Abstract and Introduction
Abstract
The objective of this article is to review current findings in the published literature on the efficacy of insulin therapy in combination with oral antidiabetic agents, with a focus on practical information that might help to provide an evidence-based template for selecting how best to combine oral agents and basal insulin in patients with type 2 diabetes.
Here we review the current oral agents used to treat type 2 diabetes, their mechanisms of action, and how they can be combined with insulin therapy to help patients achieve guideline-recommended glycemic goals. While practical advice exists for initiating a therapeutic regimen comprised of basal insulin and oral agent(s), direction as to appropriate therapy for individual patients with differing physiologic requirements is needed. Oral antidiabetic therapy in combination with insulin provides an effective therapeutic option for patients who are unable to achieve or maintain glycemic goals on oral therapy alone.
Introduction
Despite the well-documented consequences of long-term uncontrolled hyperglycemia, the majority of patients with type 2 diabetes are not achieving the degree of glycemic control recommended by currently accepted guidelines. The 2006 American Diabetes Association (ADA) recommendations for glycemic control are listed as a glycosylated hemoglobin A1C (A1C) level < 7.0% and fasting plasma glucose (FPG) levels of 90–130 mg/dL (5.0–7.2 mmol/L), or as close to physiologic levels as possible without unacceptable hypoglycemia.[1] The American Association of Clinical Endocrinologists (AACE) has an even more stringent recommendation for an A1C level of ≤ 6.5%.[2] Despite this expert guidance, the National Health and Nutrition Examination Survey demonstrated that only 37% of adults with diabetes in the United States are achieving target A1C levels.[3]
When diet and exercise fail to provide adequate glucose control in type 2 diabetes, oral antidiabetic agents are usually prescribed as initial therapy. Although many patients initially attain control, over the long term there is generally a requirement for intensified and multidrug regimens; ultimately, oral agents alone cannot maintain satisfactory control in many individuals and therapy must be augmented by the addition of insulin. Indeed, newly diagnosed individuals with A1C levels > 10.0% are not likely to achieve goals on oral therapy alone and insulin therapy should be initiated.[2]
Normal pancreatic insulin secretion comprises 2 components: (1) a constant low level of secretion (basal secretion) to suppress glucose production between meals and overnight, and (2) spikes of higher-level secretion in response to rising glucose levels after meals (postprandial secretion). Therapy with basal insulin is a strategy that attempts to approximate the basal component of normal pancreatic insulin secretion that, in combination with oral therapy, may help patients achieve recommended glycemic goals.
Basal insulin most commonly is administered at night (in this article, we will refer to neutral protamine Hagedorn [NPH] insulin, insulin detemir, and insulin glargine as the agents administered as basal insulin). Because basal insulin activity is the primary source of fasting glucose control, basal insulin treatment is often thought of as only impacting the fasting glucose. However, effective basal insulin therapy can concomitantly lower meal-associated glucose levels through overall improvement in metabolic control[4] and can be a relatively simple and highly effective approach to insulin therapy, particularly early in the course of diabetes.[5] The benefits of combining basal insulin with oral agents for improved glycemic control are well established. Although there is expert guidance available for the primary care clinician on addressing hyperglycemia in type 2 diabetes patients,[6] how and when to combine the therapeutic options and which agents among the classes are best suited for combination are expanded upon in this article.
A number of different oral antidiabetic agents with distinct mechanisms of action are currently available. Practical information and guidance is needed for clinicians to make clinical decisions about use of these agents in combination with basal insulin.
This article outlines currently available oral antidiabetic agents and basal insulins and provides practical advice based on current clinical literature for choosing appropriate oral agents for use in combination therapy with basal insulin. Specifically, this report addresses appropriate agents and therapeutic options only for patients with type 2 diabetes, because oral agents are not appropriate treatment for patients with type 1 diabetes. The differing attributes of oral agents currently available for use in combination with insulin provide an opportunity for the physician to better individualize care for patients with type 2 diabetes.
Currently Available Oral Agents
Among the major classes of currently available oral agents, the therapeutic actions of insulin secretagogues and insulin sensitizers address the 2 core defects in type 2 diabetes: a deficit of endogenous insulin secretion and insulin resistance in the skeletal muscle and hepatic and adipose compartments. Additionally, the alpha-glucosidase inhibitors slow glucose uptake from many dietary sources. Notably, any combination of currently available agents is unlikely to reduce A1C levels by more than approximately 3.0% (Table 1).[2] In addition, dosing and precaution information for currently available oral agents is listed in Table 2 .
Table 1.
Fasting and Postprandial Blood Glucose and A1C Responses to Pharmacologic Treatment in Patients With Type 2 Diabetes[2]
| Drug | Decrease in FBG (mg/dL [mmol/L]) | 1-Hr PPG (Decrease From Baseline) (mg/dL [mmol/L]) | Decrease in A1C (From Baseline) (%) |
|---|---|---|---|
| Sulfonylureas | 40–60 (2.2–3.3) | – | 1.0–2.0 |
| Repaglinide/nateglinide | 30.3 (1.7) | 56.5 (3.1) | 1.1 |
| Metformin | 53 (2.9) | – | 1.4 |
| Rosiglitazone (across dose range) | 25–55 (1.4–3.0) | – | 0.1–0.7 |
| Pioglitazone | 20–55 (1.1–3.0) | – | 0.3–0.9 |
| Alpha-glucosidase inhibitors | 20–30 (1.1–1.7) | 20–74 (1.1–4.1) | 0.5–1.0 |
A1C = glycosylated hemoglobin A1C; FBG = fasting blood glucose; PPG = postprandial plasma glucose.
