Abstract
During routine assessment of the hormonal phenotype of breast carcinomas, we detected expression of the estrogen receptor (ER) in the germinal centers of reactive lymphoid follicles surrounding malignant foci. To confirm and extend this finding, we compared ER-α, progesterone receptor (PR), and androgen receptor (AR) immunostaining in hyperplastic or metastatic lymph nodes obtained from patients with various pathology, disease location, gender and age. Irrespective of these parameters, we found that: 1) ER-α-positive cells were located prevalently in germinal centers, 2) the PR was weakly expressed by cells within and surrounding germinal centers, and 3) the androgen receptor was undetectable. Transcripts for ER-α and PR were also detected by reverse transcription-polymerase chain reaction on laser-microdissected lymph node germinal centers. Morphologically, the ER-positive cells resemble dendritic cells and by double immunostaining were found to express both CD21 and CD23, which is characteristic of follicular dendritic cells. Finally, we assessed the effects of Tamoxifen treatment by comparing the numbers of ER-positive follicular dendritic cells in lymph nodes obtained from breast cancer patients before and after treatment. The results show that Tamoxifen treatment generated larger germinal centers with more abundant ER+/CD21+/CD23+ cells. Taken together, these results open new perspectives on the effects of sex steroids and their antagonists on the human response in cancer and inflammation.
Dendritic cells (DC) play a pivotal role as antigen-presenting cells in the immune response. The heterogeneity of DC subsets and lineages, and the functional plasticity of these cells at the immature stages, are the basis of the diverse functions of DC. 1
The endocrine system participates in regulating the processes of maturation of different DC subtypes, eg, thyroid-stimulating hormone (TSH) induces a stimulatory effect on phagocytosis and cytokine production in murine DC, which are known to express functionally active TSH receptors. 2 In addition, mRNAs for estrogen receptor (ER)-α and -β have been demonstrated by reverse transcription-polymerase chain reaction (RT-PCR) analysis in CD14+ monocytes, cultured immature CD1a+ cells and mature CD83+ cells. 3 In culture, bone marrow stromal cells give rise to extensive and long-term production of DC 4 which are influenced by androgen and estrogen, and contain splice-variant transcripts of ER-α and ER-β mRNAs. 5,6
Two different subtypes of DC have been described within the germinal centers of lymphoid follicles: follicular dendritic cells (FDC) and so-called germinal center dendritic cells (GCDC). FDC are capable of binding and retaining antigen in the form of immune complexes for future presentation to B cells and their subsequent rescue from apoptosis. 7 FDC express the low-affinity IgE receptor (CD23), and the C3d (CD21) and C3b (CD35) complement receptors. GCDC are distinct from FDC because they have a different immunophenotype (CD4+, CD11c+, and CD3−) and function. 8
During the immunocytochemical evaluation of the ER status in samples of breast carcinoma tissue, we observed weak ER-α expression in germinal center cells present within tumor-infiltrating lymphoid cells organized in follicles. The morphological features of these ER+ cells suggested their DC origin. 9 By double immunostaining, we confirmed that ER expression mostly coincided with CD21+ CD23+ FDC of germinal centers in reactive lymph nodes and in lymphoid-associated tumor infiltrates in breast cancer. Given the above results, we also evaluated the effects of anti-estrogen treatment in breast cancer patients on the process of FDC maturation and germinal center formation.
Materials and Methods
Specimens
Reactive and metastatic lymph nodes were obtained from the pathology archives of the Department of Biomedical Science and Human Oncology, University of Turin Medical School. The main criterion adopted in selecting lymph nodes for steroid hormone receptor analysis was the morphological identification, in hematoxylin and eosin (H&E)-stained sections, of at least one secondary follicle with germinal centers. From 20 patients with either cancer or inflammatory disease, we examined a total of 264 lymph nodes and 43 were selected for immunocytochemical analysis. Details of the patients’ clinical data, anatomical site, and disease affecting the lymph node are listed in Table 1 ▶ . Also included in this study but not listed in Table 1 ▶ are three patients with primary breast carcinomas (see Tamoxifen-treated cases) and two patients with FDC sarcoma, one splenic and one nodal, in which steroid hormone receptor expression was studied.
Table 1.
