Abstract
Results of recent studies have indicated that bone marrow cells can differentiate into various cells of ectodermal, mesodermal, and endodermal origins when transplanted into the body. However, the problems associated with those experiments such as the long latent period, rareness of the event, and difficulty in controlling the processes have hampered detailed mechanistic studies. In the present study, we examined the potency of mouse bone marrow cells to differentiate into cells comprising skin tissues using a skin reconstitution assay. Bone marrow cells from adult green fluorescent protein (GFP)-transgenic mice were transplanted in a mixture of embryonic mouse skin cells (17.5 days post-coitus) onto skin defects made on the backs of nude mice. Within 3 weeks, fully differentiated skin with hair was reconstituted. GFP-positive cells were found in the epidermis, hair follicles, sebaceous glands, and dermis. The localization and morphology of the cells, results of immunohistochemistry, and results of specific staining confirmed that the bone marrow cells had differentiated into epidermal keratinocytes, sebaceous gland cells, follicular epithelial cells, dendritic cells, and endothelial cells under the present conditions. These results indicate that this system is suitable for molecular and cellular mechanistic studies on differentiation of stem cells to various epidermal and dermal cells.
Until recently, it was a well-established concept that even multi-potent stem cells give rise to only ontogenically closely-related cell types. This classical view has drastically been altered by findings in the past decade that some adult somatic cells show unexpectedly broad plasticity 1 and hence possess fertile potency to form various tissues for clinical applications. The plasticity, however, may be a double-edged sward. Instability of cellular phenotypes may lead to deterioration of the structure and/or function of tissues made of the cells and may eventually give rise to malignant cells. It is of the utmost importance, therefore, to thoroughly study the plasticity and stability of cell types that are of potential therapeutic use. Bone marrow cells show the broadest differentiation potential among adult somatic cell populations. 1-3 This, together with relatively easy availability, makes bone marrow one of the most promising cell sources for clinical applications.
The skin has a complex tissue architecture composed of a large variety of cell types of ectodermal and mesodermal origins. Most of these cells are produced from corresponding progenitor/stem cells by tightly regulated mechanisms. A subpopulation of cells located in the bulge regions of hair follicles has been shown to give rise to epidermal keratinocytes as well as hair follicles. 4-7 These cells have been shown to have characteristics of typical stem cells, including the capacity to produce different cell lineages, the slow-cycling nature identified as label-retaining cells, 8,9 and the high proliferation potential of these cells in culture. 7,10-11 A cell population having at least some of the characteristics of stem cells has also been found in the basal layer of the epidermis. 12,13
Recently, Krause et al 3 showed that a single bone marrow-derived hematopoietic stem cell could differentiate into a number of different cell types, including epidermis, in recipient mouse tissues. About 2% of epidermal cells were thought to be of the donor origin when observed 11 months after transplantation. In female patients transplanted with hematopoietic stem cells from male donors, some cytokeratin-expressing cells with Y-chromosomes were detected in the skin and liver. 14 These results indicate that a subpopulation of bone marrow cells can differentiate into skin cells. However, the problems associated with those experiments such as the long latent period, rareness of the event, and difficulty in controlling the processes have hampered detailed mechanistic studies.
In this study, we examined the repertoire of skin cell types to which mouse bone marrow cells can differentiate using a skin reconstitution assay. 15,16 Adult mouse bone marrow cells gave rise to epidermal keratinocytes, follicular epithelial cells, sebaceous gland cells, dendritic cells, and endothelial cells within 3 weeks after transplantation.
Materials and Methods
Animals
ICR and C57BL/6 mice with confirmed pregnancy were purchased from Nippon SLC (Hamamatsu, Japan). Green fluorescent protein (GFP)-transgenic mice were provided by Dr. Masaru Okabe (Osaka University) and were maintained in the animal center of our university. BALB/c nude mice were purchased from Nihon Charles River (Yokohama, Japan).
