Abstract
Proteolysis of the thrombin receptor, protease activated receptor-1 (PAR1), may enhance normal and pathological cellular invasion, and indirect evidence suggests that activation of PAR1 expressed by invasive extravillous trophoblasts (EVTs) influences human placentation. Here we describe PAR1, PAR2, and PAR3 protein distribution in the developing human placenta and implicate PAR1 and PAR2 activation in functions central to EVT invasion. PAR1, PAR2, and PAR3 are expressed in cultured 8- to 13-week-old EVTs, and in situ in 18- to 20-week-old placental syncytiotrophoblasts and invasive trophoblasts. Thrombin, but not the PAR2 agonist peptide SLIGKV, inhibited proliferation in cultured EVTs, although both agonists stimulated phosphoinositide hydrolysis and EVT invasion through Matrigel barriers. Thrombin-induced phosphoinositide hydrolysis was completely inhibited and the thrombin effect on proliferation was prevented when PAR1 cleavage was first blocked with specific monoclonal antibodies, indicating that PAR1 is the predominant thrombin receptor on EVTs. Together these results support a role for PAR1, and potentially PAR2 and PAR3 in the invasive phase of human placentation.
The development of the placenta is primarily dependent on the differentiation of trophoblast cells along two pathways. 1 In one pathway, mononucleated cytotrophoblast cells cease proliferation and then fuse to form the terminally differentiated multinucleated syncytiotrophoblast. As pregnancy progresses, cytotrophoblast cells become more sparse within the villi and the syncytiotrophoblast forms the only continuous layer separating the maternal intervillous space and the fetal capillary endothelium. In the other pathway, a subset of undifferentiated cytotrophoblast cells in anchoring villi invades maternal tissues securing the attachment of the placenta to the maternal uterine wall and development of an adequate vascular supply. Trophoblast invasion into the uterine wall occurs in two waves, the first wave occurring during the first 10 weeks of pregnancy and the second wave of invasion occurring between 14 and 20 weeks of gestation. 2 Shallow invasion by extravillous trophoblasts (EVTs) into the uterine wall results in reduced placental perfusion and placental dysfunction, which has been associated with adverse reproductive outcomes including spontaneous miscarriage, fetal growth restriction, and pre-eclampsia. 3,4
Proteases, particularly matrix metalloproteases and coagulation factors, are known to be involved in cellular invasion, but the proteases that are essential for human trophoblast invasion are unknown. 5,6 Indeed, studies of normally invasive cells in other tissues have been primarily limited to studies of macrophages, leukocytes, cancer cells, and endothelial cells, and these studies have implicated multiple proteases, protease inhibitors, and classes of cell-surface protease receptors in the invasive process. 7,8 The temporal and anatomical distribution of coagulation and matrix-remodeling proteases and their inhibitors in normal placental and uterine tissues, combined with alterations in their distribution patterns in gestational diseases, supports the hypothesis that these enzymes are critical to trophoblast invasion and differentiation. For example, elevated expression of thrombin receptor transcripts were reported in invasive placental trophoblast cells compared to differentiated noninvasive trophoblast cells. 9 Also, the timing of expression and placental distribution of thrombomodulin in normal and complicated pregnancies suggests tight regulation of the coagulation cascade in placentation. An endothelial cell membrane protein, thrombomodulin, binds thrombin with high affinity and alters its substrate specificity, locally reducing coagulation and fibrinolysis. Thrombomodulin expression is elevated in term syncytiotrophoblast microvilli compared to first trimester placenta, and is elevated in pre-eclampsia, which is associated with shallow trophoblast invasion. 10,11 These and other findings suggest a link between coagulation proteases and trophoblast invasion, and led us to examine the role of protease-activated receptors (PARs) in this process. 12-14
PARs are members of the G-protein-coupled receptor superfamily that are activated by the proteolytic cleavage of their large amino terminal domain. Activating cleavage leads to the exposure of a new N-terminus containing a tethered ligand sequence that activates the receptor through interactions with its extracellular surface. 15 Currently four PARs are known, three of which (PAR1, PAR3, and PAR4) are activated by thrombin. The fourth receptor, PAR2, can be cleaved and activated by trypsin, tryptases, and other trypsin-like serine proteases including components of the coagulation cascade (eg, the tissue factor/VIIa complex and factor Xa), but it is not a proteolytic substrate for thrombin. 16 PAR1, PAR2, and PAR4, but not PAR3, can also be activated by synthetic peptides corresponding to their respective tethered ligand. Despite common signaling pathways including the activation of phospholipase C (PLC), PAR activation leads to a range of cellular responses that vary by species and tissue. 17 These variations can result from differences in cellular expression of different PAR subtypes, because cells can simultaneously express more than one PAR. Varied PAR-mediated protease responses can also occur in the presence of different posttranslational modifications of PAR amino-termini, or if PAR-expressing cells are first exposed to inactivating proteases that cleave the receptor and render it insensitive to subsequent exposures to activating proteases. 18 The latter of these phenomena has been demonstrated for PAR1, PAR2, and PAR3. 19-22
Several recent studies examined PAR function in tumor cell invasion, but no consistent role emerged despite similar experimental strategies. 9,23-25 Even-Ram and colleagues examined PAR1 mRNA expression in early placentation, an approach that likely circumvents many of the pitfalls associated with transformed cell models of invasion. 9 The authors showed that PAR1 mRNA expression was not detectable in placental biopsies during the first 6 weeks of gestation, increased dramatically between weeks 7 and 10, and then dropped precipitously after week 11, a pattern that correlates with the first invasive phase of placentation. 2,9 To date however, neither functional data nor evidence of PAR1 protein expression has been reported for placental tissues. Moreover, expression and function of PAR2 and/or the other known thrombin receptors (PAR3 and PAR4) have also not been described in human placentation. We used specific monoclonal antibodies to PAR1 and PAR2, and developed novel PAR3-specific antibodies to characterize the expression of these receptors at various stages of human placental development. We also used previously developed methods to characterize the relative contribution of different PARs to thrombin responses in primary EVT cultures. 17,26-28 Results from these functional studies support the hypothesis that thrombin receptor activation plays a role in EVT invasion during normal placentation.
Materials and Methods
Reagents
Highly purified α-thrombin (∼3000 U/mg, 1 U/ml, ∼10 nmol/L) was provided by Dr. John Fenton (New York State Department of Health, Albany, NY). The PAR2 agonist peptide SLIGKV (residues 37 to 42 of human PAR-2); 29 SLIGKVDGTSHVTG (residues 37 to 50 of human PAR2), the peptide against which monoclonal antibody SAM11 was raised; 30,31 KYEPFWEDEEKNES (residues 51 to 64 of human PAR1), 32 the peptide immunogen against which antibody WEDE15 was raised; 33 and peptides CQSGMENDTNNLAK, TFRGAPPNSFEE, and IKCPEESASHLHVK (residues 19 to 32, 39 to 51, and 69 to 82, respectively, of human PAR3), 34 used for PAR3 monoclonal antibody epitope mapping were synthesized and high performance liquid chromatography purified at the University of Pennsylvania Medical Center Protein Chemistry laboratory. [3H] Myoinositol (specific activity, 22.2 Ci/mmol) was obtained from NEN Life Sciences, Boston, MA.