Table 2.
| Class | Dosing | Precaution |
|---|---|---|
| Sulfonylureas | General: hypoglycemia | |
| Glimepiride | Starting: 1–2 mg once daily with first main meal Maintenance: 1–4 mg once daily Maximum: 8 mg once daily |
In renally impaired patients (creatinine clearance < 22 mL/min), a glimepiride dose of 1.0 mg may be sufficient |
| Glipizide | Starting: 5 mg once daily with breakfast Maintenance: 5–10 mg once daily Maximum: 20 mg once daily |
May need to reduce glipizide dose in patients with impaired renal or hepatic function |
| Glyburide | Starting: 2.5–5.0 mg once daily with first meal Maintenance: 1.25–20.0 mg, once daily or divided twice daily Maximum: 20 mg |
Initiate dose at 1.25 mg/day in patients with impaired renal or hepatic function |
| Chlorpropamide | Starting: 250 mg once daily Maintenance: 250 mg once daily (100–125 mg with less severe hyperglycemia) Maximum: 500 mg, maintenance doses > 750 mg should be avoided |
Certain reactions associated with idiosyncrasy or hypersensitivity have occurred; these include jaundice, skin eruptions less frequently progressing to erythema multiforme, and exfoliative dermatitis. Reactions are mild, occur usually within 6 wk of initial therapy and resolve with drug discontinuation |
| Meglitinides | General: Titrate upward with care in patients with impaired hepatic function | |
| Repaglinide | Starting: A1C < 8.0%, 0.5 mg with each meal; A1C ≥ 8.0%, 1–2 mg dose Maintenance: 0.5–4.0 mg with meals Maximum: 16 mg total once daily |
Initial repaglinide dose should be 0.5 mg with each meal and carefully titrated upward in patients with severe renal impairment |
| Nateglinide | Starting/maintenance: 120 mg 3 times daily, before meals; 60-mg dose if near A1C goal | (See general precautions for meglitinides) |
| Thiazolidinediones | General: Weight gain and edema are primary side effects | |
| Pioglitazone | Starting: 15 mg or 30 mg once daily Maintenance: 15–45 mg once daily Maximum: 45 mg once daily |
Use with caution in patients with existing edema |
| Avoid use in patients at risk for heart failure | ||
| Rosiglitazone | Starting: 4 mg once daily or divided twice daily Maximum: 8 mg once daily or divided twice daily |
Therapy should not be initiated in patients with serum transaminase levels 2.5–3.0 times the upper limit of normal and/or evidence of liver disease |
| Monitor liver function tests periodically | ||
| Biguanides (metformin) | General: High levels can cause lactic acidosis | |
|
Immediate-release formula Starting: 850 mg once daily or 500 mg twice daily; titrate increases at 500 mg/wk or 850 mg every 2 wks Maintenance: 1500–2000 mg daily in divided doses, with meals Maximum: 2550 mg/day Extended-release formula Starting: 500 mg once daily with evening meal; titrate increases at 500 mg/wk Maximum: 2000 mg once daily or divided twice daily (if higher doses are required, use immediate-release formula) Liquid formula Starting: 500 mg twice daily or 850 mg once daily with meals; increase dose 500 mg/wk or 850 mg/2 wk Maintenance: 1500 mg-2000 mg once daily divided among meals Maximum: 2550 mg divided into 3 doses |
Contraindicated when serum creatinine levels exceed 1.4 (women) to 1.5 (men) mg/dL | |
| Other contraindications: congestive heart failure, significant liver disease, or any condition predisposing hypoperfusion | ||
| When first taken, may cause nausea, diarrhea; liquid form may aid gradual titration | ||
| Alpha-glucosidase inhibitors | General: Side effects include intestinal gas, abdominal cramps, and diarrhea; symptoms usually subside with time | |
| Acarbose | Starting: 25 mg 3 times daily with first bite of each meal; to minimize side effects, titrate from 25 mg once daily Maintenance: 25–50 mg 3 times daily Maximum: ≤ 60 kg, 50 mg 3 times daily; > 60 kg, 100 mg 3 times daily |
Serum transaminase may be elevated with acarbose; should be checked every 3 months during the first year of therapy; consider dose reduction or discontinuation if elevated |
| Acarbose is not recommended for patients with renal impairment until more clinical trial data are available for this population | ||
| Miglitol | Starting: 25 mg 3 times daily with first bite of each meal to minimize side effects; after 4–8 weeks, titrate gradually to 50 mg 3 times daily Maintenance: 50 mg 3 times daily Maximum: 100 mg 3 times daily |
Miglitol may accumulate in patients with renal impairment |
Insulin Secretagogues
Sulfonylureas (eg, glyburide, glipizide, and glimepiride) lower blood glucose primarily by stimulating insulin release from the pancreas. These agents demonstrate similar efficacy for glucose control. With the exception of long-acting formulations, sulfonylureas are characterized by rapid onset. All secretagogues can produce hypoglycemia, particularly if caloric intake is inadequate or meals are not eaten regularly.[7–9] Although older, longer-acting sulfonylureas (eg, chlorpropamide) are used less commonly since a longer half-life can cause prolonged hypoglycemia, these agents may be considered for persons of marginal economic resources if hypoglycemia is a lesser concern.[9–12] Weight gain is a common side effect of sulfonylureas, but is seen less often with newer-generation agents (eg, glimepiride) than with traditional long-acting formulations.[13]
The meglitinides, repaglinide and nateglinide, also stimulate pancreatic insulin secretion. Meglitinides have a more rapid onset and shorter duration of action compared with sulfonylureas; therefore, they are indicated for control of postprandial serum glucose excursions. Meglitinides may also be associated with a lower risk of hypoglycemic events due to their limited duration of action.[14,15] Because of the potential for hypoglycemia when a meal is skipped, patients need careful instruction to use meglitinides only when a meal is imminent (ie, administer 15–30 minutes before a meal is begun).
