Abstract
Galectin-1 (Gal-1), the prototype of a family of β-galactoside-binding proteins, has been shown to attenuate experimental acute and chronic inflammation. In view of the fact that endothelial cells (ECs), but not human polymorphonuclear leukocytes (PMNs), expressed Gal-1 we tested here the hypothesis that the protein could modulate leukocyte-EC interaction in inflammatory settings. In vitro, human recombinant (hr) Gal-1 inhibited PMN chemotaxis and trans-endothelial migration. These actions were specific as they were absent if Gal-1 was boiled or blocked by neutralizing antiserum. In vivo, hrGal-1 (optimum effect at 0.3 μg equivalent to 20 pmol) inhibited interleukin-1β-induced PMN recruitment into the mouse peritoneal cavity. Intravital microscopy analysis showed that leukocyte flux, but not their rolling velocity, was decreased by an anti-inflammatory dose of hrGal-1. Binding of biotinylated Gal-1 to resting and postadherent human PMNs occurred at concentrations inhibitory in the chemotaxis and transmigration assays. In addition, the pattern of Gal-1 binding was differentially modulated by PMN or EC activation. In conclusion, these data suggest the existence of a previously unrecognized function of Gal-1, that is inhibition of leukocyte rolling and extravasation in experimental inflammation. It is possible that endogenous Gal-1 may be part of a novel anti-inflammatory loop in which the endothelium is the source of the protein and the migrating PMNs the target for its anti-inflammatory action.
Galectins are a growing family of protein defined by their affinity for β-galactosides and by conserved sequence elements in their carbohydrate recognition domains. 1 At present, 14 mammalian galectins have been identified 2 and found distributed in a variety of tissues. Galectins can be detected in the cytosol, nucleus, and membrane compartments of producing cells, suggesting that they might mediate a wide range of biological functions. 2
Galectin-1 (Gal-1), the earliest described member of the family is a homodimeric protein with a carbohydrate recognition domain of 134 amino acids. 3 Expression of Gal-1 has been specially identified in lymphoid organs such as the thymus, the lymph nodes, in activated macrophages, and T cells and in immune-privileged sites such as placenta and cornea, suggesting an important role in generating and maintaining immune tolerance. The precise in vivo functions of Gal-1 are currently unclear because targeted disruption of the Gal-1 gene in null-mutant mice resulted in the absence of major phenotypic abnormalities, perhaps because of compensatory phenomena. 4,5 However, the effects produced by exogenous administration of human recombinant Gal-1 suggest a key role in a variety of biological events involving cell-cell and cell-extracellular matrix interactions, cell growth regulation, metastasis, and immunomodulation. 6 In an experimental model of arthritis, Gal-1 has been found to possess anti-inflammatory activity probably in relation to its ability to promote Th1 cell apoptosis, switching the immune response toward a Th2 phenotype. 7 Recently, it has been shown that administration of Gal-1 inhibited the acute inflammatory response in a mouse model of paw edema. 8 Interestingly, the latter study reported that Gal-1 anti-edema effect was associated with a reduced polymorphonuclear leukocyte (PMN) influx into the inflamed paws.
In both acute and chronic inflammation, the migration of blood-borne leukocyte across the postcapillary venule endothelium represents a necessary and important step in the cellular response to the inflammatory insult. Leukocytes firstly interact with the endothelium by tethering and rolling mechanisms, mediated by the selectin family of adhesion molecules. This is followed by firm adhesion and transmigration across the endothelial layer mediated by the integrin family of adhesion molecules. 9,10 Given that Gal-1 possesses anti-inflammatory activities and that its expression in endothelial cells (ECs) can be up regulated by proinflammatory mediators, 11,12 we hypothesize that Gal-1 could have the potential to inhibit leukocyte migration both under normal and, most significantly, inflammatory conditions. Therefore, the aim of the present study was to investigate the anti-migratory potential of Gal-1, using a combination of in vitro and in vivo models of PMN-EC interaction.
Materials and Methods
Materials
Human recombinant (hr) Gal-1 was prepared as previously described. 13 Briefly, plasmid pH14Gal was constructed from the plasmid pUC540 (KanR) and a cDNA for Gal-1 derived from a human lung cDNA library. Escherichia coli strains of SCS1 and Y1090 were then transformed with pH14Gal and Gal-1 expression was assessed by Western blot analysis. Finally, the recombinant protein was purified by affinity chromatography on an asialofetuin-agarose column. Endotoxin content of the purified sample was <60 ng/mg protein. Human recombinant mutant CS2 was produced by a site-directed mutagenesis experiment in which the cysteine residue at position 2 in the N-terminus was substituted with a serine as described previously. 14 The preparation of the rabbit polyclonal anti-human Gal-1 antibody has previously been described. 13 Human recombinant interleukin (IL)-8 was a generous gift of Dr. A Rot (Novartis Forschungsinstitut, Vienna, Austria).
Preparation of Peripheral Human PMNs
Human PMNs were freshly prepared from healthy volunteers by histopaque 1191/1117 gradient as previously described. 15 Blood was first diluted (1:1) with RPMI 1640 (Sigma, Poole, UK) medium before being added to the gradient, and centrifuged at 1200 rpm for 30 minutes. PMNs were collected and the erythrocytes were removed by hypotonic lysis. Cells were further washed twice in RPMI medium before experimentation.
PMN Chemotaxis Assay
A Neuroprobe ChemoTxplate (Receptor Technologies Ltd., Adderbury, UK) with polycarbonate membrane filters of 3-μm membrane pores was used, using a protocol recently described for eosinophil chemotaxis. 16 Briefly, purified PMNs were diluted to 4 × 106cells/ml in RPMI and 0.1% fetal calf serum (Sigma), and incubated with Gal-1 (concentration range, 0.04 to 4 μg/ml corresponding to ∼2.7 nmol/L to 0.27 μmol/L) for 10 minutes at 37°C. Human IL-8 (CXCL8; Novartis, Vienna, Austria) was placed in the bottom well of the Neuroprobe plate, the polycarbonate filter was placed on top and 25 μl of PMNs were placed on top of the filter. Preliminary experiments determined that a concentration of 30 ng/ml of IL-8 produced optimal PMN chemotaxis. 15 Plates were incubated for 2 hours in a humidified incubator at 37°C with 5% CO2. Cells remaining on top of the filter were absorbed off and the filter tops were carefully washed to ensure removal of nonmigrated cells. The plates were centrifuged (1200 rpm, 5 minutes at room temperature) to pellet cells on the underside of the filters. The filter was removed and cells in the bottom wells were stained in Turk’s solution (0.01% crystal violet in 3% acetic acid) and counted by light microscopy.
PMN Apoptosis
In selected experiments, PMNs (1 × 106/ml) were incubated with hrGal-1 (0.04 to 4 μg/ml) for 2 hours before quantification of cell apoptosis using the fluorescein isothiocyanate-labeled annexin V and propidium iodide (PI) protocol, as previously described. 17
Ea.hy926 Culture Conditions and Transmigration Assay
Ea.hy926 cells [a hybridoma between human umbilical vein endothelial cells (HUVECs) and the epithelioma A549] were provided by Dr. C-J Edgell, Department of Pathology, School of Medicine, University of North Carolina, Chapel Hill, NC. Ea.hy926 cells retain most of the features of HUVECs, including the expression of endothelial adhesion molecules and human factor VIII-related antigen. 18 Ea.hy926 cells were cultured in DMEM-F12 (Sigma) supplemented with 10% fetal calf serum and antibiotics (cultured medium) and subcultured every 3 days using cell dissociation solution (Sigma). The transmigration assay was performed using a protocol modified from a previous study. 15 Cells were first washed in phosphate-buffered saline (PBS), and added (5 × 105 cells in 1 ml of cultured medium) onto Biocoat 24-well plates inserts (3 μm) precoated with fibronectin (Marathon Laboratory Supplies, London, UK) and cultured for 24 hours in a humidified incubator at 37°C with 5% CO2. On the day of the experiment, the inserts were washed with fresh media to removed nonadherent cells. PMNs (1 × 106 in 0.5 ml) incubated in the absence or presence of increasing concentrations of hrGal-1, were then added to the inserts, while 30 ng/ml of IL-8 were added in the bottom compartment. After 2 hours at 37°C in 5% CO2, cells that had migrated through the filters were retrieved from the lower compartment, stained in Turk’s solution (0.01% crystal violet in 3% acetic acid) and counted by light microscopy. The anti-migratory effect of Gal-1 was also tested in a slightly different experimental setting, in which Ea.hy926 cells were incubated for 24 hours with 10 ng/ml of human recombinant tumor necrosis factor-α (PeproTech EC Ltd., London, UK), a procedure known to up-regulate ICAM-1 and promote PMN transmigration. 19
Western Blotting for Gal-1 Expression
Three types of human ECs were used: namely Ea.hy926 (hybridoma cell line, see above); human bone marrow-derived ECs, provided by Dr. Babette Weksler, Cornell University, New York, USA); 20 primary human umbilical vein ECs (HUVECs; provided by Dr. P Vo, William Harvey Research Institute (WHRI), London UK). Human PMNs as well as U937 cells and RAW macrophages, as positive control, were also used to detect Gal-1 expression. 21 In all cases, pelleted cells were lysed in Tris-HCl (10 mmol/L, pH 7.4) containing 1 μmol/L leupeptin, pepstatin A, and aprotinin; 200 μmol/L phenylmethyl sulfonyl fluoride; 100 mmol/L sodium fluoride and sodium orthovanadate (Sigma). Cell extracts were centrifuged at 13,000 rpm for 5 minutes and supernatants collected. Total protein concentration was determined according to Bradford. 22 To detect Gal-1, protein extract (25 μg per lane) was loaded onto a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis for electrophoresis together with appropriate molecular weight markers (Amersham Pharmacia Biotech, Buckinghamshire, UK) and transferred to ECL Hybond nitrocellulose membrane. Reversible protein staining of the membranes with 0.1% Ponceau-S (Sigma) in 5% acetic acid was used to verify even protein transfer. Membranes were incubated overnight in 5% nonfat dry milk before the addition of a polyclonal antibody to Gal-1 (1:5000) 7 in PBS with 0.1% Tween 20 (PBST). This was followed by 30 minutes of washing with PBST and incubation for 60 minutes at room temperature with peroxidase-conjugated goat anti-rabbit IgG (1:2000; DAKO, Cambridgeshire, UK). Membranes were again washed twice for 15 minutes with PBST and immunoreactive proteins were detected using an enhanced chemiluminescence (ECL) kit (Amersham Pharmacia Biotech). In some experiments, membrane was striped and reprobed with α-tubulin antibody (Sigma).