Monoclonal Antibodies
WEDE15 and ATAP2 are IgG1 monoclonal antibodies directed against epitopes within the amino terminus of PAR-1 that correspond to the hirudin-like domain and activating peptide domains of PAR-1, respectively. 33,35 Combinations of these antibodies inhibit PAR1 cleavage by thrombin. 26 SAM11 is a mouse IgG2a monoclonal antibody raised against human PAR-2. 16 Cytokeratin 18, an EVT marker, was detected with monoclonal anti-cytokeratin peptide 18 antibody (clone CY-90; Sigma, St. Louis, MO). 36 Vimentin is a marker not present in EVTs, but present in potential contaminating cells. A monoclonal antibody against vimentin (clone V9) was obtained from Sigma. Mouse monoclonal antibodies to the amino terminus of human PAR3 were generated in cooperation with the hybridoma core facility of the University of Pennsylvania School of Medicine Cancer Center. Mice were immunized with a fusion protein consisting of human PAR3 residues 21 to 94 followed by a 6xHIS epitope tag, a generous gift from Dr. Shaun Coughlin (University of California at San Francisco). This region of PAR3 begins at the carboxyl end of the predicted PAR3 signal peptide, spans the thrombin cleavage site and hirudin-like domains, and ends just before the beginning of the predicted first transmembrane domain of the receptor. Hybridoma culture supernatants were screened for PAR3 peptide-reactive antibodies by enzyme-linked immunosorbent assay as previously described for the development of PAR1 and PAR2 monoclonal antibodies, then screened for antibody binding to the PAR3 amino terminus on the surface of transfected fibroblasts as described. 26,31,33,35 Briefly, transfected cells were analyzed for surface expression of a PAR3NT-CXCR4 receptor chimera by flow cytometry using the monoclonal antibody 12G5. PAR3NT-CXCR4 is an engineered cell-surface receptor made up of the amino terminal exodomain of human PAR3 fused to the first putative transmembrane domain of the human receptor for stromal derived factor-1 (CXCR4). 26 In separate reactions, hybridoma supernatants were analyzed for immunoreactivity with the PAR3 amino terminal exodomain. Antibodies that bound to cells transfected with the expression vector pRK7 or to cells transfected with chimeric receptors with the PAR1, PAR2, or PAR4 amino terminal exodomain fused to CXCR4 were excluded from the screen. PAR3 immunoreactive antibodies used in these studies were purified from ascites by protein A affinity chromatography using the MAPS II kit (Bio-Rad, Richmond, CA). Epitope mapping of PAR3-specific monoclonal antibodies was performed by enzyme-linked immunosorbent assay using synthetic peptides corresponding to three different PAR3 amino terminal domains. 37 Because some of the PAR3 monoclonals developed in this manner reacted with the immunogen, but not shorter synthetic PAR3 peptides, epitope mapping of those antibodies was limited to determining the sensitivity of antibody-reactive PAR3 epitopes to thrombin treatment. This cleavage sensitivity was assessed using flow cytometry of PAR3NT-CXCR4-transfected fibroblasts before and after thrombin treatment as described previously for the PAR1NT-CXCR4 receptor chimera. 26
Cell Culture
EVTs isolated and propagated from first trimester placental tissues (8 to 13 weeks of gestation) according to the method of Graham and colleagues, 38 were the generous gift of Dr. Colin MacCalman (University of British Columbia, Vancouver, Canada). Cells were grown at 37°C under 5% CO2 in a humidified incubator on plastic surfaces in complete growth medium consisting of Dulbecco’s modified Eagle’s medium (DMEM) (Life Technologies, Inc., Grand Island, NY) supplemented with 10% fetal calf serum (Hyclone, Logan, UT), 100 U/ml penicillin, 100 μg/ml streptomycin, 0.6 mmol/L l-glutamine. Canonical stocks were established from first passage cells and frozen under liquid N2 in 90% fetal calf serum, 10% dimethyl sulfoxide. Cells were thawed, propagated, and used in these experiments through four passages. Cells grown under these conditions showed no loss of invasive or proliferative phenotype, and exhibited 100% immunoreactivity to cytokeratin 18 and no vimentin immunoreactivity. Megakaryoblastic DAMI cells were cultured in RPMI 1640 supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. 39 HEK 293T, BeWo, and JEG3 cells were grown as previously described. 27,40
Northern Blotting
To determine whether EVTs, BeWo, or JEG3 cells expressed PAR1, PAR2, PAR3, or PAR4, we analyzed receptor mRNA expression by Northern blotting. Total RNA (10 μg) was loaded onto 1% denaturing agarose gels and, after electrophoresis, blotted onto nylon membranes and probed with radiolabeled human PAR cDNAs, specific for each receptor. Probes for the PARs were derived from the following restriction fragments of their fully sequenced cDNAs: PAR1 PstI-EcoRI (nucleotides 764 to 2123), PAR2 BamHI (nucleotides 1 to 1266), PAR3 HindIII-KpnI (nucleotides 1 to 405). Probes were labeled with 32P using the random primer labeling kit (Prime-It II kit; Stratagene, La Jolla, CA). Membranes were analyzed, then stripped in 0.1% sodium dodecyl sulfate at 90°C and rehybridized with a probe for 18S rRNA to assess RNA loading.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
To confirm the negative Northern results obtained for PAR-4, we examined PAR expression by the more sensitive RT-PCR procedure. Control reactions were prepared using total RNA from human cell lines known to express overlapping subsets of the known PARs, and included reactions without reverse transcription to control for contamination by genomic DNA. Briefly, total Dami, human umbilical vein endothelial cell, or EVT RNAs (5 μg) were reverse-transcribed and subjected to PCR for PAR4 and PAR1 (positive control) as previously described. 26
Immunohistochemistry
Paraffin-embedded placental tissues from three different 18- to 20-week gestational women who had undergone elective pregnancy terminations were obtained from the University of Pennsylvania Department of Pathology and Laboratory Medicine. Immunohistochemical detection of PAR1 and PAR2 was performed as previously described. 41 Briefly, before immunohistochemistry, placental sections were deparaffinized and incubated in a graded series of ethanol washes to rehydrate the tissue. Subsequently, immunohistochemistry slides were blocked with normal blocking serum (Vector Laboratories, Burlingame, CA) for 10 minutes, rinsed with phosphate-buffered saline (PBS), and then incubated with 5 μg/ml of the primary antibody in PBS for 30 minutes at room temperature in a humidified chamber. Slides were washed and incubated for 30 minutes with biotinylated secondary antibody (horse anti-mouse, Vector Laboratories), rinsed in PBS, and then incubated with avidin-horseradish peroxidase-biotin complex reagent (ABC; Vector Laboratories) for 30 minutes. Slides were washed and developed for 5 minutes twice with diaminobenzidine (Biomeda, Foster City, CA), rinsed in distilled water, counterstained with hematoxylin, dehydrated, and coverslipped in Permount (Fisher Scientific, Pittsburgh, PA). Negative control slides were prepared by preadsorption of the primary antibody with its immunizing peptide (200-fold molar excess overnight at 4°C) and, in the case of PAR3 antibodies, by performing the staining procedure in the absence of primary antibody or separately with an isotype-matched control antibody. Slides were photographed using a Nikon Eclipse TE300 microscope outfitted with a Roper Scientific MicroMax Camera (Princeton Instruments, Trenton, NJ) and the Universal Imaging System (Universal Imaging Corp., White Plains, NY) using Metamorph imaging software (Downingtown, PA). PAR3 antibodies were screened for their utility in immunohistochemistry by examining the expression of PAR3 in transfected cells. Briefly, HEK 293T cells were transfected with 10 μg of plasmid DNA, either empty expression vector pRK7 (mock transfection), or the PAR3-CXCR4 chimeric receptor as described previously. 26 One pool each of transfected or mock-transfected cells was analyzed by flow cytometry with the cleavage-insensitive anti-PAR3 monoclonal antibody PAR3–22 or anti-CXCR4 monoclonal antibody 12g5 to confirm expression of the PAR3-CXCR4 chimera. To assess the impact of fixation on PAR3 epitopes, another aliquot of each pool of detached cells was fixed in 10% neutral buffered formalin for 4, 24, or 48 hours, embedded in paraffin blocks, cut into 5-mm sections using a cryostat, mounted on glass slides, and examined by immunocytochemistry with PAR3-specific monoclonal antibodies or isotype-matched control monoclonal antibodies. Alternatively, transfected cells were prepared for immunostaining by plating in chamber slides to a concentration of 5 × 10 4 cells/well, allowed to grow 48 hours after transfection to ∼70% confluence, rinsed in warm PBS, fixed in 10% formalin in PBS for 10 minutes, and washed three times in PBS.
