Abstract
Having previously shown that previous immunity to one virus can influence the host response to a subsequent unrelated virus, we questioned whether the outcome to a given virus infection would be altered in similar or different ways by previous immunity to different viruses, and whether immunity to a given virus would have similar effects on all subsequent infections. In mouse models of respiratory viral infections, immunity to lymphocytic choriomeningitis virus (LCMV), murine cytomegalovirus (MCMV), or influenza A virus enhanced both Th1-type cytokine responses and viral clearance in the lung on vaccinia virus infection. A common pathological feature was the presence of chronic mononuclear infiltrates instead of the acute polymorphonuclear response seen in the infected nonimmune mice, but some pathologies such as enhanced bronchus-associated lymphoid tissue and bronchiolitis obliterans were unique for the immunizing virus, LCMV. Immunity to influenza virus influenced subsequent infections diversely, inhibiting vaccinia virus but enhancing LCMV and MCMV titers and completely altering cytokine profiles. Influenza virus immunity enhanced the mild mononuclear responses usually observed during acute infections with MCMV or LCMV in nonimmune mice, but unique features such as enhanced bronchiolization and mononuclear consolidation occurred during MCMV infection of influenza virus-immune mice. Heterologous immunity induced two patterns of disease outcome dependent on the specific virus infection sequence: improved, if the acute response switched from a neutrophilic to a lymphocytic response or worsened, if it switched from a mild to a severe lymphocytic response. Heterologous immunity thus occurs between many viruses, resulting in altered protective immunity and lung immunopathology, and this is influenced by the specific virus infection sequence.
Lung pathology associated with viral infections can range from mild pneumonitis to severe pneumonia, which can involve secondary invasion by bacteria. Certain types of viruses, such as hantaviruses, characteristically cause severe pneumonias, 1 whereas others, such as cytomegalovirus (CMV), will occasionally cause pneumonitis, most frequently in immunodeficient individuals. 2 Other viruses such as influenza viruses have variation between strains, differing markedly in their virulence and in their ability to cause a wide range of lung pathology from mild to severe. Pneumonia during an influenza virus infection is often accompanied by bacterial invasion and pronounced neutrophilic infiltrates, although the 1918 epidemic strain appeared to be lethal without bacterial invasion. 3
These distinct virus-induced pathologies in the lung can involve discrete sites of inflammation and predominance of different types of immune system cells. 4-13 Respiratory syncytial virus can cause a pneumonia involving strong eosinophilic infiltrates associated with Th2-like CD4 T-cell immune responses. 14,15 Complex lymph node-like clustering of lymphocytes, known as bronchus-associated lymphoid tissue (BALT), is seen during some human infections of the lung, particularly during chronic or recurrent pneumonias. 16 In cases of recurrent pneumonias BALT has been reported to develop into a low-grade B-cell lymphoma. 16 It is unclear which pathogens drive the induction of BALT or lymphocytic interstitial pneumonitis, another term used to define this pathology. 16 Unique pathologies such as bronchiolitis obliterans, a blockage of the airways with organized fibrous material and inflammatory cells, may develop during some viral and intracellular bacterial infections. 17 Interestingly, enhanced BALT has been associated with stable graft function after lung transplantation, whereas bronchiolitis obliterans is strongly associated with lung transplant rejection. 16,18
What is quite clear, however, is that not everyone who experiences a viral infection of the lung has comparable severity of infection or comparable pathology. These differences are thought to be related to such issues as the genetic and physiological status of the host and the dose of virus received, but recent work in animal models has indicated that the pathogenesis of viral infections in the lung may be related in part to the host’s experience with putatively unrelated pathogens. In our earlier work, we have shown that intranasal vaccinia virus (VV) infection of lymphocytic choriomeningitis virus (LCMV)-immune mice reactivates LCMV-specific memory CD8 T cells, which play a role in augmenting VV clearance, enhancing survival and inducing a very different immunopathology than in a naïve host. 19
In the present study, we questioned whether the pathogenic response to an infection with a given virus would be altered in similar or different ways by previous immunity to different viruses, and whether immunity to a given virus would have similar effects on subsequent infections with different viruses. We examined the viral titers, cytokine response, and pathology in mice inoculated intranasally with LCMV (a small RNA arenavirus), murine cytomegalovirus (MCMV; a large DNA herpesvirus), or influenza virus (a small RNA orthomyxovirus) and later challenged with VV (a large DNA poxvirus), or in mice infected with influenza virus and later challenged with VV, LCMV, or MCMV. We chose these four viruses because they are phylogenetically unrelated and because they are naturally spread through infection of the respiratory mucosa and induce significant inflammation in the lung. 5-13 LCMV and VV, which are usually not studied in respiratory models, are in nature spread via the respiratory route. VV, used for vaccination against smallpox, is a model for poxviruses, which are naturally transmitted via the respiratory tract and frequently associated with induction of pneumonia. 20-22 LCMV, which induces a flu-like illness in humans, is a common pathogen, with 5 to 14% of the general population being serologically positive. 23,24 All four of these viruses used here are known to induce distinctive lung pathologies in mice which are easily defined and followed. 5-13 The pathological changes we observed in our study during acute infections of naïve mice with all four viruses were consistent with those observed by other investigators. 5-13 VV and influenza induced potent acute, neutrophilic inflammatory responses. VV infection of the lung induced severe pulmonary edema and acute necrotizing bronchiolitis, whereas influenza virus caused severe acute inflammatory pneumonic consolidations. In contrast, LCMV and MCMV induced mild disease with chronic mononuclear infiltrates in the interstitium. Using this baseline knowledge we could then assess what effect heterologous immunity would have on lung pathology. Our results here document that the cytokine response and resulting pathology to a given virus is dramatically and uniquely altered by previous infections with distinct heterologous viruses. Notably, we also find that a history of infection with influenza virus can lead to either decreased or enhanced viral titers on challenge with heterologous viruses. Heterologous immunity also resulted in two different patterns of disease outcome dependent on the specific virus history: a change from a neutrophilic response to a lymphocytic response associated with rapid clearance of virus or a change from a mild lymphocytic response to a severe lymphocytic response associated with enhanced virus load. The implication of these studies is that the host response to viral infections in the lung is in part a function of the infection history of the host.
Materials and Methods
Mice
C57BL/6 (B6, H2b) male mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and used at 2 to 18 months of age.
Viruses
LCMV, strains Armstrong and clone 13, an RNA virus in the Old World arenavirus family, was propagated in BHK21 baby hamster kidney cells. 25 The WR strain of VV, a DNA virus in the poxvirus family, was propagated in L929 cells. 25 MCMV, strain Smith, a DNA virus in the herpesvirus family, was extracted from salivary glands of infected BALB/c mice. 25 The mouse adapted influenza A virus A/PR/8/34 (H1N1), a RNA virus in the orthomyxovirus family, was grown in the allantoic fluid of 10-day-old embryonated chicken eggs (SPAFAS, Preston CT). For acute virus infections, metofane-anesthetized mice were challenged intranasally with 4 × 105 plaque-forming units (PFU) of LCMV, 400 PFU of MCMV to generate MCMV-immune mice, 105 PFU of MCMV for acute challenge, or 70 or 200 PFU of influenza virus. Various VV doses (1 × 103 to 1 × 105 PFU) were used because there was some variability in virulence among different VV stocks.
Cell Lines
American Type Culture Collection vero cells were cultured in Eagle’s minimal essential medium (Life Technologies, Inc., Grand Island, NY), supplemented with 100 U/ml penicillin G, 100 μg/ml streptomycin sulfate, 2 mmol/L l-glutamine, 10 mmol/L HEPES, and 10% heat-inactivated (56°C, 30 minutes) fetal bovine serum (Sigma, St. Louis, MO). GT-KO cells are mouse embryonic fibroblast lines cultured from α-1–3-galactosyltransferase (GT)-deficient transgenic mice. They were cultivated as monolayers in Dulbecco’s modified Eagle’s medium (Life Technologies, Inc.) supplemented with penicillin, streptomycin, glutamine, pyruvate, and 10% fetal bovine serum (D10 medium) as described above. 26
Infection Protocol
For acute infection with a single virus, metofane-anesthetized mice were intranasally inoculated with a sublethal dose of the virus. At various times after the inoculation, the lungs were harvested for histological evaluation. For other studies, metofane-anesthetized mice were immunized intranasally with a sublethal dose of one virus. After the rise and fall of the acute T-cell response, and when the immune system had returned to homeostasis (usually 6 weeks or later), the mice were challenged with the second heterologous virus. Five to 7 days after the second virus infection, the spleen, lung, and/or mediastinal lymph nodes were harvested, homogenized, and titrated in virus plaque assays, as previously described. 27 In the control and LCMV-immune mice challenged with VV, natural killer cells were depleted to demonstrate that natural killer cells were not involved in the differences in virus titers early in infection. Other experiments not shown here without natural killer depletion showed similar differences between these two groups. 19 In other experiments the lungs were also harvested, fixed, and sectioned for histological evaluation. To decrease the concentration of impurities in virus preparations phosphate-buffered saline (PBS) was used to dilute LCMV, influenza virus, and MCMV as previously described. 19,27 VV culture supernatants were purified over sucrose gradients and then diluted in PBS. Control naïve mice were sham-immunized intranasally with either PBS or PBS-diluted tissue culture media sedimented like virus over a sucrose gradient. All mice used were healthy with no evidence of any underlying disease. The immune mice were always age-matched to the control mice and housed under exactly the same pathogen-free conditions and for the same time period.
