rat Smurf2 selectively disappeared after pretreatment of the antibody with the blocking peptide. B: The levels of Smad2, Smurf2, phospho-Smad2, and β-actin in the glomeruli isolated from rats with ATS nephritis on days 4, 5, 6, and 7, and from control animals (day 0) were measured by Western blot analysis. C: Smad2-ubiquitination activity in the glomerular extracts isolated from rats in B was assayed by an in vitro ubiquitination system using recombinant GST-Smad2 protein. The reaction mixtures were immunoprecipitated with anti-Smad2 antibody and the precipitated proteins were subjected to immunoblot analysis using anti-ubiquitin antibody (upper panel). The anti-Smad2-immunoprecipitated proteins in the reaction mixtures were also immunoblotted using anti-Smad2 antibody (lower panel).
To investigate the relationship between the levels of Smad2 and the ubiquitination of Smad2, we subjected the glomerular extracts to an assay for ubiquitination activity directed against GST-Smad2. Markedly increased ubiquitination activity directed against exogenous recombinant GST-Smad2 was noted in the glomerular extracts from ATS nephritic rats on days 5, 6, and 7 (Figure 7C) ▶ . These data suggest that the ubiquitination of Smad2 correlated with the increased levels of Smurf2 in the glomeruli of rats with ATS nephritis.
Discussion
It is well recognized that the overexpression of TGF-β in the kidney underlies the development of glomerular and tubulointerstitial pathological matrix accumulation in nephritis. 2 We ( 6,28 and the present study) and other investigators 4,30 have demonstrated increased glomerular expression of TGF-β in rats with ATS nephritis, which peaks on day 7 when mesangial-matrix synthesis is maximal. Critical downstream targets of TGF-β signal transduction are TβR and members of the Smad family. Several recent studies reported that the Smad-mediated intracellular TGF-β signaling pathway is involved in the progression of kidney disease. 23,25,26 However, the regulation of the TGF-β-Smad signaling pathway in kidney disease has not been elucidated.
A marked decrease in Smad2 protein was noted in the glomeruli of rats with ATS nephritis, which reached a minimum between days 5 and 6. Because the levels of Smad2 mRNA in the nephritic glomeruli did not significantly differ from those in control glomeruli, it is unlikely that the decrease in Smad2 was due to the reduced expression of Smad2. Decreased translation of Smad2 may also contribute to the low Smad2 protein levels and this cannot be ruled out by the present study. Degradation of Smad proteins has recently been reported to be mediated by the ubiquitin-proteasome pathway. 16-19 It is now also understood that protein ubiquitination is carried out by a sequence of E1 ubiquitin-activating enzymes, E2 ubiquitin-conjugating enzymes, and E3 ubiquitin ligases, and that the E3 ubiquitin ligases play a crucial role in defining the substrate specificity of and subsequent protein degradation by the 26S proteasomes. In the present study, we demonstrated for the first time that levels of Smurf2, an E3 ubiquitin ligase that targets Smad2, 18 were increased, reached a maximum on day 5, and were inversely proportional to the decrease in Smad2 in the ATS nephritic glomeruli. Increases in Smad2 ubiquitination, as well as an increased degradation of Smad2, were also noted in the nephritic glomeruli. We further demonstrated that degradation of Smad2 in the glomerular extracts from ATS nephritic kidneys was inhibited by the addition of proteasome inhibitor. These data suggest that accelerated degradation of Smad2 by the Smurf2-mediated ubiquitin-proteasome pathway contributes to the decrease in Smad2 in the glomeruli of rats with ATS nephritis. Recently, we also found a similar decrease in Smad2 associated with increased expression of Smurf2 in the cortices of obstructed kidneys of mice with unilateral ureteral obstruction (manuscript in preparation), a model of progressive tubulointerstitial fibrosis in which the increased expression of TGF-β1 plays a pathogenic role. 31 We could not identify the cells that express Smurf2 because the antibodies we used were not suitable for the immunohistochemical detection of rat Smurf2.