Preparation of Embryonic Skin Cells
Dorsal skin tissues were obtained from mouse embryos (30 to 40 embryos for one transplantation experiment) at 17.5 days post-coitus (dpc) and incubated in 0.05% collagenase (Wako, Osaka, Japan) in a keratinocyte growth medium 17 without growth factors at 4°C overnight. The epidermis was separated from the dermis under a dissecting microscope, and a cell suspension was prepared by gentle pipetting followed by filtration with a #150 mesh (Ikemoto, Tokyo, Japan).
Preparation of Bone Marrow Cells
Two femurs were dissected from the GFP-transgenic mice for one transplantation experiment and washed thoroughly to avoid possible contamination with cells outside the bones. Then both ends of each femur were cut and the bone marrow was washed out with phosphate-buffered saline (PBS). Cells were suspended by gentle pipetting.
Transplantation of Cells
A mixed cell slurry containing approximately 5 × 106 each of epidermal and dermal cells was prepared. To examine the differentiation capacity of bone marrow cells, 5 × 106 bone marrow cells were mixed into the cell slurry. Silicon chambers (Figure 1) ▶ were purchased from Renner (Darmstadt, Germany). An area of back skin of about 1 cm in diameter was removed from each nude mouse under anesthesia, and the lower chamber was inserted and sewn onto the muscle fascia. After applying the cell slurry, the upper chamber was placed on the lower chamber and fixed with a gluey tape. The chamber was removed 1 week after the transplantation and the tissue sections were made 3 or 4 weeks after the transplantation.
Figure 1.
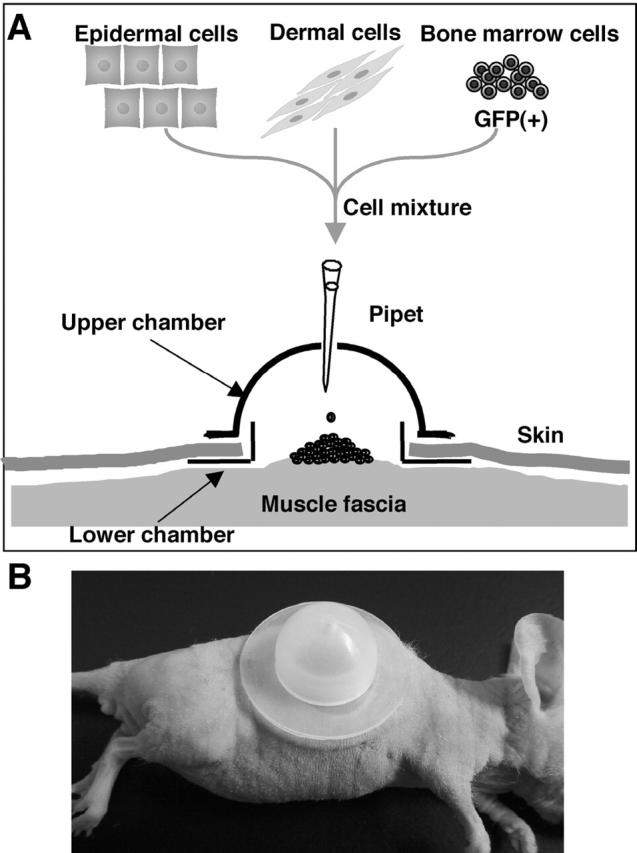
Skin reconstitution assay using a silicon chamber. Epidermal and dermal cell suspensions were prepared from embryonic mouse skin and transplanted as a mixed slurry with or without cells from adult bone marrow. Skin defects were made on the backs of nude mice.
Immunohistochemistry
Paraffin sections were made from the reconstituted skin tissues 3 weeks after transplantation. For observation of GFP-positive cells, the tissues were embedded in Technovit 8100 (Heraeus Kulzer, Wehrheim, Germany) after fixation with 4% paraformaldehyde to avoid heating steps, which weaken the fluorescence of GFP. Sections made from these blocks were also used for staining with Oil Red O. After dipping in 60% isopropyl alcohol, the sections were stained with Oil Red O (1.8 mg/ml, Wako) and then washed with 60% isopropyl alcohol. For detecting cytokeratins, rabbit polyclonal anti-mouse cytokeratin K1-antibody or anti-mouse cytokeratin K6-antibody (Covance, Richmond, CA) and TRITC-conjugated anti-rabbit IgG (Sigma) were used as the first and second antibodies, respectively. For detecting von Willebrand factor, the sections were treated with monoclonal anti-human von Willebrand factor (DakoCytomation, Copenhagen, Denmark) followed by ENVISION+ (DakoCytomation) and visualized by the use of Simple Stain AEC solution (Nichirei, Tokyo, Japan).