Virus Titration
A 10% homogenate of lung tissue from each individual mouse was serially diluted and titrated on the appropriate cell line to quantify the viral titers. American Type Culture Collection vero cells were used for LCMV and VV titration and GT-KO cells were used for MCMV titration. Titers were reported as the arithmetic means of log10 PFU for whole lungs that had been individually titrated from four to five mice per group.
RNase Protection Assay
Total RNA was extracted from whole lung tissue using Trizol agent 3 days after virus challenge. Detection and quantitation of a variety of murine cytokine mRNAs were accomplished with the multiprobe RNase protection assay system as per the manufacturer’s instructions (PharMingen, La Jolla, CA). During the 4 years these experiments were performed three different mouse cytokine multiprobe template sets were used: 1) mCK-1b containing interleukin (IL)-4, IL-5, IL-10, IL-13, IL-15, IL-9, IL-2, IL-3, interferon (IFN)-γ, L32, and GAPDH; 2) mCK-2b containing IL-12p35, IL-12p40, IL-10, IL-1α, IL-1β, IL-1Rα, IGIF, IL-6, IFN-γ, MIF, L32, and GAPDH; 3) mCK3b containing tumor necrosis factor-β, LTβ, tumor necrosis factor-α, IL-6, IFN-γ, IFN-β, transforming growth factor-β1, transforming growth factor-β2, transforming growth factor-β3, MIF, L32, and GAPDH. The specific cytokine bands were identified on the basis of their individual migration patterns in comparison with the undigested probes. The bands were quantified by densitometric analysis with Image Quant densitometric software.
Lung Histological Evaluation
Lungs were harvested from mice either in the naïve state, the immune state (2.5 to 18 months after a single infection), or 5 to 7 days after an acute infection. The lungs were then fixed in 10% neutral buffered formaldehyde and paraffin embedded. Tissue sections (5 μm) were stained with hematoxylin and eosin and analyzed microscopically. Two independent pathologists blinded to the experimental status of the lung samples scored all of the lung pathology. Lung pathology was graded based on the distribution and the severity of disease from 0 to 3 (0, within normal limit; 1, 1 to 9% involvement of the lung parenchyma; 2, 10 to 49% involvement of the lung parenchyma; 3, ≥50% involvement of the lung parenchyma). The consistency of this scoring scheme was assessed by having these same two reviewers blindly regrade 20 of the slides 1 year later and the scoring was similar to the earlier score.
Results
The Specific History of Heterologous Virus Infections Determines Whether Viral Clearance Is Enhanced or Impeded
To determine what influence, if any, previous exposure to a heterologous virus had on protective immunity in the lung, adult C57BL/6 mice were initially infected intranasally with LCMV, MCMV, or influenza virus. More than 6 weeks after the acute response had resolved to the first virus, and when the hosts were in a resting immune state, the immune and control naïve mice were challenged with a second virus, as seen in Table 1 ▶ . Two distinct patterns emerged on how previous infections influenced viral clearance in the lung. Immunity to LCMV resulted in an 80% reduction in VV titer early in infection, a result consistent with our previous observations. 19 Similarly, previous infection with MCMV and influenza virus resulted in reduced VV titers (68% and 84%, respectively) (Table 1A) ▶ . This protective effect lasted for long periods of time after the original virus infection because mice either 12 or 30 weeks after LCMV infection and 6 or 12 weeks after influenza infection were equally efficient at resisting VV challenge.
Table 1.
Specific History of Heterologous Virus Infections Determines Whether Viral Clearance Is Enhanced or Impeded
| Immunizing virus | Challenge virus* | Days after challenge | Virus titer (mean log10 PFU/lung ± SEM)† | Virus titer change (immune versus control)‡ | ||||
|---|---|---|---|---|---|---|---|---|
| A. Previous heterologous virus infections protect against VV | ||||||||
| Control§ | VV (3) | 6 | 6.2 ± 0.3 | |||||
| LCMV§ | 5.5 ± 0.4 | 80% ↓ (P = 0.03) | ||||||
| Control | VV (1) | 5 | 7.5 ± 0.1 | |||||
| MCMV | 7.0 ± 0.1 | 68% ↓ (P = 0.004) | ||||||
| Control | VV (2) | 5 | 6.2 ± 0.1 | |||||
| Influenza | 5.4 ± 0.4 | 84% ↓ (P < 0.1) | ||||||
| B. Prior immunity to influenza virus can impede or enhance viral clearance | ||||||||
| Control | VV (2) | 5 | 7.5 ± 0.1 | |||||
| Influenza | 7.1 ± 0.2 | 60% ↓ (P = 0.03) | ||||||
| Control | LCMV (3) | 7 | 5.4 ± 0.4 | |||||
| Influenza | 6.0 ± 0.2 | 4× ↑ (P = 0.04) | ||||||
| Control | MCMV (7) | 7 | 5.0 ± 0.3 | |||||
| Influenza | 5.9 ± 0.1 | 8× ↑ (P = 0.02) | ||||||
The infection of mice was done as described in Materials and Methods.
* The number in parentheses represents the number of experiments done.
† Data are presented as mean ± SEM log10PFU/lung from a representative experiment. There were three to five mice per treatment group.
‡ Significance of the difference between the control and immunized groups of mice (t-test).
§ LCMV-immune mice acutely infected with VV were natural killer cell-depleted by intraperitoneal injection of 100 μl (200 μg) of anti-NK1.1 (clone PK136) on day 0 and 4 after infection.
A different pattern was observed in influenza virus-immune mice. A history of influenza virus infection was protective against VV challenge but impeded the clearance of both LCMV and MCMV on subsequent challenge. There was a significant fourfold increase in LCMV and an eightfold increase in MCMV titers in the lungs of influenza virus-immune mice when compared to controls (Table 1B) ▶ . Within this same group of influenza virus-immune mice there was still a 60% decrease in VV titers as compared to controls. These experiments were performed 6 to 24 weeks after influenza infection, indicating that this enhanced LCMV and MCMV replication appeared to be independent of the time after initial immunization with influenza virus. These studies suggest that exposure to heterologous viruses can be beneficial, resulting in protective immunity, but, depending on the sequence of virus infections, can also result in enhanced virus replication.
The Specific Heterologous Virus Infection Sequence Determines the Cytokine Profile
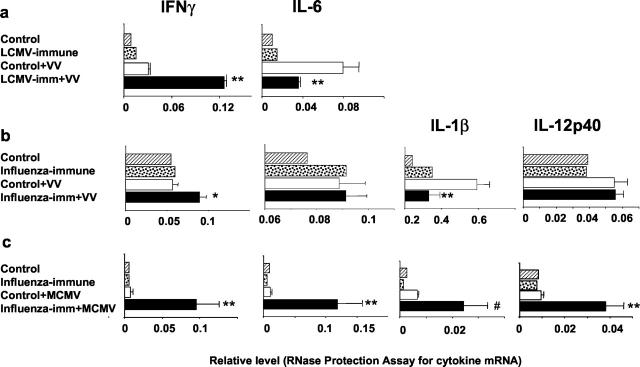
We questioned whether previous immunity to heterologous viruses could mediate changes in early cytokine responses and whether the cytokine profile differed during the protective and detrimental effects of heterologous immunity. The lungs of LCMV-immune mice early after VV challenge or of influenza virus-immune mice early after VV or MCMV challenge were assessed for their cytokine mRNA levels by RNase protection assays (Figure 1) ▶ . We had previously shown that LCMV-specific memory CD8 T cells produce enhanced levels of the Th1-type cytokine, IFN-γ, on VV infection and that IFN-γ was important in mediating protective immunity against VV. 19 In the present study our results also show that at 3 days after VV infection in LCMV-immune mice lungs there were fourfold higher levels of IFN-γ mRNA (Figure 1a) ▶ . At the same time, on VV challenge there was also a 50% reduction in the mRNA levels of the proinflammatory cytokine, IL-6, in the LCMV-immune mouse lungs as compared to the controls. In one experiment we examined levels of the Th2-type cytokines, IL-4 and IL-5, and there was little change in LCMV-immune mouse lungs compared to controls (relative level of IL-4: control, 0.03 ± 0.001; LCMV-immune, 0.026 ± 0.002; relative level of IL-5: control, 0.03 ± 0.003; LCMV-immune, 0.02 ± 0.002; n = 3 mice/group; cytokine template set mCK-1b).