Non-phosphorylated Smad2 is retained in the cytoplasm, and its retention is mediated by interactions with SARA (Smad anchor for receptor activation). 32 Receptor-mediated phosphorylation of Smad2 increases its affinity for Smad4, and promotes its release from SARA and the accumulation of phosphorylated Smad2 in the nucleus. 33 Although cytoplasmic ubiquitination and degradation of Smad1 20 and cytoplasmic ubiquitination of Smad3 which targets HEF1 for degradation 34 have been reported, most studies indicated that the ubiquitination of Smad2 progresses in the nucleus by the action of Smurf2. 16-18 Therefore, it is suggested that the decrease in Smad2 in ATS nephritic glomeruli resulted from the accelerated proteasomal degradation of Smad2, which was released from SARA, imported into the nucleus and ubiquitinated by Smurf2 on TβR-mediated phosphorylation.
The proteolytic degradation of intracellular proteins by the ubiquitin-proteasome pathway is essential for diverse processes, such as signal transduction, cell-cycle progression, and endocytosis. 35 Recently, the TGF-β-Smad signaling pathway-mediated proteolysis of p57kip2, a cyclin-dependent kinase inhibitory protein that acts as a negative regulator of the cell cycle and regulates cell differentiation, has been demonstrated in osteoblastic cells. 36 In ATS nephritic glomeruli, the levels of Smad3, another R-Smad involved in TGF-β signaling, and Co-Smad4 did not change significantly, which presents a striking contrast to Smad2. The decrease in Smad2 resulted from enhanced ubiquitination and proteasomal degradation may lead to a relative predominance of Smad3-mediated TGF-β signaling, and to a repressionof that mediated by Smad2. In fact, increased expression of plasminogen-activator inhibitor-1 (PAI-1), a protease inhibitor that suppresses tissue plasminogen activator (tPA), a matrix-degrading enzyme, and sustains extracellular matrix deposition, has been shown in ATS nephritic glomeruli. 37 And further, it has been reported that PAI-1 expression is regulated by TGF-β signaling mediated by Smad3-Smad4 complexes. 38 An increase in Smad3-mediated TGF-β signaling, demonstrated by an increased nuclear accumulation of Smad3, was also reported in the glomerular and tubulointerstitial cells of the db/db mouse, a genetic model of type 2 diabetes, in which renal expression of TGF-β and TβRII are augmented concomitantly. 26
In the present study, we did not directly demonstrate whether phosphorylated Smad3 dominantly mediated the TGF-β signals in ATS nephritic glomeruli. However, based on the dramatic decrease in total Smad2 in ATS nephritic glomeruli, we consider that it could influence, at least in part, the level of phosphorylated Smad2. Actually, only a slight increase in phosphorylated Smad2 was observed in ATS nephritic glomeruli, in which markedly enhanced TGF-β activity has been demonstrated. 4 Additionally, these data also suggest that not all TGF-β signaling mediated by Smad2 was eliminated by the enhanced ubiquitin-dependent degradation of Smad2 in ATS nephritic glomeruli.
