Abstract
Various epidemiological studies have demonstrated a relatively low incidence of cardiovascular events in premenopausal women and its marked increment after menopause. In addition, estrogens have been postulated to exert direct anti-atherogenic effects via binding to estrogen receptors in vascular smooth muscle cells (VSMCs). However, not all postmenopausal women develop atherosclerosis despite decreased levels of serum estrogen. Therefore, we believe it is important to examine the status of estrogen metabolism in situ in the human cardiovascular system. Estrone sulfate (E1S) is a major circulating plasma estrogen that is converted into the biologically active estrogen, estrone (E1) by steroid sulfatase (STS). E1 is also sulfated and reverted into E1S by estrogen sulfotransferase (EST). These two enzymes have recently been shown to play important roles in the in situ estrogen actions of estrogen-dependent human tissues and various sex steroid-dependent tumors. STS and EST, however, have not been studied in detail in the human vascular system associated with atherosclerotic changes. In the present study, we evaluated the relative abundance of STS- and EST-immunoreactive protein and mRNA expression in human aorta using immunohistochemistry and reverse transcription followed by quantitative polymerase chain reaction in addition to enzyme activity. STS expression levels were found to be significantly higher in the VSMCs obtained from female aortas with mild atherosclerotic changes than in those with severe atherosclerotic changes and in male aortas regardless of atherosclerotic changes. EST expression levels in the VSMCs of these aortas, however, were significantly higher in female aortas with severe atherosclerotic changes and in male aortas than in female aortas with mild atherosclerotic changes. We believe it is important to examine factors regulating the expression and activity of these estrogen-metabolizing enzymes in the human aorta. Various cytokines have been proposed to function as regulators of these enzymes in other tissues. In the present study, we studied the effects of interleukin (IL)-1β, known to be produced in human atherosclerotic lesions, on the expression of these enzymes using cultured human VSMCs originally obtained from a female patient. IL-1β markedly inhibited the expression of STS mRNA and enzyme activity, but stimulated the expression of EST mRNA and enzyme activity. In addition, IL-1β also reduced E2 production from E1S and E1 in VSMCs. Results from the present study seem to suggest that the expression levels of both STS and EST mRNA and activity may be significantly associated with the degree of atherosclerotic changes in the female aorta, which may be related to cytokines produced in situ, such as IL-1β, in human atherosclerotic lesions.
Various epidemiological studies have reported a relatively low incidence of cardiovascular events in premenopausal women and its marked increment after menopause. 1 Estrogen has, therefore, been proposed as a cardioprotective agent, especially in women. 2 In addition, results of several experimental studies have demonstrated that estrogen is predominantly involved in the developmental suppression of atherosclerosis in various animal models, suggesting that estrogens may have direct anti-atherogenic effects on the cardiovascular system of these animals. 3-8 Estrogens are believed to exert direct anti-atherogenic effects through an initial interaction with the estrogen receptor (ER) in vascular smooth muscle cells (VSMCs). Furthermore, numerous clinical and epidemiological studies have demonstrated that the lack of estrogen is one of the risk factors for the development of atherosclerosis in postmenopausal women. 2,9 However, it is also true that not all postmenopausal women develop atherosclerosis despite decreased levels of serum estrogen. 10 In addition, the great majority of studies to date have failed to demonstrate a correlation between the total levels of circulating estrogens or their metabolites and the degree of atherosclerosis both in men and postmenopausal women. 10,11 Therefore, we believe it is important to examine the status of estrogen metabolism in situ and estrogenic effects in human atherosclerotic aorta.
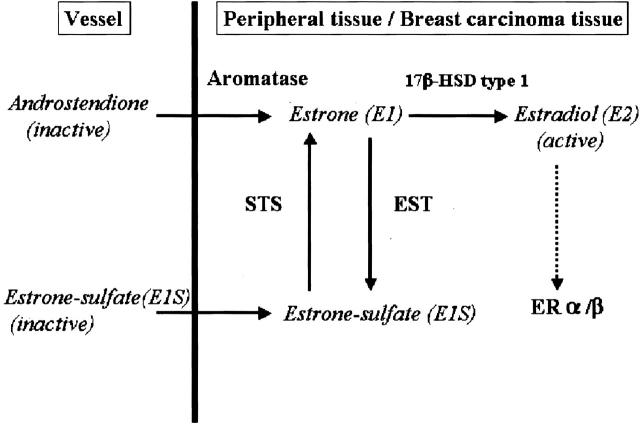
In premenopausal women, the ovary is the main source of circulating estrogen, but in men and postmenopausal women, estrogen biosynthesis is mainly peripheral via conversion of androstenedione or C19 steroids derived from the adrenal cortex, ovary, and/or testis. 12 However, a major circulating form of plasma estrogen is estrone sulfate (E1S), a biologically inactive form of estrogen. E1S has a relatively long half-life in the peripheral blood, 13 whereas serum levels of E1S are known to be 10-fold higher than those of unconjugated estrone (E1) or estradiol (E2). 14 Figure 1 ▶ represents the pathways of in situ estrogen production in human normal peripheral tissues and/or breast carcinomas. 15,16 E1 is sulfated into E1S by the cytosolic enzyme, estrogen sulfotransferase (EST). 17 EST, SULT 1E1 or STE gene, is a member of the superfamily of cytosolic steroid sulfotransferases. EST enzyme activity has been reported in several human tissues. 18 It is also well known that marked differences in EST expression and/or activity exist in tissues depending on species, sex, age, development, and the physiological status of laboratory animals. 19-21 E1S is transformed into a biologically active form, E1, by steroid (estrone) sulfatase (STS). 22-24 STS expression has been examined in estrogen-dependent neoplasia such as breast cancer and endometrial carcinoma. 22-24 In the human cardiovascular system, the expression of STS has been demonstrated in cultured VSMCs. 25 Moreover, mRNA and enzyme activity for both STS and EST have been demonstrated in the human aorta. 26 However, to date, the expression of these enzymes has not been examined in the human cardiovascular system with atherosclerosis. It is also important to examine the factors regulating the expression of these estrogenic metabolizing enzymes. Various cytokines have been proposed as major regulators of these enzymes in other normal and malignant tissues. Reed and Purohit 27 demonstrated that in situ estrogen synthesis in breast carcinoma cells were regulated by various cytokines, including interleukin (IL)-1, IL-6, tumor necrosis factor-α, that were produced in situ by carcinoma or parenchymal cells and stromal cells. IL-1β is well known to exert effects on estrogenic enzyme expression in human tissues. 28-31 In advanced atherosclerotic lesions, the number of lymphocytes and macrophages producing various cytokines was, in general, more abundant than in early atherosclerotic lesions. 32 Among these cytokines, IL-1β has been shown to be produced in situ in many types of the cells present in human atherosclerotic lesions, including lymphocytes, macrophages, endothelial cells, and VSMCs. 32-34 When compared to other cytokines, IL-1β has been demonstrated to play very important roles in neointimal growth of atherosclerotic lesions. 32,35-37
Figure 1.
Scheme representing local production of estrogens in human peripheral tissues and/or breast carcinoma. High concentration of circulating inactive steroids, androstenedione, and estrone-sulfate, are major precursor substrates of local estrogen production in those tissues. Aromatase catalyzes androstenedione into estrone, and STS hydrolyzes estrone-sulfate to estrone. Estrone is subsequently converted to potent estradiol by 17β-HSD-1, and acts on target cells via ERα/β. EST sulfonates estrogens (predominantly estrone) to biologically inactive estrone-sulfates.
In the present study, we examined the relative level of immunoreactive protein and mRNA transcripts for STS and EST by immunohistochemistry and real-time polymerase chain reaction (PCR) in specimens of human aorta. Furthermore, STS and EST enzymatic activities were analyzed to confirm the presence of bioactive conversion of E1 and E1S in the human aorta. We then correlated the level of STS and EST expression with the degree of atherosclerosis, sex, and other features of the samples to further elucidate if in situ estrogen metabolism in the human aorta may have clinical significance, especially in relation to the development of atherosclerosis. We further examined the possible effects of IL-1β on the expression of STS and EST mRNA and activities using cultured VSMCs.