Figure 1.
Specific heterologous virus infection sequence determines early cytokine profile. Total RNA was isolated from the whole lung tissue 3 days after virus challenge. Detection and quantification of a variety of murine cytokine mRNAs were accomplished as outlined in Materials and Methods. There were three mice in virus-challenged immune and control groups and one mouse in the control and immune groups. The results were presented as relative 32P intensities of the signals for individual cytokines, which were normalized to the levels of housekeeping gene expression. *, P < 0.1; **, P < 0.05; #, P = 0.07. a: The cytokine template set mCK-3b was used. b and c: The cytokine template set mCK-2b was used. These results are representative of two to three similar experiments. One mouse was used in the control and immune groups because preliminary experiments indicated that the variability between individual mice in theses groups was negligible.
Cytokine profiles on VV infection of influenza virus-immune mice mirrored the ones observed in LCMV-immune mice. There was a 60% higher level of IFN-γ mRNA expression in the lungs at day 3 after VV infection in influenza virus-immune mice as compared to VV-infected controls (Figure 1b) ▶ . Concomitantly, these same influenza virus-immune mice showed a significant 50% reduction in mRNA levels of the proinflammatory cytokine IL-1β, although IL-6 levels remained similar to control mice (Figure 1b) ▶ . Thus, when previous immunity resulted in protective immunity against VV infection, there were enhanced Th1 type responses and reduced proinflammatory responses on VV challenge.
A very different cytokine profile was observed in influenza virus-immune mouse lungs challenged with MCMV, in which previous immunity had a negative impact on clearance of the second virus. There was a dramatic, 12-fold increase in IFN-γ mRNA level when compared to control mice 3 days after MCMV challenge (Figure 1c) ▶ . This was accompanied with a 2.2-fold increase in tumor necrosis factor-α, a second Th1-type cytokine (relative level: control, 0.5 ± 0.1; influenza virus-immune, 1.1 ± 0.3, P = 0.08; n = 2 mice/group; data from one of two similar experiments). Unlike the influenza virus or LCMV-immune mice challenged with VV, the MCMV-infected influenza virus-immune mice had significantly increased levels of proinflammatory cytokines, including 11-fold, fourfold, and fourfold increases in IL-6, IL-1β, and IL-12p40, respectively (Figure 1c) ▶ . Interestingly, in three separate experiments there were no measurable levels of Th2-type cytokine IL-4 or IL-5 mRNA (cytokine template set mCK-1b) in the lung of either nonimmune or influenza virus-immune mice challenged with MCMV.
Distinctive Lung Immunopathology during Acute Virus Infection of Nonimmune Mice
To examine whether previous infections with heterologous viruses could influence lung pathology on subsequent infections, we needed to document baseline lung pathology during acute infection in nonimmune mice. Each acute virus infection in naive mice induced distinct pathologies in the lung that returned to essentially normal architecture with minimal changes in the resting immune state (Figure 2 ▶ , Tables 2 and 3 ▶ ). Figure 2a ▶ shows a naïve mouse lung section, characterized by clean and open bronchioles, thin-walled alveoli, normal vasculature, and flat pleura. Acute LCMV or MCMV infections induced relatively mild lung disease with predominantly mild mixed mononuclear (MN) (macrophages and lymphocytes) interstitial infiltrates (Figure 2, b and c ▶ ; Table 3 ▶ ). Acute LCMV infection induced mild to moderate transient BALT, characterized as nodules of lymphoid tissue in the bronchial lamina propria near the branch points of an airway or between the bronchus and an artery. 28 Although present in the normal mouse lung, BALT is not usually visible but can be induced by infection (Figure 2b ▶ and Table 3 ▶ ). Both human CMV (HCMV) and MCMV are known to infect endothelial cells. 29 Therefore, it was not surprising to observe vasculitis and endothelial activation on infection with MCMV (Table 3) ▶ .
Figure 2.

Distinct lung pathology with different viruses can be altered by previous immunity to heterologous viruses. Lung sections from mice were stained with H&E. Column 1: Naïve control (a) and single virus acute infection (day 7) [LCMV (b), MCMV (c), influenza virus (d)]. Column 2: Single virus immune [VV (e), LCMV (f), MCMV (g), influenza virus (h)]. Column 3: Immune mice infected with VV [naïve + VV day 7 (i), LCMV-immune + VV day 7 (j), MCMV-immune + VV day 5 (k), influenza virus-immune + VV day 5 (l)]. Distinctive pathology occurred with each acute viral infection. Acute LCMV (b) and MCMV (c) induced moderate to severe interstitial disease with MN infiltrates, but MCMV also induced vascular lymphocytic tufting. f and g: These changes completely resolved in the immune state except for very mild lymphocytic infiltrates in the interstitium. Acute influenza virus (d) and VV (i) infections cause more severe diseases with mixed PMN and MN infiltrates, associated with pneumonic consolidation in influenza virus-infected mice and necrotizing bronchiolitis and pulmonary edema in VV-infected mice. e: BALT developed late during VV infection and persisted in VV-immune mice. h: There were some residual areas of consolidation in influenza virus-immune lungs. Acute VV challenge of immune mice led to altered pathology: no edema but massive lymphocytic responses with enhanced BALT in LCMV-immune mice (j), or decrease in edema and enhanced chronic MN infiltrates in the interstitium and vasculature in MCMV (k) and influenza virus-immune (l) mice. Original magnifications, ×60.
Table 2.
Prior Heterologous Virus Exposure Induces Variations in Chronic Mononuclear Infiltrates in VV-Infected Mouse Lungs
| Lung compartments | Pathological features | Distribution and severity of disease‡ | |||||
|---|---|---|---|---|---|---|---|
| Day 5 | Day 7 | ||||||
| Control + VV (n = 4)§ | LCMV-immune∥ + VV (n = 4) | MCMV-immune∥ + VV (n = 2) | Influenza-immune + VV (n = 2) | Control + VV (n = 11) | LCMV-immune + VV (n = 9) | ||
| Alveoli | Edema | 2.8 (2–3)¶ | 1.5 (0–3) | 2.5 (2–3) | 2 | 2 (0–3) | 0.9 (0–2) |
| AMI* | 1 | ||||||
| BALT | Extent of BALT | 1.8 (1–3) | 1 | 0.5 (0–1) | 0.5 (0–2) | 2.1 (0–3) | |
| Airways | Necrotizing bronchiolitis | 1.8 (0–3) | 0 | 1 | 2 (1–3) | 1.4 (0–3) | 1.3 (0–3) |
| Bronchiolitis | 0.8 (0–2) | 0.3 (0–1) | 1 | 1 | 1.5 (0–3) | 0.6 (0–3) | |
| Peribronchial AMI | 2 (1–3) | 0.5 (0–1) | 1 | 1.2 (0–3) | |||
| Peribronchial CMI | 0.5 (0–1) | ||||||
| Bronchiolitis obliterans | 0.6 (0–2) | ||||||
| Bronchiolization | 0.5 (0–1) | ||||||
| Interstitium | Consolidation | 0.5 (0–1) | |||||
| CMI† | 1.8 (1–2) | 1 | 1.5 (1–2) | 1.4 (0–3) | |||
| AMI | 1.5 (0–3) | 1.5 (1–2) | 0.5 (0–1) | 0.5 (0–3) | 0.3 (0–1) | ||
| Pleura | CMI | 0.8 (0–1) | 1 | 1.5 (1–2) | 0.2 (0–1) | 1 | |
| Vasculature | Lymphocytic cuffing and tufting | 1 (0–2) | 0.5 (0–1) | 1.5 (0–1) | 0.7 (0–2) | ||
| Necrotizing vasculitis | 0.3 (0–2) | 0.4 (0–1) | |||||
| Endothelial activation | 0.5 (0–1) | ||||||
| Perivascular CMI | 0.5 (0–1) | 3 | 0.4 (0–1) | ||||
| Perivascular AMI | 2 (0–3) | 1.5 (0–3) | 1 (0–2) | 0.8 (0–3) | |||
| Edema | 0.3 (0–1) | 0.6 (0–3) | |||||
Five or 7 days after VV infection mouse lungs were harvested, sectioned, and stained (H&E), as described in Materials and Methods.
