Abstract
Bronchiolitis obliterans organizing pneumonia (BOOP) is a clinical syndrome characterized by perivascular/peribronchiolar leukocyte infiltration leading to the development of intraalveolar fibrosis. We have developed an animal model of BOOP where CBA/J mice infected with 1 × 106 plaque-forming units (PFU) reovirus 1/L develop follicular bronchiolitis and intraalveolar fibrosis similar to human BOOP. In this report, we demonstrate a role for T cells in the development of intraluminal fibrosis associated with BOOP. Corticosteroid treatment of reovirus 1/L-infected mice both inhibited the development of fibrotic lesions when administered early in the time-course and promoted the resolution of fibrotic lesions when corticosteroid administration was delayed. Further, the depletion of either CD4+ or CD8+ T cells before reovirus 1/L infection also inhibited fibrotic lesion development. Both corticosteroid treatment and depletion of CD4+ or CD8+ T cells also resulted in decreased expression of the proinflammatory and profibrotic cytokines, interferon (IFN)-γ and monocyte chemoattractant protein-1 (MCP-1). Further, treatment of mice with a neutralizing monoclonal antibody to IFN-γ also significantly inhibited the development of fibrosis. Taken together, these results suggest a significant role for T cells in the development of reovirus 1/L-induced BOOP fibrotic lesions in CBA/J mice and suggests that TH1-derived cytokines, especially IFN-γ, may play a key role in fibrotic lesion development.
Bronchiolitis obliterans organizing pneumonia (BOOP), first described in 1985, is a pattern of injury characterized histologically as “patchy plugs of fibrous tissue (Masson bodies) filling bronchiolar lumens (bronchiolitis obliterans) and alveolar ducts and spaces (organizing pneumonis).” 1-3 This patchy fibrosis may begin as focal lesions within the alveoli and the terminal bronchioles of the lung and progress bilaterally over time. 1 Other histological features include clusters of mononuclear inflammatory cells, chronic inflammation in the walls of the surrounding alveoli with reactive type II cells, increased numbers of foamy macrophages in the alveoli, and preserved lung architecture. 2,4 The development of BOOP is often of unknown etiology (idiopathic BOOP) but BOOP has also been associated as a consequence of lung injury due to environmental toxins, bacterial infections, viral infections, and lung or bone marrow transplantation. 3,5 BOOP is responsive to corticosteroids and treatment with prednisone continues to be the primary treatment for patients with symptomatic and progressive disease. 2-4
Since infiltrating lymphocytes are associated with the initiation of BOOP lesions, 6,7 it is possible that these cells play an active role in the progression of inflammatory foci into lesions that are progressively dominated by fibroblasts. In patients with BOOP, there is an increase of activated bronchoalveolar lavage (BAL) lymphocytes with up to 80% to 95% of this cellular infiltrate being comprised of cytotoxic/suppressor CD8+ T cells. 6-9 These cells may be involved in the inflammation and subsequent fibrosis occurring in BOOP patients. 2,5,7,10-12 Several studies have shown that the infiltration of T lymphocytes may be important in the development of other forms of pulmonary fibrosis, although the data from both animal models and patients has been equivocal. 13-18 Although these existing models of experimental pulmonary fibrosis have been useful for histopathological and functional investigations of other types of fibrotic events in the lung, the process of fibrotic lesion development in these models may be distinct from BOOP lesion development. Thus, differences in the phenotype of the inflammatory cell infiltrate, expression of soluble mediators, and response to various treatments may be different and, therefore, may fail to accurately reflect the intraluminal and fibroblastic nature of the bronchoalveolar obliteration observed in BOOP lesions. 19-21
We have described a spectrum of inflammatory lung diseases after respiratory infection with reovirus serotype 1, strain Lang (reovirus 1/L), which is dependent on the strain of mice used. 22-27 In this spectrum CBA/J mice infected with 1 × 106 PFU reovirus 1/L develop a histologically severe inflammation characterized by an infiltration of lymphocytes organized adjacent to the pulmonary vasculature of the lung. 23 This pattern of mononuclear cell organization without the involvement of intraluminal fibrosis results in lesions histopathologically consistent with the non-fibrotic human syndrome termed follicular bronchiolitis (FB). However, accompanying the development of FB in CBA/J mice are the presence of foamy macrophages and the elicitation of a non-specific fibrotic response of the lung characteristic of BOOP fibrotic lesions. 23 To investigate the role of the inflammatory cell infiltrate, especially T cells, in the development of reovirus 1/L-induced fibrotic lesions, the effect of either corticosteroid treatment or the depletion of CD4+ or CD8+ T cells before reovirus 1/L infection was determined. Our results indicate that corticosteroid treatment of reovirus 1L-infected mice both inhibited the development of fibrotic lesions when administered early in the time-course and promoted the resolution of fibrotic lesions when corticosteroid administration was delayed. In addition, the depletion of either CD4+ or CD8+ T cells before reovirus 1/L infection also inhibited fibrotic lesion development. Both corticosteroid treatment and depletion of CD4+ or CD8+ T cells also resulted in decreased expression of the proinflammatory and profibrotic cytokines, interferon (IFN)-γ and monocyte chemoattractant protein-1 (MCP-1). Finally, treatment of mice with a neutralizing monoclonal antibody to IFN-γ also significantly inhibited the development of fibrosis. Taken together, the results suggest a significant role for T cells in the development of reovirus 1/L-induced BOOP fibrotic lesions in CBA/J mice and that TH1 derived cytokines, especially IFN-γ, may play a key role in fibrotic lesion development.
Materials and Methods
Animals
Four- to 5-week-old female CBA/J mice (The Jackson Laboratory, Bar Harbor, ME) were maintained in microisolator cages under specific pathogen-free conditions in a BL-2 facility. Cages were housed in an HEPA-filtered animal isolator clean room (Nuaire Inc., Plymouth, MN). All animal manipulations were performed in class II biological safety cabinets. Virally primed mice were kept physically isolated from all other mice.
Virus
Reovirus 1/L was originally obtained from Dr. W. Joklik (Duke University School of Medicine, Durham, NC). Third-passage gradient-purified stocks were obtained by re-cloning and amplifying parental stocks on L-929 fibroblast cells [American Type Culture Collection (ATCC), Rockville, MD] as previously described. 23 Following the purification of new stocks, infectious viral titers were obtained by limiting dilution on L-929 monolayers. 23
Inoculation Protocol
Animals were lightly anesthetized with an i.p. injection of 0.08 ml of 20% ketamine (Vetalar 100 mg/ml; Fort Dodge Laboratories, Inc., Fort Dodge, IA) and 2% PromAce (acepromazine maleate 10 mg/ml; Ayerst Laboratories, New York, NY) before immunization. Animals were infected by the intranasal (i.n.) application of 1 × 106 PFU of reovirus 1/L in 30 μl (15 μl in each nostril) in sterile injectable grade 0.9% NaCl (Baxter Healthcare Corp., Deerfield, IL). Control animals were inoculated with 30 μl (15 μl in each nostril) of sterile injectable grade 0.9% NaCl. After the indicated timepoints, animals were sacrificed with an i.p. injection of 0.2 ml sodium Nembutal (50 mg/ml; Abbott Laboratories, North Chicago, IL).
Methylprednisolone Administration
As an initial dosing regimen either 10 mg/kg or 20 mg/kg methylprednisolone (MPS) (∼0.1 to 0.2 mg/mouse) (Sigma Chemicals, St. Louis, MO) dissolved in PBS was administered i.p. to mice beginning on either days 0, 5, 10, or 14 post-reovirus 1/L infection and given daily until the completion of the time-course. Since these initial studies indicated that treatment with MPS (20 mg/kg) either beginning at day 0 or day 5 post infection or administration of MPS (10 mg/kg) before day 5 post reovirus 1/L infection (beginning at day 0) resulted in an increased mortality rate as compared to that observed in untreated, reovirus 1/L-infected mice (Table 1) ▶ , all additional studies were performed using MPS at a concentration of 10 mg/kg beginning 5 days post-reovirus 1/L infection unless otherwise noted.
Table 1.
Modulation of Reovirus 1/L-Induced BOOP Fibrotic Lesions in CBA/J Mice
| Day* | Dose† | % Mortality‡ | FB§ | Fibrosis¶ |
|---|---|---|---|---|
| Untreated | 0.0 mg/kg | 20% | +++ | ++++ |
| Day 0∥ | 20 mg/kg | 60% | +++ | ND |
| Day 0∥ | 10 mg/kg | 40% | +++ | ND |
| Day 5∥ | 20 mg/kg | 40% | ++ | + |
| Day 5** | 10 mg/kg | 20% | ++ | + |
| Day 10†† | 10 mg/kg | 20% | +++ | ++ |
| Day 14†† | 10 mg/kg | 20% | +++ | ++ |
| Untreated‡‡ CD4− | 0.0 mg/kg | 0% | +§§ | + |
| Untreated‡‡ CD8− | 0.0 mg/kg | 0% | ++§§ | + |
*Day treatment with methylprednilosone was begun postinfection with 1 × 106 PFU reovirus 1/L.
†Dose of methylprednilosone administered daily i.p. in 100 μl PBS.
‡Percent mortality on day 14 postreovirus 1/L infection.
§Follicular bronchiolitis severity on day 14 postreovirus 1/L infection.
¶Fibrotic lesion severity on day 21 postreovirus 1/L infection.
∥Experiment performed once with two mice per timepoint.
**Experiment performed five times with two mice per timepoint.
††Experiment performed twice with two to four mice per timepoint.