Electron Microscopy Analysis to Monitor Gal-1 Expression in EA.hy926 Cells
Ea.hy926 cells were prepared for electron microscopy by standard methods as previously described. 23 Briefly, adherent cells were removed using cell dissociation solution and washed with PBS. After resuspension in 0.5 ml of 4% paraformaldehyde and 0.5% glutaraldehyde, 0.1% sodium cacodylate buffer (pH 7.4) for 24 hours at 4°C, they were then washed in sodium cacodylate, dehydrated through a graded series of ethanol (70 to 100%), and embedded in LR Gold (London Resin Co., Reading, Berkshire, UK). Sections (∼90 nm) were cut on an ultramicrotome (Reichert Ultracut; Leica, Austria) and placed on nickel grids for immunogold labeling. These sections were incubated with the following reagents at room temperature: 1) 0.1 mol/L of PBS containing 1% egg albumin (PBEA); 2) 0.1 mol/L of PBS containing 5% egg albumin for 30 minutes; 3) the rabbit serum anti-Gal-1 (final dilution of 1:300 in PBEA) for 2 hours; 4) after four washes (5 minutes each) in PBEA, with a goat anti-rabbit IgG (Fc fragment-specific) antibody (Ab) (1:50 in PBEA) conjugated to 15-nm colloidal gold (British Biocell, Cardiff, UK). After 1 hour, sections were extensively washed in PBEA and then in distilled water. Sections were stained with uranyl acetate and lead citrate before examination on a Zeiss LEO 906 electron microscope.
Analysis of Cell Surface Gal-1-Binding Sites by Fluorescence-Activated Cell Sorting Analysis
HrGal-1 was biotinylated using the ECL protein biotinylation module (Amersham Pharmacia Biotech). Two different cell conditions were used: freshly prepared human PMNs or postadherent PMNs and ECs prepared in parallel the same day (PMNs from the same donor) as described previously. 15 For the latter condition, PMNs were added to monolayers of Ea.hy926 cell in six-well plates, and PMN adhesion promoted with 100 nmol/L of phorbol 12-myristate 13-acetate (PMA) for 30 minutes at 37°C. At the end of the incubation period, postadherent cells were harvested with cell dissociation medium and diluted in wash buffer (containing PBS, 0.1% bovine serum albumin, and 1 mmol/L CaCl2). Aliquots (0.5 to 1 × 106 cells in 20 μl) were added to a 96-well plate together with 20 μl of wash buffer or biotinylated Gal-1 (0.04 to 4 μg/ml, corresponding to a concentration range of 2.7 to 270 nmol/L). In some wells, unlabeled Gal-1 (0.04 to 0.4 μg/ml) was added. After 1 hour at 4°C, and three washes, cells were incubated with 20 μl of phycoerythrin-conjugated to streptavidin (Serotec, Oxford, UK) for a further 1 hour on ice. In another set of experiments, the effects of Gal-1 binding were examined in the presence of lactose (30 mmol/L). In all cases, samples were analyzed on a Becton Dickinson FACscan using Cell Quest software. For the co-culture samples, PMNs and Ea.hy926 cells were clearly distinguished for their forward and side scatter characteristics. At least 5000 events were analyzed for each sample. Data are expressed as median fluorescence intensity units as measured in the FL2 channel, set to a photomultiplicator value of 600.
In Vivo Models of Inflammation
Animal work was performed according to Home Office regulations (guidance on the operation on animals was from the Scientific Procedures act 1986). IL-1β-induced peritonitis. 24 Male Swiss Albino mice (20 to 22 g body weight) were injected intraperitoneally with mouse recombinant IL-1β (5 ng in 0.5 ml) alone or together with hrGal-1 (0.01 to 1 μg 0.5 ml per mouse). Another group of mice was treated with dexamethasone (1 mg/kg s.c.) 1 hour before IL-1β. In all cases animals were sacrificed 4 hours later and the peritoneal cavities were washed with PBS and heparin (25 U/ml), before quantification of PMN recruitment after staining with Turk’s solution and light microscopy. The content of prostaglandin E2 (PGE2) in the cell-free lavage fluids was determined with a specific Enzyme Immune Assay (EIA) (Amersham Pharmacia Biotech). Concentrations of the murine CXC chemokine KC (CXCL1) in the lavage fluids were determined with a specific enzyme-linked immunosorbent assay (R&D Systems, Abingdon, UK).
Intravital microscopy. 25 The mesenteric vascular bed of anesthetized mice was exteriorized and the preparation was mounted on a Zeiss Axioskop FS microscope equipped with a ×40 water immersion objective. The preparation was monitored using a color camera and recorded for subsequent offline analysis. Mesenteries were superfused with bicarbonate-buffered solution at 37°C (mmol/L: NaCl, 131; KCl, 3.35; MgSO4, 0.57; NaHCO3, 17.9; and CaCl2, 1.49, pH 7.4, gassed with 5% CO2/95% N2) at a rate of 2 ml/min. Centerline red blood cell velocity was measured in venules by using an optical Doppler velocimeter (Microcirculation Research Institute, Texas A&M University, Dallas, TX). Venular blood flow was calculated from the product of mean red blood cell velocity (Vmean = centerline velocity/1.6) and microvascular cross-sectional area, assuming a cylindrical geometry. Wall shear rate was calculated by the Newtonian definition: shear rate = 8,000x (Vmean/diameter). One to three randomly selected postcapillary venules (diameter between 20 and 40 μm; length of at least 100 μm) were observed for each mouse.
Animals received either saline, mouse recombinant IL-1β (5 ng i.p.) alone, or together with 0.3 μg of Gal-1, and the mesenteric vascular bed was prepared for microscopic observation 2 hours later. The extent of the inflammatory response elicited by IL-1β was analyzed by measuring the rolling phenomenon as white blood cell velocity (VWBC) and cell flux (number of rolling cells passing through a given point per minute). Leukocyte adhesion was quantified by counting the number of adherent cells (stationary for >30 seconds) in 100-μm vessel length, whereas leukocyte emigration from the microcirculation into the tissue was quantified by counting the number of cells in the perivascular tissue up to 50 μm away from the vessel wall. A previous study indicated that the majority of leukocytes interacting with the activated endothelium were PMNs. 25
Data Handling and Statistical Analysis
In vitro experiments were conducted in triplicate and repeated with at least three distinct cell preparations (chemotaxis and transmigration; biotinylated Gal-1 binding). Data for chemotaxis and transmigration are reported as mean ± SEM of migration index (MI), calculated as follows: (number of cells migrating to chemoattractant)/(number of cells migrating to vehicles). Western blotting experiments were repeated at least two or three times with different cell preparations. In vivo, data (mean ± SEM) are reported as percent of IL-1β induced migration, n = number of animals per group. In all cases, potential differences among the experimental groups were determined by one-way analysis of variance followed by the Dunnett’s test taking a P value <0.05 as significant.