Materials and Methods
Specimens
Human abdominal aorta were collected at the time of autopsy at Tohoku University Hospital, Sendai, Japan, within 3 hours postmortem from 39 patients (17 male, 22 female; mean age, 57.7 ± 3.8 years). The Ethics’ Committee at Tohoku University School of Medicine approved the research protocol for this study. The classification of atherosclerosis defined by the American Heart Association (AHA) in 1995 38 was used in this study to semiquantify the degree of atherosclerotic changes in each specimen, using macroscopic findings at the time of autopsy and subsequent microscopic findings. This classification was based on the fact that in the groups with advanced atherosclerotic changes, clinical complications may develop suddenly compared to those with early atherosclerotic changes. 38 Aortic specimens were tentatively classified into the following five groups: A, B, C, D, and E based on the sex, degree of atherosclerosis, and in female specimens, pre/postmenopausal status (group A: male, normal or mild atherosclerosis, corresponding to group I to III in the AHA classification; group B: male, advanced atherosclerosis, corresponding to group IV to VI in the AHA classification; group C: premenopausal female, normal or mild atherosclerosis; group D: postmenopausal female, advanced atherosclerosis; and group E: postmenopausal female, normal or mild atherosclerosis). Specimens used in the immunohistochemical study were grouped as follows: A, nine cases; B, eight cases; C, six cases; D, nine cases; and E, seven cases. (Table 1) ▶ . In addition, 28 cases (8 male, 20 female; mean age, 55.1 ± 4.7 years) of fresh tissues were available for real-time reverse transcriptase (RT)-PCR studies. The distribution of the cases among these groups was summarized as follows: A, four cases; B, four cases; C, six cases; D, eight cases; and E, six cases (Table 1) ▶ . Thirteen samples of human aorta (group A, two cases; group B, two cases; group C, three cases; group D, three cases; and group E, three cases; mean age, 54.9 ± 6.4 years) were obtained for enzyme assay and RT-PCR study (Table 1) ▶ . No cases of premenopausal female aorta with severe atherosclerotic change were available for examination in this study. Fresh tissues were not always available for examination in all of the cases. In these cases, tissue specimens used for RT-PCR and enzyme assay were collected from the areas adjacent to the specimens for histopathological examination. The adventitia and fat tissues surrounding the aorta and blood were immediately and carefully removed using clean surgical scissors, forceps, and towels at the time of autopsy, after which specimens used for RT-PCR and enzyme assay were immediately frozen in liquid nitrogen and stored at −80°C until use.
Table 1.
Subjects for Immunohistopathology, RT-PCR, and Enzyme Assay Analysis
| Group | Sex | Degree of atherosclerosis | Mense | Immunohistopathology | RT-PCR | Enzyme assay | |||
|---|---|---|---|---|---|---|---|---|---|
| n | Age | n | Age | n | Age | ||||
| A | Male | Mild* | 9 | 41.2 ± 8.2 | 4 | 48.6 ± 8.4 | 2 | 49.5 ± 17.5 | |
| B | Male | Severe† | 8 | 69.4 ± 2.4 | 4 | 69.4 ± 2.4 | 2 | 61.0 ± 10.0 | |
| C | Female | Mild | Premenopause | 6 | 25.0 ± 5.5 | 6 | 25.0 ± 5.5 | 3 | 32.7 ± 9.1 |
| D | Female | Severe | Postmenopause | 9 | 74.7 ± 2.9 | 8 | 69.5 ± 4.1 | 3 | 65.0 ± 9.0 |
| E | Female | Mild | Postmenopause | 7 | 71.9 ± 4.0 | 6 | 70.7 ± 4.6 | 3 | 63.0 ± 5.3 |
| Total | 39 | 57.7 ± 3.8 | 28 | 55.1 ± 4.7 | 13 | 54.9 ± 6.4 | |||
*Mild atherosclerosis means the samples which belonged to group I to III in the AHA classification.
†Severe atherosclerosis means the subjects which belonged to group IV to VI in the AHA classification.
Immunohistochemical Staining
Antibodies used in this study are summarized as follows; rabbit polyclonal antibody for EST (PV-P2237) was purchased from Medical Biological Laboratory (Nagoya, Japan), and was raised against the synthetic N-terminal peptide of human EST corresponding to amino acids 1 to13. The affinity-purified monoclonal antibody for STS, KM1049, was raised against the enzyme purified from human placenta, which recognizes the STS peptide corresponding to amino acids 420 to 428. Utilization of the STS antibody in the evaluation of human breast cancer has been previously reported. 39 In addition, 17β-hydroxysteroid dehydrogenase type 1 (17β-HSD-1) antibody was a rabbit polyclonal antibody against the enzyme purified from human placenta and was kindly provided by Dr. Van Luu-The (Le Centre Hospitalier de l’Universite Laval, Quebec, Canada). The dilutions of primary antibodies were as follows: STS, 1:3000; EST, 1:750; and 17β-HSD-1, 1: 600.
Details of immunohistochemical procedures have been previously described by Sasano and colleagues. 40-42 These sections were immunostained by the biotin-streptavidin method. Slides were deparaffinized in xylene and ethanol, and then rinsed in phosphate-buffered saline (PBS). Sections were deparaffinized with xylene and microwaved (500 W) for 15 minutes in citric acid buffer (2 mmol/L citric acid and 9 mmol/L trisodium citrate dehydrate, pH 6.0) for EST antigen retrieval. After these procedures, reacted tissue sections were rinsed in PBS, and normal rabbit or goat serum was added for 30 minutes. After drainage of blocking agents, the primary antibody was applied, and the tissue slides were incubated at 4°C overnight. Sections were then rinsed in PBS, and endogenous peroxidase activity was then blocked with the application of a solution consisting of 150 ml of methyl alcohol plus 1.5 ml of 3% hydrogen peroxidate (H2O2) for 30 minutes. Slides were rinsed with PBS, and a bridging antibody was applied for 30 minutes at room temperature, after which a solution of peroxidase-anti-peroxidase was added for 30 minutes after a wash with PBS. For STS immunostaining, specimens were incubated with EnVision secondary antibody (DAKO Co. Ltd., Carpinteria, CA) for 1 hour at room temperature. For EST and 17β-HSD-1 immunostaining, specimens were incubated with the Histofine SAB-PO (R) kit secondary antibody (Nichirei Co. Ltd., Tokyo, Japan) for 1 hour at room temperature. A chromogen solution, which consists of diaminobenzidine and H2O2, was then applied to the sections. The reaction was terminated when the positive control tissues demonstrated color development while the negative controls remained colorless. As a positive control, normal liver and full-term placenta were used for the detection of EST, STS, and 17β-HSD-1, respectively. 26,40 Negative controls, in which primary antibody was replaced with 0.01 mol/L of PBS, were performed with each staining run. In addition, we used double-immunostaining with diaminobenzidine and Vector-blue for these proteins and α-smooth muscle actin (α-SMA), respectively, to further characterize the peptide-positive cells in human aorta. 43 In addition, we used monoclonal antibodies against CD34 antigen for endothelial cells and CD68 antigen for macrophages in adjacent tissue sections to examine whether these proteins were expressed in these cells. The hematoxylin and eosin and modified Masson-Goldner’s method was used to identify the internal and external elastic laminas. Examination of these tissue sections provided the anatomical landmarks of each aortic specimen necessary to document the degree of atherosclerosis. After completely reviewing the immunohistochemical sections, relative immunoreactivity for STS, EST, and 17β-HSD-1 in each sample of human aorta was classified into the following groups by blind ranking of each slide by three of the authors (YM, TS, and HS) independently: 2 = strongly positive, 1 = weakly positive, and 0 = negative. 44 Disconcordant results among the observers were re-evaluated together, using a multiheaded light microscope, and statistical significance was evaluated among the above groups.
