Abstract
Macrophages accumulate in ischemic areas of such pathological tissues as solid tumors, atherosclerotic plaques and arthritic joints. Studies have suggested that hypoxia alters the phenotype of macrophages in a way that promotes these lesions. However, the genes up-regulated by macrophages in such hypoxic tissues are poorly characterized. Here, we have used cDNA array hybridization to investigate the effects of hypoxia on the mRNAs of 1185 genes in primary human monocyte-derived macrophages. As shown previously in other cell types, mRNA levels for vascular endothelial growth factor (VEGF) and glucose transporter 1 (GLUT-1) were up-regulated by hypoxia. However, the mRNAs of other genes were also up-regulated including matrix metalloproteinase-7 (MMP-7), neuromedin B receptor, and the DNA-binding protein inhibitor, Id2. The promoters of GLUT-1 and MMP-7 confer hypoxic inducibility on a reporter gene in RAW 264.7 macrophages, indicating that the hypoxic up-regulation of these mRNAs may occur, at least in part, at the transcriptional level. GLUT-1 and MMP-7 mRNA were also shown to be up-regulated in hypoxic macrophages in vitro by real-time RT-PCR, and these proteins were elevated in hypoxic macrophages in vitro and in hypoxic areas of human breast tumors. The hypoxia up-regulated genes identified could be important for the survival and functioning of macrophages in hypoxic diseased tissues, and their promoters could prove useful in macrophage-delivered gene therapy.
The presence of areas of low oxygen tension (hypoxia) is a feature common to malignant tumors, 1 wounds, 2 arthritic joints 3 and atherosclerotic plaques. 4 These areas form when the local blood supply is poorly organized, occluded, or simply unable to keep pace with the growth and/or infiltration of cells in a given area. Macrophages accumulate in large numbers in such hypoxic/ischemic tissues 5 and respond to hypoxia by up-regulating a number of transcription factors. Among the most prominent of these are the hypoxia-inducible factors (HIFs) 1 and 2, increased levels of which are seen in macrophages in ischemic areas of malignant tumors, 6,7 the inflamed synovial lining of joints with rheumatoid arthritis 8 and human dermal wounds. 9 Interestingly, conditional ablation of HIF-1α in macrophages renders them incapable of migrating into damaged/diseased tissue and performing their normal inflammatory and microbicidal functions in such sites. 10
HIFs 1 and 2 are heterodimers consisting of different hypoxia-inducible α subunits and a common, constitutively expressed β subunit. Under normal oxygen tensions (“normoxia”), the α subunits are rapidly ubiquitinated and degraded by the proteasome. Hypoxia inhibits ubiquitination of HIFs, causing them to accumulate in the nucleus, where they bind to short DNA sequences called hypoxia response elements (HREs) near oxygen-sensitive genes, 11 stimulating their transcription. Such genes include vascular endothelial growth factor (VEGF). 12 Expression of VEGF by macrophages is markedly increased by exposure to hypoxia in vitro 13 and in poorly vascularized areas of malignant tumors 14 where it promotes the formation of new blood vessels, increasing the supply of oxygen and nutrients to the area. Macrophages are also known to release PR-39, a 39 amino acid peptide that inhibits the degradation of HIF-1α protein by neighboring cells, thereby stimulating their expression of VEGF. 15 Together, these observations may explain previous reports showing that the presence of high numbers of tumor-associated macrophages (TAM) correlates with increased tumor angiogenesis and/or reduced patient survival in various forms of cancer. 14,16
The ability of macrophages to accumulate preferentially in ischemic/hypoxic areas of diseased tissues and up-regulate hypoxia-inducible transcription factors has led to the proposal that they may have utility as delivery vehicles to target gene therapy to hypoxic tissues. Indeed, we demonstrated recently that primary human macrophages engineered to carry the gene for the pro-drug activating enzyme, cytochrome p450 2B6, under the control of a trimerized HRE, are capable of migrating into the hypoxic centers of tumor spheroids (small, spherical tumor masses grown in vitro), and activating expression of the enzyme. This produced marked tumor cell killing in the presence of the non-toxic pro-drug, cyclophosphamide. 17 In addition, the murine macrophage-like cell line, ANA-1, has been engineered to express interferon-γ (IFN-γ) in hypoxia by transfecting cells with the IFN-γ gene under the control of the HRE from the inducible nitric oxide synthase (iNOS) gene. In this way, macrophages were made to selectively secrete this potent, pro-inflammatory cytokine (and thus a series of other, IFN-γ responsive gene products) in hypoxia. 18 Such an approach could be used to target high-level expression of such inflammatory mediators to diseased sites. 19 However, the HREs used in these studies were originally identified by virtue of their ability to confer hypoxic-inducibility on reporter genes in tumor cell lines rather than macrophages. As the activity of individual HREs varies markedly between different cell types, we contend that the most appropriate HRE for use in macrophage-based gene therapies would be derived from a gene highly up-regulated in hypoxic macrophages.
One study used randomly primed PCR followed by sequencing of the products to identify genes up-regulated by macrophages following a brief exposure to hypoxia (1.5 hours). However, the cellular responses observed were likely to represent only the very early stages of the hypoxic response. This, together with the limitations of the PCR-based method applied, might explain why only one gene, MAPK phosphatase 1 (MKP-1), was identified as being up-regulated in hypoxic macrophages. 20
In the present study we have used cDNA array hybridization to identify changes in mRNA levels in primary human monocyte-derived macrophages (MDMs) caused by 16 hours of exposure to hypoxia. Along with those of such known hypoxia-regulated genes as VEGF and the glucose transporter, GLUT-1, three other mRNA transcripts are shown for the first time to be up-regulated by hypoxia, the most highly induced being the enzyme matrix metalloproteinase 7 (MMP-7, or matrilysin). These findings were confirmed using real-time RT-PCR, and then immunohistochemistry used to show the up-regulation of both MMP-7 and GLUT-1 proteins in hypoxic human MDMs in vitro and by macrophages in hypoxic areas of human breast tumors.
We then investigated the possibility of the MMP-7 and GLUT-1 genes being up-regulated by hypoxia at the transcriptional level using reporter gene constructs regulated by parts of the promoters of these two genes. Both up-regulated luciferase gene expression in the macrophage cell line RAW 264.7 following exposure to hypoxia. We confirmed the essential role of HIF-1 in the hypoxic induction of the GLUT-1 promoter in macrophages by showing that its hypoxic inducibility in RAW 264.7 cells was lost when the HIF-1 binding site was ablated. Although a putative HRE was identified in the MMP-7 promoter, a trimer of this sequence was not able to up-regulate luciferase gene expression in hypoxic RAW 264.7 cells, suggesting that other, as yet undetermined, enhancers in this promoter may mediate the effects of hypoxia on the transcription of this gene in such cells.
Materials and Methods
Cell Culture
Peripheral blood mononuclear cells were isolated from platelet-depleted buffy coats provided by the Blood Transfusion Service, Sheffield, UK, using Ficoll-Paque Plus (Amersham Pharmacia Biotech, St. Albans, UK). Mononuclear cells were cultured in Iscove’s modified Dulbecco’s medium (Gibco BRL, Paisley, UK) supplemented with 2.5% human AB serum (Sigma, Poole, UK), penicillin (100 IU/ml), streptomycin (100 μg/ml) and Fungizone (1.25 μg/ml). Initially, 75-cm2 flasks were seeded with 4 × 107 to 1 × 108 cells. Glass coverslips (1.5-cm diameter) were also placed in 12-well plates and seeded with 4 × 106 mononuclear cells.
After 2 hours, the medium containing non-adherent cells was removed and replaced with fresh medium. Adherent cells (monocytes) were used in experiments following a further 6 days of culture. Immunohistochemical staining for the pan macrophage marker, CD68, was used to identify macrophages at this stage, as previously described. 16 This indicated that >95% of cells were monocyte-derived macrophages (MDMs). The murine macrophage-like cell line, RAW 264.7, was obtained from the European Collection of Animal Cell Cultures and cultured according to their recommendations.