Clinical Data, Primary Lesion, and Histopathology of the Regional Lymph Nodes Selected for Immunocytochemical Analysis of ER Expression in GC Cells of 20 Patients
| Patient number | Sex | Age | Primary pathology | Number and pathology of LN with GC | Mean number of ER+ cells per GC |
|---|---|---|---|---|---|
| 1 | F | 86 | Colon adenocarcinoma | 3/Hyperplasia | 32 ± 5 |
| 2 | F | 45 | Breast IDC | 2/Metastasis | 12 ± 2 |
| 3 | F | 67 | Breast IDC | 1/Metastasis | 15 ± 2 |
| 4 | F | 51 | Breast IDC | 2/Metastasis | 28 ± 9 |
| 5 | F | 54 | Breast IDC | 3/Hyperplasia | 23 ± 3 |
| 6 | M | 58 | Skin melanoma | 3/Hyperplasia | 35 ± 7 |
| 7 | M | 66 | Gastric adenocarcinoma | 4/Hyperplasia | 8 ± 2 |
| 8 | M | 46 | Skin melanoma | 2/Hyperplasia | 14 ± 6 |
| 9 | F | 69 | Breast IDC | 4/Hyperplasia | 13 ± 7 |
| 10 | F | 79 | Breast ILC | 2/Metastasis | 41 ± 10 |
| 11 | F | 69 | Breast ILC | 2/Hyperplasia | 18 ± 2 |
| 12 | F | 63 | Breast IDC | 3/Hyperplasia | 49 ± 7 |
| 13 | F | 70 | Pancreas adenocarcinoma | 3/Hyperplasia | 28 ± 4 |
| 14 | F | 80 | Breast IDC | 1/Hyperplasia | 10 ± 2 |
| 15 | F | 36 | Latero cervical adenopathy | 1/Hyperplasia | 30 ± 8 |
| 16 | M | 23 | Peritoneal inflammation | 1/Hyperplasia | 11 ± 5 |
| 17 | F | 32 | Thyroid papillary carcinoma | 2/Hyperplasia | 36 ± 7 |
| 18 | M | 79 | Colon adenocarcinoma | 2/Hyperplasia | 20 ± 5 |
| 19 | M | 60 | Pancreas adenocarcinoma | 1/Hyperplasia | 21 ± 3 |
| 20 | F | 77 | Gallbladder inflammation | 1/Hyperplasia | 24 ± 3 |
IDC, infiltrating ductal carcinoma; ILC, infiltrating lobular carcinoma; GC, germinal centers; LN, lymph node.
Tamoxifen-Treated Cases
To evaluate the effects of Tamoxifen on FDC, we analyzed expression of the ER, CD21, and CD23 by immunocytochemistry in lymph nodes obtained before and after Tamoxifen therapy in three patients (designated Tam-1, Tam-2, and Tam-3) with primary breast carcinomas. Patient Tam-1 was treated for 5 years with Tamoxifen alone for an ER+ invasive ductal carcinoma without lymph node metastases. During therapy the patient developed a contralateral ER+ invasive ductal carcinoma and underwent tumor excision together with sentinel lymph node biopsy. Patients Tam-2 and Tam-3 received chemotherapy plus Tamoxifen for an ER+ lobular invasive carcinoma with lymph node metastases; both patients (Tam-2 after 4 years, Tam-3 after 5 years) developed an invasive lobular carcinoma in the contralateral breast and underwent axillary lymph node dissection.
Immunocytochemistry
All specimens were fixed in 4% formalin and embedded in paraffin. For immunocytochemistry, the endogenous peroxidase in formalin-fixed, paraffin-embedded tissue was inhibited by incubating specimens with 3% H2O2 for 5 minutes before incubation with the primary monoclonal antibody (mAb). Sections were incubated with a primary anti-ER-α mAb clone 1D5, diluted 1:100 (DAKO, Glostrup, Denmark) whose epitope maps to the N-terminal region of the ER, 10 and anti-progesterone receptor (PR) mAb clone 1A6, diluted 1:10, and anti-androgen receptor (AR) mAb clone F39.4.1, pre-diluted, both from Biogenex (San Ramon, CA). Specimens that reacted with the anti-ER-α mAb were further tested with the anti-ER-α polyclonal antibody MC-20, diluted 1:1000 (Santa Cruz Biotechnology, Santa Cruz, CA) whose epitope maps to the C-terminal region of the ER. 10
Immunophenotyping of ER+ Cells
In selected cases, two-step double immunostaining was performed to evaluate which cell type expressed ER-α. The immunophenotype of the ER+ germinal center cells was studied with antibodies specific for the ER, CD21 (clone 1F8), diluted 1:20; CD20, a B-cell marker (clone L26), diluted 1:200; and CD3, a T-cell marker (polyclonal), diluted 1:50, all purchased from DAKO; CD23 (clone BU.38; The Binding Site, San Diego, CA), pancytokeratin (clone KL1) and CD1a (Immunotech, Marseille Cedex, France).
In step one, ER binding was revealed with a 3,3′-diaminobenzidine (DAB) solution without nickel (brown precipitate). The sections were then washed in phosphate-buffered saline (PBS), incubated for 30 minutes at 37°C in 0.01 mol/L HCl and washed thoroughly with water (∼2 hours) to remove unbound antibody that could potentially interfere with the second part of the immunocytochemical reaction. In the second step, binding of KL1, CD23, and CD3 was visualized with a solution of DAB and 0.3% NiCl2 in 50 ml of PBS pH 7.4 (black precipitate). Double staining was also performed by inverting the ER (black precipitate) and CD21 and CD20 (brown precipitate) revealing solutions.
Microdissection and RNA Extraction
Expression of the genes for ER-α and PR in germinal center cells was evaluated by specific RT-PCR analysis of total RNA extracted from germinal centers microdissected from two reactive, non-metastatic lymph nodes from patient 8 (cutaneous melanoma). Lymph nodes were longitudinally bisected through the hilus; one half was immediately snap-frozen and the other was fixed in 4% formalin so that ER expression could first be evaluated by immunocytochemistry to confirm the presence of the protein. The frozen lymph node halves were microdissected to select pure cell populations of germinal centers. Five-μm-thick sections were collected on thin glass coverslips, briefly stained with H&E, and all microscopically recognizable germinal centers were isolated by microdissection with a laser micromanipulator (Olympus/Cell Robotics Inc., Albuquerque, NM). Both microdissected germinal centers and the residual diffuse lymphoid tissue were collected in separate microcentrifuge tubes and total RNA was extracted using Trireagent solution (Genomed, Celbio, Pero, Italy) according to the manufacturer’s instructions. RNA concentration was measured spectrophotometrically by determination at 260/280 nm, its purity and integrity checked by electrophoresis on a 1.5% agarose gel.