We have previously reported that the glomeruli isolated from rats with ATS nephritis were partially refractory to growth inhibition by TGF-β1, and that the expression of TβRII is decreased in the proliferating mesangial cells of ATS nephritic glomeruli. 28 In addition to the modulation of TβRII expression in proliferating mesangial cells, there is a relative predominance of Smad3, associated with the decrease in Smad2 that results from increased Smad2 ubiquitination and degradation. This may also contribute to the attenuated mitoinhibitory responses to TGF-β1 in ATS nephritic glomeruli. Smad3 overexpression has been shown to stimulate cell proliferation and decrease differentiation in the preadipocyte cell line, 3T3-F442A. 39 Reisdorf et al 40 reported that the nuclear activity of Smad3 is a limiting factor in the myofibroblast response to TGF-β1. Modulation of TGF-β signaling via Smad2 and Smad3, which resulted in differential TGF-β responsiveness during the activation of transdifferentiation from quiescent hepatic satellite cells to myofibroblasts, has been demonstrated in liver injury. 41,42 Although it is quite conceivable that the targeted destruction of TGF-β-activated Smad2 leads to the irreversible termination of Smad2-mediated intracellular TGF-β signaling, 16 the precise biological role of the decrease in Smad2 in ATS nephritic glomeruli remains unknown. It seems plausible that the expression of TGF-β and TβR, as well as the Smads, may not simply reflect the expected pathological cell response to TGF-β, if the post-receptor regulation of TGF-β signaling, including the ubiquitination and degradation of Smad2 mediated by Smurf2, is modulated in the lesions. By considering their roles in the intracellular regulation of the TGF-β-Smad signaling network in vivo, an understanding of the ubiquitination and proteasomal degradation of the Smads could shed new light on the mechanisms underlying the development of kidney disease.
Acknowledgments
We thank Dr. Hidetoshi Hayashi, Nagoya City University, for kindly providing us with plasmid vector pGEX-4T-1, having human full-length Smad2 cDNA and the GST gene. We also thank Dr. Xin-Hua Feng, Baylor College of Medicine, and Dr. Kohei Miyazono, University of Tokyo, for kindly providing us with the plasmid vector pcDNA3, having human full-length Smurf2 cDNA. We also thank Dr. Tomoyasu Isobe and Sayuri Suzuki for technical assistance.
Footnotes
Address reprint requests to Akashi Togawa, M.D., First Department of Medicine, Hamamatsu University School of Medicine, 1–20-1 Handayama, Hamamatsu, Shizuoka 431-3192, Japan. E-mail: akasito@gb3.so-net.ne.jp.
Supported by a Grant-in-Aid for Scientific Research (number 12671035 to T. Y.) from the Ministry of Education, Science and Culture, Japan.
References
- 1.Sporn MB, Roberts AB: Transforming growth factor-β in disease: recent progress and new challenges. J Cell Biol 1992, 119:1017-1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Border WA, Noble NA: Transforming growth factor-β in tissue fibrosis. N Engl J Med 1994, 331:1286-1292 [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA: Expression of transforming growth factor-β is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA 1993, 90:1814-1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okuda S, Languino L, Ruoslahti E, Border WA: Elevated expression of transforming growth factor-β and proteoglycan production in experimental glomerulonephritis: possible role in expansion of the mesangial extracellular matrix. J Clin Invest 1990, 86:453-462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshioka K, Takemura T, Murakami K, Okada M, Hino S, Miyamoto H, Maki S: Transforming growth factor-β protein and mRNA in glomeruli in normal and diseased human kidneys. Lab Invest 1993, 68:154-163 [PubMed] [Google Scholar]
- 6.Yamamoto T, Noble NA, Miller DE, Border WA: Sustained expression of TGF-β1 underlies development of progressive kidney fibrosis. Kidney Int 1994, 45:916-927 [DOI] [PubMed] [Google Scholar]