Hypoxic Induction Studies
Cells were incubated for 16 hours at 37°C in humidified Heto multi-gas incubators containing either air (20.9% oxygen; normoxia), or 0.5% oxygen; equivalent to pathological levels of hypoxia which range from 0 to 1% oxygen in vivo. 1-4 Five percent CO2 was used in all normoxic and hypoxic incubators, with the balance being nitrogen in hypoxic incubations. Separate Analox oxygen meters were used in all incubators to monitor oxygen levels throughout hypoxic induction studies.
cDNA Array Hybridization
Total RNA was prepared using the RNeasy Purification System (Qiagen, Crawley, UK). RNA produced using this kit is considered to be largely free of contaminating DNA. However, a DNase step was performed to ensure total removal of contaminating DNA. This involved incubating 500 μg of total RNA with 50 units of DNase I at 37°C for 30 minutes followed by phenol chloroform extraction and ethanol precipitation, as described in the Atlas Pure Total RNA Labeling System Kit (Clontech, Basingstoke, UK). The production of radiolabeled cDNA probe mixture by reverse transcription was performed with the same kit, using a mixture of primers supplied by the manufacturer to allowed the production of cDNAs from 1185 different mRNAs. The probe mixture was then scintillation counted, and 3 × 106 cpm of each probe hybridized to Clontech Atlas Human 1.2 nylon arrays containing DNA from these 1185 genes overnight at 68°C in 5 ml of ExpressHyb solution (Clontech). Arrays were washed at 68°C, four times with 2X SSC (1X SSC is 150 mmol/L NaCl, 15 mmol/L sodium citrate, pH 7.0) with 1% SDS, and once with 0.1X SSC with 0.5% SDS, as per the manufacturer’s instructions. Arrays were then exposed to Kodak BioMax MS film at −70°C using an intensifying screen.
Macrophage isolation and exposure to normoxia/hypoxia, RNA preparation, and hybridization was carried out twice, on separate occasions, using cells from different donors and different pairs of array membranes, to confirm the results. Autoradiographs of the gene spots on normoxic and hypoxic arrays were scanned in TIFF format and quantified using Atlas Image Version 1.5 software (Clontech). Searching for the presence of putative transcription factor binding sites in HREs was done using the program MatInspector Version 2.2, available at the internet site http://transfac.gbf.de.
Real-Time RT PCR
RNA was prepared from primary human macrophages using the Qiagen RNeasy Mini Kit, and was quantified by UV spectrometry at 260 nm. Reverse transcription was carried out using the First Strand cDNA Synthesis Kit for PCR from Roche Applied Science (Mannheim, Germany). Real-time PCR reactions were carried out using the Roche Light Cycler, utilizing Roche SYBR Green reagents according to the manufacturer’s instructions. Amplification of PCR products was quantified during PCR by measurement of fluorescence associated with binding of double-stranded DNA to the SYBR Green dye incorporated in the reaction mix. The sequences of the oligonucleotides used to PCR-amplify the cDNAs of interest were: MMP-7 primer 1: ATG GAC TTC CAA AGT GGT CAC CTA CAG, primer 2: GGA TAC ATC ACT GCA TTA GGA TCA GAG; GLUT-11 primer 1: CAA CTG GAC CTC AAA TTT CAT TGT GGG, primer 2: CGG GTG TCT TAT CAC TTT GGC TGG; VEGF primer 1: CAG CGC AGC TAC TGC CAT CCA ATC GAG A, primer 2: GCT TGT CAC ATC TGC AAG TAC GTT CGT TTA. Housekeeping genes (G6PDH, glucose-6-phosphate dehydrogenase; ALAS, 5-aminolevulinate synthase gene; HPRT, hypoxanthine phosphoribosyltransferase; and PBGD, porphobilinogen deaminase) were quantified using the LightCycler-h-Housekeeping Gene Selection Set (Roche) according to the manufacturer’s instructions. This allows accurate and specific quantification of amplified product by measurement of fluorescence produced by FRET (fluorescence resonance energy transfer) following the simultaneous hybridization of two gene-specific fluorophore-labeled DNA oligonucleotides to adjacent sequences within the amplified product. Following an initial denaturation step of 95°C for one minute, 45 cycles of 95°C for 10 seconds, 60°C for 1 second, and 72°C for 10 seconds were used for all MMP-7, GLUT-1, and VEGF PCRs. For housekeeping genes, an initial denaturation step of 95°C for 10 minutes (to activate the “Hotstart” Taq polymerase), followed by 45 cycles of 95°C for 10 seconds, 57.5°C for 15 seconds, and 72°C for 15 seconds was used.
Reporter Gene Constructs
Two forms of GLUT-1 enhancer were used: GLUT-1 N (wild-type) and GLUT-1 mol/L (bearing a mutated, non-functional HIF-1 binding site), 21 consisting of a 184-bp sequence from the rat GLUT-1 1 promoter cloned into the KpnI/NheI sites of the pGL3 promoter luciferase reporter construct (Promega, Southampton, UK). GLUT-1 N and M were kind gifts from Dr. A. Maity, University of Pennsylvania Medical Center, Philadelphia, PA, USA. The OB-HRE1 construct contained a trimer of a modified version of the murine PGK 1 HRE, with defined spacer sequences added. 22 This was used as a positive control in the reporter studies as it is known to be induced in hypoxic macrophages in vitro. 7,17 The OB-HRE1 trimer was cloned into the NheI site of the pGL3 promoter vector. This vector contains an enhancerless SV40 promoter driving luciferase (LUC) expression. An MMP-7 promoter construct, 23 consisting of a 2.3-kb sequence from the human MMP-7 promoter cloned into the pGL2 basic luciferase reporter vector, (which contains no promoter), was a gift from Dr. H. Crawford, Vanderbilt University Medical Center, Nashville, TN, USA.
Our examination of the promoter of the MMP-7 gene (available in GenBank, Accession No. L22525) identified a sequence (−617 to −590 relative to the transcription start site) showing marked similarity to HREs found near other hypoxia-regulated genes (shown in Table 1 ▶ ). This putative HRE contains the HIF-1 binding sequence, 5′-ACGTG-3′, as well as a sequence, 5′-CACAG-3′, previously defined as the HIF ancillary site (HAS) in the VEGF promoter. 24 This was shown to be essential for the full hypoxic-induction of the VEGF HRE, although the spacing between the HBS and HAS in the case of MMP-7 (13 bp) differs from the reported 8 or 9 bp. 24 A concatamerized trimer of the putative MMP-7 HRE was synthesized and cloned into the pGL3 promoter vector. A trimer was used because previous studies have shown that such multiple copies of HREs give good hypoxic inducibility in human macrophages. 17,18 The trimer was made by synthesizing an oligonucleotide containing three copies of the putative HRE plus additional bases incorporating MluI and XhoI restriction sites for cloning and a BssHII site for screening of clones. Positive strand MMP-7-HRE trimer: 5′ CGCGCGC GGG ACG TGG AAG GTG AGG GGA CAC AGC/GGG ACG TGG AAG GTG AGG GGA CAC AGC/GGG ACG TGG AAG GTG AGG GGA CAC AGC 3′ (the underlined bases were added to introduce an MluI overhang and a BssHII site, and the individual HRE repeats are separated by slashes). Negative strand MMP-7-HRE trimer: 5′ TCGA GCT GTG TCC CCT CAC CTT CCA CGT CCC/GCT GTG TCC CCT CAC CTT CCA CGT CCC/GCT GTG TCC CCT CAC CTT CCA CGT CCC GCG 3′ (the underlined bases at the 5′ end introduce an XhoI overhang, and those at the 3′ end introduce a BssHII site). When annealed these primers gave an MluI overhang at the 5′ end, and an XhoI overhang at the 3′ end, which allowed cloning into the MluI/XhoI sites upstream of the minimal SV40 promoter in pGL3 promoter, resulting in the loss of the MluI site, which is replaced with a BssHII site for screening purposes. The XhoI site was maintained.
Table 1.