RT-PCR
ER and PR were amplified by RT-PCR as previously described. 11,12 Briefly, 1 μg of each RNA was reverse transcribed at 42°C for 45 minutes, using 2 U avian myeloblastosis virus reverse transcriptase (AMV-RT; Finnzymes Oy, Espoo, Finland) in the presence of a gene-specific antisense primer downstream of the region to be amplified. These primers were: rt-ER 5′-ACTCCAGAATTAAGC-3′ [corresponding to nucleotides (nt) 1665–1651 of the human cDNA sequence (GenBank accession number: M12674)]; rt-PR 5′-CGAAAACCTGGC-3′, corresponding to nt 2394–2383 of the cDNA sequence (GenBank accession number M15716). Using 10% of the RT reaction, PCR was carried out with 2.5 U/tube TaqDNA polymerase (Finnzymes) and the following primers: ER forward primer: 5′-CATAACGACTATATGTGTCCAGCC-3′ (nt 938–961); ER reverse primer: 5′-AACCGAGATGATGTAGCCAGCAGC-3′ (nt 1596–1573); PR forward primer: 5′- TGCTCAAGGAGGGCCTGCCGCAGGT-3′ (nt 1761–1785); PR reverse primer 5′-CTACTGAAAGAAGTTGCCTCTCGCC-3′ (nt 2363–2339). Amplification was carried out for 35 cycles of denaturation at 94°C, annealing at 65°C and extension at 72°C, for 30 seconds each. RT-PCR for β-actin was performed as control. PCR products were separated by electrophoresis on a 1.5% agarose gel stained with ethidium bromide. Gels were photographed and bands analyzed with the PC program Kodak Science 1D Image System (Kodak, Rochester, NY). The net intensity of receptor bands was normalized to the intensity of the β-actin band before comparing bands obtained from two different samples.
Results
Immunolocalization of Sex Steroid Hormone Receptors
To evaluate ER expression in germinal centers from lymph nodes affected by malignant or inflammatory diseases, 43 lymph nodes with morphological evidence of secondary follicles were obtained from 20 patients as detailed in Table 1 ▶ . Specific nuclear expression of ER-α was observed in germinal center cells by immunohistochemistry using either the 1D5 mAb or the MC-20 polyclonal antibody. PR+ cells were both rare and barely visible, inside and outside the germinal centers, while AR expression could not be detected. As shown in Table 1 ▶ , the results were not affected by the primary pathology, the anatomical site of the lymph node, the patient’s gender, or age. The ER+ cells were constantly clustered in areas corresponding to germinal centers in secondary follicles; in contrast, the perisinusoidal and interfollicular areas showed only rare ER+ and PR+ cells. Primary follicles, composed of unstimulated naïve B lymphocytes, were ER−. The number of ER+ cells within germinal centers ranged from 8 ± 2 to 49 ± 7 (Table 1) ▶ , their number increasing with the increasing diameter of the germinal center. Morphologically, ER+ cells displayed clear thin cytoplasm and evident nuclear incisions or multiple nuclei, strongly resembling DC (Figure 1, A and B) ▶ .
Figure 1.
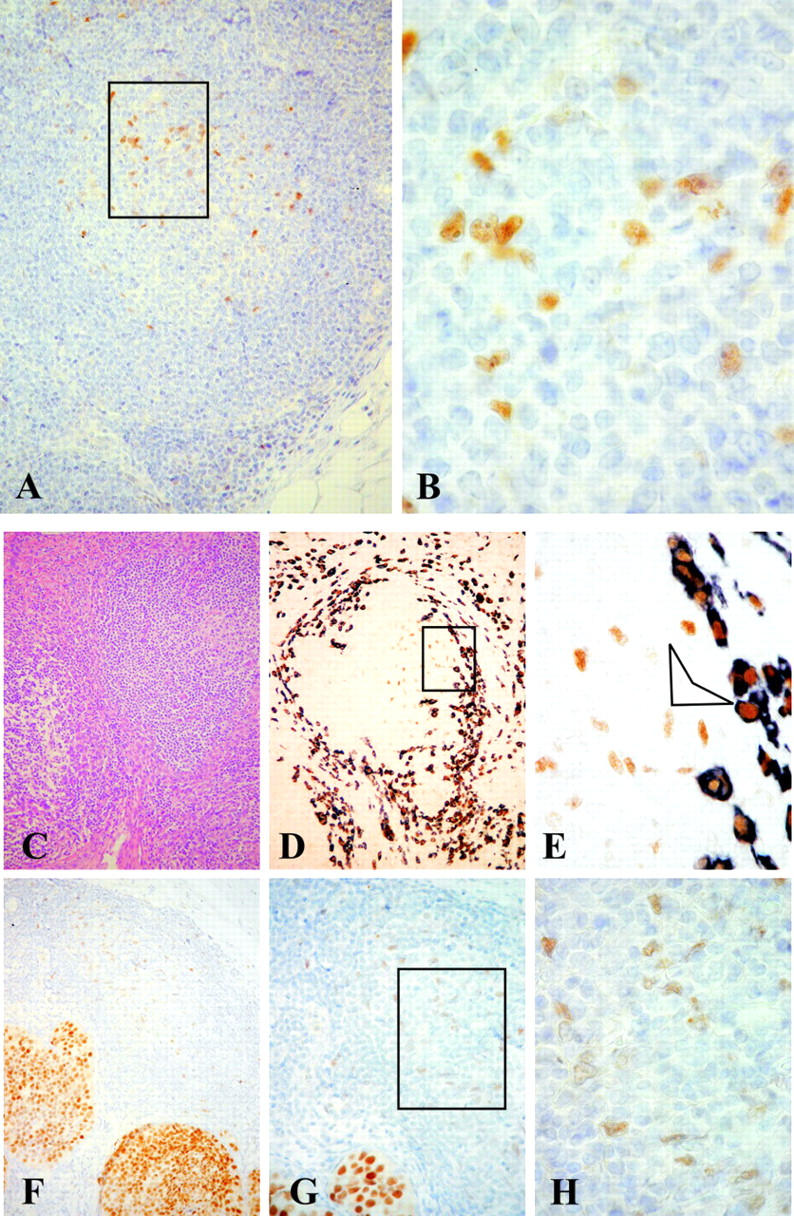
ER-α immunolocalization in hyperplastic lymph node (patient 6, cutaneous melanoma). A: ER+ cells are clustered in areas corresponding to secondary follicles with prominent germinal centers (inset). B: ER+ cells display clear thin cytoplasm with evident nuclear incisions or multiple nuclei. Original magnification, ×4 (A) and ×100 (B). Phenotypic analysis of ER+ cells in metastatic lymph node (patient 10, in situ lobular breast carcinoma). C: H&E. D and E: Double immunostaining for keratin (black, cytoplasmic) and ER-α (brown, nuclear). Keratin+ metastatic cells, surrounding a germinal