- 7.Border WA, Noble NA: TGF-β in kidney fibrosis: a target for gene therapy. Kidney Int 1997, 51:1388-1396 [DOI] [PubMed] [Google Scholar]
- 8.Taniguchi Y, Yorioka N, Masaki T, Yamashita K, Ito T, Ueda H, Yamakido M: Role of transforming growth factor-β1 in glomerulonephritis. J Int Med Res 1997, 25:71-80 [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto T, Watanabe T, Ikegaya N, Fujigaki Y, Matsui K, Masaoka H, Nagase M, Hishida A: Expression of Types I, II, and III TGF-β receptors in human glomerulonephritis. J Am Soc Nephrol 1998, 9:2253-2261 [DOI] [PubMed] [Google Scholar]
- 10.Moustakas A, Souchelnytskyi S, Heldin CH: Smad regulation in TGF-β signal transduction. J Cell Sci 2001, 114:4359-4369 [DOI] [PubMed] [Google Scholar]
- 11.Massagué J: How cells read TGF-β signals. Nat Rev Mol Cell Biol 2000, 1:169-178 [DOI] [PubMed] [Google Scholar]
- 12.Ten Dijke P, Goumans MJ, Itoh F, Itoh S: Regulation of cell proliferation by Smad proteins. J Cell Physiol 2002, 191:1-16 [DOI] [PubMed] [Google Scholar]
- 13.Datto MB, Frederick JP, Pan L, Borton AJ, Zhuang Y, Wang XF: Targeted disruption of Smad3 reveals an essential role in transforming growth factor-β mediated signal transduction. Mol Cell Biol 1999, 19:2495-2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Attisano L, Wrana JL: Smads as transcriptional co-modulators. Curr Opin Cell Biol 2000, 12:235-243 [DOI] [PubMed] [Google Scholar]
- 15.Ciechanover A, Orian A, Schwartz AL: Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays 2000, 22:442-451 [DOI] [PubMed] [Google Scholar]
- 16.Lo RS, Massagué J: Ubiquitin-dependent degradation of TGF-β-activated Smad2. Nat Cell Biol 1999, 1:472-478 [DOI] [PubMed] [Google Scholar]
- 17.Lin X, Liang M, Feng XH: Smurf2 is a ubiquitin E3 ligase mediating proteasome-dependent degradation of Smad2 in transforming growth factor-β signaling. J Biol Chem 2000, 275:36818-36822 [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Chang C, Gehling DJ, Hemmati-Brivanlou A, Derynck R: Regulation of Smad degradation and activity by Smurf2, an E3 ubiquitin ligase. Proc Natl Acad Sci USA 2001, 98:974-979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuchi M, Imamura T, Chiba T, Ebisawa T, Kawabata M, Tanaka K, Miyazono K: Ligand-dependent degradation of Smad3 by a ubiquitin ligase complex of ROC1 and associated proteins. Mol Biol Cell 2001, 12:1431-1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu H, Kavsak P, Abdollah S, Wrana JL, Thomsen GH: A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature 1999, 400:687-693 [DOI] [PubMed] [Google Scholar]
- 21.Bonni S, Wang HR, Causing CG, Kavsak P, Stroschein SL, Luo K, Wrana JL: TGF-β induces assembly of Smad2-Smurf ubiquitin ligase complex that targets SnoN for degradation. Nat Cell Biol 2001, 3:587-595 [DOI] [PubMed] [Google Scholar]
- 22.Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH, Wrana JL: Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF-β receptor for degradation. Mol Cell 2000, 6:1365-1375 [DOI] [PubMed] [Google Scholar]
- 23.Poncelet AC, de Caestecher MP, Schnaper HW: The transforming growth factor-β/SMAD signaling pathway is present and functional in human mesangial cells. Kidney Int 1999, 56:1354-1365 [DOI] [PubMed] [Google Scholar]
- 24.Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, ten Duke P: Identification of Smad7, a TGF-β-inducible antagonist of TGF-β signaling. Nature 1997, 389:631-635 [DOI] [PubMed] [Google Scholar]
- 25.Uchida K, Nitta K, Kobayashi H, Kawachi H, Shimizu F, Yumura W, Nihei H: Localization of Smad6 and Smad7 in the rat kidney and their regulated expression in the anti-Thy-1 nephritis. Mol Cell Biol Res Commun 2000, 4:98-105 [DOI] [PubMed] [Google Scholar]