* AMI, acute mixed infiltrate (polymorphornuclear and mononuclear cells).
† CMI, chronic mononuclear infiltrate (lymphocytes and macrophages).
‡ Grading of the pathological changes in the lung, based on the distribution and the severity of disease from 0 to 3 (0 or blank, within normal limit; 1, 1 to 9%, 2, 10 to 49%, and 3, ≥50% involvement of the lung parenchyma).
§ The number of mice in each group.
¶ Data are presented as average scores from multiple mice per treatment, and the number in parentheses after each average score represents the range of scores from mice challenged with the virus indicated.
∥ LCMV-immune and MCMV-immune mouse lungs had normal architecture except for the following minimal changes, LCMV-immune: interstitial and subpleural CMI 0.8 (0.5–1), n = 4; MCMV-immune: interstitial and subpleural CMI 1, perivascular CMI 0.5 (0–1), n = 2.
Table 3.
Prior Immunity to Influenza Virus Enhances the Usual Mild Mononuclear Responses in the Lung upon LCMV and MCMV Infection
| Lung compartments | Pathological features | Distribution and severity of disease‡ | |||||
|---|---|---|---|---|---|---|---|
| Influenza-acute (n = 3)§ | Influenza-immune (n = 5) | Control + MCMV (n = 6) | Influenza-immune + MCMV (n = 6) | Control + LCMV (n = 4) | Influenza-immune + LCMV (n = 4) | ||
| Alveoli | Edema | 1.3 (1–2)¶ | 0 | 0 | 0.2 (0–1) | 0.5 (0–1) | 0.4 (0–1) |
| AMI* | 3 | ||||||
| BALT | Extent of BALT | 0.6 (0–1) | 0.6 (0–2) | 0.8 (0–1) | 2.6 (1–3) | 0 | 0.5 (0–2) |
| Airways | Necrotizing bronchiolitis | 1 (0–2) | |||||
| Bronchiolitis | 1.6 (0–3) | 0 | 0.2 (0–1) | 0.2 (0–1) | 0 | 0 | |
| Peribronchial AMI | 0.3 (0–1) | ||||||
| Peribronchial CMI | 0.2 (0–1) | ||||||
| Bronchiolization | 0 | 0.4 (0–1) | 0 | 1.5 (1–2) | 0 | 1.8 (1–2) | |
| Interstitium | Consolidation | 2 (0–3) | 0.6 (0–1) | 0 | 2.5 (1–3) | 0 | 1.3 (1–2) |
| CMI† | 3 | 1.8 (1–3) | 2.2 (2–3) | 2.8 (2–3) | 1.3 (1–2) | 1.8 (1–2) | |
| AMI | 2.6 (2–3) | ||||||
| Pleura | CMI | 2.3 (1–3) | 1.2 (1–2) | 2.2 (2–3) | 2.8 (2–3) | 1.3 (1–2) | 1.3 (1–2) |
| AMI | 2.3 (1–3) | ||||||
| Vasculature | Lymphocytic cuffing and tufting | 1 | 0 | 2.2 (2–3) | 0.8 (0–2) | 0.3 (0–1) | 0 |
| Necrotizing vasculitis | 0.3 (0–1) | ||||||
| Perivascular CMI | 0 | 0.4 (0–1) | 0 | 0 | 0.3 (0–3) | 0.8 (0–1) | |
| Perivascular AMI | 3 | ||||||
| Endothelial activation | 0 | 0 | 1.3 (0–2) | 0.6 (0–2) | 0 | 0 | |
| Edema | 1 (0–3) | ||||||
Seven days after infection mouse lungs were harvested, sectioned, and stained (H&E), as described in Materials and Methods.
* AMI, acute mixed infiltrate (polymorphornuclear and mononuclear cells).
† CMI, chronic mononuclear infiltrate (lymphocytes and macrophages).
‡ Grading of the pathological changes in the lung, based on the distribution and the severity of disease from 0 to 3 (0 or blank, within normal limit; 1, 1 to 9%, 2, 10 to 49%, and 3, ≥50% involvement of the lung parenchyma).
§ The number of mice in each group.
¶ Data are presented as average scores from multiple mice per treatment, and the number in parentheses after each average score represents the range of scores from mice challenged with the virus indicated.
Influenza virus or VV infections at much lower doses of virus than LCMV or MCMV caused much more severe acute lung damage, with some long-term residual changes in the lung. Influenza virus induced severe acute inflammation, with many mixed polymorphonuclear (PMN) and MN cells around airways, in alveolar spaces and interstitium; this inflammation was often so severe that it led to areas of pneumonic consolidation. Moderate edema, necrotizing vasculitis, and perivascular mixed PMN and MN infiltrates were also present (Figure 2d ▶ and Table 3 ▶ ). After the acute influenza virus infection resolved, the lung architecture was relatively normal, but on occasion small areas of residual consolidation and mild BALT persisted in the influenza virus-immune mouse lung (Figure 2h) ▶ . Also, very mild bronchiolization occasionally occurred in the influenza virus-immune mouse lung (Table 3) ▶ . Bronchiolization represents a repair process whereby alveolar walls become lined by cells resembling, but not necessarily identical with, bronchiolar epithelium. 30
During acute VV infection, the mouse lungs developed necrotizing bronchiolitis, defined by the presence of necrotic bronchiolar epithelium with PMNs and debris, and severe alveolar edema (Figure 2i ▶ , Figure3a ▶ , and Table 2 ▶ ). This accumulation of extravascular fluid in the air spaces could significantly disturb gas exchange and contribute to the mortality observed with VV infection. 19 In addition, the VV-infected mouse lungs mainly showed acute, mixed inflammatory infiltrates with PMN and MN cells present in the peribronchial areas and interstitium, as well as in the perivascular areas (as shown at higher magnifications in Figure 3, a and b ▶ , and Table 2 ▶ ). Enhanced BALT could develop but only later, more than 15 days after VV infection (not shown). Mild BALT could persist for at least 3 months in the VV-immune mouse lung (Figure 2e) ▶ .
Figure 3.
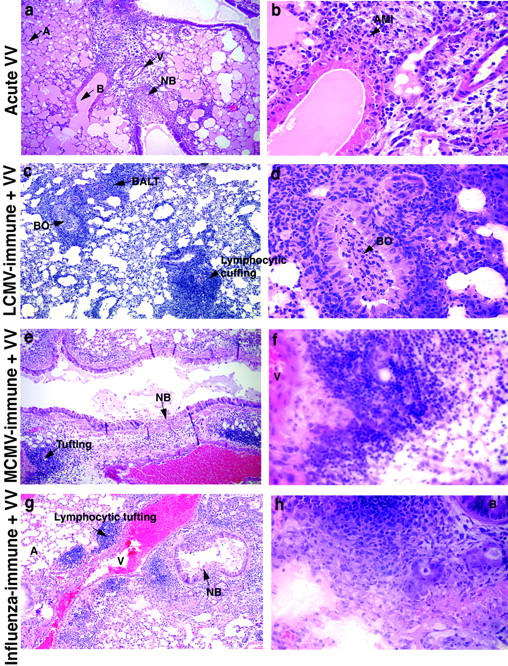
Patterns of enhanced chronic mononuclear (lymphocytic) infiltrates in VV-infected mouse lungs vary depending on previous heterologous virus exposure. Higher magnifications of the lung sections shown in Figure 2 (i, j, k, and l) ▶ . These figures demonstrate in greater detail the alteration in lung pathology of naïve mice (a, b) as compared to LCMV-immune (c, d), MCMV-immune (e, f), and influenza virus-immune (g, h) upon VV challenge (day 7). a and b: VV-infected control mouse lungs showed severe alveolar edema (pinkish material in air spaces) and acute mixed inflammation (AMI) with PMN and MN infiltrates. c and d: VV-infected LCMV-immune mouse lungs had increased BALT, bronchiolitis obliterans (BO), and perivascular lymphocytic cuffing. VV-infected MCMV (e and f) and influenza virus-immune (g and h) lungs had perivascular lymphocytic tufting. Necrotizing bronchiolitis (NB) was one of the main lesions caused by VV infection; it was seen in all acute VV-infected mice. A, alveoli; B, bronchiole; V, vessel. Original magnifications: ×180 (a, c, e, and g); ×720 (b, d, f, and h).