center, show intense ER nuclear staining. In contrast, keratin− DC within the germinal center show light ER nuclear staining (D, inset; E, arrow), indicating lower expression of the receptor. Original magnification, ×4 (C and D), and ×100 (E). ER expression in extra-nodal lymphatic tissue (patient 10, in situ breast carcinoma). F to G: ER immunostaining. Nuclei of germinal center cells show light staining, confirming weak expression of the receptor in these cells compared to the intense nuclear expression of ER in carcinoma cells. Original magnification, ×4 (F), ×20 (G), and ×100 (H).
As shown in Table 1 ▶ , the lymph nodes analyzed from patients 2, 3, 4, and 10 (all breast carcinomas) still showed residual secondary follicles despite the presence of metastases (Figure 1C) ▶ . In patients 3 and 10, both the nodal metastases and the primary breast carcinomas expressed ER. By double immunostaining, the metastatic cells were outlined by cytoplasmic keratin staining and showed intense ER nuclear expression (Figure 1, D and E) ▶ . In contrast, DC were negative for keratin immunostaining and ER nuclear expression was weak (Figure 1D ▶ , inset, and E, arrow). Similar low-level expression of ER-α was found in germinal centers of follicles in tumor-infiltrating lymphoid tissue present around ER+ (Figure 1, F to H) ▶ and ER− foci of primary invasive and in situ breast carcinomas additionally studied (data not shown). These results suggest that DC are characterized by lower ER expression than epithelial cells. No steroid hormone receptors were detected in the two CD21+ FDC sarcomas tested.
Immunophenotype of ER+ Cells
The immunophenotype of ER+ cells was defined by means of double-immunocytochemical staining with the anti-ER mAb in combination with one of the following mAbs: CD20, CD3, CD1a, CD21, and CD23. Firstly, by double immunostaining with either CD20 or CD3, the ER+ cells were found to be mostly confined within the B-cell follicles (Figure 2, A to H) ▶ whereas no ER expression was observed in CD1a+ interdigitating DC outside the germinal centers (data not shown). Most ER+ cells within germinal centers were negative for CD3 and CD20 (Figure 2, A to D) ▶ , most were CD21+ (Figure 2, E and F) ▶ and 10% to 50% were CD23+ (Figure 2, G and H) ▶ . These data confirm that at least a fraction of ER+ cells were FDC.
Figure 2.

Phenotype analysis of GC ER+ cells by double-immunocytochemical staining with antibodies for CD20, CD3, CD21, and CD23 in hyperplastic lymph node (patient 20, gall bladder inflammation). A and B: The ER+ cells (black nuclear stain) within the B-cell follicle are CD20− (brown cytoplasmic stain). C and D: CD3+ cells (black cytoplasmic stain) do not show any expression of ER (brown nuclear stain). E and F: ER+ cells (black nuclear stain) express CD21 (brown cytoplasmic stain), suggesting an FDC phenotype. G and H: In 10% to 50% of ER+ cells (brown nuclear stain) CD23 (black cytoplasmic stain) is co-expressed, confirming that at least a fraction are FDC. Original magnification, ×10 (A, C, E, G) and ×100 (B, D, F, H). RT-PCR analysis of ER- and PR-RNA in a reactive lymph node (patient 8, cutaneous melanoma). I: Germinal center (GC) with clustered ER+ cells (circle) and residual lymphoid tissue (LT) containing single ER+ cells (boxes) before microdissection. Original magnification, ×20. J: Gel photograph of RT-PCR analysis of RNA from GC and LT for ER-α, PR, and β-actin, from left to right. MW represents molecular weight marker 100-bp ladder.