Insulin Sensitizers
Metformin is the only biguanide currently approved for use in the United States. Metformin decreases hepatic glucose production, increases hepatic sensitivity to insulin, and may also have a mild effect on sensitivity to insulin in muscle.[16] Metformin is as effective as sulfonylureas in reducing A1C levels (approximately 1.0% to 2.0% [ Table 1 ]). Metformin is weight neutral and therefore may be useful in treating obese patients.[17]
Pioglitazone and rosiglitazone are the 2 thiazolidinediones currently approved for use in the United States. Thiazolidinediones enhance glucose uptake and disposal by increasing glucose transporters in peripheral tissues.[16] Weight gain and edema are the primary side effects of thiazolidinediones.[18,19] Edema may occur more frequently in patients treated with thiazolidinediones in combination with insulin[18,19] and should be used cautiously in patients with preexisting edema. These agents should be avoided in patients exhibiting signs and symptoms of heart failure (New York Heart Association Class III or IV), including orthopnea, dyspnea, unexplained cough or fatigue, or pedal edema.[20]
Alpha-Glucosidase Inhibitors
Acarbose and miglitol slow glucose uptake from many dietary sources by inhibiting the alpha-glucosidase-dependent breakdown of complex carbohydrates as well as simple sugars in the brush border of the proximal small intestine epithelium.[21,22] The mechanism of action is not glucose absorption blockade; indeed, long-term therapy is not associated with weight loss. Rather, simply by “spreading out” the digestion of carbohydrates through the small intestine, alpha-glucosidase inhibitors decrease postprandial as well as fasting glucose concentrations. Of interest, a recent meta-analysis of 7 randomized studies of patients with type 2 diabetes suggested a reduction in the risk of myocardial infarction (hazard ratio = 0.36 [95% CI, 0.16–0.80]; P = .0120) associated with acarbose compared with placebo.[23] Gastrointestinal symptoms are the most common side effects associated with alpha-glucosidase inhibitors, however, slow titration and careful instruction can help address these effects.
Rationale for Combination Therapy
As the disease progresses, many patients with type 2 diabetes will eventually be unable to adequately achieve or maintain glycemic control, whether monotherapy or combination oral therapies are employed. The reason for diminishing antihyperglycemic effects with oral agents over time is multifactorial and includes progressive loss of beta-cell function,[24,25] comorbidities, lifestyle factors, and possibly glucotoxicity. In most cases, patients on oral antidiabetic therapy will require not only an increase in dose but also the addition of a second or third oral agent.[26,27] As the number and dosage of oral agents increases, the side-effect profile, regimen complexity, and expense rise commensurately.
One fundamental obstacle to achieving glycemic control with oral agents is their limited capacity to reduce overall A1C levels in patients presenting with relatively high levels (> 10.0%). The expected reduction in A1C with maximum doses of oral antidiabetic agents in combination therapy is approximately 3.0% (Table 1). Accordingly, it is unlikely that patients presenting with A1C > 10.0% will achieve treatment goals on oral agents alone. These agents should, however, be added sequentially without delay. Physicians should also not delay in advancing therapy if it becomes apparent that combination therapy is either not reducing hyperglycemia quickly enough or that the efficacy of individual agents has reached its maximum benefit and can no longer contribute to improving glycemic parameters. An algorithm for treating patients with type 2 diabetes with insulin and oral therapy is shown in the Figure.[28]
In contrast to the diminishing benefits obtained from therapy with multiple oral antidiabetic agents, combining insulin therapy with oral agents may allow patients to reach and maintain glycemic goals.[29,30] The combination of oral agents with insulin may sometimes mitigate hypoglycemia and weight gain observed with insulin monotherapy.[31,32] Another possible benefit of combining oral agents with insulin is improvement of lipid profiles.[32,33] A recent position statement from the Implementation Conference for the American College of Endocrinology Outpatient Diabetes Mellitus Consensus Conference[34] indicated that insulin in combination with oral agents or basal-bolus regimens (ie, basal insulin in combination with bolus insulin, such as aspart, glulisine, or lispro) should be considered at an early stage in type 2 diabetes if glycemic goals are not readily attained and maintained with oral agents alone. Premixed insulin preparations were recommended to be used only in special situations.
Patients should be made aware at the inception of type 2 diabetes therapy that, because it is generally a progressive disorder, at some time in their treatment programs it is likely that insulin will be employed due to its advantage of attaining control when other methods can no longer maintain control. Rather than posture insulin treatment as being instituted because “they” (the patients) have failed or “the drugs” have failed, incorporating a stance that fosters progressive treatment selection based on the likelihood of clinical success (ie, achievement of goal A1C) prepares patients for the possibility that insulin may be a “right” choice at any point in the treatment pathway. An analysis of data from published clinical studies supports the use of bedtime insulin combined with ≥ 1 oral antidiabetic agents for patients poorly controlled on other regimens.[4,29]
Currently Available Insulins
Basal Insulins
Exogenous basal insulin replacement (ie, with NPH, insulin detemir, or insulin glargine) suppresses hepatic gluconeogenesis between meals and overnight. This effect may reduce the insulin resistance associated with type 2 diabetes.[35] Once-daily basal insulin administered at bedtime was initially shown to be effective for achieving glycemic control in small studies of patients with type 2 diabetes who were failing sulfonylurea therapy.[36,37] In a recent large, randomized, multicenter study by Riddle and colleagues,[29] a single bedtime injection of long-acting insulin (insulin glargine or NPH) was given to patients with inadequate glycemic control on 1 or 2 oral agents. Existing oral agents were continued, and the insulin dose was systematically titrated toward a fasting glucose target ≤ 100 mg/dL (5.6 mmol/L) (Table 3). By using this forced-titration algorithm, a majority of patients were able to reach an A1C goal ≤ 7.0% on once-daily basal insulin combined with oral agents. Although the insulin titration regimen used in this clinical trial was successful, some clinicians or their patients were daunted by the suggested increment of as much as 8 U of basal insulin for a fasting glucose > 180 mg/dL (10 mmol/L). An alternate dosing strategy that has been successfully implemented in recent trials is to increase the basal insulin by 2 U every 3 days until FPG ≤ 100 mg/dL (5.5 mmol/L).[38,39]
Table 3.
Forced-Titration Schedule for Insulin Glargine[29]
| Start with 10 U/day basal insulin dose and adjust weekly | |
|---|---|
| Self-monitored FPG* | ↑ in insulin dose (U/day) |
| ≥ 180 mg/dL (9.9 mmol/L) | 8 |
| ≥ 140 but < 180 mg/dL (≥ 7.7 but < 9.9 mmol/L) | 6 |
| ≥ 120 but < 140 mg/dL (≥ 6.6 but < 7.7 mmol/L) | 4 |
| ≥ 100 but < 120 mg/dL (≥ 5.5 but < 6.6 mmol/L) | 2 |
FPG = fasting plasma glucose
Two consecutive days with no episodes of severe hypoglycemia or plasma glucose levels < 72 mg/dL (4.0 mmol/L).
Small ↓ (2–4 U/day adjustment) in dose allowed when self-monitored plasma glucose level is < 56 mg/dL (3.1 mmol/L) or severe hypoglycemic episode occurs. Individual titration schedules should be implemented to minimize risk of hypoglycemia.