Comparison of the Double-Stranded DNA Sequences of the Putative HRE from the MMP-7 Gene with Two Other, Well-Characterized HREs
| HRE | DNA sequence | HAS sequence | HRE position in native gene | Reference |
|---|---|---|---|---|
| MMP-7 | GGACGTGGAAGGTGAGGGGA CACAGCGCCTGCACCTTCCAC TCCCCTGTGTCGC | CACAG | −617 to −590 | Present study |
| OB-HRE1 | GTCGTGCAGGACGTGACA TCTAGTCAGCACGTCCTGCA CTGTAGATCA | None | −299 to −272 (in murine PGK1 gene) | 22 |
| EPO | CCTACGTGCTGTCTCACACAG CCTGGATGCACGACAGAGTGT GTCGGA | CACAG | +3067 to +3089 | 24 |
A modified form of the murine PGK1 HRE, “OB-HRE1”, 22 which is known to be hypoxia-inducible in macrophage cell lines, and primary macrophages, 7,17 and the human erythropoietin HRE (EPO) which is hypoxia-inducible in various cell types. 11,24 The consensus HIF binding site (5′-ACGTG-3′) is underlined, and bases highlighted in italics on the OB-HRE1 sequence are the spacer sequence used in this OB-HRE1 timer. 22 HRE positions in the native genes are shown relative to the A of the ATG translation initiation codon (designated as +1),
Cell Transfections
All DNA was prepared using Qiagen endotoxin-free maxi prep kits. RAW 264.7 cells were trypsinized and plated in plastic 6-well tissue culture plates at a density of 2 × 105 cells per well 24 hours before transfection, and then transfected using 6 μl of the Fugene 6 transfection reagent (Roche, Lewes, UK) according to the manufacturer’s instructions, using 1 μg of plasmid DNA per well, and serum-free medium to bring the volume of the mixture up to 100 μl per well. Medium was not removed before or after addition of the Fugene/DNA mixture. Following incubation of transfected cells in normoxic or hypoxic conditions for 16 hours, medium was removed and the monolayers lysed with 200 μl of 1X luciferase assay passive lysis buffer (Promega). Cells were rocked at room temperature for 30 minutes, freeze-thawed once at −70°C, centrifuged at 10,000 × g for 2 minutes to remove cell debris, and then assayed for luciferase activity. Luminometry was performed using luciferase assay reagents from Promega, and an MLX microtiter plate luminometer (Dynex Technologies, Ashford, UK).
Immunolocalization of CD68, MMP-7, GLUT-1, and HIF-1α Protein
Primary human monocyte-derived macrophages grown on coverslips and exposed to normoxia (20.9% O2) or hypoxia (0.5% O2) for 16 hours were removed from incubators and fixed for 10 minutes in 10% formalin in PBS. Ten ductal invasive breast carcinomas were obtained from the archives of the Academic Unit of Pathology, University of Sheffield, Royal Hallamshire Hospital, Sheffield, UK, and four sequential 3-μm sections were cut from each block. For each set of sections, one section was stained for CD68 (for macrophages), the second for HIF-1α, the third for MMP-7, and the last used as the negative control (see below).
After de-waxing and rehydration of tissue sections (not needed for cells grown on coverslips), endogenous peroxidase was blocked using 3% H2O2 in methanol for 20 minutes followed by the appropriate antigen retrieval methods (in the case of tissue sections): for MMP-7 and GLUT-1, microwaving (800W) in 0.1 mol/L tri-sodium citrate (pH 7.4) for 8 minutes; for CD68, 0.01% protease type XXIV (Sigma) in PBS at 37°C for 20 minutes; and for immunostaining for HIF-1α, target retrieval solution (DAKO Ltd, UK) at 95°C for 45 minutes.
After washing in PBS and incubation with normal horse serum (Vector Laboratories, Peterborough, UK), sections/coverslips were incubated for 16 hours at 4°C in either a negative control monoclonal antibody (of the same IgG isotype and concentration as MMP-7 antibody), a monoclonal anti-human MMP-7 antibody which detects both latent and active forms (CN Biosciences, Nottingham, UK) at a dilution of 1:100, a monoclonal anti-human HIF-1α (NB100–123; Novus Biologicals Inc., Littleton, CO) at a dilution of 1:100, a monoclonal anti-human CD68 antibody (KP-1, Dako Ltd, UK) at 1:100, or a monoclonal anti-human GLUT-1 antibody (Chemicon, Harrow, UK) at a dilution of 1:100. For the HIF-1α immunostaining, the Catalyzed Amplification System (CAS; Dako Ltd, UK) was used as the detection system. This is based on the streptavidin-biotin horseradish peroxidase-complex formation. For all other antibodies a standard three-stage immunoperoxidase technique was used (Vector Laboratories Elite ABC Kit) together with either the red chromagen, AEC, or the brown chromagen, DAB. Slides/coverslips were then dehydrated, cleared, and mounted using aquamount (BDH, Poole, UK).
Results
cDNA Array Analysis of the Effect of Hypoxia on Macrophage Gene Expression
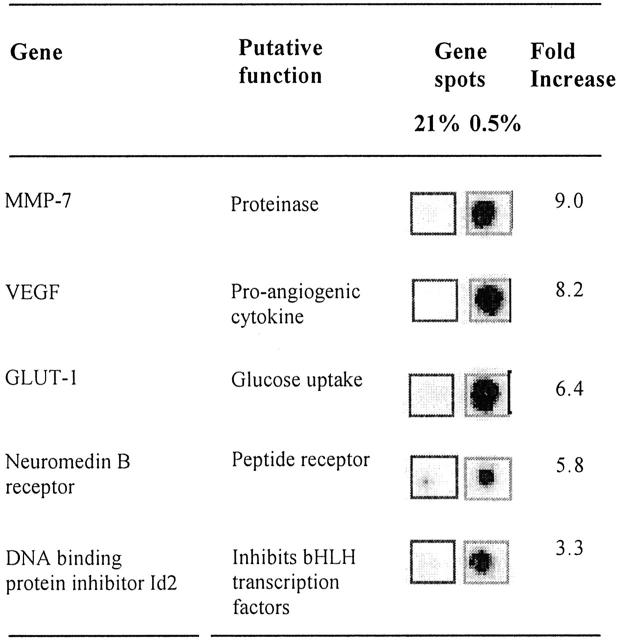
Typical array images generated following hybridization with probes derived from normoxic (21% O2) and hypoxic (0.5% O2) macrophages are shown in Figure 1 ▶ . The fold inductions (hypoxic divided by normoxic level) for the five most highly up-regulated mRNA transcripts are given in Figure 2 ▶ . These mRNAs were confirmed as hypoxia-inducible using a second pair of arrays hybridized to probes derived from a different batch of MDMs (data not shown). Two of the up-regulated genes, (GLUT-1 and VEGF) have been previously reported to be hypoxia-responsive in a range of cell types, including macrophages 10,12,13,25-27 thus confirming the efficacy of the hypoxic induction and RNA preparation methods used. Three other novel, hypoxia-induced mRNA transcripts were identified: MMP-7 (matrilysin), neuromedin B receptor (NMBR), and DNA-binding protein inhibitor Id2. In addition, the mRNA levels for a number of other genes were found to show reproducible down-regulation in hypoxia: RAB7 (a member of the RAS oncogene family; 3.8-fold inhibition), matrix metalloproteinase 14 (3-fold), IL-1β precursor (2.8-fold), and cathepsin C (2.5-fold) (data not shown).
Figure 1.
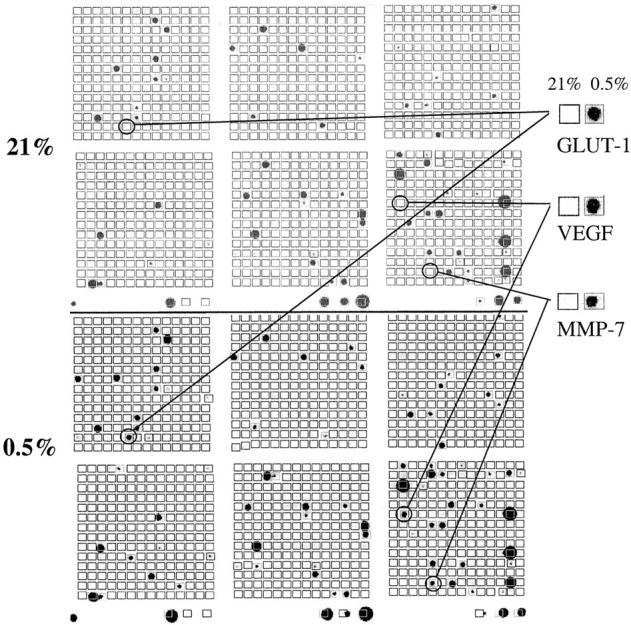
Genes expressed by human macrophages as detected by cDNA array analysis of mRNA levels. Two representative cDNA array autoradiographs are shown, after hybridization with radiolabeled probes from human MDMs exposed to normoxia (21% oxygen) or hypoxia (0.5% oxygen), respectively, for 16 hours. Enlarged versions of the array spots of the three most highly up-regulated genes are shown. Essentially similar results were different preparations of macrophage mRNA were used on separate arrays.
Figure 2.