Phosphoinositide Hydrolysis
Activation of PARs results in increases in intracellular calcium and causes a concomitant stimulation of phosphoinositide hydrolysis. 15 The latter response can be measured as the accumulation of phosphorylated inositides over time, thus providing a sensitive indicator of PAR activation. To assess PAR subtype-specific activation, EVTs were loaded overnight with 4 μCi/ml of [3H] myoinositol in complete growth medium. Cells were washed once and then serum-starved for 2 hours in DMEM. Fifteen minutes before treatments were administered, 20 mmol/L of LiCl2 was added to the cultures, followed by the addition of PBS alone (buffer control), thrombin (20 nmol/L), or SLIGKV peptide (50 μmol/L). Before agonist addition, some samples were preincubated for 10 minutes with either the anti-PAR-1 antibody mix (WEDE 15 + ATAP2) or isotype-matched control antibodies before agonist addition. 26 Cells were incubated for 45 minutes at 37°C, then extracted in perchloric acid/ethylenediaminetetraacetic acid, and neutralized. Total inositol phosphates were measured by ion exchange chromatography on Dowex columns followed by scintillation counting. 19,42
Measurement of Cellular Proliferation
Cell viability and proliferation was measured using the WST-1 reagent (Promega, Madison, WI), a tetrazolium dye that is cleaved to formazan by cellular enzymes, according to the manufacturer’s recommendations. Briefly, early passage EVTs were seeded at 10,000 cells per well in 96-well tissue-culture dishes in DMEM supplemented with 2% Nutridoma-SP (Roche Molecular Biochemicals, Indianapolis, IN) with agonist or buffer as described in the figure legend. Cells were treated as described, incubated 16 hours, and WST-1 was added to each well and incubated for 3 hours according to the manufacturer’s recommendations. The conversion of WST-1 to formazan was measured spectrophotometrically on a 96-well plate reader. Inhibition of PAR1-induced responses using PAR1 antibodies was performed as described previously. 26-28
Invasion Assays
Early passage EVTs (n = 50,000) were seeded onto Matrigel-coated 8-μm filter chambers (Becton Dickinson, Fullerton, CA) in DMEM/Nutridoma as above, supplemented with agonist or buffer as described above. DMEM supplemented with 2% fetal calf serum was added to the lower cell as a chemoattractant, and agonist was added at equal concentrations to both the upper and lower chambers. After 16 hours at 5% CO2 and 37°C, the Matrigel was removed with a cotton swab, and the filters were fixed in 4% paraformaldehyde and stained with hematoxylin. Cells that had traversed the Matrigel and passed through the pores of the filter were counted by two observers who were blinded to the nature of agonist treatments.
Statistical Analysis
Phosphoinositide data were analyzed by one-way analysis of variance for independent samples and pairwise comparisons were made using Tukey’s honestly significantly different (HSD) test. Proliferation data were ln transformed to normalize for heterogeneous variance, then analyzed by one-way analysis of variance for three independent samples and Tukey’s honestly significantly different test.
Results
PAR mRNA Expression in Cultured EVTs
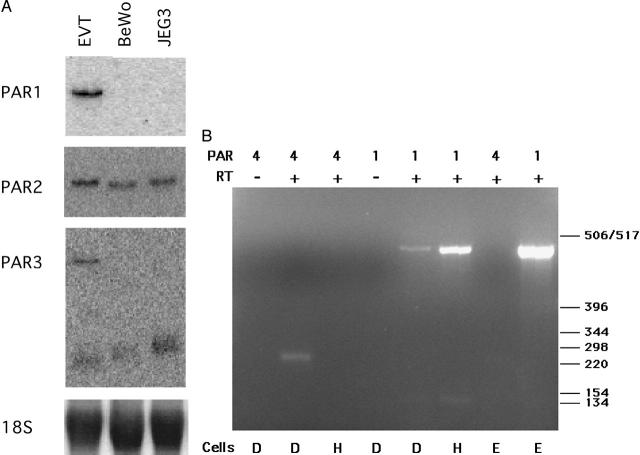
To determine the complement of PARs expressed in cultured EVTs, we first analyzed the abundance of transcripts encoding the known PARs. Northern analysis for PAR1, PAR2, and PAR3 mRNA expression in cultured EVTs indicated the presence of all three transcripts (Figure 1A) ▶ . The transcript sizes agree with previously published reports. 26,32,34 PAR1 was detected in the EVTs, but was not present in the BeWo and JEG3 choriocarcinoma cell lines. A single transcript was detected for PAR2 in EVT, BeWo, and JEG3 cells. Consistent with the initial characterization of PAR3 mRNA expression in human tissues 34,43 two PAR3 transcripts were detected by Northern blot in the EVTs. BeWo and JEG3 cells exhibited only the lower band (Figure 1A) ▶ . The absence of PAR4 transcripts in these blots was confirmed using RT-PCR (Figure 1B) ▶ . Using primers specific for PAR1 and the PAR4 amino-terminus, we were able to detect PAR1 and PAR4 transcripts in Dami cells (positive control). This megakaryoblastic line was previously shown to express PAR1, PAR3, and PAR4. 44 As with EVTs, no PAR4 mRNA was detected in human umbilical vein endothelial cells, previously reported to express PAR1, PAR2, and PAR3 but not PAR4 mRNA. 26,31,43 Conversely, PAR1 (RNA loading control) was detected in both EVT and human umbilical vein endothelial cells. From these experiments, we conclude that cultured EVTs express mRNA encoding PAR1, PAR2, and PAR3, but not PAR4.