Results
Reconstitution of Skin Tissues Including Hair Follicles by Embryonic Skin Cells
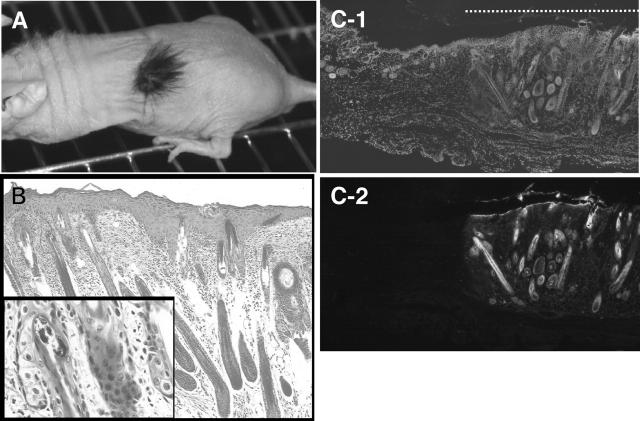
A mixture of 5 × 106 cells each of epidermal cells and dermal cells prepared from C57BL/6 mouse embryos at 17.5 dpc was transplanted into a skin defect made on the back of each nude mouse (Figure 1) ▶ . Within 2 weeks, the defect was completely re-shielded with apparently normal epidermis with short hair. Fully-grown black hair was observed 4 weeks after the transplantation (Figure 2A) ▶ . Histological examination revealed that epidermis and hair follicles (Figure 2B) ▶ with sebaceous glands (Figure 2B ▶ , inset) had been formed. It is known that the mouse embryonic epidermis at 17.5 dpc is multi-layered and fully differentiated to form a cornified layer, but that hair follicles are still developing and only hair pegs are observed. 18 This indicates that the transplanted epidermal and dermal cells in our experiments segregated from each other and followed the development program in the new environment, as reported by Lichti et al 16,19 Using epidermal and dermal cells prepared from GFP-transgenic mice, we confirmed that the reconstituted skin tissues were composed of transplanted cells and not of host cells that had migrated from the surrounding tissues (Figure 2, C-1 and C-2) ▶ . Cells from ICR mouse embryos also reconstituted skin tissues in a similar manner to those from C57BL/6 mice (data not shown).
Figure 2.
Skin tissues reconstituted by a mixture of epidermal and dermal cells. A: Active hair growth in a region transplanted with embryonic epidermal and dermal cells observed 3 weeks after transplantation. B: Histology of the reconstituted skin stained with hematoxilin/eosin. Epidermis and hair follicles with sebaceous glands (inset) were seen. Bar, 50 μm (inset, 10 μm). C: Skin reconstituted by epidermal and dermal cells derived from GFP-transgenic mice. C-1: DAPI staining of nuclei. C-2: Fluorescent microscopic image. The dotted line in C-1 indicates the part of reconstituted skin. Bar, 200 μm.