Results
Expression of Endogenous Gal-1
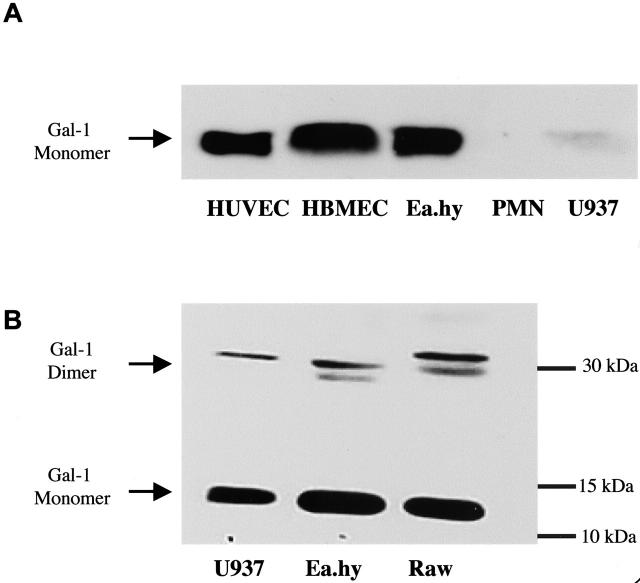
Initially, we confirmed Gal-1 expression in a range of human ECs. As reported in other culture conditions, 11,12 resting Ea.hy926, human bone marrow-derived ECs and HUVECs expressed Gal-1 (Figure 1) ▶ . Interestingly, human PMNs did not express detectable amounts of the protein, whereas Gal-1 was expressed by U937 cells.
Figure 1.
Expression of Gal-1 in primary and immortalized cells. Cell extracts were subject to electrophoresis and endogenous Gal-1 expression monitored by Western blotting analysis. Extracts were prepared from U937, human umbilical vein endothelial cells (HUVECs), Ea.hy926, human bone marrow endothelial cells, human PMNs, or mouse macrophages (RAWs). A and B are representative of three distinct experiments, and show the expression of Gal-1 monomer (∼15 kd) and dimer (∼30 kd).
Ultrastructural analysis by electron microscopy was further performed with EA.hy926 cells. Gal-1 was present in the cytosol, vacuoles, and in the nucleus as well as on the plasma membrane (Figure 2) ▶ . Prompted by the uneven expression of Gal-1 between several ECs and human PMNs, we tested the novel hypothesis that the protein could modulate EC-PMN interaction in inflammatory settings.
Figure 2.
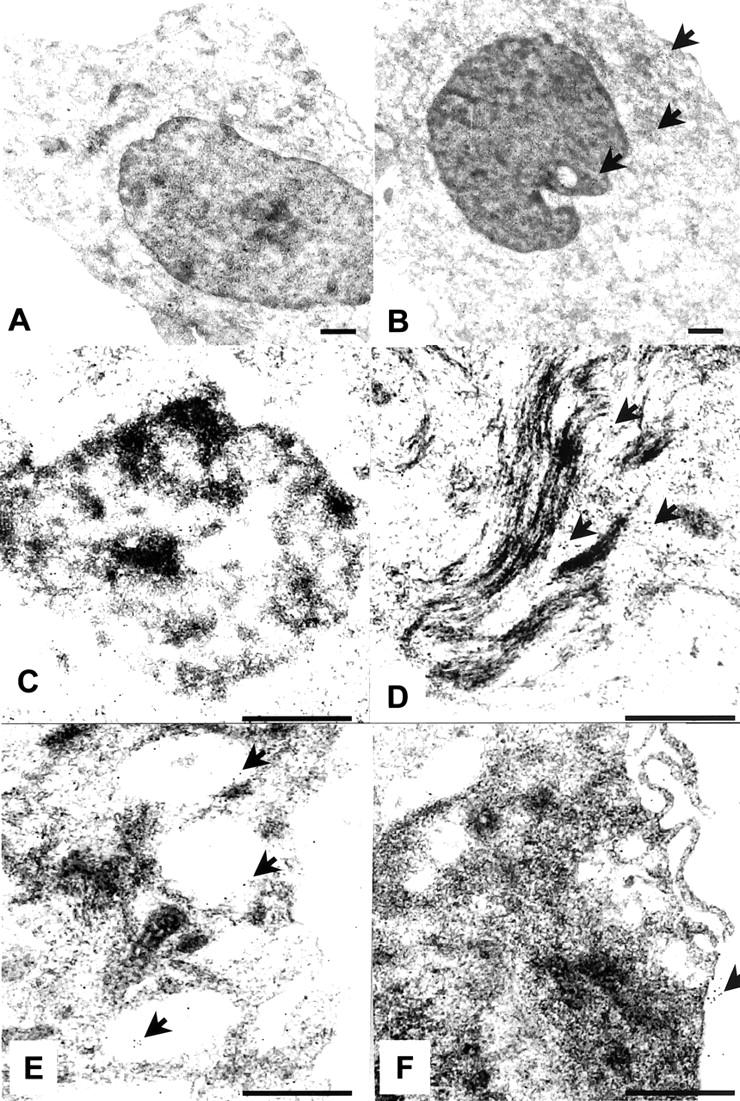
Ultrastructural analysis of Gal-1 in Ea.hy926 cells. A: Absence of gold particles in sections incubated with the control nonimmune rabbit serum. B: Widespread distribution of Gal-1 throughout the cell. The subcellular location is more evident at higher magnification, with detection of immunogold particles in the nucleolus (C), associated with cytoskeletal proteins (D), cytoplasmic vacuoles (E), and in association with the plasma membrane (F). Arrowheads highlight the presence of gold particles. Pictures are representative of 10 distinct cells. Scale bars, 0.5 μm.
Gal-1 Inhibited IL-8-Induced PMN Chemotaxis and trans-Endothelial Migration
Gal-1 was previously proposed to have an inhibitory effect on leukocyte migration in an in vivo model of acute inflammation. 8 However, a direct effect on the PMNs was never tested. Here we assessed its activity on PMN chemotaxis. Incubation of human PMNs with hrGal-1 inhibited IL-8-induced PMN chemotaxis in a concentration-dependent manner (Figure 3A) ▶ . The inhibitory effect of hrGal-1 was significant at all concentrations studied.
Figure 3.
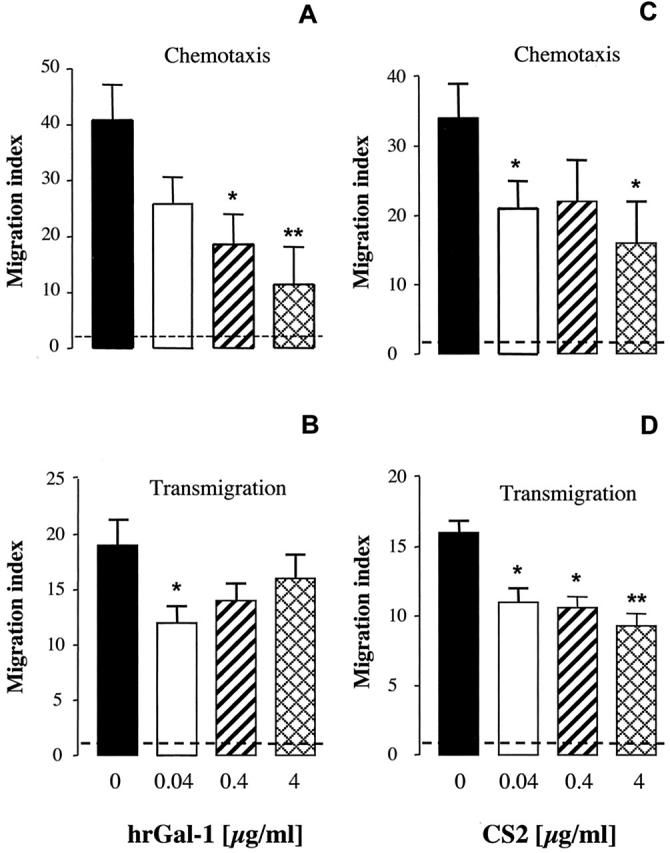
Gal-1 and CS2 inhibited IL-8-induced PMN chemotaxis and transmigration. Mean ± SEM of chemotaxis index showing the effects of Gal-1 and CS2 on PMN chemotaxis and transmigration. Purified PMNs were incubated for 10 minutes in the absence or presence of increasing concentrations of hrGal-1 (A and B) or CS2 (C and D) before being added to the ChemoTx plate or to Biocoat insert containing monolayers of Ea.hy926 cells. In both cases human recombinant IL-8 (30 ng/ml) was added to the lower chambers, and the number of PMNs migrated to the bottom well was assessed after 2 hours. In the chemotaxis assay, 679 ± 88 PMNs migrated to vehicle (no IL-8; negative control, n = 8), whereas the addition of IL-8 produced a chemotactic response of 45.2 ± 7.3 × 103 cells (positive control, n = 8). In the transmigration assay, 15 ± 0.8 × 103 PMNs migrated to vehicle (negative control, n = 10), whereas IL-8 produced a migratory response of 280 ± 54 × 103 cells (positive control, n = 10). Migration index was calculated by dividing the number of PMNs migrated to IL-8 by the number of cells migrated to vehicle. Dotted line represents chemotaxis index of 1 or basal level of chemotaxis. Data are from cells prepared from three to four different donors. *, P < 0.05 and **, P < 0.01 versus IL-8 alone.