Alterations in VV-Induced Lung Pathology Dependent on the Specific History of Heterologous Virus Exposure
When the LCMV-, MCMV-, or influenza virus-immune mice were challenged with VV, the pathology varied dramatically not only from the VV-infected controls but also from each other (Figures 2 and 3) ▶ . One of the main lesions of acute VV infection, necrotizing bronchiolitis, was present in the lungs of all of the VV-infected groups: controls, LCMV-, MCMV-, or influenza virus-immune mice (Figure 3; b, e, and g ▶ ; and Table 2 ▶ ). However, compared to controls, the lungs of LCMV-immune mice challenged with VV showed very little alveolar edema but had a very prominent lymphocytic response instead of an acute inflammatory response. Most strikingly, the LCMV-immune mouse lungs had very prominent BALT as early as 5 and 7 days after VV infection (Figure 2j ▶ ; Figure 3, c and d ▶ ; and Table 2 ▶ ). Also prominent was chronic MN infiltration (CMI), with lymphocytes and macrophages present in the interstitium and pleura. In addition, the LCMV-immune lungs showed perivascular lymphocytic cuffing, as defined by a significant lymphocyte accumulation around the vessels (Figure 2j ▶ ; Figure 3, c and d ▶ ; and Table 2 ▶ ). Interestingly, by day 7 of VV infection, LCMV-immune mouse lungs also displayed the less common pathology of bronchiolitis obliterans, showing obstruction of bronchioles by plugs of fibrin and inflammatory cells (Figure 3, c and d ▶ ; and Table 2 ▶ ).
On VV challenge the lungs of MCMV- or influenza virus-immune mice were like those of LCMV-immune mice, showing a prominent chronic mononuclear (macrophages and lymphocytes) response (Figure 2, k and l ▶ ; Figure 3, e and g ▶ ; and Table 2 ▶ ). Compared to LCMV-immune mice, both MCMV- or influenza virus-immune mice had a greater accumulation of lymphocytes and other mononuclear cells around the vessels, ie, tufting and cuffing. However, there was less of an increase in BALT than was observed in LCMV-immune mice (Figure 2, j, k, and l ▶ ; Figure 3, c, e, g ▶ ; and Table 2 ▶ ). Alveolar edema was decreased in MCMV-, influenza virus-, and LCMV-immune mice as compared to controls, but immunity to LCMV had the greatest impact on lessening edema (Table 2) ▶ . The major difference between influenza virus-immune mice and MCMV-immune mice on VV challenge was the presence of mild residual areas of consolidation in VV-infected influenza virus-immune mouse lungs, whereas VV-infected MCMV-immune mouse lungs demonstrated greater endothelial activation (Table 2) ▶ .
Clearly, previous immunity to LCMV, MCMV, or influenza virus led to significant alterations in VV-induced immunopathology in almost all of the lung compartments. Although the common feature of this altered pathology was an enhanced chronic response (lymphocytes and macrophages), immunity to each virus resulted in these cells being distributed differently in various lung compartments (Table 2 ▶ and Figure 3 ▶ ). Previous immunity to each virus also led to some unique features with the subsequent acute VV infection, such as endothelial activation in MCMV-immune mice and bronchiolitis obliterans in LCMV-immune mice.
Enhanced Chronic Mononuclear (Macrophages and Lymphocytes) Infiltrates in Acute LCMV- or MCMV-Infected Lungs of Influenza Virus-Immune Mice
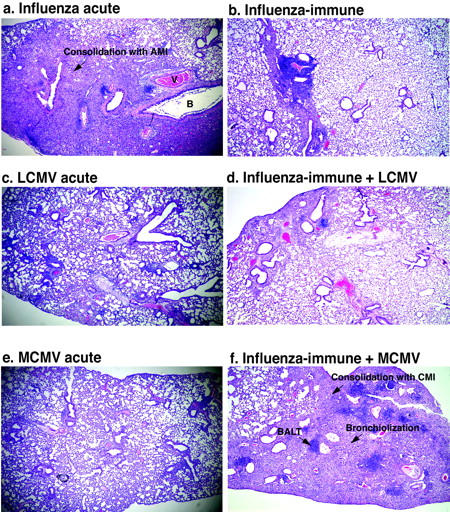
Because immunity to influenza virus enhanced MCMV and LCMV titers in the lung, we questioned what influence immunity to influenza virus had on lung pathology. The most prominent feature of acute influenza virus infection was severe pneumonic consolidation mostly with PMNs (Figure 4a ▶ ; Figure 5, a and b ▶ ; and Table 3 ▶ ). When this resolved, the mouse lung was left with some minimal residual areas of consolidation infiltrated with chronic mononuclear cells as long as 24 months after infection (Figure 4b ▶ ; Figure 5, c and d ▶ ; and Table 3 ▶ ).
Figure 4.
Previous immunity to influenza virus markedly enhances the usual mild mononuclear infiltrates in the lung upon LCMV and MCMV infection. Lung sections from mice were stained with H&E. a: Acute influenza virus infection (day 7) caused consolidation with acute mixed inflammatory infiltrates (PMN and MN infiltrates). b: Influenza virus-immune lungs were essentially normal with residual areas of consolidation. As compared with LCMV-infected lung (c) (day 7) with mild lymphocytic response around airways and in the interstitium, LCMV-infected influenza virus-immune mice (d) showed a slightly enhanced lymphocytic response and consolidation with MN cells. e: Acute MCMV infection (day 7) induced interstitial disease with MN infiltrates and vascular lymphocytic tufting. f: MCMV-infected influenza virus-immune mice had increased BALT and consolidation of the interstitium. B, bronchiole; V, vessel. Original magnifications, ×84.
Figure 5.
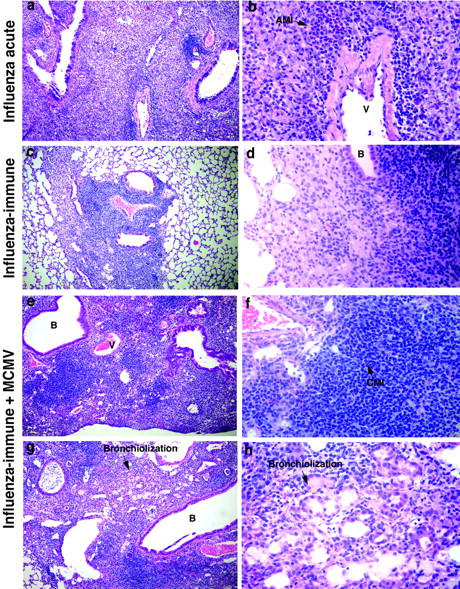
Unique immunopathology in MCMV-infected mouse lungs induced by previous immunity to influenza virus. Higher magnifications of the lung sections in Figure 4 (a, b, e, and f) ▶ . a and b: Influenza virus-infected lung 7 days after challenge. c and d: Influenza virus-immune mouse lung (2 months). e and f: MCMV-infected influenza virus-immune mouse lung showing very prominent chronic MN inflammation in a large, densely consolidated area. g and h: Another MCMV-infected influenza virus-immune mouse lung, showing an area of alveolar bronchiolization. Original magnifications: ×184 (a, c, e, and g); ×736 (b, d, f, and h).
When influenza virus-immune mice were infected with MCMV, the lung pathology was very different from that of the acute MCMV infection of naive mice. Acute MCMV infection of naïve mice induced a mild mononuclear (lymphocytes and macrophages) infiltrate around bronchioles, vessels, and in the interstitium (Figure 4e) ▶ , and endothelial activation in the vessels of the lung (Table 3) ▶ . On MCMV infection of influenza virus-immune mice, the lymphocytic response in the lung was much greater than in nonimmune control mice. There was a dramatic increase in BALT and in the consolidation that was already present in a mild residual form in the influenza virus-immune lung. However, the consolidation in influenza virus-immune mice challenged with MCMV consisted of chronic mononuclear infiltrates rather than the acute neutrophilic infiltrates observed during acute influenza virus infection (Figure 4f ▶ ; Figure 5, e and f ▶ ; and Table 3 ▶ ). There was also an increase in alveolar bronchiolization, otherwise only occasionally seen in a very mild form in influenza virus-immune control mouse lungs (Figure 4f ▶ ; Figure 5, g and h ▶ ; and Table 3 ▶ ).