Differentiation of Adult Bone Marrow Cells to Various Cell Types Composing Skin
When adult bone marrow cells from GFP-transgenic mice were transplanted in a mixture with epidermal and dermal cells from ICR mouse embryos at 17.5 dpc, skin tissues were reconstituted with a similar time course to that in the case of skin cells only. In sections of the reconstituted skin 3 weeks after transplantation, many GFP-positive cells were observed in the epidermis, hair follicles, sebaceous glands, and dermis (Figure 3A) ▶ . Among 372 hair follicles counted in the reconstituted area, 18 hair follicles (4.8%) contained GFP-positive cells. The GFP-positive cells were estimated to account for 10% to more than 90% of the cells in such hair follicles. GFP-positive cells were found in 10 sebaceous glands among 87 sebaceous glands counted, accounting for 25% to more than 90% of the cells in the GFP-positive glands. Some hair follicles were mostly composed of GFP-positive cells (Figure 3, C and D) ▶ . To characterize the skin cells derived from the bone marrow cells, we performed immunohistochemistry and specific staining. Suprabasal GFP-positive cells in the epidermis (Figure 3, B-1 to 3) ▶ and in the lower parts of sebaceous glands (Figure 3, F-1 to 3) ▶ expressed cytokeratin K1, a representative differentiation marker of epidermal keratinocytes and their related cells. The GFP-positive cells in outer root sheath of hair follicles expressed cytokeratin K6 (Figure 3, E-1 to 3) ▶ . Oil Red O staining showed that at least some of the sebaceous glands were derived from the transplanted bone marrow cells and that they accumulated lipid in cytoplasm (Figure 3, G-1 to 2) ▶ . No GFP-positive melanocytes were observed.
Figure 3.
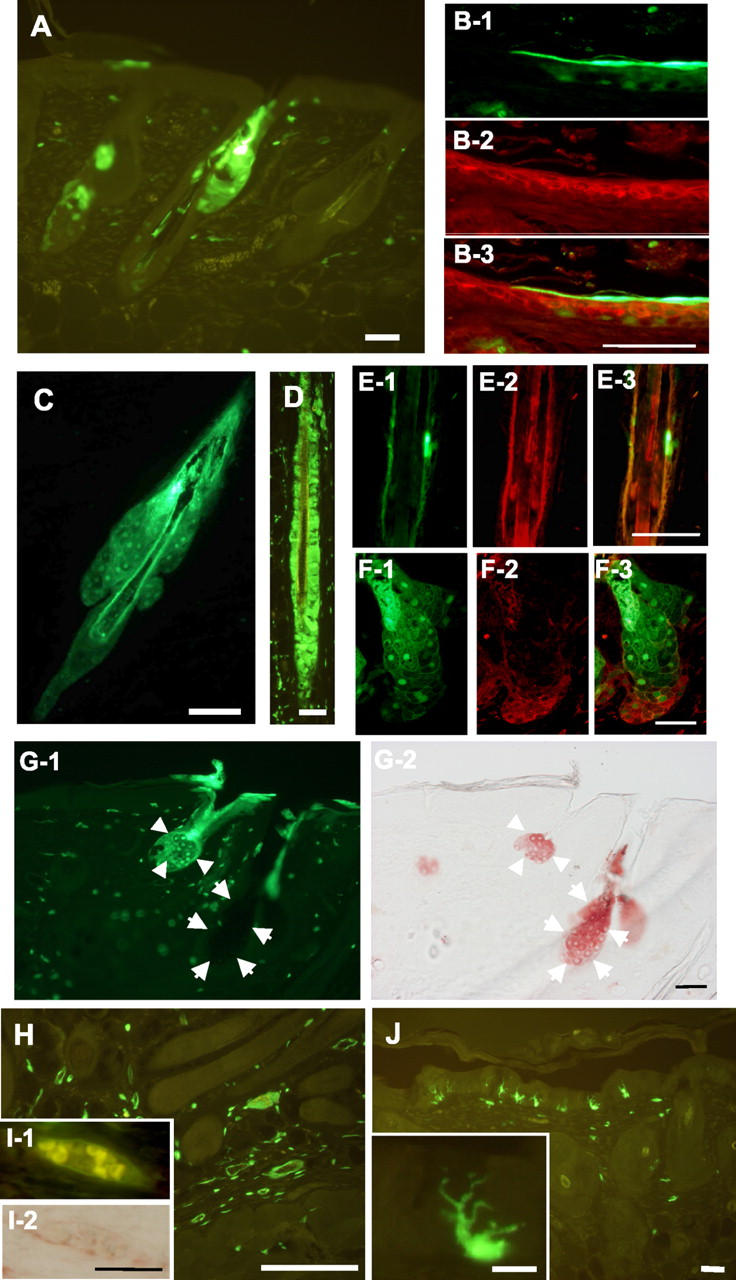
Participation of bone marrow cells in the reconstitution of skin. Bone marrow cells derived from GFP-transgenic mice were transplanted with epidermal and dermal cells from ICR mouse embryos. A: GFP-positive cells were observed in the epidermis, hair follicles, and sebaceous glands 3 weeks after transplantation. B-1 to 3: Epidermal cells. B-1, GFP-positive bone marrow-derived cells; B-2, immunostained for cytokeratin K1; B-3, merged figure. C: A nearly whole hair follicle including sebaceous glands was positive for GFP. D: GFP-positive cells in outer and inner root sheaths of a hair follicle. E-1 to 3: A hair follicle. E-1, GFP-positive bone marrow-derived cells; E-2, immunostained for cytokeratin K6; E-3, merged figure. F-1 to 3: A sebaceous gland. F-1, GFP-positive bone marrow-derived cells; F-2, immunostained for cytokeratin K1; F-3, merged figure. G-1 and 2: A skin section showing sebaceous glands. G-1, GFP-positive bone marrow-derived cells; G-2, staining with Oil Red O (shown in red). Sebaceous glands derived from bone marrow cells and embryonic skin cells are indicated by arrowheads and arrows, respectively. H: GFP-positive cells scattered in the dermis. Some of the dermal GFP-positive cells formed a circular structure in which erythrocytes were observed (I-1). I-2: Immunohistochemistry for von Willebrand factor showing a cross-section of a blood vessel (shown in red). J: Some GFP-positive cells in the epidermis have dendritic shapes. Inset, a dendritic cell at a higher magnification. Bars in A, C, H, and J, 100 μm. Bars in B, E, F, G, I, and the inset of J, 50 μm. Bars in D, 20 μm.