cDNA Synthesis and Real-Time Polymerase Chain Reaction (PCR)
Total RNA was extracted by homogenizing frozen tissue samples in 1 ml of TRIzol reagent (Life Technologies, Inc., Grand Island, NY) followed by a phenol-chloroform phase extraction and isopropanol precipitation. All RNA samples were quantified by spectrophotometry and stored at −80°C until processed for reverse transcription (RT). The Superscript Preamplification system RT kit (Life Technologies, Inc.) was used in the synthesis of complementary DNA. cDNA was synthesized from total RNA (2 μg) using 25 ng/μL of Oligo (dT)12-18 Primer (Life Technologies, Inc., Gaithersburg, ND) on a PTC-200 Peltier Thermal Cycler DNA Engine (MJ Research, Inc., Watertown, MA). To test for the presence of genomic DNA contamination, we performed the RT step in the absence of Superscript II RNase H− Reverse Transcriptase (Life Technologies, Inc.) followed by PCR. RT-PCR products lacking reverse transcriptase in the initial RT step were run on an ethidium-bromide-stained 2% agarose gel. No bands were observed in these samples (data not shown). The resulting cDNA was used as a template for real-time PCR. Real-time PCR was performed with the Light Cycler System (Roch Diagnostics GmbH, Mannheim, Germany) using the DNA binding dye SYBER Green I (Roch Diagnostics GmbH) for the detection of PCR products. 45,46 PCR was set up using 2 mmol/L MgCl2, 10 pmol/L of each primer (Table 2) ▶ , and 2.5 U TaqDNA polymerase (Life Technologies, Inc.). An initial denaturing step of 95°C for 1 minute was followed by 40 cycles, respectively, of 95°C for 0 seconds, 15 seconds annealing at 60°C [STS, aromatase, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)], 58°C (EST); and extension for 15 seconds at 72°C. The fluorescence intensity of the double-strand-specific SYBER Green I, which reflects the amount of formed specific PCR products, was read by the Light Cycler at 85°C at the end of each extension step. After PCR, these products were resolved on a 2% agarose-ethidium-bromide gel. Images were captured with Polaroid film (Polaroid, Cambridge, MA) under UV transillumination. In initial experiments, PCR products were purified and subjected to direct sequencing (ABI PRISM BigDye Terminator Cycle Sequencing Ready Reaction kit and ABI PRISM 310 Genetic Analyzer; Perkin-Elmer Corp. PE Applied Biosystems, Foster City, CA) to verify amplification of the correct sequences. Negative control experiments lacked cDNA substrate to check for the presence of exogenous contaminant DNA. No amplified products were observed under these conditions. The mRNA levels for STS, EST, and aromatase in each case were summarized as a ratio with respect to GAPDH, and evaluated as a ratio (%) compared with that of each plasmid cDNA, described below.
Table 2.
Primer Sequences Used in RT-PCR Analysis
| cDNA | Sequence | Size (bp) |
|---|---|---|
| STS | Forward 5′-AGGGTCTGGGTGTGTCTGTC-3′ | 290 |
| Reverse 5′-ACTGCAACGCCTACTTAAATG-3′ | ||
| EST | Forward 5′-AGAGGAGCTTGTGGACAGGA-3′ | 114 |
| Reverse 5′-GGCGACAATTTCTGGTTCAT-3′ | ||
| Aromatase | Forward 5′-GTGAAAAAGGGGACAAACTA-3′ | 215 |
| Reverse 5′-ACACTTCTGAGACGATTCCA-3′ | ||
| 17β-HSD-1 | Forward 5′-AGGGCCGCGTGGACGTGCTGGTGTGTAAC-3′ | 200 |
| Reverse 5′-CCATCAATCCTCCCACGCTCCCGG-3′ | ||
| GAPDH | Forward 5′-TGAACGGGAAGCTCACTGG-3′ | 307 |
| Reverse 5′-TCCACCACCCTGTTGCTGTA-3′ |
Synthesis of STS, Aromatase, EST, and Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) Plasmids
To determine the quantity of target cDNA transcripts, cloned plasmid DNAs for STS, aromatase, EST, and GAPDH were used to generate standard curves for real-time quantitative PCR. The cDNAs for STS, aromatase, EST, and GAPDH were amplified by conventional PCR using reverse-transcribed total RNA from placenta (STS, aromatase, and GAPDH), 26,47 and liver (HuH7 human hepatocellular carcinoma cells) (EST), respectively. 26 PCR products were cloned into the pGEM-T Easy vector. The plasmids were purified by a standard miniprep, and the respective inserts released from the vector with a single EcoRI digest and subjected to direct sequencing to verify amplification of the correct sequences. Purified plasmid DNA concentrations were assessed by spectrophotometry at 260 nm and then serially diluted.
Enzyme Assay
EST was assayed as described previously. 48 Briefly, frozen samples were homogenized in a reaction buffer at 4°C and centrifuged for 15 minutes at 1000 × g. The upper layer was used as the enzyme source. Approximately 0.2 mg of protein were added in each assay and the reaction contained 50 mmol/L Tris-HCl, pH 7.4, 7 mmol/L MgCl2, and E1 contained [3H] E1 at 20 nmol/L. Reactions were started with the addition of 3′-phospho-adenosine 5′-phospho-sulfate (PAPS) to a final concentration of 20 μmol/L, in a final volume of 0.125 ml. The reaction mixtures were incubated at 37°C for 30 minutes. Reactions were terminated with the addition of 4.0 ml of chloroform, followed by the addition of 0.375 ml of 0.25 mol/L Tris-HCl, pH 8.7, to alkalinize the solution. The reaction mixtures were centrifuged at 600 × g for 5 minutes to separate the aqueous and organic phases. Synthesis of the tritiated E1S was determined with a liquid scintillation counter (LC-6500; Beckman). The STS activity was assayed according to Utaaker and colleagues 49 with slight modifications. Briefly, enzyme solution (∼0.2 mg protein) was mixed with E1S containing [6,7-3H] E1S (1.6 × 105 dpm, 0.5 pmol/L) at 20 μmol/L, and added to a reaction volume up to 0.15 ml with PBS (−) containing 25 mmol/L sucrose and 4 mmol/L of nicotinamide. The reaction mixture was incubated at 37°C for 60 minutes in a shaking water bath. The enzyme reaction was terminated with the addition of toluene and mixed with a vortex for 1 minute. The reaction mixtures were centrifuged at 600 × g for 5 minutes to separate the aqueous and organic phases. The toluene layer was collected and [3H] radioactivity was measured via a liquid scintillation counter (LC-6500, Beckman), which is equivalent to the amount of E1 formed. Incubation conditions of these assays were designed so that the formation of product was linear.
Analysis with Cultured VSMCs
Cell Culture
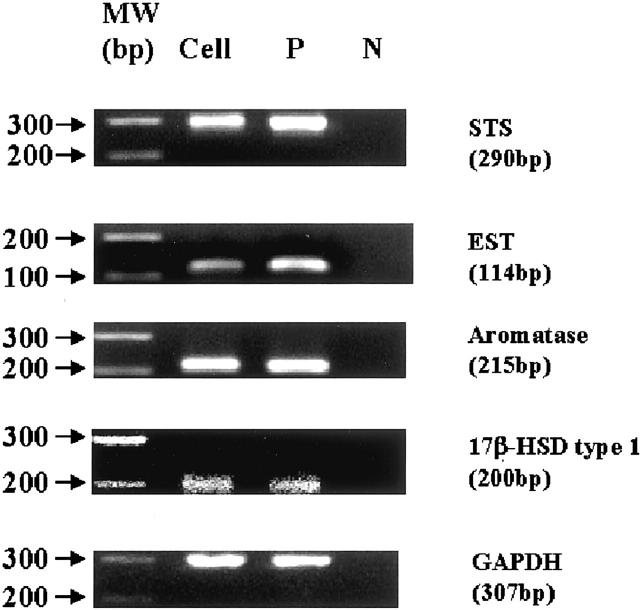
Human female aortic smooth muscle cell lines CRL-1999 were purchased from the American Type Culture Collection (ATCC) (Manassas, VA). They were cultured to subconfluence (24,000 cells/cm2) in a 75-cm2 flask in F12-K medium (ATCC), at 37°C in a 5% CO2 atmosphere. The expression of STS, EST, aromatase, and 17β-HSD-1 was confirmed in these cells using RT-PCR analysis (Figure 2) ▶ .
Figure 2.
Results of real-time RT-PCR analysis for STS, EST, aromatase, and 17β-HSD-1 in cultured human VSMCs. These results demonstrate that VSMCs are positive for STS, EST, aromatase, and 17β-HSD-1. Cell, vascular smooth muscle cells; P, positive controls; N, negative controls.