Immunity to influenza virus had a similar effect on the immunopathology in response to another virus, LCMV, although not as striking as the effect on the MCMV response. Acute infection with LCMV in a control mouse results predominantly in a mild interstitial mononuclear infiltrate (Figure 4c ▶ and Table 3 ▶ ). In influenza virus-immune mice LCMV infection induced an increase in the accumulation of lymphocytes and other mononuclear cells especially around airways and vessels. There was also some enhancement of the residual chronic mononuclear consolidation already present in mild form in influenza virus-immune mice lungs (Figure 4d ▶ and Table 3 ▶ ). Thus, previous immunity to influenza virus resulted in a marked increase of the usually mild lymphocytic and mononuclear infiltration occurring on LCMV or MCMV infection.
Discussion
This study demonstrates that previous immunity to a heterologous virus infection can alter the outcome of a subsequent virus infection, influencing viral clearance, early cytokine responses, and immunopathology in the lung. These changes in disease outcome were dependent on the specific history of heterologous virus infections, whether we examined the acute response to VV in mice immune to any of three different viruses (LCMV, MCMV, or influenza virus) or the effect immunity to influenza virus had on the acute responses to subsequent infections with three different viruses (VV, MCMV, or LCMV). We were able to identify two patterns of disease outcome dependent on the specific sequence of virus infections. When previous immunity changed an acute neutrophilic response to a lymphocytic response (LCMV plus VV, MCMV plus VV, influenza virus plus VV) there was a decreased virus load; when it changed a mild lymphocytic to a severe lymphocytic response (influenza virus plus LCMV, influenza virus plus MCMV) there was an enhancement in viral titers (Table 4) ▶ . These results would suggest that there might be a predictable component to disease outcome as influenced by heterologous immunity based on knowledge of an individual’s history of infection and virus-specific immune responses.
Table 4.
Sequence of Heterologous Virus Infections Influences Viral Clearance, Early Cytokine Profiles, and Histopathology in the Lung
| Virus titer | Cytokine profile | Histopathology | |
|---|---|---|---|
| LCMV-immune + VV versus Acute VV | ↓ | IFN-γ ↑ | · Dramatically decreased edema |
| IL-6 ↓ | · Increased chronic mononuclear (lymphocytic) response | ||
| · Decreased acute inflammatory (PMN) response | |||
| · Early very prominent BALT (lymphocytic) | |||
| · Bronchiolitis obliterans | |||
| MCMV-immune + VV versus Acute VV | ↓ | Not done | · Decreased edema |
| · Increased chronic mononuclear (lymphocytic) response, especially perivascular | |||
| · Decreased acute inflammatory (PMN) response | |||
| · Moderate BALT | |||
| · Endothelial activation | |||
| Influenza-immune + VV versus Acute VV | ↓ | IFN-γ ↑ | · Decreased edema |
| IL-1β ↓ | · Increased chronic mononuclear (lymphocytic) response | ||
| · Decreased acute inflammatory (PMN) response | |||
| · Mild BALT | |||
| · Mild consolidation | |||
| Influenza-immune + LCMV versus Acute LCMV | ↑ | Not done | · Slightly increased BALT |
| · Slightly increased mononuclear infiltrates in the interstitium and around vessels | |||
| · Moderate consolidation with mononuclear cells | |||
| · Increased bronchiolization | |||
| Influenza-immune + MCMV versus Acute MCMV | ↑ | IFN-γ ↑ | · Increased BALT (in the consolidated areas) |
| TNF-α ↑ | · Massive consolidation with lymphocytes and | ||
| IL-6 ↑ | mononuclear cells | ||
| IL-1β ↑ | · Increased bronchiolization | ||
| IL-12 ↑ |
The enhanced early protective immunity to VV in mice immune to LCMV, MCMV, or influenza virus is most likely mediated by the pronounced CD8 T-cell response and increased Th1-type cytokine, IFN-γ, 19 as VV is known to be very sensitive to IFN-γ. 31 Our earlier work 19 would suggest that IFN-γ also is playing an important role in the accumulation of mononuclear cells instead of neutrophils in the lungs of these immune mice. However, the pattern of the lymphocyte and macrophage accumulation was noticeably different between the groups, with greater amounts of BALT surrounding the airways in LCMV-immune mice and greater amounts of perivascular tufting and cuffing in MCMV- and influenza virus-immune mice. On VV challenge, the LCMV-immune mice developed bronchiolitis obliterans, the influenza virus-immune mice had mild residual areas of consolidation, whereas the MCMV-immune mouse lungs demonstrated endothelial activation (Table 4) ▶ . The factors contributing to these variations in VV-induced pathology have yet to be elucidated.
Interestingly, although immunity to influenza virus protected against VV, it enhanced MCMV or LCMV virus titers early in infection. Both VV and MCMV induced high levels of IFN-γ early after infection of influenza virus-immune mice, but MCMV also increased levels of IL-1β, a proinflammatory cytokine, and IL-6, which is both a Th2 and proinflammatory cytokine. In contrast, VV-challenged influenza virus-immune mice had decreased levels of the proinflammatory cytokine IL-1β. IFN-γ is known to enhance IL-1 and IL-6 production by macrophages and other mononuclear cells during a delayed type hypersensitivity (DTH) reaction, which is classically mediated by a potent CD4 T-cell response. 32 Potentially, IFN-γ produced by heterologous memory T cells may result in an overwhelming DTH-like response in the lung. It is possible that the enhanced chronic mononuclear infiltration of the lung seen in MCMV or LCMV infections in influenza virus-immune mice represents a form of overwhelming CD4-mediated DTH-like response instead of a potent Th1-type CD8 T-cell response, and perhaps this type of environment may be detrimental to viral clearance (Table 4) ▶ . Also, the massive induction of IL-6, a Th2 cytokine important in the differentiation of CD4 T cells into Th2 cells in the presence of IL-4 and the differentiation of B cells into plasma cells, 32 may play a role in the negative outcome to MCMV in mice immune to influenza virus. Possibly, MCMV infection in influenza-immune mice may drive a CD4 Th2 type response and/or potent production of high-antibody levels early in infection, instead of the purely Th1-type response observed on VV infection of influenza virus-immune or LCMV-immune mice. The possibility that an early potent Th2 rather than a Th1 response may be detrimental to clearance of virus in this model needs investigation. The enhanced endothelial activation and vasculitis that is observed in influenza virus-immune mice challenged with MCMV may be enhanced by the increased levels of IL-1β, which is also an important mediator of immune complex formation, an essential mechanism in induction of vasculitis. 33 However, it is also possible that the reverse is true and that the increased virus load is driving the increase in both of these proinflammatory cytokines.
There are several possible mechanisms that could result in the enhancement of MCMV and LCMV replication in influenza virus-immune mice. Immune enhancement of virus replication, caused by increased uptake of antibody-coated virus by FcγR+ cells, has been observed in some viral infections. 34-36 This mechanism could occur if there were early high levels of virus-specific antibodies, which could be driven by the high levels of IL-6 demonstrated in influenza virus-immune mice. Several cytokines, such as tumor necrosis factor-α, IL-1, and IFN-γ, also augment CMV replication in humans, rats, and mice. 37-42 These cytokines can transcriptionally activate nuclear factor-κB, 43,44 which has been shown to activate the CMV enhancer through a viral transactivator. 45 It is therefore possible that these elevated cytokines contributed to the enhanced MCMV replication. Furthermore, both MCMV 46,47 and LCMV (clone 13) 48 replicate well in macrophages. It is conceivable that the massive infiltration with mononuclear cells (lymphocytes and macrophages) in MCMV- or LCMV-infected influenza virus-immune lungs might support enhanced replication of these viruses.
Several features of the lung pathology observed in the present study have implications for our understanding of immune-mediated diseases in the human lung. LCMV-immune mice infected with VV developed two interesting pathologies, enhanced BALT and bronchiolitis obliterans. In humans and mice BALT is not a constitutive structure of the lung, but it can be initiated and vigorously expanded on infection to become a highly integrated mucosal immune system along the respiratory tract. 16,28 It is not clear what the significance of enhanced BALT means in humans. Generally, increased BALT is observed in children, in adults who smoke, in some individuals with autoimmune diseases, and in very athletic cross-country skiers. 16 In humans, the etiology of bronchiolitis obliterans 17 is also not well understood, but is thought to be immune-mediated. Bronchiolitis obliterans is known to occur in association with viral and intracellular bacterial infections 17 as well as being the most significant cause of morbidity and mortality after lung transplantation, 18 in which it is strongly associated with acute rejection. 49 MCMV-infected influenza virus-immune mice also developed another interesting pathology, enhanced bronchiolization. Bronchiolization is a repair process whereby the alveolar walls are lined by cells resembling bronchiolar epithelium; it can be observed after a variety of insults such as respiratory infection, exposure to chemical irritants, and even circulatory disturbances involving the lung. 30 If such modified alveolar tissues are exposed to carcinogens, bronchiolar carcinoma could develop, and the influence of pathogenic viruses on cancers has been indicated. 50 We would like to suggest that an individual’s history of previous infections may influence whether they develop these conditions. Further studies using the heterologous virus model in mice may lead to new insights on these poorly understood human conditions.