In the dermis, some GFP-positive cells had formed a circular structure (Figure 3H) ▶ . As shown in Figures 3I-1 ▶ , erythrocytes were observed inside a circular structure of dermal GFP-positive cells. These cells expressed von Willebrand factor (Figure 3I-2) ▶ and were distinct from cells expressing α-smooth muscle actin (data not shown). GFP-positive cells with typical dendritic shapes were observed in the epidermis (Figure 3J) ▶ . We repeated the transplantation seven times and obtained essentially similar results. These results indicate that the transplanted bone marrow cells differentiated into various cells to compose skin tissues.
Discussion
In this study, we examined the multi-potency of adult mouse bone marrow cells using a skin-reconstitution assay. The localization in reconstituted skin, the morphology, and the expression of respective marker proteins of the bone marrow-derived cells (Figure 3) ▶ indicate that adult bone marrow cells can differentiate into epidermal keratinocytes, sebaceous gland cells, follicular epithelial cells, dendritic cells, and endothelial cells within 3 weeks.
Realization of dormant differentiation potency of a given cell partly depends on the environment/biological context in which the cell resides. Embryonic stem cells participate in the formation of almost all normal tissues when injected into blastocysts, while they form teratomas when subcutaneously injected into adult mice. Even teratocarcinoma cells were found to contribute to the formation of some normal tissues when injected into blastocysts. 20 Another aspect to note in the formation of tissues from progenitor/stem cells in vivo is that adult organs are often self-sufficient, ie, they can compensate cell loss without any supply of cells from other organs. Thus, significant repopulation of exogenous hepatocyte progenitor cells in the liver was observed only when the liver was severely damaged. 21,22 The characteristics of the system we used are that the cells in a mixed suspension segregate from each other and actively form a tissue architecture through cell-cell and cell-environment interactions. They do not simply restore the structure from which the cells originate but they proceed in the expected developmental program to form mature tissues. Being placed in these environments, the bone marrow cells were instructed by the embryonic skin cells and efficiently manifested their potency to differentiate into skin-composing cells. When only bone marrow cells were transplanted, the lesion was not epithelialized and was rapidly re-shielded by the surrounding skin tissues on removal of the silicon chamber (data not shown). Due to these features and the fact that the composition of cell suspension can be easily modulated, the present assay is one of the most appropriate systems for examining differentiation potency of a given cell population.