Analysis of STS and EST mRNA, and E1/E2 Concentration in Cell Culture Medium
These cells were seeded in a 75-cm2 flask at an initial concentration of 100,000 cells/well containing 10% fetal bovine serum per well and cultured until a confluent state was obtained. The medium was then replaced with serum- and phenol red-free medium (modified Eagle’s medium; Sigma Co., St. Louis, MO). Serum- and phenol red-free modified Eagle’s medium was again replaced at 24 hours in the presence of E1S (500 pg/ml) and IL-1β (10 pg/ml) or vehicle (diluted water). The level of E1S in the serum of postmenopausal women was used as a reference for calculating the physiological concentration range of E1S. 15,24 We examined the concentration of E1 and E2 in the medium at 8 hours (treated by E1S) and 24 hours after treatment (treated by E1S alone or by E1S with IL-1β) using a standard radioimmunoassay. In addition, using a standard radioimmunoassay we confirmed that the concentrations of E1 and E2 were 0 pg/ml in the serum- and phenol red-free modified Eagle’s medium alone and/or in medium with IL-1β but not with E1S. We then examined the expression levels of both STS and EST mRNA in VSMCs (treated by E1S alone and by E1S with IL-1β) 24 hours after treatment, according to the previous study. 28
Statistical Analysis
Values for the results were presented as mean ± SE of means (SEM). Immunohistochemistry data were analyzed using the nonparametric Kruskal-Wallis test. The data of quantitative RT-PCR, enzyme assay, and analysis with cultured VSMCs were analyzed using one-way analysis of variance followed by Tukey-Kramer HSD multiple comparisons posttest for comparisons between two groups. P values less than 0.05 were considered significant in this study.
Results
Immunohistochemistry
Results are summarized in Table 3 ▶ and Figure 3 ▶ . Immunoreactivity for both EST and STS was detected in VSMCs but not in endothelial cells and/or inflammatory cells including macrophages. Immunoreactivity for both EST and STS demonstrated significant differences among the five groups (P < 0.05). Immunoreactivity of STS was markedly stronger in VSMCs of the premenopausal (1.83 ± 0.17) and/or postmenopausal female aorta with mild atherosclerotic changes (1.57 ± 0.30) compared to those of the postmenopausal female aorta with severe atherosclerotic changes (0.22 ± 0.15). In contrast, EST immunoreactivity was marked in the postmenopausal female aorta with severe atherosclerotic changes (1.78 ± 0.15) compared to those of the premenopausal female aortas (0.83 ± 0.17) and/or postmenopausal female aortas with mild atherosclerotic changes (1.00 ± 0.31). STS immunoreactivity was lower in male aortas with mild atherosclerotic (0.22 ± 0.15) and severe atherosclerotic changes (0.25 ± 0.16) compared to that in premenopausal and postmenopausal female aortas with mild atherosclerotic changes. EST immunoreactivity tended to be higher in male aortas with mild atherosclerotic changes (1.11 ± 0.26) and/or severe atherosclerotic changes (1.50 ± 0.27) compared to that in female aortas with mild atherosclerotic changes. Relative immunoreactivity for both EST and STS was not correlated with the degree of atherosclerosis in the male aorta. In addition, relative immunoreactivity for 17β-HSD-1 tended to be stronger in VSMCs of the premenopausal (1.60 ± 0.25) and/or postmenopausal female aorta with mild atherosclerotic changes (1.00 ± 0.19) compared to those of the postmenopausal female aorta with severe atherosclerotic changes (0.40 ± 0.25), but the differences did not reach statistical significance among these five groups. Figure 3 ▶ shows representative illustrations of an abdominal aorta specimen obtained from a 63-year-old woman with a mild degree of atherosclerosis (group E), and those of a 65-year-old woman with a severe degree of atherosclerosis (group D).
Table 3.
Results of Immunoreactivity for STS, EST, and 17β-HSD-1 in VSMCs of Human Aorta
| Group | n | STS* | EST* | 17β-HSD-1 |
|---|---|---|---|---|
| Group A | 9 | 0.22 ± 0.15 | 1.11 ± 0.26 | 0.63 ± 0.32 |
| Group B | 8 | 0.25 ± 0.16 | 1.50 ± 0.27 | 0.38 ± 0.18 |
| Group C | 6 | 1.83 ± 0.17 | 0.83 ± 0.17 | 1.60 ± 0.25 |
| Group D | 9 | 0.22 ± 0.15 | 1.78 ± 0.15 | 0.40 ± 0.25 |
| Group E | 7 | 1.57 ± 0.30 | 1.00 ± 0.31 | 1.00 ± 0.19 |
| Total | 39 | 0.64 ± 0.14 | 1.28 ± 0.12 | 0.77 ± 0.13 |
Data are the mean ± SEM. Relative immunoreactivity for each sample was classified into the following groups: 2, strongly positive; 1, weakly positive; and 0, negative. Statistical significance was evaluated among the groups using Kruskal-Wallis test.
*P < 0.05.
Figure 3.
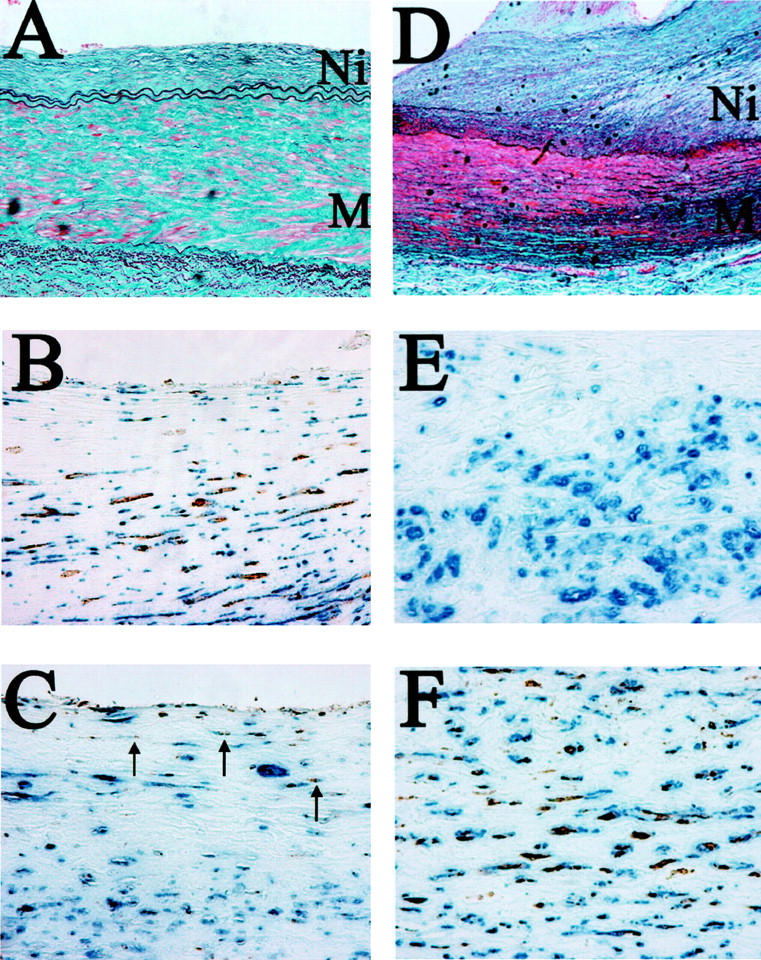
Modified Masson-Goldner’s stains (A) and double-immunohistochemical staining illustrations for α-SMA and STS (B), for α-SMA and EST (C) in an abdominal aorta specimen obtained from a 63-year-old woman with a mild degree of atherosclerosis (group E). In addition, modified Masson-Goldner’s stains (D) and double-immunohistochemical staining illustrations for α-SMA and STS (E), and for α-SMA and EST (F) of an abdominal aorta specimen obtained from a 65-year-old woman with a severe degree of atherosclerosis (group D). Immunopositive cells for STS and EST appear brown as a result of diaminobenzidine colorimetric reaction. Immunopositive cells for α-SMA appear blue as a result of Vector-blue colorimetric reaction. Double-immunopositive cells are confirmed, respectively. These figures demonstrate that the expression of STS is abundantly expressed, whereas that of EST is comparatively weaker (arrows) than that of STS in VSMCs of female aorta with mild atherosclerotic changes. In contrast, these figures also show that the expression of immunoreactive EST is abundantly expressed, whereas that of STS is negative in VSMCs of female aorta with severe atherosclerotic changes. Ni, neointima; M, media. Original magnifications: ×100 for modified Masson-Goldner’s stains; ×400 for double-immunohistochemical staining, respectively.