‡‡Mice were depleted of CD4+ or CD8+ cells prior to reovirus 1/L infection as described in Materials and Methods.
§§Follicular bronchiolitis severity on day 21 post-reovirus 1/L infection.
ND, not determined.
CD4 and CD8 Depletion
Adult CBA/J mice were treated i.p. with either 0.5 mg of purified GK1.5 monoclonal antibody (mAb) 28 for depletion of CD4+ lymphocytes or 0.25 mg of purified 53–6.72 mAb 29 for depletion of CD8+ lymphocytes for three consecutive days. Depleted mice were then infected i.n. with 1 × 106 PFU of reovirus 1/L in 30 μl (15 μl in each nostril) in sterile injectable grade 0.9% NaCl. Control, depleted animals were inoculated with 30 μl (15 μl in each nostril) of sterile injectable grade 0.9% NaCl. The depleted state was maintained by treating with either 0.5 mg purified GK1.5 or 0.25 mg of purified 53–6.72 mAb every 6 days. Depletion of the appropriate subset of T cells was verified by flow cytometry of cells obtained from the lymph node and spleen before infection with reovirus 1/L on day 0 and on days 7 and 14 postinfection. Depleted mice were evaluated for the development of BOOP fibrotic lesions at day 21 post-reovirus 1/L infection.
In Vivo Interferon-γ Depletion
An anti-IFN-γ mAb (R4–6A2, rat IgG1, ATCC HB170) was obtained from ATCC 30-32 and ascites fluid was generated for in vivo use (Strategic Biosolutions, Newark, NJ). Reovirus 1/L-infected (106 PFU BOOP) CBA/J mice were treated i.p. every 3 days beginning on day 3 postinfection with either 100 μg anti-IFN-γ antibody in PBS or 100 μg normal rat IgG (Sigma) in PBS. Mice were evaluated on days 14 and 21 postinfection for the development of fibrotic lesions by hematoxylin and eosin (H&E) and Mason’s trichrome stain.
Bronchoalveolar Lavage (BAL)
BAL was performed in situ by injecting and withdrawing a 0.5 ml aliquot of Hank’s balanced salt solution (HBSS) twice through an intubation needle (21 gauge). A total of 1.5 ml of HBSS was used. BAL fluid was frozen at −70°C until use. Cells collected by BAL were washed three times with HBSS containing 5% fetal calf serum (FCS) and 0.05% azide, and resuspended at 1 × 106 cells/ml.
Histology
Lungs were inflated in situ with 10% neutral buffered formalin (0.5 mls) (Richard-Allan Scientific, Kalamazoo, MI) by intratracheal (i.t.) intubation, removed, and suspended in an additional 10% neutral buffered formalin overnight before being embedded in paraffin. H&E stain and Mason’s trichrome stain, which was used to visualize collagen deposition, were performed on 4-μm sections. Inflammatory infiltration with the development of FB, which is defined as a mononuclear cell infiltrate that condenses into prominent peribronchiolar lymphoid accumulations, was blindly evaluated. FB was scored on a scale of 0 to 3: 0, normal; 1, mild (< 4 follicles per lobe); 2, moderate (between 5 and 8 follicles per lobe); 3, severe (> 8 follicles per lobe). Fibrosis was scored on a scale of 0 to 4: 0, normal; 1, mild; 2, moderate; 3, severe; 4, very severe.
Hydroxyproline (HP) Assay
The extent of pulmonary fibrosis was also determined by estimating total lung collagen as reflected by the measurement of the HP content of the lung as previously described. 25,26,33 Mice were sacrificed at various intervals after infection with reovirus 1/L and the lungs were removed, lyophilized, and weighed. Differences between groups were examined for statistical significance using two-tailed Student’s t-test. A P value less than 0.05 was considered significant.
Antibodies
The following monoclonal antibodies were used in this study: Cy-Chrome-conjugated rat anti-mouse CD45 (30-F11, leukocyte common antigen, Ly-5); fluorescein isothiocyanate (FITC)-conjugated hamster anti-mouse CD3 (145–2C11, CD3 ε chain); FITC-conjugated rat anti-mouse CD8a (53–6.7, Ly-2); R-phycoerythrin (PE)-conjugated rat anti-mouse CD4 (GK1.5, L3T4) (Caltag, Burlingame, CA); R-PE-conjugated rat anti-mouse Pan-NK cells (DX5); FITC-conjugated rat anti-mouse CD45R/B220 (RA3–6B2); R-PE-conjugated rat anti-mouse CD11b (M1/70, integrinαm chain, Mac-1 α chain); and FITC-conjugated rat anti-mouse Ly6G (RB6–8C5, Gr-1, neutrophils) (Pharmingen, San Diego, CA); hamster anti-rat CD3 (ε-chain, 48–2B) (Santa Cruz Biotechnology, Santa Cruz, CA); rat anti-mouse CD11b (Mac-1 α chain) (Serotec, Westbury, NY); and rat anti-mouse neutrophil (MCA 771F) (Serotec).
Flow Cytometric Analysis
Cells collected by BAL were washed three times with HBSS containing 5% FCS and 0.05% azide, and resuspended at 1 × 106 cells/ml. Cells were stained for cell surface marker expression as previously described except that all cells were also stained with anti-CD45 (30-F11), leukocyte common antigen Ly-5, and only anti-CD45-positive cells were acquired for analysis. 24,26 Isotype-matched controls were run for each sample (Caltag and Pharmingen). The total number of PMNs was obtained by adding the anti-Gr-1 (Ly6G) single-positive cells and the anti-Gr-1/anti-Mac-1 (integrinαm chain) double-positive cells. The total number of B cells was obtained by adding the anti-B220 (CD45R) single-positive cells plus the anti-B220/anti-Mac-1 double-positive cells. The total number of macrophages was obtained by enumerating those cells stained only with anti-Mac-1. Flow cytometric analysis was performed using a dual-laser FACS Caliber flow cytometer and the Cell Quest acquisition and analysis software program (BD Biosciences, San Jose, CA).
RNase Protection Assay
Total cellular RNA was isolated from whole lungs by guanidium denaturation using TRI-reagent (Molecular Research Center, Cincinnati, OH). Riboquant multiprobe ribonuclease protection assay (RPA) mouse template sets mCK-1b, mCK-2b, mCK-3b and mCK-5 were purchased from Pharmingen. Template set mCK-1b contained probes for Interleukin (IL)-2 - 5, -9, -10, -13, -15, and IFN-γ. Template set mCK-2b contained probes for IL-1α, -1β, -1Ra, -6, -10, -12, IFN-γ inducing factor (IGIF), IFN-γ, and migration inhibitory factor (MIF). Template set mCK-3b contained probes for tumor necrosis factor (TNF)-β, lymphotoxin (LT)-β, TNF-α, IL-6, IFN-γ, IFN-β, transforming growth factor (TGF)-β1, TGF-β2, TGF-β3, and MIF. Template set mCK-5 contained probes for the chemokines, lymphotactin (Ltn), regulated on activation normal T cells expressed and secreted (RANTES), eotaxin, macrophage inflammatory protein (MIP)-1β, MIP-1α, MIP-2, interferon inducible protein (IP)-10, monocyte chemoattractant protein (MCP)-1, and T-cell activation factor (TCA)-3. All template sets also contained probes for the control genes GAPDH and L32. RPA analysis was performed as previously described 26 using radiolabeled RNA transcripts from the multiprobe sets generated by in vitro transcription (Pharmingen) following the manufacturer’s instructions. Gels were dried and exposed to Fuji RX film at −70°C with DuPont Cronex Quanta III intensifying screens for 1 to 5 days. Band intensities on scanned RPA gels were analyzed using the public domain NIH Image program developed at the U.S. National Institutes of Health. Specific cytokine or chemokine band intensities were normalized to L32 controls to account for differences in total RNA loading in each sample. The mean ± SD of the densitometric measurements from two independent experiments with two mice per time point (four independent autoradiographs) over the indicated timepoints were determined. Differences in expression level between uninfected controls and reovirus 1/L-infected groups were examined for statistical significance using a two-tailed Student’s t-test. A P value less than 0.05 was considered significant.
ELISA
100 μl of BAL fluid was analyzed for mouse IFN-γ and MCP-1 in duplicate using the R&D Systems Quantikine M immunoassay systems (R&D Systems, Minneapolis, MN). The results were expressed as the mean ± SD. Differences between groups were examined for statistical significance using a two-tailed Student’s t-test. A P value less than 0.05 was considered significant.
Results
Methylprednisolone Modulates the Development of Fibrosis After i.n. Infection with Reovirus 1/L
To determine the efficacy of treatment with MPS on the development of reovirus 1/L-induced BOOP fibrotic lesions, CBA/J mice were infected i.n with 1 × 106 PFU reovirus 1/L and treated with various doses of MPS daily at various timepoints post-reovirus 1/L infection. Mice inoculated with saline and treated with MPS beginning either on day 0, 5, 10, or 14 postinoculation did not develop any abnormalities and 100% of treated animals survived (data not shown). In reovirus 1/L-infected mice, when treatment with MPS was begun simultaneously to infection with reovirus 1/L at day 0, a three-fold increase in mortality of reovirus 1/L-infected animals (60% versus 20%) was observed when animals were treated with 20 mg/kg MPS daily (Table 1) ▶ . Even when the dose of MPS was reduced to 10 mg/kg daily, a two-fold increase in mortality of reovirus 1/L-infected animals (40% versus 20%) was observed as compared to untreated, reovirus 1/L-infected animals (Table 1) ▶ . Due to the high degree of mortality associated with these treatment regimens, we were unable to evaluate fibrotic lesion development at day 21 postinfection (Table 1) ▶ . However, FB formation, which is characterized as a mononuclear cell infiltrate that condenses into peribronchiolar lymphoid accumulations, was prominent.