Figure 1.
Cultured EVTs (8 to 10 weeks of gestation) express PAR1, PAR2, and PAR3 mRNA. A: Northern blot analysis of cultured EVTs, and the choriocarcinoma cell lines, BeWo and JEG3, was performed to detect PAR mRNAs as described in Materials and Methods. For PAR3, note the appearance of the ∼2.0-kb transcript in all lanes and the ∼4.0-kb band in only the EVT lane. PAR4 blots were negative in all cells (not shown). 18S rRNA serves as a control for sample loading. B: RT-PCR confirms the absence of PAR4 in EVTs. Total RNA from megakaryoblastic Dami cells (D, bottom) that express PAR1 and PAR4, pooled primary human umbilical vein endothelial cells (H) that express PAR1 and not PAR4, and EVTs (E) was reverse-transcribed and PAR4 and PAR1 transcripts were amplified by PCR as described in Materials and Methods. Primer pairs for PAR1 and PAR4 are indicated in the top row of text, below which is an indication of the inclusion (+) or omission (−) of reverse transcriptase (RT) in the amplification reaction. PAR4 transcripts appear as a faint band in the Dami RT+ lane only, while all three cell types expressed PAR1 mRNA. This figure is representative of results obtained in two identical experiments.
Immunohistochemical Analysis of PAR1, PAR2, and PAR3 in 18- to 20-Week Placental Tissue
Based on the results presented above we conclude that PAR1, PAR2, and PAR3 are expressed in cultured EVTs. We sought to confirm these results at the protein level in placental tissues. Using previously developed monoclonal antibodies to PAR1 and PAR2, and a thrombin cleavage-insensitive PAR3-specific antibody developed for these experiments, we examined first and second trimester placental tissues. Figure 2 ▶ shows that EVTs within 18- to 20-weeks of gestational age placental tissue stained positively for PAR1, PAR2, and PAR3. Cytokeratin 18 staining confirmed the identity of these cells as EVTs (Figure 2G) ▶ . Figure 2 ▶ also shows that syncytiotrophoblast stained positively for PAR1, PAR2, and PAR3, whereas stromal cells within the villi showed only modest staining for these receptors. Consistent with previous reports of endothelial cell PAR expression, capillaries within the villi also stained positively for PAR1, PAR2, and PAR3 (for examples see Figure 2, B and D ▶ ). 26,31,43 In the case of PAR1 and PAR2, preabsorption of each antibody with its respective immunizing peptide caused an almost complete loss of signal, indicating that the staining was specific [Figure 2, A ▶ (bottom inset) and F]. Both substitution of the PAR3 antibody with an isotype-matched control antibody and omission of the primary antibody yielded no staining (Figure 2C ▶ , bottom inset). PAR1, PAR2, and PAR3 immunoreactivity included substantial cytoplasmic staining (see magnified insets).
Figure 2.
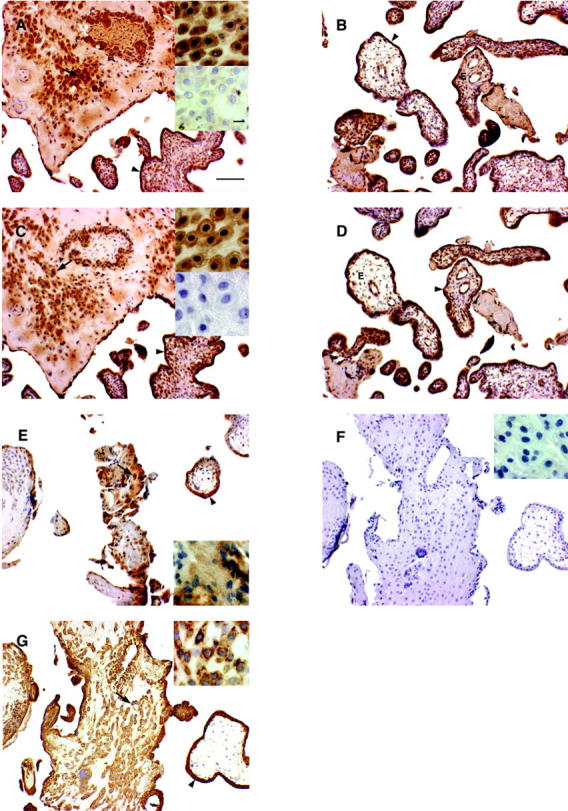
Immunoreactivity of the PAR1, PAR2, PAR3, and cytokeratin 18 in placental tissues derived from elective terminations of pregnancy at 18 to 20 weeks of gestation. Tissue sections obtained from three patients were probed with specific monoclonal antibodies as described in Materials and Methods. PAR1 immunoreactivity is shown in A and B, PAR3 staining in C and D, PAR2 in E preabsorbed PARZ antibody in F, and cytokeratin 18 staining in G. Representative EVTs are denoted with arrows (A, C, E, and G) and the syncytiotrophoblast is marked with arrowheads (B, D, and E). Note the cytoplasmic staining of PARs (top insets in A, C, and E) and the staining of the capillary endothelium (denoted with E) within the villi (examples in B and D). The bottom insets in A and C are representative staining reactions in which the PAR1-specific antibody was preincubated with excess immunizing peptide (A, bottom inset) or with only the secondary detection antibody (C, bottom inset). Results in the bottom inset of C were comparable when an isotype-matched primary antibody was used (data not shown). F: A representative staining reaction in which the PAR2-specific antibody was preincubated with excess immunizing peptide. The scale bars in A, C, and E and their insets represent 100 and 10 μm, respectively, and are consistent across all similarly sized panels in this figure.