Real-Time PCR
Results are summarized in Figure 4 ▶ . The relative abundance of STS mRNA determined by real-time PCR analysis was found to be higher in the premenopausal and postmenopausal female aorta with a mild degree of atherosclerosis (8.0 ± 1.9% and 32.3 ± 8.7%, respectively) than in the postmenopausal female aorta with a severe degree of atherosclerotic change (2.0 ± 1.3%) (P < 0.05). The relative abundance of EST mRNA with respect to GAPDH was lower in the premenopausal female aorta and in the postmenopausal aorta with a mild degree of atherosclerotic change (0.6 ± 0.5% and 0.9 ± 0.3%, respectively) than in the postmenopausal female aorta with a severe degree of atherosclerosis (12.0 ± 4.5%) (P < 0.05). Relative mRNA levels for STS in the male aorta with severe atherosclerosis (0.4 ± 0.3%) was significantly lower than in those of premenopausal and postmenopausal female aortas with a mild degree of atherosclerotic change (P < 0.05). In addition, the relative mRNA levels for EST in the male aorta with mild atherosclerotic changes (7.9 ± 1.8%) and with severe atherosclerotic changes (4.3 ± 0.9%) were significantly higher than in premenopausal and postmenopausal female aortas with a mild degree of atherosclerotic changes (P < 0.05). In contrast, the relative mRNA abundance for both EST and STS in the male aorta determined by real-time PCR analysis was not correlated with the degree of atherosclerosis. In addition, the relative mRNA abundance of aromatase in both the male and female aorta determined by real-time PCR analysis was not correlated with the degree of atherosclerosis.
Figure 4.
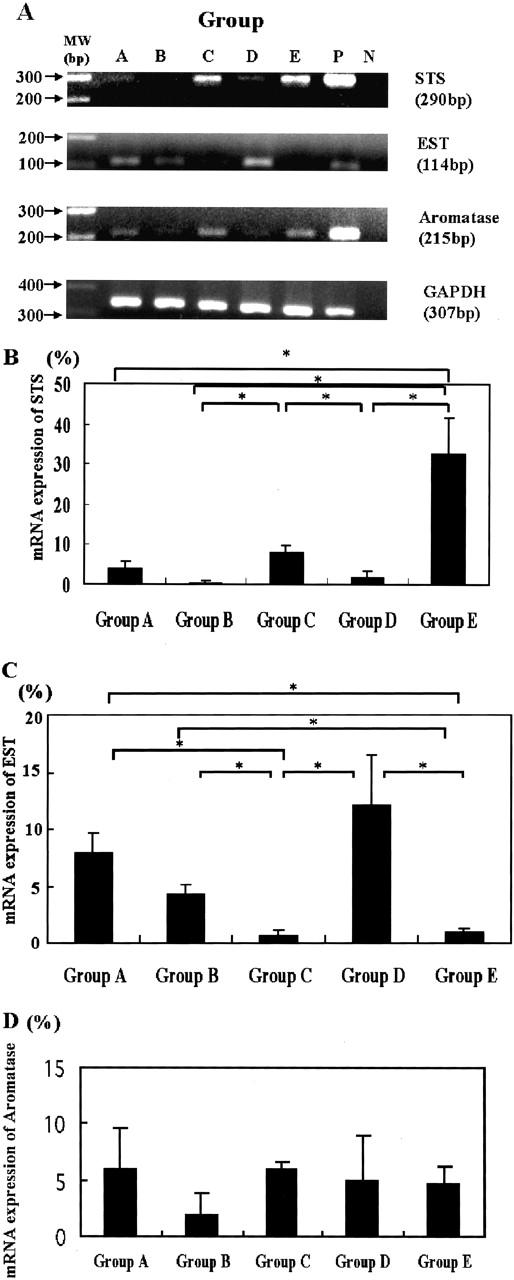
A: Results of real-time RT-PCR analysis for STS, EST, and aromatase in human aortas. In all samples in which mRNA expression was found to be expressed, the bands for these mRNAs were detected as specific single bands (290 bp for STS, 114 bp for EST, 215 bp for aromatase, and 307 bp for GAPDH). The amplified products were run on a 2% agarose gel stained with ethidium bromide. Representative gel photos are shown. Group A, The aorta of a 32-year-old man with mild atherosclerotic change; group B, the aorta of a 67-year-old man with severe atherosclerotic change; group C, the aorta of a 38-year-old premenopausal woman with mild atherosclerotic change; group D, the aorta of 65-year-old postmenopausal woman with severe atherosclerotic change; group E, the aorta of 63-year-old postmenopausal woman with mild atherosclerotic change; P, positive controls (placenta or HuH7 cell lines); N, negative controls (no cDNA substrates). B–D: The results for relative mRNA expression for STS (B), EST (C), and aromatase (D) adjusted with respect to GAPDH (asterisk, P < 0.05).
Enzyme Assay
Results are summarized in Figure 5 ▶ . The enzyme activity for STS was higher in premenopausal and postmenopausal female aortas with mild atherosclerotic changes (0.68 ± 0.07 nmol/mg*hour, and 1.01 ± 0.14 nmol/mg*hour, respectively) than in female aortas with severe atherosclerotic changes (0.37 ± 0.01 nmol/mg*hour) (P < 0.05). Enzyme activity for EST was higher in female aortas with severe atherosclerotic changes (7.21 ± 1.12 nmol/mg*hour) than in premenopausal and postmenopausal female aortas with mild atherosclerotic changes (1.98 ± 0.36 nmol/mg*hour and 3.14 ± 0.76 nmol/mg*hour, respectively) (P < 0.05). However, relative enzyme activity levels for both EST and STS in the male aorta were not correlated with the degrees of atherosclerosis.
Figure 5.
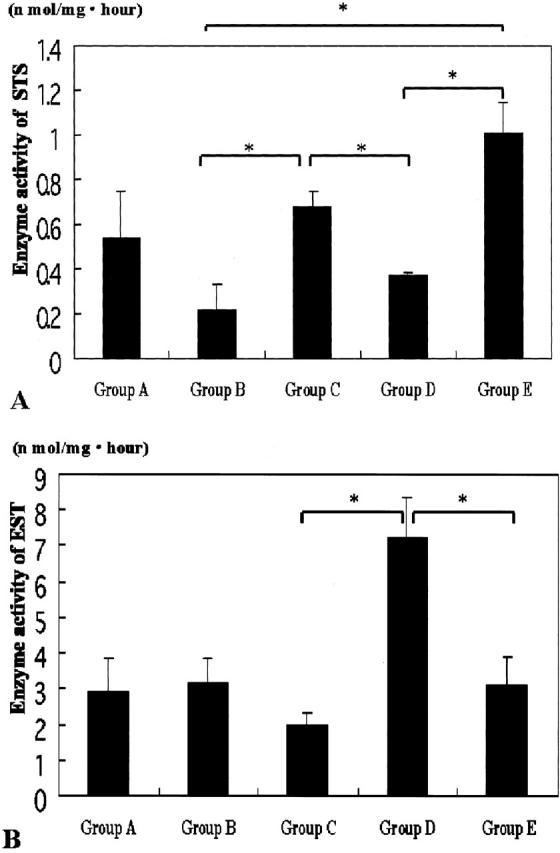
The results of STS (A) and EST (B) activity in enzyme assay analysis (asterisk, P < 0.05).
Cell Culture Study
Results are summarized in Figure 6 ▶ .
Figure 6.