These studies demonstrate that heterologous immunity may have a dramatic impact on the subsequent outcome of infections, but the mechanisms behind this phenomenon still need to be clarified. Previous studies with LCMV-immune mice challenged with VV or Pichinde virus suggested that memory CD8 T cells play an important role during heterologous infections. 19,25,27,51-54 LCMV-specific memory CD8 T cells can be reactivated by heterologous viruses in a cross-reactive manner 25 and we have identified three potentially cross-reactive VV peptides recognized by LCMV-specific CD8 T cells. For the other sequences of virus infections described in this article we have yet to define the specific mechanisms involved in mediating the changes observed in disease outcome, but the differences observed in T-cell cytokines are consistent with memory T cells being involved.
Other studies support the concept that heterologous immunity may be of importance in the pathogenesis of viral infections. One study showed that Sendai virus-immune mice challenged with influenza virus had 90% lower influenza virus titers in their lungs than nonimmune controls, although this was not discussed by the authors. 55 Moreover, influenza virus-infected Sendai virus-immune mice had altered pathology, with an increase in persistent alveolitis and hydroxyproline-associated collagen deposition 60 days after the influenza virus infection. In a second study, mice infected with influenza virus before vaccination with VV–respiratory syncytial virus G protein developed a Th1-type response on respiratory syncytial virus challenge instead of the expected Th2 response and cleared respiratory syncytial virus without developing severe eosinophilia. 15,56 Thus, heterologous memory T cells may affect the Th1 or Th2 bias of subsequent infections. For instance, mice immunized with Mycobacterium bovis-bacillus Calmette-Guerin, which induces a strong Th1 response, had a suppressed Th2 response and considerably reduced lung eosinophilia when exposed to an allergen. 57
These experimental models imply that heterologous immunity in humans might be a determining factor between a clinical and subclinical, or between a lethal and nonlethal infection. For instance, it is possible that cross-reactive memory T-cell responses to influenza virus variants can become pathogenic to an immune human population after the viruses develop mutations in, or reassortments of, their HA gene, which makes them resistant to antibody-mediated neutralization. 58,59 Many viruses, such as Epstein-Barr virus, and varicella-zoster virus, cause much more severe infections in teenagers and young adults than they do in younger children. 60,61 Such a difference may be because of immunopathology occurring as a consequence of the reactivation of memory cells, which may be more diverse and prominent in a more immunologically mature individual.
Acknowledgments
We thank Dr. Harriet Robinson (Vaccine Research Center and Yerkes Regional Primate Research Center, Emory University, Atlanta, Georgia) for providing us with influenza A virus (A/Pr/8/34), Y. Liu for technical assistance, and E. Szomolanyi-Tsuda and M. Cornberg for their discussions and comments on the manuscript.
Footnotes
Address reprint requests to Liisa K. Selin, Department of Pathology, University of Massachusetts Medical School, 55 Lake Avenue North, Worcester, MA 01655. E-mail: liisa.selin@umassmed.edu.
Supported by the National Institutes of Health (research grants AR-35506 and CA-34461 to R. M. W., AI-46578 to L. K. S., and supported in part by Center Grant DK32520 to I. J.).
The contents of this publication are solely the responsibility of the authors and do not represent the official view of the National Institutes of Health.
References
- 1.Ennis FA, Cruz J, Spiropoulou CF, Waite D, Peters CJ, Nichol ST, Kariwa H, Koster FT: Hantavirus pulmonary syndrome: CD8+ and CD4+ cytotoxic T lymphocytes to epitopes on Sin Nombre virus nucleocapsid protein isolated during acute illness. Virology 1997, 238:380-390 [DOI] [PubMed] [Google Scholar]
- 2.Salomon N, Perlman DC: Cytomegalovirus pneumonia. Semin Respir Infect 1999, 14:353-358 [PubMed] [Google Scholar]
- 3.Oxford JS: Influenza A pandemics of the 20th century with special reference to 1918: virology, pathology and epidemiology. Rev Med Virol 2000, 10:119-133 [DOI] [PubMed] [Google Scholar]
- 4.Ely KH, Cauley LS, Roberts AD, Brennan JW, Cookenham T, Woodland DL: Nonspecific recruitment of memory CD8+ T cells to the lung airways during respiratory virus infections. J Immunol 2003, 170:1423-1429 [DOI] [PubMed] [Google Scholar]
- 5.Walker DH, Murphy FA: Pathology and pathogenesis of arenavirus infections. Curr Top Microbiol Immunol 1987, 133:89-113 [DOI] [PubMed] [Google Scholar]
- 6.Martinez Peralta LA, Laguens M, Ponzinibbio C, Laguens RP: Infection of guinea pigs with two strains of lymphocytic choriomeningitis virus. Medicina (B Aires) 1990, 50:225-229 [PubMed] [Google Scholar]
- 7.Jordan MC: Interstitial pneumonia and subclinical infection after intranasal inoculation of murine cytomegalovirus. Infect Immun 1978, 21:275-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staczek J: Murine cytomegaloviruses. Granoff A Webster RG eds. Encyclopedia of Virology. 1999:pp 363-369 Academic Press London
- 9.Lemercier G, Ponsot JF, Mouretin G, Mavet S, Fontanges R: Morphometric study of the bronchopulmonary lymphoid system in the normal mouse and the mouse infected with influenza virus. Ann Anat Pathol (Paris) 1979, 24:301-304 [PubMed] [Google Scholar]
- 10.Mackenzie CD, Taylor PM, Askonas BA: Rapid recovery of lung histology correlates with clearance of influenza virus by specific CD8+ cytotoxic T cells. Immunology 1989, 67:375-381 [PMC free article] [PubMed] [Google Scholar]
- 11.Loosli CG, Stinson SF, Ryan DP, Hertweck MS, Hardy JD, Serebrin R: The destruction of type 2 pneumocytes by airborne influenza PR8-A virus; its effect on surfactant and lecithin content of the pneumonic lesions of mice. Chest 1975, 67:7S-14S [DOI] [PubMed] [Google Scholar]
- 12.Yap KL, Braciale TJ, Ada GL: Role of T-cell function in recovery from murine influenza infection. Cell Immunol 1979, 43:341-351 [DOI] [PubMed] [Google Scholar]
- 13.Jakab GJ, Astry CL, Warr GA: Alveolitis induced by influenza virus. Am Rev Respir Dis 1983, 128:730-739 [DOI] [PubMed] [Google Scholar]
- 14.Graham BS, Rutigliano JA, Johnson TR: Respiratory syncytial virus immunobiology and pathogenesis. Virology 2002, 297:1-7 [DOI] [PubMed] [Google Scholar]
- 15.Varga SM, Wang X, Welsh RM, Braciale TJ: Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4(+) T cells. Immunity 2001, 15:637-646 [DOI] [PubMed] [Google Scholar]
- 16.Tschernig T, Pabst R: Bronchus-associated lymphoid tissue (BALT) is not present in the normal adult lung but in different diseases. Pathobiology 2000, 68:1-8 [DOI] [PubMed] [Google Scholar]
- 17.Schlesinger C, Meyer CA, Veeraraghavan S, Koss MN: Constrictive (obliterative) bronchiolitis: diagnosis, etiology, and a critical review of the literature. Ann Diagn Pathol 1998, 2:321-334 [DOI] [PubMed] [Google Scholar]
- 18.McKane BW, Trulock EP, Patterson GA, Mohanakumar T: Lung transplantation and bronchiolitis obliterans: an evolution in understanding. Immunol Res 2001, 24:177-190 [DOI] [PubMed] [Google Scholar]
- 19.Chen HD, Fraire AE, Joris I, Brehm MA, Welsh RM, Selin LK: Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat Immunol 2001, 2:1067-1076 [DOI] [PubMed] [Google Scholar]
- 20.Fenner F, Henderson DA, Arital I, Ladnyi ID, Jezek Z: Smallpox and Its Eradication. 1988. World Health Organization Geneva
- 21.Wehrle PF, Posch J, Richter KH, Henderson DA: An airborne outbreak of smallpox in a German hospital and its significance with respect to other recent outbreaks in Europe. Bull World Health Organ 1970, 43:669-679 [PMC free article] [PubMed] [Google Scholar]