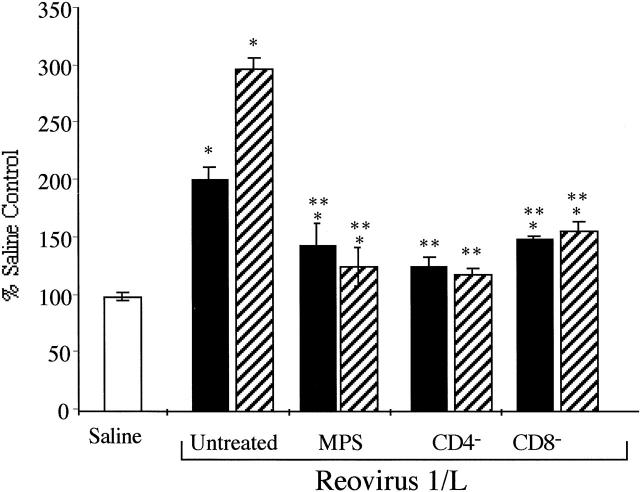
To prevent the increase in mortality of MPS-treated, reovirus 1/L-infected mice that was observed, and to determine whether MPS treatment effects fibrotic lesion development, the administration of MPS was delayed until day 5 postinfection. Mice treated with 20 mg/kg MPS daily still exhibited an increase in mortality (40% versus 20%) (Table 1) ▶ . However, mice treated with 10 mg/kg MPS daily exhibited a similar mortality rate of 20% as compared to untreated, reovirus 1/L-infected mice (Table 1) ▶ . As determined by H&E (Figure 1, A and C) ▶ and Mason’s trichrome staining (Figure 1, B and D) ▶ , a significant inhibition of fibrotic lesion development was observed in MPS-treated, reovirus 1/L-infected mice (Figure 1, C and D) ▶ , as compared to untreated, reovirus 1/L-infected mice (Figure 1, A and B) ▶ . However, both FB and foamy macrophages are still prominently observed in the lungs of MPS-treated reovirus 1/L-infected mice at days 14 and 21 postinfection (Figure 1, C and D) ▶ . In addition, to support the histological evaluation, total lung collagen was estimated by the biochemical measurement of HP content of the lungs on days 14 and 21 from reovirus 1/L-infected mice to evaluate the extent of pulmonary fibrosis. Values were expressed as the percentage of that obtained in control mice. As shown in Figure 2 ▶ , a two-fold (day 14) to three-fold (day 21) increase in HP accumulation in the lungs was observed postinfection with reovirus 1/L as compared to saline, inoculated controls. In contrast, less than a 1.5-fold increase in HP content was observed in MPS-treated, reovirus 1/L-infected mice as compared to untreated, reovirus 1/L-infected mice on either day 14 or day 21 post-reovirus 1/L infection (Figure 2) ▶ . The significant decrease in HP content in the lungs of MPS treated, reovirus 1/L-infected mice is consistent with the observation of limited areas of fibrotic polyps observed in Figure 1, C and D ▶ . Therefore, our results demonstrate both histologically and biochemically that the administration of MPS beginning on day 5 postinfection to mice receiving 1 × 106 PFU reovirus 1/L was effective at inhibiting fibrosis associated with reovirus 1/L-induced BOOP. While fibrotic lesion development was significantly inhibited by MPS treatment beginning on day 5 postinfection, prominent FB lesions (condensing lymphoid follicles) were still present (Table 1) ▶ .
Figure 1.
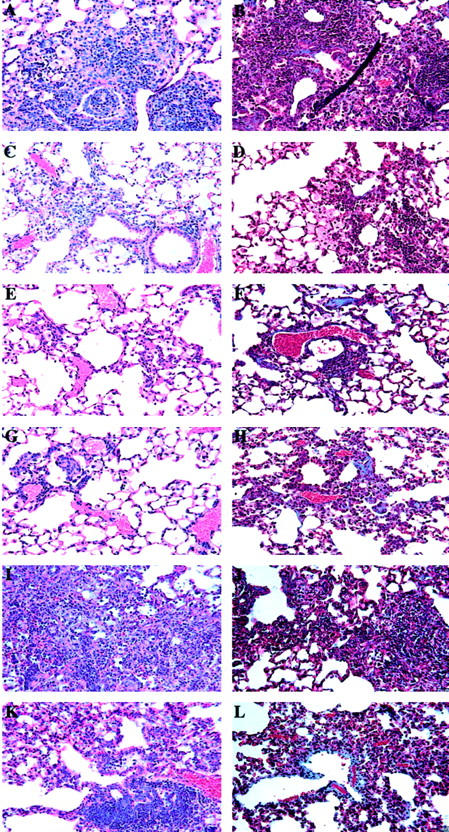
Inhibition of fibrosis associated with BOOP after corticosteroid treatment or after depletion of CD4+ or CD8+ T cells. Normal (A, B, I, J), MPS-treated (C, D, K, L), CD4-depleted (E, F), and CD8-depleted (G, H) CBA/J mice were i.n. infected with reovirus 1/L and paraffin-embedded lung sections were stained with H&E (A, C, E, G, I, K) or Mason’s trichrome (B, D, F, H, J, L) for determination of collagen deposition on days 14 (J) or 21 (B, D, F, H, L) post-reovirus 1/L infection. With Mason’s trichrome the nuclei stain a dark blue/purple, muscle stains red, and collagen stains blue. Reovirus 1/L-infected CBA/J lung on day 21 postinfection (A and B); Reovirus 1/L-infected CBA/J mice treated daily with MPS (beginning on day 5 postinfection) on day 21 postinfection (C and D); CD4-depleted, reovirus 1/L-infected CBA/J lung (E and F); CD8-depleted, reovirus 1/L-infected CBA/J lung (G and H); Reovirus 1/L-infected CBA/J lung on day 14 postinfection (I and J); Reovirus 1/L-infected CBA/J mice treated daily with MPS (beginning on day 14 postinfection) on day 21 postinfection (K and L). Representative of four independent experiments containing 2 mice per timepoint (A to D, I, J). Representative of one independent experiment containing 2 mice per timepoint (E to H). Representative of two independent experiments containing 4 mice per timepoint (K and L). Objective magnification, ×20.
Figure 2.
Modulation of total hydroxyproline content in the lungs of reovirus 1/L-infected mice. CBA/J mice were i.n. infected with 1 × 106 PFU reovirus 1/L and HP content as a measurement of total collagen content in the lungs was determined. Results are expressed as a percentage of HP as compared to saline-inoculated control mice (open bar) on either day 14 (solid bar) or day 21 (striped bar) post-reovirus 1/L infection. Each data-point represents the mean ± SD of four mice. *P < 0.05 as compared to saline-inoculated control mice. **P < 0.05 as compared to reovirus 1/L-infected mice.
To evaluate the effect of MPS administration on the resolution of BOOP lesions, mice were i.n. infected with 1 × 106 PFU of reovirus 1/L, and MPS treatment was begun 10 or 14 days postinfection when fibrotic lesion development had already begun. Treatment with MPS at 10 mg/kg daily beginning on days 10 or 14 post-reovirus 1/L infection also demonstrated 20% mortality (Table 1) ▶ . At day 21 postinfection, although fibrotic lesions are still observed in mice treated with MPS beginning on day 14 postinfection (Figure 1, K and L) ▶ , these areas of fibrosis are smaller, more discrete, and less severe then the fibrotic lesions observed in untreated, reovirus 1/L-infected mice on day 14 (Figure 1, I and J) ▶ or day 21 (Table 1 ▶ ; Figure 1, A and B ▶ ). In both untreated and MPS-treated, reovirus 1/L-infected mice, both fibrotic lesions and FB resolved by day 28 to 35 postinfection (data not shown) 23 .
Depletion of Either CD4+ or CD8+ T Cells Before Reovirus 1/L Infection Inhibits the Development of Fibrotic Lesions
To determine whether the infiltration of T cells plays a significant role in reovirus 1/L-induced BOOP fibrotic lesions, CBA/J mice were depleted of either CD4+ or CD8+ T cells before infection with 1 × 106 PFU reovirus 1/L. Verification of depletion of either CD4+ or CD8+ T cells was determined by flow cytometry before infection with reovirus 1/L and maintenance of the depleted state was verified at both days 7 (data not shown) and 14 postinfection (Figure 4E) ▶ . Both CD4- and CD8-depleted mice inoculated with saline did not develop any abnormalities and 100% of the animals survived through day 21 (data not shown). In addition, no mortality was observed in either CD4- or CD8-depleted reovirus 1/L-infected mice (Table 1) ▶ . As determine by H&E (Figure 1, E and G) ▶ and Mason’s trichrome (Figure 1, F and H) ▶ staining, a significant inhibition of fibrotic lesion development on day 21 was observed in both reovirus 1/L-infected CD4- (Figure 1, E and F) ▶ and CD8-depleted (Figure 1, G and H) ▶ mice, as compared to untreated, reovirus 1/L-infected mice (Figure 1, A and B) ▶ . However, both FB and foamy macrophages are still observed in the lungs of CD4- and CD8-depleted reovirus 1/L-infected mice at day 21 postinfection (Figure 1, E and G) ▶ , although these lesions were not prominent. In addition, the measurement of total lung collagen on either day 14 or 21 from CD4- or CD8-depleted reovirus 1/L-infected mice was significantly lower than that observed in reovirus 1/L-infected mice (Figure 2) ▶ . This significant decrease in HP content in the lungs of CD4- or CD8-depleted reovirus 1/L-infected mice is consistent with the observation of limited areas of fibrotic polyps observed in Figure 1, E and G ▶ .