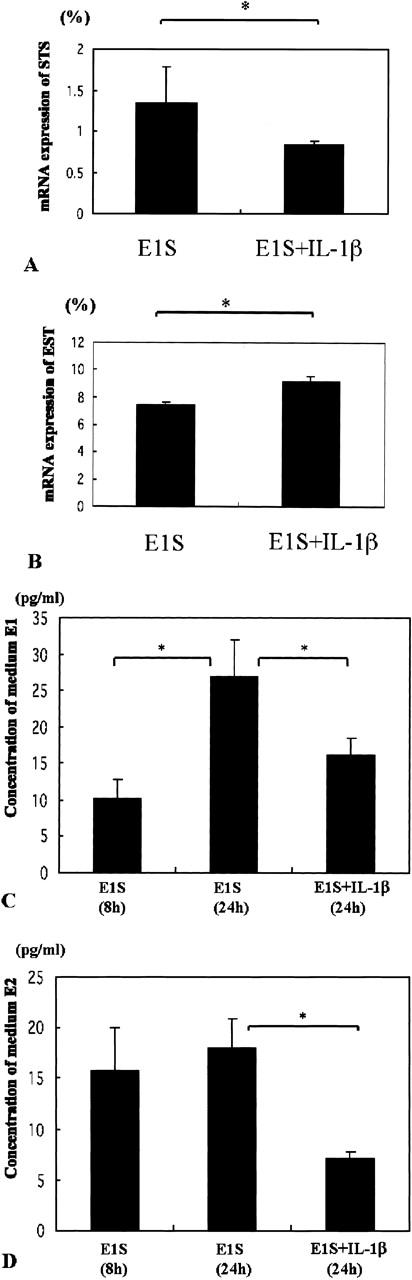
A and B: The relative levels of STS (A) and EST (B) mRNA expression between VSMCs treated with E1S alone and those treated with E1S and IL-1β after 24 hours (asterisk, P < 0.05). C and D: The relative levels of E1 (C) and E2 (D) concentrations in modified Eagle’s medium medium. We measured the levels of E1 and E2 in VSMCs treated with E1S alone at 8 and 24 hours, and in VSMCs treated with E1S and IL-1β after 24 hours (asterisk, P < 0.05).
STS and EST mRNA, and E1/E2 Concentration
The relative abundance of STS mRNA was lower in VSMC cells treated with E1S and IL-1β (0.8 ± 0.1%) than in VSMCs treated with E1S alone (1.4 ± 0.2%) (P < 0.05). The relative abundance of EST mRNA was higher in VSMCs treated with E1S and IL-1β (9.1 ± 0.4%) than in medium of VSMCs treated with E1S alone (7.4 ± 0.2%) (P < 0.05). The concentration of E1 in the medium of VSMCs treated by E1S for 24 hours (26.9 ± 5.0 pg/ml) was significantly higher than cells treated for 8 hours (10.2 ± 2.6 pg/ml) and VSMCs treated by E1S and IL-1β (16.3 ± 2.3 pg/ml) (P < 0.05). In addition, the concentration of E2 in VSMCs treated by E1S for 24 hours (18.1 ± 2.8 pg/ml) was significantly higher than VSMCs treated by E1S and IL-1β for 24 hours (7.1 ± 0.6 pg/ml) (P < 0.05).
Discussion
This is the first study demonstrating the presence of STS and EST and its possible biological significance in human aorta and its lesions. The status of atherosclerosis is well known to be different between males and females. In this study, a correlation between STS and EST enzymes with the degree of atherosclerosis was detected only in the female aorta, and not in male aorta. In addition, we further demonstrated that IL-1β, one of the cytokines produced in situ in atherosclerotic lesions, may be involved in alterations of the enzymes associated with atherosclerosis. Results of several recent studies have demonstrated that estrogen inhibits proliferation and migration of these transformed VSMCs in vitro described above. 50,51 These findings as well as the results from the present study seem to suggest that estrogens may play an important role in protecting blood vessels such as the abdominal aorta from developing atherosclerosis by possibly suppressing the activity of transformed VSMCs via the actions of ERs. ERβ has been reported to be predominantly expressed in VSMCs and is thus likely to be involved in the actions of estrogens in these vascular tissues. 52
One of the major pathways in providing peripheral sources of estrone is through the aromatization of testosterone or androstenedione to E1 or E2. Aromatase, an enzyme involved in the conversion of androgens into estrogens, has been reported in VSMCs of the human aorta. 53-55 Therefore, estrogens produced in situ are considered to play very important roles in the prevention of atherosclerosis by directly acting on VSMCs. However, previous studies have reported that the levels of aromatase expression in the human aorta were not necessarily correlated with gender and/or the degree of atherosclerosis in human aorta. 54 Results from the present study were also consistent with the report documented by Murakami and colleagues. 54 Conversion of E1S to E1 by STS has also been postulated to be an important source of peripheral estrogen production. STS enzyme has also been shown to be relatively high in abundance in the human adult liver and adrenal gland. 26 This enzyme protein was also demonstrated in cultured human VSMCs. 25 After the conversion of E1S to E1 by STS, E1 was also converted to E2 × 17β-HSD-1. E1S was reported to be easily converted not only to E1, but also to E2. 56,57 The expression of 17β-HSD-1 was also confirmed in cultured VSMCs used in this study. Therefore, E2 was postulated to be produced from E1S and E1 in human VSMCs, and may exert direct effects on vessels in anti-atherogenesis, which is consistent with the result of a previous report. 25 The amount of STS was more abundant in female aorta with mild atherosclerotic changes than those with severe atherosclerotic changes. Therefore, in situ production of estrogens in VSMCs of female aorta via STS may possibly exert suppression of VSMC proliferation in the initial phase of atherosclerotic change. In contrast, EST is known to consist of a number of isoenzyme forms. 58 Dooley and colleagues 59 recently reported that estrogen sulfotransferase gene (1E1) transcripts are present in various human normal tissues. Results of a recent study using mRNA analysis, immunohistochemistry, and activity demonstrated that EST was present in a wide range of human tissues. 26 These results seem to suggest that EST plays an important role in the regulation of estrogen metabolism in various peripheral tissues in humans. 26 In liver, kidney, and gastrointestinal tract tissues, EST is believed to be involved in metabolizing excessive amounts of biologically active estrogens to biologically inactive sulfonated forms with marked hydrophilic properties, with the involvement of 17β-HSD-2. 26 Sulfation of estrogens can indirectly influence estrogenic actions by regulating the levels of unconjugated estrogens that can bind to ERs. EST activity in cultured normal cells has been reported to be markedly high, more than that of breast cancer cell lines. 60 These findings together with the results from the present study seem to suggest that EST plays very important roles in the regulation of estrogen metabolism in VSMCs of the human aorta. However, regardless of menopausal status of the patients, the expression of EST in female aorta was significantly lower in mild atherosclerotic aorta than in severe atherosclerotic aorta. In the female aorta with mild atherosclerotic changes, EST expression may be suppressed so that locally synthesized estrogens could not be metabolized, and can maintain relatively high concentrations of estrogen in situ, sufficient to exert estrogenic actions on VSMCs. When the degree of atherosclerotic changes advance, the expression levels of EST may increase because of the effects of cytokines as the result of a previous report demonstrating that vascular endothelial growth factor up-regulated the expression level of EST in myometrial endothelial cells. 61 However, it awaits further investigations for clarification.
In this study, we demonstrated that STS activity and mRNA expression in VSMCs of human aorta are significantly suppressed by IL-1β but that IL-1β accelerates EST expression in VSMCs. These findings indicate that cytokines produced in situ including IL-1β may decrease STS activity and increase EST expression, which may subsequently decrease local estrogen biosynthesis in severe atherosclerotic lesions of female aortas. IL-1β in the human endometrium and in breast cancer has also been shown to regulate the local concentration of E2 by repressing STS activity. 28-30 In addition, IL-1β has been reported to inhibit EST expression in the Leydig cells of mice. 31 Results describing STS suppression by IL-1β are consistent with the results of the present study. In male aorta both with mild and severe atherosclerotic changes, STS and EST activity and levels were not significantly correlated with the degree of atherosclerosis. In addition, the status of expression and activity of these enzymes in the male aorta regardless of atherosclerotic changes was not different from that of female aortas with severe atherosclerosis. Moreover, it has been reported that the amount of aromatase mRNA in tissues from female patients has been reported to be significantly higher than that of tissues from male patients. 54 These findings suggest that estrogens may be less involved in the prevention of atherosclerosis in the male aorta compared to that of the female aorta, which may be related to higher risks of progression of atherosclerosis than in men. However, Nathan and colleagues 62 previously reported that testosterone attenuates early atherogenesis possibly by being converted to estrogens by aromatase expressed in the vessel wall of male aorta. In addition, this may be beneficial for the prevention of progression of atherosclerosis process, because males have much higher steady-state levels of testosterone compared with females, and can acquire constant steady levels of E2 in the vascular tissue after conversion of testosterone by aromatase. 62 This may be one of the reasons that could account for the results of our study, but it awaits further investigations for clarification. In our study, we could not examine premenopausal patients with severe atherosclerotic changes because of the unavailability of these specimens. Therefore, further studies are required to clarify the significance of these enzymes and their involvement in the development and/or pathogenesis of atherosclerosis between males and females.