Functional PAR in EVT Cells: Cell Signaling Cascades Induced by PAR Activation
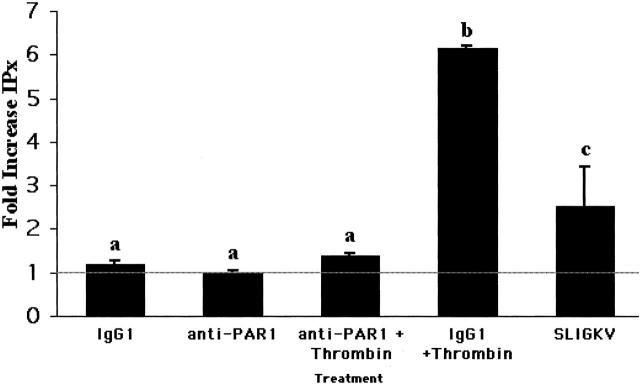
We next examined EVT responses to PAR agonists to determine whether our mRNA profiling correlated with the expression of functional PARs. Figure 3 ▶ shows that cultured EVTs respond to PAR agonists with increased phosphoinositide hydrolysis indicative of increased phospholipase C activity, a hallmark of PAR activation. 15 Because thrombin cleaves and activates PAR1 and PAR3, and because both mRNA species were detected in EVTs, we used antibodies that block PAR1 cleavage and tested for a residual thrombin response. EVTs responded equally to thrombin alone or in the presence or absence of 50 μg/ml of IgG1 control antibody, consistent with previous results in fibroblasts and human umbilical vein endothelial cells (data not shown). 26,28,37 Figure 3 ▶ shows that when EVTs were pretreated with IgG1 control antibody, followed by 20 nmol/L of thrombin, phosphoinositide hydrolysis increased more than sixfold over basal levels (P < 0.0001). In contrast, when EVTs were first incubated with PAR1-blocking antibodies and then treated with thrombin, thrombin-induced phosphoinositide hydrolysis was reduced by 94 ± 3%, a level not significantly different from buffer or IgG1 treatment (P < 0.01), and indicative of an absence of an independent functional PAR3 response by these cells. Because previous studies demonstrated that, in cells expressing PAR1 and PAR4, residual thrombin-induced increases in intracellular calcium and phosphoinositide hydrolysis are detectable in the presence of PAR1-blocking antibodies, the data presented here also support the conclusion that cultured EVTs do not express PAR4. 37
Figure 3.
PAR1 and PAR2 signal via phospholipase C in EVTs. Phosphoinositide hydrolysis in response to thrombin or the PAR2 agonist peptide SLIGKV was measured as described in the Materials and Methods. Adherent EVTs (n = 3, three different preparations/patients) were pretreated with buffer (line), an isotype control monoclonal antibody (IgG1), or PAR1-blocking antibodies (anti-PAR1). Cells were then exposed to thrombin (20 nmol/L) or SLIGKV peptide (50 μmol/L) for 45 minutes before harvesting and measurement of phosphoinositide hydrolysis. The results shown are mean ± SEM of three studies of triplicate samples expressed as the fold increase in total [3H] inositol phosphate formation compared with the results obtained in each experiment in which cells were pretreated and stimulated with buffer. a,b, Means ± SEM with different superscripts are different (P < 0.05).
Proliferation of EVTs in Response to PAR Stimulation
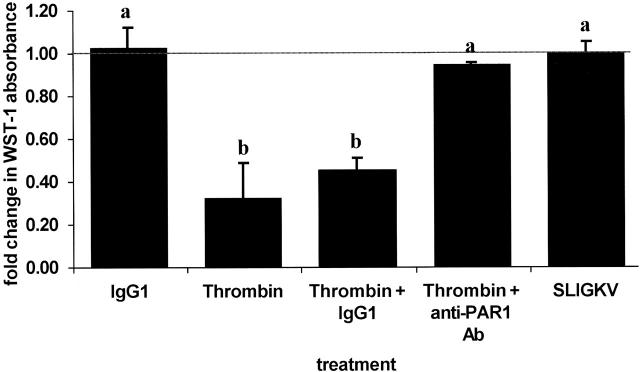
The presence of functional PAR1 and PAR2 in cultured EVTs suggests that these receptors may mediate processes in EVTs that are critical to placentation. We used WST-1, a tetrazolium dye, to assess EVT viability in culture as an indirect indicator of EVT proliferation, one such characteristic process. 45 EVT proliferation was examined after stimulation with thrombin or the PAR2-activating peptide SLIGKV at concentrations above the EC50 for activation of their respective receptors. 29,32 Figure 4 ▶ shows that EVT proliferation in culture is inhibited ∼54% by thrombin stimulation, but is unaffected by treatment with SLIGKV under the conditions tested (P < 0.05). This thrombin effect was completely prevented if the cells were first pretreated with antibodies that block PAR1 cleavage, whereas isotype-matched control antibodies had no effect on cell proliferation (P < 0.05). Measures of cellular proliferation obtained with the WST-1 reagent agree with visual cell counts of selected samples, and no evidence for thrombin- or PAR2 agonist peptide-induced cell death or toxicity was observed as monitored by trypan blue exclusion (data not shown).
Figure 4.
Thrombin treatment inhibits EVT proliferation. Cell proliferation was determined with the WST-1 reagent as described in Materials and Methods. Adherent EVTs (n = 3 or 4/treatment) were stimulated with buffer (line), an isotype control monoclonal antibody or anti-PAR1 antibody (IgG1), 10 nmol/L thrombin, thrombin in the presence of either IgG1 or the anti-PAR1-blocking antibodies, or 50 μmol/L SLIGKV peptide for 20 hours. Cells were then exposed to WST-1 reagent for 4 hours before absorbance levels were determined. The data are expressed as the fold increase in absorbance compared to buffer-treated cells. a,b, Means ± SEM with different superscripts are significantly different (P < 0.05).
Thrombin and the PAR-2 Agonist Peptide SLIGKV Stimulate EVT Invasion
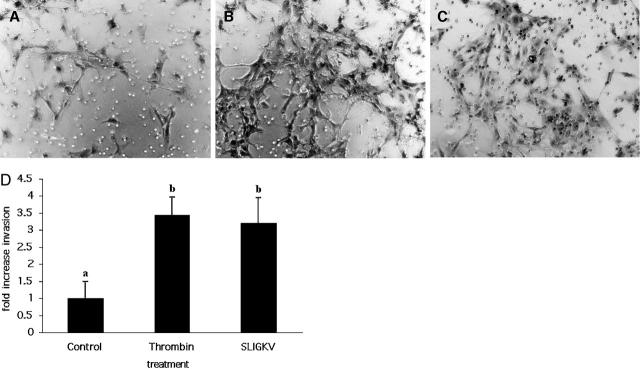
Invasive activity is central to EVT function in vivo. In culture, EVTs retain their invasive phenotype, as seen by their ability to invade artificial extracellular matrix barriers. 46 We examined the invasive capacity of EVTs cultured on Matrigel barriers under the same conditions used for the proliferation experiments described above. Figure 5 ▶ shows that both agonists stimulated EVT invasion above basal levels (Figure 5 ▶ , compare B and C with control panel A), with thrombin causing a 3.4-fold increase in EVT invasion, and SLIGKV stimulating a 3.2-fold increase (P < 0.05, Figure 5D ▶ ).
Figure 5.
Thrombin and PAR2 agonist peptide stimulate EVT invasion across Matrigel barriers. EVTs were grown in Boyden chambers, stimulated with thrombin (20 nmol/L) or SLIGKV (50 μmol/L) and invasion was quantified as described in Materials and Methods. A–C: EVTs that have traversed the Matrigel barrier and emerged on the underside of the transwell membrane after buffer (A), thrombin (B), or SLIGKV (C) treatment. Note the greater number of cells visible in the thrombin-treated (B) and PAR2 agonist-treated (C) wells compared to the buffer control well (A). D: Quantification of EVT invasion after treatment with thrombin (n = 4) or SLIGKV (n = 3) compared to control (buffer alone, n = 4). Treatments were completed in triplicate for each experiment (ie, different EVT preparations) and average cell counts for each experiment was determined by two independent observers blinded to the agonist treatment and these values were used to create the mean ± SEM shown. a,b, Means ± SEM with different superscripts are significantly different (P < 0.05).