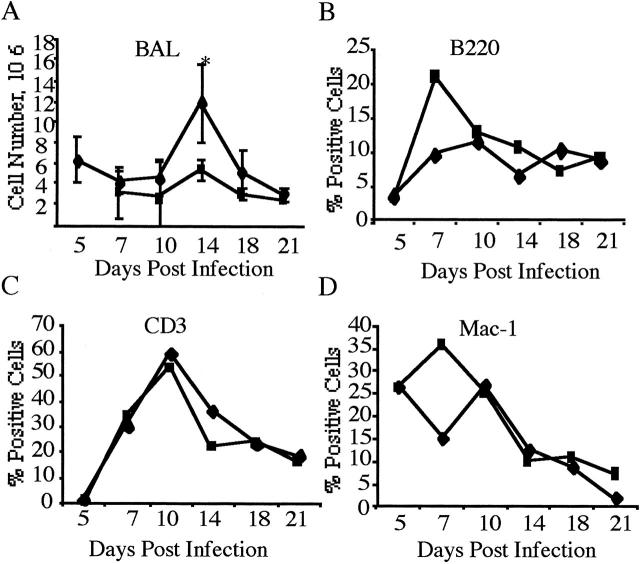
Figure 4.
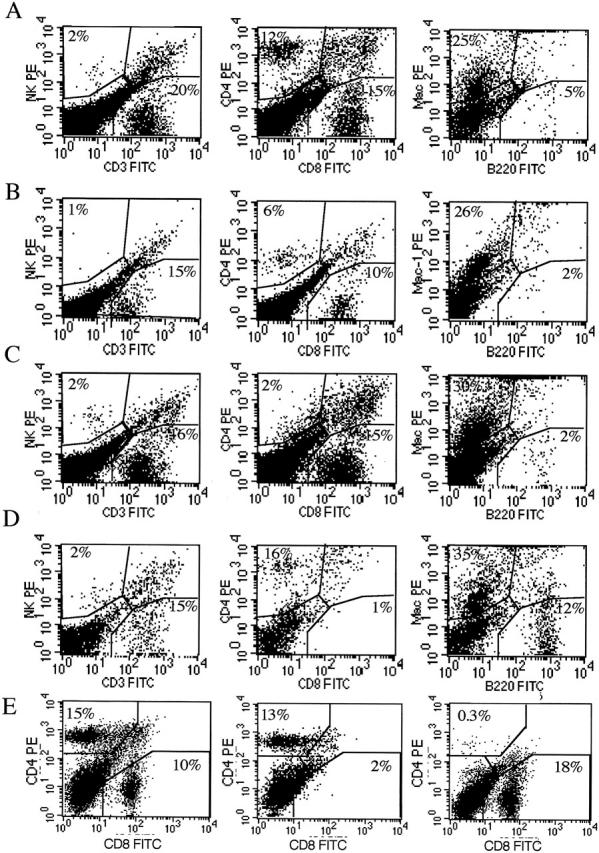
Phenotype of cells in the BAL fluid of reovirus 1-L-infected CBA/J mice. CBA/J mice were i.n. infected with 1 × 106 PFU reovirus 1/L and two-color flow cytometric analysis of the infiltrating cell populations in the BAL were analyzed on day 14 postinfection. Reovirus 1/L-infected CBA/J mice (A); MPS-treated, reovirus 1/L-infected CBA/J mice (B); CD4-depleted, reovirus 1/L-infected CBA/J mice (C); CD8-depleted, reovirus 1/L-infected CBA/J mice (D); spleen cells from normal, CD8- or CD4-depleted reovirus 1/L-infected animals (E). Representative of two independent experiments containing pooled cells from three mice per timepoint (A and B). Representative of one independent experiment containing two mice per time point (C to E).
The Administration of MPS or Depletion of Either CD4+ or CD8+ T Cells Modifies the Inflammatory Response to Reovirus 1/L
To determine the percentage over time of different leukocyte subsets present in the inflammatory infiltrate after MPS treatment beginning 5 days postinfection with 1 × 106 PFU reovirus 1/L cells obtained from the BAL were analyzed by flow cytometry using monoclonal antibodies specific for T-cell subsets (CD3, CD4, CD8), B cells (B220), macrophages (CD11b), and NK cells (pan-NK). BAL cells were stained with the leukocyte common antigen (Ly-5) anti-CD45 mAb and only anti-CD45-positive cells were acquired for analysis. Isotype-matched controls were run for each sample (data not shown).
Over the course of the infection, the total cell number recovered from the BAL fluid in MPS-treated mice was significantly decreased only on day 14 post infection (Figure 3A) ▶ (12 × 105 in untreated reovirus 1/L-infected mice versus 6 × 105 in MPS-treated, reovirus-infected mice). In addition, over the time course of 21 days in either MPS-treated or untreated mice the predominant cell types found in the BAL were T cells, B cells, and macrophages (Figure 3, B to D) ▶ . However, at day 14, MPS-treated reovirus 1/L infected mice demonstrated a decrease in both CD4 (6% versus 12%) and CD8 (10% versus 15%) populations. No significant infiltration of PMNs was observed in either MPS-treated or -untreated reovirus 1/L-infected animals (data not shown). Similar to the cellular response observed from cells obtained by BAL, few differences in the percentages of T or B lymphocytes and macrophages were observed in the interstitial areas of untreated reovirus 1/L-infected mice versus MPS-treated, reovirus 1/L-infected mice (data not shown).
Figure 3.
Modulation of cellular infiltration and phenotype post reovirus 1/L infection. Untreated (♦) or MPS-treated (▪) CBA/J mice were infected i.n. with 1 × 106 PFU reovirus 1/L for a time-course of 21 days. Cells were harvested and counted from the BAL fluid (A). Each data-point represents the average ± SD from two experiments with three mice per timepoint. *P < 0.05 reovirus 1/L-infected mice as compared to reovirus 1/L-infected, MPS-treated CBA/J mice. Cells were pooled and stained for surface phenotype expression using antibodies to B220 (B), CD3 (C), Mac-1 (D). Each data point represents the percent positive cells from the average of two independent experiments with pooled cells from three mice per timepoint.
In either CD4- or CD8-depleted reovirus 1/L-infected mice, the predominant cell types found in the BAL were macrophages (30% - 35% in CD4- or CD8-depleted reovirus 1/L-infected mice versus 25% reovirus 1/L-infected mice or 26% MPS-treated reovirus 1/L infected mice) (Figure 4) ▶ . CD4-depleted, reovirus 1/L-infected mice showed an infiltration of CD8+ cells (15%) without a significant infiltration of CD4+ cells (2%) (Figure 4C) ▶ in the BAL fluid. A similar response was observed with CD8-depleted, reovirus 1/L-infected mice (16% CD4+ cells versus 1% CD8+ cells) (Figure 4, C and D) ▶ . No significant infiltration of PMNs was observed in either CD4- or CD8-depleted reovirus 1/L-infected animals (data not shown). Depletion of either CD4+ or CD8+ cells was verified on both day 7 (data not shown) and day 14 (Figure 4E) ▶ post-reovirus 1/L infection. As shown in Figure 4E ▶ , the phenotype of spleen cells from normal mice was 15% CD4+ and 10% CD8+cells, while animals depleted of either CD8, or CD4 demonstrated the appropriate phenotype (CD8-depleted animals: 13% CD4+ and 2% CD8+ cells; CD4-depleted animals: 0.3% CD4+ and 18% CD8+ cells).
Cytokine and Chemokine Expression are Modulated in the Lungs of MPS-Treated CBA/J Mice Receiving 1 × 106 PFU Reovirus 1/L
Cytokine and chemokine mRNA expression in total lung tissue was evaluated by RPA. Three cytokine RPA template sets (Figure 5, A to C) ▶ and one chemokine RPA template set (Figure 5D) ▶ were evaluated in MPS-treated or untreated CBA/J mice after i.n. inoculation with 1 × 106 PFU of reovirus 1/L over a time-course of 21 to 28 days. Together, these four template sets allow an analysis of the expression and modulation of mRNAs for cytokines and chemokines which have been implicated to play a role in the pulmonary fibrotic process (such as MCP-1, IL-6, IFN-γ) as well as others contained within the template sets.
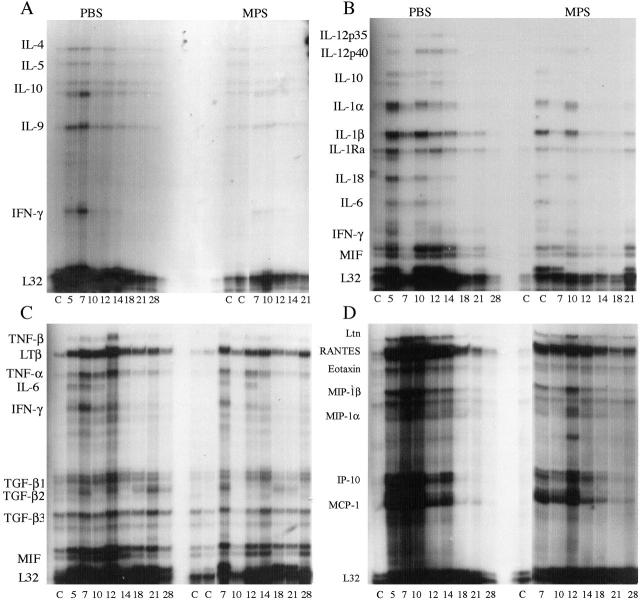
Figure 5.
