Abstract
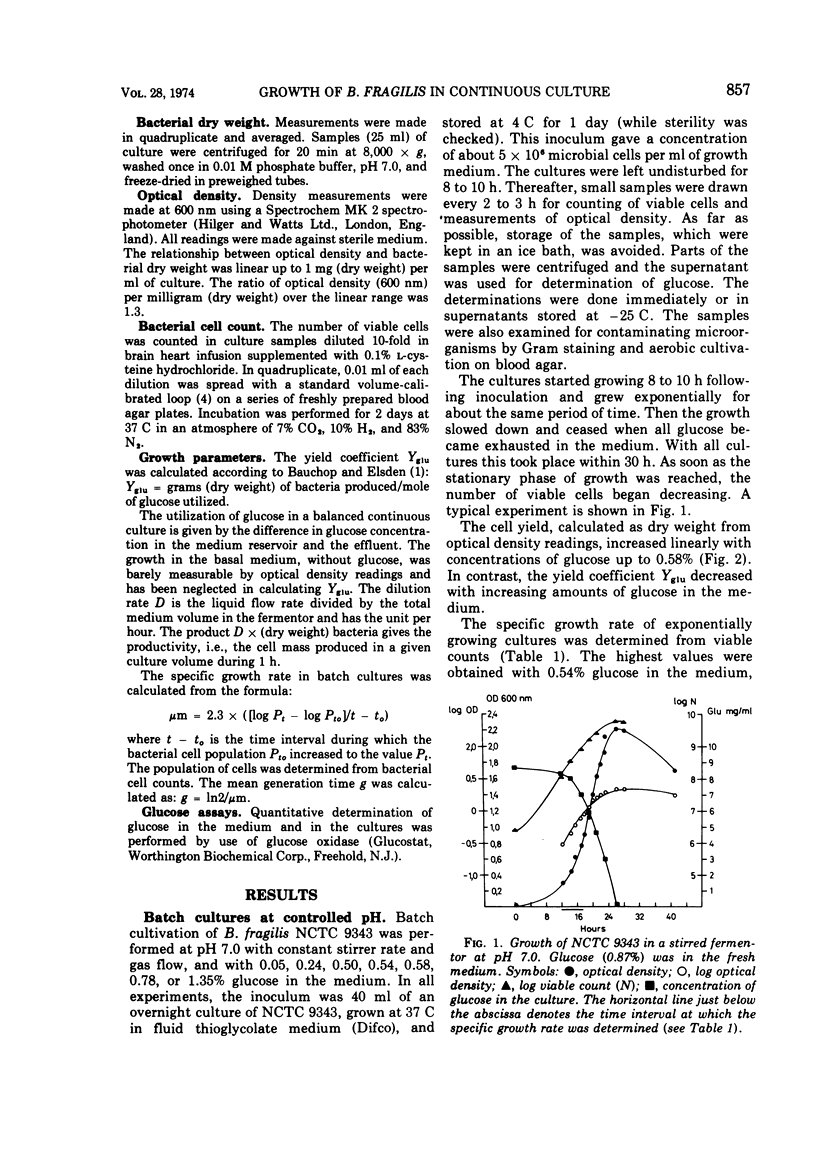
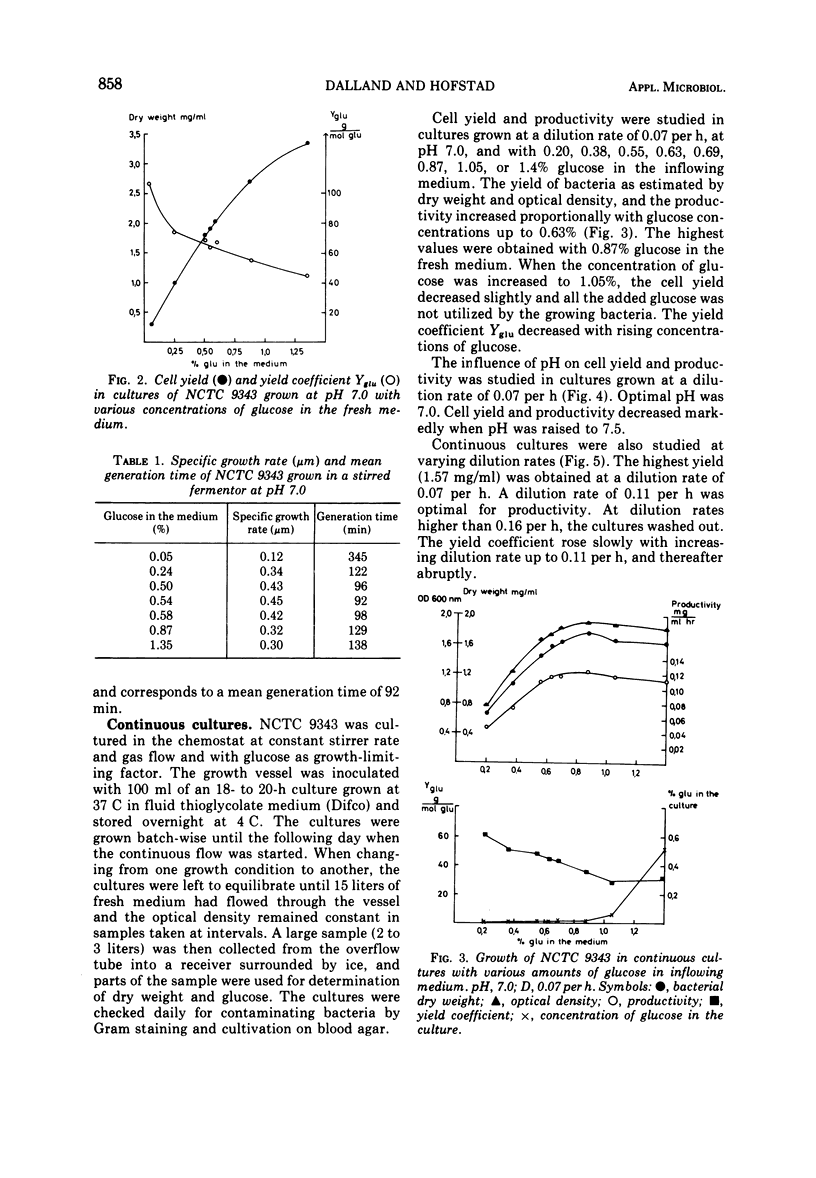
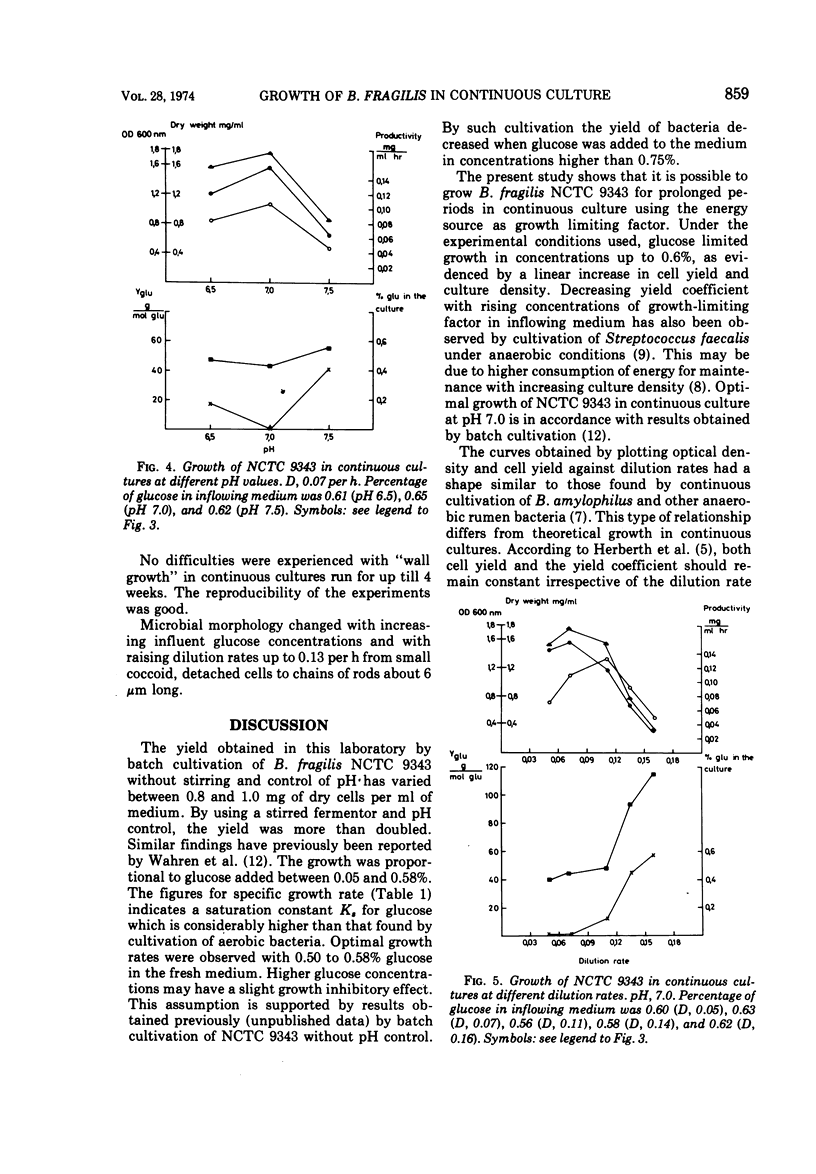
Bacteroides fragilis NCTC 9343 has been grown in continuous cultures with glucose as growth-limiting factor. At pH 7.0 and at a dilution rate of 0.07 per h, glucose limited growth in concentrations up to 0.6%. Maximal cell yield and productivity were obtained with 0.87% glucose in the inflowing medium. A pH of 7.0 was optimal for growth. With 0.6% glucose in the fresh medium and at pH 7.0, cell yield and productivity were highest at a dilution rate of 0.07 per h and 0.11 per h, respectively. At dilution rates higher than 0.07 per h, glucose was no longer growth limiting, and at dilution rates above 0.11 per h, another compound seemed to have replaced glucose also as energy source. When grown in batch cultures at pH 7.0, the best yields of B. fragilis was achieved with 0.6% glucose in the fresh medium. The highest specific growth rate (μm) determined from viable counts was 0.45, corresponding to a mean generation time of 92 min.