NPH is the conventional intermediate-acting insulin. NPH has an onset of action of 2–4 hours, peak activity at 4–10 hours, and a duration of action of 10–16 hours.[40] The pattern of peak activity of NPH insulin can lead to a risk of nocturnal hypoglycemia, even if administered at bedtime. Conversely, waning insulin activity in the morning can cause some patients to develop hyperglycemia. The lente insulins are long-acting insulins that have been used effectively for basal insulin coverage for many years in patients with type 1 and type 2 diabetes. These insulin preparations, however, were recently discontinued by the manufacturer.[41]
The development of insulin analogs represents an important advancement in the treatment of diabetes. Insulin analogs are generated by substituting or rearranging the amino acid sequence of human insulin. These alterations lead to changes in the pharmacokinetic/pharmacodynamic properties of the insulin molecule. Insulin glargine is the only long-acting basal insulin analog currently in widespread use in both the United States and Europe, while insulin detemir has recently become available in the United States but was approved for use in Europe in June 2004. Insulin glargine has a steady absorption profile that allows consistent systemic delivery from the site of injection. As a result, insulin glargine provides a sustained metabolic effect over nearly 24 hours without a pronounced peak of activity.[42] In a study comparing the pharmacokinetic/pharmacodynamic profiles of insulin glargine, NPH, and continuous subcutaneous insulin infusion (CSII), insulin glargine demonstrated lower intersubject variability than NPH and appeared to more closely resemble the profile of CSII.[42] The sustained, consistent action of insulin glargine may, in part, be responsible for the lower incidence of nocturnal hypoglycemia and weight gain associated with its use in patients with type 2 diabetes compared with NPH insulin therapy.[4,29,43] Because both NPH and insulin glargine were equally effective in reaching target A1C in the largest trial to date, either is a rational choice. It should be noted, however, that episodes of hypoglycemia in this study were higher with NPH insulin. The lesser expense of NPH makes it preferable for some patients, while less weight gain and hypoglycemia associated with insulin glargine makes it preferable by other patients. A recent study assessed the cost consequence associated with NPH vs insulin glargine in a managed care setting. In this setting, it was found that the savings associated with reduced hypoglycemic events in patients taking insulin glargine more than offset the increased acquisition cost associated with glargine use relative to NPH.[44]
Insulin detemir is also a soluble insulin analog that recently has been approved in the United States.[45,46] As with insulin glargine, the pharmacodynamic profile of insulin detemir is associated with low interpatient variability compared with NPH insulin.[47] This new insulin analog also has a peak in its time-action profile.[48] However, published pharmacodynamic results for insulin detemir show that it is comparable to NPH insulin for blood glucose control and may be associated with a reduced risk of hypoglycemia.[49] Similar to NPH insulin, insulin detemir can be dosed once to twice daily with the majority of patients requiring twice-daily administration to achieve glycemic control.[50] Pharmacokinetic studies that directly compare insulin detemir with insulin glargine will be needed to accurately address any time-action profile similarities or differences between these 2 basal insulin analogs.
Similar to the Treat-To-Target study by Riddle and colleagues, a recent study also used a forced titration algorithm to examine the addition of basal insulin to existing oral agent regimens, but compared insulin detemir with NPH insulin over 26 weeks.[51] This study demonstrated that insulin detemir and NPH insulin similarly reduced A1C but that weight gain and hypoglycemia, including nocturnal hypoglycemia, was significantly reduced with insulin detemir compared with NPH insulin (P < .001).
The efficacy of insulin detemir has been evaluated in patients with type 1 diabetes as well. In a multicenter, randomized study involving 448 patients with type 1 diabetes who received basal insulin detemir and insulin aspart at meals, A1C concentrations were identical (7.6%) after 6 months of treatment with insulin detemir or NPH insulin. Overall, FPG levels were slightly but not significantly lower in patients treated with insulin detemir than in patients who received NPH insulin. Nightly plasma glucose profiles were significantly more stable and smoother in the insulin detemir group than in the NPH insulin group, and early-morning FPG levels were significantly lower in the patients who received insulin detemir than in the patients who received NPH insulin. A comparison of pharmacodynamic profiles suggests a slightly delayed onset of action with insulin detemir compared with NPH insulin. The risks of hypoglycemia and nocturnal hypoglycemia were significantly lower with insulin detemir than with NPH insulin (22% vs 34%), and weight gain was significantly higher in patients receiving NPH insulin.[52]
A similar study also used a forced titration algorithm in examining the addition of basal insulin to existing oral agent regimens, but compared insulin detemir with NPH insulin over 24 weeks. This study demonstrated that insulin detemir and NPH insulin similarly reduced A1C but that weight gain and hypoglycemia, including nocturnal hypoglycemia, were significantly reduced with insulin detemir compared with NPH insulin (P < .001).