To simulate a more physiological environment we examined next the effect of hrGal-1 in the PMN trans-endothelial migration assay. Addition of IL-8 at 30 ng/ml stimulated ∼30% of human PMNs to transmigrate within the 2-hour period, which was inhibited by incubation with hrGal-1 in a dose-dependent manner, selectively at the concentration of 0.04 μg/ml (Figure 3B) ▶ . To address whether the effect of Gal-1 on PMNs was stimulus-dependent, we tested its activity under a different experimental setting. EA.hy926 cells were activated for 24 hours with human recombinant tumor necrosis factor-α (10 ng/ml), a procedure known to up-regulate ICAM-1 and promote PMN transmigration. 19 In this experimental setting, ∼20% of PMNs transmigrated after 2 hours and the migration indexes in the presence hrGal-1 were reduced from a control value of 6.2 ± 0.8 (n = 4) to 3 ± 0.8 (P < 0.05), 3.2 ± 0.43 (P < 0.05) and 4.5 ± 0.7 (P > 0.05), for 0.04, 0.4, and 4 μg/ml of hrGal-1, respectively. Thus, using two distinct stimuli (IL-8 and tumor necrosis factor-α), hrGal-1 appeared to be more effective in inhibiting PMN trans-migration at low concentrations.
We then tested the effect of Gal-1 mutant CS2 on PMN chemotaxis and transmigration. CS2 had a cysteine residue substituted by serine at position 2 on the N-terminus and therefore cannot covalently link. 14 Our data showed that CS2 was equally effective against PMN chemotaxis at all concentrations tested (Figure 3C) ▶ . In partial analogy to hrGal-1, mutant CS2 was also effective in the PMN transmigration assay at all concentrations tested (Figure 3D) ▶ .
The specificity of Gal-1 effects was confirmed in two different manners. Figure 4 ▶ showed that boiled Gal-1 was inactive in the chemotaxis assay. In addition, a neutralizing antiserum reverted the inhibitory action produced by the protein, whereas equal dilution of a control rabbit serum was ineffective (Figure 4) ▶ .
Figure 4.
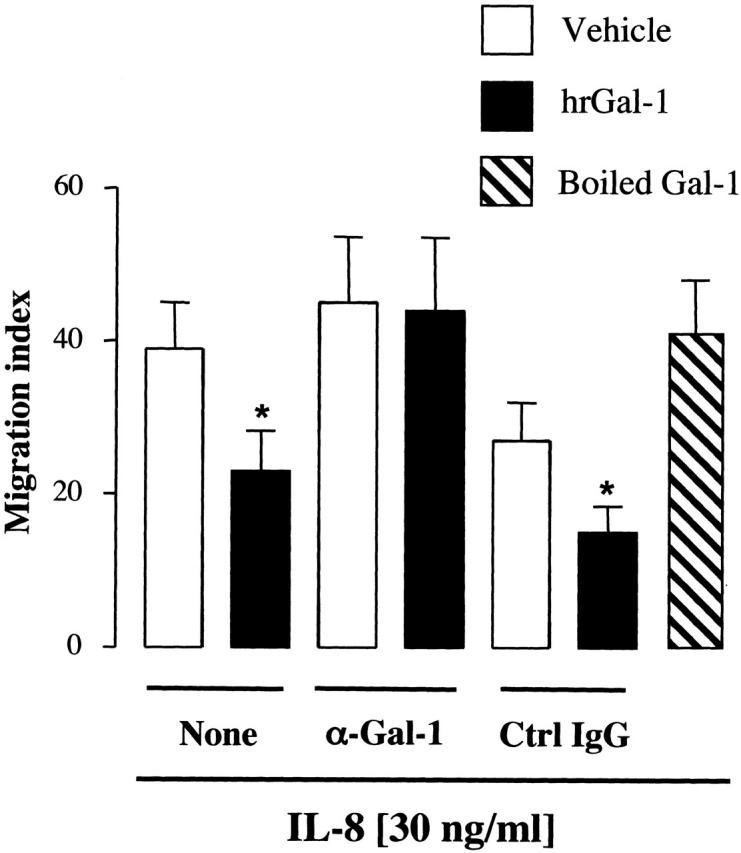
Validation of hrGal-1 effects in the chemotaxis assay. Purified PMNs were incubated for 5 minutes in the absence or presence of a specific anti-Gal-1 (α-Gal-1) or nonimmune IgG before the addition of 0.4 μg/ml of hrGal-1. A preparation of boiled hrGal-1 (0.4 μ/ml) was also tested. In both cases human recombinant IL-8 (30 ng/ml) was added to the lower chambers, and the number of PMNs migrated to the bottom well was assessed after 2 hours. Chemotaxis index was calculated as described in Figure 3 ▶ . Data are from cells prepared from three to four different donors. *, P < 0.05 versus respective vehicle group.
This inhibitory action of hrGal-1 on PMN locomotion occurred in the absence of cell apoptosis. Human PMNs cultured for 2 hours showed a modest degree of fluorescein isothiocyanate-annexin V binding and this was not modified by cell incubation with 0.04 to 4 μg/ml of hrGal-1. Data (percent of annexin V-positive cells) at the 2-hour time point were as follows: 12.7 ± 1.6%, 12.5 ± 2.5%, 10.5 ± 1.3%, and 13.8 ± 1.2% in the absence and presence of 0.04, 0.4, and 4 μg/ml of hrGal-1, respectively.
Effects of hrGal-1 on IL-1β-Induced Peritonitis
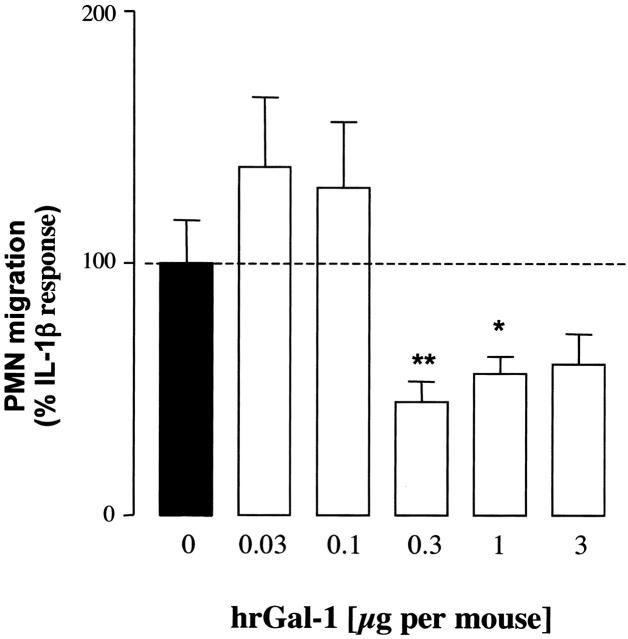
To provide relevance to inflammatory conditions, we used a mouse model of peritonitis in which it is well known that injection of IL-1β produced migration of leukocyte with a majority (>80%) being PMNs. 24 Simultaneously injection of mice with IL-1β and lower doses of Gal-1 (0.03 to 0.1 μg per mouse) had no effect on PMN migration, whereas a significant inhibition of this cellular response was measured at doses of 0.3 to 1 μg/mouse (20 to 66 pmol) (Figure 5) ▶ . This model of acute inflammation is also characterized by an increase in prostaglandin and KC release, however the reduction of PMN migration produced by hrGal-1 was not associated with a parallel reduction in mediator production, except for an inhibition of PGE2 levels at the dose of 1 μg (Table 1) ▶ . The co-administration of CS2 (0.3 μg i.p.) with IL-1β also significantly reduced PMN recruitment from 2.9 ± 0.4 (control IL-1β) to 0.8 ± 0.1 × 106 PMNs per mouse (n = 5; P < 0.05). A similar inhibitory effect was observed with dexamethasone both on PMN migration (−70%, n = 6, P < 0.05) and mediator release (Table 1) ▶ .
Figure 5.