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- Carter B. L., Bull A. T., Rowley B. I., Pirt S. J. Relationship between energy substrate utilization and specific growth rate in Aspergillus nidulans. J Bacteriol. 1971 Oct;108(1):309–313. doi: 10.1128/jb.108.1.309-313.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBERT D., ELSWORTH R., TELLING R. C. The continuous culture of bacteria; a theoretical and experimental study. J Gen Microbiol. 1956 Jul;14(3):601–622. doi: 10.1099/00221287-14-3-601. [DOI] [PubMed] [Google Scholar]
- HOBSON P. N. CONTINUOUS CULTURE OF SOME ANEROBIC AND FACULTATIVELY ANAEROBIC RUMEN BACTERIA. J Gen Microbiol. 1965 Feb;38:167–180. doi: 10.1099/00221287-38-2-167. [DOI] [PubMed] [Google Scholar]
- Haugen J., Strom O., Ostervold B. Bacterial counts in urine. 1. The reliability of the loop technique. Acta Pathol Microbiol Scand. 1968;74(3):391–396. [PubMed] [Google Scholar]
- Hobson P. N., Summers R. The continuous culture of anaerobic bacteria. J Gen Microbiol. 1967 Apr;47(1):53–65. doi: 10.1099/00221287-47-1-53. [DOI] [PubMed] [Google Scholar]
- Pirt S. J. The maintenance energy of bacteria in growing cultures. Proc R Soc Lond B Biol Sci. 1965 Oct 12;163(991):224–231. doi: 10.1098/rspb.1965.0069. [DOI] [PubMed] [Google Scholar]