Genes regulated by hypoxia in human macrophages as detected by cDNA array analysis of mRNA levels. Insets show the matched array spots of the five most highly up-regulated genes from a representative cDNA array autoradiograph after hybridization with radiolabeled cDNA probes derived from human MDMs exposed to normoxia (21% oxygen) or hypoxia (0.5% oxygen) for 16 hours. Fold induction values were calculated as the hypoxic values divided by normoxic values for the same gene.
Around 120 of the 1185 genes represented on the arrays used in this study were expressed at detectable levels by MDMs in culture. In contrast to the relatively small number of these identified here as being induced or repressed by hypoxia, the majority were unaffected. A small proportion of these genes were expressed at very high levels, in both normoxia and hypoxia. The 20 most abundant mRNAs are shown in Table 2 ▶ . Interestingly, three of these code for thymosins, a group of small peptides known to perform a wide range of important functions. 28,29
Table 2.
The Most Highly Expressed Genes in Both Normoxic and Hypoxic MDM in Vitro
| Gene | Relative mRNA level |
|---|---|
| Thymosin-β-10 | 27 |
| Thymosin-β-4 | 21.9 |
| HLA class I | 21.8 |
| Alpha-1-acid glycoprotein 1 precursor | 19.4 |
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | 15.3 |
| Ubiquitin | 15.2 |
| Tax responsive enhancer binding protein (Taxreb 107, 60S RP) | 10 |
| ICAM-1 | 9.1 |
| CD18, Cell surface/LFA1 | 8.7 |
| 27KDa heat shock protein | 8.1 |
| Nuclease sensitive element DNA-binding protein | 8.1 |
| Apolipoprotein E precursor | 6.7 |
| Migration inhibitory factor related protein 8 | 6.6 |
| Matrix metalloproteinase 9 (MMP-9) | 6.4 |
| Transforming protein RHOA H12 | 6.3 |
| 40S ribosomal protein | 5.5 |
| Adenylate cyclase-stimulating G alpha protein (GNAS) | 5.3 |
| Prothymosin (PTMA) | 5.2 |
| Migration inhibitory factor related protein 14 | 5.1 |
| Cathepsin D precursor | 4.8 |
Levels are expressed as signal relative to cytoplasmic β-actin mRNA (arbitrarily defined as 1 in this table) as determined by image analysis of cDNA array gene spots.
Confirmation of Key Array Results Using Real-Time RT-PCR
Up-regulation of the three mRNAs shown by the arrays to be most highly induced by hypoxia in primary human macrophages, GLUT-1, MMP-7, and VEGF, was confirmed using real time RT-PCR (Figure 3) ▶ . All three mRNAs were up-regulated in three independent experiments, carried out on different days using macrophages obtained from three different donors. Average fold inductions were 16.4-fold for GLUT-1, 6.4-fold for VEGF, and 5.6-fold for MMP-7. Relative quantification of four housekeeping genes was used to confirm that equal amounts of normoxic and hypoxic RNA had been used for reverse transcription in each experiment. In contrast to the three genes of interest, mRNA levels of the four housekeeping genes were found to be either unaffected or slightly reduced by hypoxia (Figure 3B) ▶ . The high degree of accordance between the four housekeeping genes in normoxia and hypoxia shows that the RNA samples used for the RT-PCRs were accurately quantified and that the hypoxia-induced differences for MMP-7, GLUT-1, and VEGF mRNAs observed in the array and real time RT-PCR experiments were genuine.
Figure 3.
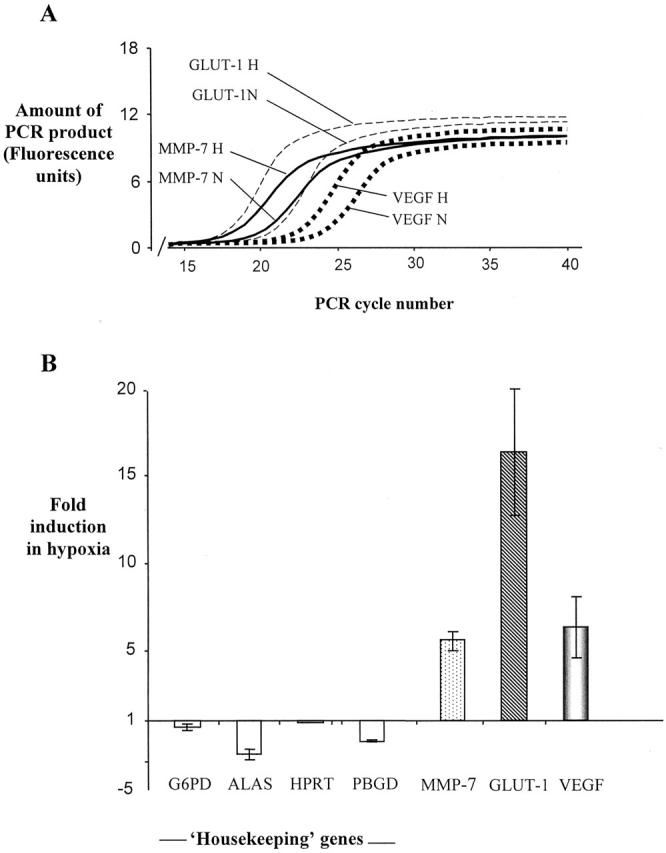
Effect of hypoxia on mRNA level determined using real-time RT-PCR. A: A representative experiment illustrating PCR curves for amplifications of GLUT-1, MMP-7, and VEGF, which are used by the Lightcycler software to calculate fold induction data, based on the PCR cycle at which amplification becomes exponential for each individual test gene. B: Fold induction in hypoxia relative to normoxia for each test gene and four housekeeping genes. Negative values indicate a level of mRNA under hypoxia lower than that under normoxia. Data shown is the mean of three independent experiments carried out on three different RNA preparations. Bars represent standard errors of the mean. MMP-7, matrix metalloproteinase 7; GLUT-1, glucose transporter 1; VEGF, vascular endothelial growth factor; β2 mol/L, β2 microglobulin; G6PDH, glucose-6-phosphate dehydrogenase; ALAS, 5-aminolevulinate synthase gene; HPRT, hypoxanthine phosphoribosyltransferase; PBGD, porphobilinogen deaminase.
Induction Studies
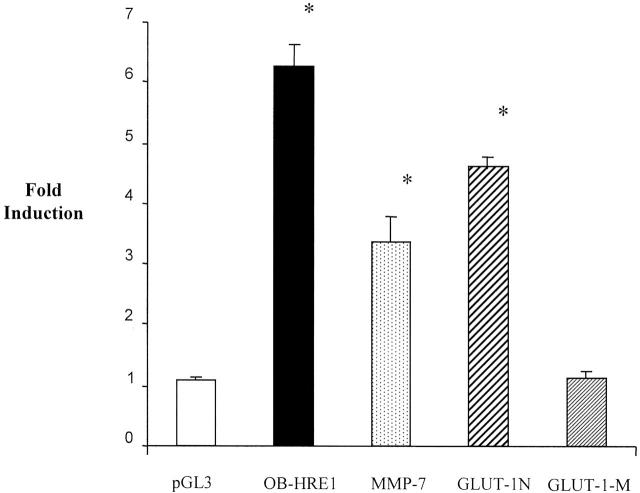
Luciferase reporter constructs driven by either the HRE from the rat GLUT-1 promoter (used because the human GLUT-1 promoter has not been characterized) or the human MMP-7 promoter were inducible by hypoxia in the RAW 264.7 macrophage cell line (Figure 4) ▶ . As expected, the construct driven by the GLUT-1 HRE bearing a point-mutated, non-functional HIF-1 binding site 21 was not induced by hypoxia. This indicates that HIF-1 is likely to be responsible for induction of the GLUT-1 promoter in these cells. The data indicate that the MMP-7 promoter is up-regulated by hypoxia at the transcriptional level, at least in this cell line. The hypoxia-induced increases observed in MMP-7 mRNA observed in primary macrophages by cDNA array and RT-PCR analyses are therefore likely to be due to transcriptional up-regulation, at least in part, although other factors such as enhanced mRNA stability under hypoxia could also play a part. The MMP-7 promoter construct, although inducible by hypoxia, was expressed at generally lower levels compared with the other constructs used (average LUC relative light unit values under hypoxia were: pGL3 20,057; OB-HRE1 614,350; GLUT-1 N 761,215; GLUT-1 mol/L 96,184; MMP-7 promoter 146). The markedly lower activity of the MMP-7 construct is likely to be due to the fact that it is the only construct which consists of a native promoter, unlike the OB-HRE1 and GLUT-1 constructs which consist of HRE enhancer sequences cloned into the strong SV40 viral promoter found in the pGL3 promoter vector.