Anti-migratory actions of hrGal-1 in IL-1β-induced peritonitis. Mice were injected intraperitoneally with IL-1β (5 ng in 0.1 ml of saline) in the absence or presence of different doses of hrGal-1. After 4 hours, peritoneal cavities were washed and PMN migration quantified. Data are reported as percentage of IL-1β response (3 ± 0.9 × 106 PMNs per cavity). Data are mean ± SEM of 6 to 10 mice per group. *, P < 0.05 and **, P < 0.001 versus IL-1β alone.
Table 1.
Effect of hrGal-1 on Exudate KC and PGE2 Levels
| Treatment | KC (pg/ml) | PGE2 (pg/ml) |
|---|---|---|
| Saline | 35 ± 8 | 449 ± 83 |
| IL-1β | 131 ± 32 | 920 ± 250 |
| IL-1β+ Gal-13 μg | 102 ± 13 | 769 ± 268 |
| IL-1β+ Gal-11 μg | 86 ± 32 | 337 ± 20* |
| IL-1β+ Gal-10.3 μg | 120 ± 34 | 1921 ± 125 |
| IL-1β+ dexamethasone | 66 ± 4* | 550 ± 35* |
The peritoneal exudates of mice injected with 5 ng of murine IL-1β in the absence or presence of the reported doses of Gal-1 were centrifuged and the supernatants assayed for KC and PGE2 levels. Dexamethasone (1 mg/kg s.c.) was given 1 hour before the cytokine. IL-1β significantly increased KC and PGE2 release (P < 0.05 compared to saline group). Data are mean ± SEM of 6 to 10 mice per group.
*P < 0.05 versus IL-1β alone.
Intravital Microscopy
To further shed light on the site and mechanism of Gal-1 action, we monitored leukocyte rolling, adhesion, and emigration in the mouse mesenteric microcirculation by intravital microscopy. Stimulation of the mesenteric vascular bed with IL-1β produced the expected changes in the microcirculation, 25 with an increase in white blood cell flux and the induction of the leukocyte rolling phenomenon, detected by a sharp reduction in VWBC (Figure 6, A and B) ▶ . This was followed by an increase in the extent of cell adhesion and emigration, compared to saline-treated mice (Figure 6, C and D) ▶ . Treatment of mice with hrGal-1 (at the dose of 0.3 μg, chosen from the experiments of peritonitis; see Figure 5 ▶ ) produced a selective attenuation of IL-1β-induced cellular response. In particular, hrGal-1 significantly reduced the effect of the cytokine on cell flux, cell adhesion, and emigration. However, the lower number of cells that entered into the rolling phenomenon in the IL-1β plus hrGal-1 group did roll with a VWBC not significantly different from that measured in the IL-1β group (Figure 6B) ▶ .
Figure 6.
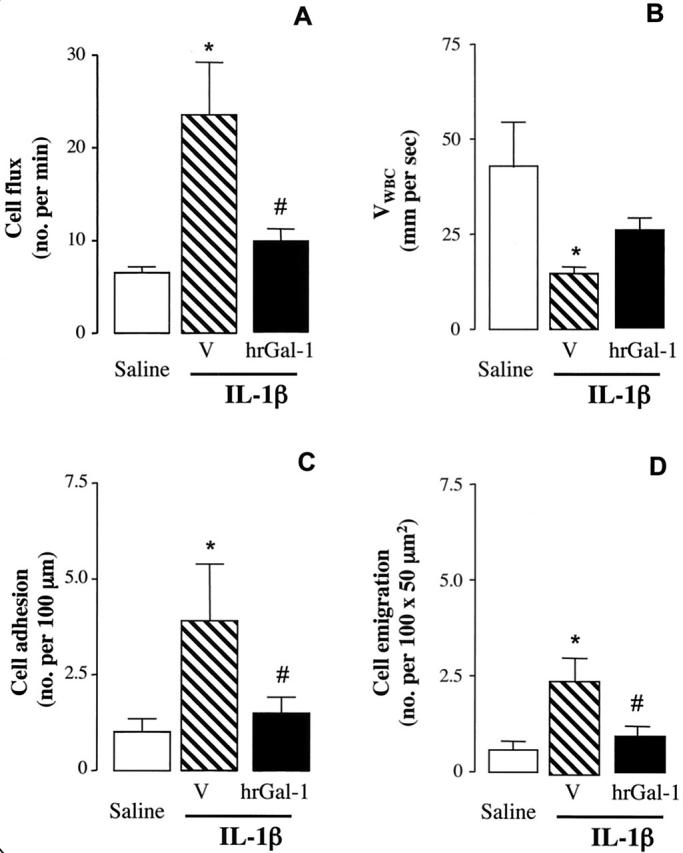
Effects of hrGal-1 on leukocyte rolling, adhesion, and emigration in the mesenteric microcirculation. Mice received either saline (0.5 ml), IL-1β (5 ng i.p.) alone or together with 0.3 μg of Gal-1. The mesenteric vascular bed was externalized and prepared for microscopic observation 2 hours later. The following parameters were measured in the microcirculation: cell flux (A), expressed as number of cells passing per minute; VWBC in μm/second (B); cell adhesion (C); and cell emigration (D). Data are mean ± SEM of four to six mice per group. *, P < 0.05 versus saline control group. #, P < 0.05 versus IL-1β alone.
These effects on the microcirculation, as those in the experiments of peritonitis, are unlikely to be because of indirect systemic and local actions of the protein. In fact, anti-inflammatory doses of hrGal-1 did not modify circulating blood cell numbers (Table 2) ▶ . In addition, direct observation of the potential effect of an hrGal-1 intravenous injection on venular and arteriolar blood flow did not reveal any significant and long-lasting changes in centerline blood erythrocyte flow and vessel diameter (Table 3) ▶ .
Table 2.
Systemic Treatment with hrGal-1 Does Not Affect Peripheral Leukocyte Counts
| Cell types | Saline (×106/ml) | Gal-1 (×106/ml) |
|---|---|---|
| PMN | 0.82 ± 0.23 | 0.76 ± 0.13 |
| Monocytes | 0.37 ± 0.08 | 0.52 ± 0.07 |
| Lymphocytes | 1.02 ± 0.19 | 1.42 ± 0.19 |
| Total | 2.22 ± 0.43 | 2.78 ± 0.23 |
Mice were injected intraperitoneally (I.P.) with either saline or 0.3 μg of hrGal-1. Two hours later, animals were sacrificed and different leukocytes were counted. Data are mean ± SEM of three mice per group.
Table 3.
Lack of Effect of hrGal-1 on Arterial and Venular Flow and Shear Rate
| Parameter | Saline | IL-1β | IL-1β + hrGal-1 | Saline + hrGal-1 |
|---|---|---|---|---|
| Arterial diameter (μm) | 70.3 ± 12 | 68.1 ± 7.3 | 78.1 ± 9.5 | 72.38 ± 7.5 |
| Arterial shear/second | 3482 ± 674 | 2767 ± 384 | 2791 ± 403 | 3020 ± 571 |
| Venular diameter (μm) | 109.7 ± 4.6 | 109.5 ± 2.3 | 108.6 ± 2.6 | 113.3 ± 0.95 |
| Venular shear/second | 350 ± 33.9 | 486.3 ± 56.5 | 487.8 ± 67.6 | 413.6 ± 37.2 |
Mice were injected with either saline (100 μl) or IL-1β (10 ng i.p.). Two hours later, the mesenteric was exposed, animals were then received a bolus injection of 0.3 μg Gal-1 (i.v.): arterial and venular diameter, centreline erythrocyte velocity, and shear rate were measured in the subsequent 10 minutes. Values (mean ± SEM of three mice per group) refer to the measurements at 10 minutes.
Gal-1-Binding Sites by Fluorescence-Activated Cell Sorting Analysis
A concentration-dependent binding of biotinylated Gal-1 was detected on both human PMNs and EA.hy926 cells (Figure 7) ▶ , with an interesting difference in relation to cell activation. The binding was significantly augmented when tested on postadherent PMNs (Figure 7A) ▶ , whereas a significant reduction was seen on EA.hy926 cells that had been used as an adhesion substrate (Figure 7B) ▶ .
Figure 7.