RT-PCR Analysis of ER-α and PR mRNA
To further validate the above observations, ER-α and PR mRNA expression was analyzed by RT-PCR using laser-microdissected germinal centers (Figure 2, I and J ▶ , lanes labeled GC) and the surrounding lymphoid tissue (Figure 2, I and J ▶ , lanes labeled LT) obtained from patient 8 (reactive lymph node in cutaneous melanoma). Amplification of the ER yielded the expected 0.65-kb pair band corresponding to the full-length ER-α. An additional, weaker 0.5-kb band was also detected, which probably corresponds to an ER splice variant. PR amplification yielded the expected 0.6-kb band. By visual inspection, the β-actin control bands were of similar intensity in the GC and LT lanes and though not quantitative, the ER-α band in germinal center microdissected samples was reproducibly ∼2-fold more intense than the ER-α band obtained from residual diffuse lymphoid tissue samples. On the other hand, PR bands were weak but detectable and equivalent in intensity in both specimens.
Effects of Tamoxifen Treatment on ER+ FDC
The effects of Tamoxifen on ER+ FDC was indirectly evaluated by comparing lymph nodes obtained before and after anti-estrogen treatment in patients with consecutive (∼4 to 5 years apart) bilateral breast carcinomas. Before Tamoxifen treatment, we observed few, small GC in lymph nodes from patient Tam-1 with rare ER+/CD21+ and ER+/CD23+ FDC. No lymph node metastases were observed. The sentinel lymph node obtained after 5 years of treatment with Tamoxifen alone showed diffuse hyperplasia of the lymphoid tissue with enlargement of the sinuses but no germinal center formation. Consequently, neither CD23+ nor CD21+ FDC were observed (Figure 3, A and B) ▶ . A breast cancer ER+ micrometastasis was present in the sentinel lymph node (Figure 3C) ▶ .
Figure 3.
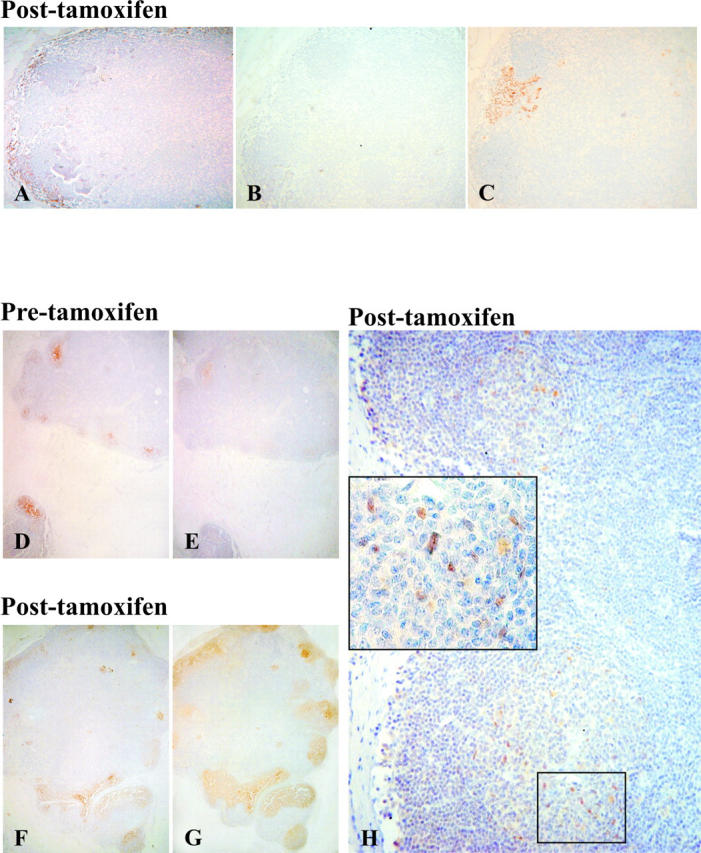
Effects of Tamoxifen treatment on FDC. A to C: Sections of a lymph node after Tamoxifen treatment (patient Tam-1). After 5 years treatment with Tamoxifen alone the lymph node shows diffuse hyperplasia with enlargement of the sinuses, without GC formation and absence of FDC as demonstrated by negative immunostaining for CD21 (A) and CD23 (B). C: The same lymph node presents a breast cancer micrometastasis with intense nuclear staining for the ER, whereas lymphoid cells are negative. Original magnification, ×4 (A to C). D to H: Sections of a lymph node before and after Tamoxifen treatment (patient Tam-2). Before treatment, germinal centers are small as outlined by immunoreactivity for CD23 (D) and CD21 (E). In a lymph node from the same patient, excised during Tamoxifen treatment, CD21 (F) and CD23 (G) immunostaining outlines large germinal centers. H: Inset, ER+ cells are present inside and outside the GC. Original magnification, ×10 (D to H); ×40 (H, inset).