Figure 4.
Effect of hypoxia on luciferase reporter constructs after transfection into the murine macrophage-like cell line, RAW 264.7. Cells were exposed to 20.9% (normoxia) or 0.5% O2 (hypoxia) for 16 hours. pGL3, the luciferase reporter construct pGL3 promoter containing the minimal, enhancerless SV40 promoter; OB-HRE1, 22 a luciferase reporter construct controlled by a trimer of the HRE monomer described in Table 1 ▶ (cloned into the multiple cloning site of pGL3 promoter); MMP-7 promoter, 23 a 2.3-kb sequence containing the promoter of the human MMP-7 gene, cloned into the multiple cloning site of pGL2 basic (Promega), a promoterless luciferase reporter construct. GLUT-1-N 21 contains a 184-bp sequence from the rat GLUT-1 promoter cloned into the multiple cloning site of pGL3 promoter (which contains the viral SV40 promoter), and GLUT-1 mol/L is identical apart from a 4-bp mutation (CGTG to ACAT) resulting in ablation of the HRE (HIF binding site). Fold induction values refer to luciferase values obtained under hypoxia divided by those seen under normoxia. Fold inductions (hypoxic luciferase values divided by normoxic values) from four independent transfections are shown. Means ± SEM. *, P < 0.05 compared to pGL3 promoter (Mann Whitney U-test).
The MMP-7 promoter construct was also tested in the human macrophage cell line, U937, but the low transfection efficiency achieved with this cell line and the low level of expression from the MMP-7 promoter construct meant that there was insufficient luciferase production (ie, above background), in normoxia or hypoxia, to assess whether hypoxic induction of transcription of the MMP-7 construct was taking place (data not shown).
An examination of the sequence of the human MMP-7 promoter shows the presence of a 27-bp putative HRE-like sequence (Table 1) ▶ . To investigate the possible ability of this 27-bp sequence to act as a hypoxia-inducible enhancer, a trimerized version of it was inserted into a luciferase reporter construct (pGL3 promoter, which contains a minimal enhancerless promoter). However, no hypoxia inducibility was conferred on the luciferase reporter gene by this trimer in either the RAW 264.7 (Figure 4) ▶ or U937 (data not shown) cell lines.
Immunolocalization of GLUT-1 and MMP-7 Protein
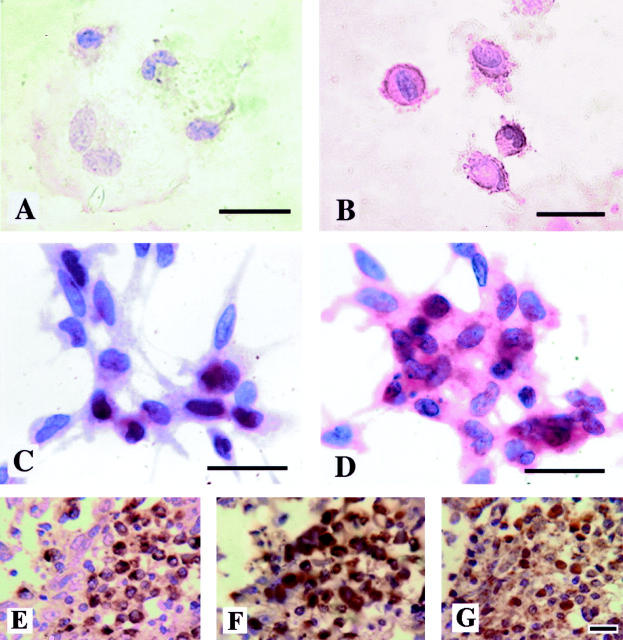
MDMs expressed immunoreactive MMP-7 at low levels in normoxia, but increased their expression of both GLUT-1 and MMP-7 markedly following exposure to 0.5% O2 for 16 hours (Figure 5 A–D ▶ ). MMP-7 was immunolocalized in the cytoplasm, and GLUT-1 on the surface of hypoxic MDM. Immunostaining of sections of malignant human breast tumors showed the presence of clusters of CD68-positive macrophages expressing MMP-7 in hypoxic areas (ie, where the macrophages and tumor cells express HIF-1α; Figure 5, E–G ▶ ). Tumor cells as well as macrophages expressed detectable levels of MMP-7 protein. The size and number of these focal areas of positive TAMs was variable between individual tumors, as was the presence of immunoreactivity to MMP-7 protein in tumor cells. No immunostaining was evident in cell preparations or on tissue sections when the MMP-7 antibody was replaced by an IgG isotype-matched control antibody.
Figure 5.
Immunodetection of GLUT-1 and MMP-7 in human macrophages in vitro and in vivo. These proteins were immunolocalized in/on human MDM grown on coverslips (GLUT-1, A and B; MMP-7, C and D) following exposure to normoxia (20.9% oxygen, A and C) or hypoxia (0.5% oxygen, B and D) for 16 hours in vitro. Although low-level expression of immunoreactive GLUT-1 (brown) and MMP-7 (red) can just be seen in the cytoplasm of these cells in normoxia, this was markedly increased following exposure to hypoxia. Immunoreactive CD68 (E), MMP-7 (F), and HIF-1α (G) proteins were also detectable in macrophages in focal areas of malignant human breast carcinomas (brown color reaction). CD68 and MMP-7 staining was cytoplasmic, whereas HIF-1α staining was seen in both the cytoplasm and nuclei of cells. Sections exposed to a negative control antibody showed no staining reaction (not shown). Bar, 20 μm.
Discussion
This study has examined the effects of hypoxia on the mRNA levels of 1185 genes in human macrophages. Of these, approximately 120 were expressed at detectable levels in normoxic human monocyte-derived macrophages in vitro, with the majority of these being unaffected by exposure to hypoxia. A number of mRNA species were found at high levels in macrophages in normoxia, and to be maintained in hypoxia. Three members of the thymosin family were particularly prominent. These peptides are known to be expressed by macrophages 30 and to promote cell migration and angiogenesis, activation of metalloproteinases and immuno-modulation. 28,29 As our data show that these continue to be expressed at extremely high levels in hypoxia, it is possible that thymosin release could contribute to the pro-angiogenic activities of macrophages in tumors, arthritic joints, and healing wounds.
However, a small panel of mRNA transcripts was shown to be consistently up-regulated by hypoxia and thus may play an important role in determining the activities of macrophages in ischemic/hypoxic diseased tissues. Levels of mRNA for two genes previously known to be regulated by hypoxia, VEGF and GLUT-1, were seen to be elevated in hypoxic macrophages. Induction of VEGF was predicted given the report by Harmey and her co-workers 13 showing hypoxic stimulation of VEGF release by IFN-γ-primed primary human macrophages in vitro, and our demonstration of VEGF expression by macrophages in hypoxic sites in breast carcinomas in vivo. 14 Hypoxic induction of VEGF release by macrophages may be an important part of the pro-angiogenic activities of macrophages in human tumors. 5,16
VEGF was the only one of the seven mRNA species previously found to be markedly up-regulated by hypoxia in squamous cell carcinoma cell lines in vitro, 31 which was also found by us to be up-regulated in hypoxic macrophages. Since most of these seven previously identified genes were present on the arrays used by us in the present study, the lack of accordance suggests that the effect of hypoxia on gene expression may be cell-type specific, and that there is therefore no single “hypoxic phenotype.”