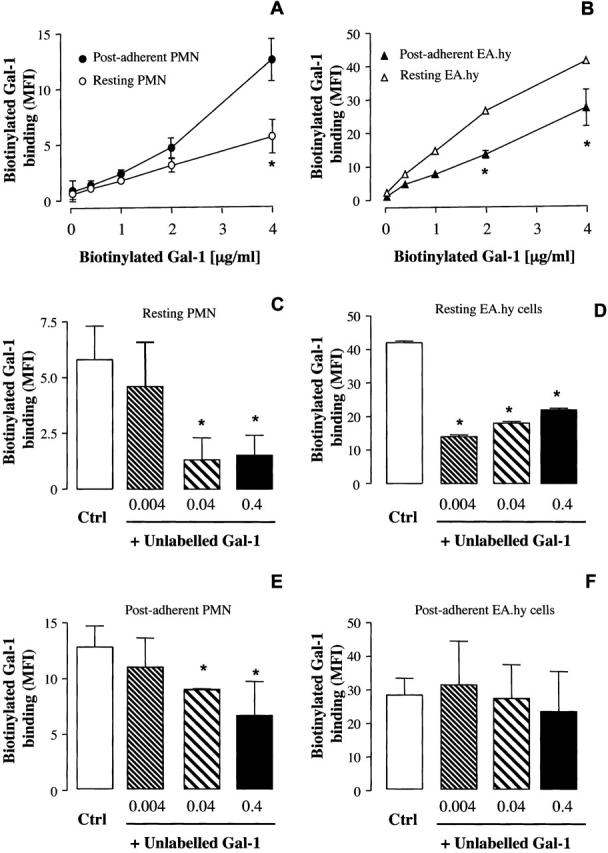
Flow cytometry analysis of Gal-1-binding sites on resting or postadherent PMNs and Ea.hy926 cells. A and B: Human PMNs were left either untreated or activated by PMA-induced adhesion (30 minutes, 37°C) to Ea.hy926 cell monolayers. The same samples were used to assess binding to postadherent ECs. The binding of biotinylated Gal-1 (4 μg/ml) to human PMNs was inhibited by the addition of increasing concentrations of unlabeled Gal-1 in resting (C) and postadherent cells (E). The binding of biotinylated Gal-1 to Ea.hy926 cells was inhibited by the addition of increasing concentrations of unlabeled Gal-1 in basal (D), but not in Ea.hy926 cells after PMN adhesion (F). Data are mean ± SEM of three to four donors. *, P < 0.05 versus control biotinylated Gal-1 alone.
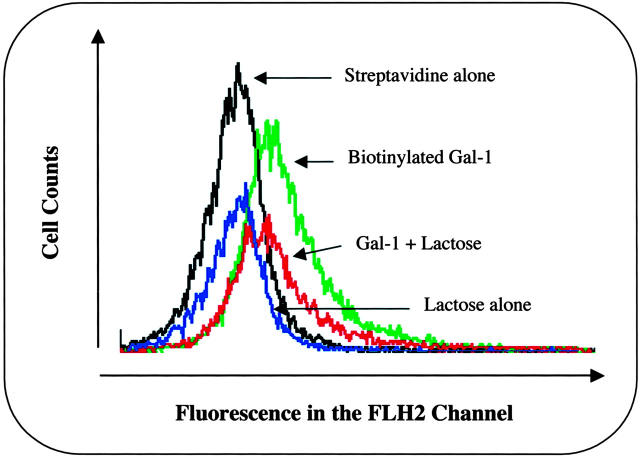
Competition experiments demonstrated two further points: hrGal-1 binding to human PMNs was in the range of concentrations that displayed biological actions (see Figure 4 ▶ ), with a significant displacement of biotinylated Gal-1 being produced by 0.4 and 0.04 μg/ml of cold hrGal-1 (Figure 7C) ▶ . With some experimental variation, this was also the case for postadherent PMNs (Figure 7E) ▶ . In contrast, modulation of Gal-1 binding site(s) appeared to be different with EA.hy926 cells, with a virtual absence of specific binding in activated ECs (Figure 7F) ▶ . Finally, binding of biotinylated Gal-1 to postadherent PMNs was partially reversed by 30 mmol/L of lactose, a well-known inhibitor against Gal-1 action, which competed for Gal-1-binding sites (Figure 8) ▶ . Out of three experiments, after correction for phycoerythrin-conjugated streptavidin binding, mean ± SEM of 8.5 ± 0.7 and 5.0 ± 0.5 median fluorescence intensity units were calculated for biotinylated Gal-1 binding in the absence and presence of lactose (P < 0.05). Thus, a portion of biotinylated Gal-1 binding is insensitive to the sugar.
Figure 8.
Effect of lactose on biotinylated Gal-1 binding to postadherent PMNs. Histograms represent fluorescence intensity in the FL2 channel as a result of cell surface Gal-1 binding to human PMNs, in the presence and absence of 30 mmol/L of lactose. Histograms are representative of three experiments.
Discussion
The major observation of this study is that Gal-1 can interfere with the initial interaction of blood PMNs with the postcapillary venule endothelium. In addition, Gal-1 effect seems to be specific and brought about through interaction with PMN binding site(s). Based on the facts that: 1) endogenous Gal-1 is expressed in ECs (Figure 1) ▶ , 2) its expression can be modulated by inflammatory cytokines 11 (M La, unpublished data), and 3) Gal-1-binding sites on PMNs and ECs are modulated by proinflammatory mediators (Figure 7) ▶ , we propose that this protein may play a role in the down-regulation of the host inflammatory response (phase of resolution). This control mechanism would be relatively novel as the PMNs did not seem to express the protein, as detected by Western blotting analysis.
We were able to confirm Gal-1 expression in ECs, 11,12 whereas resting human PMNs did not appear to contain detectable levels of the protein. Thus, we hypothesized that endothelial-derived Gal-1 could be an inhibitory mediator on the process of PMN extravasation. We first tested the effect of hrGal-1 on PMN chemotaxis and trans-endothelial migration, finding that in both assays the PMN response was sensitive to the inhibitory effect of the protein. Low concentrations of hrGal-1 were required to bring about these inhibitory actions. Furthermore, the specificity of these effects was confirmed by testing boiled hrGal-1, that was inactive, and by the blockage produced by a neutralizing antibody. Importantly, Gal-1 in vitro inhibition of PMN locomotion occurred in the absence of detectable proapoptotic effects. Similarly hrGal-1 (0.04 to 4 μg/ml) was not chemotactic for human PMNs (M La, unpublished data).
Gal-1 can exist in a reversible monomer-dimer equilibrium. 26 Although the protein contains free cysteine residues with the potential to form covalent bonds, there is evidence suggesting that dimerization can also occur via the formation of noncovalent bonds forms by extended β-sheet interactions across two monomeric subunits. 27 In any case, it has been proposed that the extent of dimer isoform predominates at high concentrations: this hypothesis is based on the observation that overexpression of recombinant Gal-1 in Chinese hamster ovary cells occurs in the monomeric form that can reversibly dimerize in a concentration-dependent manner. 26 However, whether the monomer-dimer equilibrium occurs for all mammalian Gal-1 remains unknown. A recent study used size exclusion chromatography to show that hrGal-1 mainly exists as a dimer even at low concentrations. 28 In our experimental conditions, we partially addressed this issue by testing the effects of the mutant CS2. Human recombinant CS2 is a Gal-1 mutant produced by the replacement of the cysteine residue at position 2 with serine, and shown to exist as a monomer under nondenaturing conditions. 14 In contrast, hamster recombinant Gal-1 mutated at position 2 was found to behave similar to Gal-1 in its ability to dimerize. 29 Thus, species specificity may also govern the ratio Gal-1 monomer/dimer. In our study, CS2 appeared to be less potent than the parent protein in vitro (and showed activity at 4 μg/ml) but it was almost equally active in vivo. Nevertheless, the fact that mutant CS2 reduced PMN activation in vitro and in vivo supported the concept that Gal-1 monomer is the active form, at least on PMN-EC interaction.