Footnotes
Address reprint requests to Yasuhiro Nakamura, M.D., Department of Pathology, Tohoku University School of Medicine, 2-1 Seiryo-machi, Aoba-ku, Sendai, 980-8575 Japan. E-mail:nakamura@patholo2.med.tohoku.ac.jp.
References
- 1.Glendy RE, Levine SA, White PD: Coronary disease in youth: comparison of 100 patients under 40 with 300 persons past 80. JAMA 1937, 109:1775-1781 [Google Scholar]
- 2.Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH: Postmenopausal estrogen therapy and cardiovascular disease. N Engl J Med 1991, 325:756-762 [DOI] [PubMed] [Google Scholar]
- 3.Stamler J, Pick R, Katz LN: Prevention of coronary atherosclerosis by estrogen-androgen administration in the cholesterol-fed chick. Circ Res 1953, 4:94-98 [DOI] [PubMed] [Google Scholar]
- 4.Weigensberg BI, Lough J, More RH, Katz E, Pugash E, Peniston C: Effects of estradiol on myointimal thickenings from catheter injury and on organizing white mural non-occlusive thrombi. Atherosclerosis 1984, 52:253-265 [DOI] [PubMed] [Google Scholar]
- 5.Adams MR, Kaolan JR, Manuck SB, Koritnik DR, Parks JS, Wolfe MS, Clarkson TB: Inhibition of coronary artery atherosclerosis by 17β-estradiol in ovariectomized monkeys: lack of an effect of added progesterone. Arteriosclerosis 1990, 10:1051-1057 [DOI] [PubMed] [Google Scholar]
- 6.Hough IL, Zilversmit DB: Effect of 17β-estradiol cholesterol content and metabolism in cholesterol-fed rabbits. Arteriosclerosis 1986, 6:57-63 [DOI] [PubMed] [Google Scholar]
- 7.Sullivan TR, Karas RH, Aronovitz M, Faller GT, Ziar JP, Smith JJ, O’Donnell TF, Mendelson ME: Estrogen inhibits the response-to-injury in a mouse carotid artery model. J Clin Invest 1995, 96:2482-2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodgin JB, Krege JH, Reddick RL, Korach KS, Smithies O, Maeda N: Estrogen receptor α is a major mediator of 17b-estradiol’s anti-atherogenic effects on lesion size in Apoe−/− mice. J Clin Invest 2001, 107:333-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett-Conner E, Bush TL: Estrogen and coronary heart disease in women. JAMA 1991, 265:1861-1867 [PubMed] [Google Scholar]
- 10.Cauley JA, Gutni JP, Glynn NW, Paternostro-Bayles M, Cottington E, Kuller LH: Serum estrone concentrations and coronary artery disease in postmenopausal women. Arterioscler Thromb 1992, 14:14-18 [DOI] [PubMed] [Google Scholar]
- 11.Phillips GB, Pinkernell BH, Jing TY: The association of hypotestosteronemia with coronary artery disease in men. Arterioscler Thromb 1994, 14:701-706 [DOI] [PubMed] [Google Scholar]
- 12.Sasano H, Harada N: Intratumoral aromatase in human breast, endometrial, and ovarian malignancies. Endocrine Rev 1998, 19:593-607 [DOI] [PubMed] [Google Scholar]
- 13.Ruder HJ, Loriaux L, Lipsett MB: Estrone sulfate: production rate and metabolism in man. J Clin Invest 1972, 51:1020-1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samojlik E, Santen RJ, Worgul TJ: Plasma estrone sulfate: assessment of reduced estrogen production during treatment of metastatic breast carcinoma. Steroids 1982, 39:497-507 [DOI] [PubMed] [Google Scholar]
- 15.Santner SJ, Leszczynski D, Wright C, Manni A, Feil PD, Santen RJ: Estrone sulfate: a potential source of estradiol in human breast cancer tissues. Breast Cancer Res Treat 1986, 7:35-44 [DOI] [PubMed] [Google Scholar]
- 16.Suzuki T, Moriya T, Ishida T, Kimura M, Ohuchi N, Sasano H: In situ production of estrogens in human breast carcinoma. Jpn Breast Cancer 2002, 9:296-302 [DOI] [PubMed] [Google Scholar]
- 17.Falany CN: Enzymology of human cytosolic sulfotransferase. EMBO J 1997, 11:206-216 [DOI] [PubMed] [Google Scholar]
- 18.Hobkirk R: Steroid sulfotransferase and steroid sulfatase: characteristics and biological roles. Can J Biochem Cell Biol 1985, 63:1127-1144 [DOI] [PubMed] [Google Scholar]
- 19.Hobkirk R, Glasier MA: Estrogen sulfotransferase distribution of mouse and guinea pig: steroidal inhibition of the guinea pig enzyme. Biochem Cell Biol 1992, 70:712-715 [DOI] [PubMed] [Google Scholar]
- 20.Hobkirk R, Cardy CA, Saidi F, Kennedy TG: Development and characteristics of an oestrogen sulphotransferase in placenta and uterus of the pregnant mouse. Biochem J 1983, 216:451-457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancini MA, Song CS, Rao TR, Chatterjee B, Roy AK: Spatiotemporal expression of estrogen sulfotransferase within the hepatic lobule of male rats: implication of in situ estrogen inactivation in androgen action. Endocrinology 1992, 131:1541-1546 [DOI] [PubMed] [Google Scholar]
- 22.Dao TL, Hayes C, Liddy PR: Steroid sulfatase activities in human breast tumors. Proc Soc Exp Biol Med 1974, 146:381-384 [DOI] [PubMed] [Google Scholar]
- 23.Pasqualini JR, Gelly C, Lecerf F: Estrogen sulfatase: biological and ultrastructural responses and metabolism in MCF-7 human breast cancer cells. Breast Cancer Res Treat 1986, 8:233-240 [DOI] [PubMed] [Google Scholar]
- 24.Naitoh K, Honjo H, Yamamoto T, Urabe M, Ogino Y, Yasumura T, Nambara T: Estrone sulfatase and sulfotransferase activity in human breast cancer and endometrial cancer. J Steroid Biochem 1989, 33:1049-1054 [DOI] [PubMed] [Google Scholar]
- 25.Kikuchi N, Urabe M, Iwasa K, Okubo T, Tsuchiya H, Hosoda T, Tatsumi H, Honjo H: Atheroprotective effect of estriol and estrone sulfate on human vascular smooth muscle cells. J Steroid Biochem 2000, 72:71-78 [DOI] [PubMed] [Google Scholar]
- 26.Miki Y, Nakata T, Suzuki T, Darnel AD, Moriya T, Kaneko C, Hidaka K, Shiotsu Y, Kusaka H, Sasano H: Systemic distribution of steroid sulfatase and estrogen sulfotransferase in human adult and fatal tissues. J Clin Endocrinol Metab 2002, 87:5760-5768 [DOI] [PubMed] [Google Scholar]
- 27.Reed MJ, Purohit A: Breast cancer and the role of cytokines in regulating estrogen synthesis: an emerging hypothesis. Endocr Rev 1997, 18:701-715 [DOI] [PubMed] [Google Scholar]
- 28.Matsuoka R, Yanaihara A, Saito H, Furusawa Y, Toma Y, Shimizu Y, Yanaihara T, Okai T: Regulation of estrogen activity in human endometrium: effect of IL-1beta on steroid sulfatase activity in human endometrial stromal cells. Steroids 2002, 67:655-659 [DOI] [PubMed] [Google Scholar]
- 29.Newman SP, Purohit A, Ghilchik MW, Potter BV, Reed MJ: Regulation of steroid sulphatase expression and activity in breast cancer. J Steroid Biochem Mol Biol 2000, 75:259-264 [DOI] [PubMed] [Google Scholar]
- 30.Honma S, Shimodaira K, Shimizu Y, Tsuchiya N, Saito H, Yanaihara T, Okai T: The influence of inflammatory cytokines on estrogen production and cell proliferation in human breast cancer cells. Endocr J 2002, 49:371-377 [DOI] [PubMed] [Google Scholar]