A different morphological pattern was observed in lymph nodes obtained from patients Tam-2 and Tam-3 who were initially treated with Tamoxifen plus chemotherapy and subsequently with Tamoxifen alone for 4 and 5 years, respectively. Before medical treatment, lymph node germinal centers were small (Figure 3, D and E) ▶ . Contralateral axillary lymph nodes excised from patient Tam-2 during Tamoxifen treatment were all free of metastasis while in patient Tam-3, three of 18 nodes had metastases. In both patients, reactive lymph nodes showed a mean of six germinal centers per lymph node. Germinal centers were larger than before therapy, as demonstrated by CD21 and CD23 immunostaining (Figure 3, F and G) ▶ , and ER+ cells were present inside and outside the germinal center (Figure 3H) ▶ .
Discussion
Much of what is known about the presence of the ER in dendritic cells has been learned from in vitro DC culture models and is limited to the demonstration of the specific mRNA transcript by RT-PCR. 3,13 In the present work, we demonstrated the nuclear expression of ER in germinal center DC within activated follicles in both lymph nodes and tissue-infiltrating lymphocytes surrounding breast carcinoma foci. The specificity of the reaction was supported by the fact that the ER was reproducibly detected using both mono- and polyclonal antibodies that bind different domains of the receptor. Furthermore, our observations are not part of a generalized feature of sex hormone receptors, being mostly limited to the ER while PR expression was barely detectable by immunocytochemistry and AR expression was not observed at all. Concurrently, RT-PCR amplification confirmed the presence of ER-α mRNA in microdissected germinal centers where amplified bands were reproducibly stronger than in the interfollicular areas of lymph nodes containing ER+ cells in the presence of equivalently intense β-actin control bands in the two specimens.
We also showed that the majority of ER+ cells in GC were CD21+ FDC, whereas only a fraction was CD23+. FDC have heterogeneous morphology and immunophenotype, depending on their localization within the follicle and on the stage of the germinal center reaction. The FDC probably involved in antigen presentation inside and outside the germinal center are characterized by CD21 expression, while the functionally more mature FDC within the germinal center express CD23, a molecule involved in later stages of the immune response. 14 The two FDC sarcomas studied here were ER-negative. Our results suggest that the ER is already expressed in the early stages of FDC maturation, while it is lost in transformed FDC. However, in a patient with Hodgkin’s disease, Maia et al 15 described specific ER immunoreactivity in Reed-Sternberg cells and in cells from non-neoplastic reactive germinal centers, which were interpreted as variably sized lymphocytes. Interestingly, other authors have suggested that Reed-Sternberg cells originate from FDC in a small percentage of Hodgkin’s disease. 16,17
In previous studies that evaluated the presence of DC in primary tumors, immature CD1a+ DC were found to reside within the tumor mass in breast and colon carcinomas, whereas mature CD83+ DC were located in peritumoral areas. 18,19 In agreement with a recent report, 20 we demonstrated that FDC can also be detected in the peritumoral area of a primary breast cancer, when tissue-infiltrating lymphocytes are arranged in follicular-like structures with germinal centers. Recently, it was shown that seeding with FDC or their precursors represents the critical step in germinal center formation at extranodal sites. 21
The expression of the ER-α in germinal center FDC was not affected by either the age or sex of the patient. Furthermore, ER-α expression was observed in germinal centers of lymph nodes excised because of both non-neoplastic and neoplastic diseases, the latter for staging tumors of different histological type (carcinomas and melanomas) and anatomical origin (breast, GI tract, skin). A previous study described a cell population, located in germinal centers and the subcapsular and cortical sinuses, which showed immunoreactivity for an ER-associated protein in lymph nodes from breast cancer patients. Immunofluorescence phenotyping of these cells characterized the germinal center cells as FDC; the others were macrophages. 22
The role exerted by the ER-α on DC on stimulation by specific hormones or by non-steroidal modulators has not yet been fully clarified. For example, it is known that the presence of ER-α in bone marrow stroma is necessary for the maintenance of normal proportions of B lymphocytes, possibly by mediating the production of growth factors and/or cytokines. 23 It has also been reported that the ER can interact with the transcription factor NF-κB, 24 which is a key regulator of the inflammatory response and an effective target for blocking DC antigen presentation and inhibiting T-cell-dependent immune responses. In addition, in rheumatoid arthritis, where FDC have been described in inflamed synovial stroma, 25 Tamoxifen, a non-steroidal anti-estrogen, inhibits the differentiation of synovial macrophages into DC and the capacity of synovial macrophage-derived DC to stimulate allogeneic T cells. 13 Anti-estrogens can also inhibit terminal maturation of CD14+ DC. 3 Depending on the target cells, Tamoxifen can act either as an estrogen agonist or antagonist. In breast cancer, anti-estrogens are widely used because their inhibitory effect on cancer cell proliferation antagonizes the estrogenic stimulatory effect.