We transplanted freshly-isolated unfractionated bone marrow cells. At present, no information on the origin of cells that give rise to skin cells is available. Adult bone marrow is thought to contain several different stem cells, including hematopoietic and mesenchymal stem cells. 23 It has been reported that hepatocytes were induced in vivo from mesenchymal stem cells 24 as well as from hematopoietic stem cells. 25 There is evidence for the presence of a common progenitor cell population of hematopoietic and mesenchymal stem cells. 26,27 Cells in normal bone marrow that expressed some differentiation marker proteins of muscle cells 28 or hepatocytes 29 have been found. Further extensive studies are needed, however, to gain clear insights into stem cell populations, their plasticity, and the interrelationships among them in bone marrow. To elucidate these issues, the development of appropriate assay systems, such as our system, for demonstrating differentiation/proliferation potential of cells is needed.
Acknowledgments
We thank Dr. U. Lichti for her kind teaching of the transplantation technique and Miss Chikako Kinoshita, a medical student of Okayama University, for her technical assistance.
Footnotes
Address reprint requests to Nam-ho Huh, M.D., Department of Cell Biology, Okayama University Graduate School of Medicine and Dentistry, 2–5-1 Shikatachou, Okayama 700-8558, Japan. E-mail: namu@md.okayama-u.ac.jp.
Supported in part by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan and from the Japan Society for the Promotion of Science.
References
- 1.Poulsom R, Alison MR, Forbes SJ, Wright NA: Adult stem cell plasticity. J Pathol 2002, 197:441-456 [DOI] [PubMed] [Google Scholar]
- 2.Goodell MA, Jackson KA, Majka SM, Mi T, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK: Stem cell plasticity in muscle and bone marrow. Ann NY Acad Sci 2001, 938:208-218; 218220 [DOI] [PubMed] [Google Scholar]
- 3.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ: Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001, 105:369-377 [DOI] [PubMed] [Google Scholar]
- 4.Lyle S, Christofidou-Solomidou M, Liu Y, Elder DE, Albelda S, Cotsarelis G: The C8/144B monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. J Cell Sci 1998, 111:3179-3188 [DOI] [PubMed] [Google Scholar]
- 5.Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM: Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 2000, 102:451-461 [DOI] [PubMed] [Google Scholar]
- 6.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W: β-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105:533-545 [DOI] [PubMed] [Google Scholar]
- 7.Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y: Morphogenesis and renewal of hair follicles from adult multi-potent stem cells. Cell 2001, 104:233-245 [DOI] [PubMed] [Google Scholar]
- 8.Cotsarelis G, Sun TT, Lavker RM: Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990, 61:1329-1337 [DOI] [PubMed] [Google Scholar]
- 9.Morris RJ, Potten CS: Highly persistent label-retaining cells in the hair follicles of mice and their fate following induction of anagen. J Invest Dermatol 1999, 112:470-475 [DOI] [PubMed] [Google Scholar]
- 10.Yang JS, Lavker RM, Sun TT: Upper human hair follicle contains a subpopulation of keratinocytes with superior in vitro proliferative potential. J Invest Dermatol 1993, 101:652-659 [DOI] [PubMed] [Google Scholar]
- 11.Rochat A, Kobayashi K, Barrandon Y: Location of stem cells of human hair follicles by clonal analysis. Cell 1994, 76:1063-1073 [DOI] [PubMed] [Google Scholar]
- 12.Jones PH, Harper S, Watt FM: Stem cell patterning and fate in human epidermis. Cell 1995, 80:83-93 [DOI] [PubMed] [Google Scholar]