Although both insulin glargine and insulin detemir may be useful for basal therapy, there are differences between the two. Insulin detemir is designed to bind to serum albumin. Serum protein binding can be a vital point of interaction between drugs with high intrinsic binding affinity. Displacement of bound drug by a competing substance may significantly increase the free concentration of the drug and potentiate its actions. However, in vitro data have not shown insulin detemir to have interactions with other drugs known to bind to albumin.[53] Clinical trials directly comparing insulin glargine with insulin detemir have not been completed but are currently under way and will provide insight to additional potential differences between these 2 basal insulins.[54]
Use of Oral Antidiabetic Agents in Combination With Insulin in Patients With Type 2 Diabetes: Clinical Data
Therapy with either oral agents or insulin alone can be suboptimal for many patients with type 2 diabetes, and there is an abundance of clinical evidence that supports combining insulin with oral agents to improve glycemic control. In the Veterans Affairs Cooperative Study–Diabetes Mellitus, results demonstrated that a single injection of evening NPH insulin in combination with glipizide, as part of an intensive insulin therapy regimen in patients poorly controlled on other pharmacologic therapies, maintained near-normal glycemic control.[55] A meta-analysis of 16 randomized, placebo-controlled studies revealed significant reductions in A1C as well as decreased insulin requirements with insulin plus sulfonylurea therapy vs insulin monotherapy.[56] Data from the United Kingdom Progressive Diabetes Study have provided the most definitive evidence demonstrating the inadequacy of oral agent therapy alone and the eventual requirement for insulin to maintain glycemic control in most patients.[24]
In a 24-week study examining the efficacy of metformin added to insulin therapy in patients with type 2 diabetes uncontrolled on insulin alone, A1C was significantly reduced compared with intensive insulin therapy alone (P < .04). Less weight gain and a decrease in insulin dose were also observed in the insulin-metformin group.[57] Another study, involving patients poorly controlled on sulfonylurea plus insulin therapy, showed that metformin plus once-daily insulin was significantly more effective than insulin plus glyburide, insulin plus glyburide and metformin, or additional insulin (a second injection).[31] The insulin-plus-metformin group also had less symptomatic and biochemical cases of hypoglycemia and did not experience weight gain.[31]
Acarbose was shown to be an effective adjunct therapy in combination with insulin. After 24 weeks of treatment, A1C was reduced by 0.40%, and insulin requirements were substantially lowered in patients in whom acarbose was added to their insulin regimen vs those remaining on insulin monotherapy.[58] A similar trial demonstrated a 0.69% A1C reduction as well as significant reductions in postprandial glucose and triglyceride levels with the addition of acarbose to existing insulin therapy.[59]
Pioglitazone added to insulin regimens, in which patients are not optimally controlled, has also been shown to provide a significant decrease in A1C and FPG vs placebo and has the added benefit of increasing high-density lipoprotein and decreasing triglyceride levels. However, hypoglycemia and edema were more common in patients in whom pioglitazone was added to their existing insulin regimen in this study.[33]
Addition of rosiglitazone to the regimens of patients who are inadequately controlled on insulin monotherapy (A1C approximately 9.0%) has also been demonstrated to be effective in reducing glycemic parameters when compared with placebo.[60] A dose response in lowering A1C over 26 weeks was seen between 4-mg and 8-mg daily doses of rosiglitazone (0.7%, and 1.3%, reductions, respectively; P < .0001 for both compared with placebo). This study and the pioglitazone study discussed above indicate that addition of a thiazolidinedione to insulin monotherapy regimens that are not adequately controlling hyperglycemia can contribute to overall A1C reduction by more than 1% in a dose-dependent manner.
The initiation of basal insulin has demonstrated efficacy for improving glycemic parameters in combination with oral agents. Basal insulin replacement improves not only FPG but also results in improved postprandial glucose. Since postprandial glucose excursions are, effectively, superimposed peaks on basal (interprandial) glucose levels, the efficacy of insulin glargine in lowering basal glucose levels favorably impacts postprandial levels throughout the day.[61] In trials comparing insulin glargine with NPH insulin added to existing oral therapy, both insulins were equally effective in achieving target A1C. However, insulin glargine produced less hypoglycemia and weight gain than NPH insulin.[29,62,63] In a recent study of 371 insulin-naive patients inadequately controlled on a sulfonylurea plus metformin, target A1C ≤ 7.0% was achieved by a significantly larger proportion of patients who received once-daily insulin glargine (plus glimepiride and metformin) relative to patients who received 70% NPH and 30% regular human insulin (with no oral agents) (P = .0003). Similarly, more patients achieved target FPG ≤ 100 mg/dL (5.5 mmol/L) in the insulin glargine treatment group and fewer patients in this group experienced confirmed hypoglycemic events (P = .0001 and P < .0001, respectively).[30]
Practical Suggestions for Combination Therapy
Factors Governing Choice of Oral Agent to Use in Combination With Basal Insulin
When oral therapy fails to attain or maintain glycemic goals, it is appropriate to add basal insulin to the regimen without discontinuing oral agents. Cessation of oral therapy while titrating insulin is less desirable, since the basal insulin is not targeted to be the primary modulator of postprandial glucose excursions. When initiating basal insulin, 10 U of either NPH insulin, insulin detemir, or insulin glargine is an appropriate beginning dose, titrated as discussed above. The average dose ultimately required to attain the A1C goal in a 100-kg person is generally around 40–45 U of basal insulin daily. Therefore, a generalized recommendation is 0.5 U/kg of body weight. Using typical titration schemes (eg, 2-U increments once or twice weekly until fasting glucose goals are reached), it can be anticipated that a duration of 4 months or longer may be required to reach the full therapeutic dose of basal insulin. Table 4 provides a summary of factors that may affect choice of oral agents and suggested choices for oral therapies.
Table 4.
Candidates for Antidiabetic Oral Agents in Combined Therapy With Insulin According to Clinically Mitigating Factors
| Clinical Factor | Oral Agent Candidate(s) for Combined Therapy With Insulin |
|---|---|
| Efficacy | Sulfonylureas, metformin, and thiazolidinediones |
| Minimizing cost | Least expensive: sulfonylureas; most expensive: thiazolidinediones |
| Weight gain | Metformin and/or alpha-glucosidase inhibitors |
| Least risk of hypoglycemia | Once-daily basal insulin with glargine and metformin, thiazolidinediones, alpha-glucosidase inhibitors |
| Improvement of insulin resistance | Metformin and thiazolidinediones |
| Patient and provider effort | Once-daily metformin or sulfonylureas |
| Hepatic impairment | Metformin, sulfonylureas, alpha-glucosidase inhibitors |
| Abnormal renal function or congestive heart failure | Sulfonylurea or other secretagogue (caution should be used in patients with renal impairment), alpha-glucosidase inhibitors, thiazolidinediones should be avoided |
| Convenience (frequency of dosing) | Sulfonylureas, thiazolidinediones, metformin, or combination of metformin and glyburide; or combination of metformin and rosiglitazone |
There are many factors the physician should take into account when making clinical decisions regarding choice of basal insulin for combination therapy. These factors can include convenience, hypoglycemia incidence, control of postprandial glucose levels, avoiding weight gain, and achieving a more physiologic insulin profile. Convenience may be incorporated into insulin regimens by use of once-daily options.