Differential cytokine and chemokine mRNA expression from the lungs of untreated and MPS-treated reovirus 1/L-infected CBA/J mice. CBA/J mice were i.n. infected with 1 × 106 PFU reovirus 1/L and treated with MPS i.p. daily beginning on day 5 postinfection. RNA was harvested from the whole lungs at the indicated timepoints, and probed by RPA analysis using the following multiprobe template sets: mCK-1b (T-cell-derived cytokine panel) (A); mCK-2b (inflammatory cytokine panel) (B); mCK-3b (cytokines associated with fibrosis) (C); and mCK-5 (chemokine panel) (D). The position of individual cytokine or chemokine mRNA expression is indicated on the left side of each panel. Representative of three independent experiments containing two mice per timepoint.
Substantial expression of mRNA for a number of cytokines and chemokines were observed in the lungs of CBA/J mice infected i.n. with 1 × 106 PFU reovirus 1/L. (Figure 5) ▶ . These include IL-9 (Figure 5A) ▶ , the proinflammatory cytokines IL-1α, IL-1 β, IL-1 Ra, IL-6 (Figure 5B) ▶ , IFN-γ, (Figure 5, A to C) ▶ , MIF (Figure 5, B and C) ▶ , and the chemokines, MIP-1β, MIP-1α, MCP-1, RANTES, and IP-10 (Figure 5D) ▶ . In comparison to CBA/J mice infected with 1 × 106 PFU reovirus 1/L, MPS-treated reovirus 1/L-infected i.n. CBA/J mice, in general, demonstrated a decreased expression of most of the cytokines and chemokines present on the four template sets. Expression of the following cytokines and chemokines was observed in MPS-treated reovirus 1/L-infected CBA/J mice: IL-9 (Figure 5A) ▶ IL-1α, IL-1 β, IL-1 Ra, IL-6 (Figure 5B) ▶ , IFN-γ, (Figure 5, A to C) ▶ , MIF (Figure 5, B and C) ▶ , MCP-1, RANTES, and IP-10 (Figure 5D) ▶ . Cytokine mRNA expression was not induced for a number of cytokine genes in either MPS-treated or untreated reovirus 1/L-infected mice. These included IL-2, IL-3, and IL-13. In addition, mRNA expression for the chemokines eotaxin, MIP-2, and TCA-3 were not significantly induced.
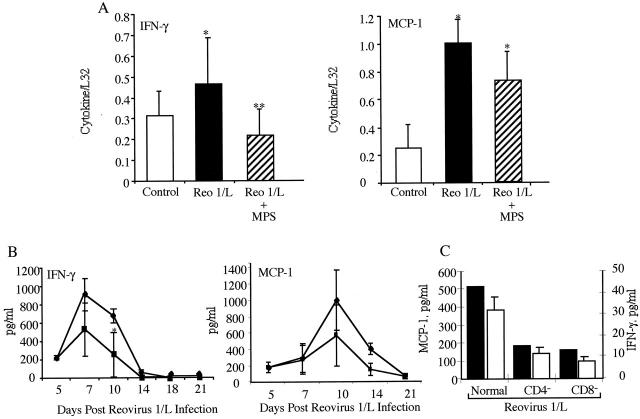
Since both IFN-γ and MCP-1 have been implicated in the fibrotic process induced by reovirus 1/L, 26,27 we quantitated the autoradiographs and found a significant up-regulation of both IFN-γ and MCP-1 (Figure 6A) ▶ mRNA in CBA/J mice infected with 1 × 106 PFU reovirus 1/L. However, in CBA/J mice infected with 1 × 106 PFU reovirus 1/L and treated with MPS beginning on day 5 postinfection, a significant decrease in the mRNA expression of IFN-γ (Figure 6A) ▶ was observed. Although the expression of MCP-1 was decreased after MPS treatment, this decrease as compared to control mice was not statistically significant (Figure 6A) ▶ . In a similar manner, while a significant increase in IFN-γ protein in the BAL fluid was observed in both MPS treated and untreated reovirus 1/L-infected mice as compared to uninfected controls (Figure 6B) ▶ , a decrease in IFN-γ protein expression was observed between MPS-treated and untreated, reovirus 1/L-infected mice (Figure 6B) ▶ . Similar to the expression of mRNA for MCP-1, a decrease (although not statistically significant) in the protein expression of MCP-1 in the BAL fluid of MPS-treated reovirus 1/L-infected was observed (Figure 6B) ▶ . In contrast, in both CD4- and CD8-depleted mice, a reduction in the protein expression of both MCP-1 and IFN-γ was observed (Figure 6C) ▶ . In all cases IFN-γ and MCP-1 was not detected in control, saline-inoculated mice (data not shown).
Figure 6.
Expression of IFN-γ and MCP-1 in corticosteroid-treated or CD4- or CD8-depleted CBA/J mice infected with 1 × 106 PFU reovirus 1/L. Relative differences in mRNA expression of either IFN-γ or MCP-1 (A) over time were determined by comparing the ratio of cytokine/chemokine mRNA with the housekeeping gene, L32 from RPA autoradiograms. IFN-γ expression was evaluated between days 5 and 10 postinfection. MCP-1 expression was evaluated between days 3 and 10 postinfection. Control, uninfected mice (open bar); reovirus 1/L-infected CBA/J mice (solid bar); MPS-treated, reovirus 1/L-infected CBA/J mice (striped bar). The mean ± SD of the densitometric measurements from two independent experiments with two mice per timepoint (four independent autoradiographs) over the indicated timepoints are presented. Detection of IFN-γ or MCP-1 protein by ELISA in the BAL fluid over time from reovirus 1/L-infected CBA/J mice (♦) and MPS-treated reovirus 1/L-infected CBA/J mice (▪) (B). The mean ± SD of two independent experiments, evaluated in duplicate, is shown. Detection of MCP-1 (▪) or IFN-γ (□) proteins by ELISA in the BAL fluid evaluated on day 14 post-reovirus 1/L infection from reovirus 1/L-infected normal, CD4-depleted, or CD8-depleted CBA/J mice (C). Representative of one experiment with two animals per timepoint, performed in duplicate *P < 0.05 as compared to saline-inoculated control mice. **P < 0.05, as compared to reovirus 1/L-infected mice.
To demonstrate a role for IFN-γ in the development of fibrotic lesions in reovirus 1/L induced BOOP, reovirus 1/L-infected (106 PFU BOOP) CBA/J mice were treated i.p. every 3 days beginning on day 3 postinfection with either 100 μg anti-IFN-γ antibody (R4–6A2) in PBS (Figure 7, C and D) ▶ or 100 μg normal rat IgG (Figure 7, A and B) ▶ . Mice were evaluated on days 14 (Figure 7, A and C) ▶ and 21 (Figure 7, B and D) ▶ postinfection for the development of fibrotic lesions by H&E staining. As can be observed with H&E staining, significant fibrotic lesion development was observed in reovirus 1/L-infected mice who were treated with normal rat IgG on both days 14 and 21 (Figure 7, A and B) ▶ . However, in reovirus 1/L-infected, anti-IFN-γ treated mice, although follicular bronchiolitis (FB) lesions were observed on day 14 and to a lesser extent on day 21 (Figure 7D) ▶ postinfection, this was accompanied by little to no fibrotic lesion development (Figure 7, C and D) ▶ .
Figure 7.
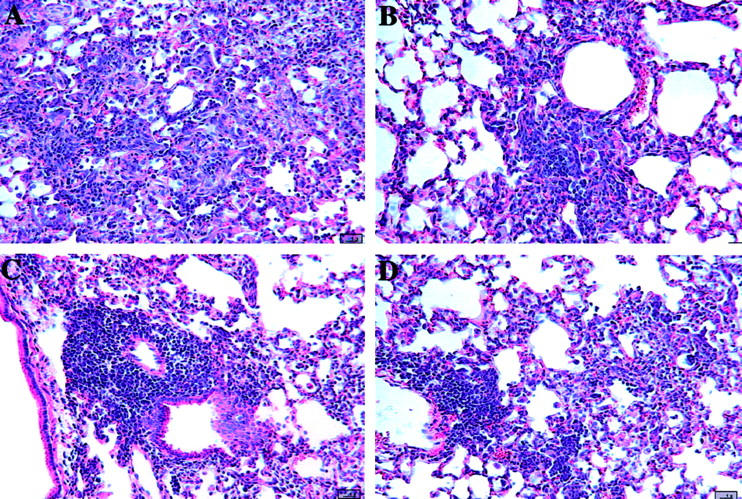
Inhibition of fibrosis associated with BOOP after treatment with a neutralizing monoclonal antibody to interferon-γ. CBA/J mice were i.n. infected with reovirus 1/L and treated i.p. every 3 days beginning on day 3 postinfection with either 100 μg normal rat IgG (A and B) or 100 μg anti-IFN-γ antibody (C and D) in PBS. Mice were evaluated both on days 14 (A and C) and 21 (B and D) postinfection for the development of fibrotic lesions by H&E stain. A and B: Reovirus 1/L-infected CBA/J mice treated with normal Rat IgG. C and D: Reovirus 1/L-infected CBA/J mice treated with an anti-IFN-γ antibody. Representative of one independent experiment containing four mice per timepoint (magnification ×20).