GLUT-1 was also seen to be up-regulated by hypoxia at both the mRNA and protein level. This protein is a glucose transporter expressed ubiquitously in normal tissues but at higher levels in a number of tumors. 32 Up-regulation of GLUT-1 by hypoxia is likely to promote macrophage survival in ischemic tissues by facilitating glucose uptake for glycolytic production of energy during periods of oxygen deprivation. The expression and activity of GLUT-1 have been shown to be coupled to energy status in other cell types, such that inhibition of oxidative phosphorylation following exposure to hypoxia leads to stimulation of GLUT-1 levels and thus glucose transport. 33 Ablation of hypoxic induction using a GLUT-1 promoter containing a mutated HRE in our reporter gene studies indicates that the presence of a fully functional HIF binding site, and thus presumably HIF-1 (or another HIF), is required for hypoxic induction of this gene. This accords well with previous studies showing the effects of hypoxia on the GLUT-1 promoter to be HIF-1-dependent in murine macrophages 10 and in various tumor cell lines. 25,26 Our real-time RT-PCR data, showing a 16.4-fold induction in GLUT-1 mRNA by hypoxia, closely matches recent data generated using the same technique showing a 13.5-fold increase in GLUT-1 mRNA in hypoxic murine peritoneal macrophages. 10
We also show, for the first time, that hypoxia stimulates an increase in mRNA coding for MMP-7 (matrilysin) as quantified by cDNA array and real-time RT-PCR analyses. As with GLUT-1, hypoxia not only induced MMP-7 mRNA but also MMP-7 protein in primary macrophages grown on coverslips. To our knowledge this is only the second report of an MMP being regulated by hypoxia. The other was in a recent paper showing MMP-2 up-regulation by hypoxia in mouse embryonic stem cells, a phenomenon shown to involve activation of the MMP-2 promoter by HIF-1. 34 A gene spot for MMP-2 was present on the cDNA arrays used in the present study, but the hybridization signal was below the limit of detection in both normoxia and hypoxia, so no conclusion can be drawn as to whether MMP-2 is also regulated by hypoxia in human macrophages.
MMP-7, the smallest MMP, lacks the C-terminal domain restricting the substrate specificity of other enzymes of this type, 35 and so is able to efficiently digest many components of the basement membrane and extracellular matrix including proteoglycans, elastin, laminin, fibronectin, versican, and entactin. 36-37 Furthermore, MMP-7 is important for its role in the proteolytic processing and activation of other molecules such as tumor necrosis factor, 38 urokinase plasminogen activator, 39 α-defensins, 40 and MMPs 1, 2, and 9. 41 To date, only macrophages, 42-44 glandular epithelial cells, and tumor cells 35 have been shown to be capable of expressing MMP-7. MMP-7 is abundantly expressed in malignant colon, stomach, prostate, lung, and brain tumors, 45-48 and contributes to proliferation, angiogenesis, invasive tumor behavior, and metastasis. 47-51 MMP-7 mRNA and protein have been localized to both the stromal and malignant components of human tumors, 45,46 and MMP-7 expression has recently been linked to resistance to doxorubicin chemotherapy, owing to the ability of MMP-7 to cleave Fas ligand from the surface of tumor cells, blocking the action of the drug. 52
In the present study, tumor-associated macrophages were shown to express high levels of MMP-7 protein in hypoxic areas of human breast carcinomas, suggesting that the hypoxic induction of this protease in macrophages may contribute to the resistance of hypoxic tumor sites to treatment with doxorubicin. 53 Collectively, these studies indicate that increased expression of MMP-7 in tumors could have important implications for tumor growth, spread, and response to some forms of therapy. In this context it is interesting to note that highly necrotic (hypoxic) breast carcinomas often show high levels of macrophage infiltration, and that these two features are associated with poor prognosis in this disease. 16,54
Induction of MMP-7 mRNA/protein expression by hypoxia may explain, in part, the high levels of this enzyme expressed by macrophages in human atherosclerotic lesions, 45 as these sites are known to be ischemic and thus low in oxygen. 4 The fact that MMP-7 levels are high at sites of plaque rupture suggests that it may be an important feature in the progression of certain cardiovascular diseases. 46
The hypoxic induction of a reporter construct, containing the human MMP-7 promoter linked to the luciferase gene, in the RAW 264.7 macrophage cell line suggests that hypoxic induction of MMP-7 mRNA detected by cDNA array and RT-PCR analyses of primary macrophages may be due, at least in part, to transcriptional up-regulation. However, this finding must be treated with caution, as it is based on results from just one cell line, and other mechanisms such as increases in mRNA stability under hypoxia could also play a role. Our attempts to examine the hypoxic inducibility of the MMP-7 promoter in a second macrophage cell line, U937, proved inconclusive due to the low general level of luciferase gene expression produced by this construct in these cells (data not shown). This could be due to the fact that U937 cells (unlike primary macrophages and RAW 264.7 cells, according to our studies) failed to express detectable levels of MMP-7 protein unless stimulated to differentiate in vitro using the phorbol ester, PMA. 55 This suggests that the differentiation and/or activation status of a macrophage cell line may determine the activity of the MMP-7 promoter.
Given the recent demonstration of MMP-2 gene induction by HIF-1 in hypoxic stem cells, 34 we were interested to know whether HIF-1 also played a part in the hypoxic induction of the MMP-7 promoter in RAW 264.7 cells. We noted that the human MMP-7 promoter contains a sequence closely resembling a HRE at position −617 to −590 (Table 1) ▶ , containing a consensus HIF binding site (HBS; 5′-ACGTG’3) and a cis-acting sequence described as the “HIF ancillary site” (HAS). 24 The latter has been found to consist of the core sequences 5′-CACA-3′ or 5′-CACG-3′, and is thought to bind to constitutively expressed transcription factors and/or form an inverted repeat structure by base pairing with the HBS, and to play an important role in the HIF transactivation of such hypoxia-inducible genes as VEGF. 24 However, a trimer of the HRE-like sequence from the MMP-7 promoter was found to be incapable of conferring hypoxic inducibility by acting as an enhancer on the pGL3 promoter reporter construct, which contains a minimal enhancerless promoter, in RAW cells. This indicates that the putative HRE in the MMP-7 promoter is unlikely to regulate the hypoxic inducibility of this gene, although it is possible that cis-acting elements required for this HRE to function were absent from the trimer construct used and are essential for the hypoxic induction of the putative MMP-7 HRE. The apparent lack of functionality of the putative HRE could also be due to the fact that the sequence separating the putative HBS and HAS sites within it is longer (13 bases) than in other well-characterized HREs (usually 8 or 9 bases), 24
Other possibilities are that the hypoxic inducibility of MMP-7 transcription is mediated by another HIF binding site within the promoter, of which there are several, although none have HAS-like sequences nearby. Alternatively, other elements in the MMP-7 promoter could be responsible for the observed hypoxic induction of MMP-7 transcription. This promoter contains 12 potential binding sites for the transcription factor CCAAT/enhancer-binding protein β (C/EBPβ or NFIL-6). The insertion of short sequences containing C/EBPβ sites confers hypoxic inducibility on certain promoters in both endothelial cells in vitro and in a transgenic mouse model. 56-58 An alternative candidate for mediating transcriptional up-regulation of MMP-7 in response to hypoxia is Ets-1. The MMP-7 promoter contains two binding sites for Ets-1, and is known to be up-regulated by over-expression of this transcription factor. 59 Furthermore, the Ets-1 promoter contains a HIF binding site and has been shown to be up-regulated by HIF-1 in a human bladder cancer cell line subjected to hypoxia, 60 suggesting that hypoxia could act on the MMP-7 promoter via HIF-1-mediated up-regulation of Ets-1.
The last two hypoxically up-regulated mRNAs identified by cDNA array analysis of MDMs were those coding for Id2 and the receptor for neuromedin B. Id2 is a helix-loop-helix (HLH) protein 61 which is involved in the proliferation and differentiation of a number of different cell types. 62 Id2 regulates the activity of basic HLH (bHLH) transcription factors by heterodimerizing with them via its HLH domain. Because Id2 lacks the basic domain required for DNA binding, the bound bHLH transcription factor is inactivated. Up-regulation of Id2 by hypoxia therefore represents a potential novel mechanism of gene regulation in hypoxic macrophages, and possibly other cell types, to facilitate their activities and survival in hypoxia. Neuromedin B receptor (NMBR), a member of the bombesin receptor family, is widely distributed in cells of the central nervous system and alimentary tract. 63 NMBR is a high affinity G-protein-coupled receptor which binds Neuromedin B, a small amidated peptide which mediates a wide range of effects, including activation of macrophages. 64 The effect of up-regulation of this receptor on hypoxic macrophages would presumably be to render macrophages more susceptible to activation by neuromedin (or related molecules) under conditions of hypoxia. The functional significance of this remains unclear.
In summary, this study shows that mRNA levels for VEGF, GLUT-1, MMP-7, Id2, and NMBR are increased in primary human monocyte-derived macrophages during exposure to hypoxia, and that the increases in GLUT-1 and MMP-7 mRNAs are likely to be due, at least in part, to transcriptional up-regulation by hypoxia. The products of these up-regulated genes may contribute to the activities of macrophages in many types of ischemic tissue. Of particular interest, given the important role of the protein in many pathological processes, is the finding that MMP-7 is up-regulated by hypoxia. The data we present here also have implications for the design of hypoxia-responsive promoters for use in transcriptional targeting of macrophage-delivered gene therapies. 17 It is clear that the promoters of VEGF, MMP-7, and particularly GLUT-1, are highly hypoxia-inducible in macrophages, and elements from these promoters may prove useful in the future development of hypoxia-inducible therapeutic constructs optimized for use in macrophages.