- 13.Kunimura C, Kikuchi K, Ahmed N, Shimizu A, Yasumoto S: Telomerase activity in a specific cell subset co-expressing integrinβ1/EGFR but not p75NGFR/bcl2/integrin β4 in normal human epithelial cells. Oncogene 1998, 17:187-197 [DOI] [PubMed] [Google Scholar]
- 14.Korbling M, Katz RL, Khanna A, Ruifrok AC, Rondon G, Albitar M, Champlin RE, Estrov Z: Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med 2002, 346:738-746 [DOI] [PubMed] [Google Scholar]
- 15.Fusenig NE, Breitkreutz D, Dzarlieva RT, Boukamp P, Bohnert A, Tilgen W: Growth and differentiation characteristics of transformed keratinocytes from mouse and human skin in vitro and in vivo. J Invest Dermatol 1983, 81:168s-175s [DOI] [PubMed] [Google Scholar]
- 16.Weinberg WC, Goodman LV, George C, Morgan DL, Ledbetter S, Yuspa SH, Lichti U: Reconstitution of hair follicle development in vivo: determination of follicle formation, hair growth, and hair quality by dermal cells. J Invest Dermatol 1993, 100:229-236 [DOI] [PubMed] [Google Scholar]
- 17.Tsunenaga M, Kohno Y, Horii I, Yasumoto S, Huh NH, Tachikawa T, Yoshiki S, Kuroki T: Growth and differentiation properties of normal and transformed human keratinocytes in organotypic culture. Jpn J Cancer Res 1994, 85:238-244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sengel P: Morphogenesis of Skin. 1976:pp 5-57 Cambridge University Press New York
- 19.Lichti U, Weinberg WC, Goodman L, Ledbetter S, Dooley T, Morgan D, Yuspa SH: In vivo regulation of murine hair growth: insights from grafting defined cell populations onto nude mice. J Invest Dermatol 1993, 101:124S-129S [DOI] [PubMed] [Google Scholar]
- 20.Illmensee K, Mintz B: Totipotency and normal differentiation of single teratocarcinoma cells cloned by injection into blastocysts. Proc Natl Acad Sci USA 1976, 73:549-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overturf K, Al-Dhalimy M, Tanguay R, Brantly M, Ou CN, Finegold M, Grompe M: Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat Genet 1996, 12:266-273 [DOI] [PubMed] [Google Scholar]
- 22.Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA: Hepatocytes from non-hepatic adult stem cells. Nature 2000, 406:257. [DOI] [PubMed] [Google Scholar]
- 23.Prockop DJ: Marrow stromal cells as stem cells for non-hematopoietic tissues. Science 1997, 276:71-74 [DOI] [PubMed] [Google Scholar]
- 24.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM: Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002, 418:41-49 [DOI] [PubMed] [Google Scholar]
- 25.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M: Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med 2000, 6:1229-1234 [DOI] [PubMed] [Google Scholar]
- 26.Huss R: Isolation of primary and immortalized CD34-hematopoietic and mesenchymal stem cells from various sources. Stem Cells 2000, 18:1-9 [DOI] [PubMed] [Google Scholar]
- 27.Hall FL, Han B, Kundu RK, Yee A, Nimni ME, Gordon EM: Phenotypic differentiation of TGF-β1-responsive pluripotent premesenchymal prehematopoietic progenitor (P4 stem) cells from murine bone marrow. J Hematother Stem Cell Res 2001, 10:261-271 [DOI] [PubMed] [Google Scholar]
- 28.Corti S, Strazzer S, Del Bo R, Salani S, Bossolasco P, Fortunato F, Locatelli F, Soligo D, Moggio M, Ciscato P, Prelle A, Borsotti C, Bresolin N, Scarlato G, Comi GP: A subpopulation of murine bone marrow cells fully differentiates along the myogenic pathway and participates in muscle repair in the mdx dystrophic mouse. Exp Cell Res 2002, 277:74-85 [DOI] [PubMed] [Google Scholar]
- 29.Miyazaki M, Akiyama I, Sakaguchi M, Nakashima E, Okada M, Kataoka K, Huh NH: Improved conditions to induce hepatocytes from rat bone marrow cells in culture. Biochem Biophys Res Commun 2002, 298:24-30 [DOI] [PubMed] [Google Scholar]