Discussion
In this study, we have used a reovirus 1/L-induced model of BOOP to study cellular and molecular events important in the development of this type of pulmonary fibrosis. Using reovirus 1/L-infected mice that were either treated with corticosteroids or depleted of either CD4+ or CD8+ T cells, our results demonstrate that T cells play a major role in the development of intraluminal fibrosis associated with BOOP. Corticosteroid treatment of reovirus 1/L-infected mice both inhibited the development of fibrotic lesions when administered early in the time-course and promoted the resolution of fibrotic lesions when corticosteroid administration was delayed. Daily treatment with corticosteroids inhibited the total cellular infiltration into the lung post reovirus 1/L infection, and also resulted in an inhibition of the proinflammatory and profibrotic cytokines, IFN-γ and MCP-1. Further, the depletion of either CD4+ or CD8+ T cells before reovirus 1/L infection also inhibited fibrotic lesion development as well as IFN-γ and MCP-1 protein expression. Consistent with these results, T-cell-deficient neonatally thymectomized (nTx) CBA/J mice do not develop intraluminal fibrosis after infection with 1 × 106 PFU reovirus 1/L and both the phenotype of the infiltrating cells and the expression of both IFN-γ and MCP-1 were significantly altered in these nTx CBA/J mice as compared to normal CBA/J mice. 27 Taken together, this study combined with our previous studies suggest a significant role for T cells in the development of reovirus 1/L-induced BOOP fibrotic lesions and suggests that TH1 derived cytokines, especially IFN-γ, may play a key role in fibrotic lesion development.
Currently few small animal models of BOOP exist. However, several studies have shown that the infiltration of T lymphocytes may be important in the development of other forms of pulmonary fibrosis although the data from both animal models and patients has been equivocal. Intratracheal administration of bleomycin in rodents, a model for idiopathic pulmonary fibrosis (IPF), results in interstitial fibrosis accompanied by a significant infiltration of both T and B lymphocytes. 17,18 In this model, some reports have demonstrated that the inhibition or depletion of lymphocytes by anti-lymphocyte antibody, mAb to T-cell subsets, or treatment with steroids inhibited the development of bleomycin-induced fibrosis, 34-36 while other studies found no effect of T-cell depletion on fibrotic lesions induced by bleomycin instillation. 37-39 Similarly, in nude mice lacking T cells or SCID mice deficient in both T and B cells, conflicting evidence for a role of T cells in the fibrotic process has been reported. 40-42 A role for T cells has also been proposed for Bronchiolitis Obliterans Syndrome (BOS) which is the major limitation to survival postlung transplantation and is characterized by persistent peribronchiolar inflammation that leads to airway fibrosis/obliteration. 3,16 Models of BOS demonstrate increases in CD4+ and CD8+ T cells, B cells, and macrophages post allograft transplantation. 15,16,43,44 Further, in SCID allograft models of BOS no influx of lymphocytes or fibrosis was observed, suggesting that lymphocytes may play an important role in the development of fibrosis during chronic graft rejection. 16 While these models have been used to evaluate potential mechanisms related to fibrosis, they may not fully recapitulate what occurs in similar human pathologies. As an example, while bleomycin-induced fibrosis in rodents is used as a model for human IPF, many features of bleomycin-induced fibrosis are not shared with the human condition. 45 However, in this regard, the reovirus 1/L-induced model of BOOP recapitulates the histological (intraluminal fibrosis) and phenotypic characteristics of human BOOP and thus, is an excellent small animal model of human BOOP. Furthermore, unlike other animal models for pulmonary fibrosis, reovirus-induced pulmonary fibrosis offers the advantage in that it also provides a model that recapitulates the response of human disease to clinical treatments currently in use. Since these lesions occur in a well-defined temporal sequence that proceeds from initial peribronchiolar inflammatory lesions to characteristic, fibrotic cellular BOOP lesions, this model can be used to evaluate the cellular and molecular signals that may lead to fibrotic lesion development in human BOOP.
The results described in this manuscript as well as in our previous publications all consistently demonstrate a clear dependence of T cells on the fibrotic process associated with reovirus 1/L-induced BOOP. 26 Thus, we analyzed cytokines and chemokines that have previously been associated with fibrotic lesion development. We believe that our data supports a role for IFN-γ in the fibrotic process and that it acts as a profibrotic agent in the spectrum of fibrosis induced in reovirus 1/L-infected mice. 22,25-27 Our data demonstrate that when the inflammatory and fibrotic process in BOOP is inhibited either by corticosteroid treatment or CD4 or CD8 depletion, the concentration of IFN-γ in the BAL fluid decreases. Both a decrease in IFN-γ expression and limited fibrotic lesion development was also demonstrated in T-cell-deficient nTx mice that were infected with reovirus 1/L-induced BOOP. 27 However, in reovirus 1/L-induced ARDS, the fibrotic component was not inhibited and IFN-γ expression levels remained high in either corticosteroid-treated or nTx animals. 25-27 Further, treatment of reovirus 1/L-induced BOOP with an anti-IFN-γ neutralizing monoclonal antibody inhibited fibrotic lesion development on both days 14 and 21 postinfection, further supporting our hypothesis that IFN-γ plays a significant role in fibrotic lesion development. Our data also demonstrate an increased expression of MCP-1 in reovirus-1/L induced BOOP that was modified after corticosteroid treatment or within CD4- or CD8-depleted mice. This data are also in agreement with our previous studies that demonstrated an increase in MCP-1 expression in reovirus 1/L-induced ARDS whose fibrosis is not modified either in nTx mice or by corticosteroid treatment. 26,27 However, in reovirus 1/L-induced BOOP a decrease in MCP-1 expression as well as fibrosis was observed in nTx mice. 27 Taken together, these data and our previously published results demonstrate a direct correlation of proinflammatory cytokine expression such as IFN-γ and MCP-1 and the development of fibrosis.
Our results, demonstrating a role for IFN-γ and MCP-1 in reovirus 1/L-induced BOOP are in agreement with other studies in which both IFN-γ and MCP-1 have been implicated in the fibrotic process in both patients and animal models. 46-48 A role for IFN-γ in bleomycin-induced interstitial fibrosis is supported by the observations that susceptible versus non-susceptible mouse strains produce high amounts of IFN-γ, 42 depletion of T cells down-regulates both IFN-γ expression and fibrosis, 35,49 and high levels of IFN-γ expression and fibrosis are observed in SCID mice, which are susceptible to bleomycin-induced fibrosis. 42 Bleomycin treatment of IFN-γ knockout mice (IFN-γ -/-) also resulted in both a significant inhibition of pulmonary inflammation and fibrosis. 50 IFN-γ has also been implicated in the fibrotic process associated with the tracheal transplant model of BOS. 11,16,51,52 Expression of TH1 cytokines including IFN-γ and IL-10 were up-regulated to a greater extent than TH2 cytokines (IL-4), suggesting that although cytokine production by all T-lymphocyte subsets (TH1, TH2) may be involved in the development of BOS, the TH1 cytokine products may be more important in the development of fibrosis. 52 INF-γ has also been implicated as a profibrotic factor in lung fibrosis that occurs in patients or animal models with fibrosing alveolitis, IPF, sarcoidosis, chronic beryllium disease, silicosis, and lung allograft fibrosis. 53-58 MCP-1 is also expressed in a number of inflammatory conditions in patients that demonstrate a fibrotic component including ARDS, IPF, systemic sclerosis, and BOOP 59-63 as well as in a number of mouse models of fibrosis. 63-65 In addition, depletion of MCP-1 by treatment with anti-MCP-1 antibodies or by loss of CCR2 signaling results in a significant reduction in fibro-obliteration. 63,64 Taken together these data also suggest a potential role for MCP-1 in the fibrotic process.
Although the process and underlying mechanisms of fibrosis have not been clearly elucidated, the release of cytokines and chemokines from inflammatory cells has been implicated in the development and regulation of fibrosis. Our data clearly support a role for T cells in the development of intraluminal fibrosis associated with BOOP since we observed the lack of fibrotic lesion development in reovirus 1/L-infected nTx CBA/J, 27 in CD4- or CD8-depleted CBA/J mice as well as in corticosteroid-treated mice. In contrast to BOOP, a definitive role for T cells in the development of fibrosis associated with reovirus 1/L-induced ARDS has not been established. In reovirus 1/L-induced ARDS, both infection of nTx mice and corticosteroid treatment have shown little effect in both the early inflammatory response as well as the later fibrotic response. 26,27 We have previously proposed that a positive feedback loop exists between the expression of MCP-1 and IFN-γ in the fibrotic response associated with reovirus 1/L-induced BOOP and ARDS. 27 We suggest that while the initial infection of resident epithelial cells by reovirus 1/L leads to MCP-1 expression and cellular infiltration, the sustained production of MCP-1 is the result of an autocrine or paracrine mechanism driven by the secretion of IFN-γ. This sustained production of MCP-1 leads to the continued recruitment of inflammatory cells, eventually leading to fibrotic development. In support of this model, IFN-γ has been shown to induce the expression of MCP-1 from a number of cell types including macrophages, epithelial cells, vascular endothelial cells, and fibroblasts. 65-69 Although histopathologically similar fibrotic lesions are associated with reovirus 1/L-induced BOOP and ARDS, the mechanism of fibrotic lesion development may be distinct due to differential regulation of infiltrating cells. While T cells may be the predominant cell type producing IFN-γ in BOOP that ultimately leads to fibrosis, non-T cells such as NK cells may be responsible for the production of IFN-γ in ARDS that ultimately leads to fibrosis. Therefore, treatment methods focused only on one aspect of fibrosis may be inefficient at modulating the development of fibrosis in both of these diseases since their disease processes are distinct.