- 31.Qian YM, Song WC: Regulation of estrogen sulfotransferase expression in Leydig cells by cyclic adenosine 3′,5′-monophosphate and androgen. Endocrinology 1999, 140:1048-1053 [DOI] [PubMed] [Google Scholar]
- 32.Hansson GK, Jonasson L, Seifert PS, Stemme S: Immune mechanisms in atherosclerosis. Arteriosclerosis 1989, 9:567-578 [DOI] [PubMed] [Google Scholar]
- 33.Lusis AJ: Atherosclerosis. Nature 2000, 407:233-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross R: Atherosclerosis: an inflammatory disease. N Engl J Med 1999, 340:115-126 [DOI] [PubMed] [Google Scholar]
- 35.Libby P: Differential effects of human interleukin-1 on growth of human fibroblasts and vascular smooth cells. Atherosclerosis 1985, 5:186-191 [DOI] [PubMed] [Google Scholar]
- 36.Saegusa Y, Ziff M, Welkovich L, Cavender D: Effect of cytokines on human endothelial cell proliferation. J Cell Physiol 1990, 142:488-495 [DOI] [PubMed] [Google Scholar]
- 37.Cozzolino F, Torcia M, Aldinucci D, Ziche M, Almerigogna F, Bani D, Stern DM: Interleukin 1 is an autocrine regulator of human endothelial cell growth. Proc Natl Acad of Sci USA 1990, 87:6487-6491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW: A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. Circulation 1995, 92:1355-1374 [DOI] [PubMed] [Google Scholar]
- 39.Saeki T, Takashima S, Sasaki H, Hanai N, Salomon DS: Localization of estrone sulfatase in human breast carcinomas. Breast Cancer 1999, 6:331-337 [DOI] [PubMed] [Google Scholar]
- 40.Sasano H, Frost AR, Saito R, Harada N, Poutanen M, Vihko R, Bulun SE, Silverberg SG, Nagura H: Aromatase and 17β-hydroxysteroid dehydrogenase type 1 in human breast carcinoma. J Clin Endocrinol Metab 1996, 81:4042-4046 [DOI] [PubMed] [Google Scholar]
- 41.Sasano H: Functional pathology of human ovarian steroidogenesis, normal cycling ovary and steroid producing neoplasms. Endo Pathol 1994, 5:81-89 [DOI] [PubMed] [Google Scholar]
- 42.Sasano H, Kimura M, Shizawa S, Kimura S, Nagura H: Aromatase and steroid receptors in gynecomastia and male breast carcinoma: an immunohistochemical study. J Clin Endocrinol Metab 1996, 81:3063-3067 [DOI] [PubMed] [Google Scholar]
- 43.Mori M, Kurane I, Janus J, Ennis FA: Cytokine production by dengue virus antigen-responsive human T lymphocytes in vitro examined using a double immunocytochemical technique. J Leukoc Biol 1997, 61:338-345 [DOI] [PubMed] [Google Scholar]
- 44.Suzuki T, Takahashi K, Darnel AD, Moriya T, Murakami O, Narasaka T, Takeyama J, Sasano H: Chicken ovalbumin upstream promoter transcription factor II in the human adrenal cortex and its disorders. J Clin Endocrinol Metab 2000, 85:2752-2757 [DOI] [PubMed] [Google Scholar]
- 45.Read S: Recovery efficiencies of nucleic acid extraction kits as measured by quantitative LightCycler PCR. J Clin Pathol Mol Pathol 2001, 54:86-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niino Y, Irie T, Takaishi M, Hosono T, Nam-ho Huh, Tachikawa T, Kuroki T: PKCθII, a new isoform of protein kinase C specifically expressed in the seminiferous tubules of mouse testis. J Biol Chem 2001, 276:36711-36717 [DOI] [PubMed] [Google Scholar]
- 47.Pasanen M: The expression and regulation of drug metabolism in human placenta. Adv Drug Deliv Rev 1999, 38:81-97 [DOI] [PubMed] [Google Scholar]
- 48.Falany CN, Krasnykh V, Falany JL: Bacterial expression and characterization of a cDNA for human liver estrogen sulfotransferase. J Steroid Biochem Mol Biol 1995, 52:529-539 [DOI] [PubMed] [Google Scholar]
- 49.Utaaker E, Støa KF: Oestrone sulfatase activity of the rat uterus in different hormonal states. Hormone Res 1980, 13:180-185 [DOI] [PubMed] [Google Scholar]
- 50.Seeger H, Walwiener D, Mueck AO: Effect of medroxyprogesterone acetate and norethisterone on serum-stimulated and estradiol-inhibited proliferation of human coronary artery smooth muscle cells. Menopause 2001, 8:5-9a [DOI] [PubMed] [Google Scholar]
- 51.Okubo T, Urabe M, Tsuchiya H, Iwasa K, Yokota K, Kikuchi N, Yamamoto T, Honjo H: Effect of estrogen and progesterone on gene expression of growth regulatory molecules and proto-oncogene in vascular smooth muscle cells. Endocr J 2000, 47:205-214 [DOI] [PubMed] [Google Scholar]
- 52.Hodges YK, Tung L, Yan XD, Graham JD, Horwitz KB, Horwitz LD: Estrogen receptors alpha and beta: prevalence of estrogen receptor beta mRNA in human vascular smooth muscle and transcriptional effects. Circulation 2000, 101:1792-1798 [DOI] [PubMed] [Google Scholar]
- 53.Diano S, Horvath TL, Mor G, Register T, Adams M, Harada N, Naftolin F: Aromatase and estrogen receptor immunoreactivity in the coronary arteries of monkeys and human subjects. Menopause 1999, 6:21-28 [PubMed] [Google Scholar]
- 54.Murakami H, Harada N, Sasano H: Aromatase in atherosclerotic lesions of human aorta. J Steroid Biochem Mol Biol 2001, 79:67-74 [DOI] [PubMed] [Google Scholar]
- 55.Harada N, Sasano H, Murakami H, Ohkuma T, Nagura H, Takagi Y: Localized expression of aromatase in human vascular tissues. Circ Res 1999, 84:1285-1291 [DOI] [PubMed] [Google Scholar]
- 56.Bhavnani BR: Pharmacokinetics and pharmacodynamics of conjugated equine estrogens: chemistry and metabolism. Proc Soc Exp Biol Med 1998, 217:6-16 [DOI] [PubMed] [Google Scholar]
- 57.Runder HJ, Loriaux DL, Lipsett MB: Estrone sulfate: production rate and metabolism in man. J Clin Invest 1972, 51:1020-1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strott CA: Steroid sulfotransferase. Endocr Rev 1996, 17:670-697 [DOI] [PubMed] [Google Scholar]
- 59.Dooley TP, Haldeman-Cahill R, Joiner J, Wilborn TW: Expression profiling of human sulfotransferase and sulfatase gene superfamilies in epithelial tissues and cultured cells. Biochem Biophys Res Commun 2000, 277:236-245 [DOI] [PubMed] [Google Scholar]
- 60.Anderson E, Howell A: Oestrogen sulphotransferase in malignant and normal human breast tissue. Endocr Relate Cancer 1995, 2:227-233 [Google Scholar]
- 61.Weston GC, Haviv I, Rogers PA: Microarray analysis of VEGF-responsive genes in myometrial endothelial cells. Mol Hum Reprod 2002, 8:855-863 [DOI] [PubMed] [Google Scholar]
- 62.Nathan L, Shi W, Dinh H, Mukherjee TK, Wang X, Lusis AJ, Chaudhuri G: Testosterone inhibits early atherogenesis by conversion to estradiol: critical role of aromatase. Proc Natl Acad Sci USA 2001, 98:3589-3593 [DOI] [PMC free article] [PubMed] [Google Scholar]