Conclusion
Combining basal insulin with oral therapy may help patients achieve the goals for glycemic control recommended by the ADA and the AACE. Each oral therapeutic class can provide specific benefits according to its respective intrinsic pharmacologic properties. Ideally, combination therapy should be individualized to match patient lifestyle, baseline A1C, comorbidities, insulin requirements, economics, personal preference, and patient convenience. Utilizing oral therapies with complementary mechanisms of action, eg, combining secretagogues with sensitizers, can maximize efficacy and minimize adverse events. Treatment with insulin provides replacement therapy that complements the insulin-sensitizing action of biguanides and thiazolidinediones. Secretagogues can work with exogenous insulin by raising endogenous insulin levels. Alpha-glucosidase inhibitors generally have a somewhat more modest add-on efficacy than other classes of oral agents but may be useful in patients who are very close to goal A1C (< 7.0%) and could benefit from an incremental reduction in postprandial glucose. The multiplicity of oral drugs available for use with insulin need not be viewed as confounding, but rather as an opportunity to individualize and intensify care for patients with type 2 diabetes.
Figure 1.
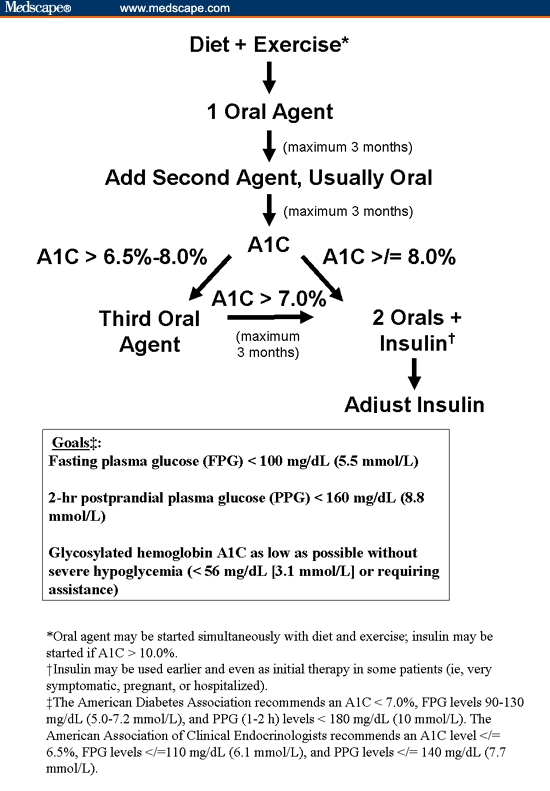
Algorithm for management of type 2 diabetes.
Acknowledgments
I greatly appreciate the editorial participation of Paul Ruest, PhD, who provided editorial guidance in the preparation and development of this article.
The author gratefully acknowledges the Embryon scientific staff, who assisted in the preparation of a first draft of this article based on an author-approved outline, and also assisted in implementing author revisions. Embryon supports the Good Publications Practice Working Group guidelines on the role of medical writers in developing scientific publications. This content has been edited and peer-reviewed by Medscape General Medicine.
Funding Information
This article was supported by sanofi-aventis U.S.
Footnotes
Readers are encouraged to respond to George Lundberg, MD, Editor of MedGenMed, for the editor's eye only or for possible publication via email: glundberg@medscape.net
References
- 1.American Diabetes Association. Standards of medical care in diabetes-2006. Diabetes Care. 2006;29(Suppl 1):S4–S42. [PubMed] [Google Scholar]
- 2.American Association of Clinical Endocrinologists. The American Association of Clinical Endocrinologists Medical Guidelines for the Management of Diabetes Mellitus: The AACE System of Intensive Diabetes Self-Management-2002 Update. Endocr Pract. 2002;8(suppl 1):40–82. [PubMed] [Google Scholar]
- 3.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335–342. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 4.Yki-Järvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. Diabetes Care. 2000;23:1130–1136. doi: 10.2337/diacare.23.8.1130. [DOI] [PubMed] [Google Scholar]
- 5.Vinik AI. Benefits of early initiation of insulin therapy to long-term goals in type 2 diabetes mellitus. Insulin. 2006;1:2–12. [Google Scholar]
- 6.Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care. 2006;29:1963–1972. doi: 10.2337/dc06-9912. [DOI] [PubMed] [Google Scholar]
- 7.Amaryl [package insert] Bridgewater, NJ: Aventis Pharmaceuticals Inc.; 2005. [Google Scholar]
- 8.Micronase [package insert] Kalamazoo, Mich: Pharmacia & Upjohn Company; 2002. [Google Scholar]
- 9.Diabeta [package insert] Bridgewater, NJ: Aventis Pharmaceuticals Inc.; 2004. [Google Scholar]
- 10.Glucotrol XL [prescribing information] New York, NY: Pfizer Inc; 2003. [Google Scholar]
- 11.Berelowitz M, Fischette C, Cefalu W, Schade DS, Sutfin T, Kourides IA. Comparative efficacy of a once-daily controlled-release formulation of glipizide and immediate-release glipizide in patients with NIDDM. Diabetes Care. 1994;17:1460–1464. doi: 10.2337/diacare.17.12.1460. [DOI] [PubMed] [Google Scholar]
- 12.Birkeland KI, Furuseth K, Melander A, Mowinckel P, Vaaler S. Long-term randomized placebo-controlled double-blind therapeutic comparison of glipizide and glyburide. Glycemic control and insulin secretion during 15 months. Diabetes Care. 1994;17:45–49. doi: 10.2337/diacare.17.1.45. [DOI] [PubMed] [Google Scholar]
- 13.Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA. 2002;287:360–372. doi: 10.1001/jama.287.3.360. [DOI] [PubMed] [Google Scholar]
- 14.Malaisse WJ. Pharmacology of the meglitinide analogs: new treatment options for type 2 diabetes mellitus. Treat Endocrinol. 2003;2:401–414. doi: 10.2165/00024677-200302060-00004. [DOI] [PubMed] [Google Scholar]
- 15.Luna B, Feinglos MN. Oral agents in the management of type 2 diabetes mellitus. Am Fam Physician. 2001;63:1747–1756. [PubMed] [Google Scholar]
- 16.Inzucchi SE, Maggs DG, Spollett GR, et al. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338:867–872. doi: 10.1056/NEJM199803263381303. [DOI] [PubMed] [Google Scholar]