The majority of the investigations in the literature have reported Gal-1 anti-inflammatory effects in models driven by T-cell activation. Gal-1 ability to induce Th1 cell apoptosis has been proposed as the mechanism responsible for its efficacy in experimental models of arthritis. 7 With the exception of a recent study, 8 the potential effect of Gal-1 on the phenomenon of PMN recruitment in vivo has not been investigated. In the study of Rabinovich and colleagues, 8 local injection of 0.5 to 8 μg of Gal-1 reduced phospholipase A2-induced paw edema and PMN infiltration. However, this model is a relatively complex response in which several soluble mediators and cells are involved. 30 Therefore, we complemented the in vitro observations on PMN chemotaxis and migration, with a simpler in vivo model of PMN recruitment. 24
We chose IL-1β as a stimulus for two reasons: firstly, IL-1β-induced PMN extravasation is much more specific in its mechanisms (ie, it is not associated with the multiple activation that characterizes the inflammatory response produced by phospholipases or insoluble polymers such as carrageenin); 31 secondly, IL-1β-induced PMN tissue infiltration is not only relevant to acute inflammation, but it is also functional during the active phases of chronic inflammatory diseases including rheumatoid arthritis. 32
Both hrGal-1 and mutant CS2 inhibited IL-1β-induced PMN infiltration into the mouse peritoneal cavity, indicating a possible association between the mechanisms of action underlying the effects in vitro with those quantified in vivo. Interestingly, the native protein produced a bell-shaped dose-response curve, with a maximal effect at a dose as low as 0.3 μg (20 pmol) per mouse. It is well known that IL-1β induces the synthesis and/or release of chemokines and nonprotein chemoattractants, these together with endothelial adhesion molecule up-regulation, promote PMN extravasation. 33-36 As the Gal-1 anti-migratory effect did not seem to be linked to an inhibition of IL-1β induced mediator generation, we tested the hypothesis that Gal-1 could affect a specific step in the PMN interaction with the activated endothelium. We chose the mouse mesentery for intravital microscopy to test this hypothesis because it has been shown that in this model IL-1β attracts predominantly blood-borne PMNs. 25 Treatment of mice with hrGal-1 produced a selective interference with the PMN-capturing phenomenon, whereas it did not alter the speed at which recruited cells rolled on the activated endothelium. PMN capturing is sustained by endothelial P-selectin and/or PMN L-selectin. 37 It is therefore possible that Gal-1 interferes with the expression or binding of either selectin. However, if an identical mechanism is responsible for Gal-1 inhibition of PMN activities in vitro and in vivo, then it is more likely that the protein interferes with an L-selectin event. Importantly, hrGal-1 inhibition of cell capturing, indirectly demonstrated by the decreased cell flux, was associated with downstream reduction in white blood cell adhesion to and migration through the inflamed postcapillary venule endothelium. Equally important, these in vivo anti-inflammatory actions of Gal-1 appeared to be genuinely because of an interference with PMN-EC interactions, and not secondary to other systemic or local effects. For instance, the anti-inflammatory doses of hrGal-1 (0.3 μg/mouse equivalent to 20 pmol) did not modify the number of circulating leukocytes, nor provoked alteration in arterial or venule shear rates (which indirectly could have affected white blood cell rolling).
Recently, Gal-1 has been proposed to have a proinflammatory profile, although this assumption was solely based on its ability to activate human PMN NADPH oxidase in vitro. 38 In addition, this response was achieved when cells were stimulated with a high amount of the protein (40 μg/ml), well above the concentrations used here to display PMN inhibitory effects. In addition, the in vivo data discussed above clearly indicate an anti-inflammatory, rather than proinflammatory profile of Gal-1, at least in the context of acute experimental inflammation. The data here present are more in line with other in vivo investigations. 7,8 Finally, a histological study of Gal-1 expression in the nasal polyps positively associated Gal-1 expression with inhibition of eosinophil accumulation, also in response to corticosteroid treatment. 39 Thus, the anti-migratory effect we discussed here for Gal-1 may be common to human and rodent granulocytes. It is yet to be investigated whether inhibition of actin polymerization, demonstrated in the eosinophil, 39 is the common intracellular molecular mechanism.
In the final part of the study, we sought to gain information on potential Gal-1-binding sites and target cells. Complex sugars are known to modulate PMN extravasation. For instance, inhibition of selectin binding to sialyl Lewis X by the polysaccharide fucoidin, results in reduction of PMN rolling and extravasation. 40,41 These sugars may be expressed on several membrane proteins, 42 including those mediating L-selectin and P-selectin binding to their counterligands. 37 It is of interest that a recent study has reported a role for complex sugars in mediating the anti-inflammatory effects of heparin derivatives. 43
The key structure recognized by Gal-1 is the disaccharide unit, O-linked N-acetyl-lactosamine (Gal-β1–4GlcNAc). 44 This structure is found in a variety of glycoconjugates and in addition, it can serve as a backbone for various molecules including blood group determinants and Lewis antigens. 45 For example, polymerized Gal-β1–4GlcNAc structures are found in glycoconjugates such as laminin and fibronectin. At present, little is know about Gal-1 ligands on PMNs. We could demonstrate presence of a specific binding on human PMNs, in view of the displacement produced by unlabeled Gal-1, and the inhibition exerted by lactose. Displacement by unlabeled Gal-1 occurred at concentrations identical to those required for inhibition of PMN chemotaxis and trans-EC migration. Compared to unlabeled Gal-1, higher concentrations of biotinylated Gal-1 were required to achieve the same level of binding. This decreased affinity may be a consequence of the biotinylation. Nonetheless, in most situations, binding of the biotinylated molecule could be displaced by the unlabeled molecule, indicating that the two preparations bind to the same receptor(s).
The binding experiments revealed modulation in relation to the status of cell activation inasmuch as PMN adhesion led to an increase in binding. This pattern was opposite to the one detected in ECs. An adhesion event was necessary here, because PMA addition to PMNs in the test tube did not augment Gal-1 binding, whereas it did if PMA-stimulated cells were allowed to adhere to plastic wells (M La, unpublished data). These findings suggest that the expression of binding sites for Gal-1 on human PMNs can be increased after cell adhesion, likely through a process of granule fusion with the plasma membrane, whereas it is reduced in activated ECs, possibly because of a proteolytic event. Future studies will test these hypotheses. Experiments of over-lay using PMN extracts indicated the existence of multiple potential Gal-1-binding proteins. 38 We have reproduced, at least in part, this data and shown an alteration of these bands in relation to the status of PMN activation (M La, unpublished data). Future studies will address the nature of these binding site(s), whether for instance they are analogous to those reported for Gal-3. 46
In summary, we have demonstrated a novel effect for hrGal-1 that is a specific inhibitory action on the initial steps governing PMN-endothelium interaction. Supposing these effects are common to those elicited by EC-derived Gal-1, it is tempting to propose a model in which ECs will be the major producer of the protein, and the extravasating PMNs will be the target, with a down-stream anti-inflammatory end point.
Footnotes
Address reprint requests to Mauro Perretti, William Harvey Research Institute, Bart’s and the London, Queen Mary SMD, University of London, Charterhouse Square, London EC1M 6BQ, UK. E-mail: m.perretti@qmul.ac.uk.
Supported by the Wellcome Trust United Kingdom (grants 062367/Z/00 and 061757) and the Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil (to S. M. O. and G. C.).
M. P. is a Senior Fellow of the Arthritis Research Campaign (UK).
References
- 1.Barondes SH, Cooper DN, Gitt MA, Leffler H: Galectins: structure and function of a large family of animal lectins. J Biol Chem 1994, 269:20807-20810 [PubMed] [Google Scholar]
- 2.Rabinovich GA, Baum LG, Tinari N, Paganelli R, Natoli C, Liu FT, Iacobelli S: Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response? Trends Immunol 2002, 23:313-320 [DOI] [PubMed] [Google Scholar]
- 3.Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K, Leffler H, Liu F, Lotan R, Mercurio AM, Monsigni M, Pillal S, Poirer F, Raz A, Rigby PWJ, Rini JM, Wang JL: Galectins: a family of animal beta-galactoside-binding lectins. Cell 1994, 76:597-598 [DOI] [PubMed] [Google Scholar]
- 4.Colnot C, Fowlis D, Ripoche MA, Bouchaert I, Poirier F: Embryonic implantation in galectin 1/galectin 3 double mutant mice. Dev Dyn 1998, 211:306-313 [DOI] [PubMed] [Google Scholar]
- 5.Poirier F, Robertson EJ: Normal development of mice carrying a null mutation in the gene encoding the L14 S-type lectin. Development 1993, 119:1229-1236 [DOI] [PubMed] [Google Scholar]