Acknowledgments
We thank Dr. A. Maity, for the gift of constructs GLUT-1 N and GLUT-1 mol/L, and Dr. H. Crawford for the gift of the MMP-7 promoter construct.
Footnotes
Address reprint requests to Professor Claire Lewis, Head, Tumor Targeting Group, Section of Oncology and Pathology, Division of Genomic Medicine, University of Sheffield Medical School, Sheffield S10 2RX, United Kingdom. E-mail: claire.lewis@sheffield.ac.uk.
Supported by a Yorkshire Cancer Research, United Kingdom, grant.
Dr. B. Burke’s current address is the Department of Microbiology and Immunology, Medical Sciences Building, University of Leicester, LE1 9HN, United Kingdom.
References
- 1.Vaupel P, Kallinowski F, Okunieff P: Blood flow, oxygen and nutrient supply, and metabolic environment of human tumors: a review. Cancer Res 1989, 49:6449-6465 [PubMed] [Google Scholar]
- 2.Kivisaari J: Oxygen and carbon dioxide tensions in healing tissue. Acta Chir Scand 1975, 141:693-696 [PubMed] [Google Scholar]
- 3.Stevens CR, Williams RB, Farrell AJ, Blake DR: Hypoxia and inflammatory synovitis: observations and speculation. Ann Rheum Dis 1991, 50:124-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjornheden T, Levin M, Evaldsson M, Wiklund O: Evidence of hypoxic areas within the arterial wall in vivo. Arterioscler Thromb Vasc Biol 1999, 19:870-876 [DOI] [PubMed] [Google Scholar]
- 5.Lewis JS, Lee J, Underwood JCE, Harris AL, Lewis CE: Macrophage responses to hypoxia: implications for disease mechanisms. J Leukoc Biol 1999, 66:889-900 [DOI] [PubMed] [Google Scholar]
- 6.Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL: The expression and distribution of the hypoxia-inducible factors HIF-1α and HIF-2α in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol 2000, 157:411-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke B, Tang N, Corke KP, Tazzyman D, Ameri K, Wells M, Lewis CE: Expression of HIF-1α by human macrophages: implications for the use of macrophages in hypoxia-regulated cancer gene therapy. J Pathol 2002, 196:204-212 [DOI] [PubMed] [Google Scholar]
- 8.Hollander AP, Corke KP, Freemont AJ, Lewis CE: Expression of HIF-1α by macrophages in the rheumatoid synovium: implications for targeting hypoxia-regulated gene therapy to the inflamed joint. Arthritis Rheum 2001, 44:1540-1544 [DOI] [PubMed] [Google Scholar]
- 9.Crowther M, Brown NJ, Bishop ET, Lewis CE: Macrophage regulation of angiogenesis in wounds and malignant tumors: a response to common microenvironmental stress factors. J Leukoc Biol 2001, 70:478-490 [PubMed] [Google Scholar]
- 10.Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber H-P, Ferrara N, Johnson RS: HIF-1 is essential for myeloid cell-mediated inflammation. Cell 2003, 112:645-657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wenger RH: Mammalian oxygen sensing, signaling, and gene regulation. J Exp Biol 2000, 203:1253-1263 [DOI] [PubMed] [Google Scholar]
- 12.Semenza GL: HIF-1: using two hands to flip the angiogenic switch. Cancer Metastasis Rev 2000, 19:59-65 [DOI] [PubMed] [Google Scholar]
- 13.Harmey JH, Dimitriadis E, Kay E, Redmond HP, Bouchier-Hayes D: Regulation of macrophage production of VEGF by hypoxia and TGF β-1. Ann Surg Oncol 1998, 5:271-278 [DOI] [PubMed] [Google Scholar]
- 14.Lewis JS, Landers RJ, Underwood JCE, Harris AL, Lewis CE: Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol 2000, 192:150-158 [DOI] [PubMed] [Google Scholar]
- 15.Li J, Post M, Volk R, Gao Y, Li M, Metais C, Sato K, Tsai J, Aird W, Rosenberg RD, Hampton TG, Sellke F, Carmeliet P, Simons M: PR39, a peptide regulator of angiogenesis. Nat Med 2000, 6:49-55 [DOI] [PubMed] [Google Scholar]
- 16.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL: Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 1996, 56:4625-4629 [PubMed] [Google Scholar]
- 17.Griffiths L, Binley K, Iqball S, Kan O, Maxwell P, Ratcliffe P, Lewis CE, Harris AL, Kingsman S, Naylor SM: The macrophage: a novel system to deliver gene therapy to pathological hypoxia. Gene Ther 2000, 7:255-262 [DOI] [PubMed] [Google Scholar]
- 18.Carta L, Pastorino S, Melillo G, Bosco MC, Massazza S, Varesio L: Engineering of macrophages to produce interferon-γ in response to hypoxia. J Immunol 2001, 166:5374-5380 [DOI] [PubMed] [Google Scholar]
- 19.Andreesen R, Hennemann B, Krause SW: Adoptive immunotherapy of cancer using monocyte-derived macrophages: rationale, current status, and perspectives. J Leukoc Biol 1998, 64:419-426 [DOI] [PubMed] [Google Scholar]
- 20.Grimshaw MJ, Balkwill FR: Inhibition of monocyte and macrophage chemotaxis by hypoxia and inflammation: a potential mechanism. Eur J Immunol 2001, 31:480-489 [DOI] [PubMed] [Google Scholar]
- 21.Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A: Regulation of GLUT-1 mRNA by hypoxia inducible factor-1: interaction between H-ras and hypoxia. J Biol Chem 2001, 276:9519-9525 [DOI] [PubMed] [Google Scholar]
- 22.Boast K, Binley K, Iqball S, Price T, Spearman H, Kingsman S, Kingsman A, Naylor S: Characterization of physiologically regulated vectors for the treatment of ischemic disease. Hum Gene Ther 1999, 10:2197-2208 [DOI] [PubMed] [Google Scholar]
- 23.Crawford HC, Fingleton B, Gutsavson MD, Kurpios N, Wagenaar RA, Hassell JA, Matrisian LM: The PEA3 subfamily of ets transcription factors synergizes with β-catenin-LEF1 to activate matrilysin transcription in intestinal tumours. Mol Cell Biol 2001, 21:1370-1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura H, Weisz A, Ogura T, Hitomi Y, Kurashima Y, Hashimoto K, D’Aquisto F, Makuuchi M, Esumi H: Identification of hypoxia-inducible factor 1 ancillary sequence and its function in vascular endothelial growth factor gene induction by hypoxia and nitric oxide. J Biol Chem 2000, 276:2292-2298 [DOI] [PubMed] [Google Scholar]
- 25.Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A: Regulation of GLUT-1 mRNA by hypoxia inducible factor-1: interaction between H-ras and hypoxia. J Biol Chem 2001, 276:9519-9525 [DOI] [PubMed] [Google Scholar]
- 26.Williams KJ, Telfer BA, Airley RE, Peters HP, Sheridan MR, van der Kogel AJ, Harris AL, Stratford IJ: A protective role for HIF-1 in response to redox manipulation and glucose deprivation: implications for tumorigenesis. Oncogene 2002, 2:282-290 [DOI] [PubMed] [Google Scholar]
- 27.Zhang JZ, Behrooz A, Ismail-Beigi F: Regulation of glucose transport by hypoxia. Am J Kidney Dis 1999, 34:189-202 [DOI] [PubMed] [Google Scholar]
- 28.Malinda KM, Goldstein AL, Kleinman HK: Thymosin β 4 stimulates directional migration of human umbilical vein endothelial cells. EMBO J 1997, 11:474-481 [DOI] [PubMed] [Google Scholar]
- 29.Huff T, Muller CS, Otto AM, Netzker R, Hannappel E: β-Thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol 2001, 33:205-220 [DOI] [PubMed] [Google Scholar]
- 30.Gondo H, Kudo J, White JW, Barr C, Selvanayagam P, Saunders GF: Differential expression of the human thymosin-β 4 gene in lymphocytes, macrophages, and granulocytes. J Immunol 1987, 139:3840-3848 [PubMed] [Google Scholar]