- 22.Tufariello J, Cho S, Horwitz MS: The adenovirus E3 14.7-kilodalton protein which inhibits cytolysis by tumor necrosis factor increases the virulence of vaccinia virus in a murine pneumonia model. J Virol 1994, 68:453-462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehmann-Grube F: Lymphocytic choriomeningitis virus. Braude AL Davis CE Fierer J eds. Infectious Diseases and Medical Microbiology. 1986:pp 1076 W. B. Saunders Co. Philadelphia
- 24.Marrie TJ, Saron MF: Seroprevalence of lymphocytic choriomeningitis virus in Nova Scotia. Am J Trop Med Hyg 1998, 58:47-49 [DOI] [PubMed] [Google Scholar]
- 25.Selin LK, Nahill SR, Welsh RM: Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J Exp Med 1994, 179:1933-1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welsh RM, O’Donnell CL, Reed DJ, Rother RP: Evaluation of the Galalpha1–3Gal epitope as a host modification factor eliciting natural humoral immunity to enveloped viruses. J Virol 1998, 72:4650-4656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selin LK, Varga SM, Wong IC, Welsh RM: Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J Exp Med 1998, 188:1705-1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pabst R: Is BALT a major component of the human lung immune system? Immunol Today 1992, 13:119-122 [DOI] [PubMed] [Google Scholar]
- 29.Grefte A, van der Giessen M, van Son W, The TH: Circulating cytomegalovirus (CMV)-infected endothelial cells in patients with an active CMV infection. J Infect Dis 1993, 167:270-277 [DOI] [PubMed] [Google Scholar]
- 30.Nettesheim P, Szakal AK: Morphogenesis of alveolar bronchiolization. Lab Invest 1972, 26:210-219 [PubMed] [Google Scholar]
- 31.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M: Functional role of type I and type II interferons in antiviral defense. Science 1994, 264:1918-1921 [DOI] [PubMed] [Google Scholar]
- 32.Janeway CA, Travers P, Walport M, Shlomchik M: Immunobiology. 2001. Garland Publishing New York
- 33.Mulligan MS, Ward PA: Immune complex-induced lung and dermal vascular injury. Differing requirements for tumor necrosis factor-alpha and IL-1. J Immunol 1992, 149:331-339 [PubMed] [Google Scholar]
- 34.Littaua R, Kurane I, Ennis FA: Human IgG Fc receptor II mediates antibody-dependent enhancement of dengue virus infection. J Immunol 1990, 144:3183-3186 [PubMed] [Google Scholar]
- 35.Jolly PE, Weiss HL: Neutralization and enhancement of HIV-1 infection by sera from HIV-1 infected individuals who progress to disease at different rates. Virology 2000, 273:52-59 [DOI] [PubMed] [Google Scholar]
- 36.Battegay M, Moskophidis D, Waldner H, Brundler MA, Fung-Leung WP, Mak TW, Hengartner H, Zinkernagel RM: Impairment and delay of neutralizing antiviral antibody responses by virus-specific cytotoxic T cells. J Immunol 1993, 151:5408-5415 [PubMed] [Google Scholar]
- 37.Haagmans BL, Stals FS, van der Meide PH, Bruggeman CA, Horzinek MC, Schijns VE: Tumor necrosis factor alpha promotes replication and pathogenicity of rat cytomegalovirus. J Virol 1994, 68:2297-2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mutimer DJ, Shaw J, O’Donnell K, Elias E: Enhanced (cytomegalovirus) viral replication after transplantation for fulminant hepatic failure. Liver Transpl Surg 1997, 3:506-512 [DOI] [PubMed] [Google Scholar]
- 39.Yerkovich ST, Olver SD, Lenzo JC, Peacock CD, Price P: The roles of tumour necrosis factor-alpha, interleukin-1 and interleukin-12 in murine cytomegalovirus infection. Immunology 1997, 91:45-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haagmans BL, van der Meide PH, Stals FS, van den Eertwegh AJ, Claassen E, Bruggeman CA, Horzinek MC, Schijns VE: Suppression of rat cytomegalovirus replication by antibodies against gamma interferon. J Virol 1994, 68:2305-2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murayama T, Mukaida N, Khabar KS, Matsushima K: Potential involvement of IL-8 in the pathogenesis of human cytomegalovirus infection. J Leukoc Biol 1998, 64:62-67 [DOI] [PubMed] [Google Scholar]
- 42.Hatch WC, Freedman AR, Boldt-Houle DM, Groopman JE, Terwilliger EF: Differential effects of interleukin-13 on cytomegalovirus and human immunodeficiency virus infection in human alveolar macrophages. Blood 1997, 89:3443-3450 [PubMed] [Google Scholar]
- 43.Christman JW, Lancaster LH, Blackwell TS: Nuclear factor kappa B: a pivotal role in the systemic inflammatory response syndrome and new target for therapy. Intensive Care Med 1998, 24:1131-1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baud V, Karin M: Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol 2001, 11:372-377 [DOI] [PubMed] [Google Scholar]
- 45.Sambucetti LC, Cherrington JM, Wilkinson GW, Mocarski ES: NF-kappa B activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J 1989, 8:4251-4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pottathil R, Pottathil IR, Cheung KS, Lang DJ: Enhanced replication of murine cytomegalovirus in murine leukemic lymphocytes. Cancer Res 1986, 46:124-126 [PubMed] [Google Scholar]
- 47.Shanley JD, Pesanti EL: Murine peritoneal macrophages support murine cytomegalovirus replication. Infect Immun 1983, 41:1352-1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matloubian M, Kolhekar SR, Somasundaram T, Ahmed R: Molecular determinants of macrophage tropism and viral persistence: importance of single amino acid changes in the polymerase and glyco-protein of lymphocytic choriomeningitis virus. J Virol 1993, 67:7340-7349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belperio JA, Keane MP, Burdick MD, Lynch JP, III, Xue YY, Li K, Ross DJ, Strieter RM: Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J Immunol 2002, 169:1037-1049 [DOI] [PubMed] [Google Scholar]
- 50.Kotin P: The influence of pathogenic viruses on cancers induced by inhalation. Proc Can Cancer Conf 1966, 6:475-498 [PubMed] [Google Scholar]
- 51.Brehm MA, Pinto AK, Daniels KA, Schneck JP, Welsh RM, Selin LK: T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat Immunol 2002, 3:627-634 [DOI] [PubMed] [Google Scholar]
- 52.Kim SK, Brehm MA, Welsh RM, Selin LK: Dynamics of memory T cell proliferation under conditions of heterologous immunity and bystander stimulation. J Immunol 2002, 169:90-98 [DOI] [PubMed] [Google Scholar]
- 53.Selin LK, Lin MY, Kraemer KA, Pardoll DM, Schneck JP, Varga SM, Santolucito PA, Pinto AK, Welsh RM: Attrition of T cell memory: selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity 1999, 11:733-742 [DOI] [PubMed] [Google Scholar]
- 54.Welsh RM, Selin LK: No one is naive: the significance of heterologous T-cell immunity. Nat Rev Immunol 2002, 2:417-426 [DOI] [PubMed] [Google Scholar]
- 55.Jakab GJ: Sequential virus infections, bacterial superinfections, and fibrogenesis. Am Rev Respir Dis 1990, 142:374-379 [DOI] [PubMed] [Google Scholar]
- 56.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE: An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol 1969, 89:405-421 [DOI] [PubMed] [Google Scholar]
- 57.Erb KJ, Holloway JW, Sobeck A, Moll H, Le Gros G: Infection of mice with Mycobacterium bovis-bacillus Calmette-Guerin (BCG) suppresses allergen-induced airway eosinophilia. J Exp Med 1998, 187:561-569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jameson J, Cruz J, Ennis FA: Human cytotoxic T-lymphocyte repertoire to influenza A viruses. J Virol 1998, 72:8682-8689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doherty PC, Zinkernagel RM: T-cell-mediated immunopathology in viral infections. Transplant Rev 1974, 19:89-120 [DOI] [PubMed] [Google Scholar]
- 60.Weinstein L, Meade RH: Respiratory manifestations of chickenpox. Arch Intern Med 1956, 98:91-99 [DOI] [PubMed] [Google Scholar]
- 61.Rickinson AB, Kieff E: Epstein-Barr virus. Fields BN Knipe DM Howley PM Chanock RM Melnick JL Monath TP Roizman B Straus SS eds. Virology. 1996:pp 2397-2446 Lippincott-Raven Publishers Philadelphia