A major problem in the treatment of breast cancer is Tamoxifen resistance, which has been suggested to be linked to splicing of wild-type ER mRNA. Many ER splicing variants, lacking one or more exons, have been identified in normal and breast cancer tissues. 26 The exact functional role of these splice variants has not been established but several lines of evidence suggest that some may be involved in estrogen-induced signal transduction. 27,28 An ER variant lacking exon 5 has been detected not only in breast tissue but also in peripheral blood mononuclear cells 29 while the exon 4-deleted variant is strongly expressed in breast tissue. Our experiments of ER-α amplification yielded a major 0.6-kb band and a minor 0.5-kb band, which could be interpreted as a splice variant band in RNA from germinal centers not detectable in the surrounding diffuse lymphoid tissue. This preliminary result sheds little light on the functional significance of the expression of an ER splicing variant in germinal center cells. On the other hand, the data on Tamoxifen-treated patients indirectly suggest a stimulatory effect of Tamoxifen on FDC mediated by the ER. The interaction between Tamoxifen and the ER can be inferred by the fact that in reactive lymph nodes without germinal center formation (eg, in patient Tam-1), Tamoxifen has no effect because the target cells are absent. On the contrary, the data obtained in the other two cases suggest that when ER+ target cells are present, long-term Tamoxifen treatment may be responsible for the formation of large germinal centers, rich in CD21+ and CD23+ cells. This finds some analogy in the enhanced proliferation of activated T cells induced by Tamoxifen and another anti-estrogen, Toremifene, through increased surface expression of tumor necrosis factor receptor 2. 30 However, one should also entertain the possibility of an interaction between the drugs that are part of the therapeutic protocols for advanced breast cancer and Tamoxifen because all patients with increased FDCs had received chemotherapy and Tamoxifen.
Our results suggest a possible role of the ER in FDC clustering and germinal center formation, hinting to a novel set of immune-endocrine interactions where the ER may play a role in immune surveillance, either by inhibiting or by activating the functional differentiation of FDC.
Acknowledgments
We thank Carla Pecchioni and Milena Cerrato for expert technical assistance.
Footnotes
Address reprint requests to Anna Sapino, M.D., Department of Biomedical Science and Human Oncology, University of Torino, Via Santena 7, 10126 Torino, Italy. E-mail: anna.sapino@unito.it.
Supported by grants from Ministry of University, Instruction and Research (MIUR), Rome, Italy and by the Special Project “Oncology” Compagnia San Paolo/FIRMS, Torino, Italy.
L.R. is member of a Ph.D. program in Human Oncology.
References
- 1.Liu YJ: Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell 2001, 106:259-262 [DOI] [PubMed] [Google Scholar]
- 2.Bagriacik EU, Klein JR: The thyrotropin (thyroid-stimulating hormone) receptor is expressed on murine dendritic cells and on a subset of CD45RB high lymph node T cells: functional role for thyroid-stimulating hormone during immune activation. J Immunol 2000, 164:6158-6165 [DOI] [PubMed] [Google Scholar]
- 3.Komi J, Lassila O: Nonsteroidal anti-estrogens inhibit the functional differentiation of human monocyte-derived dendritic cells. Blood 2000, 95:2875-2882 [PubMed] [Google Scholar]
- 4.Hagihara M, Li C, Gansuvd B, Munkhbat B, Inoue H, Shimakura Y, Tsuchiya T, Ueda Y, Oki M, Ando K, Kato S, Hotta T: Extensive and long-term ex vivo production of dendritic cells from CD34-positive umbilical cord blood or bone marrow cells by novel culture system using mouse stroma. J Immunol Methods 2001, 253:45-55 [DOI] [PubMed] [Google Scholar]
- 5.Smithson G, Medina K, Ponting I, Kincade PW: Estrogen suppresses stromal cell-dependent lymphopoiesis in culture. J Immunol 1995, 155:3409-3417 [PubMed] [Google Scholar]
- 6.Smithson G, Couse JF, Lubahn DB, Korach KS, Kincade PW: The role of estrogen receptors and androgen receptors in sex steroid regulation of B lymphopoiesis. J Immunol 1998, 161:27-34 [PubMed] [Google Scholar]
- 7.Kim HS, Zhang X, Klyushnenkova E, Choi YS: Stimulation of germinal center B lymphocyte proliferation by an FDC-like cell line, HK. J Immunol 1995, 155:1101-1109 [PubMed] [Google Scholar]
- 8.Grouard G, Durand I, Filgueira L, Banchereau J, Liu YJ: Dendritic cells capable of stimulating T cells in germinal centres. Nature 1996, 384:364-367 [DOI] [PubMed] [Google Scholar]
- 9.Gerdes J, Stein H, Mason DY, Ziegler A: Human dendritic reticulum cells of lymphoid follicles: their antigenic profile and their identification as multinucleated giant cells. Virchows Arch B Cell Pathol Incl Mol Pathol 1983, 42:161-172 [DOI] [PubMed] [Google Scholar]
- 10.Huang A, Leygue E, Snell L, Murphy LC, Watson PH: Expression of estrogen receptor variant mRNAs and determination of ER status in human breast cancer. Am J Pathol 1997, 150:1827-1833 [PMC free article] [PubMed] [Google Scholar]