- 31.Koong AC, Denko NC, Hudson KM, Schindler C, Swiersz L, Koch C, Evans S, Ibrahim H, Le QT, Terris DJ, Giaccia AJ: Candidate genes for the hypoxic tumor phenotype. Cancer Res 2000, 60:883-887 [PubMed] [Google Scholar]
- 32.Smith TA: FDG uptake, tumour characteristics, and response to therapy: a review. Nucl Med Commun 1998, 19:97-105 [DOI] [PubMed] [Google Scholar]
- 33.Ouiddir A, Planes C, Fernandes I, VanHesse A, Clerici C: Hypoxia up-regulates activity and expression of the glucose transporter GLUT-1 in alveolar epithelial cells. Am J Respir Cell Mol Biol 1999, 21:710-718 [DOI] [PubMed] [Google Scholar]
- 34.Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghavi P, Semenza GL: Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res 2003, 63:1138-1143 [PubMed] [Google Scholar]
- 35.Massova I, Kotra LP, Fridman R, Mobashery S: Matrix metalloproteinases: structures, evolution, and diversification. EMBO J 1998, 12:1075-1095 [PubMed] [Google Scholar]
- 36.Murphy G, Cockett MI, Ward RV, Docherty AJ: Matrix metalloproteinase degradation of elastin, type IV collagen, and proteoglycan: a quantitative comparison of the activities of 95 kDa and 72 kDa gelatinases, stromelysins-1 and -2, and punctuated metalloproteinase (PUMP). Biochem J 1991, 277:277-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sang OX: Complex role of matrix metalloproteinases in angiogenesis. Cell Res 1998, 8:171-178 [DOI] [PubMed] [Google Scholar]
- 38.Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements JM, Crimmin M, Davidson AH, Drummond AH, Galloway WA, Gilbert R: Matrix metalloproteinases and processing of pro-TNF-α. J Leukoc Biol 1995, 57:774-777 [DOI] [PubMed] [Google Scholar]
- 39.Wilson CL, Matrisian LM: Matrilysin: an epithelial matrix metalloproteinase with potentially novel functions. Int J Biochem 1996, 28:123-136 [DOI] [PubMed] [Google Scholar]
- 40.Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, Lopez-Boado YS, Stratman JL, Hultgren SJ, Matrisian LM, Parks WC: Regulation of intestinal α-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 1999, 286:113-117 [DOI] [PubMed] [Google Scholar]
- 41.Rudek MA, Venitz J, Figg WD: Matrix metalloproteinase inhibitors: do they have a place in anticancer therapy? Pharmacotherapy 2002, 22:705-720 [DOI] [PubMed] [Google Scholar]
- 42.Busiek DF, Ross FP, McDonnell S, Murphy G, Matrisian LM, Welgus HG: The matrix metalloprotease, matrilysin (PUMP), is expressed in developing human mononuclear phagocytes. J Biol Chem 1992, 267:9087-9092 [PubMed] [Google Scholar]
- 43.Halpert I, Sires UI, Roby JD, Potter-Perigo S, Wight TN, Shapiro SD, Welgus HG, Wickline SA, Parks WC: Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc Natl Acad Sci USA 1996, 93:9748-9753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah PK: Role of inflammation and metalloproteinases in plaque disruption and thrombosis. Vasc Med 1998, 3:199-206 [DOI] [PubMed] [Google Scholar]
- 45.Yamashita K, Azumano I, Mai M, Okada Y: Expression and tissue localization of matrix metalloproteinase 7 (matrilysin) in human gastric carcinomas: implications for vessel invasion and metastasis. Int J Cancer 1998, 79:187-206 [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto H, Itoh F, Iku S, Adachi Y, Fukushima H, Sasaki S, Mukaiya M, Hirata K, Imai K: Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human pancreatic adenocarcinomas: clinicopathologic and prognostic significance of matrilysin expression. J Clin Oncol 2001, 19:1118-1127 [DOI] [PubMed] [Google Scholar]
- 47.Shapiro SD: Diverse roles of macrophage matrix metalloproteinases in tissue destruction and tumor growth. Thromb Haemost 1999, 82:846-849 [PubMed] [Google Scholar]
- 48.Fingleton BM, Heppner Goss KJ, Crawford HC, Matrisian LM: Matrilysin in early stage intestinal tumorigenesis. APMIS 1999, 107:102-110 [DOI] [PubMed] [Google Scholar]
- 49.Nishizuka I, Ichikawa Y, Ishikawa T, Kamiyama M, Hasegawa S, Momiyama N, Miyazaki K, Shimada H: Matrilysin stimulates DNA synthesis of cultured vascular endothelial cells and induces angiogenesis in vivo. Cancer Lett 2001, 173:175-182 [DOI] [PubMed] [Google Scholar]
- 50.Huo N, Ichikawa Y, Kamiyama M, Ishikawa T, Hamaguchi Y, Hasegawa S, Nagashima Y, Miyazaki K, Shimada H: MMP-7 (matrilysin) accelerated growth of human umbilical vein endothelial cells. Cancer Lett 2002, 177:95-100 [DOI] [PubMed] [Google Scholar]
- 51.Matrisian LM, Wright J, Newell K, Witty JP: Matrix-degrading metalloproteinases in tumor progression. Princess Takamatsu Symp 1994, 24:152-161 [PubMed] [Google Scholar]
- 52.Mitsiades N, Yu W, Poulaki V, Tsokos M, Stamenkovic I: Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res 2001, 61:577-581 [PubMed] [Google Scholar]
- 53.Sanna K, Rofstad EK: Hypoxia-induced resistance to doxorubicin and methotrexate in human melanoma cell lines in vitro. Int J Cancer 1994, 58:258-262 [DOI] [PubMed] [Google Scholar]
- 54.Leek RD, Landers RJ, Harris AL, Lewis CE: Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer 1999, 79:991-995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jormsjo S, Whatling C, Walter DH, Zeiher AM, Hamsten A, Eriksson P: Allele-specific regulation of matrix metalloproteinase-7 promoter activity is associated with coronary artery luminal dimensions among hypercholesterolemic patients. Arterioscler Thromb Vasc Biol 2001, 21:1834-1839 [DOI] [PubMed] [Google Scholar]
- 56.Yan SF, Tritto I, Pinsky D, Liao H, Huang J, Fuller G, Brett J, May L, Stern D: Induction of interleukin 6 (IL-6) by hypoxia in vascular cells: central role of the binding site for nuclear factor-IL-6. J Biol Chem 1995, 270:11463-11471 [DOI] [PubMed] [Google Scholar]
- 57.Roesler WJ: The role of C/EBP in nutrient and hormonal regulation of gene expression. Ann Rev Nutr 2001, 21:141-165 [DOI] [PubMed] [Google Scholar]
- 58.Yan SF, Zou YS, Mendelsohn M, Gao Y, Naka Y, Du Yan S, Pinsky D, Stern D: Nuclear factor interleukin 6 motifs mediate tissue-specific gene transcription in hypoxia. J Biol Chem 1997, 272:4287-4294 [DOI] [PubMed] [Google Scholar]
- 59.Ozaki I, Mizuta T, Zhao G, Yotsumoto H, Hara T, Kajihara S, Hisatomi A, Sakai T, Yamamoto K: Involvement of the Ets-1 gene in overexpression of matrilysin in human hepatocellular carcinoma. Cancer Res 2000, 60:6519-6525 [PubMed] [Google Scholar]
- 60.Oikawa M, Abe M, Kurosawa H, Hida W, Shirato K, Sato Y: Hypoxia induces transcription factor Ets-1 via the activity of hypoxia-inducible factor-1. Biochem Biophys Res Commun 2001, 289:39-43 [DOI] [PubMed] [Google Scholar]
- 61.Sun XH, Copeland NG, Jenkins NA, Baltimore D: Id proteins Id1 and Id2 selectively inhibit DNA binding by one class of helix-loop-helix proteins. Mol Cell Biol 1991, 11:5603-5611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andrews-Barquin PJ, Hernandez MC, Israel MA: Id2 genes in nervous system development. Histol Histopathol 2000, 15:603-618 [DOI] [PubMed] [Google Scholar]
- 63.Kroog GS, Jensen RT, Battey JF: Mammalian bombesin receptors. Med Res Rev 1995, 15:389-417 [DOI] [PubMed] [Google Scholar]
- 64.Ohki-Hamazaki H: Neuromedin B. Prog Neurobiol 2000, 62:297-312 [DOI] [PubMed] [Google Scholar]