Acknowledgments
We thank Margaret Romano for the preparation of histological sections, and the Medical University of South Carolina and the Hollings Cancer Center for support of the MUSC Analytical Flow Cytometry Facility.
Footnotes
Address reprint requests to Lucille London, Ph.D., Department of Microbiology and Immunology, Medical University of South Carolina, P.O. Box 250504, 173 Ashley Avenue, Charleston, South Carolina 29425. E-mail: londonl@musc.edu.
Supported by a U.S. Public Health Service grant AI R01 40175 and a grant from the American Lung Association (both to L. L.).
References
- 1.Epler GR, Colby TV, McLoud TC, Carrington CB, Gaensler EA: Bronchiolitis obliterans organizing pneumonia. N Engl J Med 1985, 312:152-158 [DOI] [PubMed] [Google Scholar]
- 2.Epler GR: Bronchiolitis obliterans organizing pneumonia. Arch Intern Med 2001, 161:158-164 [DOI] [PubMed] [Google Scholar]
- 3.Schlesinger C, Koss MN: Bronchiolitis: update 2001. Curr Opin Pulm Med 2002, 8:112-116 [DOI] [PubMed] [Google Scholar]
- 4.Epler GR: Bronchiolitis obliterans organizing pneumonia: definition and clinical features. Chest 1992, 102(Suppl 1):2S-6S [DOI] [PubMed] [Google Scholar]
- 5.Colby TV: Pathologic aspects of bronchiolitis obliterans organizing pneumonia. Chest 1992, 102(Suppl 1):38S-43S [DOI] [PubMed] [Google Scholar]
- 6.Costabel U, Teschler H, Guzman J: Bronchiolitis obliterans organizing pneumonia (BOOP): the cytological and immunocytological profile of bronchoalveolar lavage. Eur Respir J 1992, 5:791-797 [PubMed] [Google Scholar]
- 7.Mukae H, Kadota J, Kohno S, Matsukura S, Hara K: Increase of activated T cells in BAL fluid of Japanese patients with bronchiolitis obliterans organizing pneumonia and chronic eosinophilic pneumonia. Chest 1995, 108:123-128 [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto M, Jna Y, Kitaichi M, Haraswa M, Tamura M: Clinical features of BOOP in Japan. Chest 1992, 102(Suppl 1):21S-25S [DOI] [PubMed] [Google Scholar]
- 9.Ogata K, Koga T, Yagawa K: Interferon-related bronchiolitis obliterans organizing pneumonia. Chest 1994, 106:612-613 [DOI] [PubMed] [Google Scholar]
- 10.Ross DJ, Jordan SC, Nathan SD, Kass RM, Koerner SK: Delayed development of obliterative bronchiolitis syndrome with OKT3 after unilateral lung transplantation: a plea for multicenter immunosuppressive trials. Chest 1996, 109:870-873 [DOI] [PubMed] [Google Scholar]
- 11.Ross DJ, Moudgil A, Bagga A, Toyoda M, Marchevsky AM, Kass RM, Jordan SC: Lung allograft dysfunction correlates with γ-interferon gene expression in bronchoalveolar lavage. J Heart Lung Transplant 1999, 18:627-636 [DOI] [PubMed] [Google Scholar]
- 12.Costabel U, King TE: International consensus statement on idiopathic pulmonary fibrosis. Eur Respir J 2001, 17:163-167 [DOI] [PubMed] [Google Scholar]
- 13.du Bois RM, Wells AU: Cryptogenic fibrosing alveolitis/idiopathic pulmonary fibrosis. Eur Respir J 2001, 18(Suppl 32):43S-55S [PubMed] [Google Scholar]
- 14.Crystal RG, Bitterman PB, Mossman B, Schwarz MI, Sheppard D, Almasy L, Chapman HA, Friedman SL, King TE, Jr, Leinwand LA, Liotta L, Martin GR, Schwartz DA, Schultz GS, Wagner CR, Musson RA: Future research directions in idiopathic pulmonary fibrosis: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 2002, 166:236-246 [DOI] [PubMed] [Google Scholar]
- 15.Ikonen T, Uusitalo M, Taskinen E, Korpela A, Salminen US, Morris RE, Harjula AL: Small airway obliteration in a new swine heterotopic lung and bronchial allograft model. J Heart Lung Tranplant 1998, 17:945-953 [PubMed] [Google Scholar]
- 16.Neuringer IP, Mannon RB, Coffman TM, Parsons M, Burns K, Yankaskas JR, Aris RM: Immune cells in a mouse airway model of obliterative bronchiolitis. Am J Respir Cell Mol Biol 1998, 19:379-386 [DOI] [PubMed] [Google Scholar]
- 17.Lazo JS, Hoyt DG, Sebti SM, Pitt BR: Bleomycin: a pharmacologic tool in the study of the pathogenesis of interstitial pulmonary fibrosis. Pharmcol Ther 1990, 47:347-358 [DOI] [PubMed] [Google Scholar]
- 18.Zhu J, Cohen DA, Goud SN, Kaplan AM: Contribution of T lymphocytes to the development of bleomycin-induced pulmonary fibrosis. Ann NY Acad Sci 1996, 796:194-202 [DOI] [PubMed] [Google Scholar]
- 19.Piguet PF, Collart MA, Grau GE, Sappino AP, Vassalli P: Requirement of tumour necrosis factor for development of silica-induced pulmonary fibrosis. Nature 1990, 344:245-247 [DOI] [PubMed] [Google Scholar]
- 20.Zhang HY, Gharaee-Kermani M, Zhang K, Karmiol S, Phan SH: Lung fibroblast α-smooth muscle actin expression and contractile phenotype in bleomycin-induced pulmonary fibrosis. Am J Pathol 1996, 148:527-537 [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes PJ: Chronic obstructive pulmonary disease. N Engl J Med 2000, 343:269-280 [DOI] [PubMed] [Google Scholar]
- 22.Bellum SC, Hamamdzic DH, Thompson AH, Harley RA, London SD, London L: Experimental reovirus serotype 1/strain Lang infection of the lung: a model for the study of the lung in the context of mucosal immunity. Lab Invest 1996, 74:221-231 [PubMed] [Google Scholar]
- 23.Bellum SC, Dove D, Harley RA, Greene WB, Judson MA, London L, London SD: Respiratory reovirus 1/L induction of intraluminal fibrosis: a model for the study of bronchiolitis obliterans organizing pneumonia. Am J Pathol 1997, 150:2243-2254 [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson AH, London L, Bellum SC, Hamamdzic D, Harley RA, London SD: Respiratory-mucosal lymphocyte populations induced by reovirus serotype 1 infection. Cell Immunol 1996, 169:278-287 [DOI] [PubMed] [Google Scholar]
- 25.London L, Majeski EI, Paintlia MK, Harley RA, London SD: Respiratory reovirus 1/L induction of diffuse alveolar damage: a model of acute respiratory distress syndrome. Exper Mol Pathol 2002, 72:24-36 [DOI] [PubMed] [Google Scholar]
- 26.London L, Majeski EI, Altman-Hamamdzic S, Enockson C, Paintlia MK, Harley RA, London SD: Respiratory reovirus 1/L induction of diffuse alveolar damage: pulmonary fibrosis is not modulated by corticosteroids in acute respiratory distress syndrome in mice. Clin Immunol 2002, 103:284-295 [DOI] [PubMed] [Google Scholar]
- 27.Majeski EI, Harley RS, Bellum SC, London SD, London L: Differential role for T cells in the development of fibrotic lesions associated with reovirus 1/L induced BOOP versus ARDS. Am J Respir Cell Mol Bio 2003, 28:208-217 [DOI] [PubMed] [Google Scholar]
- 28.Dialynas DP, Quan ZS, Wall KA, Pierres A, Quintans J, Loken MR, Pierre M, Fitch FW: Characterization of the murine T cell surface molecule designated L3T4, identified by monoclonal antibody GK1.5: similarities to the human Leu3/T4 molecule. J Immunol 1983, 131:2445-2451 [PubMed] [Google Scholar]
- 29.Ledbetter JA, Herzenberg LA: Xenogeneic antibodies to mouse lymphoid differentiation antigens. Immuno Rev 1979, 47:63-90 [DOI] [PubMed] [Google Scholar]
- 30.Spitalny GL, Havell EA: Monoclonal antibody to murine γ interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med 1984, 159:1560-1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tay CH, Welsh RM: Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J Virol 1997, 71:267-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sayles PC, Johnson LL: Intact immune defenses are required for mice to resist the ts-4 vaccine strain of Toxoplasma gondii. Infect Immun 1996, 64:3088-3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy GK, Enwemeka CS: A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem 1996, 29:225-229 [DOI] [PubMed] [Google Scholar]
- 34.Phan SH, Thrall RS, Williams C: Bleomycin-induced pulmonary fibrosis: effects of steroid on lung collagen metabolism. Am Rev Respir Dis 1981, 124:428-434 [DOI] [PubMed] [Google Scholar]
- 35.Sharma SK, MacLean JA, Pinto C, Kradin RL: The effect of an anti-CD3 monoclonal antibody on bleomycin-induced lymphokine production and lung injury. Am J Respir Crit Care Med 1996, 154:193-200 [DOI] [PubMed] [Google Scholar]