- ROSENBERGER R. F., ELSDEN S. R. The yields of Streptococcus faecalis grown in continuous culture. J Gen Microbiol. 1960 Jun;22:726–739. doi: 10.1099/00221287-22-3-726. [DOI] [PubMed] [Google Scholar]
- SCHULZE K. L., LIPE R. S. RELATIONSHIP BETWEEN SUBSTRATE CONCENTRATION, GROWTH RATE, AND RESPIRATION RATE OF ESCHERICHIA COLI IN CONTINUOUS CULTURE. Arch Mikrobiol. 1964 Apr 2;48:1–20. doi: 10.1007/BF00406595. [DOI] [PubMed] [Google Scholar]
- Tempest D. W., Hunter J. R., Sykes J. Magnesium-limited growth of Aerobacter aerogenes in a chemostat. J Gen Microbiol. 1965 Jun;39(3):355–366. doi: 10.1099/00221287-39-3-355. [DOI] [PubMed] [Google Scholar]
- Wahren A., Berholm K., Holme T. Formation of proteolytic activity in continuous culture of Sphaerophorus necrophorus. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(3):391–398. doi: 10.1111/j.1699-0463.1971.tb00078.x. [DOI] [PubMed] [Google Scholar]
- Wahren A., Holme T. Growth of Bacteroidaceae in stirred fermentors. Appl Microbiol. 1969 Aug;18(2):235–239. doi: 10.1128/am.18.2.235-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