- 17.Paolisso G, Amato L, Eccellente R, et al. Effect of metformin on food intake in obese subjects. Eur J Clin Invest. 1998;28:441–446. doi: 10.1046/j.1365-2362.1998.00304.x. [DOI] [PubMed] [Google Scholar]
- 18.Actos [package insert] Lincolnshire, Ill: Takeda Pharmaceutical Company Limited; 2004. [Google Scholar]
- 19.Avandia [package insert] Research Triangle Park, NC: GlaxoSmithKline; 2005. [Google Scholar]
- 20.Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2004;27:256–263. doi: 10.2337/diacare.27.1.256. [DOI] [PubMed] [Google Scholar]
- 21.Precose [prescribing information] West Haven, Conn: Bayer Pharmaceuticals Corporation; 2003. [Google Scholar]
- 22.Glyset [prescribing information] Kalamazoo, Mich: Pharmacia & Upjohn; 2004. [Google Scholar]
- 23.Hanefeld M, Cagatay M, Petrowitsch T, Neuser D, Petzinna D, Rupp M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J. 2004;25:10–16. doi: 10.1016/s0195-668x(03)00468-8. [DOI] [PubMed] [Google Scholar]
- 24.Wright A, Burden ACF, Paisey RB, Cull CA, Holman RR for the U.K. Prospective Diabetes Study Group. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57) Diabetes Care. 2002;25:330–336. doi: 10.2337/diacare.25.2.330. [DOI] [PubMed] [Google Scholar]
- 25.Turner RC, Cull CA, Frighi V, Holman RR for the UK Prospective Diabetes Study Group (UKPDS) Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49) JAMA. 1999;281:2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 26.Banerjee S, Sinharoy K, Singh AK. Oral hypoglycaemic agent failure. J Indian Med Assoc. 2002;100:452–456. [PubMed] [Google Scholar]
- 27.LeRoith D. Beta-cell dysfunction and insulin resistance in type 2 diabetes: role of metabolic and genetic abnormalities. Am J Med. 2002;113:3S–11S. doi: 10.1016/s0002-9343(02)01276-7. [DOI] [PubMed] [Google Scholar]
- 28.Dailey G. A timely transition to insulin: identifying type 2 diabetes patients failing oral therapy. Formulary. 2005;40:114–130. [Google Scholar]
- 29.Riddle MC, Rosenstock J, Gerich J on behalf of the Insulin Glargine Study Investigators. The Treat-to-Target Trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–3086. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 30.Janka HU, Plewe G, Riddle MC, Kliebe-Frisch C, Schweitzer MA, Yki-Järvinen H. Comparison of basal insulin added to oral agents versus twice-daily premixed insulin as initial insulin therapy for type 2 diabetes. Diabetes Care. 2005;28:254–259. doi: 10.2337/diacare.28.2.254. [DOI] [PubMed] [Google Scholar]
- 31.Yki-Järvinen H, Ryysy L, Nikkilä K, Tulokas T, Vanamo R, Heikkilä M. Comparison of bedtime insulin regimens in patients with type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med. 1999;130:389–396. doi: 10.7326/0003-4819-130-5-199903020-00002. [DOI] [PubMed] [Google Scholar]
- 32.Bergenstal R, Johnson M, Whipple D, et al. Advantages of adding metformin to multiple dose insulin therapy in type 2 diabetes [abstract 0347] Diabetes. 1998 Abstract 0347. [Google Scholar]
- 33.Rosenstock J, Einhorn D, Hershon K, Glazer NB, Yu S. Efficacy and safety of pioglitazone in type 2 diabetes: a randomised, placebo-controlled study in patients receiving stable insulin therapy. Int J Clin Pract. 2002;56:251–257. [PubMed] [Google Scholar]
- 34.American Association of Clinical Endocrinologists. Implementation Conference for ACE Outpatient Diabetes Mellitus Consensus Conference Recommendations: Position Statement; February 2, 2005. Available at: http://www.aace.com/pub/pdf/guidelines/OutpatientImplementationPositionStatement.pdf. Accessed March 14, 2005. [Google Scholar]
- 35.Scarlett JA, Gray RS, Griffin J, Olefsky JM, Kolterman OG. Insulin treatment reverses the insulin resistance of type II diabetes mellitus. Diabetes Care. 1982;5:353–363. doi: 10.2337/diacare.5.4.353. [DOI] [PubMed] [Google Scholar]
- 36.Taskinen M-R, Sane T, Helve E, Karonen S-L, Nikkilä EA, Yki-Järvinen H. Bedtime insulin for suppression of overnight free-fatty acid, blood glucose, and glucose production in NIDDM. Diabetes. 1989;38:580–588. doi: 10.2337/diab.38.5.580. [DOI] [PubMed] [Google Scholar]
- 37.Shank ML, Del Prato S, DeFronzo RA. Bedtime insulin/daytime glipizide: effective therapy for sulfonylurea failures in NIDDM. Diabetes. 1995;44:165–172. doi: 10.2337/diab.44.2.165. [DOI] [PubMed] [Google Scholar]
- 38.Ryysy L, Yki-Järvinen H, Hänninen J. Simplifying treat to target - the LANMET study. Program and abstract of the 40th European Association for the Study of Diabetes Annual Meeting; September 7, 2004. September 5–9, 2004; Munich, Germany. Abstract 749. [Google Scholar]
- 39.Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R Study Group. AT.LANTUS trial investigating treatment algorithms for insulin glargine (LANTUS®): results of the Type 2 Study [Abstract 1980-PO] Diabetes. 2004;53(suppl)(2):A473. [Google Scholar]
- 40.American Diabetes Association. Resource Guide: Insulin. Diabetes Forecast. 2006;(suppl):RG14–RG20. [Google Scholar]
- 41. Humulin® U Ultralente® and Humulin® L Lente® Insulins will no longer be available. Internet. 2005; Available at: http://www.lillydiabetes.com/pdf/Lente_Info_Sheet.pdf. Accessed October 27, 2006.