- 36.Koenig WJ, Cross CE, Hesterberg TW, Last JA: The smoking gun: mechanism of methylprednisolone prevention of bleomycin-induced pulmonary fibrosis. Chest 1983, 83:5S-7S [DOI] [PubMed] [Google Scholar]
- 37.Sterling KM, DiPetrillo T, Cutroneo KR, Prestayko A: Inhibition of collagen accumulation by glucocorticoids in rat lung after intratracheal bleomycin instillation. Cancer Res 1982, 42:405-408 [PubMed] [Google Scholar]
- 38.Janick-Buckner D, Ranges GE, Hacker MP: Effect of cytotoxic monoclonal antibody depletion of T-lymphocyte subpopulations on bleomycin-induced lung damage in C57BL/6J mice. Toxicol Appl Pharm 1989, 100:474-484 [DOI] [PubMed] [Google Scholar]
- 39.Khalil N, Whitman C, Zuo L, Danielpour D, Greenberg A: Regulation of alveolar macrophage transforming growth factor-β secretion by corticosteroids in bleomycin-induced pulmonary inflammation in the rat. J Clin Invest 1993, 92:1812-1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szapiel SV, Elson N, Fulmer JD, Hunninghake GW, Crystal RG: Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis 1979, 120:893-899 [DOI] [PubMed] [Google Scholar]
- 41.Schrier DJ, Phan SH, McGarry BM: The effects of the nude (nu/nu) mutation on bleomycin-induced pulmonary fibrosis: a biochemical evaluation. Am Rev Respir Dis 1983, 127:614-617 [DOI] [PubMed] [Google Scholar]
- 42.Lake-Bullock HM, Zhu V, Hao J, Cohen H, Kaplan AM: T cell independence of bleomycin-induced pulmonary fibrosis. J Leuk Biol 1999, 65:187-195 [DOI] [PubMed] [Google Scholar]
- 43.Koskinen P, Kallio EA, Bruggeman CA, Lemstrom KB: Cytomegalovirus infection enhances experimental obliterative bronchiolitis in rat tracheal allografts. Am J Respir Crit Care Med 1997, 155:2078-2088 [DOI] [PubMed] [Google Scholar]
- 44.Boehler A, Chamberlain D, Kesten S, Slutsky AS, Liu M, Keshavjee S: Lymphocytic airway infiltration as a precursor to fibrous obliteration in a rat model of bronchiolitis obliterans. Transplantation 1997, 64:311-317 [DOI] [PubMed] [Google Scholar]
- 45.Cooper JAD, III: Pulmonary fibrosis: pathways are slowly coming onto light. Am J Respir Cell Mol Biol 2000, :520-523 [DOI] [PubMed] [Google Scholar]
- 46.Lukacs NW, Hogaboam C, Chensue SW, Blease K, Kunkel SL: Type 1/type 2 cytokine paradigm and the progression of pulmonary fibrosis. Chest 2001, 120(Suppl 1):5S-8S [DOI] [PubMed] [Google Scholar]
- 47.Kuwano K, Hagimoto N, Hara N: Molecular mechanisms of pulmonary fibrosis and current treatment. Curr Mol Med 2001, 1:551-573 [DOI] [PubMed] [Google Scholar]
- 48.Lasky JA, Brody AR: Interstitial fibrosis and growth factors. Environ Health Perspect 2000, 108(Suppl 4):751-762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tager A, Luster A, Kradin R: T-cell chemokines interferon-inducible protein-10 and monokine induced by interferon-γ are upregulated in bleomycin-induced lung injury. Chest 1999, 116:90S. [PubMed] [Google Scholar]
- 50.Chen ES, Greenlee BM, Wills-Karp M, Moller DR: Attenuation of lung inflammation and fibrosis in interferon-γ-deficient mice after intratracheal bleomycin. Am J Respir Cell Mol Biol 2001, 24:545-555 [DOI] [PubMed] [Google Scholar]
- 51.Boehler A, Bai XH, Liu M, Cassivi S, Chamberlain D, Slutsky AS, Keshavjee S: Upregulation of T-helper 1 cytokines and chemokine expression in post-transplant airway obliteration. Am J Respir Crit Care Med 1999, 159:1910-1917 [DOI] [PubMed] [Google Scholar]
- 52.Neuringer IP, Walsh SP, Mannon RB, Gabriel S, Aris RM: Enhanced T cell cytokine gene expression in mouse airway obliterative bronchiolitis. Transplantation 2000, 69:399-405 [DOI] [PubMed] [Google Scholar]
- 53.Davis GS, Pfeiffer LM, Hemenway DR: Interferon-γ production by specific lung lymphocyte phenotypes in silicosis in mice. Am J Respir Cell Mol Biol 2000, 22:491-501 [DOI] [PubMed] [Google Scholar]
- 54.Robinson BW, Rose AH: Pulmonary γ interferon production in patients with fibrosing alveolitis. Thorax 1990, 45:105-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shaw RJ, Benedict SH, Clark RA, King TE, Jr: Pathogenesis of pulmonary fibrosis in interstitial lung disease: alveolar macrophage PDGF(B) gene activation and up-regulation by interferon-γ. Am Rev Respir Dis 1991, 143:167-173 [DOI] [PubMed] [Google Scholar]
- 56.Awad M, Pravica V, Perrey C, El Gamel A, Yonan N, Sinnott PJ, Hutchinson IV: CA repeat allele polymorphism in the first intron of the human interferon-γ gene is associated with lung allograft fibrosis. Human Immunol 1999, 60:343-346 [DOI] [PubMed] [Google Scholar]
- 57.Shigehara K, Shijubo N, Ohmichi M, Takahashi R, Kon S, Okamura H, Kurimoto M, Hiraga Y, Tatsuno T, Abe S, Sato N: IL-12 and IL-18 are increased and stimulate IFN-γ production in sarcoid lungs. J Immunol 2001, 166:642-649 [DOI] [PubMed] [Google Scholar]
- 58.Wallace WAH, Howie SEM: Immunoreactive interleukin 4 and interferon-γ expression by type II alveolar epithelial cells in interstitial lung disease. J Pathol 1999, 187:475-480 [DOI] [PubMed] [Google Scholar]
- 59.Iyonaga K, Takeya M, Saita N, Sakamoto O, Yoshimura T, Ando M, Takahashi K: Monocyte chemoattractant protein-1 in idiopathic pulmonary fibrosis and other interstitial lung diseases. Hum Pathol 1994, 25:455-463 [DOI] [PubMed] [Google Scholar]
- 60.Suga M, Iyonaga K, Ichiyasu H, Saita N, Yamasaki H, Ando M: Clinical significance of MCP-1 levels in BALF and serum in patients with interstitial lung diseases. Eur Respir J 1999, 14:376-382 [DOI] [PubMed] [Google Scholar]
- 61.Hasegawa M, Sato S, Takehara K: Augmented production of chemokines (monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α) and MIP-1β) in patients with systemic sclerosis: mCP-1 and MIP-1α may be involved in the development of pulmonary fibrosis. Clin Exper Immunol 1999, 117:159-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Car BD, Meloni F, Luisetti M, Semenzato G, Gialdroni-Grassi G, Walz A: Elevated IL-8 and MCP-1 in the bronchoalveolar lavage fluid of patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am J Respir Crit Care Med 1994, 149:655-659 [DOI] [PubMed] [Google Scholar]
- 63.Belperio JA, Keane MP, Burdick MD, Lynch JP, 3rd, Xue YY, Berlin A, Ross DJ, Kunkel SL, Charo IF, Strieter RM: Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. J Clin Invest 2001, 108:547-556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith RE, Strieter RM, Phan SH, Kunkel SL: C-C chemokines: novel mediators of the profibrotic inflammatory response to bleomycin challenge. Am J Respir Cell Mol Biol 1996, 15:693-702 [DOI] [PubMed] [Google Scholar]
- 65.Moore BB, Paine R, III, Christensen PJ, Moore TA, Sitterding S, Ngan R, Wilke CA, Kuziel WA, Toews GB: Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol 2001, 167:4368-4377 [DOI] [PubMed] [Google Scholar]
- 66.Andoh A, Takaya H, Makino J, Sato H, Bamba S, Araki Y, Hata K, Shimada M, Okuno T, Fujiyama Y, Bamba T: Cooperation of interleukin-17 and interferon-γ on chemokine secretion in human fetal intestinal epithelial cells. Clin Exper Immunol 2001, 125:56-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van der Velden VH, Verheggen MM, Bernasconi S, Sozzani S, Naber BA, van der Linden-van Beurden CA, Hoogsteden HC, Mantovani A, Versnel M: Interleukin-1β and interferon-γ differentially regulate release of monocyte chemotactic protein-1 and interleukin-8 by human bronchial epithelial cells. Eur Cytokine Netw 1998, 9:269-277 [PubMed] [Google Scholar]
- 68.Bauermeister K, Burger M, Almanasreh N, Knopf HP, Schumann RR, Schollmeyer P, Dobos GJ: Distinct regulation of IL-8 and MCP-1 by LPS and interferon-γ-treated human peritoneal macrophages. Nephrol Dial Transplant 1998, 13:1412-1419 [DOI] [PubMed] [Google Scholar]
- 69.Brown Z, Gerritsen M, Carley WW, Strieter RM, Kunkel SL, Westwick J: Chemokine gene expression and secretion by cytokine-activated human microvascular endothelial cells: differential regulation of monocyte chemoattractant protein-1 and interleukin-8 in response to interferon-γ. Am J Pathol 1994, 145:913-921 [PMC free article] [PubMed] [Google Scholar]