- 11.Pfeffer U, Fecarotta E, Vidali G: Coexpression of multiple estrogen receptor variant messenger RNAs in normal and neoplastic breast tissues and in MCF-7 cells. Cancer Res 1995, 55:2158-2165 [PubMed] [Google Scholar]
- 12.Cassoni P, Catalano MG, Sapino A, Marrocco T, Fazzari A, Bussolati G, Fortunati N: Oxytocin modulates estrogen receptor α expression and function in MCF7 human breast cancer cells. Int J Oncol 2002, 21:375-378 [PubMed] [Google Scholar]
- 13.Komi J, Mottonen M, Luukkainen R, Lassila O: Non-steroidal anti-oestrogens inhibit the differentiation of synovial macrophages into dendritic cells. Rheumatology (Oxford) 2001, 40:185-191 [DOI] [PubMed] [Google Scholar]
- 14.Bagdi E, Krenacs L, Krenacs T, Miller K, Isaacson PG: Follicular dendritic cells in reactive and neoplastic lymphoid tissues: a reevaluation of staining patterns of CD21, CD23, and CD35 antibodies in paraffin sections after wet heat-induced epitope retrieval. Appl Immunohistochem Mol Morphol 2001, 9:117-124 [DOI] [PubMed] [Google Scholar]
- 15.Maia DM, Sciarrotta J, Abendroth K, Blatt J: Sex steroid receptors in Hodgkin’s disease. Leuk Lymphoma 2000, 39:365-371 [DOI] [PubMed] [Google Scholar]
- 16.Nakamura S, Nagahama M, Kagami Y, Yatabe Y, Takeuchi T, Kojima M, Motoori T, Suzuki R, Taji H, Ogura M, Mizoguchi Y, Okamoto M, Suzuki H, Oyama A, Seto M, Morishima Y, Koshikawa T, Takahashi T, Kurita S, Suchi T: Hodgkin’s disease expressing follicular dendritic cell marker CD21 without any other B-cell marker: a clinicopathologic study of nine cases. Am J Surg Pathol 1999, 23:363-376 [DOI] [PubMed] [Google Scholar]
- 17.Delsol G, Meggetto F, Brousset P, Cohen-Knafo E, al Saati T, Rochaix P, Gorguet B, Rubin B, Voigt JJ, Chittal S: Relation of follicular dendritic reticulum cells to Reed-Sternberg cells of Hodgkin’s disease with emphasis on the expression of CD21 antigen. Am J Pathol 1993, 142:1729-1738 [PMC free article] [PubMed] [Google Scholar]
- 18.Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, Valladeau J, Davoust J, Palucka KA, Banchereau J: In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med 1999, 190:1417-1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki A, Masuda A, Nagata H, Kameoka S, Kikawada Y, Yamakawa M, Kasajima T: Mature dendritic cells make clusters with T cells in the invasive margin of colorectal carcinoma. J Pathol 2002, 196:37-43 [DOI] [PubMed] [Google Scholar]
- 20.Coronella JA, Spier C, Welch M, Trevor KT, Stopeck AT, Villar H, Hersh EM: Antigen-driven oligoclonal expansion of tumor-infiltrating B cells in infiltrating ductal carcinoma of the breast. J Immunol 2002, 169:1829-1836 [DOI] [PubMed] [Google Scholar]
- 21.Takemura S, Braun A, Crowson C, Kurtin PJ, Cofield RH, O’Fallon WM, Goronzy JJ, Weyand CM: Lymphoid neogenesis in rheumatoid synovitis. J Immunol 2001, 167:1072-1080 [DOI] [PubMed] [Google Scholar]
- 22.Laguens G, Di Girolamo V, Spinelli O, Laguens RP: Identification of a tumor antigen associated to the estrogen receptor in axillary lymph nodes of breast cancer patients. Medicina (B Aires) 1996, 56:269-272 [PubMed] [Google Scholar]
- 23.Thurmond TS, Murante FG, Staples JE, Silverstone AE, Korach KS, Gasiewicz TA: Role of estrogen receptor α in hematopoietic stem cell development and B lymphocyte maturation in the male mouse. Endocrinology 2000, 141:2309-2318 [DOI] [PubMed] [Google Scholar]
- 24.Stein B, Yang MX: Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-κ B and C/EBP β. Mol Cell Biol 1995, 15:4971-4979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindhout E, van Eijk M, van Pel M, Lindeman J, Dinant HJ, de Groot C: Fibroblast-like synoviocytes from rheumatoid arthritis patients have intrinsic properties of follicular dendritic cells. J Immunol 1999, 162:5949-5956 [PubMed] [Google Scholar]
- 26.van Dijk MA, Hart AA, van’t Veer LJ: Differences in estrogen receptor α variant messenger RNAs between normal human breast tissue and primary breast carcinomas. Cancer Res 200, 60:530-533 [PubMed] [Google Scholar]
- 27.Castles CG, Fuqua SA, Klotz DM, Hill SM: Expression of a constitutively active estrogen receptor variant in the estrogen receptor-negative BT-20 human breast cancer cell line. Cancer Res 1993, 53:5934-5939 [PubMed] [Google Scholar]
- 28.Fuqua SA, Schiff R, Parra I, Friedrichs WE, Su JL, McKee DD, Slentz-Kesler K, Moore LB, Willson TM, Moore JT: Expression of wild-type estrogen receptor β and variant isoforms in human breast cancer. Cancer Res 1999, 59:5425-5428 [PubMed] [Google Scholar]
- 29.Moutsatsou P, Kassi E, Creatsas G, Coulocheri S, Scheller K, Sekeris CE: Detection of oestrogen receptor variants in endometrium, myometrium, leiomyoma and peripheral blood mononuclear cells: comparison to variants present in breast cancer. J Cancer Res Clin Oncol 1998, 124:478-484 [DOI] [PubMed] [Google Scholar]
- 30.Komi J, Lassila O: Antioestrogens enhance tumour necrosis factor receptor 2 (TNF-R2) expression and TNF-R2-mediated proliferation in activated T cells. Scand J Immunol 1998, 48:254-260 [DOI] [PubMed] [Google Scholar]