- 6.Perillo NL, Marcus ME, Baum LG: Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med 1998, 76:402-412 [DOI] [PubMed] [Google Scholar]
- 7.Rabinovich GA, Daly G, Dreja H, Tailor H, Riera CM, Hirabayashi J, Chernajovsky Y: Recombinant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T cell apoptosis. J Exp Med 1999, 190:385-398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabinovich GA, Sotomayor CE, Riera CM, Bianco I, Correa SG: Evidence of a role for galectin-1 in acute inflammation. Eur J Immunol 2000, 30:1331-1339 [DOI] [PubMed] [Google Scholar]
- 9.Panes J, Granger DN: Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology 1998, 114:1066-1090 [DOI] [PubMed] [Google Scholar]
- 10.Smith CW: Introduction: functional polarity of motile neutrophils. Blood 2000, 95:2459-2461 [PubMed] [Google Scholar]
- 11.Baum LG, Seilhamer JJ, Pang M, Levine WB, Beynon D, Berliner JA: Synthesis of an endogeneous lectin, galectin-1, by human endothelial cells is up-regulated by endothelial cell activation. Glycoconj J 1995, 12:63-68 [DOI] [PubMed] [Google Scholar]
- 12.Lotan R, Belloni PN, Tressler RJ, Lotan D, Xu XC, Nicolson GL: Expression of galectins on microvessel endothelial cells and their involvement in tumour cell adhesion. Glycoconj J 1994, 11:462-468 [DOI] [PubMed] [Google Scholar]
- 13.Hirabayashi J, Ayaki H, Soma G, Kasai K: Production and purification of a recombinant human 14 kDa beta-galactoside-binding lectin. FEBS Lett 1989, 250:161-165 [DOI] [PubMed] [Google Scholar]
- 14.Hirabayashi J, Kasai K: Effect of amino acid substitution by sited-directed mutagenesis on the carbohydrate recognition and stability of human 14-kDa beta-galactoside-binding lectin. J Biol Chem 1991, 266:23648-23653 [PubMed] [Google Scholar]
- 15.Perretti M, Croxtall JD, Wheller SK, Goulding NJ, Hannon R, Flower RJ: Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat Med 1996, 2:1259-1262 [DOI] [PubMed] [Google Scholar]
- 16.Lim LH, Flower RJ, Perretti M, Das AM: Glucocorticoid receptor activation reduces CD11b and CD49d levels on murine eosinophils: characterization and functional relevance. Am J Respir Cell Mol Biol 2000, 22:693-701 [DOI] [PubMed] [Google Scholar]
- 17.Solito E, de Coupade C, Canaider S, Goulding NJ, Perretti M: Transfection of annexin 1 in monocytic cells produces a high degree of spontaneous and stimulated apoptosis associated with caspase-3 activation. Br J Pharmacol 2001, 133:217-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edgell CJ, McDonald CC, Graham JB: Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA 1983, 80:3734-3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wheller SK, Perretti M: Dexamethasone inhibits cytokine-induced intercellular adhesion molecule-1 up-regulation on endothelial cell lines. Eur J Pharmacol 1997, 331:65-71 [DOI] [PubMed] [Google Scholar]
- 20.Schweitzer KM, Vicart P, Delouis C, Paulin D, Drager AM, Langenhuijsen MM, Weksler BB: Characterization of a newly established human bone marrow endothelial cell line: distinct adhesive properties for hematopoietic progenitors compared with human umbilical vein endothelial cells. Lab Invest 1997, 76:25-36 [PubMed] [Google Scholar]
- 21.Rabinovich GA, Iglesias MM, Modesti NM, Castagna LF, Wolfenstein-Todel C, Riera CM, Sotomayor CE: Activated rat macrophages produce a galectin-1-like protein that induces apoptosis of T cells: biochemical and functional characterization. J Immunol 1998, 160:4831-4840 [PubMed] [Google Scholar]
- 22.Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976, 72:248-254 [DOI] [PubMed] [Google Scholar]
- 23.Oliani SM, Paul-Clark MJ, Christian HC, Flower RJ, Perretti M: Neutrophil interaction with inflamed postcapillary venule endothelium alters annexin 1 expression. Am J Pathol 2001, 158:603-615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ajuebor MN, Gibbs L, Flower RJ, Das AM, Perretti M: Investigation of the functional role played by the chemokine monocyte chemoattractant protein-1 in interleukin-1-induced murine peritonitis. Br J Pharmacol 1998, 125:319-326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLean PG, Ahluwalia A, Perretti M: Association between kinin B(1) receptor expression and leukocyte trafficking across mouse mesenteric postcapillary venules. J Exp Med 2000, 192:367-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho M, Cummings RD: Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells. II. Localization and biosynthesis. J Biol Chem 1995, 270:5207-5212 [DOI] [PubMed] [Google Scholar]
- 27.Lobsanov YD, Gitt MA, Leffler H, Barondes SH, Rini JM: X-ray crystal structure of the human dimeric S-Lac lectin, L-14-II, in complex with lactose at 2.9-A resolution. J Biol Chem 1993, 268:27034-27038 [DOI] [PubMed] [Google Scholar]
- 28.Giudicelli V, Lutomski D, Levi-Strauss M, Bladier D, Joubert-Caron R, Caron M: Is human galectin-1 activity modulated by monomer/dimer equilibrium? Glycobiology 1997, 7:viii-x [DOI] [PubMed] [Google Scholar]
- 29.Cho M, Cummings RD: Characterization of monomeric forms of galectin-1 generated by site-directed mutagenesis. Biochemistry 1996, 35:13081-13088 [DOI] [PubMed] [Google Scholar]
- 30.Cirino G, Peers SH, Flower RJ, Browning JL, Pepinsky RB: Human recombinant lipocortin 1 has acute local anti-inflammatory properties in the rat paw edema test. Proc Natl Acad Sci USA 1989, 86:3428-3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vinegar R, Truax JF, Selph JL, Johnston PR, Venable AL, McKenzie KK: Pathway to carrageenan-induced inflammation in the hind limb of the rat. Fed Proc 1987, 46:118-126 [PubMed] [Google Scholar]
- 32.Quayle JA, Adams S, Bucknall RC, Edwards SW: Interleukin-1 expression by neutrophils in rheumatoid arthritis. Ann Rheum Dis 1995, 54:930-933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nourshargh S, Larkin SW, Das A, Williams TJ: Interleukin-1-induced leukocyte extravasation across rat mesenteric microvessels is mediated by platelet-activating factor. Blood 1995, 85:2553-2558 [PubMed] [Google Scholar]
- 34.Wakelin MW, Sanz MJ, Dewar A, Albelda SM, Larkin SW, Boughton-Smith N, Williams TJ, Nourshargh S: An anti-platelet-endothelial cell adhesion molecule-1 antibody inhibits leukocyte extravasation from mesenteric microvessels in vivo by blocking the passage through the basement membrane. J Exp Med 1996, 184:229-239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahluwalia A, De Felipe C, O’Brien J, Hunt SP, Perretti M: Impaired IL-1beta-induced neutrophil accumulation in tachykinin NK1 receptor knockout mice. Br J Pharmacol 1998, 124:1013-1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tailor A, Tomlinson A, Salas A, Panes J, Granger DN, Flower RJ, Perretti M: Dexamethasone inhibition of leucocyte adhesion to rat mesenteric postcapillary venules: role of intercellular adhesion molecule 1 and KC. Gut 1999, 45:705-712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuhlbrigge RC, Alon R, Puri KD, Lowe JB, Springer TA: Sialylated, fucosylated ligands for L-selectin expressed on leukocytes mediate tethering and rolling adhesions in physiologic flow conditions. J Cell Biol 1996, 135:837-848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almkvist J, Dahlgren C, Leffler H, Karlsson A: Activation of the neutrophil nicotinamide adenine dinucleotide phosphate oxidase by galectin-1. J Immunol 2002, 168:4034-4041 [DOI] [PubMed] [Google Scholar]
- 39.Delbrouck C, Doyen I, Belot N, Decaestecker C, Ghanooni R, de Lavareille A, Kaltner H, Choufani G, Danguy A, Vandenhoven G, Gabius HJ, Hassid S, Kiss R: Galectin-1 is overexpressed in nasal polyps under budesonide and inhibits eosinophil migration. Lab Invest 2002, 82:147-158 [DOI] [PubMed] [Google Scholar]
- 40.Granert C, Raud J, Xie X, Lindquist L, Lindbom L: Inhibition of leukocyte rolling with polysaccharide fucoidin prevents pleocytosis in experimental meningitis in the rabbit. J Clin Invest 1994, 93:929-936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindbom L, Xie X, Raud J, Hedqvist P: Chemoattractant-induced firm adhesion of leukocytes to vascular endothelium in vivo is critically dependent on initial leukocyte rolling. Acta Physiol Scand 1992, 146:415-421 [DOI] [PubMed] [Google Scholar]
- 42.Shimaoka M, Ikeda M, Iida T, Taenaka N, Yoshiya I, Honda T: Fucoidin, a potent inhibitor of leukocyte rolling, prevents neutrophil influx into phorbol-ester-induced inflammatory sites in rabbit lungs. Am J Respir Crit Care Med 1996, 153:307-311 [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Brown JR, Varki A, Esko JD: Heparin’s anti-inflammatory effects require glucosamine 6-O-sulfation and are mediated by blockade of L- and P-selectins. J Clin Invest 2002, 110:127-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rabinovich GA, Rubinstein N, Fainboim L: Unlocking the secrets of galectins: a challenge at the frontier of glyco-immunology. J Leukoc Biol 2002, 71:741-752 [PubMed] [Google Scholar]
- 45.Kelm S, Schauer R, Crocker PR: The sialoadhesins—a family of silica acid-dependent cellular recognition molecules within the immunoglobulin superfamily. Glycoconj J 1996, 13:913-926 [DOI] [PubMed] [Google Scholar]
- 46.Feuk-Lagerstedt E, Jordan ET, Leffler H, Dahlgren C, Karlsson A: Identification of CD66a and CD66b as the major galectin-3 receptor candidates in human neutrophils. J Immunol 1999, 163:5592-5598 [PubMed] [Google Scholar]