- 42.Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142–2148. doi: 10.2337/diabetes.49.12.2142. [DOI] [PubMed] [Google Scholar]
- 43.Rosenstock J, Schwartz SL, Clark CMJ, Park GD, Donley DW, Edwards MB. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24:631–636. doi: 10.2337/diacare.24.4.631. [DOI] [PubMed] [Google Scholar]
- 44.Bullano MF, Al-Zakwani IS, Fisher MD, Menditto L, Willey VJ. Differences in hypoglycemia event rates and associated cost-consequence in patients initiated on long-acting and intermediate-acting insulin products. Curr Med Res Opin. 2005;21:291–298. doi: 10.1185/030079905X26234. [DOI] [PubMed] [Google Scholar]
- 45.Leahy JL. Intensive insulin therapy in type 1 diabetes mellitus. In: Leahy JL, Cefalu WT, editors. Insulin Therapy. New York, NY: Marcel Dekker, Inc.; 2002. pp. 87–112. [Google Scholar]
- 46.Campbell RK, White JR, Levien T, Baker D. Insulin glargine. Clin Ther. 2001;23:1938–1957. doi: 10.1016/s0149-2918(01)80148-x. [DOI] [PubMed] [Google Scholar]
- 47.Pieber TR, Plank J, Goerzer E, et al. Duration of action, pharmacodynamic profile and between-subject variability of insulin detemir in subjects with type 1 diabetes [abstract] Diabetes. 2003;51(suppl 2):A53. [Google Scholar]
- 48.Heinemann L, Sinha K, Weyer C, Loftager M, Hirschberger S, Heise T. Time-action profile of the soluble, fatty acid acylated, long-acting insulin analogue NN304. Diabet Med. 1999;16:332–338. doi: 10.1046/j.1464-5491.1999.00081.x. [DOI] [PubMed] [Google Scholar]
- 49.Hermansen K, Madsbad S, Perrild H, Kristensen A, Axelsen M. Comparison of the soluble basal insulin analog insulin detemir with NPH insulin: a randomized open crossover trial in type 1 diabetic subjects on basal-bolus therapy. Diabetes Care. 2001;24:296–301. doi: 10.2337/diacare.24.2.296. [DOI] [PubMed] [Google Scholar]
- 50.Raslova K, Bogoev M, Raz I, Leth G, Gall MA, Hancu N. Insulin detemir and insulin aspart: a promising basal-bolus regimen for type 2 diabetes. Diabetes Res Clin Pract. 2004;66:193–201. doi: 10.1016/j.diabres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Hermansen K, Davies M, Derezinski T, Martinez RG, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29:1269–1274. doi: 10.2337/dc05-1365. [DOI] [PubMed] [Google Scholar]
- 52.Vague P, Selam J-L, Skeie S, et al. Insulin detemir is associated with more predictable glycemic control and reduced risk of hypoglycemia than NPH insulin in patients with type 1 diabetes on a basal-bolus regimen with premeal insulin aspart. Diabetes Care. 2003;26:590–596. doi: 10.2337/diacare.26.3.590. [DOI] [PubMed] [Google Scholar]
- 53.Kurtzhals P, Havelund S, Jonassen I, Markussen J. Effect of fatty acids and selected drugs on the albumin binding of a long-acting, acylated insulin analogue. J Pharm Sci. 1997;86:1365–1368. doi: 10.1021/js9701768. [DOI] [PubMed] [Google Scholar]
- 54.BIRDSONG trial. Nederlands trial register: a comparison of once daily insulin detemir given pre-breakfast or bedtime, according to need, with bedtime insulin glargine in people with type 2 diabetes characterized by an asymmetric insulin requirement across the day and night. Available at: http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=585. Accessed April 26, 2006.
- 55.Abraira C, Colwell JA, Nuttall FQ, et al. Veterans Affairs Cooperative Study on glycemic control and complications in type II diabetes (VA CSDM): results of the feasibility trial. Diabetes Care. 1995;18:1113–1123. doi: 10.2337/diacare.18.8.1113. [DOI] [PubMed] [Google Scholar]
- 56.Johnson JL, Wolf SL, Kabadi UM. Efficacy of insulin and sulfonylurea combination therapy in type II diabetes. A meta-analysis of the randomized placebo-controlled trials. Arch Intern Med. 1996;156:259–264. [PubMed] [Google Scholar]
- 57.Avilés-Santa L, Sinding J, Raskin P. Effects of metformin in patients with poorly controlled, insulin-treated type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;131:182–188. doi: 10.7326/0003-4819-131-3-199908030-00004. [DOI] [PubMed] [Google Scholar]
- 58.Coniff RF, Shapiro JA, Seaton TB, Hoogwerf BJ, Hunt JA. A double-blind placebo-controlled trial evaluating the safety and efficacy of acarbose for the treatment of patients with insulin-requiring type II diabetes. Diabetes Care. 1995;18:928–932. doi: 10.2337/diacare.18.7.928. [DOI] [PubMed] [Google Scholar]
- 59.Kelley DE, Bidot P, Freedman Z, et al. Efficacy and safety of acarbose in insulin-treated patients with type 2 diabetes. Diabetes Care. 1998;21:2056–2061. doi: 10.2337/diacare.21.12.2056. [DOI] [PubMed] [Google Scholar]
- 60.Raskin P, Rendell M, Riddle MC, Dole JF, Freed MI, Rosenstock J. A randomized trial of rosiglitazone therapy in patients with inadequately controlled insulin-treated type 2 diabetes. Diabetes Care. 2001;24:1226–1232. doi: 10.2337/diacare.24.7.1226. [DOI] [PubMed] [Google Scholar]
- 61.Reaven GM, Hollenbeck C, Jeng C-Y, Wu MS, Chen YD. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988;37:1020–1024. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- 62.Fritsche A, Schweitzer MA, Häring H-U. Glimepiride combined with morning insulin glargine, bedtime neutral protamine Hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2003;138:952–959. doi: 10.7326/0003-4819-138-12-200306170-00006. [DOI] [PubMed] [Google Scholar]
- 63.Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care. 1999;22:920–924. doi: 10.2337/diacare.22.6.920. [DOI] [PubMed] [Google Scholar]
- 64.Prandin [package insert] Princeton, NJ: Novo Nordisk Pharmaceuticals Inc; 2003. [Google Scholar]
- 65.Starlix [prescribing information] East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2004. [Google Scholar]
- 66.Glucophage/Glucophage XR [package insert] Princeton, NJ: Bristol-Myers Squibb Company; 2004. [Google Scholar]
- 67.Riomet [package insert] Jacksonville: Fla: Ranbaxy Pharmaceuticals Inc.; 2003. [Google Scholar]