- 26.Hong SW, Isono M, Chen S, Iglesias-De La Cruz MC, Han DC, Ziyadeh FN: Increased glomerular and tubular expression of transforming growth factor-β1, its type II receptor, and activation of the Smad signaling pathway in the db/db mouse. Am J Pathol 2001, 158:1653-1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Misra RP: Isolation of glomeruli from mammalian kidneys by graded sieving. Am J Clin Pathol 1972, 58:135-139 [DOI] [PubMed] [Google Scholar]
- 28.Watanabe T, Yamamoto T, Ikegaya N, Fujigaki Y, Suzuki H, Togawa A, Fukasawa H, Nagase M, Hishida A: Transforming growth factor-β receptors in self-limited vs. chronic progressive nephritis in rats. J Pathol 2002, 198:397-406 [DOI] [PubMed] [Google Scholar]
- 29.Kitagawa M, Hatakeyama S, Shirane M, Matsumoto M, Ishida N, Hattori K, Nakamichi I, Kikuchi A, Nakayama K, Nakayama K: An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of β-catenin. EMBO J 1999, 18:2401-2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider A, Thaiss F, Rau HP, Wolf G, Zahner G, Jocks T, Helmchen U, Stahl RA: Prostaglandin E1 inhibits collagen expression in anti-thymocyte antibody-induced glomerulonephritis: possible role of TGF-β. Kidney Int 1996, 50:190-199 [DOI] [PubMed] [Google Scholar]
- 31.Wright EJ, McCaffrey TA, Robertson AP, Vaughan ED: Chronic unilateral ureteral obstruction is associated with interstitial fibrosis and tubular expression of transforming growth factor-β. Lab Invest 1996, 74:528-537 [PubMed] [Google Scholar]
- 32.Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL: SARA, a FYVE domain protein that recruits Smad2 to the TGF-β receptor. Cell 1998, 95:779-791 [DOI] [PubMed] [Google Scholar]
- 33.Xu L, Chen YG, Massagué J: The nuclear import function of Smad2 is masked by SARA and unmasked by TGF-β-dependent phosphorylation. Nat Cell Biol 2000, 2:559-562 [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Elia AE, Law SF, Golemis EA, Farley J, Wang T: A novel ability of Smad3 to regulate proteasomal degradation of a Cas family member HEF1. EMBO J 2000, 19:6759-6769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonifacino JS, Weissman AM: Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu Rev Cell Dev Biol 1998, 14:19-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishimori S, Tanaka Y, Chiba T, Fujii M, Imamura T, Miyazono K, Ogasawara T, Kawaguchi H, Igarashi T, Fujita T, Tanaka K, Toyoshima H: Smad-mediated transcription is required for transforming growth factor-β1-induced p57(Kip2) proteolysis in osteoblastic cells. J Biol Chem 2001, 276:10700-10705 [DOI] [PubMed] [Google Scholar]
- 37.Tomooka S, Border WA, Marshall BC, Noble NA: Glomerular matrix accumulation is linked to inhibition of the plasmin protease system. Kidney Int 1992, 42:1462-1469 [DOI] [PubMed] [Google Scholar]
- 38.Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM: Direct binding of Smad3 and Smad4 to critical TGF-β-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J 1998, 17:3091-3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choy L, Skillington J, Derynck R: Roles of autocrine TGF-β receptor and Smad signaling in adipocyte differentiation. J Cell Biol 2000, 149:667-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reisdorf P, Lawrence DA, Sivan V, Klising E, Martin MT: Alteration of transforming growth factor-β1 response involves down-regulation of Smad3 signaling in myofibroblasts from skin fibrosis. Am J Pathol 2001, 159:263-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dooley S, Delvoux B, Streckert M, Bonzel L, Stopa M, ten Dijke P, Gressner AM: Transforming growth factor β signal transduction in hepatic stellate cells via Smad2/3 phosphorylation, a pathway that is abrogated during in vitro progression to myofibroblasts: TGF-β signal transduction during transdifferentiation of hepatic stellate cells. FEBS Lett 2001, 502:4-10 [DOI] [PubMed] [Google Scholar]
- 42.Tahashi Y, Matsuzaki K, Date M, Yoshida K, Furukawa F, Sugano Y, Matsushita M, Himeno Y, Inagaki Y, Inoue K: Differential regulation of TGF-β signal in hepatic stellate cells between acute and chronic rat liver injury. Hepatology 2002, 35:49-61 [DOI] [PubMed] [Google Scholar]