Discussion
Successful hemochorial placentation, which occurs in rodents and primates, depends in large part on the execution of an invasive developmental program to establish the placental circulation. We used immunohistochemical methods to describe PAR protein distribution in the developing placenta, showing PAR expression in EVTs and in the syncytiotrophoblasts, suggesting a role for PARs in both the establishment and maintenance of pregnancy. These studies are complemented by in vitro assays, demonstrating an influence of PAR activation on EVT responses related to their invasive phenotype. These results support and expand on the initial observations implicating PARs in placentation. 9 We present here the novel finding that PAR1, PAR2, and PAR3 proteins are co-expressed in EVTs in normal placentae as well as in cultured EVTs. This result was obtained for tissues from both waves of placental invasion, including EVTs in placental biopsies collected during the first wave of invasion (ie, 8 to 10 weeks of gestation; data not shown). We also found that PAR1, PAR2, and PAR3 are expressed in the syncytiotrophoblast, although their role in this tissue remains undefined. PAR1 mRNA expression has been described in human placenta, 9 but PAR3 expression has not been described. Work by other groups examining multiple tissue Northern blots showed moderate amounts of PAR4 mRNA, but not PAR2 mRNA in the human placenta and uterus. 29,30,47 In contrast, we found abundant PAR2 in EVTs and syncytiotrophoblasts, but neither PAR4 mRNA nor PAR1-independent thrombin responses were detected in cultured EVTs. In the absence of evidence for its expression in cultured EVTs, we did not examine placental tissues for PAR4. It remains possible that PAR4 expression occurs in invasive EVTs in situ, but not in explanted EVTs.
Other results presented here complement our distribution studies. We show functional responses to thrombin and PAR2 agonist peptides, both of which activate phospholipase C in cultured 8- to 10-week EVTs. Despite the expression of PAR3 transcripts in EVTs, the thrombin response is apparently mediated by PAR1, suggesting that if PAR3 is expressed on the EVT cell surface, it is not able to respond to thrombin with this prototypical PAR response. This result is consistent with previous reports in which human cells express PAR3 mRNA but have no obvious thrombin response attributable to that receptor, suggesting significant complexity in the proteolytic activation of this receptor. 26,27,48 PAR1 activation elicits EVT responses typical of invasive placental EVTs. EVT proliferation was significantly retarded after exposure to thrombin, whereas invasion through Matrigel barriers increased. This pattern is consistent with previous studies of EVTs in placentation, in which an uneven distribution of proliferation markers among different trophoblast populations in first trimester villi and a cessation of EVT proliferation during invasion of the maternal deciduas was seen. 45 This description of EVTs emerging from the proximal proliferative zone of anchoring villi suggests that a suppression of proliferation accompanies the acquisition of an invasive phenotype. Our in vitro results are consistent with such a scenario, and with other studies of proliferation in invasive EVTs, including cells cultured on Matrigel. 45,49-52 Taken together, these observations offer further support for the hypothesis that PAR1 stimulation leads to increased placental invasion.
Considering the range of PAR-cleaving proteases involved in these and other protease-dependent processes, determining the proteases that activate and inactivate PARs in placentation will require substantial effort. Trophoblast interactions with maternal tissues influence the immune response at the maternal-fetal interface. 53 In turn, maternal inflammatory responses at sites of placental invasion can have dramatic effects on coagulation. A recent report by Isermann and colleagues 54 suggests that circulating maternal proteases may activate PARs to modulate EVT proliferation and invasion, thus serving as a signal to EVTs that the maternal circulation has been breached. In mice, the thrombomodulin-protein C system was found to support early pregnancy, perhaps by moderating the effects of tissue factor on placental development and preventing PAR cleavage by thrombin. The authors hypothesize that the thrombomodulin-protein C system thus influences an autocrine loop that enhances placental growth as trophoblasts encounter maternal blood. Our data are not inconsistent with this hypothesis, but the pattern of PAR expression we describe in human EVTs suggests that such an autocrine system in humans would likely use PARs differently.
Our study adds to the understanding of cellular invasion in ways that cannot be replicated in inherently variable transformed cells or animal models of placentation. Although animal models of placentation have highlighted factors that are critical across species, abundant functional redundancies and significant differences in placental anatomy between rodents and humans have been described, making it difficult to extrapolate from rodent models to humans. 12 Primary cultures of normal invasive EVTs surmount many of these complications, because their functional programs are by necessity resistant to perturbations that give rise to many transformed cell lines. 55 In vitro models of trophoblast invasion still suffer from several drawbacks, including the necessity for the removal of cytotrophoblasts or villi from their native environments and the limitations inherent to in vitro assays. 56 Despite these potential limitations, the apparent differences in EVT responses to PAR1 and PAR2 stimulation and the absence of PAR3 responses to thrombin we report here suggest a greater complexity in protease-mediated invasion than previous hypotheses predict.
From these studies we conclude first that, based on their location and function in key placental cell types, both PAR1 and PAR2 are likely to play an important role in the establishment and maintenance of the placental circulation. In addition, we describe the distribution of a second thrombin receptor, PAR3 in the developing placenta. Although PAR3 makes no apparent contribution to thrombin responses in cultured invasive trophoblasts, at least when PAR1 cleavage is blocked, its expression in these tissues provides an intriguing area for further research. Further characterization of PAR expression in failed pregnancies, pre-eclampsia, and in cases of excessive invasion including placenta acreta and choriocarcinoma will ultimately refine our understanding of PAR function in these processes.
Acknowledgments
We thank Dr. Shaun Coughlin for the generous gift of the PAR3 fusion protein immunogen, Mena Ahuja for her assistance in hybridoma production and screening, Mike D’Andrea for his assistance in establishing optimal conditions for immunohistochemistry of PAR3, and Tanya Merdiushev for taking photomicrographs.
Footnotes
Address reprint requests to Peter J. O’Brien, Laboratory for Experimental Medicine, Eli Lilly and Company, Indianapolis, IN 46285. E-mail: obrienpj@lilly.com.
Supported by the National Institutes of Health (grants HL-40387-15 to L. F. B. and HD-06274 and HD-34612 to J. F. S.), and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (no. 12671582 to H. K.).
Current address of P. J. O: Laboratory for Experimental Medicine, Lilly Research Laboratories, Indianapolis, Indiana; current address for H. K.: Comprehensive Reproductive Medicine, Graduate School, Tokyo Medical and Dental University, Tokyo, Japan.
References
- 1.Damsky CH, Fitzgerald ML, Fisher SJ: Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J Clin Invest 1992, 89:210-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pijnenborg R, Dixon G, Robertson WB, Brosens I: Trophoblastic invasion of human deciduas from 8 to 18 weeks of pregnancy. Placenta 1980, 1:3-19 [DOI] [PubMed] [Google Scholar]
- 3.Salafia SM, Minior VK, Pezzulo JC, Popek EJ, Rosenkrantz TS, Vintzileos AM: Intrauterine growth restriction in infants of less than thirty-two weeks’ gestation: associated placental pathologic features. Am J Obstet Gynecol 1995, 173:1049-1057 [DOI] [PubMed] [Google Scholar]
- 4.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A: A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol 1994, 101:669-674 [DOI] [PubMed] [Google Scholar]
- 5.Bischof P, Meisser A, Campana A: Biochemistry and molecular biology of trophoblast invasion. Ann NY Acad Sci 2001, 943:157-162 [DOI] [PubMed] [Google Scholar]
- 6.Salamonsen LA, Nie G: Proteases at the endometrial-trophoblast interface: their role in implantation. Rev Endocr Metab Disord 2002, 3:133-143 [DOI] [PubMed] [Google Scholar]
- 7.Chang C, Werb Z: The many faces of metalloproteinases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol 2001, 11:S37-S43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Rosso M, Fibbi G, Pucci M, D’Alessio S, Del Rosso A, Magnelli L, Chiarugi V: Multiple pathways of cell invasion are regulated by multiple families of serine proteases. Clin Exp Metastasis 2002, 19:193-207 [DOI] [PubMed] [Google Scholar]
- 9.Even-Ram S, Uziely B, Cohen P, Grisaru-Granovsky S, Maoz M, Ginzburg Y, Reich R, Vlodavsky I, Bar-Shavit R: Thrombin receptor overexpression in malignant and physiological invasion processes. Nat Med 1998, 4:909-914 [DOI] [PubMed] [Google Scholar]
- 10.Fazel A, Vincenot A, Malassine A, Soncin F, Gaussem P, Alsat E, Evain-Brion D: Increase in expression and activity of thrombomodulin in term human syncytiotrophoblast microvilli. Placenta 1998, 19:261-268 [DOI] [PubMed] [Google Scholar]
- 11.Genbacev O, Zhou Y, Ludlow JW, Fisher SJ: Regulation of human placental development by oxygen tension. Science 1997, 277:1669-1672 [DOI] [PubMed] [Google Scholar]
- 12.Salamonsen LA: Role of proteases in implantation. Rev Reprod 1999, 4:11-22 [DOI] [PubMed] [Google Scholar]
- 13.Lala PK, Hamilton GS: Growth factors, proteases and protease inhibitors in the maternal-fetal dialogue. Placenta 1996, 17:545-555 [DOI] [PubMed] [Google Scholar]
- 14.Lopens KM, Blaser J, Ulm K, Schmitt M, Schneider KT, Tschesche H: Proteases and their inhibitors are indicative in gestational disease. Eur J Obstet Reprod Biol 1996, 68:59-65 [DOI] [PubMed] [Google Scholar]
- 15.Hollenberg MD, Compton SJ: International Union of Pharmacology. XXVIII. Proteinase-activated receptors. Pharm Rev 2002, 54:203-217 [DOI] [PubMed] [Google Scholar]
- 16.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J: Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci USA 1994, 91:9208-9212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Brien PJ, Molino M, Kahn M, Brass LF: Protease activated receptors: theme and variations. Oncogene 2001, 20:1570-1581 [DOI] [PubMed] [Google Scholar]
- 18.Compton SJ, Renaux B, Wijesuriya SJ, Hollenberg MD: Glycosylation and the activation or proteinase-activated receptor 2 (PAR2) by human mast cell tryptase. Br J Pharmacol 2001, 134:705-718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molino M, Blanchard N, Belmonte E, Tarver AP, Abrams C, Hoxie JA, Cerletti C, Brass LF: Proteolysis of the human platelet and endothelial cell thrombin receptor by neutrophil-derived cathepsin G. J Biol Chem 1995, 270:11168-11175 [DOI] [PubMed] [Google Scholar]
- 20.Dulon S, Cande’ C, Bunnett NW, Hollenberg MD, Chignard M, Pidard D: Proteinase-activated receptor-2 and human lung epithelial cells: disarming by neutrophil serine proteinases. Am J Respir Cell Mol Biol 2003, 28:339-346 [DOI] [PubMed] [Google Scholar]
- 21.Nakayama T, Hirano K, Shintani Y, Nishimura J, Nakatsuka A, Kuga H, Takahashi S, Kanaide H: Unproductive cleavage and the inactivation of protease-activated receptor-1 by trypsin in vascular endothelial cells. Br J Pharmacol 2003, 138:121-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cumashi A, Ansuini H, Celli N, De Blasi A, O’Brien PJ, Brass LF, Molino M: Neutrophil proteases can inactivate human PAR3 and abolish the co-receptor function of PAR3 on murine platelets. Thromb Haemost 2001, 85:533-538 [PubMed] [Google Scholar]
- 23.Henrikson KP, Salazar SL, Fenton JW, II, Pentecost BT: Role of thrombin receptor in breast cancer invasiveness. Br J Cancer 1999, 79:401-406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamath L, Meydani A, Foss F, Kuliopulos A: Signaling from protease-activated receptor-1 inhibits migration and invasion of breast cancer cells. Cancer Res 2001, 61:5933-5940 [PubMed] [Google Scholar]
- 25.Even-Ram SC, Maoz M, Pokroy E, Reich R, Katz B-Z, Gutwein P, Altevogt P, Bar-Shavit R: Tumor cell invasion is promoted by activation of protease-activated receptor-1 in cooperation with α5β1 integrin. J Biol Chem 2001, 276:10952-10962 [DOI] [PubMed] [Google Scholar]
- 26.O’Brien PJ, Prevost N, Molino M, Hollinger MK, Woolkalis MJ, Woulfe DS, Brass LF: Thrombin responses in human endothelial cells: contributions from receptors other than PAR1 include the transactivation of PAR2 by thrombin-cleaved PAR1. J Biol Chem 2000, 275:13502-13509 [DOI] [PubMed] [Google Scholar]
- 27.Huang C, De Sanctis GT, O’Brien PJ, Mizgerd JP, Friend DS, Drazen JM, Brass LF, Stevens RL: Evaluation of the substrate specificity of human mast cell tryptases βI and demonstration of its importance in bacterial infections of the lung. J Biol Chem 2001, 276:26276-26284 [DOI] [PubMed] [Google Scholar]
- 28.Riewald M, Kravchenko VV, Petrovan RJ, O’Brien PJ, Brass LF, Ulevitch RJ, Ruf W: Gene induction by coagulation factor Xa is mediated by the activation of protease-activated receptor-1. Blood 2001, 97:3109-3116 [DOI] [PubMed] [Google Scholar]
- 29.Nystedt S, Emilsson K, Larsson AK, Strömbeck B, Sundelin J: Molecular cloning and functional expression of the gene encoding the human proteinase-activated receptor 2. Eur J Biochem 1995, 232:84-89 [DOI] [PubMed] [Google Scholar]
- 30.Böhm SK, Kong W, Bromme D, Smeekens SP, Anderson DC, Connolly A, Kahn M, Nelken NA, Coughlin SR, Payan DG, Bunnett NW: Molecular cloning, expression and potential functions of the human proteinase-activated receptor-2. Biochem J 1996, 314:1009-1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molino M, Woolkalis MJ, Reavey-Cantwell J, Pratico D, Andrade-Gordon P, Barnathan ES, Brass LF: Endothelial cell thrombin receptors and PAR-2: two protease-activated receptors located in a single cellular environment. J Biol Chem 1997, 272:11133-11141 [DOI] [PubMed] [Google Scholar]
- 32.Vu T-KH, Hung DT, Wheaton VI, Coughlin SR: Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 1991, 64:1057-1068 [DOI] [PubMed] [Google Scholar]
- 33.Brass LF, Pizarro S, Ahuja M, Belmonte E, Stadel J, Hoxie JA: Changes in the structure and function of the human thrombin receptor during activation, internalization and recycling. J Biol Chem 1994, 269:2943-2952 [PubMed] [Google Scholar]
- 34.Ishihara H, Connolly AJ, Zeng D, Kahn ML, Zheng YW, Timmons C, Tram T, Coughlin SR: Protease-activated receptor 3 is a second thrombin receptor in humans. Nature 1997, 386:502-508 [DOI] [PubMed] [Google Scholar]
- 35.Hoxie JA, Ahuja M, Belmonte E, Pizarro S, Parton RG, Brass LF: Internalization and recycling of activated thrombin receptors. J Biol Chem 1993, 268:13756-13763 [PubMed] [Google Scholar]
- 36.Neudeck H, Oei SL, Steimer B, Hopp H, Graf R: Binding of antibodies against high and low molecular weight cytokeratin proteins in the human placenta with special reference to infarcts, proliferation and differentiation processes. Histochem J 1997, 29:419-430 [DOI] [PubMed] [Google Scholar]
- 37.O’Brien PJ: The role of protease-activated receptors in vascular endothelial thrombin responses [dissertation]. 2000:pp 75-95 Philadelphia University of Pennsylvania
- 38.Graham CH, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK: Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res 1993, 206:204-211 [DOI] [PubMed] [Google Scholar]
- 39.Greenberg SM, Rosenthal DS, Greeley TA, Tantravahi R, Handin RI: Characterization of a new megakaryocytic cell line: the Dami cell. Blood 1988, 72:1968-1977 [PubMed] [Google Scholar]
- 40.Koi H, Zhang J, Makrigiannakis A, Getsios S, MacCalman CD, Strauss JF, III, Parry S: Syncytiotrophoblast is a barrier to maternal-fetal transmission of herpes simplex virus. Biol Reprod 2002, 67:1572-1579 [DOI] [PubMed] [Google Scholar]
- 41.Molino M, Raghunath PN, Kuo A, Ahuja M, Hoxie JA, Brass LF, Barnathan ES: Differential expression of functional protease activated receptor-2 (PAR-2) in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 1998, 18:825-832 [DOI] [PubMed] [Google Scholar]
- 42.Dean NM, Beaven MA: Method for analysis of inositol phosphates. Anal Biochem 1989, 183:199-209 [DOI] [PubMed] [Google Scholar]
- 43.Schmidt VA, Nierman WC, Maglott DR, Cupit LD, Moskowitz KA, Wainer JA, Bahou WF: The human proteinase-activated receptor-3 (PAR-3) gene—identification within a PAR gene cluster and characterization in vascular endothelial cells and platelets. J Biol Chem 1998, 273:15061-15068 [DOI] [PubMed] [Google Scholar]
- 44.Kahn ML, Nakanishi-Matsui M, Shapiro MJ, Ishihara H, Coughlin SR: Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J Clin Invest 1999, 103:879-887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bulmer JN, Morrison L, Johnson PM: Expression of the proliferation markers Ki67 and transferrin receptor by human trophoblast populations. J Reprod Immunol 1988, 14:291-302 [DOI] [PubMed] [Google Scholar]
- 46.Ringler GE, Strauss JF, III: In vitro systems for the study of human placental endocrine function. Endocr Rev 1990, 11:105-123 [DOI] [PubMed] [Google Scholar]
- 47.Xu W-F, Andersen H, Whitmore TE, Presnell SR, Yee DP, Ching AC, Gilbert T, Davie EW, Foster DC: Cloning and characterization of human protease-activated receptor 4. Proc Natl Acad Sci USA 1998, 95:6642-6646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawabata A, Saifeddine M, Al-Ani B, Leblond L, Hollenberg MD: Evaluation of proteinase-activated receptor-1 (PAR1) agonists and antagonists using a cultured cell receptor desensitization assay: activation of PAR2 by PAR1-targeted ligands. J Pharmacol Exp Ther 1999, 288:358-370 [PubMed] [Google Scholar]
- 49.Tarrade A, Goffin F, Munaut C, Lai-Kuen R, Tricottet V, Foidart JM, Vidaud M, Frankenne F, Evain-Brion D: Effect of Matrigel on human extravillous trophoblasts differentiation: modulation of protease pattern gene expression. Biol Reprod 2002, 67:1628-1637 [DOI] [PubMed] [Google Scholar]
- 50.Enders AC: Cytodifferentiation of trophoblast in the anchoring villi and trophoblastic shell in the first half of gestation in the macaque. Micros Res Tech 1997, 38:3-20 [DOI] [PubMed] [Google Scholar]
- 51.Gerbie AB, Hathaway HH, Brewer JI: Autoradiographic analysis of normal trophoblast proliferation. Am J Obstet Gynecol 1968, 100:640-645 [DOI] [PubMed] [Google Scholar]
- 52.Blankenship T, King BF: Developmental expression of Ki-67 antigen and proliferating cell nuclear antigen in macaque placentas. Dev Dyn 1994, 201:324-333 [DOI] [PubMed] [Google Scholar]
- 53.Pijnenborg R: Implantation and immunology: maternal inflammatory and immune cellular responses to implantation and trophoblast invasion. Reprod Biomed Online 2003, 4(Suppl 3):S14-S17 [DOI] [PubMed] [Google Scholar]
- 54.Isermann B, Sood R, Pawlinski R, Zogg M, Kalloway S, Degen JL, Mackman N, Weiler H: The thrombomodulin-protein C system is essential for the maintenance of pregnancy. Nat Med 2003, 9:331-337 [DOI] [PubMed] [Google Scholar]
- 55.Paria BC, Reese J, Das SK, Dey SK: Deciphering the cross-talk of implantation: advances and challenges. Science 2002, 296:2185-2188 [DOI] [PubMed] [Google Scholar]
- 56.Bischof P, Meisser A, Campana A: Mechanisms of endometrial control of trophoblast invasion. J Reprod Fertil 2000, 55:S65-S71 [PubMed] [Google Scholar]